Summary
Children with craniofacial syndromes are at risk of sleep disordered breathing, the most common being obstructive sleep apnea. Midface hypoplasia in children with craniosynostosis and glossoptosis in children with Pierre Robin syndrome are well recognized risk factors, but the etiology is often multifactorial and many children have multilevel airway obstruction. We examine the published evidence and explore the current management strategies in these complex patients. Some treatment modalities are similar to those used in otherwise healthy children such as as adenotonsillectomy, positive pressure ventilation and in the refractory cases, tracheostomy. However, there are some distinct approaches such as nasopharyngeal airways, tongue lip adhesion, mandibular distraction osteogenesis in children with Pierre Robin sequence, and midface advancement in children with craniosynostoses. Clinicians should have a low threshold for referral for evaluation of sleep-disordered-breathing in these patients.
Keywords: Craniofacial anomalies, sleep disordered breathing, craniosynostoses, cleft lip and palate
Introduction
Sleep disordered breathing (SDB) encompasses obstructive sleep apnea (OSA), central sleep apnea and nocturnal hypoventilation. Of these, OSA is by far the most commonly described in children with craniofacial syndromes and thus, the main focus of this review. OSA is characterized by prolonged partial upper airway obstruction and/or intermittent complete obstruction that disrupts both normal ventilation during sleep and normal neurophysiological sleep patterns (1). The reported prevalence in children with craniofacial syndromes ranges from 7–67% depending on the stringency of the diagnostic criteria and population studied (2). OSA can result in a wide array of morbidities, including cardiovascular, metabolic, neurocognitive and behavioral sequelae, significantly impacting on quality of life (3–6).
To illustrate the typical presentation and clinical course of SDB in children with craniofacial syndromes, we will start by describing the case of a child with Crouzon syndrome with severe OSA, and use this to illustrate the common management problems encountered in these complex patients. As it is not possible in a targeted review to cover all genetically derived craniofacial syndromes, we have concentrated on the first two categories in the Whitaker classification of craniofacial anomalies: I) clefts and II) synostoses (7). We discuss the unique aspects of each condition with respect to SDB, and highlight the current treatment strategies. Some of the treatment modalities are common to those used in otherwise healthy children with OSA, namely, adenotonsillectomy, continuous positive airway pressure (CPAP) or bilevel positive airway pressure (BiPAP) and in the refractory cases, tracheostomy. However, there are some distinct approaches to the management of children with craniofacial syndromes, such as insertion of nasopharyngeal airway (NPA) and surgical interventions such as midface advancement and mandibular distraction osteogenesis that will be included in the text and described in more detail.
Case study
AB was born at 34 weeks gestation. He had respiratory distress at birth, and needed admission to the neonatal intensive care unit where he received CPAP for 5 days. He was initially prevented from any oral intake, and therefore on intravenous fluids. When his respiratory distress improved, nasogastric feeding was attempted, but not even the smallest nasgastric tube could be passed through his nose. An orogastric tube was passed instead, and he was evaluated by an otolaryngologist. Craniosynostosis was suspected clinically, and the diagnosis of Crouzon syndrome was subsequently confirmed genetically. The orogastric tube was removed, and he was discharged home when 1 week old, but had a difficult post-natal course: he had severe gastroesophageal reflux, had difficulty feeding, and was a very poor sleeper. His parents discovered that he slept better when sitting up, and would carry him upright for hours on end. He had his first sleep study at the age of 6 months as part of his first assessment by the multidisciplinary craniofacial team. Evidence of moderate OSA was observed, and he underwent surgical adenoidectomy in an inpatient setting. Unfortunately, the follow-up sleep study not only had failed to improve following the procedure, but had in fact worsened. He now had an apnea hypopnea index (AHI) of 46/hTST, mean CO2 of 57mmHg and SpO2 nadir of 68%. He had a nasopharyngeal airway (NPA) inserted in the operating room (OR) by the otolaryngologist. Please note that our current hospital policy is that NPAs in children with craniosynostosis are inserted in the OR by the surgeons, under general anesthesia, as such procedures can be difficult. A sleep study with the NPA in place showed marked improvement, but clearly without normalization: AHI decreased to 12.8/hTST, average CO2 was improved at 47mmHg, but SpO2 nadir was still 70%. The child developed symptoms and signs of increased intracranial pressure (ICP), and underwent posterior vault expansion surgery at 12 months of age. During this period, he was noted to tolerate the NPA poorly, and pulled it out on several occasions, requiring repeated re-insertions under general anesthesia. The tapes on his face to hold the NPA in place were also causing his facial eczema to flare up. In the context of this constellation of problems, he therefore had a trial of positive airway pressure therapy. After a period of behavioral intervention aimed at habituation and improved adherence, he was first commenced on nasal CPAP. An overnight titration study showed that he still had obstructive events despite a positive end expiratory pressure of 16cmH20. He was therefore changed to bilevel positive pressure ventilation and pressures titrated up to 21/15 cm H20, with a backup rate of 20 breaths/min. On BiPAP, he still had moderate OSA with an AHI of 13/hTST, peak CO2 of 74mmHg and SpO2 nadir of 77%. There were problems with the interface as the mask did not fit him well. He developed a grade 3 pressure ulcer at the bridge of his nose because of pressure from the mask. He was then tried on a full face mask, with little improvement. Another side effect was abdominal distension from the BiPAP with increased emesis. There were concerns about the risk of possible aspiration should he vomit while on BiPAP. Following discussion at the multidisciplinary team meeting, it was felt that a tracheostomy was the best way forward. His parents were initially distraught, particularly because of the impact on his speech, but agreed and the procedure was performed at the age of 18 months. He had recurrent chest infections in the first year post tracheostomy tube insertion, but these have decreased in frequency as he has grown older. He is now 5 years old and doing well, he attends mainstream school and has an excellent quality of life. He has manifested catch-up growth, with his height and weight progressing nicely along the 9th centile, compared to <0.4th centile before tracheostomy. Development is appropriate for his age and he is able to vocalize normally even without a speaking valve. He had a micro laryngoscopy and bronchoscopy and underwent tonsillectomy recently in preparation for a trial of decannulation in the coming months.
I) Craniosynostoses
Craniosynostosis affects an estimated 1 in 2500 births. It occurs as part of a syndrome in approximately 40% of all cases, and the common feature is the premature fusion of one or more of the skull sutures. The more common syndromes include Pfeiffer, Apert, Crouzon, Muenke, Saethre-Chotzen and Carpenter syndromes. Mutations in the fibroblast growth factor receptor (FGFR) genes are responsible for most of the craniosynostoses (8). Pfeiffer syndrome is associated with mutations in the FGFR1 gene; Apert, Crouzon, and Pfeiffer syndromes have been associated with mutations of the FGFR2 gene, and mutations on FGFR3 have been identified for a subtype of Crouzon’s syndrome associated with acanthosis nigricans. The genotype-phenotype interactions for all these conditions remain poorly understood. The same FGFR mutations can cause different syndromes/phenotypes (9), conversely, different mutations, even on different genes, can result in the same phenotype/syndrome.
Craniosynostosis and OSA
There is a high incidence of OSA in children with craniosynostosis. Although multifactorial, a predominant contributing factor is midface hypoplasia. Driessen et al performed a prospective cohort study aiming to describe the evolution of OSA in syndromic craniosynostosis (10). Diagnosis was based on genetic analysis – Apert, Crouzon and Pfeiffer syndrome patients were analyzed as 1 group (n=50) as these patients all have midface hypoplasia. The second group (n=47) included patients with Muenke, Saethre-Chotzen syndrome and complex craniosynostosis (patients with multisuture synostosis in whom no genetic mutation had been found). The overall prevalence of OSA in all the patients was very high (68%) even in the group of patients without midface hypoplasia, though patients with midface hypoplasia had more severe OSA. Longitudinal profiles of 80 untreated patients (those without OSA or those with mild OSA) revealed that in the majority (84%), the severity of the OSA stabilized or improved with age, especially in the first few years of life, but such improvements were less likely in patients with midface hypoplasia. Alsaadi et al reported an OSA prevalence of 50% in their prospective study of 10 children with non-syndromic craniosynostosis who underwent PSG following diagnosis of craniosynostosis (11).
Moraleda-Cibrian et al studied an older population of patients with craniofacial anomalies. Of the 570 children with craniofacial anomalies, they summarized their findings in 151 who had been referred to their pediatric sleep clinic for investigation of OSA due to clinical symptoms (mean age: 8.5 years). Children who had had previous surgical procedures to improve upper airway obstruction were also excluded, such that the results are not a reflection of the true prevalence of SDB in the craniofacial patient population. Unsurprisingly, a high percentage had OSA (87%), of which the majority (76%) had mild OSA, and 24% had moderate/severe OSA (12). Children with syndromic craniofacial malformations were more likely to have more severe OSA. Positive airways pressure therapy was their first line treatment in the older school aged children, whilst adenotonsillectomy or a combination of adenotonsillectomy with CPAP was the first line treatment for younger children. Interestingly, in the 32 children who had repeated sleep studies during the course of treatment, the AHI only normalized in 2 patients and the majority had residual OSA, albeit improved compared to pre-treatment PSG findings, suggesting that adenotonsillectomy is often not curative in craniofacial patients and that CPAP can be difficult to implement efficaciously in these children.
The Canadian experience has been very similar, with 26 of 35 (74%) patients with syndromic craniosynostosis referred to a pediatric sleep clinic (mean age: 4.5 years) having OSA (13). 16 were treated with either adenotonsillectomy, cranial vault surgery, maxillary surgery or CPAP. Of the 14 who had follow-up sleep studies, 10 showed significant improvements following treatment, although only 1 achieved completely normalized respiratory patterns during sleep, and 4 either failed to improve or even worsened. Despite various interventions, complete resolution of the OSA was not achieved in the majority of this cohort.
de Jong et al reported a 18% prevalence of OSA in their group of patients with syndromic craniosynostosis (n=167). OSA was more common in patients with Apert, Crouzon and Pfeiffer syndromes than in patients with Muenke and Saethre Chotzen syndromes. However, the low prevalence reported is likely an underestimate, as they used oximetry rather than polysomnography to diagnose OSA. Based on these results, they recommended yearly screening with polysomnography in patients with Apert, Crouzon and Pfeiffer syndromes, and testing patients with Muenke and Saethre Chotzen syndromes only if they were to become symptomatic (14).
OSA has a negative impact on quality of life in these patients. A prospective study of 119 children with craniosynostosis asked their parents to complete the OSA 18 survey (a questionnaire that assesses sleep disordered breathing-related quality of life) and the Child Behavior Checklist (CBCL). OSA was associated with a lower quality of life and was also associated with the presence of behavioral problems. There was a significant correlation between the OAHI and the corresponding total CBCL scores (15).
Increased intracranial pressure (ICP) and OSA
Several factors can cause increased ICP in children with craniosynostosis, including craniocerebral disproportion, abnormal intracranial venous drainage and hydrocephalus. OSA can exacerbate these factors or even cause raised ICP by itself, due to its effects on CO2, a potent vasodilator, and its cardiovascular effects on arterial blood pressure and thus cerebral perfusion pressure (16).
One of the earliest studies to suggest a link between upper airway obstruction and raised intracranial pressure in children with craniosynostosis was by Gonsalez et al in 1997 (17). These investigators performed sleep studies while simultaneously monitoring ICP in 13 children with unoperated syndromic craniosynosotsis, and compared them with 7 control children who had isolated unicoronal synostosis and thus no facial involvement. 11 of the 13 syndromic patients had OSA compared to none of the controls. Raised ICP was documented in 10 of the syndromic patients, and in 3 of the controls. Both AHI and ICP values were more elevated during REM sleep and there was a significant correlation between OSA severity and increased ICP in REM sleep. Since then, Marucci et al have reported their experience whereby in the 20 patients with Apert syndrome who developed raised intracranial pressure, 3 were diagnosed with OSA and in 2 of these patients, treatment of the OSA alone led to resolution of the raised ICP without the need for transcranial surgery (18), and Spruijt et al have demonstrated that the presence of moderate/severe OSA significantly increases the risk of intracranial hypertension (19).
