Abstract
The kidneys play a vital role in the excretion of waste products and the regulation of electrolytes, maintenance of acid–base balance, regulation of blood pressure, and production of several hormones. Any alteration in the structure of the nephron (basic functional unit of the kidney) can have a major impact on the kidney’s ability to work efficiently. Progressive decline in kidney function can lead to serious illness and ultimately death if not treated by dialysis or transplantation. While there have been numerous studies that implicate lower nephron numbers as being an important factor in influencing susceptibility to developing hypertension and chronic kidney disease, a direct association has been difficult to establish because of three main limitations: 1) the large variation in nephron number observed in the human population; 2) no established reliable noninvasive methods to determine nephron complement; and 3) to date, nephron measurements have been done after death, which doesn’t adequately account for potential loss of nephrons with age or disease. In this review, we will provide an overview of kidney structure/function, discuss the current literature for both humans and other species linking nephron deficiency and cardio-renal complications, as well as describe the major molecular signaling factors involved in nephrogenesis that modulate variation in nephron number. As more detailed knowledge about the molecular determinants of nephron development and the role of nephron endowment in the cardio-renal system is obtained, it will hopefully provide clinicians the ability to accurately identify people at risk to develop CKD/hypertension and lead to a shift in patient care from disease treatment to prevention.
Keywords: blood pressure, kidney injury, renal hemodynamics, review, rodent
Function of the Kidney and Nephron
the basic function of the kidneys is to filter blood, reabsorb what is needed, and excrete what is not. The kidneys play a vital role in regulation of electrolytes, maintenance of acid–base balance, regulation of blood pressure (salt and water balance), and production of several hormones including calcitriol (active metabolite of vitamin D), erythropoietin (involved in red blood cell production), and renin (blood pressure control) (61). The kidney is divided into two major structures, the renal cortex and renal medulla, both containing segments of the nephron, the main functional unit of the kidney (Fig. 1). Each nephron contains a tuft of glomerular capillaries (that compose the filtration barrier via three structures: fenestrated endothelium, basement membrane, and podocytes), through which large amounts of fluid are filtered from the blood, and a long tubule (composed of distinct tubule sections) where the filtered fluid is converted into urine on its way to the renal pelvis and subsequently to the bladder (77). Kidney function is measured by glomerular filtration rate (GFR), which is defined as the volume of fluid filtered through the glomerular capillaries per unit time. Traumatic injury to the kidney can compromise renal function and lead to acute kidney injury or more subtle damage to structures of the nephron over time can lead to chronic kidney disease (CKD) or other cardiovascular complications (e.g., hypertension, cardiac hypertrophy, etc.) (59).
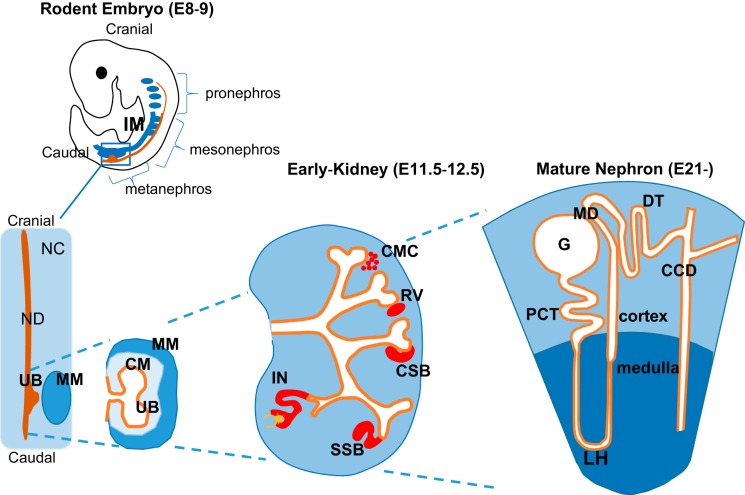
Fig. 1.
Overview of kidney organogenesis and schematic of mature nephron. During mammalian embryonic development, there are 3 paired renal organs that develop: the pronephros, the mesonephros, and the metanephros. The pronephros develop in the cervical region of embryo (cranial) and develop toward the caudal end of the intermediate mesoderm and eventually join/form the pronephric duct. The pronephric duct proceeds in a cranial-to-caudal direction. As it elongates caudally, the pronephric duct, through interaction with intermediate mesoderm (IM) form mesonephric tubules, which subsequently become the mesonephric duct (ND). The remaining IM are composed of a mesenchymal cell population called the nephrogenic cord (NC). The ND develops an outgrowth, known as the ureteric bud (UB), which invades the metanephric mesenchyme (MM), extends, and undergoes a series of branching due to reciprocal signals (Fig. 3). Around the tips of the ureteric bud tree forms the cap mesenchyme (CM), resulting from condensation of the MM. As the MM aggregates at the edge of the UB, it undergoes a mesenchymal-to-epithelial transformation and subsequently forms the renal vesicles (RV), followed by formation of comma-shaped bodies (CSB), S-shaped bodies (SSB), and finally the immature nephron (IN). Three segments emerge from the S-shaped body: the proximal segment differentiates into the glomerulus (G) and glomerular epithelial cells (podocytes); the midsection forms the proximal tubule (PCT) and loop of Henle (LH); and the distal segment becomes the distal tubule (DT). Several DT merge to form the cortical collecting duct (CCD). E, embryonic day.
Overview of Kidney and Nephron Development
The kidney has served as a model organ for developmental studies for over 40 yr, partly because of the relative uniformity with which it develops (branching morphogenesis) as well as the fact that it can be studied in culture (137). The kidney originates from a reciprocal interaction between distinct cell types within the intermediate mesoderm (IM) of which arise three successive pairs of renal structures (pronephros, mesonephros, and metanephros). These structures develop in an anterior to posterior direction (Fig. 1) (138). The ventral IM remains as a mesenchymal cell population called the nephrogenic cord (NC). The pronephros are rudimentary/transient structures that are nonfunctional in humans (but do function in lower animals). The pronephros develop from a group of cells in the IM that undergo a mesenchymal to epithelial transition [gestation day (GD) 28–35]. The epithelial cells arrange themselves in a series of tube-like structures that fuse to become the pronephric duct. After the pronephros atrophy/regress, the mesonephric duct or Wolffian duct is what remains and continues to grow until it joins the cloaca (75). Mesonephros develop by the formation of mesonephric tubules from the IM. The mesonephros becomes the main excretory structures (GD28-63) until the development of the permanent kidney. Each mesonephric tubule receives a blood supply by surrounding a capillary tuft. These structures are similar to the glomerulus of the fully developed nephron (32, 75). As development progresses, the more caudal mesonephros tubules differentiate as more cranial ones regress. The fate of the mesonephros (as the permanent kidney develops) is to become part of the epididymis, vas deferens, and seminal vesicles in males. In females, the mesonephros atrophies, leaving behind only remnants in the adult that partly compose the suspensory ligament of the ovary (160).
The development of the metanephros or definitive kidney also originates in the IM. The metanephros begins to develop around GD35 [or embryonic day (E)9.5–11.5 in rodents] as the mesonephros begins to regress (39). The metanephros begin functioning around GD63 (around the 9th week). Between the nephric duct (ND) and a specialized region of the NC, an epithelial outgrowth develops called the ureteric bud (UB). The UB invades the adjacent metanephric mesenchyme (MM) and forms the metanephric kidney (Figs. 1, 2) (29). The MM contains progenitor cells that will later form the epithelia of the metanephric nephrons and produce the inductive signals that promote and position the outgrowth of the UB from the ND (21, 82). As the MM aggregates at the edge of the UB, it undergoes a mesenchymal-to-epithelial transformation (MET) and subsequently forms the renal vesicles, followed by formation of comma- and S-shaped bodies (Fig. 1) (37, 121). Three segments emerge from the S-shaped body: the proximal segment differentiates into glomerular epithelial cells (podocytes); the midsection forms the proximal tubule and loop of Henle; and the distal segment becomes the distal tubule (37, 121). In total, these regions compose the mature nephron (Fig. 1).
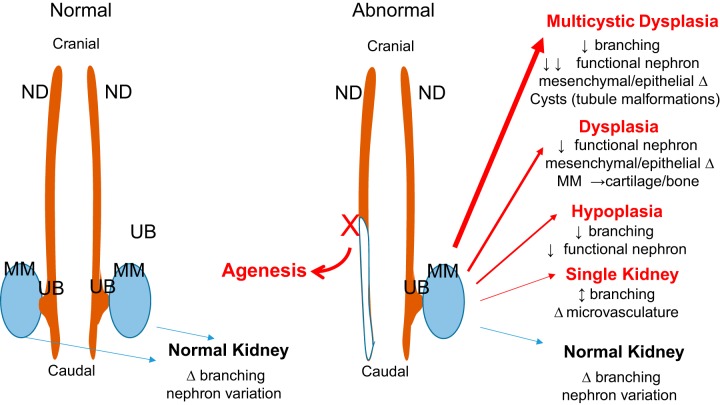
Fig. 2.
Macroview of normal and abnormal kidney development. Normal development of the kidneys involve bilateral extension of the pronephric/mesonephric duct (ND) proceeding in a cranial-to-caudal direction. Ultimately, the ureteric bud (UB) grows out from ND, invades the metanephric mesenchyme (MM), and undergoes a series of branching (as described in detail in Fig. 1). Slight changes in the timing, expression, and function of genes/proteins likely account for the observed natural variation in branching and nephron number. For conditions of abnormal development, such as failure of a kidney to develop and/or other congenital defects including ipsilateral urogenital tissues, such as vas deferens, seminal vesicle and epididymis, or uterine horn (Mullerian duct) result from a truncation of the ND. Likewise, more profound alterations in timing, expression, and function of kidney genes/proteins could lead to a variety of congenital kidney defects.
Normal Variation of Nephron Number in Humans and Animals
In humans, nephron development (nephrogenesis) occurs during the embryonic period, and thus total nephron endowment is set at birth (66). Several research groups have attempted to accurately determine the average number of nephrons that compose the human kidney. The first study to utilize an unbiased stereology method to estimate nephron numbers was Nyengaard and Bendtsen (110). In this study, a fourfold difference in nephron number (331,000–1,424,000) was observed. Subsequently, Keller et al. (79) analyzed 20 human kidneys at autopsy and observed that nephron number ranged from 531,140 to 1,959,914, also a ~4-fold difference. Variation in nephron number was confirmed by Bertram’s group (68), which found that nephron complement ranged from 210,332 to 2,026,541 (average of 884,064) or ~10-fold variation in nephron number. Unfortunately, a major limitation of these studies is that measurements were performed at autopsy and likely didn’t capture the impact of important clinical parameters (chronic hypertension, diabetes, etc.) that could explain low nephron numbers (i.e., no way to discern whether low nephron number was from birth or significant number of nephron was lost due to chronic disease). Currently, there are a number of laboratories working toward developing efficient noninvasive methods for quantitation of nephron numbers (8, 15, 117, 161). For example, Beeman et al. (15) used magnetic resonance imaging technique along with injection of cationic ferritin to measure nephron numbers. This method yielded similar results as stereology and acid maceration measurements. The major advantage is that it is nondestructive, with the ability to measure glomeruli throughout the entire kidney. However, a concern is the potential toxicity of cationic ferritin as well as the ability to label only functioning glomeruli. These types of studies, with continued improvement, could potentially provide more definite measure of nephron number across healthy individuals.
There are estimates of nephron numbers determined in several animal models, including mice, rats, primates, pigs, and sheep; however, the literature is relatively sparse. Nephron complement per kidney ranges from 9,000 to 18,000 in mice (19, 30, 150) and from 13,000 to 26,000 in rats (45, 169). Studies in baboons have shown that nephron number range from 138,078 to 427,471 (57, 58). In pigs, total nephron complement varies from 1,624,672 to 4,613,980 or a ~3-fold difference (91, 163). A fourfold difference in nephron complement is observed in sheep (200,000–800,000) (7, 49, 177). In summary, it appears that normal variation in nephron number is a common finding between species, while the degree of variation appears to be greatest in humans.
Impact of Low Nephron Number on Cardio-Renal Function: Living with a Single Kidney
The most striking example of low nephron numbers is living with a single kidney. There are several reasons why an individual may have a single kidney, including: 1) a kidney was donated to a person requiring a kidney transplant; 2) a kidney was removed due to an acute injury (e.g., physical trauma) or a disease, such as cancer; or 3) an individual developed with only one kidney (109). The health outcomes across these groups provide an opportunity to understand the potential consequences of reduced nephron endowment on health and disease. In general, the data discussed below suggest that health outcomes vary, depending upon whether the single kidney is acquired or congenital and susceptibility to other diseases.
