Abstract
Objective
Cardiac damage and vascular dysfunction are major causes of morbidity and mortality in hypertension. In the present study, we explored the beneficial therapeutic effect of endoplasmic reticulum (ER) stress inhibition on cardiac damage and vascular dysfunction in hypertension.
Methods and Results
Mice were infused with angiotensin II (400 ng/kg per minute) with or without ER stress inhibitors (taurine-conjugated ursodeoxycholic acid and 4-phenylbutyric acid) for 2 weeks. Mice infused with angiotensin II displayed an increase in blood pressure, cardiac hypertrophy and fibrosis associated with enhanced collagen I content, transforming growth factor-β1 (TGF-β1) activity, and ER stress markers, which were blunted after ER stress inhibition. Hypertension induced ER stress in aorta and mesenteric resistance arteries (MRA), enhanced TGF-β1 activity in aorta but not in MRA, and reduced endothelial NO synthase phosphorylation and endothelium-dependent relaxation (EDR) in aorta and MRA. The inhibition of ER stress significantly reduced TGF-β1 activity, enhanced endothelial NO synthase phosphorylation, and improved EDR. The inhibition of TGF-β1 pathway improved EDR in aorta but not in MRA, whereas the reduction in reactive oxygen species levels ameliorated EDR in MRA only. Infusion of tunicamycin in control mice induced ER stress in aorta and MRA, and reduced EDR by a TGF-β1–dependent mechanism in aorta and reactive oxygen species–dependent mechanism in MRA.
Conclusion
ER stress inhibition reduces cardiac damage and improves vascular function in hypertension. Therefore, ER stress could be a potential target for cardiovascular diseases.
Keywords: cardiac hypertrophy, endoplasmic reticulum stress, endothelial NO synthase, fibrosis, hypertension, oxidative stress, transforming growth factor-β1, vascular reactivity
Emerging evidence from experimental and clinical research indicates that endoplasmic reticulum (ER) stress is involved in cardiovascular diseases such as cardiac hypertrophy, heart failure, atherosclerosis, and ischemic heart disease.1–3 Although the clinical management of hypertension has advanced substantially, cardiovascular disease still constitutes a major and increasing health burden worldwide. Although treatments for hypertension have progressed, the development of novel therapies for patients with vascular complications and cardiac damage in hypertension remains a major research goal. These thoughts are supported by the observation that impaired macro- and microcirculatory heart function predicts poor outcome for patients with cardiovascular disease.4
The ER is an organelle in which membrane-bound proteins are folded into their final 3-dimensional structures where lipids and sterols are synthesized and where free calcium is stored. Various types of cellular stress (ischemia, hypoxia, gene mutation, oxidative stress, and increased protein synthesis) lead to impairment of ER function, creating a state collectively termed ER stress that leads to the activation of a complex signaling network called the unfolded protein response.5–8 The unfolded protein response is activated in the cell by 3 main signaling pathways: (1) inositol-requiring protein 1 activation, (2) the protein kinase RNA–like ER kinase activation, and (3) the activating transcription factor 6 (ATF6).9
Recent report suggests that ER stress is involved in cardiac remodeling in hypertensive animals.10 It was also shown that the inhibition of ER stress attenuates cardiovascular remodeling in aldosterone salt-treated rats.11 These observations suggest that ER stress is an important factor in cardiovascular homeostasis. The significance of ER stress and its role in vascular dysfunction and cardiac damage in hypertension are important questions that have remained unanswered. Therefore, the aim of the present study was to determine the role of ER stress in vascular endothelial dysfunction and cardiac damage in angiotensin II (ang II)–induced hypertension model.
Materials and Methods
An extensive description of materials and methods can be found in the online-only Data Supplement. A brief summary is given below.
General Protocol in Mice
One hundred mice (C57BL/6J, 8-week-old males) were purchased from Jackson Laboratories (Bar Harbor, ME).
Cardiac Fibrosis
Transverse sections of left ventricle were stained with the collagen- specific stain Sirius red (Sigma-Aldrich, St. Louis, MO).
Vascular Reactivity
In Vivo Experiments
Aorta and mesenteric resistance arteries (MRA) were mounted in a small vessel dual-chamber myograph for measurement of isometric tension.12,13 After preconstriction with phenylephrine (10−4 mol/L) and steady maximal contraction, cumulative concentration response curves were obtained for acetylcholine (1×10−8 to 3×10−5 mol/L) and sodium nitroprusside (1×10−8 to 3×10−5 mol/L).
Hypertrophy
Hearts were harvested and then atrium and all epicardial fat were removed. Cardiac hypertrophy was calculated using heart weight/ body weight ratios.
Active Transforming Growth Factor-β1 Level
Active transforming growth factor-β1 (TGF-β1) level was measured in heart, aorta, and MRA tissue lysates using TGF-β1 immunoassay (Quantikine, R&D systems, Minneapolis, MN) as previously described.14
Western Blot Analysis
Western blot analysis for endothelial NO synthase (eNOS), eukaryotic translation initiation factor 2α, TGF-β1, GRP78 (1:1000 dilution; Cell Signaling Technology, Inc), collagen I, P-Smad2/3, and Smad1/2/3 (1:250 dilution; Santa Cruz Biotechnology, Inc) was performed in lysates of aorta, MRA, and heart as previously described.15,16
Immunofluorescence
Immunostaining was performed for C/EBP homologous protein (CHOP) in paraffin sections of aorta and MRA using specific antibodies, anti-CHOP (1:200, Santa Cruz Biotechnology, Inc).
Immunohistochemistry
Aorta, MRA, and hearts were fixed in 4% paraformaldehyde followed by zinc-saturated formalin for immunohistochemical studies. Immunostaining was performed as previously described.17
Terminal Deoxynucleotidyl Transferase dUTP Nick-End Labeling Assay
Four-micrometer paraffin sections from heart were mounted using Vectashield with 4′,6-diamidino-2-phenylindole (Vector Laboratories, Inc). The images were captured using a deconvolution fluorescent scope.
