Abstract
Muscle fiber cross-sectional area (CSA) and proportion of different fiber types are important determinants of muscle function and overall metabolism. Genetic variation plays a substantial role in phenotypic variation of these traits; however, the underlying genes remain poorly understood. This study aimed to map quantitative trait loci (QTL) affecting differences in soleus muscle fiber traits between the LG/J and SM/J mouse strains. Fiber number, CSA, and proportion of oxidative type I fibers were assessed in the soleus of 334 genotyped female and male mice of the F34 generation of advanced intercross lines (AIL) derived from the LG/J and SM/J strains. To increase the QTL detection power, these data were combined with 94 soleus samples from the F2 intercross of the same strains. Transcriptome of the soleus muscle of LG/J and SM/J females was analyzed by microarray. Genome-wide association analysis mapped four QTL (genome-wide P < 0.05) affecting the properties of muscle fibers to chromosome 2, 3, 4, and 11. A 1.5-LOD QTL support interval ranged between 2.36 and 4.67 Mb. On the basis of the genomic sequence information and functional and transcriptome data, we identified candidate genes for each of these QTL. The combination of analyses in F2 and F34 AIL populations with transcriptome and genomic sequence data in the parental strains is an effective strategy for refining QTL and nomination of the candidate genes.
Keywords: skeletal muscle, muscle fiber types, genetic variation
skeletal muscle plays a broad range of biological functions including locomotion, thermoregulation, respiration, postural support, protection of bones and viscera; as well as serving as a source of amino acids in times of starvation or disease. Muscle tissue in livestock also provides an essential source of dietary proteins. In humans, there is more than a twofold difference in muscle mass between individuals of similar age and same sex (3, 33). This is the outcome of variability in the number of muscle fibers and their size (51). These differences are of clinical relevance. Variability in muscle mass significantly impacts energy expenditure (58), influencing preponderance to obesity. In addition, individuals with lower muscle mass may be more vulnerable to impairment of these vital functions due to aging and/or disease-related muscle loss. It has recently been reported that there is a positive association between muscle mass and longevity in older adults (66).
Human skeletal muscles mainly comprise a mixture of type I, IIA, and IIX muscle fibers (62). The number of fibers, their size, and varying proportions of the fiber types affect morphological and functional properties of the muscle (6). A larger diameter of the fibers and higher number of fibers typically lead to augmented muscular strength and power (25, 28). The proportion of type I muscle fibers is a factor determining success in endurance sporting events (15, 18) and overall metabolism in humans (24, 29, 44, 74). In livestock, proportion of oxidative type I fibers is associated with meat quality (65).
In humans, genetic factors account for around half of the variation in strength (19, 24, 74), and the upper limit heritability is even greater (>0.9) for muscle mass (26). Heritability estimates of proportion of type I fibers is also high, ranging between 0.4 and 0.9, indicating that genetic factors play an important role in determining muscle fiber properties (37, 63). Effects of genetic factors on muscle fibers have also been demonstrated in mouse (20, 22, 59), cattle (68), sheep (10, 38), and pig (71). However, the specific genes underlying these effects remain largely elusive.
Attempts at mapping the polygenic architecture of muscle fiber properties in mouse (11), pig (17, 43, 52, 55, 77), cattle (1), and carp (80) have been made. A number of quantitative trait loci (QTL) have been identified in these studies. However, the resolution achieved in the F2 population is not adequate for reliable nomination of the candidate genes in the majority of the QTLs of polygenic traits. The mouse soleus muscle (primarily consists of type I and IIA fiber types) closely resembles the fiber type composition of human skeletal muscles (primarily comprising type I, IIA, and IIX fiber types) and is therefore a particularly interesting experimental model. In our previous study, we mapped soleus muscle fiber traits in an F2 intercross between the LG/J and SM/J laboratory mouse strains (11). These strains differ in a number of muscular phenotypes, with the LG/J strain displaying a greater proportion of type I fibers, and a greater cross-sectional area (CSA) of type I and IIA muscle fibers. We identified in that study three significant QTLs contributing to the difference in the CSA of muscle fibers between LG/J and SM/J strains (11). Regions of conserved synteny from the identified loci were also implicated in fiber phenotypes in the pig, supporting the importance of these genomic regions in determining muscle fiber properties. However, the exact genes underlying their effects remain to be determined.
Integration of advanced study populations, high-throughput gene expression technology, and increasing availability of knockout models aid identification of causative genes. Nomination of the genes underlying QTL effects can be facilitated by improving the mapping resolution and by utilizing genomic sequence and transcriptome information. Advanced intercross lines (AIL) have been proposed as a powerful population for mapping QTLs (16). It has been demonstrated recently that a joint F2 and AIL analysis can combine the advantages of both mapping populations by increasing the power to detect QTLs and achieving a higher mapping resolution of various traits in mice (13, 47). Additionally, testing for differences in specific gene expression has led to several nominations of quantitative trait genes (30, 35). For validation of such candidate genes, phenotypic effects of relevant alleles can be examined in experimental populations where these alleles segregate albeit on a different genetic background. In addition, available knockout models offer a particularly attractive option for validation experiments.
In the present study we aimed to fine-map QTL and nominate candidate genes affecting the CSA and proportion of oxidative type I fibers in the soleus muscle in a combined analysis of F2 and F34 AIL mice, and by cross referencing QTL data with soleus transcriptome profiles in the parental strains. Further filtering of the emerged candidates was carried out in an independent AIL and a knockout model.
METHODS
Muscle Samples
This study was carried out on soleus muscles dissected from females and males of the F34 AIL of the LG/J and SM/J inbred strains. Animals were maintained as previously described (13) and killed at 94 ± 4 days. All procedures were approved by the Institutional Animal Care and Use Committee of the University of Chicago. Soleus muscle samples from F34 AIL mice described in our previous study (47) were subjected to histological analyses. The final sample size used in the present study was 334 F34 mice, 142 females and 192 males, after we discarded samples of poor tissue quality. A set of 94 F2 samples (38 females and 56 males) described in our previous study (11) was also used to increase the QTL detection power.
In addition, we also analyzed soleus muscle samples for two hypothesis-driven studies aimed at testing the effects of identified candidate genes on percentage of oxidative, type I fibers. First, we examined soleus samples from the Chd6 ATPase knockout (n = 6), heterozygous (n = 4) and wild-type (n = 4) females. The generation of the Chd6 mutant mice has been previously reported (40). Briefly, genetic manipulation generated an allele with the ATPase domain of Chd6 (exon 12) flanked by loxP sites so that the action of Cre recombinase would delete this domain. The mice were mated to a germline Cre-expressing strain (Jackson Laboratory strain 003465) to delete both exon 12 and the neomycin resistance marker used for the targeting. Subsequently, breeding generated the Chd6 ATPase knockout mice utilized in the present study. Second, solei of the advanced intercross mice (generations F9–F12), all homozygous carriers of the C57BL/6J (n = 22) or DBA/2J (n = 23) alleles at the region harboring the Alad gene, were selected from the tissue bank of our previous study (9).
Phenotype Assessment
The soleus muscles were frozen in isopentane cooled in liquid nitrogen. Transverse sections from the belly of the muscle were cut at a thickness of 10 µm with a cryotome (Leica CM1850UV) at −20°C. The muscle sections were subjected to ATPase staining (acid preincubation, pH 4.47) to distinguish between fiber types (8). Microscopic images of stained sections were taken at ×5 and ×20 magnification.
The following phenotypes were assessed: muscle fiber number (type I and IIA) and percent of type I muscle fibers, CSA of type I and type IIA fibers (Fig. 1). Muscle fiber traits were manually analyzed with ImageJ software (NIH, version 1.43). We took 25 measurements of each fiber type using the freehand selection tool at ×20 magnification to obtain a value representing the mean CSA of type I or type IIA fibers for that muscle. This was deemed as a representative sample by empirical testing as described previously (11). The total number of type I and type IIA muscle fibers was counted with the ImageJ cell counter plug-in on ×5 magnification images. As all fibers in mouse soleus pass through the belly of the muscle (69), this method provides an accurate estimate of the number of fibers constituting the muscle. We counted the total numbers of type I fibers and type IIA fibers, permitting the derivation of a percentage of type I fibers. Over the course of the study ~200,000 muscle fibers were counted and ~6,700 fibers were measured for CSA.