Acquired solitary kidney: unilateral nephrectomy.
The first reported therapeutic unilateral nephrectomy (surgical removal of one kidney) was performed in 1869 (145), and the first living kidney transplant was performed in 1954 (97). The medical benefits of unilateral nephrectomy as a cure for trauma and cancer are unquestionable (90, 153). However, for some in the medical community, the long-term outcome for living kidney donors remains a topic of concern despite a number of international surveys that report extremely low mortality of living kidney donors (0.02–0.04%) (51, 60, 94, 100). In support of these findings, a number of clinical studies have confirmed the safety of living kidney donation in adults (46, 72, 123), with some studies even demonstrating a higher life expectancy for donors compared with the general population (47). This apparent benefit of nephrectomy in donors may be related to their positive selection for low risk to develop chronic diseases (e.g., hypertension and diabetes), overall high nephron complement (in the remaining kidney), as well as careful follow-up after surgery (2, 87). In contrast, some reports have described the development of proteinuria (measure of renal injury) and hypertension after donation (135, 175), but these patients usually do not progress to end-stage renal disease (Table 1) (33, 120, 124). A recent study by Grams et al. (53) estimated that the 15 yr observed risks after donation among kidney donors were 3.5–5.3 times as high as the projected risks in the absence of donation. Similar to nephrectomy in adults, some studies that have evaluated uninephrectomy in children (43, 143) have found no significant difference in long-term outcome, while other studies identified increased risk of developing proteinuria and impaired renal function (Table 1). In summary, based on current studies, any significant negative outcomes for living kidney donors appear limited, assuming that donors are healthy and not susceptible to chronic diseases.
Table 1.
Cardio-renal implications of living with single kidney
| Proteinuria | Hypertension | Renal Impairment | |
|---|---|---|---|
| Congenital solitary kidney | |||
| Argueso et al. (5) | ++ | ++ | ++ |
| Oldrizzi et al. (112) | +++++ | ++++ | ++ |
| Kolvek et al. (83) | ++++ | ++ | ++ |
| Vu et al. (164) | + | − | − |
| Nephrectomized in childhood | |||
| Robitaille et al. (124) | + | − | − |
| Wikstad et al. (175) | + | + | − |
| Argueso et al. (4) | +++ | ++ | +++ |
| Baudoin et al. (13) | +++ | +++++ | + |
| Hegde and Coulthard (64) | + | − | − |
| Dursun et al. (43) | N/A | ++++ | + |
| Kidney donors | |||
| Fehrman-Ekholm et al. (47) | ++ | ++++ | ++ |
| Fehrman-Ekholm et al. (46) | ++ | ++++ | − |
| Talseth et al. (155) | +++++ | ++ | ++ |
| Boudville et al. (20) | N/A | + | N/A |
| Ramcharan and Matas (119) | − | − | + |
| Muzaale et al. (104) | − | − | + |
Renal impairment is based on elevated serum creatinine, decreased creatinine clearance, or formal glomerular filtration rate (GFR) measurement. +, 5–10%; ++, 11–20%; +++, 21–30%; ++++, 31–40%; +++++, >40% patients exhibiting given phenotype; N/A, no measurements were performed; −, no change between control and experimental group.
Congenital solitary kidney: born with a single kidney.
On occasion, some individuals can develop with only one kidney. This condition is commonly referred to as unilateral renal agenesis or congenital solitary kidney (CSK). In more extreme cases, it is possible that both kidneys do not develop (bilateral renal agenesis), which ultimately proves fatal. A number of malformations of the kidney are also possible, including hypoplasia, dysplasia, and multicystic dysplasia (Fig. 2). Due to common developmental pathways between kidneys and other urogenital organs, defects in the ureter [e.g., grossly dilated (megaureter)], ureter obstructions (ureterovesical junction obstruction), and/or other genital defects (absence of components of sex organs) frequently coexist with kidney malformations (147, 148). In total, these types of developmental defects are commonly referred to as congenital anomalies of the kidney and urinary tract (CAKUT) and collectively occur in 1:500 births (125).
Of particular interest are CSK individuals, because in the long term they exhibit a poorer prognosis compared with more benign forms of the CAKUT (80, 133, 178). Argueso et al. (4) reported the incidence of CSK at ~1:1,000 births, with similar incidence between males and females. This estimate is supported by several large studies that observed an incidence of CSK of ~0.15% (9, 98, 174, 182). In the short term, most CSK children usually demonstrate no clinical symptoms unless they have accompanying urinary tract malformation that require intervention. However, there are some inconsistent findings when these individuals are followed with age. Some authors report that CSK is more or less a harmless congenital malformation (164, 176), whereas other studies demonstrate that ∼30% of CSK adults require dialysis by the age of 40 yr (133). In total, 25–30% of people exhibiting CSK develop proteinuria and hypertension, and 13% experience renal insufficiency (Table 1) (1, 65).
A comparison between CSK, childhood uninephrectomy, and kidney donation individuals appears to show variation between the onset and/or progression of hypertension and impaired renal function. This variation may be due, in part, to inadequate long-term follow-up, limitation in population size, and/or the consideration of the impact of confounding factors (“second hit”), including genetic susceptibility and environmental influences. However, it does seem clear from the clinical data that there may be an important difference between developing with a single kidney and being born with two kidneys and undergoing a nephrectomy (i.e., loss/removal of kidney), as early in utero compensation or alterations in kidney development in the CSK individuals may lead to increased risk for renal impairment later in life.
Impact of Low Nephron Number on Cardio-Renal Function: Two-Kidney Individuals
Nephron number and predisposition to hypertension.
The large natural variation in nephron numbers between typical two-kidney individuals leads to an important question, “how many nephrons are needed to maintain normal kidney function?” Or conversely, “is there a minimum number or threshold at which low nephron numbers would cause a predisposition to disease?” Unfortunately, there are no definitive answers to these questions, mainly due to the inability to measure nephron numbers accurately (especially by minimally invasive techniques) as well as to account for nephron loss with age or through confounding factors, such as hypertension and CKD. The first study to examine the impact between nephron number and blood pressure was proposed almost three decades ago. In 1988, Brenner and colleagues (22) identified an association between nephron number and blood pressure in humans and hypothesized that any reduction in nephron number would be accompanied by glomerular hyperfiltration, glomerular enlargement, and culminate in systemic hypertension. This hypothesis has been well documented in different experimental animal models and humans by investigating loss of nephrons (via renal ablation and/or nephrectomy) on renal hemodynamics, blood pressure, and/or renal function measures (56, 89, 169).
More recently, another study demonstrated an inverse relationship between nephron number and blood pressure based on a German population. For normotensive individuals, the mean nephron number was ~1,429,200, whereas in hypertensive individuals, the mean nephron number was half at ~702,379 (17). This relationship has not been confirmed in African Americans (71). For Hispanic Americans and Caucasians, limited studies suggest that nephron number in hypertensive people is on average 250,000 less than in those without hypertension (69). Interestingly, a recent study in mice using blood pressure high 2 (BPH2) and their normotensive (BPN3) or low blood pressure control (BPL1) identified a significant correlation (R2 = 0.779; P < 0.0001) between nephron number and systolic blood pressure (34).
Nephron numbers and predisposition to CKD.
In contrast to hypertension, there have been relatively few direct studies that have examined the relationship between nephron number and renal pathology in humans. Similar to the association to blood pressure, an inverse association has been observed between nephron number and percent of injured glomeruli (glomerulosclerosis), although the relationship was not significant (140). In another study of 140 US individuals, a significant inverse association between nephron number and glomerulosclerosis and intimal thickening in interlobular arteries was observed (36). A direct correlation between nephron number and birth weight in both white and African Americans has also been identified (70). Using birth weight as a surrogate measure of nephron number, some studies have found that low birth weight or prematurity is associated with various measures of CKD (62) including microalbuminuria (173), reduced GFR (78), and end-stage renal disease (85).
Nephron number and cardio-renal disease: insights from animal studies.
In general, animal models have provided more direct insight into the association between nephron number and cardio-renal disease. For example, a number of mice models have been used to investigate the consequences of low nephron number. There is at least one study using a premature mouse model to investigate the association between nephron number and hypertension. The study found that mice born 2 days premature exhibited 24% fewer nephrons and subsequently developed hypertension, albuminuria, and decreased GFR compared with full-term mice (150). A number of genetically modified knockout mice models have been identified with altered nephron numbers. For example, knockout of fibroblast growth factor receptor 2 (fgfr2) leads to a 24% decrease in nephron number. By 1 yr of age, mutant mice exhibit a significant increase in systolic blood pressure and more glomerular/tubular injury vs. controls (116). The loss of one GDNF allele results in a 30% reduction in nephron number. Aged GDNF heterozygous mice present with elevated arterial pressure, glomerular hypertrophy, and hyperfiltration compared with wild-type mice (30). In contrast, transforming growth factor-β2 heterozygous [Tgfb2(+/−)] mice exhibit 30% more nephrons compared with wild-type control. Heterozygous mice exhibit lower blood pressure and mean glomerular volume compared with wild-type animals (166).
Rat models have also been utilized to investigate the association between nephron number and hypertension and renal function impairment. The Munich Wistar Frömter (MWF) rat demonstrates less nephron endowment compared with the spontaneously hypertensive rat (SHR). The transfer of region of SHR genome on rat chromosome 6 to MWF background (congenic strain) led to increased nephron number and reduced albuminuria (142). This study also provided evidence that there are genetic factors involved in nephrogenesis on RNO6, as well as a link between nephron number and renal injury. The unilateral urogenital anomalies (UUA) rat is a model of CSK that was derived from a Wistar stock. UUA animals born with CSK demonstrate 1.2- fold more nephrons in the remaining kidney, but an overall reduction of nephron numbers due to the loss of one kidney. At 50 wk of age, CSK rats have significantly higher serum creatinine and albuminuria compared with two-kidney littermates (3). Recently, we developed a new model of CSK, the HSRA (heterogeneous stock-derived model of unilateral renal agenesis) rat, which exhibits nephron deficiency (~20% fewer nephrons) in the remaining kidney, and progressive kidney injury and decline in renal function with age. The extent of injury and decline in renal function in the HSRA-S (born with single kidney) is significantly more than observed in uninephrectomized two-kidney (HSRA-UNX) and two-kidney control (HSRA-C) littermates (169).
Aside from rodent models used to study nephron number and cardio-renal disease, sheep models have also been employed. Fetal nephrectomy in the sheep resulted in the remaining kidney exhibiting a 45% increase in nephron endowment, but overall a 30% reduction compared with a sham-operated fetus with two kidneys (35). At 6 and 12 mo of age, fetal uninephrectomized sheep develop hypertension and decreased GFR vs. sham-operated animals (86, 102). Additionally, pregnant sheep exposed to betamethasone (corticosteroid/anti-inflammatory) exhibit a 26% reduction in nephron number in offspring. Later in life, these sheep develop higher blood pressure and lower GFR compared with unexposed sheep (18, 183).
Low nephron numbers and impact of “second hit.”
There are a number of animal studies that have investigated the impact of additional stressors (i.e., second hit) that induce hypertension, such as angiotensin II (ANG II), NG-nitro-l-arginine methyl ester (L-NAME), deoxycorticosterone acetate (DOCA), and salt-loading, in the context of reduced nephron numbers (136). In general, these studies demonstrate that renal injury and/or cardiovascular dysfunction is more severe than nephrectomy alone. ANG II used in combination with nephrectomy hastened glomerulosclerosis, proteinuria, and/or kidney function decline compared with sham animals (88). Interestingly, Tsukamoto et al. (158) showed ANG II infusion in uninephrectomized mice plus salt loading has a significant impact apart from renal injury as mice demonstrate hypertensive heart disease that leads to a heart failure phenotype.