Reverse Transcription Polymerase Chain Reaction Real-Time Assay
CHOP, ATF4, ATF6, and TGF-β1 mRNA levels were determined in heart, aorta, and MRA from all groups, whereas nicotinamide adenine dinucleotide phosphate (NADPH) oxidase 1 (Nox1), Nox2, and Nox4 mRNA levels were studied only in vessels.
Nitrite Levels
The amount of nitrites, the end product of NO metabolism, was measured in plasma samples by the Griess reaction.
Results
General Parameters
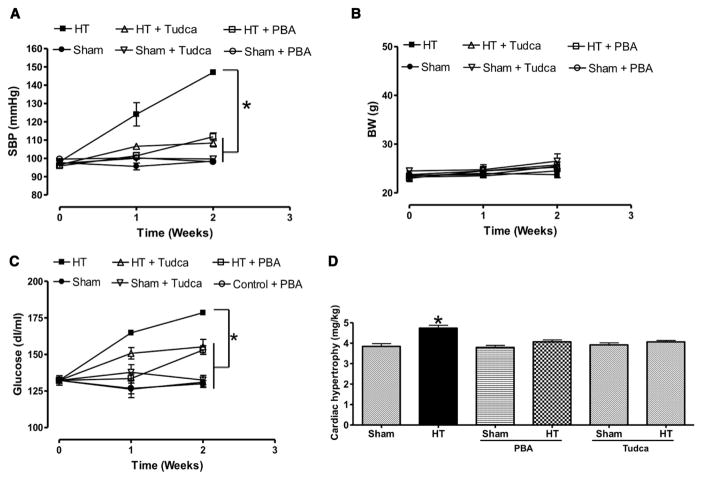
Mice infused with ang II showed a significant increase in systolic blood pressure compared with Sham (146.9±1.15 versus 100.01±0.58; P<0.05), which was significantly reduced by ER stress inhibitors (108.4±2.1 and 111.8±2.16, taurine-conjugated ursodeoxycholic acid [Tudca] and 4-phenylbutyric acid [PBA], respectively, Figure 1A). Body weight was similar in all groups of mice (Figure 1B). Blood glucose level was higher in hypertensive (178.5±1.6 mg/dL) compared with Sham mice (131±3.5 mg/dL), and was reduced by ER stress inhibitors (Figure 1C). Cardiac hypertrophy was observed in mice infused with ang II compared with Sham and mice infused with ang II treated with ER stress inhibitors (Figure 1D).
Figure 1.
Systolic blood pressure (SBP; A), body weight (BW; B), and glucose levels (mg/dL; C) measured in all groups of mice, n=10; cardiac hypertrophy index (mg/kg; D) in all groups, n=10. *P<0.05 for HT vs control, control+PBA, control+Tudca, HT+PBA, and HT+Tudca. HT indicates hypertension; PBA, 4-phenylbutyric acid; Tudca, taurine-conjugated ursodeoxycholic acid.
Cardiac Fibrosis
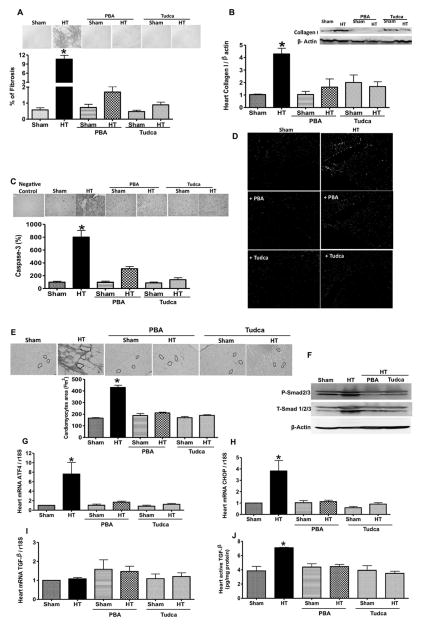
Chronic ang II infusion induces cardiac fibrosis associated with increased active caspase-3, cardiomyocyte size, and apoptotic cells (Figure 2A and 2C–2E). In addition, collagen type I expression and mRNA levels of ATF4, CHOP, and ATF6 were also increased (Figure 2B, 2G, and 2H; Figure IIA in the online-only Data Supplement). All these parameters were significantly reduced with ER stress inhibitors (Figure 2A–2E, 2G, and 2H; Figure IIA in the online-only Data Supplement). The mRNA level of TGF-β1 in heart was similar in all groups (Figure 2I) whereas active TGF-β1 level, and total and phosphorylated Smad2/3 were increased in hearts from hypertensive mice compared with Sham and hypertensive mice treated with ER stress inhibitors (Figure 2F and 2J).
Figure 2.
Representative heart sections stained with (A) Sirius red, (C) immunohistochemistry, (D) terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay (green arrows indicate the apoptotic cells), and (E) cardiomyocyte area. Quantitative data in all groups, n=6 (A, C, and E). Activating transcription factor 4 (ATF4) and C/EBP homologous protein (CHOP) mRNA levels, normalized to 18S ribosomal RNA (rRNA), in heart (G and H). Western blot analysis and quantitative data for collagen I, T-Smad1/2/3, and P-Smad2/3 in heart (B and F) from all groups, n=5. Transforming growth factor-β1 (TGF-β1) mRNA levels, normalized to 18S rRNA, in heart (I) in all groups, n=6. Active TGF-β1 levels in heart (J) in all groups, n=5. *P<0.05 for HT vs control, control+PBA, control+Tudca, HT+PBA, and HT+Tudca. HT indicates hypertension; PBA, 4-phenylbutyric acid; Tudca, taurine-conjugated ursodeoxycholic acid.
