Abstract
INTRODUCTION
This pilot study was designed to compare the effectiveness of intermittent occlusion therapy (IO-Therapy) using liquid crystal glasses versus continuous occlusion therapy using traditional adhesive patches for treating amblyopia.
DESIGN
A randomized controlled trial. Children 3–8 years of age with previously untreated, moderate, unilateral amblyopia (visual acuity of 20/40 to 20/100 in the amblyopic eye) were enrolled. Amblyopia was associated with strabismus, anisometropia, or both. All subjects had worn optimal refractive correction (if needed) for at least 12 weeks without improvement. Subjects were randomized into two treatment groups: a 4-hour IO-Therapy Group with liquid crystal glasses (Amblyz™), set at 30-second opaque/transparent intervals (occluded 50% of wear time) or a 2-hour continuous Patching Group (occluded 100% of wear time). For each patient, visual acuity was measured with the ATS-HOTV method before and after 12 weeks of treatment.
RESULTS
At the conclusion of the first 12 week-treatment interval, data from 34 patients were available for analysis. Amblyopic eye visual acuity improvement from baseline was 0.15±0.12 (95% CI=0.09 to 0.15) logMAR in the IO-Therapy Group (N=19) and 0.15±0.11 (95% CI=0.1 to 0.15) logMAR in the Patching Group (N=15). Improvements in both groups were significant. The difference between groups was not statistically significant (P=0.73). No adverse effects were reported.
CONCLUSION
In this pilot study, IO-Therapy with Amblyz liquid crystal glasses is not inferior to adhesive patching and is a promising alternative treatment for children 3–8 years of age with moderate amblyopia. A larger randomized clinical trial is needed to confirm results in future.
INTRODUCTION
Conventional occlusion treatment for unilateral amblyopia in children has been occlusion with an adhesive eye patch, which forces use of the amblyopic eye while the fellow (normal) eye is occluded. A landmark Pediatric Eye Disease Investigator Group (PEDIG) study showed that two-hours of daily patching is effective when treating children 3 to 7 years of age with moderate (visual acuity 20/40 to 20/80), unilateral amblyopia.1, 2 However, patching is not universally effective: 20% to 25% of children do not respond to patching treatment. In addition, patching is strongly disliked by patients and families and compliance has been found to be as low as 44%4–7 to 57%8, limiting the potential visual acuity improvement significantly. Furthermore, the discomfort and social stigma of patching may cause stress or anxiety, which adversely affects the child-parent relationship.9, 10. If the child’s vision does not improve at an early age, it is increasing difficult to achieve the optimal visual improvement later due to the limited critical period of vision plasticity. For all of these reasons, investigating an effective alternative amblyopia treatment for young children is critical for long-term public health.
A novel electronic device, Amblyz™ liquid crystal intermittent occlusion glasses, alternates one lens between opaque and transparent phases at 30-second intervals so that they provide effective occlusion of one eye 50% of the time they are worn. The intermittent occlusion therapy (IO-therapy) associated with these glasses is potentially an effective alternative treatment for amblyopia for the following two reasons. First, cumulative occlusion time is critical for successful treatment. A positive and predominantly linear relationship between cumulative dose and response over at least the first 400 hours of amblyopia treatment has previously been reported.11 Second, a recent study showed that splitting hours of patching is as effective as continuous hours of patching.12 Although there is no evidence as to which interval duration or style is the best, it is reasonable to postulate that the IO-Therapy glasses may work as effectively, or potentially more effectively, as compared to traditional patching. Moreover, IO-Therapy glasses avoid adhesive and are more child-friendly, potentially improving compliance. The feasibility and safety of this device for treating amblyopia in children has previously been confirmed.13
Two previous studies suggested that IO-Therapy could effectively improve visual acuity in children with amblyopia.14,15 Of note, however, neither study had a comparative patching control group; also, the occlusion dose (total daily time of occlusion) was variable among patients depending on physicians’ preference. Therefore, we do not know the true effectiveness of the IO-therapy glasses when compared with patching or how to advise physicians who would like to prescribe these glasses in a clinical setting.
Many pediatric ophthalmologists in the United States currently follow the PEDIG evidence-based recommendation to prescribe 2-hours of patching as an effective treatment for moderate amblyopia (defined as visual acuity 20/40 to 20/100 in the amblyopic eye).2, 3, 16, 17 In this study, we hypothesized that 4-hours of IO-Therapy (50% of wear time) would not be inferior to 2-hours of traditional continuous adhesive patch occlusion treatment, in improving visual acuity in 3- to 7-year-old children with moderate, unilateral amblyopia. We tested our hypothesis with a randomized, controlled, non-inferiority clinical trial using methods based upon the gold-standard PEDIG Amblyopia Treatment Study (ATS) protocols for moderate amblyopia. This paper reports results at the conclusion of a 12-week treatment period.