L-NAME, a nitric oxide synthase inhibitor, combined with subtotal nephrectomy with and without salt-loading (48, 162) generated marked glomerulosclerosis, ischemic injury, interstitial expansion, and elevated creatinine compared with controls. DOCA-salt is widely utilized to induce hypertension, mainly through volume expansion. Animal studies combining DOCA-salt treatment with nephrectomy resulted in more proteinuria, kidney injury, and myocardial remodeling (6, 74). Our work with nephron-deficient HSRA-S animals demonstrated that the model is highly susceptible to develop hypertension-induced (DOCA + 1% NaCl) renal injury, which was significantly greater than injury observed in HSRA-UNX animals (168). In contrast, the induction of hypertension in two-kidney control (HSRA-C) had little to no impact on kidney injury or kidney function. The impact of salt-loading alone, along with nephrectomy at young age was studied by Carlström et al. (25). The study demonstrated that nephrectomy performed at a young age (3 wk of age) or salt-loading separately resulted in salt-sensitive hypertension. However, the combination of young nephrectomy and long-term high-salt treatment resulted in more pronounced hypertension and salt sensitivity than either alone (25).
Age itself can also be viewed as secondary stressor. A long-term study by Rodríguez-Gómez et al. (126) demonstrated that both male and female uninephrectomized rats exhibited proteinuria and increased blood pressure after 18 mo, compared with sham animals. Male uninephrectomized rats tended to have earlier and more severe lesions than female uninephrectomized rats. In total, there is strong experimental evidence to support the idea that uninephrectomy/nephron deficiency alone can cause a predisposition to progressive kidney injury, but when combined with a secondary stressor this predisposition can be significantly accelerated.
Kidney Development and Molecular Basis of Nephrogenesis
Major molecular factors involved in nephrogenesis.
There are several key developmental events and signaling cascades in renal development that have been elucidated, mostly through studies in mice (31). However, there are likely still genes and genetic factors yet to be identified as well as a lack of understanding of the interactions and coordination of these factors to generate the complex structure of a kidney (75). In general, the development of the metanephric kidney and lower urinary tract is coordinated by complex interactions among numerous transcription/growth factors and intracellular signaling molecules (29, 37). These genes can be coexpressed in the MM, stroma, angioblasts, UB, and cloaca (95, 121). However, cells at the UB tip express many genes that are not expressed by cells in the tubular portions and vice versa (139). The discussion below will focus on providing a review of important genes and networks that function at crucial stages of renal development and are also associated with CAKUT (Fig. 3, Table 2).
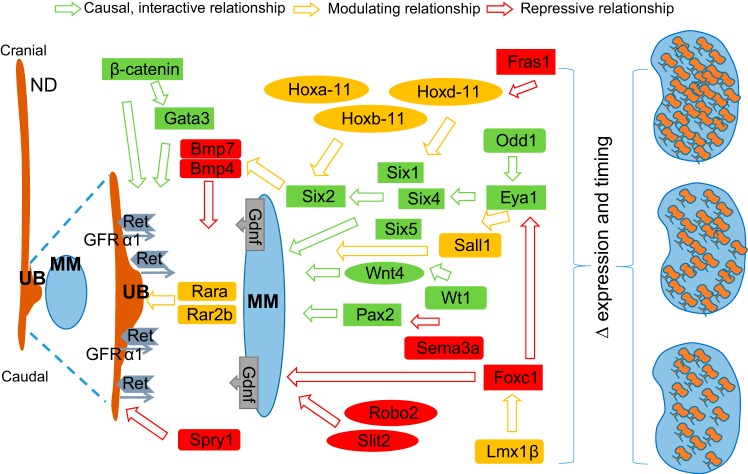
Fig. 3.
Molecular signaling involved in nephrogenesis. The development of the kidney is coordinated by the complex interactions among numerous transcription/growth factors and intracellular signaling molecules. The most important signaling pathway is GDNF-RET, which controls metanephric mesenchyme (MM) development and ureteric bud (UB) outgrowth and branching. GDNF is secreted by the MM and binds a receptor complex that includes RET and a membrane-tethered GFRA1 co-receptor located on the nephric duct (ND). GDNF signals through the RET complex to promote UB invasion of the MM. GDNF binding to the RET receptor leads to activation of signaling pathways promoting proliferation and differentiation. There are several key transcriptional regulators including Odd1, Eya1, and Six1/Six2 identified as being necessary for interaction between UB and MM. Loss of function in any of a number of genes including Pax2, Eya1, Six1, Wt1, and Hoxa11/Hoxd1 (and others) leads to a loss of Gdnf expression and either renal agenesis or renal hypoplasia. Likewise, mice knockouts of Ret or Gfra1 result in failure of the UB to form and can also result in renal agenesis. In this context, any alterations in the timing, expression, and/or function of these genes/proteins have the ability to influence nephron composition on a continuum from low to high.
Table 2.
Key genes involved in kidney development and associated with congenital abnormalities of kidney and urinary tract
| Gene | Function | Abnormalities | Reference |
|---|---|---|---|
| Gdnf, GFRα1, c-Ret | promote UB induction/branching | renal agenesis | Pichel et al., 1996; Sanchez et al., 1996 (114, 132) |
| Eya1, Six1, Six4, Six5 | induce expression and secretion of Gdnf | renal agenesis | Hoskins et al., 2007; Rodriguez-Soriano et al., 2001; Xu et al., 2003 (67, 127, 180) |
| Odd1, HoxA-11, HoxC-11, HoxD-11 | regulate Gdnf expression | renal agenesis | James et al., 2006; Patterson et al., 2001; Stricker et al., 2006 (76, 113, 151) |
| Six2 | maintain MM cells, allow continued branching | renal hypoplasia | Weber et al., 2008 (171) |
| Pax2, Sall1 | interact among UB, MM, or stroma; mesoderm differentiation | renal hypoplasia | Negrisolo et al., 2011; Weber et al., 2006 (107, 170) |
| Bmp4, Bmp7 | inhibit Wnt expression, inhibit UB branching | renal hypoplasia | Luo et al., 1995; Tabatabaeifar et al., 2009 (93, 154) |
| Fras1, Frem1 | control differentiation of stromal and mesenchymal cells | renal dysplasia | Saisawat et al., 2012 (131) |
| FoxC1, FoxC2 | DNA promoter binding to Eya1, ectopic UB budding | duplex ureters | Nakano et al., 2003 (105) |
| Robo2, Slit2 | similar to FoxC2 regulation UB budding | duplex ureters | Zu et al., 2009 (185) |
| Rara, Rar2b | upregulate c-Ret | ectopic ureter | Batourina et al., 2001 (12) |
| Spry1 | downregulate c-Ret | ectopic ureter | Rozen et al., 2009; Yosypiv et al., 2008 (129, 181) |
| WT1, Lmx1β, Sema3a | stimulate podocyte differentiation and angioblasts. | nephrotic syndrome | Chen et al., 1998; Gao et al., 2005 (26, 50) |
| REN, AGT, ACE AGTR1 | stimulate renal tubular growth | renal tubular dysgenesis | Beck et al., 2011; Mounier et al., 1987 (14, 103) |
| Wnt4 | mesenchymal signal for epithelial transformation | low nephrons | Iglesias et al., 2007 (73) |
| Hnf1β | develop renal capsule | horseshoe kidney | Nakayama et al., 2010 (106) |
| Pkd1, Pkd2 | pattern of tubular and collecting duct | polycystic kidneys | Rossetti and Harris, 2007 (128) |
The induction of the UB into the MM is directly controlled by glial-derived neurotrophic factor (Gdnf) and the tyrosine kinase receptor c-Ret, along with the co-receptor GFRα1 (27, 141). The levels and spatial expression of Gdnf play a central role in initiating budding and are regulated by multiple transcription and growth factors. Mice with inactivated Gdnf die from renal agenesis soon after birth (101). The expression of Gdnf itself is determined by a complex signaling cascade involving eyes absent homolog 1 (Eya1), and SIX homeobox (Six)1, 4, and 5 (23). Eya1 knockout mice show loss of Gdnf in the ND (179). However, Eya1 appears not to stimulate transcription directly, and the effect on Gdnf is due to the loss of expression of Six1, Six4, and Six5 in Eya1 mutant animals (111). Eya1 mutant animals also exhibit renal agenesis. Transcription factors odd skipped-related 1 (Odd1) is one of the earliest acting genes involved in metanephros formation and likely acts upstream of Eya1 in the NC to promote MM development (167). In addition to Odd1, loss of homeobox 11 (Hox11) family genes, including Hoxa-11, Hoxc-11, and Hoxd-11, leads to an arrest of MM differentiation and loss of Six and Gdnf expression (172).
Six2 is a homeodomain transcription factor expressed in the MM, which maintains MM cells in an undifferentiated state, thereby allowing continued UB branching and nephron formation to proceed (82). Paired box 2 (Pax2) is a homeobox transcription factor related to the activation of Gdnf (24). End-stage renal disease occurs in almost 100% of individuals with Pax2-associated renal hypoplasia (134). Polymorphisms in Pax2 are also associated with reduced kidney size in neonates (118). Sal-like 1 (Sall1) is expressed in the mesenchyme and recently implicated in renal hypoplasia. In Sall1-mutant animals, Gdnf and Eya1 remain expressed, indicating that Sall1 lies downstream of these signals or possibly in a separate pathway (108). Bone morphogenetic protein 4 (Bmp4) is a potent antagonist of Gdnf and plays a crucial role in inhibiting ectopic budding of the ureter (99). Homozygous Bmp4 knockout mice die early in the antenatal period but present with ectopic ureteric budding (171). Bmp7 is required for suppressing tubulogenesis and for the survival of mesenchymal cells (41). In Bmp7 knockout mice, kidneys initially form normally with appropriate UB branching, comma- and S-shaped body formation, but nephrogenesis is inhibited from E14.5 onward. Bmp7 becomes depleted in Six2 mutant kidneys (42), suggesting that Six2 lies upstream of Bmp7. Recessive mutations in human FRAS1 and FREM2 were recently detected in patients with nonsyndromic renal dysplasia. These two genes are related to extracellular matrix protein 1, which controls the differentiation of stromal and mesenchymal cells (131). In Fras1 null mutant mice, decreased FRAS1/FREM2 complex reduces the expression of transcription factors Hoxd11/Six2 and results in defective interactions between the UB and mesenchyme (115).
Additionally, studies in mice have demonstrated that genes such as forkhead box protein C (Foxc), Slit homolog 2 (Slit2), and its receptor, Roundabout homolog 2 (Robo2), confine Gdnf expression to the caudal part of the NC (84). Mutations in genes encoding these proteins lead to increased Gdnf expression to the rostral part of the embryo and can promote the outgrowth of multiple ureters (55). Mutations in Robo2 have been identified in patients with vesicoureteral junction defects and vesicoureteral reflux (16, 92). Vitamin A has also been shown to be involved in kidney signaling (52). The retinoic acid receptor-α (Rara) is expressed at low levels throughout the embryonic kidney, whereas retinoic acid receptor-β2 (Rarb) expression is restricted to stromal cells. The deletion of either Rara or Rarb does not lead to kidney defects; however, in double-null Rara and Rarb mutants, UB growth is reduced and c-Ret expression is downregulated (96). The inhibition of c-Ret expression and signaling activity is also modulated through sprouty homolog 1 (Spry1). Sprouty proteins are required for limiting branching during kidney growth (11). Mutations in Spry1 result in ectopic ureters by rendering the duct more sensitive to Gdnf levels (10).
Podocyte differentiation is regulated by transcription factors Wilms tumor 1 (WT1), LIM homeobox transcription factor 1β (Lmx1β), and semaphorin3a (Sema3a) (122, 152). Lmx1b and FoxC work together to modulate podocyte development (63). WT1 and Sema3a expression is restricted to the podocyte layer during nephron maturation as well as acting as a PAX2 repressor (130). This repression seems to be crucial since ectopic activation of PAX2 in podocytes of transgenic mice leads to severe glomerular disease (40, 165). Wingless-type integration site family 4 (Wnt4) induces MM cells to undergo MET and differentiate into nephron epithelia (149). Activation of Wnt4 seems to be controlled by a complex network of genes, including WT1 and Pax2 (144, 156). Wnt4 knockout mice almost completely lack nephrons (81).
Other important factors involved in nephrogenesis.