ER Stress and TGF-β1 in Aorta and MRA
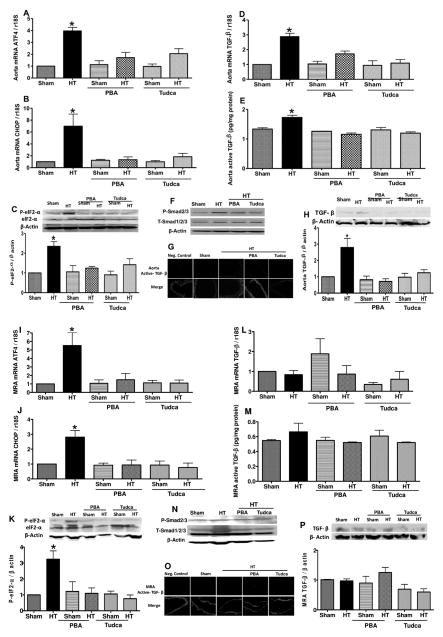
Ang II–induced hypertension increased ATF4, CHOP mRNA levels, and phosphorylated eukaryotic translation initiation factor 2α levels, which were reduced with ER stress inhibition in aorta and MRA (Figure 3A–3C, 3I–3K; Figure IIB and IIC in the online-only Data Supplement). We observed a different pattern in mRNA level, and total and active form of TGF-β1 between aorta and MRA. Thus, TGF-β1 mRNA and protein levels, its active form, and phosphorylated Smad2/3 were augmented in aorta from hypertensive mice and reduced with ER stress inhibitors (Figure 3D–3H), whereas no changes were observed in MRA in all groups of mice (Figure 3L–3P).
Figure 3.
A, B, I, and J, Activating transcription factor 4 (ATF4) and C/EBP homologous protein (CHOP) mRNA levels, normalized to 18S ribosomal RNA (rRNA), in aorta and mesenteric resistance arteries (MRA) in all groups of mice, n=6. C and K, Western blot analysis and quantitative data for eukaryotic translation initiation factor 2α (eIF2α), P-eIF2α in aorta and MRA in all groups of mice, n=5. D and L, Transforming growth factor-β1 (TGF-β1) mRNA levels, normalized to 18S rRNA, in aorta and MRA in all groups of mice, n=6. E, M, G, and O, Active TGF-β1 levels in aorta and MRA in all groups of mice, n=4. F, H, N, and P, Western blot analysis and quantitative data for TGF-β1, T-Smad1/2/3, and P-Smad2/3 in aorta and MRA in all groups, n=5.*P<0.05 for HT vs control, control+PBA, control+Tudca, HT+PBA, and HT+Tudca. HT indicates hypertension; PBA, 4-phenylbutyric acid; Tudca, taurine-conjugated ursodeoxycholic acid.
ER Stress and Vascular Reactivity in Hypertension
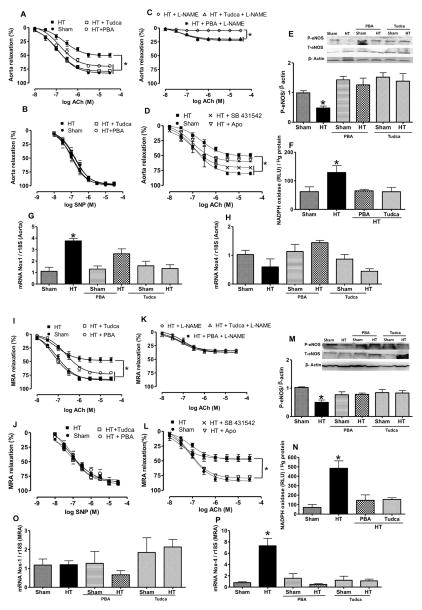
To determine the role of ER stress in vascular endothelial dysfunction in hypertension, we examined endothelium-dependent relaxation (EDR) and endothelium-independent relaxation in aorta and MRA. Ang II–induced hypertension attenuated EDR in aorta and MRA, and was significantly improved in hypertensive mice treated with Tudca and PBA compared with untreated hypertensive mice (Figure 4A and 4I). Endothelium-independent relaxation was similar in hypertensive mice treated with and without ER stress inhibitors (Figure 4B and 4J). We did not observe any change in EDR and endothelium-independent relaxation in aorta and MRA from Sham mice treated with Tudca or PBA (Figure IA, IC, ID, and IF in the online-only Data Supplement).
Figure 4.
A, B, C, I, J , and K, Endothelium-dependent and independent relaxation in response to acetylcholine (Ach) and single-nucleotide polymorphism (SNP), respectively, in aorta and mesenteric resistance arteries (MRA) with and without L-NAME in all groups, n=10. E and M, Western blot analysis and quantitative data for P-endothelial NO synthase (eNOS), T-eNOS, and β-actin in aorta and MRA in all groups, n=5. D and L, Endothelium-dependent relaxation in response to ACh with and without apocynin (Apo) and SB431542 in aorta and MRA in all groups of mice, n=5. F and N, Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase activity in aorta and MRA in all groups of mice, n=5. G, H, O, and P, NADPH oxidase 1 (Nox1) and Nox4 mRNA levels, normalized to 18S ribosomal RNA (rRNA), in aorta and MRA from all groups, n=6. *P<0.05 for HT vs control, control+PBA, control+Tudca, HT+PBA, and HT+Tudca. HT indicates hypertension; PBA, 4-phenylbutyric acid; Tudca, taurine-conjugated ursodeoxycholic acid.
The inhibition of eNOS with L-NAME reduced EDR in hypertensive mice (Figure 4C and 4K) and Sham mice (Figure IB and IE in the online-only Data Supplement). Ang II–induced hypertension reduced eNOS phosphorylation in aorta and MRA, which was restored after ER stress inhibition. In Sham mice treated with Tudca and PBA, we did not observe any effect on eNOS phosphorylation in aorta and MRA. Total eNOS expression was similar in all groups of mice (Figure 4E and 4M). In addition, nitrite levels were reduced in plasma from hypertensive mice and restored after ER stress inhibition (Figure IID in the online-only Data Supplement).
To further elucidate the mechanism of vascular endothelial dysfunction in hypertension related to ER stress, we studied the involvement of Nox and TGF-β1 pathways. Our data indicated that inhibition of Nox with apocynin slightly enhanced EDR in aorta but completely restored EDR in MRA, whereas TGF-β1 receptor antagonist significantly enhanced EDR in the aorta but no effect was observed in MRA (Figure 4D and 4L). Ang II–induced hypertension increased Nox activity in both arteries, Nox1 and Nox4 mRNA levels in aorta and MRA, respectively, which were reduced after ER stress inhibition (Figure 4F–4H, 4N–4P). These results were associated with augmented active TGF-β1 levels in aorta from hypertensive mice, which were reduced after ER stress inhibition (Figure 3E and 3G). In MRA, we did not see an increase in active TGF-β1 in all groups of mice (Figure 3M and 3O).