METHODS
This research protocol and the informed consent forms were approved by the by the Institutional Review Board of the Indiana University. It was registered in www.clinicaltrial.gov as NCT01973348. Study oversight was provided by an independent data safety monitoring committee.
Participants
We enrolled children between the ages of 3 to ≤8 years of age with untreated, moderate, unilateral amblyopia, diagnosed and cared for by pediatric ophthalmologists at Indiana University School of Medicine. Informed consent was obtained from the subject’s parent or guardian (hereafter referred to as “parent”); assent was obtained from subjects 7 to 8 years of age.
Eligibility testing included measurement of visual acuity in both eyes using the standard ATS single-surround HOTV letter protocol18 and a routine comprehensive eye exam (cycloplegic refraction, comprehensive ocular examination and a full motility examination). Inclusion and exclusion criteria are generally based on moderate amblyopia study criteria from the National Eye Institute (NEI).1, 2
Eligibility inclusion criteria
1) Age range from 3 to ≤8 years old. 2) Moderate unilateral amblyopia: best corrected visual acuity of the amblyopic eye ranging from 20/40 to 20/100.2 Visual acuity in the sound eye at least 20/40 or better. Interocular logMAR difference of at least two lines. 3) Amblyopia associated with strabismus, or anisometropia, or both. 4) Wearing of optimal spectacle correction (if needed) for a minimum of 12-weeks prior to the time of enrollment. Details of the protocol for correction of refractive error followed a previous PEDIG amblyopia treatment study guidelines of moderately amblyopic children.19
Exclusion criteria
1) Known allergy to adhesives. 2) Previous amblyopia treatment within 6 months of enrollment, except for optical correction. 3) Gestational age ≤32 weeks at birth. 4) Down’s syndrome or developmental delays. 4) Previous intraocular surgeries.
Procedure
After confirming enrollment criteria, each participant was randomly assigned to one of two treatment groups with a sealed envelope opened by the child or their parents:
IO-Therapy glasses intervention group (IO-Therapy Group)
Non-amblyopic eye received 4-hours daily wearing of 50/50 IO-therapy glasses (Amblyz™ liquid crystal glasses, XPAND 3D Group, Ljubljana, Slovenia) treatment. Glasses were set at 30-second opaque/transparent intervals for the non-amblyopic fellow eye. These glasses are rechargeable overnight and after each charge, the intermittent occlusion function works for several days. The IO-Therapy glasses also incorporate a snap-in carrier frame for individuals needing correction of refractive error with prescription lenses (Figure 1).
Figure 1.

IO-Therapy glasses. In the left column, the top image shows the liquid crystal glasses when in the occlusive, opaque mode; the bottom image demonstrates the transparent or natural state of the glasses. The image on the right side shows the snap-in carrier frame (dark red) to couple refractive correction with the IO-Therapy glasses.
Standard patching control group (Patching Group)
Non-amblyopic eye received 2-hour daily wearing of standard, latex-free, adhesive occlusion patches combined with optimal refractive correction (if needed).
To independently report compliance, all participants were provided with a calendar log, which has been used in previous patching treatment studies.3, 17 Once randomized, the Patching Group started their treatment immediately after enrollment, while the IO-Therapy Group more typically started treatment 6–10 days after enrollment due to the inherent delay of ordering and receiving the optical correction insert for the IO-Therapy glasses. Occasionally, some patients experienced accidental breakage with the IO-Therapy glasses. In these cases, we immediately shipped a new pair of glasses to them and the glasses arrived within one week.
Primary outcome at 12-week visit
Visual function
After a 12-week period of treatment, each participant returned for his or her routine eye exam and a best-corrected ATS-HOTV visual acuity was measured by a certified examiner, who was masked to the patient’s randomization treatment arm. During testing, subjects wore their regular spectacle correction.
Ocular alignment
Measurement of ocular alignment using the simultaneous prism and cover test at distance and near fixation was performed. Ocular deviation in prism diopters was recorded.