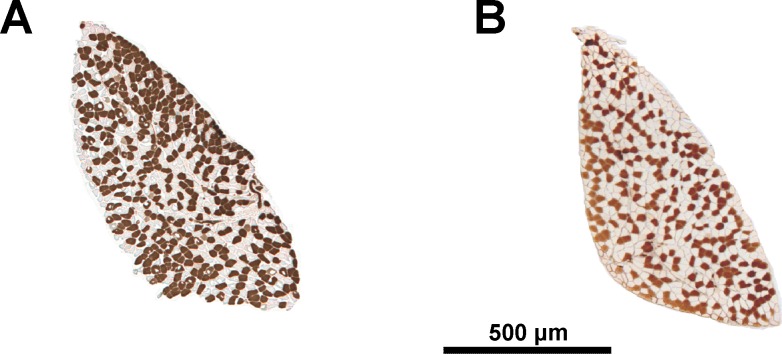
Fig. 1.
Individual variability in proportion of oxidative fibers. Solei of F34 females containing 57% (A) and 38% (B) of type I fibers. Cross sections shown following ATPase staining (acid preincubation). Dark fibers, type I; pale fibers, type IIA.
Statistical Analyses
The GraphPad Prism version 5.0 statistical package was used (GraphPad Software, La Jolla, CA). Data are presented as means ± SD unless otherwise stated. The CSA of type I and type IIA fibers were analyzed by a two-way (sex and fiber type) paired-measures (type I and type IIA fibers) ANOVA.
Genotyping and QTL Mapping
Mice were genotyped using a custom-designed single nucleotide polymorphism (SNP) array that included 4,610 polymorphic SNPs that were approximately evenly distributed across the genome, as described previously (13). The genome-wide association analysis was performed in the combined population of the F34 and recently published F2 intercrosses (11) using the R package QTLRel (12). This software accounted for the complex relationships (e.g., sibling, half-sibling, cousins) among the F34 mice by using a mixed model, as previously described (12, 13). Because of the sex differences in muscle mass in these mice (47), and the discovery of sex-specific QTL in other studies (45, 46), we included sex as an additive and interacting covariate. Threshold of significance was estimated by 1,000 permutations (14). We defined the support interval for each QTL as the 1.5-LOD drop off on either side of the peak marker. This interval was expressed in physical map position (Mb) by using the nearest genotyped SNP that flanked the support interval, based on the mouse genome build GRCm38.p3.
Transcriptome Analysis
Soleus muscle tissues from 92-day-old LG/J and SM/J females (n = 3 of each strain) were used. RNA was isolated using TRIzol (Invitrogen Life Technologies, Carlsbad, CA) followed by purification and DNase digestion using RNeasy minikits (Qiagen, Venlo, Netherlands) according to the manufacturer’s instructions. Quantification of total RNA was performed on a NanoDrop spectrophotometer (Thermo Scientific) and quality-tested on an Agilent Tapestation with R6K Screentapes (RNA integrity number ≥7.3). Generation of sense-strand cDNA from purified total RNA (Ambion WT expression kit; Ambion, Austin, Texas) followed by fragmentation and labeling (GeneChip WT labeling kit; Affymetrix, Santa Clara, CA) were performed according to the manufacturers' instructions. Hybridization, washing, staining, and scanning of microarrays were carried out on Affymetrix Mouse Gene 2.0 ST microarrays according to the manufacturer’s standard protocols using a GeneChip Fluidics station 450 and GCS3000 scanner (Affymetrix). Microarray data are available in the ArrayExpress database (http://www.ebi.ac.uk/arrayexpress) under accession number E-MTAB-5290.
Data preprocessing and quality control analysis were performed using Affymetrix GeneChip Expression Console v1.2. Probe cell intensity data on the Mouse Gene 2.0 ST array (CEL files) were processed using the RMA16 algorithm (Affymetrix), which fits a robust linear model at probe level by employing background correction, quantile normalization of log2-transformed data, and summarization to probe level data (CHP files, 41,345 probe sets).
Data was analyzed for differentially expressed genes in Partek Genomics Suite version 6.6, build 6.15.0730 (Partek, St. Louis, MO) using a Mus musculus build mm10 annotation file for Mouse Gene 2.0 ST microarrays (MoGene-2_0-st-v1.na35.mm10). CEL files (Expression Console v 1.2, Affymetrix) were imported to Partek Genomics Suite v 6.6 and processed using RMA normalization with background correction of log2-transformed data and probe set summarization by median polish. Differential expression analysis between the LG/J and SM/J strains of all genes (n = 41,345 transcript clusters) was determined by one-way ANOVA with Storey’s false discovery rate (FDR) and q-value ≤0.05 considered significant (n = 819 genes differentially expressed ≥ 1.2-fold; see Supplementary Table S1). (The online version of this article contains supplemental material.)
To assess transcription of positional candidate genes in each strain, we performed a hypothesis-driven analysis of differential gene expression between the LG/J and SM/J strains on all genes mapping to the support interval defined for each QTL in the GWAS described above. Using Partek Genomics Suite v.6.6, we identified a total of 159 genes that were represented on the mouse Gene 2.0ST microarray in M. musculus genome build GRCm38, mm10 within mapping coordinates Chr2:158908559–162608559 (26 genes), Chr3:33308451–35708451 (15 genes), Chr4:57605946–62913639 (77 genes), or Chr 11:27900000–31500000 (41 genes). One-way ANOVA identified differentially expressed genes between the LG/J and SM/J strains (P < 0.05). Fold change was calculated using the geometric mean of samples in each group.
Candidate Genes
Nomination of the candidate genes was based on the following three criteria. First, we scrutinized polymorphisms in positional candidates between the LG/J and SM/J strains. The emphasis was on the indels and SNPs that would affect the coding sequence and lead to changes in amino acids. To assess whether amino acid substitution would influence the function of a protein, we considered evolutionary conservation at the site of substitution and properties of substituted amino acids using three different bioinformatics tools as described by Nikolskiy and colleagues (56). Second, we examined expression of positional candidates across a panel of >90 mouse tissues and cell types available in the BioGPS GeneAtlas MOE430, gcrma data set (79). This analysis permits a quantitative comparison of transcript abundance of a gene between tissues. We considered that an abundant expression in skeletal muscle lineage, i.e., muscle tissue and/or C2C12 myogenic cell line, implies functional and/or structural relevance of a gene in this tissue. Third, we compared gene expression levels in the soleus muscle between the two strains as described in the previous section. Expression difference in this analysis might point at the strain-specific, genotype-dependent mechanism underlying the phenotypic difference.
RESULTS
Phenotypic Analyses
CSA.
Cross-section analysis of soleus muscle fibers was done on mice of both sexes from the F34 cohort. For muscle fiber CSA, we observed a statistically significant sex-by-fiber type interaction (P < 0.0001). In the female F34 mice there was no significant difference between type I and type IIA muscle fiber areas (913 ± 229 μm2, n = 140; and 952 ± 242 μm2, n = 140 respectively; P = 0.2). However, there was a significant difference within the males, with the type I muscle fiber area being smaller than IIA fiber area (1,084 ± 238 μm2, n = 187; and 1,215 ± 294 μm2, n = 187, respectively; P < 0.0001). Muscle fiber area was lower in females than males for type I CSA, (P < 0.0001) and type IIA CSA (P < 0.0001).
Percentage of type I fibers.
The number of type I fibers as a percentage of total fibers varied substantially between individuals (Fig. 1), ranging from 30 to 67% in females, and from 26 to 59% in males, and was greater in females than males (46 ± 8%, n = 142; and 39 ± 6%, n = 189; respectively; P < 0.0001).
Total fiber number.
No difference was observed in the total soleus fiber number between females and males (646 ± 102, n = 120, and 667 ± 105, n = 177, respectively; P = 0.0979).
QTL Analyses
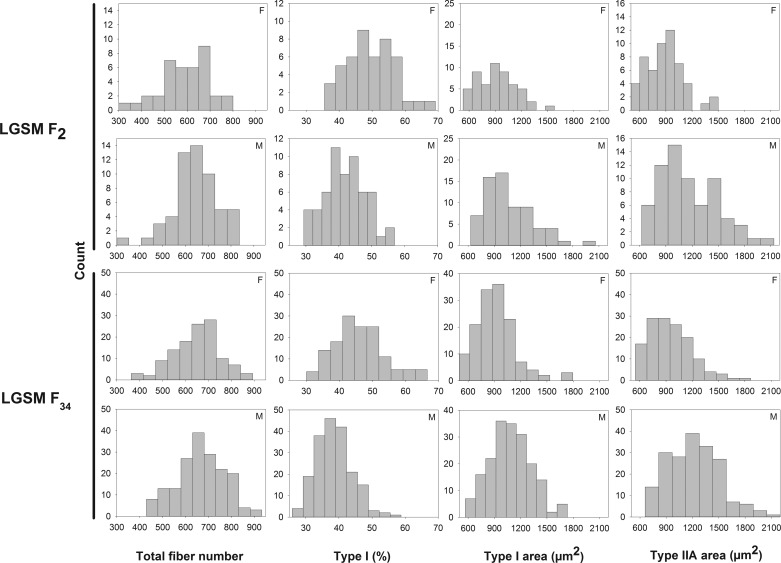
Muscle fiber traits approximated the normal distribution in both the F2 and F34 populations (Fig. 2). We identified significant QTL (at the 1 or 5% level of genome-wide statistical significance) (39) for CSA of type I and type IIA fibers and the percentage of type I fibers. We also identified chromosome-wide significant QTL for CSA of type I and type IIA fibers, the percentage of type I fibers, and total fiber number (Table 1). The size of the support interval of these QTL ranged from 0.4 to 40.7 Mb, with a median of 4.6 Mb.