Oxidative Stress, TGF-β1, and Vascular Reactivity
To further determine the relationship between ER stress and vascular dysfunction, we performed in vitro studies by incubating isolated aorta and MRA from control mice with ER stress inducer (tunicamycin) for 1 hour. The results showed that tunicamycin significantly reduced EDR in aorta and MRA, which was prevented when aorta was pretreated with Tudca or TGF-β1 pathway inhibition and when MRA was pretreated with Tudca or apocynin (Figure III in the online-only Data Supplement).
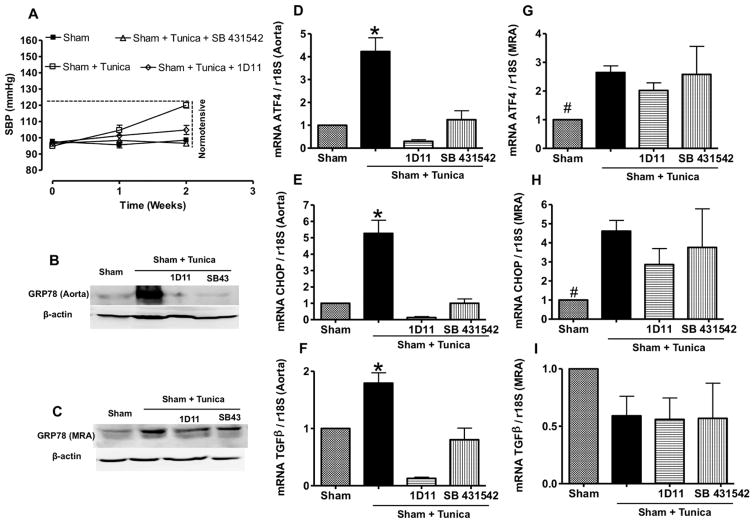
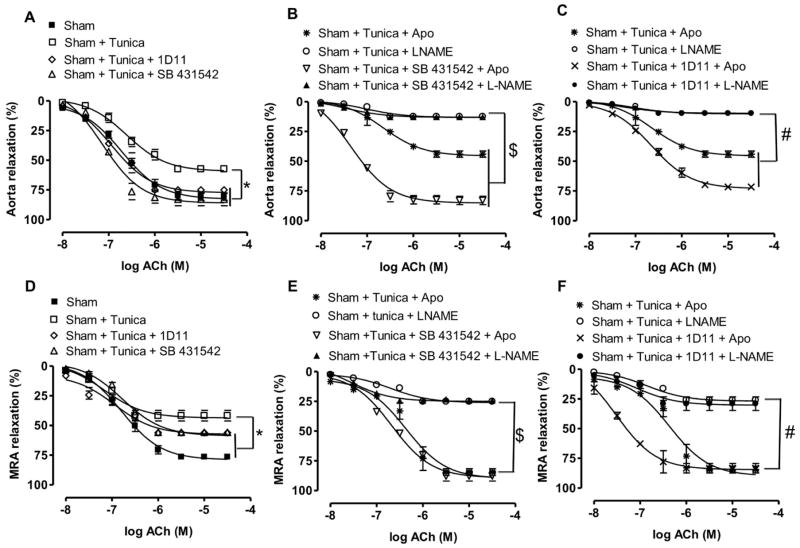
In vivo studies showed that tunicamycin injection with and without TGF-β1 pathway inhibition did not increase systolic blood pressure (Figure 5A). Tunicamycin injection increased ER stress marker expression in aorta and MRA, which was reduced only in aorta with TGF-β1 receptor antagonist (SB431542) and 1D11 neutralizing TGF-β1 antibodies (Figure 5B–5E, 5G, and 5H). The mRNA level of TGF-β1 was augmented in aorta and reduced with 1D11 and SB431542 (Figure 5F), but no change was observed in MRA (Figure 5I). Mice injected with tunicamycin showed an attenuated EDR in aorta and MRA (Figure 6A and 6D). The in vivo inhibition of TGF-β1 pathway restored EDR in aorta, whereas only a minor improvement in EDR was observed in MRA (Figure 6A and 6D). In addition, the incubation of MRA with apocynin completely restored EDR, whereas no effect was observed in aorta isolated from tunicamycin-injected mice treated with and without SB431542 or 1D11 (Figure 6B, 6C, 6E, and 6F). The L-NAME completely blocked the EDR in aorta whereas partially in MRA from control mice treated with tunicamycin with and without TGF-β1 pathway inhibition (Figure 6B, 6C, 6E, and 6F).
Figure 5.
A, Systolic blood pressure (SBP) measured in all groups, n=10. B and C, Western blotting analysis for GRP78 in aorta and mesenteric resistance arteries (MRA). D, E, F, G, H, and I, Activating transcription factor 4 (ATF4), C/EBP homologous protein (CHOP), and transforming growth factor-β1 (TGF-β1) mRNA levels, normalized to 18S ribosomal RNA (rRNA), in aorta and MRA in all groups of mice, n=6. *P<0.05 for Sham+Tunica vs Sham, Sham+Tunica+1D11, and Sham+Tunica+SB431542.
Figure 6.
A and D, Endothelium-dependent relaxation in response to acetylcholine (Ach) in aorta and mesenteric resistance arteries (MRA) from mice treated with or without Tunica with and without 1D11 or SB431542 in aorta and MRA, n=5. *P<0.05 for Sham+Tunica vs Sham, Sham+Tunica+1D11, and Sham+Tunica+SB431542. B and E, Endothelium-dependent relaxation in response to ACh in aorta and MRA from mice treated with Tunica with and without apocynin (Apo), L-NAME, and SB431542 in aorta and MRA, n=5. $P<0.05 for Sham+Tunica+L-NAME and Sham+Tunica+SB431542+L-NAME vs Sham+Tunica+Apo and Sham+Tunica+Apo+SB431542. C and F, Endothelium-dependent relaxation in response to ACh in aorta and MRA from mice treated with Tunica with and without Apo, L-NAME, and 1D11 in aorta and MRA, n=5. #P<0.05 for Sham+Tunica+L-NAME and Sham+Tunica+1D11+L-NAME vs Sham+Tunica+Apo and Sham+Tunica+Apo+1D11.