Compliance
Based on wearing events recorded in the calendar log, we evaluated compliance. Compliance was defined as the percentage of actual eye patch or IO-Therapy glasses wear hours versus the total prescribed patching or IO-Therapy glasses wear hours.5, 20 The participant’s parent was asked to comment about their child’s experiences with IO-Therapy glasses or patching and the calendar logs were collected.
Adverse events
Potential major adverse events monitored for included reverse amblyopia in the normal eye (visual acuity in the sound eye decrease by two lines), significant changes in ocular alignment (deviation changes ≥10 prism diopters), skin irritation from the adhesive patches, and any injury associated with the liquid crystal glasses and possible breakage.
Statistical analysis
The primary outcome was the visual acuity change in the amblyopic eye, in logMAR units, at the 12-week outcome visit. A paired t-test was applied to analyze visual acuities before and after treatment for each group; an independent t-test was applied to analyze visual acuity improvement between the two groups. Confidence intervals of visual acuity improvement were reported in a non-inferiority manner.21 After intervention type was considered, correlation coefficients were calculated for the treatment response (improved visual acuity in the amblyopic eye) to the variables: (i) baseline visual acuity; (ii) severity of amblyopia; and (iii) age of subject.
Sample Size
A sample size of 12 subjects per group has been shown to be effective for estimating within-group means and variances when little prior data is available.22 In this study, we were uncertain how many participants would drop from the study. Therefore, although a number of 12 would work for a pilot study, we overestimated the sample size to 20 in each group to account for attrition. Based on collected data from 34 subjects and effective size difference of 0.1, we re-estimated this study as a non-inferiority trial with continuous outcome and power is calculated as approximately 80%.
RESULTS
Between January 2014 and August 2015, 49 patients were consented and 45 entered the trial with 27 assigned to the IO-Therapy Group and 18 to the Patching Group.(Figure 2) We planned to enroll patients at 4 clinics. Randomized group assignments were distributed in sealed envelopes. Although we expected equal number of patients assigned to each treatment with randomization, uneven numbers were enrolled into the two arms because we did not randomize in blocks for each clinic site and two of the four clinics did not enroll any patients.
Figure 2.

Flow diagram of patients’ enrollment and follow-up.
Following the intent-to-treat strategy, the 12-week primary outcome examination was completed by 19 of the patients in the IO-Therapy Group and 15 patients in the Patching Group. Eighty-two percent of them were Caucasian (N=28). The baseline characteristics of the patients with follow-up in two groups were similar (Table 1), particularly in the mean age, baseline visual acuity and baseline interocular visual acuity. There were more boys in the IO-Therapy Group than in the patching group. The baseline visual acuity was on average 0.46 logMAR (approximately 20/58) for both groups.
Table 1.
Baseline characteristics according to treatment group
| Total (N= 34) |
IO-Therapy Group (N=19) |
Patching Group (N=15) |
|
|---|---|---|---|
| Gender (F/M) | 12/22 | 5/14 | 7/8 |
| Age, YR | 5.8±1.2 | 5.7±1.5 | 5.9±0.9 |
| 3–4 | 2 | 2 | 0 |
| 4–5 | 8 | 5 | 3 |
| 5–6 | 7 | 4 | 3 |
| 6–7 | 13 | 4 | 9 |
| 7–8 | 4 | 4 | 0 |
| Cause of Amblyopia | |||
| Strabismus | 12 | 9 | 3 |
| Anisometropia | 20 | 8 | 12 |
| Combined strabismus+anisometropia | 2 | 2 | 0 |
| Visual acuity in the amblyopic eye at baseline (logMAR) | 0.46±0.13 | 0.46±0.16 | 0.46±0.14 |
| Severity of amblyopia, or interocular difference of visual acuity at baseline (logMAR) | 0.39±0.12 | 0.35±0.12 | 0.38±0.14 |
Visual acuity improvement in the amblyopic eye at 12-week follow-up in groups and individuals
Following the intent-to-treat strategy, at the conclusion of the first 12-week treatment interval visual acuity in the amblyopic eye improved an average of 0.15±0.12 logMAR (non-inferiority 95% CI=0.09 to 0.15, N=19) in the IO-Therapy Group and 0.15±0.11 (non-inferiority 95% CI=0.10 to 0.15, N=15) logMAR in the Patch Group. The window for the primary outcome visit was ±1.5 weeks. The average and individual visual acuity improvement in the amblyopic eye at 12-weeks is plotted in Figure 3A and 3B. The difference between the two groups was not statistically significant (independent t-test, T= −0.14, one-sided P=0.88, Figure 3A), which indicates that the IO-therapy glasses were not inferior to adhesive patch occlusion. In the IO-Therapy Group 4 patients improved 3 lines and 3 patients did not improve at all. Similarly, in the Patching Group 4 patients improved 3 lines and 3 patients did not improve at all (Figure 3B).