Fig. 2.
Distribution of muscle fiber traits in the F2 and F34 generations of advanced intercross lines between the LG/J and SM/J strains. F, females; M, males.
Table 1.
Characteristics of muscle fiber QTL in combined analyses of the F2 and F34 intercrosses derived from the LG/J and SM/J strains
| Chr | Thr | Level | Start Mb | End Mb | Size Mb | Trait | Locus |
|---|---|---|---|---|---|---|---|
| 1 | C | 0.1 | 67.6 | 70.7 | 3.1 | % type I | |
| 1 | C | 0.1 | 193.9 | 194.3 | 0.4 | % type I, CSA2A | |
| 2 | C | 0.1 | 92.4 | 104.8 | 12.4 | % type I | |
| 2 | C | 0.05 | 139.6 | 145.6 | 6.0 | % type I | |
| 2 | G | 0.01 | 158.8 | 162.5 | 3.7 | % type I | Mfq4 (SM) |
| 3 | G | 0.05 | 33.6 | 40.0 | 6.4 | CSA1, CSA2A | Mfq5 (SM) |
| 4 | G | 0.05 | 57.7 | 62.7 | 5.0 | % type I | Mfq6 (LG) |
| 4 | C | 0.05 | 103.9 | 106.1 | 2.2 | % type I | |
| 6 | C | 0.05 | 81.9 | 84.1 | 2.2 | CSAIIA | |
| 6 | C | 0.01 | 110.8 | 116.0 | 5.2 | CSA1, CSA2A | Mfq2* (LG) |
| 7 | C | 0.05 | 138.4 | 140.0 | 1.6 | % type I | |
| 8 | C | 0.1 | 7.4 | 12.4 | 5.0 | % type I | |
| 8 | C | 0.05 | 89.0 | 92.4 | 3.4 | total | |
| 8 | C | 0.01 | 121.9 | 128.6 | 6.7 | total | |
| 10 | G | 0.1 | 120.7 | 121.3 | 0.6 | % type I | |
| 11 | C | 0.1 | 12.4 | 17.2 | 4.8 | CSAIIA | |
| 11 | C | 0.1 | 19.1 | 23.1 | 4.0 | CSAIIA | |
| 11 | G | 0.01 | 28.0 | 31.5 | 3.5 | CSA1, CSA2A | Mfq3* (LG) |
| 11 | C | 0.1 | 62.5 | 64.2 | 1.7 | % type I | |
| 11 | C | 0.1 | 70.6 | 76.2 | 5.6 | % type I | |
| 12 | C | 0.1 | 27.6 | 29.3 | 1.7 | CSA1 | |
| 13 | C | 0.01 | 5.3 | 9.9 | 4.6 | % type I | |
| 13 | C | 0.05 | 71.5 | 74.0 | 2.5 | CSAIIA | |
| 14 | C | 0.05 | 93.6 | 102.3 | 8.7 | CSAIIA | |
| 15 | C | 0.1 | 12.1 | 20.3 | 8.2 | TOTAL | |
| 16 | C | 0.05 | 68.9 | 75.1 | 6.2 | CSA1, CSA2A | |
| X | C | 0.01 | 11.8 | 52.5 | 40.7 | total |
Refined previously identified QTL in the LG/J and SM/J F2 intercross (47).
QTL, quantitative trait locus; C, chromosome-wide threshold; G, genome-wide threshold. “Level,” level of significance; Start, genomic positions based on GRCm38.p3; LG, increasing allele is LG/J; SM, increasing allele is SM/J.
The QTL at the genome-wide level of significance for CSA of type I and type IIA fibers on chromosome 3 was named Mfq5. The QTL at the genome-wide level of significance for the percentage of type I fibers on chromosome 2 and 4 were named Mfq4 and Mfq6, respectively. The SM/J allele conferred a greater percentage of type I fibers at Mfq4, and a greater CSA at Mfq5. The LG/J allele conferred a greater percentage of type I fibers at Mfq6 locus.
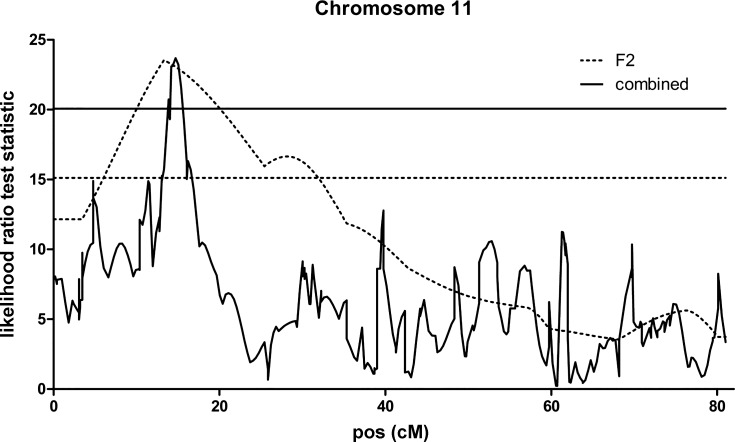
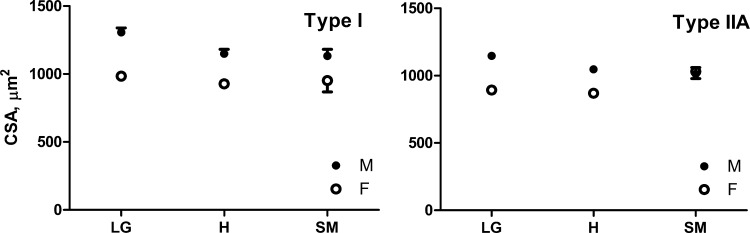
A significant QTL affecting CSA of type I and type IIA fibers was also detected on chromosome 11 (Fig. 3) within the same region as locus Mfq3, previously identified in the F2 intercross of the same parental strains (11). The QTL exhibited male specificity in both type I and IIA fibers of the F34 mice (Fig. 4). Because this QTL recapitulated properties of the Mfq3 locus, which we also found to be male specific in the F2 population, we concluded that the same locus has been refined in F34 and did not assign a new name for this QTL. The earlier-reported Mfq2 locus has been refined in a similar manner; a QTL on chromosome 6 affecting CSA of type I and type IIA fibers (at 1% chromosome specific threshold) was engulfed by the support interval of Mfq2 and also replicated its increasing allele, LG/J, in both females and males (not shown).
Fig. 3.
Type I fiber cross-sectional area quantitative trait loci (QTL) on chromosome 11. Analyses were carried out in the F2 intercross and in the combined F2 and F34 populations. x-Axis indicates the relative position in the linkage map in centimorgan (cM). The thresholds are at the level of 0.05 genome wise significance for the F2 output (dotted line) and combined output (solid line).
Fig. 4.
Sex specificity of Mfq3 locus on cross-sectional area (CSA) of soleus type I and IIA fibers in the F34 intercross. Means and SE. Genotype at the peak marker: LG, homozygous for LG/J allele; H, heterozygous; SM, homozygous for SM/J allele.
Gene Expression Analyses
We hypothesized that each identified QTL harbors one or more genetic variants that drive phenotypic differences by means of differential gene expression. Hypothesis-driven analysis of differential expression in soleus muscle was performed between LG/J and SM/J strains for the genes in the most robust QTLs affecting fiber CSA or % type I fibers (Mfq3, Mfq4, Mfq5, and Mfq6). The Mouse Gene 2.0 ST expression array contains 159 genes residing within the support intervals of these QTLs (Supplementary Table S2). Twenty genes (Table 2) showed evidence of differential expression (ANOVA, P ≤ 0.05), two of which, Alad and Hdhd3, were significant after correction for the multiple testing problem (Storey’s FDR q ≤ 0.05). Compared with other tissues and cell types, expression of differentially expressed genes Mafb, Acyp2, and Mtif2 (Table 2) is particularly enriched in skeletal muscle (BioGPS, Mouse MOE430 gene expression data).