Cranosynostosis and central apneas
Central apneas have been reported in children with craniosynostosis. The underlying mechanisms are not well understood. One hypothesis is that they are a result of pressure on the respiratory centers due to an underlying Chiari malformation or to narrowing of the craniocervical junction. Indeed, due to early fusion of the lambdoid sutures and cranial base synchondroses, Chiari malformation is a frequent finding in Crouzon (70%) and Pfeiffer (50%) syndromes, while it is an intrinsic part of Kleeblattschädel deformity (20). In contrast, in Apert syndrome, where the coronal sutures fuse before the sagittal and lambdoid sutures, Chiari malformations are rare (21).
Central apneas are a recognized complication in children with congenital Chiari malformation and improvement in the apnea severity has been demonstrated following posterior fossa decompression (22). However, the evidence is conflicting in children with craniosynostosis. Improvement of central apneas has been described in patients with hind brain compression on MRI, following posterior fossa decompression (23,24). In one report, 3 children with syndromic craniosynostosis had significant central apneas requiring treatment (13). One was treated with oxygen, one had cranial vault reconstruction, and one had cranial vault surgery and posterior fossa decompression. The CAI improved in all 3 at follow up, but normalized in only the mildest (first) patient. In contrast, Dreissen et al described just 5 out of 138 syndromic craniosynostosis patients having increased CAI, none of whom were severe enough to warrant treatment (25). The impact of hindbrain herniation is also called into question with the predominant problem in 13 craniosynostosis patients with radiologically confirmed hindbrain herniation being OSA not central apnoea. Indeed, only 2 of these patients had central apneas outside the normal range (26). Similar findings have also been described in a separate study where sleep study parameters, including both the CAI and OAHI of craniosynostosis patients with hind brain herniation were similar to those without hind brain herniation (27).
Diagnosis of OSA in patients with craniosynostosis
Bannick et al compared results from the Brouillette questionnaire with those of parental observations of the child while asleep for 30 min at home, as well as with ambulatory cardiorespiratory polygraphy in 78 patients with craniosynostosis (28). The sensitivity of the Brouillette score was lower (55%) compared to the receiver operator characteristics initially described for normal healthy children, and such disparities may be related to the fact that children with craniosynostosis tend to mouth breath and snore due to their midface hypoplasia, even if they do not have significant OSA. The negative predictive value was reasonable (90%) suggesting that severe OSA is unlikely if the questionnaire is negative. In fact, just asking a single question “Is there difficulty with breathing during sleep?” had a reasonable negative predictive value (91%) but similarly poor sensitivity (64%). Notwithstanding such findings, many of the limitations of screening approaches in otherwise healthy, non-syndromic children are still applicable in these populations, and the limitations of more restrictive multichannel recordings should not be ignored as well (29, 30).
Treatment of OSA in children with craniosynostosis
Nasopharyngeal airway
Ahmed et al studied the effectiveness of using a nasopharyngeal airway to treat OSA by bypassing the midface obstruction (31). In this case series, 27 children (mean age 12.3 months) with syndromic craniosynostosis and moderate/severe OSA were treated. Of these, 26 (96%) demonstrated an improvement in sleep severity scores following NPA insertion. The NPA was generally well tolerated, with 24 patients retaining the NPA in place for at least 6 weeks. The authors thus advocate that a NPA is an effective first-line treatment modality in the management of OSA in children with syndromic craniosynostosis. However, 3 of the cohort still had residual moderate OSA and 24 had residual mild OSA.
Adenotonsillectomy
Willington et al reported a case series of 5 children (mean age: 4 years) with syndromic craniosynostosis who underwent adenotonsillectomy for treatment of moderate/severe OSA. 1 child had resolution of her OSA, and 2 patients improved but still had residual moderate and mild OSA. Adenotonsillectomy did not make any significant difference to the remaining 2 patients and they required tracheostomy insertion with frontofacial advancement surgery (32). A slightly larger retrospective study of 27 patients (mean age: 4.5 years) with syndromic craniosynostosis showed that 16/27 (60%) patients improved symptomatically post adenotonsillectomy, and that there was overall improvement in the mean number of oxygen desaturations >4%/h, and SpO2 nadir (33). These results suggest that because the causes of airway obstruction in these patients are multifactorial, adenotonsillectomy may not result in complete resolution of the OSA. However, surgery may still confer some degree of benefit in certain children, because even a small increase in the pharyngeal airway dimensions may alter airflow dynamics and reduce the severity of the OSA.
Surgical midface advancement
Midface hypoplasia often plays a significant role in the etiology of OSA in these patients. Surgical midface advancement techniques not only improve the esthetics of these patients, but also improve OSA, re-establish normal dental occlusion and correct exorbitism (Figures 1). Since the original descriptions of the use of Le Fort III osteotomy by Tessier in craniosynostosis patients (34), surgical techniques have evolved, and the armamentarium of the modern day craniofacial surgeon includes procedures such as Le Fort III with distraction osteogenesis, monobloc advancement and facial bipartition (Figure 2).
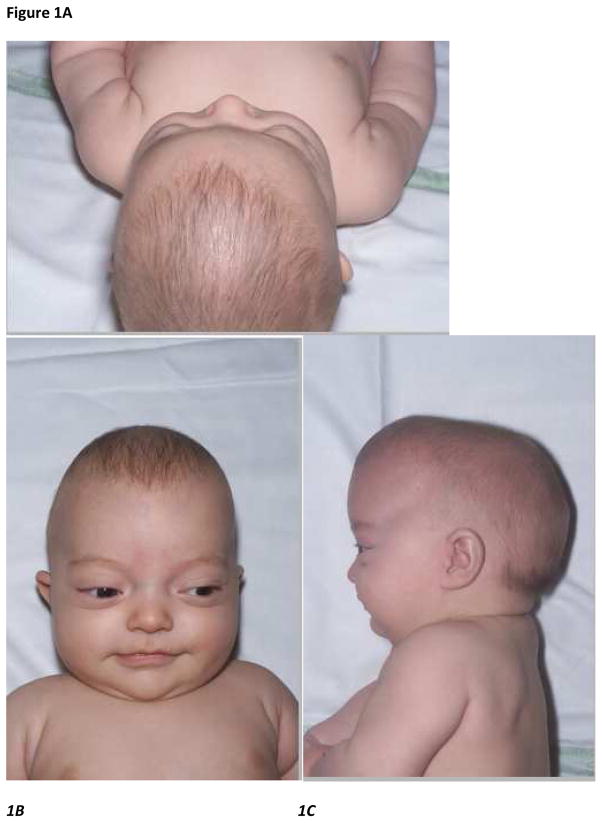
Figure 1.
Figure 1A–C. Vertical, frontal and lateral views of an infant with Crouzon syndrome aged 4 months old– note the frontal bossing and midface hypoplasia
Figure 1D–G. Multiple views of the same patient with Crouzon syndrome aged 11 years old just before monobloc distraction midfacial surgery showing typical clinical features of brachycephaly, exophthalmos & maxillary hypoplasia with mandibular prognathism
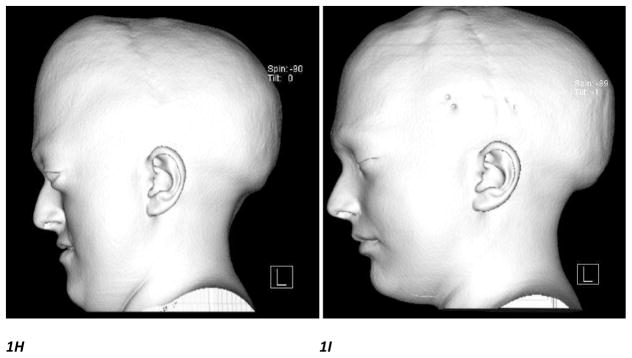
Figure 1H, I. Lateral CT scan 3D craniofacial reconstruction images performed before and after monobloc distraction surgery showing significant morphological improvement of the midfacial hypoplasia
Figure 2.
Figure 2A–C. Frontal, lateral and vertical views of a patient with Apert syndrome aged 4 years old showing typical clinical features of brachycephaly, strabismus, low set ears & midface hypoplasia with mandibular prognathism
Figure 2D, E. Frontal and lateral views of the patient with Apert syndrome following facial bipartition surgery and application of a rigid extraction device (RED) frame – note the tracheostomy tube inserted perioperatively
Figure 2F, G. Frontal and lateral views of the patient with Apert Syndrome following removal of the RED frame and tracheostomy
Figure 2H.I. Lateral CT scan 3D craniofacial reconstruction images performed before and after facial bipartition surgery showing significant morphological improvement of the midfacial hypoplasia
In the Netherlands, children with Apert, Crouzon or Pfeiffer syndromes with signs of raised ICP undergo posterior cranial vault expansion at 6–9 months. The specified preference is to perform midface advancement (monobloc or Le Fort III) in adulthood, unless there is an earlier clinical indication such as severe OSA and/or exorbitism. The majority of patients who undergo midface advancement (Le Fort I or III) show evidence of respiratory improvements, as determined by sleep study results in conjunction with clinical evaluation of the patient (35). Upper airway volume has been shown to increase following the surgical procedure (36). Results of monobloc advancement have been somewhat less promising, although numbers of reported cases are small (n=4). Upper airway volume measurements on a whole, correlate well with respiratory outcome but are not always completely reliable. The authors advocate nasopharyngoscopy prior to midface advancement to identify the level of obstruction. A retrospective review of 11 patients with syndromic craniosynostosis who had moderate/severe OSA in the Netherlands showed that in the short term, midface advancement resulted in improvement of respiratory symptoms in 6 patients, but was not effective in 5. Endoscopy showed pharyngeal collapse with obstruction of the rhino or hypopharynx. In the long term, despite midface advancement, 5/11 patients still had moderate/severe OSA, required CPAP or were unable to be decannulated (37). Taken together, it would appear that those patients who manifested improvements in PSG results following distraction procedures for documented OSA had similar linear changes in the position of the maxilla after surgery, but greater increases in an angular measurement comparable to sella-nasion-point A (SNA) angle, when compared to those children in whom no improvements or minor improvements in OSA occurred (38).
A recent systematic review of upper airway outcomes post midface distraction osteogenesis reported favorable outcomes in cephalometry, sleep study outcomes and decannulation rates in the 16 studies which met the inclusion criteria. However, it is important to note that all the studies included in this meta-analysis were retrospective, details regarding the patients who had not improved were insufficient, and long term results are still unavailable (39).
Modifications and improvements to surgical technique are constantly being reported. Recent case reports include the experience of simultaneous bimaxillary distraction (LeFort III and mandibular distraction) in a child with Crouzon who had midface hypoplasia, but also significant retrusion of the mandible and glossoptosis. There was significant improvement of the OSA from severe to mild with this intervention (40). The same group also reported promising outcomes in children who had early (mean age: 2 years 5 months) midface distraction: all 11 patients avoided tracheostomy and had significant improvements in their post-operative PSG studies and cephalogram findings (41).
II) Clefts
Cleft lip/Palate (CL/P)
Embryonic development of the primary palate (lip and alveolus) and secondary palate (soft and hard palate) occurs between weeks 6–9 of gestation. Cleft lip/palate (CL/P) results when the medial nasal process fails to contact or maintain contact with the lateral nasal and maxillary processes (42). CL/P has an incidence of approximately 1 per 1000 births, although there is substantial geographical and ethnic variation (43). An epidemiological study showed that 70% of the cases were isolated CL/P, whilst the rest were part of a syndrome or associated with other anomalies (44).
Diagnosis of OSA in children with cleft lip/palate
Children with isolated CL/P have a higher incidence of OSA. The size of their pharyngeal airways has been shown to be smaller, and their craniofacial dimensions differ from those of healthy controls (45). The oropharyngeal musculature is also disrupted by the cleft, which impacts on speech and swallow, as well as adversely affects the maintenance of airway patency, particularly during sleep. Silvestre et al screened 489 patients with isolated CL/P for OSA by asking them to complete the Pediatric Sleep Questionnaire (PSQ), and in this series, 14.7% of the respondents screened positive (46). The same group had previously reported that 32% of their 178 CL/P patients who had associated syndromes screened positive for OSA once again based on the PSQ (47). Limitations of these studies are that these are questionnaire based without PSG confirmation. Although the PSQ has been validated in otherwise healthy children (48), it may not have the same sensitivity and specificity in children with craniofacial syndromes. In fact, one study has demonstrated that the PSQ is not a good screening tool for OSA in children with craniofacial conditions with a positive predictive factor of 0.3 and negative predictive factor of 0.74 in this population (49). As previously reported, use of instruments that have not been subjected to the appropriate validation procedures using the stringent methodologies that are inevitably required to this effect, are likely to be fraught with either substantial inaccuracy, but more importantly, may lead to unnecessary over- or under-diagnosis (50, 51).