Figure 3.
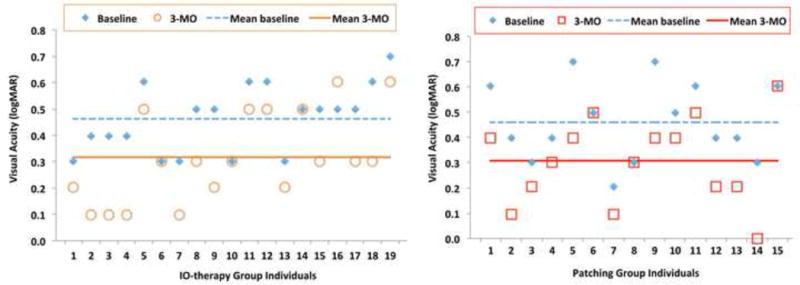
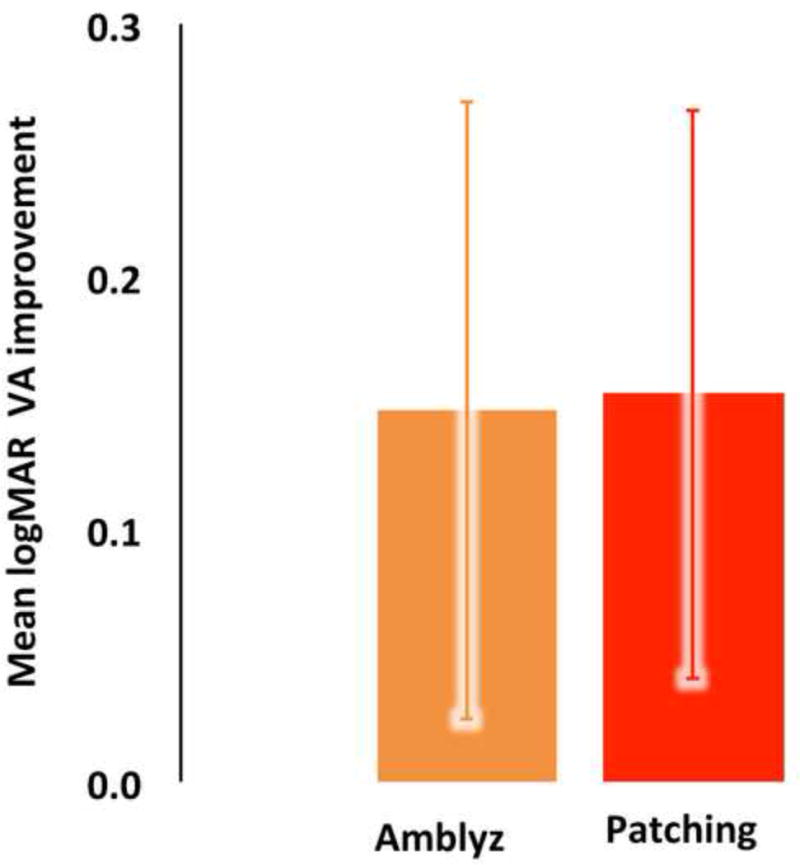
A. Visual acuity changes from the baseline in individuals for both groups at 12-week follow-up exam. Means at baseline and 3-MO follow-up were plotted. B. Mean visual acuity improvement; 12-week outcome visit compared to baseline. Both groups improved significantly from the baseline and there is no significant difference between the two groups.
One subject in each group reported that they did not apply any treatment at all, therefore we did a secondary analysis after excluding these 2 patients. At the conclusion of the first 12-week treatment interval, visual acuity in the amblyopic eye improved an average of 0.15±0.12 logMAR (non-inferiority 95% CI=0.09 to 0.15, N=18) in the IO-Therapy Group and 0.16±0.11 (non-inferiority 95% CI=0.1 to 0.16, N=14) logMAR in the Patch Group. This difference was again not statistically significant (independent t-test, T= −0.34, one-sided P=0.37). In this secondary analysis group, 4 patients improved 3 lines and 3 patients did not improve at all in the IO-Therapy Group; 4 patients improved 3 lines and 2 patients did not improve at all in the Patching Group.