Hepatocyte nuclear factor 1β (Hnf1β) is involved in renal capsule formation as defects in Hnf1β lead to kidneys fused at inferior lobes and located lower than usual (commonly referred to as horseshoe kidneys) (44). Mutations in Hnf1β are detected in 33% of children with nonsyndromic multicystic dysplastic kidney or renal hypodysplasia (159). Polycystic kidney disease (PKD) is also a congenital kidney disorder, caused by cysts that arise from tubules. Pkd1 and Pkd2 represent an example of patterning defect (146). The increased tubular cell proliferation together with decreased integration of cells into the plane of tubular epithelium or loss of oriented cell division may account for cyst formation (157). The developing mammalian metanephros expresses all components of the renin–angiotensin system (29). In humans, their expression in the embryonic kidney is as early as the fifth week of gestation when metanephric organogenesis is initiated (28). Mutations in the genes encoding for AGT (angiotensinogen), REN (renin), ACE (angiotensin-converting enzyme), or AGTR1 (AT1 receptor) lead to autosomal recessive (homozygous mutation) renal tubular dysgenesis (54, 184).
Perspective: Relevance of Understanding Molecular Determinants of Nephron Development, Endowment, and Role in Cardio-Renal Disease
The kidneys play a vital physiological role by efficiently removing waste and regulating blood pressure, but its ability to do so depends heavily on events that occur during kidney organogenesis. It is clear that kidney development requires the coordinated spatiotemporal expression of genes and numerous signaling pathways that lead to the interactive induction between UB and MM, resulting in branching morphogenesis. Thus, any alterations in the timing, expression, and/or function of genes involved in this process have the ability to influence nephron composition on a continuum from low to high (Fig. 3). Through the use of animal models (particularly mice), a significant understanding of the specific gene/proteins that play a role in this process has been achieved. However, there is still a critical need to understand how epigenetics, gene expression, and proteomic networks work together (systems biology) to result in nephrogenesis/kidney development.
There is no doubt that animal models provide a more integrative view of kidney development; however, a major limitation (regardless of species) is the ability to evaluate nephron endowment noninvasively. The ability to determine nephron number accurately early in life before the impact of disease (or environmental factors) would provide the ability to establish the exact association between nephron number and cardiovascular disease. The current data certainly suggest that there is a significant inverse relationship between nephron number and cardiovascular disease (CVD) risk (Fig. 4). Individuals that exhibit high nephron endowment and low genetic susceptibility to CVD (e.g., hypertension) are less likely to develop any impairment in renal function. These individuals would also likely not exhibit any adverse effects from either losing a kidney due to injury or serving as a kidney donor. Conversely, individuals that exhibit low nephron endowment and high genetic susceptibility to CVD are most at risk of developing significant loss of renal function with age, which would likely be exacerbated with the loss or donation of a kidney (Fig. 4). In the context of CSK, an individual could still exhibit a relatively high nephron endowment, despite only having one kidney (e.g., a CSK individual could have more nephrons than a two-kidney individual), with no adverse impact on the occurrence or progression of CKD. Thus, it is not simply a matter of having one kidney (either CSK or loss due to injury or donation) or two, but rather a measure of total nephron composition and susceptibility to confounding disease factors (“second hit”) that can lead to cardio-renal disease later in life.
Fig. 4.
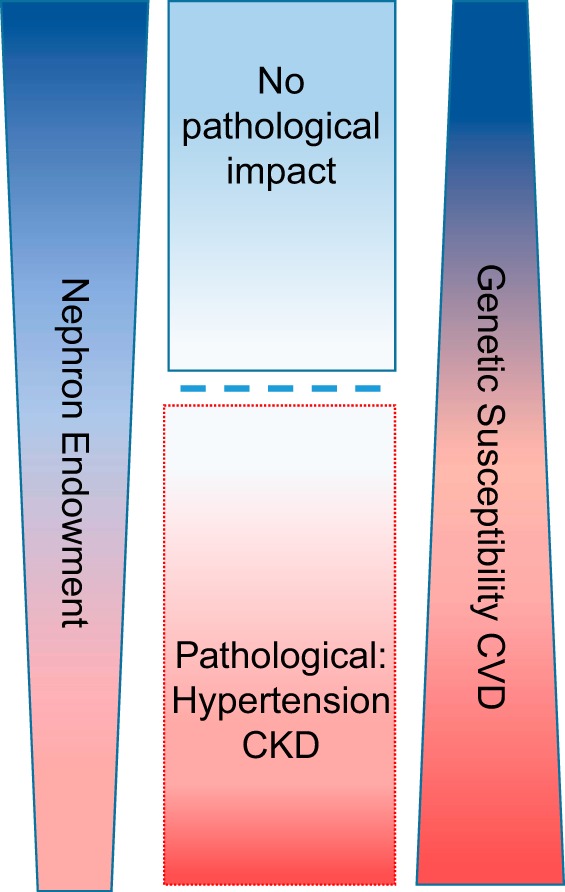
Impact of nephron number and genetic susceptibility on cardio-renal disease. Both nephron number and genetic susceptibility to cardiovascular disease (CVD) exist on a continuum from low to high. Individuals that exhibit high nephron endowment and low genetic susceptibility to CVD (e.g., hypertension) are less likely to develop any impairment in renal function, whereas individuals that exhibit low nephron endowment and high genetic susceptibility to CVD are most at risk to develop significant loss of renal function with age. There likely exists a point (threshold) at which a certain minimum number of nephrons can lead to and/or exacerbate pathological conditions, such as chronic kidney disease (CKD).
It is relatively easy to recognize the impact of low or high nephron number on CVD, but a major challenge is to identify whether there is a point or “threshold” at which normal renal function is maintained (or below a point that leads to increased risk of developing CKD and hypertension). The ability to make such determinations as well as to integrate genetic with a more extensive systems-biology understanding of the determinants of nephron development will ultimately provide clinicians the chance to identify accurately people at risk for CKD and hypertension. This, in turn, will provide physicians a powerful tool for transforming patient care, from disease treatment to preventive care.
GRANTS
M. R. Garrett is supported by National Institutes of Health (NIH) Grant HL-094446 and the Robert M. Hearin Foundation. Support was also provided through funds from the NIH, including Mississippi INBRE (P20GM-103476); Center for Psychiatric Neuroscience (CPN)-COBRE (P30GM-103328); and Obesity, Cardiorenal and Metabolic Diseases-COBRE (P20GM-104357).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
X.W. and M.R.G. prepared figures; X.W. and M.R.G. drafted manuscript; X.W. and M.R.G. edited and revised manuscript; X.W. and M.R.G. approved final version of manuscript; M.R.G. interpreted results of experiments.
REFERENCES
- 1.Abou Jaoudé P, Dubourg L, Bacchetta J, Berthiller J, Ranchin B, Cochat P. Congenital versus acquired solitary kidney: is the difference relevant? Nephrol Dial Transplant 26: 2188–2194, 2011. doi: 10.1093/ndt/gfq659. [DOI] [PubMed] [Google Scholar]
- 2.Ahmadi AR, Lafranca JA, Claessens LA, Imamdi RM, IJzermans JN, Betjes MG, Dor FJ. Shifting paradigms in eligibility criteria for live kidney donation: a systematic review. Kidney Int 87: 31–45, 2015. doi: 10.1038/ki.2014.118. [DOI] [PubMed] [Google Scholar]
- 3.Amakasu K, Suzuki K, Katayama K, Suzuki H. Age-related pathophysiological changes in rats with unilateral renal agenesis. J Vet Med Sci 73: 787–795, 2011. doi: 10.1292/jvms.10-0498. [DOI] [PubMed] [Google Scholar]
- 4.Argueso LR, Ritchey ML, Boyle ET Jr, Milliner DS, Bergstralh EJ, Kramer SA. Prognosis of children with solitary kidney after unilateral nephrectomy. J Urol 148: 747–751, 1992. [DOI] [PubMed] [Google Scholar]
- 5.Argueso LR, Ritchey ML, Boyle ET Jr, Milliner DS, Bergstralh EJ, Kramer SA. Prognosis of patients with unilateral renal agenesis. Pediatr Nephrol 6: 412–416, 1992. doi: 10.1007/BF00873996. [DOI] [PubMed] [Google Scholar]
- 6.Bae EH, Kim IJ, Joo SY, Kim EY, Kim CS, Choi JS, Ma SK, Kim SH, Lee JU, Kim SW. Renoprotective effects of sildenafil in DOCA-salt hypertensive rats. Kidney Blood Press Res 36: 248–257, 2012. doi: 10.1159/000343414. [DOI] [PubMed] [Google Scholar]
- 7.Bains RK, Sibbons PD, Murray RD, Howard CV, Van Velzen D. Stereological estimation of the absolute number of glomeruli in the kidneys of lambs. Res Vet Sci 60: 122–125, 1996. doi: 10.1016/S0034-5288(96)90005-3. [DOI] [PubMed] [Google Scholar]
- 8.Baldelomar EJ, Charlton JR, Beeman SC, Hann BD, Cullen-McEwen L, Pearl VM, Bertram JF, Wu T, Zhang M, Bennett KM. Phenotyping by magnetic resonance imaging nondestructively measures glomerular number and volume distribution in mice with and without nephron reduction. Kidney Int 89: 498–505, 2016. doi: 10.1038/ki.2015.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barakat AJ, Drougas JG. Occurrence of congenital abnormalities of kidney and urinary tract in 13,775 autopsies. Urology 38: 347–350, 1991. doi: 10.1016/0090-4295(91)80150-6. [DOI] [PubMed] [Google Scholar]
- 10.Basson MA, Akbulut S, Watson-Johnson J, Simon R, Carroll TJ, Shakya R, Gross I, Martin GR, Lufkin T, McMahon AP, Wilson PD, Costantini FD, Mason IJ, Licht JD. Sprouty1 is a critical regulator of GDNF/RET-mediated kidney induction. Dev Cell 8: 229–239, 2005. doi: 10.1016/j.devcel.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Basson MA, Watson-Johnson J, Shakya R, Akbulut S, Hyink D, Costantini FD, Wilson PD, Mason IJ, Licht JD. Branching morphogenesis of the ureteric epithelium during kidney development is coordinated by the opposing functions of GDNF and Sprouty1. Dev Biol 299: 466–477, 2006. doi: 10.1016/j.ydbio.2006.08.051. [DOI] [PubMed] [Google Scholar]
- 12.Batourina E, Gim S, Bello N, Shy M, Clagett-Dame M, Srinivas S, Costantini F, Mendelsohn C. Vitamin A controls epithelial/mesenchymal interactions through Ret expression. Nat Genet 27: 74–78, 2001. doi: 10.1038/83792. [DOI] [PubMed] [Google Scholar]
- 13.Baudoin P, Provoost AP, Molenaar JC. Renal function up to 50 years after unilateral nephrectomy in childhood. Am J Kidney Dis 21: 603–611, 1993. doi: 10.1016/S0272-6386(12)80032-1. [DOI] [PubMed] [Google Scholar]
- 14.Beck BB, Trachtman H, Gitman M, Miller I, Sayer JA, Pannes A, Baasner A, Hildebrandt F, Wolf MT. Autosomal dominant mutation in the signal peptide of renin in a kindred with anemia, hyperuricemia, and CKD. Am J Kidney Dis 58: 821–825, 2011. doi: 10.1053/j.ajkd.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beeman SC, Zhang M, Gubhaju L, Wu T, Bertram JF, Frakes DH, Cherry BR, Bennett KM. Measuring glomerular number and size in perfused kidneys using MRI. Am J Physiol Renal Physiol 300: F1454–F1457, 2011. doi: 10.1152/ajprenal.00044.2011. [DOI] [PubMed] [Google Scholar]
- 16.Bertoli-Avella AM, Conte ML, Punzo F, de Graaf BM, Lama G, La Manna A, Polito C, Grassia C, Nobili B, Rambaldi PF, Oostra BA, Perrotta S. ROBO2 gene variants are associated with familial vesicoureteral reflux. J Am Soc Nephrol 19: 825–831, 2008. doi: 10.1681/ASN.2007060692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE. Human nephron number: implications for health and disease. Pediatr Nephrol 26: 1529–1533, 2011. doi: 10.1007/s00467-011-1843-8. [DOI] [PubMed] [Google Scholar]