In other experiments, our results showed that mice injected with tunicamycin with and without Tudca did not affect systolic blood pressure or induce cardiac hypertrophy (Figure IVA and IVB in the online-only Data Supplement). However, tunicamycin injection moderately induced cardiac fibrosis that was reduced after ER stress inhibition (Figure IVC in the online-only Data Supplement). The size of cardiomyocytes did not change between groups (data not shown). Nox activity was increased in aorta and MRA from mice injected with tunicamycin, and treatment with Tudca restored the activity (Figure IVD and IVE in the online-only Data Supplement). The EDR was impaired in aorta and MRA from mice injected with tunicamycin, which was restored after ER stress inhibition (Figure VA and VD in the online-only Data Supplement). To determine the mechanism how tunicamycin impairs vascular function, aorta and MRA from mice injected with tunicamycin were incubated with apocynin. The data showed that EDR was restored in MRA, whereas no effect was observed in aorta (Figure VB and VE in the online-only Data Supplement). The L-NAME completely blocked EDR in aorta whereas partially in MRA from control mice injected with tunicamycin with or without Tudca (Figure VC and VF in the online-only Data Supplement).
To demonstrate the induction of ER stress in vascular endothelial cells, we performed immunostaining, and data indicate the presence of ER stress marker (CHOP) in endothelial cells of aorta and MRA from mice injected with tunicamycin. The induction of CHOP in vascular endothelial cells was blunted in mice injected with tunicamycin and treated with Tudca (Figure VIA and VIB in the online-only Data Supplement).
Discussion
Our study demonstrated that ER stress is an important factor in macro- and microvascular pathology and cardiac damage in hypertension. Such evidence first came from in vivo experiments in which hypertensive mice treated with ER stress inhibitors had reduced arterial blood pressure and improved EDR and cardiac damage. We further demonstrated that ER stress inhibition in hypertension differentially improves macrovascular endothelial function by TGF-β1–dependent mechanism and microvascular endothelial function by an oxidative stress–dependent mechanism. These results suggest that inhibition of ER stress could be a useful therapeutic strategy to reverse vascular complications and cardiac damage in hypertension.
Vascular pathology and cardiac damage are well characterized in hypertensive animal models and patients.18–20 Several mechanisms have been proposed, such as dysregulation of the renin angiotensin system,21,22 endothelin-1,23,24 cyclooxygenase activity,25,26 oxidative stress,27,28 calcium channels,29 immune function,17,30 and inflammation.31 Previous studies established the relationship among ER stress, diabetes mellitus, obesity, and cardiovascular diseases,32–36 but the mechanism and the significance of ER stress in hypertension-induced vascular dysfunction and cardiac damage are still unknown.
The chemical chaperone PBA and endogenous bile acids such as Tudca are known to modulate ER function, stabilize protein conformation, improve ER folding capacity, and facilitate mutant proteins trafficking.31,37 It is well established that increased arterial blood pressure is associated with cardiovascular complications, such as cardiac hypertrophy and fibrosis, renal failure, and vascular endothelial dysfunction. In the present study, we demonstrated that ang II–induced hypertension and increased blood glucose level were reduced after ER stress inhibition, indicating that ER stress is an important factor in the development of hypertension and prediabetic state. It is not surprising that the blood glucose level increased after ang II infusion because it is reported that ang II stimulates the degradation of insulin receptor substrate 1.38 In addition, it has been shown that ER stress inhibition has a beneficial effect in type 1 and type 2 diabetes mellitus in terms of glucose regulation.31,39 These results explain the relationship among hypertension, prediabetic stage, and ER stress.
Cardiac hypertrophy and fibrosis are well documented in hypertensive animals and patients.40,41 Hypertension-induced cardiac hypertrophy is a progressive event associated with myocardial remodeling characterized by fibrosis and alterations in cardiomyocytes size and function.42 In our model, ang II–induced cardiac hypertrophy and fibrosis were associated with ER stress induction. Interestingly, ER stress inhibition blunted ang II–induced cardiac hypertrophy and fibrosis suggesting that ER stress is an important factor in the development of cardiac damage in hypertension. Cardiac fibrosis is a result of the pathological accumulation of extracellular matrix components, particularly collagens I and II.43 Importantly, our data indicate that the increased collagen I expression in hypertensive mice hearts was reduced after ER stress inhibition. It is well known that in hypertension, TGF-β1 stimulates collagen synthesis.44 Our data showed that active TGF-β1 and its downstream signaling Smad2/3 were increased in mice infused with ang II and were reduced after ER stress inhibition. In addition, we observed an increase in the size of cardiomyocytes, which was reduced after ER stress inhibition. The loss of cardiomyocytes by apoptosis has emerged as an important mechanism contributing to myocardial remodeling in response to hemodynamic overload.45,46 Our data indicate that cardiomyocyte apoptosis was increased in hypertensive mice compared with Sham and hypertensive mice treated with ER stress inhibitors.
It is well known that hypertension is associated with vascular endothelial dysfunction.47,48 However, the role of ER stress in vascular endothelial dysfunction in hypertension is still unknown. Ang II–induced hypertension reduced EDR and eNOS phosphorylation and enhanced ER stress in aorta and MRA. Importantly, ER stress inhibition improved EDR in aorta and MRA, and this was associated with an increase in eNOS phosphorylation.