Severity of amblyopia
Severity of amblyopia (inter-ocular difference of visual acuity) was calculated in this study. At baseline, severity of amblyopia was 0.39±0.12 logMAR in the IO-Therapy Group and 0.35±0.12 logMAR in the Patching Group. At the 12-week visit, severity of amblyopia was 0.28±0.19 logMAR in the IO-Therapy Group and 0.23±0.13 logMAR in the Patching Group. This decrease in the severity of amblyopia in both groups was significant (Paired t-test, T=3.19, P-value=0.005 for the IO-Therapy Group; Paired t-test, T=3.81, P-value=0.002 for the Patching Group). There is no significant difference in the decrease in the severity of amblyopia between groups (T=1.00, P-Value=0.32).
Correlation with baseline visual acuity, severity of amblyopia, treatment age
In the IO-Therapy Group, the correlation coefficients of the improved visual acuity in the amblyopic eye with baseline visual acuity, severity of amblyopia and treatment age were 0.05 (P=0.83), −0.02 (P=0.94) and −0.03 (P=0.90) respectively. In the Patching Group, the correlation coefficients of the improved visual acuity in the amblyopic eye with baseline visual acuity, severity of amblyopia and treatment age, were 0.21(P=0.45), 0.41 (P=0.13) and −0.07 (P=0.80) respectively. No significant correlation was found.
Effect of treatment on visual acuity in the sound eye
At baseline, mean visual acuity of the sound eye was 0.07±0.13 logMAR in the IO-Therapy Group and 0.11±0.09 logMAR in the Patching Group (independent t-test, T=−0.86 P=0.40). At the 12-week visit, mean visual acuity in the sound eye was 0.03±0.11 logMAR in the IO-Therapy Group and 0.08±0.12 logMAR in the Patch Group (independent t-test, T=−1.23, P=0.23). At both baseline and 12-week visits, there was no significant difference between the two groups. No patients in either group had reverse amblyopia (defined as two lines worse than enrollment).
Strabismus at 12-week follow-up
No patients in either group had a significant increase in strabismus (defined as 10 prism diopters of increase at either near or distance).
Self-reported compliance
Patient compliance to the prescribed treatment was estimated with a calendar log. Compliance is calculated as the self-reported wearing hours versus the prescribed hours daily. At 12-weeks, compliance was reported by 8 patients in the IO-Therapy Group and 10 patients in the Patching Group; compliance averaged at 92% in the Patching Group and 84% in the IO-Therapy Group. This difference was not statistically different (independent t-test, P=0.38).
Patients or parents’ comments on IO-Therapy glasses
Similar to the Patching Group, children in the IO-Therapy Group struggled with some outdoor activities, but reported no issues with indoor activities while occluded. In the IO-Therapy Group, some parents reported that their child had trouble seeing outside at night secondary to the light tint inherent to the liquid crystal lenses. Additionally, some parents complained that the glasses were easily subject to damage by their child. Overall, the comments demonstrated a high level of enthusiasm from parents and children with the IO-Therapy glasses, commonly remarking that they were easy to wear for the prescribed treatment time. Several patients complained that the glasses were variably tight, loose or uncomfortable, often due to the nose pad or temple arm. For most of these complaints, our opticians were able to successfully adjust the glasses to fit the child or offer a spectacle elastic band; but occasionally these adjustments did not satisfactorily resolve the complaint and two patients in the IO-Therapy Group reported that they did not wear the glasses for the full prescribed time as directed because the glasses were still not comfortable. One patient reported a “rainbow effect” when using a pair of IO-Therapy glasses but this complaint resolved when the glasses were replaced with a new pair.
Other Adverse Effects
There were no adverse effects or injuries reported in this study. No skin irritation was reported in the Patching Group.
DISCUSSION
This study is the first pilot randomized clinical trial comparing the effectiveness of liquid crystal occlusion glasses and adhesive occlusion patches when treating children 3–8 years of age with moderate unilateral amblyopia. This is also the first hypothesis-based study to compare intermittent occlusion with continuous occlusion. The results suggest that 4-hours of intermittent occlusion (occluded 50% of treatment time) is not inferior to 2-hours of continuous occlusion.