Table 2.
Positional candidate genes differentially expressed between LG/J and SM/J soleus muscles
| Chr | QTL | Probe set ID | Gene | P Value | Fold-Change | Gene Name |
|---|---|---|---|---|---|---|
| 2 | Mfq4 | 17393868 | Mafb | 0.033 | −1.77 | v-maf musculoaponeurotic fibrosarcoma oncogene family, protein B (avian) |
| 17393910 | Chd6 | 0.042724 | −1.13 | chromodomain helicase DNA binding protein 6 | ||
| 17404652 | Gm24780 | 0.032914 | −1.98 | predicted gene Gm24780, predicted protein is B4HDV3 | ||
| 3 | Mfq5 | 17396801 | Ttc14 | 0.033387 | −1.14 | tetratricopeptide repeat domain 14 |
| 17396876 | 0.0219376 | −1.80 | there are no assigned mRNA sequences for this probe set; the probe set lies within lncRNA Sox2ot (Sox2 overlapping transcript, nonprotein coding) | |||
| 17425606 | Gm12526 | 0.046791 | −1.17 | predicted gene 12526 | ||
| 17414380 | Gm24277 | 0.00525644 | −2.07 | Gm24277, a known snRNA; the probe set also lies within an intronic region of RefSeq gene Pakap (PALM2-AKAP2), a read-through transcript on chromosome 4 | ||
| 17425701 | Mir3095 | 0.0298235 | −1.78 | Mir3095 (Entrez ID 100526502; EST ENSMUST00000175552). | ||
| 17426097 | Mup3 | 0.00304 | −1.49 | major urinary protein 3 | ||
| 17426126 | Fkbp15 | 0.018038 | −1.10 | FK506 binding protein 15 | ||
| 4 | Mfq6 | 17414545 | Slc31a1 | 0.049799 | 1.12 | solute carrier family 31, member 1 |
| 17426166 | Cdc26 | 0.021407 | −1.29 | cell division cycle 26 | ||
| 17426198 | Hdhd3 | 0.000869 | 1.86 | haloacid dehalogenase-like hydrolase domain containing 3 | ||
| 17426206 | Alad | 9.75E-05 | 1.91 | aminolevulinate, delta-, dehydratase | ||
| 17414600 | Rgs3 | 0.023096 | 1.08 | regulator of G protein signaling 3 | ||
| 17248064 | Mtif2 | 0.015517 | −1.16 | mitochondrial translational initiation factor 2 | ||
| 17261285 | LOC102637613 | 0.00432682 | 1.68 | linc RNA [AK084560 (EST)/ Gm12092 (predicted gene)]. | ||
| 17248127 | 0.00187422 | −1.46 | there are no assigned mRNA sequences for this transcript; the probe set lies within an intron of Sptbn1 | |||
| 11 | Mfq3 | 17261393 | Acyp2 | 0.011777 | 1.26 | acylphosphatase 2, muscle type |
| 17248196 | Asb3 | 0.014221 | −1.15 | ankyrin repeat and SOCS box-containing 3 |
P Value, ANOVA P value for strain effect; fold change uses SM/J as baseline (negative values indicate LG/J expression is down compared with SM/J, positive values LG/J expression up compared with SM/J); boldface indicates that gene is predominantly and/ or strongly expressed in skeletal muscle tissue (79). “Gene Name,” for probe sets not designed against an annotated gene, genes at the genomic loci of the Affymetrix probe set were identified in UCSC genome browser using mouse genome build GRCm38.
Genomic Analyses
Positional candidates with nonsynonymous polymorphisms provide a plausible genetic cause for the phenotypic differences. Based on the genomic sequence of the LG/J and SM/J strains (56), we identified 21 genes in the QTL regions with nonsynonymous polymorphisms predicted to affect protein function by at least one out of three algorithms used in the analysis (Supplementary Table S3). Four of those genes (Mfq3: Mtif2, Rtn4, Psme4, Mfq5: Dnajc19) are prioritized further because of their preferential expression in muscle lineage (differentiated muscle and/or C2C12 myoblasts) compared with other tissues and cell types. Among those, the Mtif2 gene differs by three (rs26871496, rs26871494, rs29436813) and Rtn4 by nine (rs29473364, rs29469198, rs13463765, rs29465940, rs26857726, rs26857725, rs29474377, rs26857722, rs26857721) amino acids between the two strains. At all SNPs the SM/J strain carries reference while the LG/J strain the alternative allele.
Candidate Gene Analyses
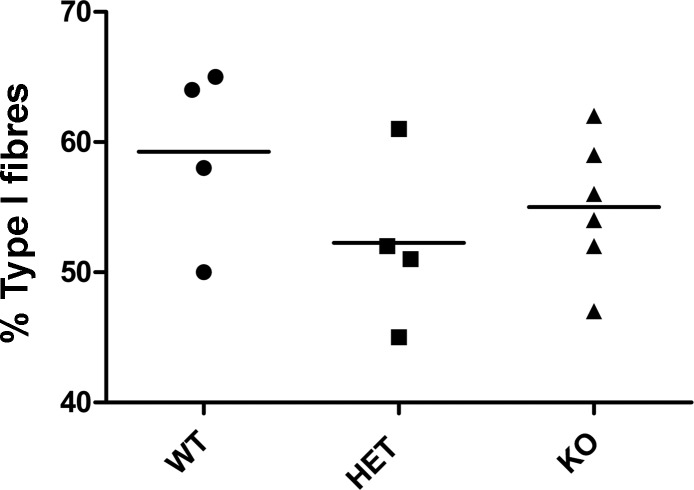
The Chd6 gene emerged as a differentially expressed positional candidate for the Mfq4 locus affecting percentage of type I fibers (Table 2). To test its effect we examined soleus muscles of Chd6 knockout, heterozygous, and wild-type littermates. This analysis however revealed that the genotype of the animals did not have a significant effect (P = 0.30) on the percentage of type I fibers (Fig. 5).
Fig. 5.
Percentage of type I fibers in the soleus muscle of 4 mo old Chd6 knockout (KO), heterozygous (HET), and wild-type (WT) females. There is no difference in percentage of type I muscle fibers in the soleus muscle between KO, HET, and WT groups (P = 0.3041). Each data point is from a single mouse; horizontal lines represent group mean.
The Alad gene emerged as a candidate for another locus affecting proportion of type I fibers, Mfq6. In the animals of an advanced intercross between the C57BL/6J and DBA/2J strains [these strains carry one or three copies of Alad, respectively (3)], we examined if the percentage of type I fibers was genotype dependent. The analysis revealed no difference in the percentage of type I fibers between the carriers of the C57BL/6J and the DBA/2J alleles, 42 ± 7 and 42 ± 8%, respectively.
DISCUSSION
A previous study on muscle weight in LG/J and SM/J strains identified a twofold difference in soleus muscle size (47). We then explored the cellular and genetic mechanisms contributing to this phenomenon, finding that the difference was largely due to the CSA of muscle fibers, and we mapped QTL affecting muscle fiber traits in an F2 intercross between the LG/J and SM/J strains (11). The present study, which utilizes the F34 advanced intercross, verified, refined, and expanded our earlier findings.
A number of studies have previously reported the effects of Stat5a and Stat5b (36), Pgc-1α (42), Ky (4), myostatin (54), leptin (61), calcineurin (76), Sod1 (5), alpha-actinin-3 (50), dystrophin (7), Tbx15 (41), and IIB myosin heavy chains (2) genes on muscle fiber area in knockout or mutant models. In addition, Pgc-1α (75), calcineurin (76), Foxo1 (34), and myostatin (20) are reported to affect the proportion of muscle fiber types. However, the genomic positions of these genes have not been linked to muscle fiber differences between the LG/J and SM/J strains, implicating involvement of novel genes.
Muscle Fiber Number
The number of fibers is an important determinant of muscle size and functional properties. It is set during embryogenesis and the first postnatal week in mice (78). The number of muscle fibers in males (667 ± 105) and females (646 ± 102) of the F34 population was comparable to that observed in the soleus of the F2 population (645 ± 102 and 595 ± 107, respectively) and within the range of the fiber count observed in solei of a variety of different strains of mice ~250–~900 fibers (32, 49, 57, 70, 72).