- 18.Bi J, Contag SA, Chen K, Su Y, Figueroa JP, Chappell MC, Rose JC. Sex-specific effect of antenatal betamethasone exposure on renal oxidative stress induced by angiotensins in adult sheep. Am J Physiol Renal Physiol 307: F1013–F1022, 2014. doi: 10.1152/ajprenal.00354.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bonvalet JP, Champion M, Courtalon A, Farman N, Vandewalle A, Wanstok F. Number of glomeruli in normal and hypertrophied kidneys of mice and guinea-pigs. J Physiol 269: 627–641, 1977. doi: 10.1113/jphysiol.1977.sp011919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boudville N, Prasad GV, Knoll G, Muirhead N, Thiessen-Philbrook H, Yang RC, Rosas-Arellano MP, Housawi A, Garg AX; Donor Nephrectomy Outcomes Research (DONOR) Network . Meta-analysis: risk for hypertension in living kidney donors. Ann Intern Med 145: 185–196, 2006. doi: 10.7326/0003-4819-145-3-200608010-00006. [DOI] [PubMed] [Google Scholar]
- 21.Boyle S, Misfeldt A, Chandler KJ, Deal KK, Southard-Smith EM, Mortlock DP, Baldwin HS, de Caestecker M. Fate mapping using Cited1-CreERT2 mice demonstrates that the cap mesenchyme contains self-renewing progenitor cells and gives rise exclusively to nephronic epithelia. Dev Biol 313: 234–245, 2008. doi: 10.1016/j.ydbio.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brenner BM, Garcia DL, Anderson S. Glomeruli and blood pressure. Less of one, more the other? Am J Hypertens 1: 335–347, 1988. doi: 10.1093/ajh/1.4.335. [DOI] [PubMed] [Google Scholar]
- 23.Brodbeck S, Englert C. Genetic determination of nephrogenesis: the Pax/Eya/Six gene network. Pediatr Nephrol 19: 249–255, 2004. doi: 10.1007/s00467-003-1374-z. [DOI] [PubMed] [Google Scholar]
- 24.Brophy PD, Ostrom L, Lang KM, Dressler GR. Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development 128: 4747–4756, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Carlström M, Sällström J, Skøtt O, Larsson E, Persson AEG. Uninephrectomy in young age or chronic salt loading causes salt-sensitive hypertension in adult rats. Hypertension 49: 1342–1350, 2007. doi: 10.1161/HYPERTENSIONAHA.107.087213. [DOI] [PubMed] [Google Scholar]
- 26.Chen H, Lun Y, Ovchinnikov D, Kokubo H, Oberg KC, Pepicelli CV, Gan L, Lee B, Johnson RL. Limb and kidney defects in Lmx1b mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat Genet 19: 51–55, 1998. doi: 10.1038/ng0598-51. [DOI] [PubMed] [Google Scholar]
- 27.Chi X, Michos O, Shakya R, Riccio P, Enomoto H, Licht JD, Asai N, Takahashi M, Ohgami N, Kato M, Mendelsohn C, Costantini F. Ret-dependent cell rearrangements in the Wolffian duct epithelium initiate ureteric bud morphogenesis. Dev Cell 17: 199–209, 2009. doi: 10.1016/j.devcel.2009.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corvol P, Schütz S, Gasc JM. Early expression of all components of the renin-angiotensin system in human development. Adv Nephrol Necker Hosp 28: 195–212, 1998. [PubMed] [Google Scholar]
- 29.Costantini F. Renal branching morphogenesis: concepts, questions, and recent advances. Differentiation 74: 402–421, 2006. doi: 10.1111/j.1432-0436.2006.00106.x. [DOI] [PubMed] [Google Scholar]
- 30.Cullen-McEwen LA, Kett MM, Dowling J, Anderson WP, Bertram JF. Nephron number, renal function, and arterial pressure in aged GDNF heterozygous mice. Hypertension 41: 335–340, 2003. doi: 10.1161/01.HYP.0000050961.70182.56. [DOI] [PubMed] [Google Scholar]
- 31.Davidson AJ. Mouse Kidney Development. Cambridge, MA: StemBook, 2008. [Google Scholar]
- 32.Davies JA, Perera AD, Walker CL. Mechanisms of epithelial development and neoplasia in the metanephric kidney. Int J Dev Biol 43: 473–478, 1999. [PubMed] [Google Scholar]
- 33.Delanaye P, Weekers L, Dubois BE, Cavalier E, Detry O, Squifflet JP, Krzesinski JM. Outcome of the living kidney donor. Nephrol Dial Transplant 27: 41–50, 2012. doi: 10.1093/ndt/gfr669. [DOI] [PubMed] [Google Scholar]
- 34.Didion SP, Wang X, Garrett MR. Direct correlation between blood pressure and nephron endowment in a genetic mouse model of hypertension (Abstract). Hypertension 68, Suppl 1: A446, 2016. [Google Scholar]
- 35.Douglas-Denton R, Moritz KM, Bertram JF, Wintour EM. Compensatory renal growth after unilateral nephrectomy in the ovine fetus. J Am Soc Nephrol 13: 406–410, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Douglas-Denton RN, McNamara BJ, Hoy WE, Hughson MD, Bertram JF. Does nephron number matter in the development of kidney disease? Ethn Dis 16, Suppl 2: S2-40–45, 2006. [PubMed] [Google Scholar]
- 37.Dressler GR. Advances in early kidney specification, development and patterning. Development 136: 3863–3874, 2009. doi: 10.1242/dev.034876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol 22: 509–529, 2006. doi: 10.1146/annurev.cellbio.22.010305.104340. [DOI] [PubMed] [Google Scholar]
- 40.Dressler GR, Wilkinson JE, Rothenpieler UW, Patterson LT, Williams-Simons L, Westphal H. Deregulation of Pax-2 expression in transgenic mice generates severe kidney abnormalities. Nature 362: 65–67, 1993. doi: 10.1038/362065a0. [DOI] [PubMed] [Google Scholar]
- 41.Dudley AT, Godin RE, Robertson EJ. Interaction between FGF and BMP signaling pathways regulates development of metanephric mesenchyme. Genes Dev 13: 1601–1613, 1999. doi: 10.1101/gad.13.12.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev 9: 2795–2807, 1995. doi: 10.1101/gad.9.22.2795. [DOI] [PubMed] [Google Scholar]
- 43.Dursun H, Bayazit AK, Cengiz N, Seydaoglu G, Buyukcelik M, Soran M, Noyan A, Anarat A. Ambulatory blood pressure monitoring and renal functions in children with a solitary kidney. Pediatr Nephrol 22: 559–564, 2007. doi: 10.1007/s00467-006-0389-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edghill EL, Bingham C, Ellard S, Hattersley AT. Mutations in hepatocyte nuclear factor-1beta and their related phenotypes. J Med Genet 43: 84–90, 2006. doi: 10.1136/jmg.2005.032854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fassi A, Sangalli F, Maffi R, Colombi F, Mohamed EI, Brenner BM, Remuzzi G, Remuzzi A. Progressive glomerular injury in the MWF rat is predicted by inborn nephron deficit. J Am Soc Nephrol 9: 1399–1406, 1998. [DOI] [PubMed] [Google Scholar]
- 46.Fehrman-Ekholm I, Dunér F, Brink B, Tydén G, Elinder CG. No evidence of accelerated loss of kidney function in living kidney donors: results from a cross-sectional follow-up. Transplantation 72: 444–449, 2001. doi: 10.1097/00007890-200108150-00015. [DOI] [PubMed] [Google Scholar]
- 47.Fehrman-Ekholm I, Elinder CG, Stenbeck M, Tydén G, Groth CG. Kidney donors live longer. Transplantation 64: 976–978, 1997. doi: 10.1097/00007890-199710150-00007. [DOI] [PubMed] [Google Scholar]
- 48.Fujihara CK, De Nucci G, Zatz R. Chronic nitric oxide synthase inhibition aggravates glomerular injury in rats with subtotal nephrectomy. J Am Soc Nephrol 5: 1498–1507, 1995. [DOI] [PubMed] [Google Scholar]
- 49.Galinsky R, Moss TJ, Gubhaju L, Hooper SB, Black MJ, Polglase GR. Effect of intra-amniotic lipopolysaccharide on nephron number in preterm fetal sheep. Am J Physiol Renal Physiol 301: F280–F285, 2011. doi: 10.1152/ajprenal.00066.2011. [DOI] [PubMed] [Google Scholar]
- 50.Gao X, Chen X, Taglienti M, Rumballe B, Little MH, Kreidberg JA. Angioblast-mesenchyme induction of early kidney development is mediated by Wt1 and Vegfa. Development 132: 5437–5449, 2005. doi: 10.1242/dev.02095. [DOI] [PubMed] [Google Scholar]
- 51.Ghods AJ. Renal transplantation in Iran. Nephrol Dial Transplant 17: 222–228, 2002. doi: 10.1093/ndt/17.2.222. [DOI] [PubMed] [Google Scholar]
- 52.Gilbert T. Vitamin A and kidney development. Nephrol Dial Transplant 17, Suppl 9: 78–80, 2002. doi: 10.1093/ndt/17.suppl_9.78. [DOI] [PubMed] [Google Scholar]
- 53.Grams ME, Sang Y, Levey AS, Matsushita K, Ballew S, Chang AR, Chow EKH, Kasiske BL, Kovesdy CP, Nadkarni GN, Shalev V, Segev DL, Coresh J, Lentine KL, Garg AX; Chronic Kidney Disease Prognosis Consortium . Kidney-failure risk projection for the living kidney-donor candidate. N Engl J Med 374: 411–421, 2016. doi: 10.1056/NEJMoa1510491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gribouval O, Gonzales M, Neuhaus T, Aziza J, Bieth E, Laurent N, Bouton JM, Feuillet F, Makni S, Ben Amar H, Laube G, Delezoide AL, Bouvier R, Dijoud F, Ollagnon-Roman E, Roume J, Joubert M, Antignac C, Gubler MC. Mutations in genes in the renin-angiotensin system are associated with autosomal recessive renal tubular dysgenesis. Nat Genet 37: 964–968, 2005. doi: 10.1038/ng1623. [DOI] [PubMed] [Google Scholar]
- 55.Grieshammer U, Le Ma, Plump AS, Wang F, Tessier-Lavigne M, Martin GR. SLIT2-mediated ROBO2 signaling restricts kidney induction to a single site. Dev Cell 6: 709–717, 2004. doi: 10.1016/S1534-5807(04)00108-X. [DOI] [PubMed] [Google Scholar]
- 56.Grønboek H, Nielsen B, Flyvbjerg A, Ørskov H. Effect of graded renal ablation on kidney and serum insulin-like growth factor-I (IGF-I) and IGF binding proteins in rats: relation to compensatory renal growth. Metabolism 46: 29–35, 1997. doi: 10.1016/S0026-0495(97)90163-3. [DOI] [PubMed] [Google Scholar]
- 57.Gubhaju L, Black MJ. The baboon as a good model for studies of human kidney development. Pediatr Res 58: 505–509, 2005. doi: 10.1203/01.PDR.0000179397.20862.73. [DOI] [PubMed] [Google Scholar]
- 58.Gubhaju L, Sutherland MR, Yoder BA, Zulli A, Bertram JF, Black MJ. Is nephrogenesis affected by preterm birth? Studies in a non-human primate model. Am J Physiol Renal Physiol 297: F1668–F1677, 2009. doi: 10.1152/ajprenal.00163.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guyton AC, Hall JE. Human Physiology and Mechanisms of Disease. Philadelphia, PA: Saunders, 1997, p. x. [Google Scholar]
- 60.Hadjianastassiou VG, Johnson RJ, Rudge CJ, Mamode N. 2509 living donor nephrectomies, morbidity and mortality, including the UK introduction of laparoscopic donor surgery. Am J Transplant 7: 2532–2537, 2007. doi: 10.1111/j.1600-6143.2007.01975.x. [DOI] [PubMed] [Google Scholar]
- 61.Hall JE, Guyton AC. Guyton and Hall Textbook of Medical Physiology. Philadelphia, PA: Saunders/Elsevier, 2011, p. xix. [Google Scholar]
- 62.Hallan S, Euser AM, Irgens LM, Finken MJ, Holmen J, Dekker FW. Effect of intrauterine growth restriction on kidney function at young adult age: the Nord Trøndelag Health (HUNT 2) Study. Am J Kidney Dis 51: 10–20, 2008. doi: 10.1053/j.ajkd.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 63.He B, Ebarasi L, Zhao Z, Guo J, Ojala JR, Hultenby K, De Val S, Betsholtz C, Tryggvason K. Lmx1b and FoxC combinatorially regulate podocin expression in podocytes. J Am Soc Nephrol 25: 2764–2777, 2014. doi: 10.1681/ASN.2012080823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hegde S, Coulthard MG. Follow-up of early unilateral nephrectomy for hypertension. Arch Dis Child Fetal Neonatal Ed 92: F305–F306, 2007. doi: 10.1136/adc.2006.104927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hegde S, Coulthard MG. Renal agenesis and unilateral nephrectomy: what are the risks of living with a single kidney? Pediatr Nephrol 24: 439–446, 2009. doi: 10.1007/s00467-008-0924-9. [DOI] [PubMed] [Google Scholar]
- 66.Hinchliffe SA, Sargent PH, Howard CV, Chan YF, van Velzen D. Human intrauterine renal growth expressed in absolute number of glomeruli assessed by the disector method and Cavalieri principle. Lab Invest 64: 777–784, 1991. [PubMed] [Google Scholar]