Table 2 demonstrates how our study differs from two previous IO-Therapy studies with liquid crystal glasses14, 15 primarily in the following aspects: (1) We studied only moderate amblyopia, which is similar to Spierer et al, while Erbagci et al included both severe and moderate amblyopia in their 14 patients. Visual acuity improvement in our study is similar to the results at the 3-month visit in the Spierer et al study14 although our daily treatment hours were less. Conversely, visual acuity improvement found in Erbagci et al is greater than our study. The most likely explanation for this difference is that their patients did not have a refractive adaptation period and many patients, particularly with refractive or refractive-accommodative amblyopia, will show significant improvement in visual acuity using optical correction treatment alone.15 (2) The occlusion time in our study is relatively less. Based on our hypothesis, and the previously published PEDIG evidence-based suggestion of 2-hours of daily patching in moderate amblyopia, we designed our study with 4-hours of IO-Therapy to achieve the 2-hours of daily occlusion (children were occluded 50% of the time while wearing the IO-Therapy glasses). In both previous studies, IO-Therapy hours were not fixed and prescribed amounts were left to the individual physicians’ preference, which varied from 4 to 12 hours14,15. (3) Our study is the only study that reporting severity of amblyopia (defined as inter-ocular difference in visual acuity) at the outcome visit. The primary reason we reported this calculation is because rapidly developing children at 3–8 years of age could improve their visual acuity solely on a basis of developmental improvement, rather than from the amblyopia treatment itself. Such a developmental improvement in acuity would be reflected in improved vision in both eyes, not just the amblyopic eye.
Table 2.
Comparison with prior IO-Therapy studies
| Spierer et al12 (2010) |
Erbagci et al13 (2015) |
Our study | |
|---|---|---|---|
| Study design | No control group | No control group | Clinical trial with patching control group |
| Sample size | 24 | 14 | 19 IO vs 15 Patch |
| Age at treatment (years) | 4–7.8 | 4.5–10 | 3–8 |
| Patients characteristics | Moderate amblyopia, previously treated or untreated | Moderate and severe amblyopia, previously untreated, no optical correction adaptation period | Moderate amblyopia, previously untreated, optical correction adapted, |
| IO-Therapy glasses occlusion setting | 40 seconds on and 20 seconds off | 30 seconds on and 30 seconds off | 30 seconds on and 30 seconds off |
| Daily IO-Therapy hours | At least 8 hours | 4–12 hours | 4 hours |
| Reported follow-up period (months) | 1.5, 3, 4.5, 6, 9 | 3 to 7 (mean 4.0±1.2) | 3 |
| Visual acuity improvement (logMAR) | 0.16±0.3 (at 3-months) | 0.3±0.2 | 0.15±0.12 |
Experiences with IO-Therapy glasses
The current version of Amblyz IO-Therapy glasses is “one-size-fits-all”. As a result, we had to exclude several children from enrollment when they did not fit into the glasses appropriately. This device may be improved in the future with the availability of additional frame sizes to fit children of different ages and shapes. As with all glasses worn by children, there were some issues with breakage but nothing that seemed out of the ordinary and there were no injuries related to the glasses. Although some local Amish parents declining allowing their children to participate in the study because of the electronic nature of the glasses, children and families were typically enthusiastic about using the “high tech” glasses and voiced no issues with the cosmetics of the sports-goggle type frames.
Compliance in this study was self-reported in both groups using a daily calendar log. The self-reported compliance in the IO-Therapy Group was similar to that in the adhesive Patching Group. This is despite the fact that the daily wearing hours were doubled for the IO-Therapy Group compared to the Patching Group. We recognize that self-reported compliance is often overestimated compared to objective compliance, however, both groups in our study used the same compliance reporting system and we found no significant difference between treatment groups.23
Power calculation of this study
Given that the children’s hospital where we enrolled patients is a tertiary referral center serving a statewide population, several patients in both groups did not keep the scheduled 12-week follow-up visit. Additionally, some children who had performed well at enrollment subsequently did not cooperate during visual acuity testing at the outcome visit. Therefore, the sample size of this study decreased from 49 to 34. However, based on available data from the sample size obtained, the power of this study as a non-inferiority trial is calculated as approximately 80%. Thus, this study has enough power to confidently reach conclusions from the 12-week outcome.