TGF-β1 and oxidative stress have been proposed as important factors in the development of vascular complications in hypertension49–51 and cardiovascular disease.52 In our model, ang II–induced hypertension enhances TGF-β1 activity and oxidative stress levels in aorta whereas only oxidative stress was increased in MRA. Interestingly, ER stress inhibition reduced oxidative stress levels in aorta and MRA, and this was associated with enhanced EDR. Similarly, the enhanced TGF-β1 activity was reduced in aorta after ER stress inhibition. Our data were supported by the reduction in Smad2/3 phosphorylation. Our findings suggest that oxidative stress and TGF-β1 are downstream signaling mechanisms of ER stress and are in agreement with previous studies reporting that ER stress induction increases reactive oxygen species.53 In addition, studies using cell cultures demonstrated that oxidative stress can also increase ER stress suggesting the possibility that ER stress and oxidative stress can activate each other.54,55
Ang II–induced hypertension induces ER stress, which enhances TGF-β1 activity and oxidative stress in aorta and MRA, respectively. TGF-β1 is a profibrotic factor involved in vascular and cardiac structural remodeling in a variety of cardiovascular diseases. The inhibition of ER stress reduced TGF-β1 activity in aorta and restored EDR. In addition, TGF-β1 inhibition improved EDR in aorta from mice infused with ER stress inducer (tunycamycin), but no effect was observed in MRA. These in vivo results were supported by in vitro studies in which the acute inhibition of the TGF-β1 activity improves EDR in aorta in the presence of ER stress. Further studies are needed to delineate the mechanism linking ER stress to the TGF-β1 pathway and EDR. The inhibition of TGF-β1 pathway did not affect EDR in MRA, indicating that TGF-β1 is most likely an important factor in large arteries than resistance arteries EDR in ang II–induced hypertension.
Our study demonstrates for the first time the importance of ER stress in the regulation of arterial blood pressure and cardiac damage, and elucidates a differential effect in macro- and microvascular function, TGF-β1, and oxidative stress–dependent mechanisms, respectively. The present study suggests that ER stress could be a potential target for a novel therapeutic strategy to reverse hypertension-induced vascular and cardiac damage.
Supplementary Material
Acknowledgments
We thank Dr Kathy Flanders (National Institutes of Health, Bethesda, MD) for providing the rabbit polyclonal antibody against active transforming growth factor-β1.
Sources of Funding
We acknowledge grant support from National Institutes of Health (1R01HL095566-PI: Dr Matrougui; 5R01HL097111-PI: Dr Trebak; and P20RR017659-COBRE-PI: Dr Navar).
Footnotes
Disclosures
None.
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.112.249318/-/DC1.
References
- 1.Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao L, Ackerman SL. Endoplasmic reticulum stress in health and disease. Curr Opin Cell Biol. 2006;18:444–452. doi: 10.1016/j.ceb.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Marciniak SJ, Ron D. Endoplasmic reticulum stress signaling in disease. Physiol Rev. 2006;86:1133–1149. doi: 10.1152/physrev.00015.2006. [DOI] [PubMed] [Google Scholar]
- 4.den Uil CA, Lagrand WK, van der Ent M, Jewbali LS, Cheng JM, Spronk PE, Simoons ML. Impaired microcirculation predicts poor outcome of patients with acute myocardial infarction complicated by cardiogenic shock. Eur Heart J. 2010;31:3032–3039. doi: 10.1093/eurheartj/ehq324. [DOI] [PubMed] [Google Scholar]
- 5.Schröder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 6.Bernales S, Papa FR, Walter P. Intracellular signaling by the unfolded protein response. Annu Rev Cell Dev Biol. 2006;22:487–508. doi: 10.1146/annurev.cellbio.21.122303.120200. [DOI] [PubMed] [Google Scholar]
- 7.Dickhout JG, Hossain GS, Pozza LM, Zhou J, Lhoták S, Austin RC. Peroxynitrite causes endoplasmic reticulum stress and apoptosis in human vascular endothelium: implications in atherogenesis. Arterioscler Thromb Vasc Biol. 2005;25:2623–2629. doi: 10.1161/01.ATV.0000189159.96900.d9. [DOI] [PubMed] [Google Scholar]
- 8.Terai K, Hiramoto Y, Masaki M, Sugiyama S, Kuroda T, Hori M, Kawase I, Hirota H. AMP-activated protein kinase protects cardiomyocytes against hypoxic injury through attenuation of endoplasmic reticulum stress. Mol Cell Biol. 2005;25:9554–9575. doi: 10.1128/MCB.25.21.9554-9575.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 10.Isodono K, Takahashi T, Imoto H, Nakanishi N, Ogata T, Asada S, Adachi A, Ueyama T, Oh H, Matsubara H. PARM-1 is an endoplasmic reticulum molecule involved in endoplasmic reticulum stress-induced apoptosis in rat cardiac myocytes. PLoS ONE. 2010;5:e9746. doi: 10.1371/journal.pone.0009746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arias-Loza PA, Hu K, Dienesch C, Mehlich AM, König S, Jazbutyte V, Neyses L, Hegele-Hartung C, Heinrich Fritzemeier K, Pelzer T. Both estrogen receptor subtypes, alpha and beta, attenuate cardiovascular remodeling in aldosterone salt-treated rats. Hypertension. 2007;50:432–438. doi: 10.1161/HYPERTENSIONAHA.106.084798. [DOI] [PubMed] [Google Scholar]