- 67.Hoskins BE, Cramer CH, Silvius D, Zou D, Raymond RM Jr, Orten DJ, Kimberling WJ, Smith RJ, Weil D, Petit C, Otto EA, Xu PX, Hildebrandt F. Transcription factor SIX5 is mutated in patients with branchio-oto-renal syndrome. Am J Hum Genet 80: 800–804, 2007. doi: 10.1086/513322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hoy WE, Douglas-Denton RN, Hughson MD, Cass A, Johnson K, Bertram JF. A stereological study of glomerular number and volume: preliminary findings in a multiracial study of kidneys at autopsy. Kidney Int Suppl 63: S31–S37, 2003. doi: 10.1046/j.1523-1755.63.s83.8.x. [DOI] [PubMed] [Google Scholar]
- 69.Hoy WE, Hughson MD, Singh GR, Douglas-Denton R, Bertram JF. Reduced nephron number and glomerulomegaly in Australian Aborigines: a group at high risk for renal disease and hypertension. Kidney Int 70: 104–110, 2006. doi: 10.1038/sj.ki.5000397. [DOI] [PubMed] [Google Scholar]
- 70.Hughson M, Farris AB III, Douglas-Denton R, Hoy WE, Bertram JF. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int 63: 2113–2122, 2003. doi: 10.1046/j.1523-1755.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 71.Hughson MD, Douglas-Denton R, Bertram JF, Hoy WE. Hypertension, glomerular number, and birth weight in African Americans and white subjects in the southeastern United States. Kidney Int 69: 671–678, 2006. doi: 10.1038/sj.ki.5000041. [DOI] [PubMed] [Google Scholar]
- 72.Ibrahim HN, Foley R, Tan L, Rogers T, Bailey RF, Guo H, Gross CR, Matas AJ. Long-term consequences of kidney donation. N Engl J Med 360: 459–469, 2009. doi: 10.1056/NEJMoa0804883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Iglesias DM, Hueber PA, Chu L, Campbell R, Patenaude AM, Dziarmaga AJ, Quinlan J, Mohamed O, Dufort D, Goodyer PR. Canonical WNT signaling during kidney development. Am J Physiol Renal Physiol 293: F494–F500, 2007. doi: 10.1152/ajprenal.00416.2006. [DOI] [PubMed] [Google Scholar]
- 74.Iyer A, Chan V, Brown L. The DOCA-salt hypertensive rat as a model of cardiovascular oxidative and inflammatory stress. Curr Cardiol Rev 6: 291–297, 2010. doi: 10.2174/157340310793566109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jain S. The many faces of RET dysfunction in kidney. Organogenesis 5: 177–190, 2009. doi: 10.4161/org.5.4.10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.James RG, Kamei CN, Wang Q, Jiang R, Schultheiss TM. Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation of kidney precursor cells. Development 133: 2995–3004, 2006. doi: 10.1242/dev.02442. [DOI] [PubMed] [Google Scholar]
- 77.Jameson JL, Loscalzo J, Harrison TR. Harrison’s Nephrology and Acid-Base Disorders. New York: McGraw-Hill Medical, 2013, p. ix. [Google Scholar]
- 78.Keijzer-Veen MG, Schrevel M, Finken MJ, Dekker FW, Nauta J, Hille ET, Frölich M, van der Heijden BJ; Dutch POPS-19 Collaborative Study Group . Microalbuminuria and lower glomerular filtration rate at young adult age in subjects born very premature and after intrauterine growth retardation. J Am Soc Nephrol 16: 2762–2768, 2005. doi: 10.1681/ASN.2004090783. [DOI] [PubMed] [Google Scholar]
- 79.Keller G, Zimmer G, Mall G, Ritz E, Amann K. Nephron number in patients with primary hypertension. N Engl J Med 348: 101–108, 2003. doi: 10.1056/NEJMoa020549. [DOI] [PubMed] [Google Scholar]
- 80.Kiprov DD, Colvin RB, McCluskey RT. Focal and segmental glomerulosclerosis and proteinuria associated with unilateral renal agenesis. Lab Invest 46: 275–281, 1982. [PubMed] [Google Scholar]
- 81.Kispert A, Vainio S, McMahon AP. Wnt-4 is a mesenchymal signal for epithelial transformation of metanephric mesenchyme in the developing kidney. Development 125: 4225–4234, 1998. [DOI] [PubMed] [Google Scholar]
- 82.Kobayashi A, Valerius MT, Mugford JW, Carroll TJ, Self M, Oliver G, McMahon AP. Six2 defines and regulates a multipotent self-renewing nephron progenitor population throughout mammalian kidney development. Cell Stem Cell 3: 169–181, 2008. doi: 10.1016/j.stem.2008.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kolvek G, Podracka L, Rosenberger J, Stewart RE, van Dijk JP, Reijneveld SA. Solitary functioning kidney in children–a follow-up study. Kidney Blood Press Res 39: 272–278, 2014. doi: 10.1159/000355804. [DOI] [PubMed] [Google Scholar]
- 84.Kume T, Deng K, Hogan BL. Murine forkhead/winged helix genes Foxc1 (Mf1) and Foxc2 (Mfh1) are required for the early organogenesis of the kidney and urinary tract. Development 127: 1387–1395, 2000. [DOI] [PubMed] [Google Scholar]
- 85.Lackland DT, Bendall HE, Osmond C, Egan BM, Barker DJ. Low birth weights contribute to high rates of early-onset chronic renal failure in the Southeastern United States. Arch Intern Med 160: 1472–1476, 2000. doi: 10.1001/archinte.160.10.1472. [DOI] [PubMed] [Google Scholar]
- 86.Lankadeva YR, Singh RR, Hilliard LM, Moritz KM, Denton KM. Impaired ability to modulate glomerular filtration rate in aged female sheep following fetal uninephrectomy. Physiol Rep 2: e00208, 2014. doi: 10.1002/phy2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.LaPointe Rudow D, Warburton KM. Selection and postoperative care of the living donor. Med Clin North Am 100: 599–611, 2016. doi: 10.1016/j.mcna.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 88.Leelahavanichkul A, Yan Q, Hu X, Eisner C, Huang Y, Chen R, Mizel D, Zhou H, Wright EC, Kopp JB, Schnermann J, Yuen PS, Star RA. Angiotensin II overcomes strain-dependent resistance of rapid CKD progression in a new remnant kidney mouse model. Kidney Int 78: 1136–1153, 2010. doi: 10.1038/ki.2010.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lenihan CR, Busque S, Derby G, Blouch K, Myers BD, Tan JC. Longitudinal study of living kidney donor glomerular dynamics after nephrectomy. J Clin Invest 125: 1311–1318, 2015. doi: 10.1172/JCI78885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li L, Lau WL, Rhee CM, Harley K, Kovesdy CP, Sim JJ, Jacobsen S, Chang A, Landman J, Kalantar-Zadeh K. Risk of chronic kidney disease after cancer nephrectomy. Nat Rev Nephrol 10: 135–145, 2014. doi: 10.1038/nrneph.2013.273. [DOI] [PubMed] [Google Scholar]
- 91.Lødrup AB, Karstoft K, Dissing TH, Pedersen M, Nyengaard JR. Kidney biopsies can be used for estimations of glomerular number and volume: a pig study. Virchows Archiv 452: 393–403, 2008. doi: 10.1007/s00428-007-0520-6. [DOI] [PubMed] [Google Scholar]
- 92.Lu W, van Eerde AM, Fan X, Quintero-Rivera F, Kulkarni S, Ferguson H, Kim HG, Fan Y, Xi Q, Li QG, Sanlaville D, Andrews W, Sundaresan V, Bi W, Yan J, Giltay JC, Wijmenga C, de Jong TP, Feather SA, Woolf AS, Rao Y, Lupski JR, Eccles MR, Quade BJ, Gusella JF, Morton CC, Maas RL. Disruption of ROBO2 is associated with urinary tract anomalies and confers risk of vesicoureteral reflux. Am J Hum Genet 80: 616–632, 2007. doi: 10.1086/512735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Luo G, Hofmann C, Bronckers AL, Sohocki M, Bradley A, Karsenty G. BMP-7 is an inducer of nephrogenesis, and is also required for eye development and skeletal patterning. Genes Dev 9: 2808–2820, 1995. doi: 10.1101/gad.9.22.2808. [DOI] [PubMed] [Google Scholar]
- 94.Matas AJ, Bartlett ST, Leichtman AB, Delmonico FL. Morbidity and mortality after living kidney donation, 1999-2001: survey of United States transplant centers. Am J Transplant 3: 830–834, 2003. [PubMed] [Google Scholar]
- 95.Mendelsohn C. Using mouse models to understand normal and abnormal urogenital tract development. Organogenesis 5: 306–314, 2009. doi: 10.4161/org.8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mendelsohn C, Batourina E, Fung S, Gilbert T, Dodd J. Stromal cells mediate retinoid-dependent functions essential for renal development. Development 126: 1139–1148, 1999. [DOI] [PubMed] [Google Scholar]
- 97.Merrill JP, Murray JE, Harrison JH, Guild WR. Successful homotransplantation of the human kidney between identical twins. J Am Med Assoc 160: 277–282, 1956. doi: 10.1001/jama.1956.02960390027008. [DOI] [PubMed] [Google Scholar]
- 98.Mihara M, Ito Y, Fukushima K, Yamashita F, Tsunosue M. Ultrasonographic screening for renal abnormalities in three-year-old children. Acta Paediatr 81: 326–328, 1992. doi: 10.1111/j.1651-2227.1992.tb12236.x. [DOI] [PubMed] [Google Scholar]
- 99.Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest 105: 863–873, 2000. doi: 10.1172/JCI8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mjøen G, Øyen O, Holdaas H, Midtvedt K, Line PD. Morbidity and mortality in 1022 consecutive living donor nephrectomies: benefits of a living donor registry. Transplantation 88: 1273–1279, 2009. doi: 10.1097/TP.0b013e3181bb44fd. [DOI] [PubMed] [Google Scholar]
- 101.Moore MW, Klein RD, Fariñas I, Sauer H, Armanini M, Phillips H, Reichardt LF, Ryan AM, Carver-Moore K, Rosenthal A. Renal and neuronal abnormalities in mice lacking GDNF. Nature 382: 76–79, 1996. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- 102.Moritz KM, Wintour EM, Dodic M. Fetal uninephrectomy leads to postnatal hypertension and compromised renal function. Hypertension 39: 1071–1076, 2002. doi: 10.1161/01.HYP.0000019131.77075.54. [DOI] [PubMed] [Google Scholar]
- 103.Mounier F, Hinglais N, Sich M, Gros F, Lacoste M, Deris Y, Alhenc-Gelas F, Gubler MC. Ontogenesis of angiotensin-I converting enzyme in human kidney. Kidney Int 32: 684–690, 1987. doi: 10.1038/ki.1987.261. [DOI] [PubMed] [Google Scholar]
- 104.Muzaale AD, Massie AB, Wang MC, Montgomery RA, McBride MA, Wainright JL, Segev DL. Risk of end-stage renal disease following live kidney donation. JAMA 311: 579–586, 2014. doi: 10.1001/jama.2013.285141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nakano T, Niimura F, Hohenfellner K, Miyakita E, Ichikawa I. Screening for mutations in BMP4 and FOXC1 genes in congenital anomalies of the kidney and urinary tract in humans. Tokai J Exp Clin Med 28: 121–126, 2003. [PubMed] [Google Scholar]
- 106.Nakayama M, Nozu K, Goto Y, Kamei K, Ito S, Sato H, Emi M, Nakanishi K, Tsuchiya S, Iijima K. HNF1B alterations associated with congenital anomalies of the kidney and urinary tract. Pediatr Nephrol 25: 1073–1079, 2010. doi: 10.1007/s00467-010-1454-9. [DOI] [PubMed] [Google Scholar]
- 107.Negrisolo S, Benetti E, Centi S, Della Vella M, Ghirardo G, Zanon GF, Murer L, Artifoni L. PAX2 gene mutations in pediatric and young adult transplant recipients: kidney and urinary tract malformations without ocular anomalies. Clin Genet 80: 581–585, 2011. doi: 10.1111/j.1399-0004.2010.01588.x. [DOI] [PubMed] [Google Scholar]
- 108.Nishinakamura R, Matsumoto Y, Nakao K, Nakamura K, Sato A, Copeland NG, Gilbert DJ, Jenkins NA, Scully S, Lacey DL, Katsuki M, Asashima M, Yokota T. Murine homolog of SALL1 is essential for ureteric bud invasion in kidney development. Development 128: 3105–3115, 2001. [DOI] [PubMed] [Google Scholar]
- 109.National Kidney and Urologic Diseases Information Clearinghouse (NKUDIC) Solitary Kidney (National Kidney and Urologic Diseases Information). (online) https://www.kidney.niddk.nih.gov [February 27, 2017].