Maclean et al performed a prospective observational study of 50 infants with CL/P. In this cohort, 35 children had isolated CL/P, 8 had Pierre Robin sequence (PRS) and 7 had an associated syndrome. Using an OMAHI >3/h as the cut off for clinically significant obstructive sleep disordered breathing, 69% of infants with isolated CL/P, 100% of infants with PRS and 86% of infants with associated syndromes met this criterion. Infants with isolated CL/P had lower AHI, OMAHI and ODI compared with infants with PRS and associated syndromes. However, with the exception of syndrome status, none of the other clinical characteristics interrogated such as patient demographics, cleft classification (cleft lip, soft palate, soft and hard palate or complete cleft lip and palate), facial measurements (including distance from otobasion inferius to soft tissue nasion, mandibular length, total facial height, etc) and symptoms of SDB were found to predict the severity of OSA (52). Importantly, SDB symptoms including snoring, difficulty breathing during sleep, observed apneas and mouth breathing were extremely common in the cohort, but did not distinguish between infants with and without OSA.
McLean and colleagues recently reported 3-year follow-up outcomes of 33 children from this original cohort. Their neurocognitive outcomes, quality of life and growth parameters (height, weight, BMI) were all within normal range. However, receptive and expressive language domain scores measured using the Bayley Scale of Infant and Toddler Development were lower in the children who had CL/P compared with controls. They also found that a higher percentage of AS/REM sleep in the initial infant PSG correlated with higher cognitive scores at 3 years, higher initial OMAHI correlated with lower global behavior scores, and higher initial AHI correlated with lower weight z-scores. It must be noted that in the follow up study, the patients were analyzed as a group and not subdivided into those with isolated CL/P (n=23), those with PRS (n=7) and those with an associated syndrome (n=3) (53).
Children with CL/P may require several surgical procedures to correct the congenital deformity. Cleft lip repair involves repair of the lip and alveolus. Palatoplasty is the main surgical technique for reconstruction of the hard and soft palates, with early repair favoring improved speech outcomes (54), whereas later repair appears to be more favorable for midfacial development, particularly the anteroposterior development of the maxillary dentoalveolus (55, 56). Velo-pharyngeal insufficiency, often resulting in hypernasal speech and dysphagia, is seen in approximately a quarter of children with CL/P. and indeed is a common complication of cleft palate repair. A number of surgical techniques such as pharyngoplasty, pharyngeal flap surgery and Furlow double opposing Z-plasty/palatoplasty can be performed to address this (57, 58). These procedures attempt to optimize speech intelligibility by improving velopharyngeal function and limiting nasal airflow (hence nasal resonance) by reducing the space between the soft palate and posterior pharyngeal wall, (59). However, OSA is a recognized complication of both pharyngoplasty and pharyngeal flap surgery (60), with severe OSA leading to cor pulmonale, and even to death following surgery, as previously described (61, 62). Madrid et al reported 30/37 (81%) of patients had OSA 1 year post Orticochea pharyngoplasty, which was severe in 8/37 (22%) (63). A study of 146 patients showed that the number of patients requiring CPAP for OSA increased from 2 to 33 after sphincter pharyngoplasty (64). Symptoms of nasal airway obstruction with mouth breathing or snoring were reported in 9/22 (41%) patients peri-operatively after hemisphincter pharyngoplasty, though all improved except 1 who continued to need CPAP (65). Clinicians should be aware of these substantial issues.
It had been thought that the frequency of OSA post pharyngoplasty was lower than in pharyngeal flap repair (66, 67). However, a recent international multicenter randomized trial did not reveal any significant difference between the 2 procedures (68). Furlow palatoplasty does however appear to have a lower OSA complication rate - in a small study of 20 patients, 1 of the 10 patients who underwent Furlow palatoplasty had mild OSA, while of the 10 patients who underwent pharyngoplasty, 2 had mild OSA, 1 moderate, and 2 severe OSA (69). This has led one center to advocate Furlow palatoplasty for overt clefts and kinetic submucous cleft palates, and pharyngeal flap to treat akinetic palates and persistent velopharyngeal insufficiency after Furlow palatoplasty (70). Hynes pharyngoplasty and Sommerlad palate re-repairs, alternative options for velopharyngeal insufficiency, may have a less detrimental effect on airway obstruction (71). There has also been promising preliminary data on the use of autologous fat injections into the posterior pharynx (72).
Treatment of OSA in children with cleft lip/palate
Adenotonsillectomy in children with cleft lip/palate
Adenotonsillectomy is currently the first line treatment recommended for otherwise healthy children with OSA who have adenotonsillar hypertrophy. However, adenoidectomy can result in velo-pharyngeal insufficiency in children with CL/P (73, 74). Most surgeons therefore advocate and opt to perform partial adenoidectomy to minimize the risk of this complication. Finkelstein et al described their experience of performing transnasal endoscopic partial adenoidectomy in 10 patients with submucous cleft palate. Preoperatively, all suffered with nasal obstruction, loud snoring and episodes of apnea. Following the procedure, nasal obstruction was relieved with preservation of velopharyngeal valve function in all patients. However, 2 patients had residual soft snoring, and 1 child re-developed nasal obstruction 10 months later and needed a repeat partial adenoidectomy (75). Tweedie et al described the use of suction diathermy to perform partial adenoidectomy in 18 patients who had previously undergone cleft palate repair in infancy (76). At median follow-up of 92 months, all demonstrated symptomatic improvement, none had developed features of velo-pharyngeal insufficiency, and there were no cases of symptomatic adenoidal re-growth. No sleep studies were performed in either study. Tonsillectomy, on the other hand, does not appear to significantly affect velo-pharyngeal competence or speech intelligibility (77).
Pierre Robin sequence
The triad of micrognathia, glossoptosis and resultant airway obstruction was described by Pierre Robin in 1923, who identified “a fall of the base of the tongue as a new cause of nasopharyngeal respiratory impairment” (78). It is commonly associated with a cleft palate: the widely accepted paradigm is that mandibular hypoplasia results in displacement of the tongue superiorly and posteriorly between the palatal shelves, thus preventing their fusion in weeks 8–10 of gestation. This aberrant process typically results in a U-shaped cleft of the soft and part of the hard palate. This sequence can occur in isolation, but in more than half of patients seen, it is part of a syndrome, such as Stickler syndrome or 22q11.2 deletion (79).
Diagnosis of OSA in infants with Pierre Robin sequence
Although it was previously thought that OSA in these patients was exclusively due to glossoptosis, nasopharyngoscopy has revealed that the etiology is in fact multifactorial. Sher et al described 4 different mechanisms of obstruction: Type 1 (most commonly seen in PRS) consists of the posterior movement of the dorsum of the tongue to the posterior pharyngeal wall; In Type 2, the tongue moves posteriorly and compresses the soft palate or the cleft palatal tags against the posterior pharyngeal wall; In Type 3, the lateral pharyngeal walls move medially and oppose one another; and in Type 4, the pharynx constricts in a circular manner (80). This is perhaps not surprising, considering oropharyngeal dysfunction has been described in patients with CL/P, impacting on speech and swallowing (81). All children with PRS should be screened for OSA because the incidence is so high, and signs may be relatively subtle: symptoms commonly indicative of OSA such as snoring are not always present and paradoxical chest and abdominal movement may be mistaken for those seen in active sleep in infants. Besides a baseline sleep study, patients should be observed clinically whilst awake, asleep, and feeding, and continue to be followed up with sleep studies at regular intervals to assess their progress.
Treatment of OSA in children with Pierre Robin sequence
Positioning
The first-line management in infants, which was also that described by Robin himself, is prone positioning. This allows the mandible and tongue to fall forwards, thus reducing obstruction at the level of the tongue-base. The reported frequency of success ranges from 25–66% depending on the patients studied (82–85). It should however be noted that besides the study which used respiratory polygraphy to confirm a 25% success rate, most of these quoted rates were not confirmed using full PSG. Some have argued that the main reason the prone position appears effective is that the visual cues, such as paradoxical chest movement and subcostal recession are less visible when prone. There is also the concern that prone position may increase the potential risk of sudden infant death syndrome
Nasopharyngeal airway
If prone positioning is ineffective, then insertion of a NPA can be attempted. The NPA breaks the “seal” between the tongue and posterior pharynx, so the child can breathe through the tube and contralateral nostril (86) (Figure 3). Typically, the NPA is an appropriately sized endotracheal tube cut to the length required, so that the tip of the NPA is just beyond the base of the tongue, but above the epiglottis, as confirmed on lateral neck radiograph or by direct visualization using endoscopy (84). The NPA needs to be upsized and its length adjusted with the infant’s growth (87). In our hospital, an NPA is tried in PRS infants with moderate to severe OSA, and if the airway obstruction is objectively shown to be relieved on a sleep study, the parents are trained to manage the NPA at home. The child is only discharged when the parents pass the required competencies, which include how to perform suctioning, how to recognize when the NPA is blocked, and how to change the NPA. The NPA is routinely changed every 4–6 weeks, or earlier if blocked. Follow-up sleep studies along with reassessment of the size of the NPA are undertaken at 2-monthly intervals. An eventual trial without the NPA is scheduled depending on clinical assessment and sleep study findings. All patients are followed up until the NPA is no longer required and a sleep study after NPA removal deemed satisfactory. Our median follow-up is 12 months (range 2–30 months) (84). The reported frequency of success ranges from 36–100% (84, 88, 89).
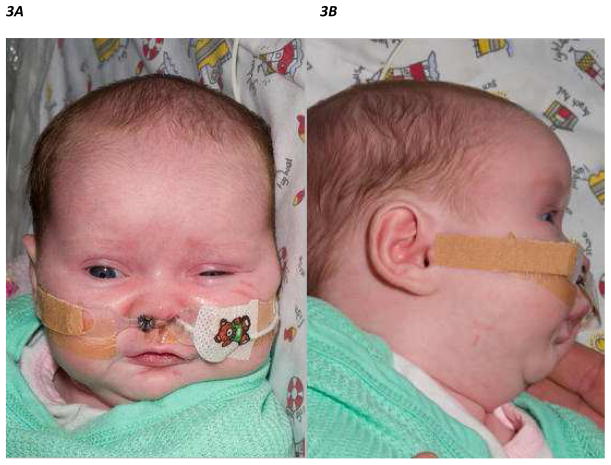
Figure 3.
Figure 3A, B. Frontal and lateral view of an infant with Pierre Robin Sequence with a nasopharyngeal airway in the right nostril secured with a holder and a nasogastric feeding tube in the left nostril
Palatal plates
A few centers have described the use of custom made palatal plates which prevent the tongue from penetrating into and obstructing the nasal airways and also facilitate the sucking action, thus potentially improving feeding difficulties. In one case series, 122/188 (65%) infants had their upper airway obstruction successfully managed in this manner, even though objective data on the extent of the airway obstruction and its resolution were not reported (90). Buchenau et al have described the use of a pre-epiglottic baton plate, a modified acrylic palatal plate in which the velar extension shifts the base of the tongue forward for the treatment of OSA in PRS infants (91). The correct length and angle are determined endoscopically. A randomized cross-over study of 11 infants with PRS was performed and the plates appeared safe and effective in reducing upper airway obstruction, with improvements in the mixed and obstructive apnea index from 13.8/hTST to 3.9/hTST (92).
Surgical interventions
There are currently no international guidelines on the surgical management of children with PRS who have failed conservative therapy. A recent online survey of American Cleft Palate–Craniofacial Association members on the practice patterns of management of upper airway obstruction in patients with PRS revealed a wide variation in personal practice (93). Mandibular distraction was the most common primary procedure, followed by tongue-lip adhesion, and then tracheostomy. This variation in practice is likely due to several factors: 1)Patients with PRS display a wide phenotypic variability, and it is likely that there is “no one surgical approach that fits all”; 2)There is no compelling evidence that one particular surgical procedure is significantly superior to another; 3)Mandibular distraction is a relatively newer procedure, and its utilization may not be as widespread; and 4)Training background of the primary surgeon, i.e., as a plastic surgeon, oromaxillofacial surgeon, or otolaryngologist is likely to influence the choice of procedure.