- 12.Kassan M, Galan M, Partyka M, Trebak M, Matrougui K. Interleukin-10 released by CD4(+)CD25(+) natural regulatory T cells improves microvascular endothelial function through inhibition of NADPH oxidase activity in hypertensive mice. Arterioscler Thromb Vasc Biol. 2011;31:2534–2542. doi: 10.1161/ATVBAHA.111.233262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- 14.Ben Mosbah I, Alfany-Fernández I, Martel C, Zaouali MA, Bintanel-Morcillo M, Rimola A, Rodés J, Brenner C, Roselló-Catafau J, Peralta C. Endoplasmic reticulum stress inhibition protects steatotic and non-steatotic livers in partial hepatectomy under ischemia-reperfusion. Cell Death Dis. 2010;1:e52. doi: 10.1038/cddis.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amin AH, Abd Elmageed ZY, Nair D, Partyka MI, Kadowitz PJ, Belmadani S, Matrougui K. Modified multipotent stromal cells with epidermal growth factor restore vasculogenesis and blood flow in ischemic hind-limb of type II diabetic mice. Lab Invest. 2010;90:985–996. doi: 10.1038/labinvest.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belmadani S, Palen DI, Gonzalez-Villalobos RA, Boulares HA, Matrougui K. Elevated epidermal growth factor receptor phosphorylation induces resistance artery dysfunction in diabetic db/db mice. Diabetes. 2008;57:1629–1637. doi: 10.2337/db07-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matrougui K, Abd Elmageed Z, Zakaria AE, Kassan M, Choi S, Nair D, Gonzalez-Villalobos RA, Chentoufi AA, Kadowitz P, Belmadani S, Partyka M. Natural regulatory T cells control coronary arteriolar endothelial dysfunction in hypertensive mice. Am J Pathol. 2011;178:434–441. doi: 10.1016/j.ajpath.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kassan M, Montero MJ, Sevilla MA. Chronic treatment with pravastatin prevents early cardiovascular changes in spontaneously hypertensive rats. Br J Pharmacol. 2009;158:541–547. doi: 10.1111/j.1476-5381.2009.00339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panza JA, Quyyumi AA, Brush JE, Jr, Epstein SE. Abnormal endothelium-dependent vascular relaxation in patients with essential hypertension. N Engl J Med. 1990;323:22–27. doi: 10.1056/NEJM199007053230105. [DOI] [PubMed] [Google Scholar]
- 20.Nava E, Lüscher TF. Endothelium-derived vasoactive factors in hypertension: nitric oxide and endothelin. J Hypertens Suppl. 1995;13:S39–S48. doi: 10.1097/00004872-199508001-00007. [DOI] [PubMed] [Google Scholar]
- 21.Warnholtz A, Nickenig G, Schulz E, Macharzina R, Bräsen JH, Skatchkov M, Heitzer T, Stasch JP, Griendling KK, Harrison DG, Böhm M, Meinertz T, Münzel T. Increased NADH-oxidase-mediated superoxide production in the early stages of atherosclerosis: evidence for involvement of the renin-angiotensin system. Circulation. 1999;99:2027–2033. doi: 10.1161/01.cir.99.15.2027. [DOI] [PubMed] [Google Scholar]
- 22.Dal-Ros S, Bronner C, Schott C, Kane MO, Chataigneau M, Schini-Kerth VB, Chataigneau T. Angiotensin II-induced hypertension is associated with a selective inhibition of endothelium-derived hyperpolarizing factor-mediated responses in the rat mesenteric artery. J Pharmacol Exp Ther. 2009;328:478–486. doi: 10.1124/jpet.108.145326. [DOI] [PubMed] [Google Scholar]
- 23.Recchia AG, Filice E, Pellegrino D, Dobrina A, Cerra MC, Maggiolini M. Endothelin-1 induces connective tissue growth factor expression in cardiomyocytes. J Mol Cell Cardiol. 2009;46:352–359. doi: 10.1016/j.yjmcc.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 24.Kemp TJ, Aggeli IK, Sugden PH, Clerk A. Phenylephrine and endothelin- 1 upregulate connective tissue growth factor in neonatal rat cardiac myocytes. J Mol Cell Cardiol. 2004;37:603–606. doi: 10.1016/j.yjmcc.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 25.Henrion D, Dechaux E, Dowell FJ, Maclour J, Samuel JL, Lévy BI, Michel JB. Alteration of flow-induced dilatation in mesenteric resistance arteries of L-NAME treated rats and its partial association with induction of cyclo-oxygenase-2. Br J Pharmacol. 1997;121:83–90. doi: 10.1038/sj.bjp.0701109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adeagbo AS, Zhang X, Patel D, Joshua IG, Wang Y, Sun X, Igbo IN, Oriowo MA. Cyclo-oxygenase-2, endothelium and aortic reactivity during deoxycorticosterone acetate salt-induced hypertension. J Hypertens. 2005;23:1025–1036. doi: 10.1097/01.hjh.0000166844.42227.5c. [DOI] [PubMed] [Google Scholar]
- 27.García-Redondo AB, Briones AM, Avendaño MS, Hernanz R, Alonso MJ, Salaices M. Losartan and tempol treatments normalize the increased response to hydrogen peroxide in resistance arteries from hypertensive rats. J Hypertens. 2009;27:1814–1822. doi: 10.1097/HJH.0b013e32832d23e6. [DOI] [PubMed] [Google Scholar]
- 28.Li JM, Shah AM. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- 29.Briones AM, Padilha AS, Cogolludo AL, Alonso MJ, Vassallo DV, Pérez-Vizcaino F, Salaices M. Activation of BKCa channels by nitric oxide prevents coronary artery endothelial dysfunction in ouabain-induced hypertensive rats. J Hypertens. 2009;27:83–91. doi: 10.1097/hjh.0b013e328317a7cf. [DOI] [PubMed] [Google Scholar]
- 30.Ait-Oufella H, Taleb S, Mallat Z, Tedgui A. Cytokine network and T cell immunity in atherosclerosis. Semin Immunopathol. 2009;31:23–33. doi: 10.1007/s00281-009-0143-x. [DOI] [PubMed] [Google Scholar]
- 31.Tycinska AM, Mroczko B, Musial WJ, Sawicki R, Kaminski K, Borowska H, Sobkowicz B, Szmitkowski M. Blood pressure in relation to neurogenic, inflammatory and endothelial dysfunction biomarkers in patients with treated essential arterial hypertension. Adv Med Sci. 2011;56:80–87. doi: 10.2478/v10039-011-0016-0. [DOI] [PubMed] [Google Scholar]