Clinical meaning and insight of this study
In 2014, the FDA approved Amblyz™ IO-Therapy glasses as a medical device (but not as a specific therapy for amblyopia). However, clinicians did not previously have an evidence-based guideline for prescribing these glasses. Our study suggests such evidence. The promising results at 12-weeks clearly warrants further investigation with a larger number of patients at multiple study centers and with longer follow-up. Moreover, this study does not simply provide evidence that 4–hours of daily IO-Therapy glasses are equally effective as compared to 2-hours of patching but, perhaps more importantly, this study suggests that it may be the cumulative time of occlusion that is important rather than what time increment it is delivered in. If 30-second intervals are good, what about 1-second intervals or even 1 millisecond intervals? This finding may have significant implications that change they way we currently treat amblyopia and may make it easier to occlude individuals with dense amblyopia, who frequently do not tolerate patching. Such potential highlights the importance of this device, which gives us another new treatment option for amblyopia in the last several decades, since atropine was induced into clinical use.24–26
CONCLUSIONS
This pilot study suggests that 4-hours of daily IO-Therapy, with liquid crystal occlusion glasses set at 50% occlusion time, was not inferior to 2-hours of daily patching when treating children 3–8 years of age with moderate, unilateral amblyopia. This promising device provides an alternative form of amblyopia treatment for children and their frequently frustrated families. These results support further study with a multicenter, randomized clinical trial to confirm our findings and delineate baseline factors that may influence the effectiveness of IO-therapy glasses versus patching, such as age, severity of amblyopia and sub-type causes of amblyopia.
Acknowledgments
The authors acknowledge Paxton Ott for proofreading and editing the manuscript. The authors sincerely appreciate the editors and anonymous reviewers’ helpful comments on improving this manuscript. Amblyz™ liquid crystal intermittent occlusion therapy glasses were provided to the study by XPAND 3D Group (Ljubljana, Slovenia). This study is supported by a Research to Prevent Blindness (RPB) Unrestricted Grant to the Glick Eye Institute. This work was supported by the grant from the National Eye Institute EY026664.
Footnotes
Trial registration: clinicaltrials.gov Identifier NCT01973348
References
- 1.Repka MX, Beck RW, Holmes JM, et al. A randomized trial of patching regimens for treatment of moderate amblyopia in children. Archives of ophthalmology. 2003;121:603–611. doi: 10.1001/archopht.121.5.603. [DOI] [PubMed] [Google Scholar]
- 2.Wallace DK, Edwards AR, Cotter SA, et al. A randomized trial to evaluate 2 hours of daily patching for strabismic and anisometropic amblyopia in children. Ophthalmology. 2006;113:904–912. doi: 10.1016/j.ophtha.2006.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holmes JM, Kraker RT, Beck RW, et al. A randomized trial of prescribed patching regimens for treatment of severe amblyopia in children. Ophthalmology. 2003;110:2075–2087. doi: 10.1016/j.ophtha.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Awan M, Proudlock FA, Gottlob I. A randomized controlled trial of unilateral strabismic and mixed amblyopia using occlusion dose monitors to record compliance. Investigative ophthalmology & visual science. 2005;46:1435–1439. doi: 10.1167/iovs.04-0971. [DOI] [PubMed] [Google Scholar]
- 5.Tjiam AM, Holtslag G, Van Minderhout HM, et al. Randomised comparison of three tools for improving compliance with occlusion therapy: an educational cartoon story, a reward calendar, and an information leaflet for parents. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2013;251:321–329. doi: 10.1007/s00417-012-2107-4. [DOI] [PubMed] [Google Scholar]
- 6.Wallace MP, Stewart CE, Moseley MJ, Stephens DA, Fielder AR. Compliance with occlusion therapy for childhood amblyopia. Investigative ophthalmology & visual science. 2013;54:6158–6166. doi: 10.1167/iovs.13-11861. [DOI] [PubMed] [Google Scholar]
- 7.Pradeep A, Proudlock FA, Awan M, Bush G, Collier J, Gottlob I. An educational intervention to improve adherence to high-dosage patching regimen for amblyopia: a randomised controlled trial. The British journal of ophthalmology. 2014;98:865–870. doi: 10.1136/bjophthalmol-2013-304187. [DOI] [PubMed] [Google Scholar]