- 110.Nyengaard JR, Bendtsen TF. Glomerular number and size in relation to age, kidney weight, and body surface in normal man. Anat Rec 232: 194–201, 1992. doi: 10.1002/ar.1092320205. [DOI] [PubMed] [Google Scholar]
- 111.Ohto H, Kamada S, Tago K, Tominaga SI, Ozaki H, Sato S, Kawakami K. Cooperation of six and eya in activation of their target genes through nuclear translocation of Eya. Mol Cell Biol 19: 6815–6824, 1999. doi: 10.1128/MCB.19.10.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Oldrizzi L, Rugiu C, De Biase V, Maschio G. The solitary kidney: a risky situation for progressive renal damage? Am J Kidney Dis 17, Suppl 1: 57–61, 1991. [PubMed] [Google Scholar]
- 113.Patterson LT, Pembaur M, Potter SS. Hoxa11 and Hoxd11 regulate branching morphogenesis of the ureteric bud in the developing kidney. Development 128: 2153–2161, 2001. [DOI] [PubMed] [Google Scholar]
- 114.Pichel JG, Shen L, Sheng HZ, Granholm AC, Drago J, Grinberg A, Lee EJ, Huang SP, Saarma M, Hoffer BJ, Sariola H, Westphal H. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature 382: 73–76, 1996. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- 115.Pitera JE, Scambler PJ, Woolf AS. Fras1, a basement membrane-associated protein mutated in Fraser syndrome, mediates both the initiation of the mammalian kidney and the integrity of renal glomeruli. Hum Mol Genet 17: 3953–3964, 2008. doi: 10.1093/hmg/ddn297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Poladia DP, Kish K, Kutay B, Bauer J, Baum M, Bates CM. Link between reduced nephron number and hypertension: studies in a mutant mouse model. Pediatr Res 59: 489–493, 2006. doi: 10.1203/01.pdr.0000202764.02295.45. [DOI] [PubMed] [Google Scholar]
- 117.Qian C, Yu X, Chen D-Y, Dodd S, Bouraoud N, Pothayee N, Chen Y, Beeman S, Bennett K, Murphy-Boesch J, Koretsky A. Wireless amplified nuclear MR detector (WAND) for high-spatial-resolution MR imaging of internal organs: preclinical demonstration in a rodent model. Radiology 268: 228–236, 2013. doi: 10.1148/radiol.13121352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Quinlan J, Lemire M, Hudson T, Qu H, Benjamin A, Roy A, Pascuet E, Goodyer M, Raju C, Zhang Z, Houghton F, Goodyer P. A common variant of the PAX2 gene is associated with reduced newborn kidney size. J Am Soc Nephrol 18: 1915–1921, 2007. doi: 10.1681/ASN.2006101107. [DOI] [PubMed] [Google Scholar]
- 119.Ramcharan T, Matas AJ. Long-term (20-37 years) follow-up of living kidney donors. Am J Transplant 2: 959–964, 2002. doi: 10.1034/j.1600-6143.2002.21013.x. [DOI] [PubMed] [Google Scholar]
- 120.Regazzoni BM, Genton N, Pelet J, Drukker A, Guignard JP. Long-term followup of renal functional reserve capacity after unilateral nephrectomy in childhood. J Urol 160: 844–848, 1998. doi: 10.1016/S0022-5347(01)62817-9. [DOI] [PubMed] [Google Scholar]
- 121.Reidy KJ, Rosenblum ND. Cell and molecular biology of kidney development. Semin Nephrol 29: 321–337, 2009. doi: 10.1016/j.semnephrol.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Reidy KJ, Villegas G, Teichman J, Veron D, Shen W, Jimenez J, Thomas D, Tufro A. Semaphorin3a regulates endothelial cell number and podocyte differentiation during glomerular development. Development 136: 3979–3989, 2009. doi: 10.1242/dev.037267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rizvi SA, Naqvi SA, Jawad F, Ahmed E, Asghar A, Zafar MN, Akhtar F. Living kidney donor follow-up in a dedicated clinic. Transplantation 79: 1247–1251, 2005. doi: 10.1097/01.TP.0000161666.05236.97. [DOI] [PubMed] [Google Scholar]
- 124.Robitaille P, Mongeau JG, Lortie L, Sinnassamy P. Long-term follow-up of patients who underwent unilateral nephrectomy in childhood. Lancet 325: 1297–1299, 1985. doi: 10.1016/S0140-6736(85)92792-8. [DOI] [PubMed] [Google Scholar]
- 125.Robson WL, Leung AK, Rogers RC. Unilateral renal agenesis. Adv Pediatr 42: 575–592, 1995. [PubMed] [Google Scholar]
- 126.Rodríguez-Gómez I, Wangensteen R, Pérez-Abud R, Quesada A, Del Moral RG, Osuna A, O’Valle F, de Dios Luna J, Vargas F. Long-term consequences of uninephrectomy in male and female rats. Hypertension 60: 1458–1463, 2012. doi: 10.1161/HYPERTENSIONAHA.112.198499. [DOI] [PubMed] [Google Scholar]
- 127.Rodríguez-Soriano J, Vallo A, Bilbao JR, Castaño L. Branchio-oto-renal syndrome: identification of a novel mutation in the EYA1 gene. Pediatr Nephrol 16: 550–553, 2001. doi: 10.1007/s004670100603. [DOI] [PubMed] [Google Scholar]
- 128.Rossetti S, Harris PC. Genotype-phenotype correlations in autosomal dominant and autosomal recessive polycystic kidney disease. J Am Soc Nephrol 18: 1374–1380, 2007. doi: 10.1681/ASN.2007010125. [DOI] [PubMed] [Google Scholar]
- 129.Rozen EJ, Schmidt H, Dolcet X, Basson MA, Jain S, Encinas M. Loss of Sprouty1 rescues renal agenesis caused by Ret mutation. J Am Soc Nephrol 20: 255–259, 2009. doi: 10.1681/ASN.2008030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ryan G, Steele-Perkins V, Morris JF, Rauscher FJ III, Dressler GR. Repression of Pax-2 by WT1 during normal kidney development. Development 121: 867–875, 1995. [DOI] [PubMed] [Google Scholar]
- 131.Saisawat P, Tasic V, Vega-Warner V, Kehinde EO, Günther B, Airik R, Innis JW, Hoskins BE, Hoefele J, Otto EA, Hildebrandt F. Identification of two novel CAKUT-causing genes by massively parallel exon resequencing of candidate genes in patients with unilateral renal agenesis. Kidney Int 81: 196–200, 2012. doi: 10.1038/ki.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sánchez MP, Silos-Santiago I, Frisén J, He B, Lira SA, Barbacid M. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature 382: 70–73, 1996. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- 133.Sanna-Cherchi S, Ravani P, Corbani V, Parodi S, Haupt R, Piaggio G, Innocenti ML, Somenzi D, Trivelli A, Caridi G, Izzi C, Scolari F, Mattioli G, Allegri L, Ghiggeri GM. Renal outcome in patients with congenital anomalies of the kidney and urinary tract. Kidney Int 76: 528–533, 2009. doi: 10.1038/ki.2009.220. [DOI] [PubMed] [Google Scholar]
- 134.Sanyanusin P, Schimmenti LA, McNoe LA, Ward TA, Pierpont ME, Sullivan MJ, Dobyns WB, Eccles MR. Mutation of the PAX2 gene in a family with optic nerve colobomas, renal anomalies and vesicoureteral reflux. Nat Genet 9: 358–364, 1995. doi: 10.1038/ng0495-358. [DOI] [PubMed] [Google Scholar]
- 135.Saran R, Marshall SM, Madsen R, Keavey P, Tapson JS. Long-term follow-up of kidney donors: a longitudinal study. Nephrol Dial Transplant 12: 1615–1621, 1997. doi: 10.1093/ndt/12.8.1615. [DOI] [PubMed] [Google Scholar]
- 136.Sarikonda KV, Watson RE, Opara OC, Dipette DJ. Experimental animal models of hypertension. J Am Soc Hypertens 3: 158–165, 2009. doi: 10.1016/j.jash.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 137.Saxén L. Organogenesis of the Kidney. Cambridge: Cambridge University Press, 1987, p. viii. doi: 10.1017/CBO9780511565083. [DOI] [Google Scholar]
- 138.Saxén L, Sariola H. Early organogenesis of the kidney. Pediatr Nephrol 1: 385–392, 1987. doi: 10.1007/BF00849241. [DOI] [PubMed] [Google Scholar]
- 139.Schmidt-Ott KM, Yang J, Chen X, Wang H, Paragas N, Mori K, Li JY, Lu B, Costantini F, Schiffer M, Bottinger E, Barasch J. Novel regulators of kidney development from the tips of the ureteric bud. J Am Soc Nephrol 16: 1993–2002, 2005. doi: 10.1681/ASN.2004121127. [DOI] [PubMed] [Google Scholar]
- 140.Schreuder MF. Safety in glomerular numbers. Pediatr Nephrol 27: 1881–1887, 2012. doi: 10.1007/s00467-012-2169-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Schuchardt A, D’Agati V, Larsson-Blomberg L, Costantini F, Pachnis V. Defects in the kidney and enteric nervous system of mice lacking the tyrosine kinase receptor Ret. Nature 367: 380–383, 1994. doi: 10.1038/367380a0. [DOI] [PubMed] [Google Scholar]
- 142.Schulte L, Schulz A, Unland J, Schulz H, Hubner N, Schmidt-Ott KM, Kreutz R. MWF rats with spontaneous albuminuria inherit a reduced efficiency of nephron induction during early nephrogenesis in comparison to SHR rats. J Hypertens 30: 2031–2038, 2012. doi: 10.1097/HJH.0b013e328356a60a. [DOI] [PubMed] [Google Scholar]
- 143.Seeman T, Patzer L, John U, Dusek J, Vondrák K, Janda J, Misselwitz J. Blood pressure, renal function, and proteinuria in children with unilateral renal agenesis. Kidney Blood Press Res 29: 210–215, 2006. doi: 10.1159/000095735. [DOI] [PubMed] [Google Scholar]
- 144.Sim EU, Smith A, Szilagi E, Rae F, Ioannou P, Lindsay MH, Little MH. Wnt-4 regulation by the Wilms’ tumour suppressor gene, WT1. Oncogene 21: 2948–2960, 2002. doi: 10.1038/sj.onc.1205373. [DOI] [PubMed] [Google Scholar]
- 145.Simon G. Chirurgie der Nieren. Stuttgart, Germany: F. Enke, 1871. [Google Scholar]
- 146.Singla V, Reiter JF. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science 313: 629–633, 2006. doi: 10.1126/science.1124534. [DOI] [PubMed] [Google Scholar]
- 147.Song R, Yosypiv IV. Genetics of congenital anomalies of the kidney and urinary tract. Pediatr Nephrol 26: 353–364, 2011. doi: 10.1007/s00467-010-1629-4. [DOI] [PubMed] [Google Scholar]
- 148.Stahl DA, Koul HK, Chacko JK, Mingin GC. Congenital anomalies of the kidney and urinary tract (CAKUT): a current review of cell signaling processes in ureteral development. J Pediatr Urol 2: 2–9, 2006. doi: 10.1016/j.jpurol.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 149.Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372: 679–683, 1994. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- 150.Stelloh C, Allen KP, Mattson DL, Lerch-Gaggl A, Reddy S, El-Meanawy A. Prematurity in mice leads to reduction in nephron number, hypertension, and proteinuria. Transl Res 159: 80–89, 2012. doi: 10.1016/j.trsl.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Stricker S, Brieske N, Haupt J, Mundlos S. Comparative expression pattern of Odd-skipped related genes Osr1 and Osr2 in chick embryonic development. Gene Expr Patterns 6: 826–834, 2006. doi: 10.1016/j.modgep.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 152.Suleiman H, Heudobler D, Raschta AS, Zhao Y, Zhao Q, Hertting I, Vitzthum H, Moeller MJ, Holzman LB, Rachel R, Johnson R, Westphal H, Rascle A, Witzgall R. The podocyte-specific inactivation of Lmx1b, Ldb1 and E2a yields new insight into a transcriptional network in podocytes. Dev Biol 304: 701–712, 2007. doi: 10.1016/j.ydbio.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 153.Sullivan LD, Westmore DD, McLoughlin MG. Surgical management of renal cell carcinoma at the Vancouver General Hospital: 20-year review. Can J Surg 22: 427– 431, 1979. [PubMed] [Google Scholar]