- 32.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myoishi M, Hao H, Minamino T, Watanabe K, Nishihira K, Hatakeyama K, Asada Y, Okada K, Ishibashi-Ueda H, Gabbiani G, Bochaton-Piallat ML, Mochizuki N, Kitakaze M. Increased endoplasmic reticulum stress in atherosclerotic plaques associated with acute coronary syndrome. Circulation. 2007;116:1226–1233. doi: 10.1161/CIRCULATIONAHA.106.682054. [DOI] [PubMed] [Google Scholar]
- 34.Xu J, Wang G, Wang Y, Liu Q, Xu W, Tan Y, Cai L. Diabetes- and angiotensin II-induced cardiac endoplasmic reticulum stress and cell death: metallothionein protection. J Cell Mol Med. 2009;13(8A):1499–1512. doi: 10.1111/j.1582-4934.2009.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Zhu H, Shen E, Wan L, Arnold JM, Peng T. Deficiency of rac1 blocks NADPH oxidase activation, inhibits endoplasmic reticulum stress, and reduces myocardial remodeling in a mouse model of type 1 diabetes. Diabetes. 2010;59:2033–2042. doi: 10.2337/db09-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minamino T, Komuro I, Kitakaze M. Endoplasmic reticulum stress as a therapeutic target in cardiovascular disease. Circ Res. 2010;107:1071–1082. doi: 10.1161/CIRCRESAHA.110.227819. [DOI] [PubMed] [Google Scholar]
- 37.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Görgün C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 38.Taniyama Y, Hitomi H, Shah A, Alexander RW, Griendling KK. Mechanisms of reactive oxygen species-dependent downregulation of insulin receptor substrate-1 by angiotensin II. Arterioscler Thromb Vasc Biol. 2005;25:1142–1147. doi: 10.1161/01.ATV.0000164313.17167.df. [DOI] [PubMed] [Google Scholar]
- 39.Raciti GA, Iadicicco C, Ulianich L, Vind BF, Gaster M, Andreozzi F, Longo M, Teperino R, Ungaro P, Di Jeso B, Formisano P, Beguinot F, Miele C. Glucosamine-induced endoplasmic reticulum stress affects GLUT4 expression via activating transcription factor 6 in rat and human skeletal muscle cells. Diabetologia. 2010;53:955–965. doi: 10.1007/s00125-010-1676-1. [DOI] [PubMed] [Google Scholar]
- 40.Kannel WB, Levy D, Cupples LA. Left ventricular hypertrophy and risk of cardiac failure: insights from the Framingham Study. J Cardiovasc Pharmacol. 1987;10(Suppl 6):S135–S140. [PubMed] [Google Scholar]
- 41.Brilla CG. Renin-angiotensin-aldosterone system and myocardial fibrosis. Cardiovasc Res. 2000;47:1–3. doi: 10.1016/s0008-6363(00)00092-4. [DOI] [PubMed] [Google Scholar]
- 42.Díez J, Fortuño MA, Ravassa S. Apoptosis in hypertensive heart disease. Curr Opin Cardiol. 1998;13:317–325. doi: 10.1097/00001573-199809000-00005. [DOI] [PubMed] [Google Scholar]
- 43.MacKenna D, Summerour SR, Villarreal FJ. Role of mechanical factors in modulating cardiac fibroblast function and extracellular matrix synthesis. Cardiovasc Res. 2000;46:257–263. doi: 10.1016/s0008-6363(00)00030-4. [DOI] [PubMed] [Google Scholar]
- 44.Murata H, Zhou L, Ochoa S, Hasan A, Badiavas E, Falanga V. TGF-beta3 stimulates and regulates collagen synthesis through TGF-beta1-dependent and independent mechanisms. J Invest Dermatol. 1997;108:258–262. doi: 10.1111/1523-1747.ep12286451. [DOI] [PubMed] [Google Scholar]
- 45.Katz AM. The cardiomyopathy of overload: an unnatural growth response in the hypertrophied heart. Ann Intern Med. 1994;121:363–371. doi: 10.7326/0003-4819-121-5-199409010-00009. [DOI] [PubMed] [Google Scholar]
- 46.Narula J, Kolodgie FD, Virmani R. Apoptosis and cardiomyopathy. Curr Opin Cardiol. 2000;15:183–188. doi: 10.1097/00001573-200005000-00011. [DOI] [PubMed] [Google Scholar]
- 47.Park JB, Charbonneau F, Schiffrin EL. Correlation of endothelial function in large and small arteries in human essential hypertension. J Hypertens. 2001;19:415–420. doi: 10.1097/00004872-200103000-00009. [DOI] [PubMed] [Google Scholar]
- 48.Pompilio G, Rossoni G, Alamanni F, Tartara P, Barajon I, Rumio C, Manfredi B, Biglioli P. Comparison of endothelium-dependent vasoactivity of internal mammary arteries from hypertensive, hypercholesterolemic, and diabetic patients. Ann Thorac Surg. 2001;72:1290–1297. doi: 10.1016/s0003-4975(01)03053-3. [DOI] [PubMed] [Google Scholar]
- 49.Ferroni P, Basili S, Paoletti V, Davì G. Endothelial dysfunction and oxidative stress in arterial hypertension. Nutr Metab Cardiovasc Dis. 2006;16:222–233. doi: 10.1016/j.numecd.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 50.Agrotis A, Saltis J, Dilley R, Bray P, Bobik A. Transforming growth factor- beta 1 and the development of vascular hypertrophy in hypertension. Blood Press Suppl. 1995;2:43–48. [PubMed] [Google Scholar]
- 51.Popovic N, Bridenbaugh EA, Neiger JD, Hu JJ, Vannucci M, Mo Q, Trzeciakowski J, Miller MW, Fossum TW, Humphrey JD, Wilson E. Transforming growth factor-beta signaling in hypertensive remodeling of porcine aorta. Am J Physiol Heart Circ Physiol. 2009;297:H2044–H2053. doi: 10.1152/ajpheart.01015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guzik TJ, West NE, Black E, McDonald D, Ratnatunga C, Pillai R, Channon KM. Vascular superoxide production by NAD(P)H oxidase: association with endothelial dysfunction and clinical risk factors. Circ Res. 2000;86:E85–E90. doi: 10.1161/01.res.86.9.e85. [DOI] [PubMed] [Google Scholar]
- 53.Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 55.Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, Kaufman RJ. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci USA. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.