- 8.Loudon SE, Fronius M, Looman CW, et al. Predictors and a remedy for noncompliance with amblyopia therapy in children measured with the occlusion dose monitor. Investigative ophthalmology & visual science. 2006;47:4393–4400. doi: 10.1167/iovs.05-1428. [DOI] [PubMed] [Google Scholar]
- 9.Choong YF, Lukman H, Martin S, Laws DE. Childhood amblyopia treatment: psychosocial implications for patients and primary carers. Eye (Lond) 2004;18:369–375. doi: 10.1038/sj.eye.6700647. [DOI] [PubMed] [Google Scholar]
- 10.Loudon SE, Passchier J, Chaker L, et al. Psychological causes of non-compliance with electronically monitored occlusion therapy for amblyopia. The British journal of ophthalmology. 2009;93:1499–1503. doi: 10.1136/bjo.2008.149815. [DOI] [PubMed] [Google Scholar]
- 11.Stewart CE, Stephens DA, Fielder AR, Moseley MJ. Modeling dose-response in amblyopia: toward a child-specific treatment plan. Investigative ophthalmology & visual science. 2007;48:2589–2594. doi: 10.1167/iovs.05-1243. [DOI] [PubMed] [Google Scholar]
- 12.Sachdeva V, Mittal V, Kekunnaya R, et al. Efficacy of split hours part-time patching versus continuous hours part-time patching for treatment of anisometropic amblyopia in children: a pilot study. The British journal of ophthalmology. 2013;97:874–878. doi: 10.1136/bjophthalmol-2012-302978. [DOI] [PubMed] [Google Scholar]
- 13.BenEzra O, Herzog R, Cohen E, Karshai I, BenEzra D. Liquid crystal glasses: feasibility and safety of a new modality for treating amblyopia. Archives of ophthalmology. 2007;125:580–581. doi: 10.1001/archopht.125.4.580. [DOI] [PubMed] [Google Scholar]
- 14.Spierer A, Raz J, Benezra O, et al. Treating amblyopia with liquid crystal glasses: a pilot study. Investigative ophthalmology & visual science. 2010;51:3395–3398. doi: 10.1167/iovs.09-4568. [DOI] [PubMed] [Google Scholar]
- 15.Erbagci I, Okumus S, Oner V, Coskun E, Celik O, Oren B. Using liquid crystal glasses to treat ambyopia in children. Journal of AAPOS: the official publication of the American Association for Pediatric Ophthalmology and Strabismus/American Association for Pediatric Ophthalmology and Strabismus. 2015;19:257–259. doi: 10.1016/j.jaapos.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 16.Holmes JM, Beck RW, Kraker RT, et al. Impact of patching and atropine treatment on the child and family in the amblyopia treatment study. Archives of ophthalmology. 2003;121:1625–1632. doi: 10.1001/archopht.121.11.1625. [DOI] [PubMed] [Google Scholar]
- 17.Repka MX, Kraker RT, Beck RW, et al. A randomized trial of atropine vs patching for treatment of moderate amblyopia: follow-up at age 10 years. Archives of ophthalmology. 2008;126:1039–1044. doi: 10.1001/archopht.126.8.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmes JM, Beck RW, Repka MX, et al. The amblyopia treatment study visual acuity testing protocol. Archives of ophthalmology. 2001;119:1345–1353. doi: 10.1001/archopht.119.9.1345. [DOI] [PubMed] [Google Scholar]
- 19.Pediatric-Eye-Disease-Investigator-Group. The clinical profile of moderate amblyopia in children younger than 7 years. Archives of ophthalmology. 2002;120:281–287. [PubMed] [Google Scholar]
- 20.Tjiam AM, Holtslag G, Vukovic E, et al. An educational cartoon accelerates amblyopia therapy and improves compliance, especially among children of immigrants. Ophthalmology. 2012;119:2393–2401. doi: 10.1016/j.ophtha.2012.05.035. [DOI] [PubMed] [Google Scholar]
- 21.Greene CJ, Morland LA, Durkalski VL, Frueh BC. Noninferiority and equivalence designs: issues and implications for mental health research. J Trauma Stress. 2008;21:433–439. doi: 10.1002/jts.20367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat. 2005;4:287–291. [Google Scholar]
- 23.Wang J. Compliance and patching and atropine amblyopia treatments. Vision research. 2015 doi: 10.1016/j.visres.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Ron A, Nawratzki I. Penalization treatment of amblyopia: a follow-up study of two years in older children. J Pediatr Ophthalmol Strabismus. 1982;19:137–139. doi: 10.3928/0191-3913-19820501-05. [DOI] [PubMed] [Google Scholar]
- 25.Repka MX, Kraker RT, Holmes JM, et al. Atropine vs patching for treatment of moderate amblyopia: follow-up at 15 years of age of a randomized clinical trial. JAMA ophthalmology. 2014;132:799–805. doi: 10.1001/jamaophthalmol.2014.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pediatric-Eye-Disease-Investigator-Group. A randomized trial of atropine vs. patching for treatment of moderate amblyopia in children. Archives of ophthalmology. 2002;120:268–278. doi: 10.1001/archopht.120.3.268. [DOI] [PubMed] [Google Scholar]