Tongue lip adhesion
Tongue lip adhesion (TLA) or glossopexy is performed by anchoring the anterior ventral tongue to the lower lip and the posterior tongue to the mandible. This procedure is clearly only going to be effective if the main level of obstruction is at the tongue base. Kirschner et al advocated this approach as the first line intervention when positioning fails, with a reported success rate of 83% (94). Dehiscence can occur, particularly if it is just adhesion to the mucosa which has been performed. Furthermore, despite a high initial success rate for correction of neonatal airway obstruction, longer term follow-up indicates that a secondary intervention is often required at a later stage (95, 96). TLA may have a detrimental impact on feeding due to its effect on tongue mobility, and thus it may impede proper bolus formation and swallowing (97). However, Cozzi et al showed an increase in weight velocity in PRS patients after TLA, suggesting that the effect of TLA is not severe enough to prevent weight gain once airway obstruction has been relieved (98). Notably however, several centers have moved away from TLA towards the newer technique of mandibular distraction in recent years.
Mandibular distraction osteogenesis
Mandibular distraction osteogenesis (MDO) was initially used as a means of achieving stabilization of the upper airway to enable successful decannulation in older PRS patients with tracheotomies who had previously failed decannulation (99). This technique involves cutting the bone, followed by a lag period during which the callus is allowed to form, before progressively advancing the segments of bone apart controlled on a rigid frame at 1–2mm/day. A consolidation phase then follows, allowing for the distracted soft callus to ossify. The pharyngeal airway size is thus increased by the gradual mandibular lengthening. Use of the technique was then expanded to infants with PRS as an alternative to tracheostomy (100). Once again, this is mainly effective for patients who have obstruction at the level of the tongue base. The distraction devices can be internal or external. External devices are easier to adjust and remove, but can be dislodged, and are associated with scarring. Internal devices tend to be better tolerated, but may require repeat general anesthetics for adjustment and removal (101). Many centers have moved towards internal devices as recent developments have meant that anesthetics for adjustment may no longer be necessary (Figure 4).
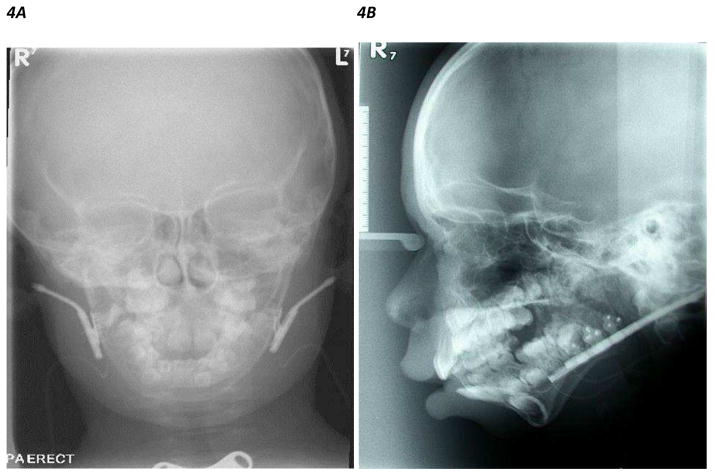
Figure 4.
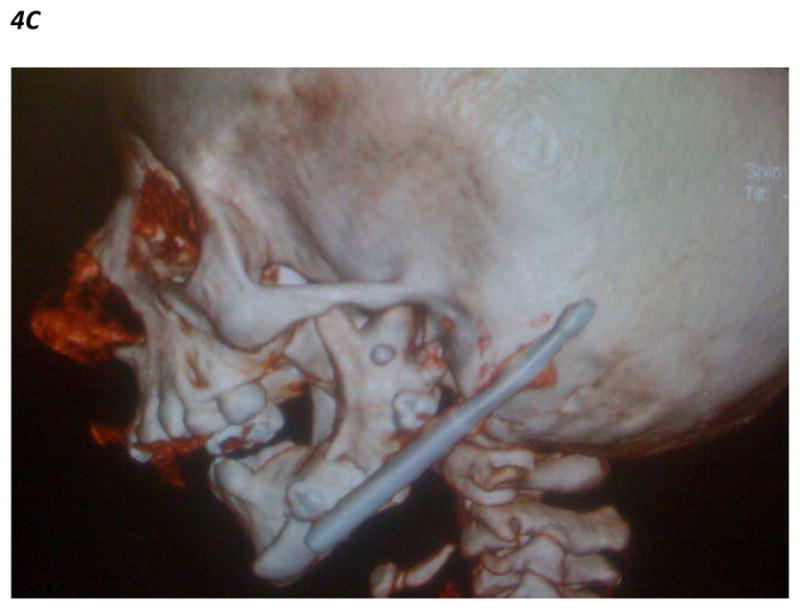
Figure 4A, B. Frontal and lateral skull X ray showing bilateral internal mandibular distraction osteogenesis devices inserted in both mandibles of a patient with mandibular hypoplasia
Figure 4C. Lateral CT scan 3D craniofacial reconstruction images showing internal mandibular distraction osteogenesis device in the left mandibular ramus of a patient with mandibular hypoplasia
PRS infants who have undergone MDO have shown clinical improvement and significant increases in distance from pharyngeal wall to tongue base, cross sectional airway and hypopharyngeal airway volumes on CT (102, 103). A published meta-analysis has shown that MDO is effective in preventing tracheostomies in 91.3% of infants, and in relieving symptoms of OSA in 97% of the affected children (104). MDO has also been shown to result in a subjective overall benefit in health-related quality of life (105).
A recent systematic review provided a good overview of the complications seen in MDO. The authors divided the complications into 6 categories: Type 1 spontaneously resolving complications including neurosensory disturbances, pain and dental problems; Type 2 medically manageable without requiring hospitalization e.g minor infection, anterior open bite, non-compliance; Type 3 surgically manageable requiring local anesthesia only; Type 4 technical complication necessitating general anesthesia for correction e.g. device failure,; Type 5 medically/surgically manageable with hospitalization or general anesthesia e.g. premature consolidation, incomplete osteotomy, surgical correction of severe open bite; Type 6 permanent sequelae, which included permanent inferior alveolar nerve neurosensory disturbance, skeletal relapse, condylar resorption, permanent damage to central incisors, chin ptosis, undesired anterior open bite, lingual nerve neurosensory disturbance (106).
Flores et al compared MDO versus TLA (107). They found that patients undergoing MDO demonstrated significantly higher oxygen saturation levels and lower AHI at 1 month and 1 year postoperatively compared with TLA (despite the MDO group showing evidence of more severe airway obstruction pre-procedure). Furthermore, although none of the patients in the MDO group required tracheostomy, 4 in the TLA group needed to proceed to tracheostomy. Surgical complication rates were similar in the 2 groups, and therefore the authors concluded that MDO appears to offer superior outcome measures compared with TLA. This was a retrospective single surgeon review, and the TLA procedures were performed between 1994–2004, whereas the MDO procedures were performed between 2004 to 2009. Potentially, other aspects of care could have changed over the years, which may be a confounding factor. Heated discussions amongst surgeons are still ongoing (108). Proponents of MDO argue that although it is a more technically challenging operation, it produces lasting skeletal correction (109) as opposed to TLA, which is more a temporizing measure while awaiting “catch up growth” of the mandible, which may not always occur (110).
A comparative cost analysis study of MDO versus tracheostomy in the US found that management with MDO resulted in significant savings over tracheostomy due to tracheostomy having greater hospital related charges (despite no difference in length of hospital stay), more frequent airway procedures, a higher incidence of gastrostomy tube feeds and increased homecare costs (111). A smaller study in the Netherlands, showed that tracheostomy had a 3-fold increase in cost, with four times more complications compared with MDO, reinforcing the current trend to avoid tracheostomy whenever possible (112).
Tracheostomy
Tracheotomy is the definitive procedure for the relief of airway obstruction. However, due to its attendant risks and complications, it is now often reserved as a last resort. There is some variation in the threshold for proceeding to tracheostomy from centre to centre. Indications include children who have multilevel airway obstruction, those who also have lower airway disease (such as chronic disease of prematurity) and thus require chronic ventilatory support, and those who have failed other treatment options.
Demke et al surveyed parents of PRS patients to evaluate their perceptions of tracheostomy morbidity and quality of life (113). Although the majority of the parental expectations regarding tracheostomy were met, most still found the overall experience difficult. The experience of the parents whose expectations were not met, further highlighted the need for better pre-procedure counseling, particularly regarding estimate of length of time the child would have the tracheostomy in place, and the likelihood of potential complications including frequent hospitalizations.
Nutrition
The importance of nutrition in PRS children cannot be overstated. PRS patients have increased energy expenditure from the increased work of breathing coupled with feeding and swallowing difficulties (114). A significant number of PRS patients will require nasogastric feeding, particularly in the first few months of life, to maintain adequate nutrition and growth (115, 116). Gastroesophageal reflux is common in these patients, and should be actively investigated for and treated (117). Early airway intervention to treat airway obstruction can have a beneficial effect on feeding (118).
Several centers have published management algorithms on the airway management of patients with PRS (79, 119–121). The common underlying theme behind these algorithms is that most clinicians aim to use the least invasive, yet most effective method for treatment of OSA. There is often a management cascade starting with conservative treatment such as prone positioning, insertion of NPA and CPAP, before surgical interventions such as mandibular distraction or tongue lip adhesion are considered. Most advocate nasopharyngolaryngoscopy and bronchoscopy prior to surgical intervention to identify the level of the obstruction. Tracheostomy tends to be reserved for patients who have failed earlier attempts at treatment or for children who have associated lower airway anomalies such as subglottic stenosis or require chronic ventilatory support. In parallel, swallow and feeding evaluation needs to take place with optimization of feeding regimes and gastroesophageal reflux disease treated if present.
In the longer term, OSA seems to improve in most patients as they grow older. A small study of 8 older PRS patients aged between 8–22 years old showed that these patients only had mild SDB, with a mean AHI of 1.8/h (compared to 0.5/h in the controls) and slightly less time in REM sleep. One patient however, had an abnormally raised CAI of 81.7/h (122).
Treacher-Collins syndrome
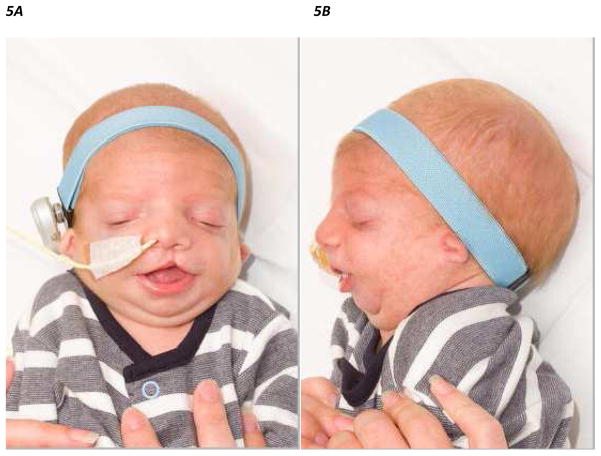
Treacher-Collins syndrome (TCS) is an autosomal-dominant disorder of craniofacial development, with an estimated incidence of 1 in every 50,000 live births. It is characterized by hypoplasia of the facial bones, cleft palate, malformation of the external ears, atresia of the external auditory canals, conductive hearing loss, downward slanting palpebral fissures, colobomas of the lower eyelids and pharyngeal hypoplasia (123) (Figure 5). The majority of patients are heterozygous for a mutation in TCOF1 (124), which is involved in rRNA transcription, by interacting with UBF, a RNA polymerase I transcription factor (125). However, patients who have mutations in the genes encoding subunits of RNA polymerases I and III have also been identified (126).
Figure 5.