From these data it emerged that males and females are born with a similar number of fibers in soleus muscle and that the sex difference in muscle weight (males have ~30% larger soleus than females) is due to the difference in fiber size. Comparison of the parental strains also revealed a similar number of fibers (11), despite the twofold difference in soleus weight (47), demonstrating that size rather than number of fibers determines variation in muscle weight between the LG/J and SM/J strains.
Fiber Area
The CSA of muscle fibers in the LG/J strain is 49–90% greater than the corresponding fibers in the SM/J strain, indicating that this variable accounts for a large portion of the muscle mass difference between the strains (47).
The area of type I (1,084 ± 238 μm2 and 913 ± 229 μm2 for males and females, respectively) and type IIA (1,215 ± 294 μm2 and 952 ± 242 μm2, respectively) of the F34 mice was comparable to the corresponding fiber area of the F2 mice of the same lineage (11), and it is within the range reported for the type I, between 920 and 1,780 μm2 (32, 57, 70), and type IIA fiber area, between 700 and 1,400 μm2 (32, 70), in various inbred mouse strains.
Percentage of Type I Fibers
The percentage of type I fibers in male (39 ± 6%) and female (47 ± 8%) F34 mice were also within the range of previous studies, which showed that percentage of type I fibers in the soleus muscle fluctuates between ~25 and ~66% (32, 57, 70).
In the F34 mice we replicated our observation in the F2 population that the percentage of type I fibers was significantly greater in females than males. This sex difference is also observed in various human muscles where, in general, women have a higher percentage of type I muscle fibers than males (27, 53, 60, 64, 67). The phenomenon is likely to be explained, at least partly, by the effect of androgens; castration leads to a higher percentage of type I fibers in the soleus of male mice (73).
Validation and Refinement of Genetic Architecture
In the present study, we validated and refined the genetic architecture of muscle fibers identified in an F2 intercross between the same parental strains (11). To increase QTL detection power, we increased sample size by combining the F34 and F2 data. The median mapping resolution of 4.6 Mb for muscle fiber QTLs was comparable with 3.7 Mb of muscle weight QTLs obtained in the same population albeit using ~1,600 fewer genetic markers than in the present analysis (47). A genome-wide significant QTL identified in the present study between 27.9 and 31.4 Mb on chromosome 11 (Table 1) overlapped with a significant QTL, Mfq3, mapped in the F2 population (11). In addition to the chromosomal location, the increasing allele of this locus (LG/J) and its male-specific effect (Fig. 4) were also replicated in F34, suggesting that the same gene(s) were involved in two different populations and permitting us to refine the Mfq3 locus from 51.6 to 3.57 Mb. The presence of two satellite QTL proximal of the refined Mfq3 (Table 1) suggests that the QTL observed in the F2 population (11) might have been an outcome of up to three linked loci.
The recently reported “mini-muscle” locus, mapped to 67.1–70.2 Mb on chromosome 11, affects muscle fiber area and proportion of fiber types (21–23). However, the mutation responsible for the mini-muscle phenotype maps to an intron of Myh4 gene located at 67.2 Mb (31), between the support intervals of two adjacent QTLs affecting fiber type between the LG/J and SM/J strains (Table 1). Together, these data suggest that a number of genes residing on chromosome 11 might be involved in the regulation of muscle fiber phenotypes.
The QTL affecting the CSA of type I and type IIA fibers on chromosome 6, albeit at 1% chromosome-wide threshold of significance (Table 1), overlapped with the Mfq2 locus found in the F2 population, characterized by the same increasing allele, LG/J. Thus, the support interval of Mfq2 could be considered to be 5.18 Mb rather than the previously reported 56.5 Mb. Importantly, the immediate proximity of the refined region (Chr 6: 110.8–116.0 Mb) to the syntenic region (Chr 6:116.0–118.0 Mb) implicated in the QTL affecting the diameter of pig IIA fibers (17) suggests that the same genes could be underlying the effects of these QTLs in mice.
A QTL affecting percentage of type I fibers (at 10% chromosome-wide threshold) on chromosome 1 (67.6–70.8 Mb) overlapped with the Mfq1 locus, which influenced the CSA of type I and type IIA fiber area in the F2 population (11). However, because the CSA and percentage of type I fibers are poorly correlated traits both in the F34 (Table 3) and the F2 mice (11), it is likely that different genes are underlying the Mfq1 locus and the QTL identified in the F34 population. Further studies are required to clarify this observation.
Table 3.
Phenotypic correlations between muscle fiber traits in F34 mice of advanced intercross lines between the LG/J and SM/J strains
| ♂\♀ | Type I, % | Total Fibers | Type I CSA | Type II CSA |
|---|---|---|---|---|
| % of type I | −0.19 | 0.11 | 0.01 | |
| Total fibers | −0.02 | −0.08 | −0.03 | |
| Type I CSA | 0.00 | 0.08 | 0.49 | |
| Type II CSA | 0.03 | 0.26 | 0.26 |
Female above, males below the diagonal.
Transcriptome Analysis
In the present study, the expressed transcriptome in soleus muscle of the parental strains was examined to facilitate nomination of the candidate genes within the refined QTL. We hypothesized that if the phenotypic effect of the QTL was brought about by the allele-specific abundance of transcripts encoded by genes within the QTL, such genes would be differentially expressed in the transcriptome between the parental strains. Comparison of expression of the genes within the four most robust QTLs identified Alad and Hdhd3 genes as potential candidates for the Mfq6 locus, which affects the proportion of type I fibers. Transcripts of both genes are more abundant in the LG/J compared with the SM/J strain. This is consistent with our findings in the TA muscle of the same strains (48). Of these two identified candidate genes, transcripts of Alad are ~20 times more abundant in the mouse muscle than Hdhd3, regardless of strain. In addition, Alad may play a role during myogenesis as its expression in C2C12 myogenic cells is fivefold higher compared with differentiated muscle (79).
Candidate Genes
The support intervals of four most robust QTLs harbor 159 genes (Supplementary Table S2). These regions were scrutinized further for genes fulfilling one of the following criteria: presence of the functional variants (i.e., nonsynonymous SNPs predicted to alter function of encoded protein); abundance of transcript in muscle lineage, particularly in comparison to other tissues and cell types; differential expression in the soleus of the two strains; and by comparing genomic sequence between the LG/J and SM/J strains a list of 21 genes was highlighted (Supplementary Table S3) with the strain-specific functional variants. Using bioinformatics, we identified four genes abundantly and/or preferentially expressed in skeletal muscle compared with other types of tissues and cells. Our own analysis of gene expression in soleus muscle highlighted a set of 20 genes differentially expressed between the two strains (Table 2). Intersection of all these lists permitted us to prioritize nine candidate genes that appeared on more than one of these lists and/or for which independent and accessible validation models were available (i.e., Chd6 and Alad). Because neither the Chd6 (Fig. 5) nor Alad gene was found to affect proportion of type I fibers in the way predicted by the QTL analyses, the list of prioritized candidates was reduced to seven genes annotated in Supplementary Table S4. Three out of four QTLs contain one (Mfq6) or more candidate genes. All candidates are abundantly transcribed in muscle lineage with Psme4, Acyp2, and Mafb showing the highest level of expression in skeletal muscle compared with other tissues and cells. None of the seven candidates have been previously implicated to affect properties of skeletal muscle fibers, although some of them have been implicated in cardiomyopathy or function as transcription factors (Supplementary Table S4). Thus, genomic and gene expression analyses permitted focusing on a limited number of positional candidates in the future validation studies for establishing the causative genes.
Conclusion
In conclusion, we have refined the genetic architecture affecting CSA of soleus muscle fibers and proportion of type I fibers in the LG/J- and SM/J-derived lineage. Integrating QTL mapping and genomic and transcriptome data from homologous muscle highlighted several candidate genes that may underpin muscle phenotypes critical to health and disease and may be worthy of follow up analyses.
GRANTS
This study was supported by National Institutes of Health Grants R01AR-056280 (A. Lionikas) and R01DA-024845 (A. A. Palmer) and Marie Curie International Reintegration Grant 249156 (A. Lionikas).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
A.L. conceived and supervised the study, A.M.C. phenotyped muscle samples, A.A.P. oversaw the breeding of the mice used in this study, provided genotypes and oversaw the QTL analyses, R.C. designed the QTLRel software used in the QTL analysis, A.M.C. carried out QTL mapping, M.E.S, C.M., and E.C.D. did transcriptome analysis, S.N.F. and J.L.F. generated and provided Chd6 knockout samples, A.L. and A.M.C. wrote the manuscript with input from all coauthors.