- 154.Tabatabaeifar M, Schlingmann KP, Litwin M, Emre S, Bakkaloglu A, Mehls O, Antignac C, Schaefer F, Weber S; ESCAPE Trial Group . Functional analysis of BMP4 mutations identified in pediatric CAKUT patients. Pediatr Nephrol 24: 2361–2368, 2009. doi: 10.1007/s00467-009-1287-6. [DOI] [PubMed] [Google Scholar]
- 155.Talseth T, Fauchald P, Skrede S, Djøseland O, Berg KJ, Stenstrøm J, Heilo A, Brodwall EK, Flatmark A. Long-term blood pressure and renal function in kidney donors. Kidney Int 29: 1072–1076, 1986. doi: 10.1038/ki.1986.109. [DOI] [PubMed] [Google Scholar]
- 156.Torban E, Dziarmaga A, Iglesias D, Chu LL, Vassilieva T, Little M, Eccles M, Discenza M, Pelletier J, Goodyer P. PAX2 activates WNT4 expression during mammalian kidney development. J Biol Chem 281: 12705–12712, 2006. doi: 10.1074/jbc.M513181200. [DOI] [PubMed] [Google Scholar]
- 157.Torres VE, Harris PC. Mechanisms of Disease: autosomal dominant and recessive polycystic kidney diseases. Nat Clin Pract Nephrol 2: 40–55, 2006. doi: 10.1038/ncpneph0070. [DOI] [PubMed] [Google Scholar]
- 158.Tsukamoto Y, Mano T, Sakata Y, Ohtani T, Takeda Y, Tamaki S, Omori Y, Ikeya Y, Saito Y, Ishii R, Higashimori M, Kaneko M, Miwa T, Yamamoto K, Komuro I. A novel heart failure mice model of hypertensive heart disease by angiotensin II infusion, nephrectomy, and salt loading. Am J Physiol Heart Circ Physiol 305: H1658–H1667, 2013. doi: 10.1152/ajpheart.00349.2013. [DOI] [PubMed] [Google Scholar]
- 159.Ulinski T, Lescure S, Beaufils S, Guigonis V, Decramer S, Morin D, Clauin S, Deschênes G, Bouissou F, Bensman A, Bellanné-Chantelot C. Renal phenotypes related to hepatocyte nuclear factor-1beta (TCF2) mutations in a pediatric cohort. J Am Soc Nephrol 17: 497–503, 2006. doi: 10.1681/ASN.2005101040. [DOI] [PubMed] [Google Scholar]
- 160.Vainio S, Müller U. Inductive tissue interactions, cell signaling, and the control of kidney organogenesis. Cell 90: 975–978, 1997. doi: 10.1016/S0092-8674(00)80363-3. [DOI] [PubMed] [Google Scholar]
- 161.Valentini RP, Langenburg S, Imam A, Mattoo TK, Zerin JM. MRI detection of atrophic kidney in a hypertensive child with a single kidney. Pediatr Nephrol 20: 1192–1194, 2005. doi: 10.1007/s00467-005-1914-9. [DOI] [PubMed] [Google Scholar]
- 162.van Koppen A, Verhaar MC, Bongartz LG, Joles JA. 5/6th nephrectomy in combination with high salt diet and nitric oxide synthase inhibition to induce chronic kidney disease in the Lewis rat. J Vis Exp: e50398, 2013. doi: 10.1159/000430475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.van Vuuren SH, Sol CM, Broekhuizen R, Lilien MR, Oosterveld MJ, Nguyen TQ, Goldschmeding R, de Jong TP. Compensatory growth of congenital solitary kidneys in pigs reflects increased nephron numbers rather than hypertrophy. PLoS One 7: e49735, 2012. doi: 10.1371/journal.pone.0049735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Vu KH, Van Dyck M, Daniels H, Proesmans W. Renal outcome of children with one functioning kidney from birth. A study of 99 patients and a review of the literature. Eur J Pediatr 167: 885–890, 2008. doi: 10.1007/s00431-007-0612-y. [DOI] [PubMed] [Google Scholar]
- 165.Wagner KD, Wagner N, Guo JK, Elger M, Dallman MJ, Bugeon L, Schedl A. An inducible mouse model for PAX2-dependent glomerular disease: insights into a complex pathogenesis. Curr Biol 16: 793–800, 2006. doi: 10.1016/j.cub.2006.02.072. [DOI] [PubMed] [Google Scholar]
- 166.Walker KA, Cai X, Caruana G, Thomas MC, Bertram JF, Kett MM. High nephron endowment protects against salt-induced hypertension. Am J Physiol Renal Physiol 303: F253–F258, 2012. doi: 10.1152/ajprenal.00028.2012. [DOI] [PubMed] [Google Scholar]
- 167.Wang Q, Lan Y, Cho ES, Maltby KM, Jiang R. Odd-skipped related 1 (Odd 1) is an essential regulator of heart and urogenital development. Dev Biol 288: 582–594, 2005. doi: 10.1016/j.ydbio.2005.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang X, Johnson AC, Sasser JM, Williams JM, Solberg Woods LC, Garrett MR. Spontaneous one-kidney rats are more susceptible to develop hypertension by DOCA-NaCl and subsequent kidney injury compared with uninephrectomized rats. Am J Physiol Renal Physiol 310: F1054–F1064, 2016. doi: 10.1152/ajprenal.00555.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wang X, Johnson AC, Williams JM, White T, Chade AR, Zhang J, Liu R, Roman RJ, Lee JW, Kyle PB, Solberg-Woods L, Garrett MR. Nephron deficiency and predisposition to renal injury in a novel one-kidney genetic model. J Am Soc Nephrol 26: 1634–1646, 2015. doi: 10.1681/ASN.2014040328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Weber S, Moriniere V, Knüppel T, Charbit M, Dusek J, Ghiggeri GM, Jankauskiené A, Mir S, Montini G, Peco-Antic A, Wühl E, Zurowska AM, Mehls O, Antignac C, Schaefer F, Salomon R. Prevalence of mutations in renal developmental genes in children with renal hypodysplasia: results of the ESCAPE study. J Am Soc Nephrol 17: 2864–2870, 2006. doi: 10.1681/ASN.2006030277. [DOI] [PubMed] [Google Scholar]
- 171.Weber S, Taylor JC, Winyard P, Baker KF, Sullivan-Brown J, Schild R, Knüppel T, Zurowska AM, Caldas-Alfonso A, Litwin M, Emre S, Ghiggeri GM, Bakkaloglu A, Mehls O, Antignac C, Network E, Schaefer F, Burdine RD. SIX2 and BMP4 mutations associate with anomalous kidney development. J Am Soc Nephrol 19: 891–903, 2008. doi: 10.1681/ASN.2006111282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wellik DM, Hawkes PJ, Capecchi MR. Hox11 paralogous genes are essential for metanephric kidney induction. Genes Dev 16: 1423–1432, 2002. doi: 10.1101/gad.993302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.White SL, Perkovic V, Cass A, Chang CL, Poulter NR, Spector T, Haysom L, Craig JC, Salmi IA, Chadban SJ, Huxley RR. Is low birth weight an antecedent of CKD in later life? A systematic review of observational studies. Am J Kidney Dis 54: 248–261, 2009. doi: 10.1053/j.ajkd.2008.12.042. [DOI] [PubMed] [Google Scholar]
- 174.Wiesel A, Queisser-Luft A, Clementi M, Bianca S, Stoll C; EUROSCAN Study Group . Prenatal detection of congenital renal malformations by fetal ultrasonographic examination: an analysis of 709,030 births in 12 European countries. Eur J Med Genet 48: 131–144, 2005. doi: 10.1016/j.ejmg.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 175.Wikstad I, Pettersson BA, Elinder G, Sökücü S, Aperia A. A comparative study of size and function of the remnant kidney in patients nephrectomized in childhood for Wilms’ tumor and hydronephrosis. Acta Paediatr Scand 75: 408–414, 1986. doi: 10.1111/j.1651-2227.1986.tb10222.x. [DOI] [PubMed] [Google Scholar]
- 176.Wilson BE, Davies P, Shah K, Wong W, Taylor CM. Renal length and inulin clearance in the radiologically normal single kidney. Pediatr Nephrol 18: 1147–1151, 2003. doi: 10.1007/s00467-003-1244-8. [DOI] [PubMed] [Google Scholar]
- 177.Wintour EM, Moritz KM, Johnson K, Ricardo S, Samuel CS, Dodic M. Reduced nephron number in adult sheep, hypertensive as a result of prenatal glucocorticoid treatment. J Physiol 549: 929–935, 2003. doi: 10.1113/jphysiol.2003.042408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Woolf AS, Hillman KA. Unilateral renal agenesis and the congenital solitary functioning kidney: developmental, genetic and clinical perspectives. BJU Int 99: 17–21, 2007. doi: 10.1111/j.1464-410X.2006.06504.x. [DOI] [PubMed] [Google Scholar]
- 179.Xu PX, Adams J, Peters H, Brown MC, Heaney S, Maas R. Eya1-deficient mice lack ears and kidneys and show abnormal apoptosis of organ primordia. Nat Genet 23: 113–117, 1999. doi: 10.1038/12722. [DOI] [PubMed] [Google Scholar]
- 180.Xu PX, Zheng W, Huang L, Maire P, Laclef C, Silvius D. Six1 is required for the early organogenesis of mammalian kidney. Development 130: 3085–3094, 2003. doi: 10.1242/dev.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Yosypiv IV, Boh MK, Spera MA, El-Dahr SS. Downregulation of Spry-1, an inhibitor of GDNF/Ret, causes angiotensin II-induced ureteric bud branching. Kidney Int 74: 1287–1293, 2008. doi: 10.1038/ki.2008.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zhang B, Wang H, Sun N, Jia LQ, Shen Y. [Incidence, diagnosis and treatment of children’s congenital abnormalities of the kidney and urinary tract detected in ultrasound screening]. Zhonghua Er Ke Za Zhi 49: 534–538, 2011. [PubMed] [Google Scholar]
- 183.Zhang J, Massmann GA, Rose JC, Figueroa JP. Differential effects of clinical doses of antenatal betamethasone on nephron endowment and glomerular filtration rate in adult sheep. Reprod Sci 17: 186–195, 2010. doi: 10.1177/1933719109351098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zivná M, Hůlková H, Matignon M, Hodanová K, Vylet’al P, Kalbácová M, Baresová V, Sikora J, Blazková H, Zivný J, Ivánek R, Stránecký V, Sovová J, Claes K, Lerut E, Fryns JP, Hart PS, Hart TC, Adams JN, Pawtowski A, Clemessy M, Gasc JM, Gübler MC, Antignac C, Elleder M, Kapp K, Grimbert P, Bleyer AJ, Kmoch S. Dominant renin gene mutations associated with early-onset hyperuricemia, anemia, and chronic kidney failure. Am J Hum Genet 85: 204–213, 2009. doi: 10.1016/j.ajhg.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Zu S, Bartik Z, Zhao S, Sillen U, Nordenskjöld A. Mutations in the ROBO2 and SLIT2 genes are rare causes of familial vesico-ureteral reflux. Pediatr Nephrol 24: 1501–1508, 2009. doi: 10.1007/s00467-009-1179-9. [DOI] [PubMed] [Google Scholar]