Figure 5A, B. Frontal and lateral view of an infant with Treacher-Collins Syndrome with previously repaired cleft lip and nasogastric feeding tube in situ
A study of 35 patients (13 children) with TCS showed that OSA was present in 54% of the children and 41% of the adult patients (127). Endoscopy of the upper airway in 11 of the patients (by sleep endoscopy, or flexible/rigid endoscopy) revealed the obstruction occurred at various anatomical levels from the nasal septum to the trachea, though most significant obstruction was at the level of the oro/hypopharynx. The authors suggested that endoscopy of the upper airway is helpful in these complex patients as it enables determination of the exact level of obstruction. Recently published 3-dimensional craniometric findings confirm that children with TCS have decreased upper airway volumes, particularly in the retroglossal region (128). In many patients, surgical treatment of just one level is unlikely to completely cure the OSA, and therefore CPAP or tracheotomy may be a better treatment approach. Of note, in children with TCS, the Brouillette score was similarly unhelpful as a screening tool for OSA with poor positive and negative predictive values (129).
Future work and unanswered questions
Most of the published literature on craniofacial abnormalities and sleep is limited by including either individual case reports or retrospective reviews that encompass relatively small patient numbers. Conclusions from such studies and reports are further hampered by the lack of objective data such as PSG findings pre and post intervention, the small number of studies that have looked at long-term outcomes, and the heterogeneous phenotype of these patients, which dilutes the already scarce experience in the evaluation and management of individual craniofacial conditions. Ideally, multicenter/multinational trials are needed for recruitment of sufficient patient numbers to allow for phenotypic matching and adequate comparison of surgical outcomes.
How aggressive should one be in treating these children? OSA has been shown to result in significant morbidities including neurocognitive and behavioral sequelae in otherwise healthy children. Although current treatment modalities undeniably reduce the severity of OSA in children with craniofacial syndromes, the reported incidence of complete resolution following treatment, unless a tracheostomy is inserted, is relatively low and difficult to predict a priori. Patients with CL/P have increased mortality and morbidity compared to unaffected individuals (130). This is particularly true in the first year of life, but also persists through childhood and adulthood (131). Although the mean IQ of children with syndromic craniosynostosis is comparable to that of the general population, there is a higher incidence of intellectual disability and behavioral problems, particularly in children with Apert and Muenke syndromes (132). It remains unclear what is the contribution of residual OSA to this morbidity and mortality.
The exposure to intermittent hypoxia in early infancy in craniofacial patients with OSA may impact on the ventilatory response to hypoxia. Infants with CL/P and high AHI (>15/hTST) demonstrated a blunted maximal ventilatory response and experienced an earlier ventilatory decline in response to hypoxia, compared with CL/P infants with low AHI (<15/hTST) (133). The strategies to augment ventilation also appear to differ: increased respiratory rate was the first initial response in infants with low AHI, while in infants with high AHI, tidal volume was the first parameter to increase. The underlying mechanisms, duration of these changes and effect of treatment are still unknown. Medical therapy in the form of leukotriene antagonists, e.g., montelukast and nasal corticosteroids has recently gained acceptance as a treatment for mild OSA. Their benefit in the treatment of OSA in craniofacial patients is unexplored. They are unlikely to be of major benefit in patients where the obstruction is predominantly due to glossoptosis or midface hypoplasia, but may have a role to play when there is lymphoid tissue hypertrophy or possibly in conjunction with other therapies.
Conclusion
The diagnosis of sleep disordered breathing should be considered in all children with craniofacial syndromes. The most common sleep disorder is OSA, though central apneas during sleep have also been reported, particularly in the craniosynostoses. The etiology of the OSA is multifactorial, and includes anatomical facial and upper airway factors such as midface hypoplasia and glossoptosis, as well as factors resulting in increased upper airway collapsibility such as oropharyngeal dysfunction.
Common treatments for OSA in this patient population include prone positioning, insertion of a nasopharyngeal airway, adenotonsillectomy, CPAP, and surgical interventions such as midface advancement in the craniosynostoses and mandibular distraction osteogenesis in children with Pierre Robin sequence. However, a significant proportion of patients have residual OSA despite treatment. Tracheostomy is the definitive method of airway management, but is fraught with higher attendant risks and morbidities. There is no doubt that these complex patients are best managed by multidisciplinary teams comprising otolaryngologists, respiratory and sleep physicians, craniofacial surgeons, orthodontists, neurosurgeons, speech therapists and specialist nurses.
Practice points.
The high prevalence of sleep disordered breathing in children with craniofacial syndromes should prompt clinicians to have a high index of suspicion and a low referral or evaluation threshold.
The most common sleep disorder in children with craniofacial anomalies is obstructive sleep apnea. Midface hypoplasia in children with craniosynostosis and glossoptosis in children with Pierre Robin syndrome are well recognized risk factors, but the etiology is often multifactorial and many children have multilevel airway obstruction.
The presence of increased intracranial pressure in children with craniosynostosis should prompt screening for sleep disordered breathing.
Most centers advocate a management cascade for infants with Pierre Robin sequence who have obstructive sleep apnea, starting with prone positioning, insertion of nasopharyngeal airway, CPAP, before surgical interventions such as mandibular distraction or tongue lip adhesion are considered. Tracheostomy should be reserved for patients who have failed other treatments or for children who have associated lower airway anomalies such as subglottic stenosis or require chronic ventilatory support.
Obstructive sleep apnea may be a complication of surgical interventions to correct velopharyngeal insufficiency in patients with cleft palate. Conversely, adenoidectomy as treatment for obstructive sleep apnea can result in velo-pharyngeal insufficiency in children with cleft lip/palate and most surgeons opt to perform partial adenoidectomy to minimize the risk of this complication.
Multidisciplinary team approaches are crucial for optimal management of these complex patients.
Research agenda.
Craniofacial syndromes are uncommon conditions with heterogeneous phenotypes. For obstructive sleep apnea treatment trials to be adequately powered in these patient groups, multicenter/multinational collaborations, with objective polysomnography measures are required.
Exposure to intermittent hypoxia in early infancy in craniofacial patients with obstructive sleep apnea may impact on the ventilatory response to hypoxia: blunted maximal ventilatory response and earlier ventilatory decline in response to hypoxia have been described. Research seeking to determine the underlying mechanisms, duration of these changes and effect of treatment would be forthcoming.
A better understanding of the contribution of residual obstructive sleep apnea to the morbidity spectrum and mortality described in craniofacial syndromes is fundamentally important, critically needed, and should guide clinical management.
The role of medical therapies such as leukotriene antagonists and nasal corticosteroids, intraoral appliances, or myofunctional therapies in the treatment of obstructive sleep apnea in these children, possibly in conjunction with other approaches may be worth exploring.
Acknowledgments
Funding Sources: LKG and DG are supported by National Institutes of Health grants HL-65270.
We would like to acknowledge Dr. P. Ayliffe, Oral and Maxillofacial surgeon at Great Ormond Street Hospital for providing some of the photos and for his feedback on the manuscript.
Abbreviations
- AHI
Apnea hypopnea index
- BiPAP
Bilevel positive airway pressure
- BMI
Body Mass Index
- CBCL
Child behavior checklist
- CL/P
Cleft lip/Palate
- CPAP
Continuous positive airway pressure
- CT
Computerized tomography
- ICP
Intracranial pressure
- FGFR
Fibroblast growth factor receptor
- MDO
Mandibular distraction osteogenesis
- MRI
Magnetic resonance imaging
- NPA
Nasopharyngeal airway
- ODI
Oxygen desaturation index
- OMAHI
Obstructive and Mixed Apnea hypopnea index
- OSA
Obstructive sleep apnea
- PRS
Pierre Robin sequence
- PSQ
Pediatric sleep questionnaire
- SDB
Sleep disordered breathing
- TCS
Treacher-Collins syndrome
- TLA
Tongue lip adhesion
- TST
Total sleep time
- UBF
Upstream binding factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012 Sep;130(3):576–84. doi: 10.1542/peds.2012-1671. [DOI] [PubMed] [Google Scholar]
- 2.Caron CJ, Pluijmers BI, Joosten KF, Mathijssen IM, van der Schroeff MP, Dunaway DJ, et al. Obstructive sleep apnoea in craniofacial microsomia: a systematic review. Int J Oral Maxillofac Surg. 2015 May;44(5):592–8. doi: 10.1016/j.ijom.2015.01.023. [DOI] [PubMed] [Google Scholar]
- 3.Marcus CL, Brooks LJ, Draper KA, Gozal D, Halbower AC, Jones J, et al. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics. 2012 Sep;130(3):e714–55. doi: 10.1542/peds.2012-1672. [DOI] [PubMed] [Google Scholar]
- 4.Tauman R, Gozal D. Obstructive sleep apnea syndrome in children. Expert Rev Respir Med. 2011 Jun;5(3):425–40. doi: 10.1586/ers.11.7. [DOI] [PubMed] [Google Scholar]
- 5.Gozal D, Capdevila OS, Kheirandish-Gozal L. Metabolic alterations and systemic inflammation in obstructive sleep apnea among nonobese and obese prepubertal children. Am J Respir Crit Care Med. 2008 May 15;177(10):1142–9. doi: 10.1164/rccm.200711-1670OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gozal D, Kheirandish-Gozal L, Bhattacharjee R, Spruyt K. Neurocognitive and endothelial dysfunction in children with obstructive sleep apnea. Pediatrics. 2010 Nov;126(5):e1161–7. doi: 10.1542/peds.2010-0688. [DOI] [PubMed] [Google Scholar]
- 7.Whitaker LA, Pashayan H, Reichman J. A proposed new classification of craniofacial anomalies. Cleft Palate J. 1981 Jul;18(3):161–76. [PubMed] [Google Scholar]
- 8.Reardon W, Winter RM, Rutland P, Pulleyn LJ, Jones BM, Malcolm S. Mutations in the fibroblast growth factor receptor 2 gene cause Crouzon syndrome. Nat Genet. 1994 Sep;8(1):98–103. doi: 10.1038/ng0994-98. [DOI] [PubMed] [Google Scholar]