Supplementary Material
ACKNOWLEDGMENTS
The authors acknowledge technical assistance from Diane Stewart, Jasmine C. Berry, and Jake T. Brown. We acknowledge the Dartmouth Mouse Modeling Shared Resource of the Norris Cotton Cancer Center (support grant P30CA023108-27), for providing Chd6 knockout mouse samples.
REFERENCES
- 1.Abe T, Saburi J, Hasebe H, Nakagawa T, Kawamura T, Saito K, Nade T, Misumi S, Okumura T, Kuchida K, Hayashi T, Nakane S, Mitsuhasi T, Nirasawa K, Sugimoto Y, Kobayashi E. Bovine quantitative trait loci analysis for growth, carcass, and meat quality traits in an F2 population from a cross between Japanese Black and Limousin. J Anim Sci 86: 2821–2832, 2008. doi: 10.2527/jas.2007-0676. [DOI] [PubMed] [Google Scholar]
- 2.Allen DL, Sartorius CA, Sycuro LK, Leinwand LA. Different pathways regulate expression of the skeletal myosin heavy chain genes. J Biol Chem 276: 43524–43533, 2001. doi: 10.1074/jbc.M108017200. [DOI] [PubMed] [Google Scholar]
- 3.Bishop TR, Miller MW, Wang A, Dierks PM. Multiple copies of the ALA-D gene are located at the Lv locus in Mus domesticus mice. Genomics 48: 221–231, 1998. doi: 10.1006/geno.1997.5183. [DOI] [PubMed] [Google Scholar]
- 4.Blanco G, Coulton GR, Biggin A, Grainge C, Moss J, Barrett M, Berquin A, Maréchal G, Skynner M, van Mier P, Nikitopoulou A, Kraus M, Ponting CP, Mason RM, Brown SD. The kyphoscoliosis (ky) mouse is deficient in hypertrophic responses and is caused by a mutation in a novel muscle-specific protein. Hum Mol Genet 10: 9–16, 2001. doi: 10.1093/hmg/10.1.9. [DOI] [PubMed] [Google Scholar]
- 5.Bordet T, Schmalbruch H, Pettmann B, Hagege A, Castelnau-Ptakhine L, Kahn A, Haase G. Adenoviral cardiotrophin-1 gene transfer protects pmn mice from progressive motor neuronopathy. J Clin Invest 104: 1077–1085, 1999. doi: 10.1172/JCI6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bottinelli R, Reggiani C. Human skeletal muscle fibres: molecular and functional diversity. Prog Biophys Mol Biol 73: 195–262, 2000. doi: 10.1016/S0079-6107(00)00006-7. [DOI] [PubMed] [Google Scholar]
- 7.Briguet A, Courdier-Fruh I, Foster M, Meier T, Magyar JP. Histological parameters for the quantitative assessment of muscular dystrophy in the mdx-mouse. Neuromuscul Disord 14: 675–682, 2004. doi: 10.1016/j.nmd.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 8.Brooke MH, Kaiser KK. Muscle fiber types: how many and what kind? Arch Neurol 23: 369–379, 1970. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- 9.Carbonetto P, Cheng R, Gyekis JP, Parker CC, Blizard DA, Palmer AA, Lionikas A. Discovery and refinement of muscle weight QTLs in B6 × D2 advanced intercross mice. Physiol Genomics 46: 571–582, 2014. doi: 10.1152/physiolgenomics.00055.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carpenter CE, Rice OD, Cockett NE, Snowder GD. Histology and composition of muscles from normal and callipyge lambs. J Anim Sci 74: 388–393, 1996. doi: 10.2527/1996.742388x. [DOI] [PubMed] [Google Scholar]
- 11.Carroll AM, Palmer AA, Lionikas A. QTL analysis of type I and type IIA fibers in soleus muscle in a cross between LG/J and SM/J mouse strains. Front Genet 2: 99, 2012. doi: 10.3389/fgene.2011.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng R, Abney M, Palmer AA, Skol AD. QTLRel: an R package for genome-wide association studies in which relatedness is a concern. BMC Genet 12: 66, 2011. doi: 10.1186/1471-2156-12-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng R, Lim JE, Samocha KE, Sokoloff G, Abney M, Skol AD, Palmer AA. Genome-wide association studies and the problem of relatedness among advanced intercross lines and other highly recombinant populations. Genetics 185: 1033–1044, 2010. doi: 10.1534/genetics.110.116863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng R, Palmer AA. A simulation study of permutation, bootstrap, and gene dropping for assessing statistical significance in the case of unequal relatedness. Genetics 193: 1015–1018, 2013. doi: 10.1534/genetics.112.146332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costill DL, Daniels J, Evans W, Fink W, Krahenbuhl G, Saltin B. Skeletal muscle enzymes and fiber composition in male and female track athletes. J Appl Physiol 40: 149–154, 1976. [DOI] [PubMed] [Google Scholar]
- 16.Darvasi A, Soller M. Advanced intercross lines, an experimental population for fine genetic mapping. Genetics 141: 1199–1207, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Estellé J, Gil F, Vázquez JM, Latorre R, Ramírez G, Barragán MC, Folch JM, Noguera JL, Toro MA, Pérez-Enciso M. A quantitative trait locus genome scan for porcine muscle fiber traits reveals overdominance and epistasis. J Anim Sci 86: 3290–3299, 2008. doi: 10.2527/jas.2008-1034. [DOI] [PubMed] [Google Scholar]
- 18.Fink WJ, Costill DL, Pollock ML. Submaximal and maximal working capacity of elite distance runners. Part II. Muscle fiber composition and enzyme activities. Ann N Y Acad Sci 301: 323–327, 1977. doi: 10.1111/j.1749-6632.1977.tb38210.x. [DOI] [PubMed] [Google Scholar]
- 19.Frederiksen H, Gaist D, Petersen HC, Hjelmborg J, McGue M, Vaupel JW, Christensen K. Hand grip strength: a phenotype suitable for identifying genetic variants affecting mid- and late-life physical functioning. Genet Epidemiol 23: 110–122, 2002. doi: 10.1002/gepi.1127. [DOI] [PubMed] [Google Scholar]
- 20.Girgenrath S, Song K, Whittemore LA. Loss of myostatin expression alters fiber-type distribution and expression of myosin heavy chain isoforms in slow- and fast-type skeletal muscle. Muscle Nerve 31: 34–40, 2005. doi: 10.1002/mus.20175. [DOI] [PubMed] [Google Scholar]
- 21.Guderley H, Houle-Leroy P, Diffee GM, Camp DM, Garland T Jr. Morphometry, ultrastructure, myosin isoforms, and metabolic capacities of the “mini muscles” favoured by selection for high activity in house mice. Comp Biochem Physiol B Biochem Mol Biol 144: 271–282, 2006. doi: 10.1016/j.cbpb.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Guderley H, Joanisse DR, Mokas S, Bilodeau GM, Garland T Jr. Altered fibre types in gastrocnemius muscle of high wheel-running selected mice with mini-muscle phenotypes. Comp Biochem Physiol B Biochem Mol Biol 149: 490–500, 2008. doi: 10.1016/j.cbpb.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 23.Hartmann J, Garland T Jr, Hannon RM, Kelly SA, Muñoz G, Pomp D. Fine mapping of “mini-muscle,” a recessive mutation causing reduced hindlimb muscle mass in mice. J Hered 99: 679–687, 2008. doi: 10.1093/jhered/esn040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernelahti M, Tikkanen HO, Karjalainen J, Kujala UM. Muscle fiber-type distribution as a predictor of blood pressure: a 19-year follow-up study. Hypertension 45: 1019–1023, 2005. doi: 10.1161/01.HYP.0000165023.09921.34. [DOI] [PubMed] [Google Scholar]
- 25.Hubal MJ, Gordish-Dressman H, Thompson PD, Price TB, Hoffman EP, Angelopoulos TJ, Gordon PM, Moyna NM, Pescatello LS, Visich PS, Zoeller RF, Seip RL, Clarkson PM. Variability in muscle size and strength gain after unilateral resistance training. Med Sci Sports Exerc 37: 964–972, 2005. [PubMed] [Google Scholar]
- 26.Huygens W, Thomis MA, Peeters MW, Vlietinck RF, Beunen GP. Determinants and upper-limit heritabilities of skeletal muscle mass and strength. Can J Appl Physiol 29: 186–200, 2004. doi: 10.1139/h04-014. [DOI] [PubMed] [Google Scholar]
- 27.Jaworowski A, Porter MM, Holmbäck AM, Downham D, Lexell J. Enzyme activities in the tibialis anterior muscle of young moderately active men and women: relationship with body composition, muscle cross-sectional area and fibre type composition. Acta Physiol Scand 176: 215–225, 2002. doi: 10.1046/j.1365-201X.2002.t01-2-01004.x. [DOI] [PubMed] [Google Scholar]
- 28.Kadi F, Eriksson A, Holmner S, Butler-Browne GS, Thornell LE. Cellular adaptation of the trapezius muscle in strength-trained athletes. Histochem Cell Biol 111: 189–195, 1999. doi: 10.1007/s004180050348. [DOI] [PubMed] [Google Scholar]
- 29.Karjalainen J, Tikkanen H, Hernelahti M, Kujala UM. Muscle fiber-type distribution predicts weight gain and unfavorable left ventricular geometry: a 19 year follow-up study. BMC Cardiovasc Disord 6: 2, 2006. doi: 10.1186/1471-2261-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karp CL, Grupe A, Schadt E, Ewart SL, Keane-Moore M, Cuomo PJ, Köhl J, Wahl L, Kuperman D, Germer S, Aud D, Peltz G, Wills-Karp M. Identification of complement factor 5 as a susceptibility locus for experimental allergic asthma. Nat Immunol 1: 221–226, 2000. doi: 10.1038/79759. [DOI] [PubMed] [Google Scholar]
- 31.Kelly SA, Bell TA, Selitsky SR, Buus RJ, Hua K, Weinstock GM, Garland T Jr, Pardo-Manuel de Villena F, Pomp D. A novel intronic single nucleotide polymorphism in the myosin heavy polypeptide 4 gene is responsible for the mini-muscle phenotype characterized by major reduction in hind-limb muscle mass in mice. Genetics 195: 1385–1395, 2013. doi: 10.1534/genetics.113.154476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kilikevicius A, Venckunas T, Zelniene R, Carroll AM, Lionikaite S, Ratkevicius A, Lionikas A. Divergent physiological characteristics and responses to endurance training among inbred mouse strains. Scand J Med Sci Sports 23: 657–668, 2013. doi: 10.1111/j.1600-0838.2012.01451.x. [DOI] [PubMed] [Google Scholar]
- 33.Kim J, Wang Z, Heymsfield SB, Baumgartner RN, Gallagher D. Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutr 76: 378–383, 2002. [DOI] [PubMed] [Google Scholar]
- 34.Kitamura T, Kitamura YI, Funahashi Y, Shawber CJ, Castrillon DH, Kollipara R, DePinho RA, Kitajewski J, Accili D. A Foxo/Notch pathway controls myogenic differentiation and fiber type specification. J Clin Invest 117: 2477–2485, 2007. doi: 10.1172/JCI32054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein RF, Allard J, Avnur Z, Nikolcheva T, Rotstein D, Carlos AS, Shea M, Waters RV, Belknap JK, Peltz G, Orwoll ES. Regulation of bone mass in mice by the lipoxygenase gene Alox15. Science 303: 229–232, 2004. doi: 10.1126/science.1090985. [DOI] [PubMed] [Google Scholar]
- 36.Klover P, Chen W, Zhu BM, Hennighausen L. Skeletal muscle growth and fiber composition in mice are regulated through the transcription factors STAT5a/b: linking growth hormone to the androgen receptor. FASEB J 23: 3140–3148, 2009. doi: 10.1096/fj.08-128215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Komi PV, Viitasalo JH, Havu M, Thorstensson A, Sjödin B, Karlsson J. Skeletal muscle fibres and muscle enzyme activities in monozygous and dizygous twins of both sexes. Acta Physiol Scand 100: 385–392, 1977. [DOI] [PubMed] [Google Scholar]
- 38.Koohmaraie M, Shackelford SD, Wheeler TL, Lonergan SM, Doumit ME. A muscle hypertrophy condition in lamb (callipyge): characterization of effects on muscle growth and meat quality traits. J Anim Sci 73: 3596–3607, 1995. doi: 10.2527/1995.73123596x. [DOI] [PubMed] [Google Scholar]
- 39.Lander E, Kruglyak L. Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results. Nat Genet 11: 241–247, 1995. doi: 10.1038/ng1195-241. [DOI] [PubMed] [Google Scholar]
- 40.Lathrop MJ, Chakrabarti L, Eng J, Rhodes CH, Lutz T, Nieto A, Liggitt HD, Warner S, Fields J, Stöger R, Fiering S. Deletion of the Chd6 exon 12 affects motor coordination. Mamm Genome 21: 130–142, 2010. doi: 10.1007/s00335-010-9248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee KY, Singh MK, Ussar S, Wetzel P, Hirshman MF, Goodyear LJ, Kispert A, Kahn CR. Tbx15 controls skeletal muscle fibre-type determination and muscle metabolism. Nat Commun 6: 8054, 2015. doi: 10.1038/ncomms9054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leick L, Hellsten Y, Fentz J, Lyngby SS, Wojtaszewski JF, Hidalgo J, Pilegaard H. PGC-1alpha mediates exercise-induced skeletal muscle VEGF expression in mice. Am J Physiol Endocrinol Metab 297: E92–E103, 2009. doi: 10.1152/ajpendo.00076.2009. [DOI] [PubMed] [Google Scholar]
- 43.Li WB, Ren J, Zhu WC, Guo BL, Yang B, Liu LT, Ding NS, Ma JW, Li L, Huang LS. Mapping QTL for porcine muscle fibre traits in a White Duroc x Erhualian F(2) resource population. J Anim Breed Genet 126: 468–474, 2009. doi: 10.1111/j.1439-0388.2009.00805.x. [DOI] [PubMed] [Google Scholar]
- 44.Lillioja S, Young AA, Culter CL, Ivy JL, Abbott WG, Zawadzki JK, Yki-Järvinen H, Christin L, Secomb TW, Bogardus C. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Invest 80: 415–424, 1987. doi: 10.1172/JCI113088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lionikas A, Blizard DA, Gerhard GS, Vandenbergh DJ, Stout JT, Vogler GP, McClearn GE, Larsson L. Genetic determinants of weight of fast- and slow-twitch skeletal muscle in 500-day-old mice of the C57BL/6J and DBA/2J lineage. Physiol Genomics 21: 184–192, 2005. doi: 10.1152/physiolgenomics.00209.2004. [DOI] [PubMed] [Google Scholar]
- 46.Lionikas A, Blizard DA, Vandenbergh DJ, Glover MG, Stout JT, Vogler GP, McClearn GE, Larsson L. Genetic architecture of fast- and slow-twitch skeletal muscle weight in 200-day-old mice of the C57BL/6J and DBA/2J lineage. Physiol Genomics 16: 141–152, 2003. doi: 10.1152/physiolgenomics.00103.2003. [DOI] [PubMed] [Google Scholar]
- 47.Lionikas A, Cheng R, Lim JE, Palmer AA, Blizard DA. Fine-mapping of muscle weight QTL in LG/J and SM/J intercrosses. Physiol Genomics 42A: 33–38, 2010. doi: 10.1152/physiolgenomics.00100.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lionikas A, Meharg C, Derry JM, Ratkevicius A, Carroll AM, Vandenbergh DJ, Blizard DA. Resolving candidate genes of mouse skeletal muscle QTL via RNA-Seq and expression network analyses. BMC Genomics 13: 592, 2012. doi: 10.1186/1471-2164-13-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luff AR, Goldspink G. Total number of fibers in muscles of several strains of mice. J Anim Sci 30: 891–893, 1970. doi: 10.2527/jas1970.306891x. [DOI] [PubMed] [Google Scholar]
- 50.MacArthur DG, Seto JT, Chan S, Quinlan KG, Raftery JM, Turner N, Nicholson MD, Kee AJ, Hardeman EC, Gunning PW, Cooney GJ, Head SI, Yang N, North KN. An Actn3 knockout mouse provides mechanistic insights into the association between alpha-actinin-3 deficiency and human athletic performance. Hum Mol Genet 17: 1076–1086, 2008. doi: 10.1093/hmg/ddm380. [DOI] [PubMed] [Google Scholar]
- 51.MacDougall JD, Sale DG, Alway SE, Sutton JR. Muscle fiber number in biceps brachii in bodybuilders and control subjects. J Appl Physiol Respir Environ Exerc Physiol 57: 1399–1403, 1984. [DOI] [PubMed] [Google Scholar]
- 52.Malek M, Dekkers JC, Lee HK, Baas TJ, Prusa K, Huff-Lonergan E, Rothschild MF. A molecular genome scan analysis to identify chromosomal regions influencing economic traits in the pig. II. Meat and muscle composition. Mamm Genome 12: 637–645, 2001. doi: 10.1007/s003350020019. [DOI] [PubMed] [Google Scholar]