- 9.Rutland P, Pulleyn LJ, Reardon W, Baraitser M, Hayward R, Jones B, et al. Identical mutations in the FGFR2 gene cause both Pfeiffer and Crouzon syndrome phenotypes. Nat Genet. 1995 Feb;9(2):173–6. doi: 10.1038/ng0295-173. [DOI] [PubMed] [Google Scholar]
- *10.Driessen C, Joosten KF, Bannink N, Bredero-Boelhouwer HH, Hoeve HL, Wolvius EB, et al. How does obstructive sleep apnoea evolve in syndromic craniosynostosis? A prospective cohort study. Arch Dis Child. 2013 Jul;98(7):538–43. doi: 10.1136/archdischild-2012-302745. [DOI] [PubMed] [Google Scholar]
- 11.Alsaadi MM, Iqbal SM, Elgamal EA, Salih MA, Gozal D. Sleep-disordered breathing in children with craniosynostosis. Sleep Breath. 2013 Mar;17(1):389–93. doi: 10.1007/s11325-012-0706-2. [DOI] [PubMed] [Google Scholar]
- 12.Moraleda-Cibrian M, Edwards SP, Kasten SJ, RS, Berger M, O’Brien LM. Obstructive Sleep Apnea Pre and Post-treatment in Symptomatic Children with Congenital Craniofacial Malformations. J Clin Sleep Med. 2014 Oct 19; doi: 10.5664/jcsm.4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Saleh S, Riekstins A, Forrest CR, Philips JH, Gibbons J, Narang I. Sleep-related disordered breathing in children with syndromic craniosynostosis. J Craniomaxillofac Surg. 2011 Apr;39(3):153–7. doi: 10.1016/j.jcms.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 14.de Jong T, Bannink N, Bredero-Boelhouwer HH, van Veelen ML, Bartels MC, Hoeve LJ, et al. Long-term functional outcome in 167 patients with syndromic craniosynostosis; defining a syndrome-specific risk profile. J Plast Reconstr Aesthet Surg. 2010 Oct;63(10):1635–41. doi: 10.1016/j.bjps.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 15.Bannink N, Maliepaard M, Raat H, Joosten KF, Mathijssen IM. Obstructive sleep apnea-specific quality of life and behavioral problems in children with syndromic craniosynostosis. J Dev Behav Pediatr. 2011 Apr;32(3):233–8. doi: 10.1097/DBP.0b013e318206d5e3. [DOI] [PubMed] [Google Scholar]
- 16.Hayward R, Gonsalez S. How low can you go? Intracranial pressure, cerebral perfusion pressure, and respiratory obstruction in children with complex craniosynostosis. J Neurosurg. 2005 Jan;102(1 Suppl):16–22. doi: 10.3171/ped.2005.102.1.0016. [DOI] [PubMed] [Google Scholar]
- *17.Gonsalez S, Hayward R, Jones B, Lane R. Upper airway obstruction and raised intracranial pressure in children with craniosynostosis. Eur Respir J. 1997 Feb;10(2):367–75. doi: 10.1183/09031936.97.10020367. [DOI] [PubMed] [Google Scholar]
- 18.Marucci DD, Dunaway DJ, Jones BM, Hayward RD. Raised intracranial pressure in Apert syndrome. Plast Reconstr Surg. 2008 Oct;122(4):1162–8. doi: 10.1097/PRS.0b013e31818458f0. discussion 9–70. [DOI] [PubMed] [Google Scholar]
- 19.Spruijt B, Joosten KF, Driessen C, Rizopoulos D, Naus NC, van der Schroeff MP. Algorithm for the management of intracranial hypertension in children with syndromic craniosynostosis. Plast Reconstr Surg. 2015 Apr; doi: 10.1097/PRS.0000000000001434. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 20.Cinalli G, Chumas P, Arnaud E, Sainte-Rose C, Renier D. Occipital remodeling and suboccipital decompression in severe craniosynostosis associated with tonsillar herniation. Neurosurgery. 1998 Jan;42(1):66–71. doi: 10.1097/00006123-199801000-00013. discussion -3. [DOI] [PubMed] [Google Scholar]
- 21.Cinalli G, Renier D, Sebag G, Sainte-Rose C, Arnaud E, Pierre-Kahn A. Chronic tonsillar herniation in Crouzon’s and Apert’s syndromes: the role of premature synostosis of the lambdoid suture. J Neurosurg. 1995 Oct;83(4):575–82. doi: 10.3171/jns.1995.83.4.0575. [DOI] [PubMed] [Google Scholar]
- 22.Spence J, Pasterkamp H, McDonald PJ. Isolated central sleep apnea in type I Chiari malformation: improvement after surgery. Pediatr Pulmonol. 2010 Nov;45(11):1141–4. doi: 10.1002/ppul.21294. [DOI] [PubMed] [Google Scholar]
- 23.Frim DM, Jones D, Goumnerova L. Development of symptomatic Chiari malformation in a child with craniofacial dysmorphism. Pediatr Neurosurg. 1990;16(4–5):228–31. doi: 10.1159/000120532. [DOI] [PubMed] [Google Scholar]
- 24.Addo NK, Javadpour S, Kandasamy J, Sillifant P, May P, Sinha A. Central sleep apnea and associated Chiari malformation in children with syndromic craniosynostosis: treatment and outcome data from a supraregional national craniofacial center. J Neurosurg Pediatr. 2013 Mar;11(3):296–301. doi: 10.3171/2012.11.PEDS12297. [DOI] [PubMed] [Google Scholar]
- 25.Driessen C, Mathijssen IM, De Groot MR, Joosten KF. Does central sleep apnea occur in children with syndromic craniosynostosis? Respir Physiol Neurobiol. 2012 May 31;181(3):321–5. doi: 10.1016/j.resp.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 26.Gonsalez SL, Thompson D, Hayward R, Lane R. Breathing patterns in children with craniofacial dysostosis and hindbrain herniation. Eur Respir J. 1998 Apr;11(4):866–72. doi: 10.1183/09031936.98.11040866. [DOI] [PubMed] [Google Scholar]
- 27.Driessen C, Joosten KF, Florisson JM, Lequin M, van Veelen ML, Dammers R, et al. Sleep apnoea in syndromic craniosynostosis occurs independent of hindbrain herniation. Childs Nerv Syst. 2013 Feb;29(2):289–96. doi: 10.1007/s00381-012-1922-6. [DOI] [PubMed] [Google Scholar]
- 28.Bannink N, Mathijssen IM, Joosten KF. Can parents predict obstructive sleep apnea in children with syndromic or complex craniosynostosis? Int J Oral Maxillofac Surg. 2010 May;39(5):421–3. doi: 10.1016/j.ijom.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Alonso-Alvarez ML, Teran-Santos J, Ordax Carbajo E, Cordero-Guevara JA, Navazo-Eguia AI, Kheirandish-Gozal L, et al. Reliability of Home Respiratory Polygraphy for the Diagnosis of Sleep Apnea in Children. Chest. 2014 Dec 24; doi: 10.1378/chest.14-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan HL, Gozal D, Ramirez HM, Bandla HP, Kheirandish-Gozal L. Overnight polysomnography versus respiratory polygraphy in the diagnosis of pediatric obstructive sleep apnea. Sleep. 2014 Feb;37(2):255–60. doi: 10.5665/sleep.3392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmed J, Marucci D, Cochrane L, Heywood RL, Wyatt ME, Leighton SE. The role of the nasopharyngeal airway for obstructive sleep apnea in syndromic craniosynostosis. J Craniofac Surg. 2008 May;19(3):659–63. doi: 10.1097/SCS.0b013e31816ae386. [DOI] [PubMed] [Google Scholar]
- 32.Willington AJ, Ramsden JD. Adenotonsillectomy for the management of obstructive sleep apnea in children with congenital craniosynostosis syndromes. J Craniofac Surg. 2012 Jul;23(4):1020–2. doi: 10.1097/SCS.0b013e31824e6cf8. [DOI] [PubMed] [Google Scholar]
- 33.Amonoo-Kuofi K, Phillips SP, Randhawa PS, Lane R, Wyatt ME, Leighton SE. Adenotonsillectomy for sleep-disordered breathing in children with syndromic craniosynostosis. J Craniofac Surg. 2009 Nov;20(6):1978–80. doi: 10.1097/SCS.0b013e3181bd2c9a. [DOI] [PubMed] [Google Scholar]
- 34.Tessier P. Total facial osteotomy. Crouzon’s syndrome, Apert’s syndrome: oxycephaly, scaphocephaly, turricephaly. Ann Chir Plast. 1967 Dec;12(4):273–86. [PubMed] [Google Scholar]
- *35.Nout E, Bannink N, Koudstaal MJ, Veenland JF, Joosten KF, Poublon RM, et al. Upper airway changes in syndromic craniosynostosis patients following midface or monobloc advancement: correlation between volume changes and respiratory outcome. J Craniomaxillofac Surg. 2012 Apr;40(3):209–14. doi: 10.1016/j.jcms.2011.04.017. [DOI] [PubMed] [Google Scholar]
- 36.Nout E, Bouw FP, Veenland JF, Hop WC, van der Wal KG, Mathijssen IM, et al. Three-dimensional airway changes after Le Fort III advancement in syndromic craniosynostosis patients. Plast Reconstr Surg. 2010 Aug;126(2):564–71. doi: 10.1097/PRS.0b013e3181de227f. [DOI] [PubMed] [Google Scholar]
- *37.Bannink N, Nout E, Wolvius EB, Hoeve HL, Joosten KF, Mathijssen IM. Obstructive sleep apnea in children with syndromic craniosynostosis: long-term respiratory outcome of midface advancement. Int J Oral Maxillofac Surg. 2010 Feb;39(2):115–21. doi: 10.1016/j.ijom.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 38.Hopper RA. New trends in cranio-orbital and midface distraction for craniofacial dysostosis. Curr Opin Otolaryngol Head Neck Surg. 2012 Aug;20(4):298–303. doi: 10.1097/MOO.0b013e3283543a43. [DOI] [PubMed] [Google Scholar]
- *39.Taylor BA, Brace M, Hong P. Upper airway outcomes following midface distraction osteogenesis: a systematic review. J Plast Reconstr Aesthet Surg. 2014 Jul;67(7):891–9. doi: 10.1016/j.bjps.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 40.Mitsukawa N, Morishita T, Saiga A, Omori N, Kubota Y, Akita S, et al. A case of Crouzon syndrome treated by simultaneous bimaxillary distraction. J Plast Reconstr Aesthet Surg. 2014 Jan;67(1):124–5. doi: 10.1016/j.bjps.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 41.Mitsukawa N, Kaneko T, Saiga A, Akita S, Satoh K. Early midfacial distraction for syndromic craniosynostotic patients with obstructive sleep apnoea. J Plast Reconstr Aesthet Surg. 2013 Sep;66(9):1206–11. doi: 10.1016/j.bjps.2013.04.061. [DOI] [PubMed] [Google Scholar]
- 42.Eppley BL, van Aalst JA, Robey A, Havlik RJ, Sadove AM. The spectrum of orofacial clefting. Plast Reconstr Surg. 2005 Jun;115(7):101e–14e. doi: 10.1097/01.prs.0000164494.45986.91. [DOI] [PubMed] [Google Scholar]
- 43.Mossey PA, Little J, Munger RG, Dixon MJ, Shaw WC. Cleft lip and palate. Lancet. 2009 Nov 21;374(9703):1773–85. doi: 10.1016/S0140-6736(09)60695-4. [DOI] [PubMed] [Google Scholar]
- 44.Calzolari E, Pierini A, Astolfi G, Bianchi F, Neville AJ, Rivieri F. Associated anomalies in multi-malformed infants with cleft lip and palate: An epidemiologic study of nearly 6 million births in 23 EUROCAT registries. Am J Med Genet A. 2007 Mar 15;143A(6):528–37. doi: 10.1002/ajmg.a.31447. [DOI] [PubMed] [Google Scholar]
- 45.Rose E, Thissen U, Otten JE, Jonas I. Cephalometric assessment of the posterior airway space in patients with cleft palate after palatoplasty. Cleft Palate Craniofac J. 2003 Sep;40(5):498–503. doi: 10.1597/1545-1569_2003_040_0498_caotpa_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 46.Silvestre J, Tahiri Y, Paliga JT, Taylor JA. Incidence of positive screening for obstructive sleep apnea in patients with isolated cleft lip and/or palate. Can J Plast Surg. 2014 Winter;22(4):259–63. doi: 10.4172/plastic-surgery.1000886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Silvestre J, Tahiri Y, Paliga JT, Taylor JA. Screening for obstructive sleep apnea in children with syndromic cleft lip and/or palate. J Plast Reconstr Aesthet Surg. 2014 Nov;67(11):1475–80. doi: 10.1016/j.bjps.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 48.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric sleep questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000 Feb 1;1(1):21–32. doi: 10.1016/s1389-9457(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 49.Cielo CM, Silvestre J, Paliga JT, Maguire M, Gallagher PR, Marcus CL, et al. Utility of screening for obstructive sleep apnea syndrome in children with craniofacial disorders. Plast Reconstr Surg. 2014 Sep;134(3):434e–41e. doi: 10.1097/PRS.0000000000000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spruyt K, Gozal D. Development of pediatric sleep questionnaires as diagnostic or epidemiological tools: a brief review of dos and don’ts. Sleep Med Rev. 2011 Feb;15(1):7–17. doi: 10.1016/j.smrv.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spruyt K, Gozal D. Pediatric sleep questionnaires as diagnostic or epidemiological tools: a review of currently available instruments. Sleep Med Rev. 2011 Feb;15(1):19–32. doi: 10.1016/j.smrv.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *52.MacLean JE, Fitzsimons D, Fitzgerald DA, Waters KA. The spectrum of sleep-disordered breathing symptoms and respiratory events in infants with cleft lip and/or palate. Arch Dis Child. 2012 Dec;97(12):1058–63. doi: 10.1136/archdischild-2012-302104. [DOI] [PubMed] [Google Scholar]
- 53.Smith CB, Walker K, Badawi N, Waters KA, MacLean JE. Impact of sleep and breathing in infancy on outcomes at three years of age for children with cleft lip and/or palate. Sleep. 2014 May;37(5):919–25. doi: 10.5665/sleep.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rohrich RJ, Rowsell AR, Johns DF, Drury MA, Grieg G, Watson DJ, et al. Timing of hard palatal closure: a critical long-term analysis. Plast Reconstr Surg. 1996 Aug;98(2):236–46. doi: 10.1097/00006534-199608000-00005. [DOI] [PubMed] [Google Scholar]
- 55.Friede H, Enemark H. Long-term evidence for favorable midfacial growth after delayed hard palate repair in UCLP patients. Cleft Palate Craniofac J. 2001 Jul;38(4):323–9. doi: 10.1597/1545-1569_2001_038_0323_lteffm_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 56.Liao YF, Cole TJ, Mars M. Hard palate repair timing and facial growth in unilateral cleft lip and palate: a longitudinal study. Cleft Palate Craniofac J. 2006 Sep;43(5):547–56. doi: 10.1597/05-119. [DOI] [PubMed] [Google Scholar]
- 57.MacLean JE, Hayward P, Fitzgerald DA, Waters K. Cleft lip and/or palate and breathing during sleep. Sleep Med Rev. 2009 Oct;13(5):345–54. doi: 10.1016/j.smrv.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 58.Abdel-Aziz M, El-Hoshy H, Ghandour H. Treatment of velopharyngeal insufficiency after cleft palate repair depending on the velopharyngeal closure pattern. J Craniofac Surg. 2011 May;22(3):813–7. doi: 10.1097/SCS.0b013e31820f3691. [DOI] [PubMed] [Google Scholar]
- 59.Nasser M, Fedorowicz Z, Newton JT, Nouri M. Interventions for the management of submucous cleft palate. Cochrane Database Syst Rev. 2008;(1):CD006703. doi: 10.1002/14651858.CD006703.pub2. [DOI] [PubMed] [Google Scholar]
- 60.Liao YF, Chuang ML, Chen PK, Chen NH, Yun C, Huang CS. Incidence and severity of obstructive sleep apnea following pharyngeal flap surgery in patients with cleft palate. Cleft Palate Craniofac J. 2002 May;39(3):312–6. doi: 10.1597/1545-1569_2002_039_0312_iasoos_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 61.Kravath RE, Pollak CP, Borowiecki B, Weitzman ED. Obstructive sleep apnea and death associated with surgical correction of velopharyngeal incompetence. J Pediatr. 1980 Apr;96(4):645–8. doi: 10.1016/s0022-3476(80)80730-x. [DOI] [PubMed] [Google Scholar]
- 62.Robson MC, Stankiewicz JA, Mendelsohn JS. Cor pulmonale secondary to cleft palate repair. Case report. Plast Reconstr Surg. 1977 May;59(5):754–7. doi: 10.1097/00006534-197705000-00035. [DOI] [PubMed] [Google Scholar]
- 63.Madrid JR, Ortega VG, Echeverry P, Velasquez NL. Prevalence of Obstructive Sleep Apnea After Orticochea Pharyngoplasty for Velopharyngeal Insufficiency Management. Cleft Palate Craniofac J. 2013 Aug 16; doi: 10.1597/12-049. [DOI] [PubMed] [Google Scholar]
- 64.Ettinger RE, Oppenheimer AJ, Lau D, Hassan F, Newman MH, Buchman SR, et al. Obstructive sleep apnea after dynamic sphincter pharyngoplasty. J Craniofac Surg. 2012 Nov;23(7 Suppl 1):1974–6. doi: 10.1097/SCS.0b013e31825b3ba9. [DOI] [PubMed] [Google Scholar]
- 65.Lin WN, Wang R, Cheong EC, Lo LJ. Use of hemisphincter pharyngoplasty in the management of velopharyngeal insufficiency after pharyngeal flap: an outcome study. Ann Plast Surg. 2010 Aug;65(2):201–5. doi: 10.1097/SAP.0b013e3181c71063. [DOI] [PubMed] [Google Scholar]
- 66.de Serres LM, Deleyiannis FW, Eblen LE, Gruss JS, Richardson MA, Sie KC. Results with sphincter pharyngoplasty and pharyngeal flap. Int J Pediatr Otorhinolaryngol. 1999 Apr 25;48(1):17–25. doi: 10.1016/s0165-5876(99)00006-3. [DOI] [PubMed] [Google Scholar]
- 67.Liao YF, Noordhoff MS, Huang CS, Chen PK, Chen NH, Yun C, et al. Comparison of obstructive sleep apnea syndrome in children with cleft palate following Furlow palatoplasty or pharyngeal flap for velopharyngeal insufficiency. Cleft Palate Craniofac J. 2004 Mar;41(2):152–6. doi: 10.1597/02-162. [DOI] [PubMed] [Google Scholar]
- 68.Abyholm F, D’Antonio L, Davidson Ward SL, Kjoll L, Saeed M, Shaw W, et al. Pharyngeal flap and sphincterplasty for velopharyngeal insufficiency have equal outcome at 1 year postoperatively: results of a randomized trial. Cleft Palate Craniofac J. 2005 Sep;42(5):501–11. doi: 10.1597/03-148.1. [DOI] [PubMed] [Google Scholar]
- 69.Madrid JR, Eduardo Nieto L, Gomez V, Echeverry P, Tavera MC, Oliveros H. Palatoplasty as the technique of choice for prevention of obstructive sleep apnea secondary to surgery for velopharyngeal insufficiency. Cleft Palate Craniofac J. 2011 Mar;48(2):145–9. doi: 10.1597/09-178. [DOI] [PubMed] [Google Scholar]
- 70.Rottgers SA, Ford M, Cray J, Smith D, Kinsella C, Grunwaldt L, et al. An algorithm for application of furlow palatoplasty to the treatment of velocardiofacial syndrome-associated velopharyngeal insufficiency. Ann Plast Surg. 2011 May;66(5):479–84. doi: 10.1097/SAP.0b013e3182185ccb. [DOI] [PubMed] [Google Scholar]
- 71.Mehendale FV, Lane R, Laverty A, Dinwiddie R, Sommerlad BC. Effect of palate re-repairs and hynes pharyngoplasties on pediatric airways: an analysis of preoperative and postoperative cardiorespiratory sleep studies. Cleft Palate Craniofac J. 2013 May;50(3):257–67. doi: 10.1597/11-198. [DOI] [PubMed] [Google Scholar]
- 72.Cao Y, Ma T, Wu D, Yin N, Zhao Z. Autologous fat injection combined with palatoplasty and pharyngoplasty for velopharyngeal insufficiency and cleft palate: preliminary experience. Otolaryngol Head Neck Surg. 2013 Aug;149(2):284–91. doi: 10.1177/0194599813490893. [DOI] [PubMed] [Google Scholar]
- 73.Saunders NC, Hartley BE, Sell D, Sommerlad B. Velopharyngeal insufficiency following adenoidectomy. Clin Otolaryngol Allied Sci. 2004 Dec;29(6):686–8. doi: 10.1111/j.1365-2273.2004.00870.x. [DOI] [PubMed] [Google Scholar]
- 74.Witzel MA, Rich RH, Margar-Bacal F, Cox C. Velopharyngeal insufficiency after adenoidectomy: an 8-year review. Int J Pediatr Otorhinolaryngol. 1986 Feb;11(1):15–20. doi: 10.1016/s0165-5876(86)80023-4. [DOI] [PubMed] [Google Scholar]
- 75.Finkelstein Y, Wexler DB, Nachmani A, Ophir D. Endoscopic partial adenoidectomy for children with submucous cleft palate. Cleft Palate Craniofac J. 2002 Sep;39(5):479–86. doi: 10.1597/1545-1569_2002_039_0479_epafcw_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 76.Tweedie DJ, Skilbeck CJ, Wyatt ME, Cochrane LA. Partial adenoidectomy by suction diathermy in children with cleft palate, to avoid velopharyngeal insufficiency. Int J Pediatr Otorhinolaryngol. 2009 Nov;73(11):1594–7. doi: 10.1016/j.ijporl.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 77.Paulson LM, Macarthur CJ, Beaulieu KB, Brockman JH, Milczuk HA. Speech outcomes after tonsillectomy in patients with known velopharyngeal insufficiency. Int J Otolaryngol. 2012;2012:912767. doi: 10.1155/2012/912767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robin P. A drop of the base of the tongue considered as a new cause of nasopharyngeal respiratory impairment. Bull Acad Natl Med (Paris) 1923;89:37–41. [Google Scholar]
- *79.Evans KN, Sie KC, Hopper RA, Glass RP, Hing AV, Cunningham ML. Robin sequence: from diagnosis to development of an effective management plan. Pediatrics. 2011 May;127(5):936–48. doi: 10.1542/peds.2010-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sher AE, Shprintzen RJ, Thorpy MJ. Endoscopic observations of obstructive sleep apnea in children with anomalous upper airways: predictive and therapeutic value. Int J Pediatr Otorhinolaryngol. 1986 Apr;11(2):135–46. doi: 10.1016/s0165-5876(86)80008-8. [DOI] [PubMed] [Google Scholar]
- 81.Nagaoka K, Tanne K. Activities of the muscles involved in swallowing in patients with cleft lip and palate. Dysphagia. 2007 Apr;22(2):140–4. doi: 10.1007/s00455-006-9067-y. [DOI] [PubMed] [Google Scholar]
- 82.Meyer AC, Lidsky ME, Sampson DE, Lander TA, Liu M, Sidman JD. Airway interventions in children with Pierre Robin Sequence. Otolaryngol Head Neck Surg. 2008 Jun;138(6):782–7. doi: 10.1016/j.otohns.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 83.Caouette-Laberge L, Bayet B, Larocque Y. The Pierre Robin sequence: review of 125 cases and evolution of treatment modalities. Plast Reconstr Surg. 1994 Apr;93(5):934–42. [PubMed] [Google Scholar]
- *84.Abel F, Bajaj Y, Wyatt M, Wallis C. The successful use of the nasopharyngeal airway in Pierre Robin sequence: an 11-year experience. Arch Dis Child. 2012 Apr;97(4):331–4. doi: 10.1136/archdischild-2011-301134. [DOI] [PubMed] [Google Scholar]
- 85.Schaefer RB, Gosain AK. Airway management in patients with isolated Pierre Robin sequence during the first year of life. J Craniofac Surg. 2003 Jul;14(4):462–7. doi: 10.1097/00001665-200307000-00011. [DOI] [PubMed] [Google Scholar]
- 86.Parhizkar N, Saltzman B, Grote K, Starr J, Cunningham M, Perkins J, et al. Nasopharyngeal airway for management of airway obstruction in infants with micrognathia. Cleft Palate Craniofac J. 2011 Jul;48(4):478–82. doi: 10.1597/09-263. [DOI] [PubMed] [Google Scholar]
- 87.Mondini CC, Marques IL, Fontes CM, Thome S. Nasopharyngeal intubation in Robin sequence: technique and management. Cleft Palate Craniofac J. 2009 May;46(3):258–61. doi: 10.1597/08-042.1. [DOI] [PubMed] [Google Scholar]
- 88.Marques IL, de Sousa TV, Carneiro AF, Barbieri MA, Bettiol H, Gutierrez MR. Clinical experience with infants with Robin sequence: a prospective study. Cleft Palate Craniofac J. 2001 Mar;38(2):171–8. doi: 10.1597/1545-1569_2001_038_0171_cewiwr_2.0.co_2. [DOI] [PubMed] [Google Scholar]
- 89.Heaf DP, Helms PJ, Dinwiddie R, Matthew DJ. Nasopharyngeal airways in Pierre Robin Syndrome. J Pediatr. 1982 May;100(5):698–703. doi: 10.1016/s0022-3476(82)80567-2. [DOI] [PubMed] [Google Scholar]
- 90.Bütow KW, Hoogendijk CF, Zwahlen RA. Pierre Robin sequence: appearances and 25 years of experience with an innovative treatment protocol. J Pediatr Surg. 2009 Nov;44(11):2112–8. doi: 10.1016/j.jpedsurg.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 91.Bacher M, Sautermeister J, Urschitz MS, Buchenau W, Arand J, Poets CF. An oral appliance with velar extension for treatment of obstructive sleep apnea in infants with Pierre Robin sequence. Cleft Palate Craniofac J. 2011 May;48(3):331–6. doi: 10.1597/09-091. [DOI] [PubMed] [Google Scholar]