- 53.Mannion AF, Dumas GA, Cooper RG, Espinosa FJ, Faris MW, Stevenson JM. Muscle fibre size and type distribution in thoracic and lumbar regions of erector spinae in healthy subjects without low back pain: normal values and sex differences. J Anat 190: 505–513, 1997. doi: 10.1046/j.1469-7580.1997.19040505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-beta superfamily member. Nature 387: 83–90, 1997. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 55.Nii M, Hayashi T, Mikawa S, Tani F, Niki A, Mori N, Uchida Y, Fujishima-Kanaya N, Komatsu M, Awata T. Quantitative trait loci mapping for meat quality and muscle fiber traits in a Japanese wild boar x Large White intercross. J Anim Sci 83: 308–315, 2005. doi: 10.2527/2005.832308x. [DOI] [PubMed] [Google Scholar]
- 56.Nikolskiy I, Conrad DF, Chun S, Fay JC, Cheverud JM, Lawson HA. Using whole-genome sequences of the LG/J and SM/J inbred mouse strains to prioritize quantitative trait genes and nucleotides. BMC Genomics 16: 415, 2015. doi: 10.1186/s12864-015-1592-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nimmo MA, Wilson RH, Snow DH. The inheritance of skeletal muscle fibre composition in mice. Comp Biochem Physiol A 81: 109–115, 1985. doi: 10.1016/0300-9629(85)90275-0. [DOI] [PubMed] [Google Scholar]
- 58.Pourhassan M, Bosy-Westphal A, Schautz B, Braun W, Glüer CC, Müller MJ. Impact of body composition during weight change on resting energy expenditure and homeostasis model assessment index in overweight nonsmoking adults. Am J Clin Nutr 99: 779–791, 2014. doi: 10.3945/ajcn.113.071829. [DOI] [PubMed] [Google Scholar]
- 59.Rehfeldt C, Ott G, Gerrard DE, Varga L, Schlote W, Williams JL, Renne U, Bünger L. Effects of the compact mutant myostatin allele Mstn (Cmpt-dl1Abc) introgressed into a high growth mouse line on skeletal muscle cellularity. J Muscle Res Cell Motil 26: 103–112, 2005. doi: 10.1007/s10974-005-1099-7. [DOI] [PubMed] [Google Scholar]
- 60.Roepstorff C, Donsmark M, Thiele M, Vistisen B, Stewart G, Vissing K, Schjerling P, Hardie DG, Galbo H, Kiens B. Sex differences in hormone-sensitive lipase expression, activity, and phosphorylation in skeletal muscle at rest and during exercise. Am J Physiol Endocrinol Metab 291: E1106–E1114, 2006. doi: 10.1152/ajpendo.00097.2006. [DOI] [PubMed] [Google Scholar]
- 61.Sáinz N, Rodríguez A, Catalán V, Becerril S, Ramírez B, Gómez-Ambrosi J, Frühbeck G. Leptin administration favors muscle mass accretion by decreasing FoxO3a and increasing PGC-1alpha in ob/ob mice. PLoS One 4: e6808, 2009. doi: 10.1371/journal.pone.0006808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schiaffino S. Fibre types in skeletal muscle: a personal account. Acta Physiol (Oxf) 199: 451–463, 2010. doi: 10.1111/j.1748-1716.2010.02130.x. [DOI] [PubMed] [Google Scholar]
- 63.Simoneau JA, Bouchard C. Genetic determinism of fiber type proportion in human skeletal muscle. FASEB J 9: 1091–1095, 1995. [DOI] [PubMed] [Google Scholar]
- 64.Simoneau JA, Bouchard C. Human variation in skeletal muscle fiber-type proportion and enzyme activities. Am J Physiol Endocrinol Metab 257: E567–E572, 1989. [DOI] [PubMed] [Google Scholar]
- 65.Sosnicki A. Histopathological observation of stress myopathy in M. longissimus in the pig and relationships with meat quality, fattening and slaughter traits. J Anim Sci 65: 584–596, 1987. doi: 10.2527/jas1987.652584x. [DOI] [PubMed] [Google Scholar]
- 66.Srikanthan P, Karlamangla AS. Muscle mass index as a predictor of longevity in older adults. Am J Med 127: 547–553, 2014. doi: 10.1016/j.amjmed.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Staron RS, Hagerman FC, Hikida RS, Murray TF, Hostler DP, Crill MT, Ragg KE, Toma K. Fiber type composition of the vastus lateralis muscle of young men and women. J Histochem Cytochem 48: 623–629, 2000. doi: 10.1177/002215540004800506. [DOI] [PubMed] [Google Scholar]
- 68.Stavaux D, Art T, McEntee K, Reznick M, Lekeux P. Muscle fibre type and size, and muscle capillary density in young double-muscled blue Belgian cattle. Zentralbl Veterinarmed A 41: 229–236, 1994. doi: 10.1111/j.1439-0442.1994.tb00089.x. [DOI] [PubMed] [Google Scholar]
- 69.Timson BF, Bowlin BK, Dudenhoeffer GA, George JB. Fiber number, area, and composition of mouse soleus muscle following enlargement. J Appl Physiol (1985) 58: 619–624, 1985. [DOI] [PubMed] [Google Scholar]
- 70.Totsuka Y, Nagao Y, Horii T, Yonekawa H, Imai H, Hatta H, Izaike Y, Tokunaga T, Atomi Y. Physical performance and soleus muscle fiber composition in wild-derived and laboratory inbred mouse strains. J Appl Physiol (1985) 95: 720–727, 2003. doi: 10.1152/japplphysiol.00946.2002. [DOI] [PubMed] [Google Scholar]
- 71.Van den Maagdenberg K, Stinckens A, Lefaucheur L, Buys N, De Smet S. The effect of mutations in the insulin-like growth factor-II and ryanodine receptor-1 genes on biochemical and histochemical muscle fibre characteristics in pigs. Meat Sci 79: 757–766, 2008. doi: 10.1016/j.meatsci.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 72.van der Laarse WJ, Diegenbach PC, Maslam S. Quantitative histochemistry of three mouse hind-limb muscles: the relationship between calcium-stimulated myofibrillar ATPase and succinate dehydrogenase activities. Histochem J 16: 529–541, 1984. doi: 10.1007/BF01041353. [DOI] [PubMed] [Google Scholar]
- 73.Vaughan HS, Aziz-Ullah, Goldspink G, Nowell NW. Sex and stock differences in the histochemical myofibrillar adenosine triphosphatase reaction in the soleus muscle of the mouse. J Histochem Cytochem 22: 155–159, 1974. doi: 10.1177/22.3.155. [DOI] [PubMed] [Google Scholar]
- 74.Wade AJ, Marbut MM, Round JM. Muscle fibre type and aetiology of obesity. Lancet 335: 805–808, 1990. doi: 10.1016/0140-6736(90)90933-V. [DOI] [PubMed] [Google Scholar]
- 75.Wang YX, Zhang CL, Yu RT, Cho HK, Nelson MC, Bayuga-Ocampo CR, Ham J, Kang H, Evans RM. Regulation of muscle fiber type and running endurance by PPARdelta. PLoS Biol 2: e294, 2004. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res 94: 110–118, 2004. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 77.Wimmers K, Fiedler I, Hardge T, Murani E, Schellander K, Ponsuksili S. QTL for microstructural and biophysical muscle properties and body composition in pigs. BMC Genet 7: 15, 2006. doi: 10.1186/1471-2156-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wirtz P, Loermans HM, Peer PG, Reintjes AG. Postnatal growth and differentiation of muscle fibres in the mouse. I. A histochemical and morphometrical investigation of normal muscle. J Anat 137: 109–126, 1983. [PMC free article] [PubMed] [Google Scholar]
- 79.Wu C, Orozco C, Boyer J, Leglise M, Goodale J, Batalov S, Hodge CL, Haase J, Janes J, Huss JW III, Su AI. BioGPS: an extensible and customizable portal for querying and organizing gene annotation resources. Genome Biol 10: R130, 2009. doi: 10.1186/gb-2009-10-11-r130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Y, Xu P, Lu C, Kuang Y, Zhang X, Cao D, Li C, Chang Y, Hou N, Li H, Wang S, Sun X. Genetic linkage mapping and analysis of muscle fiber-related QTLs in common carp (Cyprinus carpio L.). Mar Biotechnol (NY) 13: 376–392, 2011. doi: 10.1007/s10126-010-9307-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.