- 92.Buchenau W, Urschitz MS, Sautermeister J, Bacher M, Herberts T, Arand J, et al. A randomized clinical trial of a new orthodontic appliance to improve upper airway obstruction in infants with Pierre Robin sequence. J Pediatr. 2007 Aug;151(2):145–9. doi: 10.1016/j.jpeds.2007.02.063. [DOI] [PubMed] [Google Scholar]
- 93.Collins B, Powitzky R, Robledo C, Rose C, Glade R. Airway management in pierre robin sequence: patterns of practice. Cleft Palate Craniofac J. 2014 May;51(3):283–9. doi: 10.1597/12-214. [DOI] [PubMed] [Google Scholar]
- 94.Kirschner BS. Differences in the management of inflammatory bowel disease in children and adolescents compared to adults. Neth J Med. 1998 Dec;53(6):S13–8. doi: 10.1016/s0300-2977(98)00118-1. [DOI] [PubMed] [Google Scholar]
- 95.Denny AD, Amm CA, Schaefer RB. Outcomes of tongue-lip adhesion for neonatal respiratory distress caused by Pierre Robin sequence. J Craniofac Surg. 2004 Sep;15(5):819–23. doi: 10.1097/00001665-200409000-00023. [DOI] [PubMed] [Google Scholar]
- 96.Evans AK, Rahbar R, Rogers GF, Mulliken JB, Volk MS. Robin sequence: a retrospective review of 115 patients. Int J Pediatr Otorhinolaryngol. 2006 Jun;70(6):973–80. doi: 10.1016/j.ijporl.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 97.Cruz MJ, Kerschner JE, Beste DJ, Conley SF. Pierre Robin sequences: secondary respiratory difficulties and intrinsic feeding abnormalities. Laryngoscope. 1999 Oct;109(10):1632–6. doi: 10.1097/00005537-199910000-00016. [DOI] [PubMed] [Google Scholar]
- 98.Cozzi F, Totonelli G, Frediani S, Zani A, Spagnol L, Cozzi DA. The effect of glossopexy on weight velocity in infants with Pierre Robin syndrome. J Pediatr Surg. 2008 Feb;43(2):296–8. doi: 10.1016/j.jpedsurg.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 99.Denny AD, Talisman R, Hanson PR, Recinos RF. Mandibular distraction osteogenesis in very young patients to correct airway obstruction. Plast Reconstr Surg. 2001 Aug;108(2):302–11. doi: 10.1097/00006534-200108000-00004. [DOI] [PubMed] [Google Scholar]
- 100.Denny A, Amm C. New technique for airway correction in neonates with severe Pierre Robin sequence. J Pediatr. 2005 Jul;147(1):97–101. doi: 10.1016/j.jpeds.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 101.Cicchetti R, Cascone P, Caresta E, Papoff P, Miano S, Cerasaro C, et al. Mandibular distraction osteogenesis for neonates with Pierre Robin sequence and airway obstruction. J Matern Fetal Neonatal Med. 2012 Oct;25(Suppl 4):141–3. doi: 10.3109/14767058.2012.715011. [DOI] [PubMed] [Google Scholar]
- 102.Roy S, Munson PD, Zhao L, Holinger LD, Patel PK. CT analysis after distraction osteogenesis in Pierre Robin Sequence. Laryngoscope. 2009 Feb;119(2):380–6. doi: 10.1002/lary.20011. [DOI] [PubMed] [Google Scholar]
- 103.Looby JF, Schendel SA, Lorenz HP, Hopkins EM, Aizenbud D. Airway analysis: with bilateral distraction of the infant mandible. J Craniofac Surg. 2009 Sep;20(5):1341–6. doi: 10.1097/SCS.0b013e3181ae4139. [DOI] [PubMed] [Google Scholar]
- 104.Ow AT, Cheung LK. Meta-analysis of mandibular distraction osteogenesis: clinical applications and functional outcomes. Plast Reconstr Surg. 2008 Mar;121(3):54e–69e. doi: 10.1097/01.prs.0000299285.97379.35. [DOI] [PubMed] [Google Scholar]
- 105.Hong P, McNeil M, Kearns DB, Magit AE. Mandibular distraction osteogenesis in children with Pierre Robin sequence: impact on health-related quality of life. Int J Pediatr Otorhinolaryngol. 2012 Aug;76(8):1159–63. doi: 10.1016/j.ijporl.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 106.Verlinden CR, van de Vijfeijken SE, Jansma EP, Becking AG, Swennen GR. Complications of mandibular distraction osteogenesis for congenital deformities: a systematic review of the literature and proposal of a new classification for complications. Int J Oral Maxillofac Surg. 2015 Jan;44(1):37–43. doi: 10.1016/j.ijom.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 107.Flores RL, Tholpady SS, Sati S, Fairbanks G, Socas J, Choi M, et al. The surgical correction of Pierre Robin sequence: mandibular distraction osteogenesis versus tongue-lip adhesion. Plast Reconstr Surg. 2014 Jun;133(6):1433–9. doi: 10.1097/PRS.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 108.Gosain AK. Discussion: The surgical correction of Pierre Robin sequence: mandibular distraction osteogenesis versus tongue-lip adhesion. Plast Reconstr Surg. 2014 Jun;133(6):1440–2. doi: 10.1097/PRS.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 109.Denny AD. Discussion: The surgical correction of Pierre Robin sequence: mandibular distraction osteogenesis versus tongue-lip adhesion. Plast Reconstr Surg. 2014 Jun;133(6):1443–4. doi: 10.1097/PRS.0000000000000182. [DOI] [PubMed] [Google Scholar]
- 110.Figueroa AA, Glupker TJ, Fitz MG, BeGole EA. Mandible, tongue, and airway in Pierre Robin sequence: a longitudinal cephalometric study. Cleft Palate Craniofac J. 1991 Oct;28(4):425–34. doi: 10.1597/1545-1569_1991_028_0425_mtaaip_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- 111.Runyan CM, Uribe-Rivera A, Karlea A, Meinzen-Derr J, Rothchild D, Saal H, et al. Cost analysis of mandibular distraction versus tracheostomy in neonates with Pierre Robin sequence. Otolaryngol Head Neck Surg. 2014 Nov;151(5):811–8. doi: 10.1177/0194599814542759. [DOI] [PubMed] [Google Scholar]
- 112.Paes EC, Fouche JJ, Muradin MS, Speleman L, Kon M, Breugem CC. Tracheostomy versus mandibular distraction osteogenesis in infants with Robin sequence: a comparative cost analysis. Br J Oral Maxillofac Surg. 2014 Mar;52(3):223–9. doi: 10.1016/j.bjoms.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 113.Demke J, Bassim M, Patel MR, Dean S, Rahbar R, van Aalst JA, et al. Parental perceptions and morbidity: tracheostomy and Pierre Robin sequence. Int J Pediatr Otorhinolaryngol. 2008 Oct;72(10):1509–16. doi: 10.1016/j.ijporl.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 114.Caron CJ, Pluijmers BI, Joosten KF, Mathijssen IM, van der Schroeff MP, Dunaway DJ, et al. Feeding difficulties in craniofacial microsomia: a systematic review. Int J Oral Maxillofac Surg. 2015 Jun;44(6):732–7. doi: 10.1016/j.ijom.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 115.Elliott MA, Studen-Pavlovich DA, Ranalli DN. Prevalence of selected pediatric conditions in children with Pierre Robin sequence. Pediatr Dent. 1995 Mar-Apr;17(2):106–11. [PubMed] [Google Scholar]
- 116.Li HY, Lo LJ, Chen KS, Wong KS, Chang KP. Robin sequence: review of treatment modalities for airway obstruction in 110 cases. Int J Pediatr Otorhinolaryngol. 2002 Aug 1;65(1):45–51. doi: 10.1016/s0165-5876(02)00131-3. [DOI] [PubMed] [Google Scholar]
- 117.Marques IL, Monteiro LC, de Souza L, Bettiol H, Sassaki CH, de Assumpcao Costa R. Gastroesophageal reflux in severe cases of Robin sequence treated with nasopharyngeal intubation. Cleft Palate Craniofac J. 2009 Jul;46(4):448–53. doi: 10.1597/08-120.1. [DOI] [PubMed] [Google Scholar]
- 118.Lidsky ME, Lander TA, Sidman JD. Resolving feeding difficulties with early airway intervention in Pierre Robin Sequence. Laryngoscope. 2008 Jan;118(1):120–3. doi: 10.1097/MLG.0b013e31815667f3. [DOI] [PubMed] [Google Scholar]
- 119.Gangopadhyay N, Mendonca DA, Woo AS. Pierre robin sequence. Semin Plast Surg. 2012 May;26(2):76–82. doi: 10.1055/s-0032-1320065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dauria D, Marsh JL. Mandibular distraction osteogenesis for Pierre Robin sequence: what percentage of neonates need it? J Craniofac Surg. 2008 Sep;19(5):1237–43. doi: 10.1097/SCS.0b013e3181843293. [DOI] [PubMed] [Google Scholar]
- 121.Schaefer RB, Stadler JA, 3rd, Gosain AK. To distract or not to distract: an algorithm for airway management in isolated Pierre Robin sequence. Plast Reconstr Surg. 2004 Apr 1;113(4):1113–25. doi: 10.1097/01.prs.0000110323.50084.21. [DOI] [PubMed] [Google Scholar]
- 122.Spier S, Rivlin J, Rowe RD, Egan T. Sleep in Pierre Robin syndrome. Chest. 1986 Nov;90(5):711–5. doi: 10.1378/chest.90.5.711. [DOI] [PubMed] [Google Scholar]
- 123.Shprintzen RJ, Croft C, Berkman MD, Rakoff SJ. Pharyngeal hypoplasia in Treacher Collins syndrome. Arch Otolaryngol. 1979 Mar;105(3):127–31. doi: 10.1001/archotol.1979.00790150017005. [DOI] [PubMed] [Google Scholar]
- 124.Positional cloning of a gene involved in the pathogenesis of Treacher Collins syndrome. The Treacher Collins Syndrome Collaborative Group. Nat Genet. 1996 Feb;12(2):130–6. doi: 10.1038/ng0296-130. [DOI] [PubMed] [Google Scholar]
- 125.Valdez BC, Henning D, So RB, Dixon J, Dixon MJ. The Treacher Collins syndrome (TCOF1) gene product is involved in ribosomal DNA gene transcription by interacting with upstream binding factor. Proc Natl Acad Sci U S A. 2004 Jul 20;101(29):10709–14. doi: 10.1073/pnas.0402492101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dauwerse JG, Dixon J, Seland S, Ruivenkamp CA, van Haeringen A, Hoefsloot LH, et al. Mutations in genes encoding subunits of RNA polymerases I and III cause Treacher Collins syndrome. Nat Genet. 2011 Jan;43(1):20–2. doi: 10.1038/ng.724. [DOI] [PubMed] [Google Scholar]
- 127.Plomp RG, Bredero-Boelhouwer HH, Joosten KF, Wolvius EB, Hoeve HL, Poublon RM, et al. Obstructive sleep apnoea in Treacher Collins syndrome: prevalence, severity and cause. Int J Oral Maxillofac Surg. 2012 Jun;41(6):696–701. doi: 10.1016/j.ijom.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 128.Ma X, Forte AJ, Persing JA, Alonso N, Berlin NL, Steinbacher DM. Reduced three-dimensional airway volume is a function of skeletal dysmorphology in treacher collins syndrome. Plast Reconstr Surg. 2015 Feb;135(2):382e–92e. doi: 10.1097/PRS.0000000000000993. [DOI] [PubMed] [Google Scholar]
- 129.Plomp RG, Joosten KF, Wolvius EB, Hoeve HL, Poublon RM, van Montfort KA, et al. Screening for obstructive sleep apnea in Treacher-Collins syndrome. Laryngoscope. 2012 Apr;122(4):930–4. doi: 10.1002/lary.23187. [DOI] [PubMed] [Google Scholar]
- 130.Christensen K, Juel K, Herskind AM, Murray JC. Long term follow up study of survival associated with cleft lip and palate at birth. BMJ. 2004 Jun 12;328(7453):1405. doi: 10.1136/bmj.38106.559120.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hujoel PP, Bollen AM, Mueller BA. First-year mortality among infants with facial clefts. Cleft Palate Craniofac J. 1992 Sep;29(5):451–5. doi: 10.1597/1545-1569_1992_029_0451_fymaiw_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- *132.Maliepaard M, Mathijssen IM, Oosterlaan J, Okkerse JM. Intellectual, behavioral, and emotional functioning in children with syndromic craniosynostosis. Pediatrics. 2014 Jun;133(6):e1608–15. doi: 10.1542/peds.2013-3077. [DOI] [PubMed] [Google Scholar]
- *133.MacLean JE, Tan S, Fitzgerald DA, Waters KA. Assessing ventilatory control in infants at high risk of sleep disordered breathing: a study of infants with cleft lip and/or palate. Pediatr Pulmonol. 2013 Mar;48(3):265–73. doi: 10.1002/ppul.22568. [DOI] [PubMed] [Google Scholar]