Abstract
Inhibition of α-amylase and α-glucosidase, advanced glycation end products (AGEs) formation, and oxidative stress by isolated active constituents of Osmanthus fragrans flowers (9,12-octadecadienoic acid and 4-(2,6,6-trimethyl-1-cyclohexenyl)-3-buten-2-one) and their structural analogues were evaluated. 9,12-Octadecadienoic acid was 10.02 and 22.21 times more active against α-amylase and α-glucosidase, respectively, than acarbose and ascorbic acid, followed by 9,12,15-octadecatrienoic acid, 9-octadecenoic acid, 4-(2,6,6-trimethyl-1-cyclohexenyl)-3-buten-2-one, 4-(2,6,6-trimethyl-2-cyclohexenyl)-3-buten-2-one, 1-heptadecanecarboxylic acid, and 1-pentadecanecarboxylic acid. Concerning the inhibition of AGEs formation, similar with data for 2,2’-diphenyl-1-picrylhydrazl radical scavenging activities, 9,12-octadecadienoic acid was 3.54 times more active than aminoguanidine, followed by 9,12,15-octadecatrienoic acid, and 9-octadecenoic acid. These results indicate that 4-(2,6,6-trimethyl-1-cyclohexenyl)-3-buten-2-one, 9,12-octadecadienoic acid and their analogues inhibit α-amylase and α-glucosidase, AGEs formation, and oxidative stress have potential value in alleviating diabetic pathological conditions.
Diabetes mellitus (DM) is a metabolic disease that can be accompanied by abnormal plasma blood levels and complications including cardiovascular diseases, neuropathy, and retinopathy1. DM affects approximately 2% of the world population2. About 90% of those with diabetes have the non-insulin dependent type 2 form (T2 DM)2. T2 DM is characterized by relative or complete deficiencies in insulin action and secretion, leading to metabolic disorders and hyperglycemia2,3. Hyperglycemia can play a leading role as a factor of tissue damage through several mechanisms, including increased flux of glucose and other sugars through the polyol pathway, increased formation of AGEs, increased expression of the AGEs receptor and its activating ligands, activation of protein kinase C isoforms, and over-activity of the hexosamine pathway4,5,6.
Five mechanisms are activated by mitochondrial overproduction of reactive oxygen species5. T2 DM associated chronic hyperglycemia can be discerned by examining the postprandial blood glucose level (PBGL)7. PBGL has been recently implicated as an important factor in the development and beginning of T2 DM8. A sudden rise in PBGLs causing chronic hyperglycemia happens due to the rapid uptake of glucose by intestinal α-glucosidases and hydrolysis of starch by α-amylase. Therapy involves decreasing PBGL by delaying glucose absorption through the inhibition of α-glucosidase and α-amylase, which are carbohydrate hydrolytic enzymes8. Inhibition of AGEs formation is another therapeutic option for diabetes that is not dependent on the control of PBGL, and could be useful in the prevention or reduction of diabetic complications. Studies have been performed to develop more effective inhibitors of α-amylase and α-glucosidase, AGEs formation, and oxidative stress from biomaterials to cure diabetes and its complications4,5,6,8.
Osmanthus fragrans (Oleaceae family) has been domesticated as a local herb in East Asia and is the source of medicinal compounds9. O. fragrans flowers are also used as additives in foods and beverages9, and are considered natural essences and are commonly used in expensive cosmetics and perfumes9. O. fragrans flowers are used to alleviate pain and coughing, have antioxidant activity, and can provide neuroprotection10. Various compounds isolated from O. fragrans flowers, including tyrosyl acetate, phillygenin, ligustroside, rutin, and verbascoside findings, indicate that O. fragrans flowers may have important pharmacological properties11.
Little is known of the potential inhibitory effects of the active constituents isolated from O. fragrans flowers on α-amylase and α-glucosidase activities, AGEs formation, and oxidative stress. In this study, the active constituents of O. fragrans flowers were identified, and their inhibitory activities were evaluated.
Results and Discussion
Inhibition of α-amylase and α-glucosidase by the hexane, chloroform, ethyl acetate, butanol, and distilled water fractions partitioned from the methanol extract of O. fragrans flowers were evaluated (Table 1). The IC50 values for α-amylase and α-glucosidase inhibition were 275.6 and 134.5 μg/mL, respectively. Among the five fractions, the respective IC50 value of the chloroform fraction against α-amylase and α-glucosidase was 134.5 and 60.5 μg/mL. The IC50 values of the hexane fraction were 250.2 and 120.4 μg/mL, respectively. The inhibitory effect of the chloroform fraction against α-amylase and α-glucosidase was 1.18 and 1.25 times higher than that of the acarbose positive control (IC50, 158.4 and 75.5 μg/mL), respectively. A prior study reported strong inhibitory activity (IC50 12.5 μg/mL) of O. fragrans extract against α-glucosidase compared with acarbose (IC50 1,081.27 μg/mL)12. Treatment with O. fragrans extract can decrease PBGL and fasting blood glucose12. In the same study, treatment with O. fragrans extract (500 mg/kg) significantly decreased the content of serum malondialdehyde and increased the level of superoxide dismutase in diabetic rats, and oral administration of 160 mg/kg of the extract significantly decreased the level of serum triglyceride and serum cholesterol in diabetic rats, and significantly increased liver glycogen content12. The present findings bolster the idea that the chloroform fraction derived from O. fragrans flowers could efficiently inhibit α-amylase and α-glucosidase, and could possibly play a role in treatment of hypoglycemia through oxidative mechanisms.
Table 1. IC50 values of five fractions partitioned from the methanol extract of O. fragrans.
| Materials | IC50 values (μg/ml, means ± SE) |
|||
|---|---|---|---|---|
| AGE | α–Amylase | DPPH | α–Glucosidase | |
| Methanol extract | 185.8 ± 1.6 | 275.6 ± 2.1 | 69.8 ± 2.0 | 134.5 ± 1.9 |
| Hexane fraction | 152.8 ± 1.8 | 250.2 ± 2.1 | 62.5 ± 1.4 | 120.4 ± 2.3 |
| Chloroform fraction | 110.5 ± 2.5 | 134.5 ± 1.7 | 60.7 ± 2.1 | 60.5 ± 1.6 |
| Ethyl acetate fraction | 258.8 ± 1.7 | 290.4 ± 1.9 | 75.2 ± 0.5 | 143.5 ± 2.1 |
| Butanol fraction | >500 | 325.5 ± 1.2 | 76.4 ± 0.6 | 184.3 ± 2.3 |
| Distilled water fraction | >500 | 0 | 129.8 ± 1.2 | 0 |
| Acarbose | 158.4 ± 1.4 | 75.5 ± 1.8 | ||
| Ascorbic acid | 25.5 ± 0.4 | |||
| Aminoguanidine | 54.5 ± 0.7 | |||
Acarbose was used as the positive control for α–glucosidase and α–amylase. Ascorbic acid was the positive control for DPPH. Aminoguanidine was the positive control for the inhibition of AGEs formation.
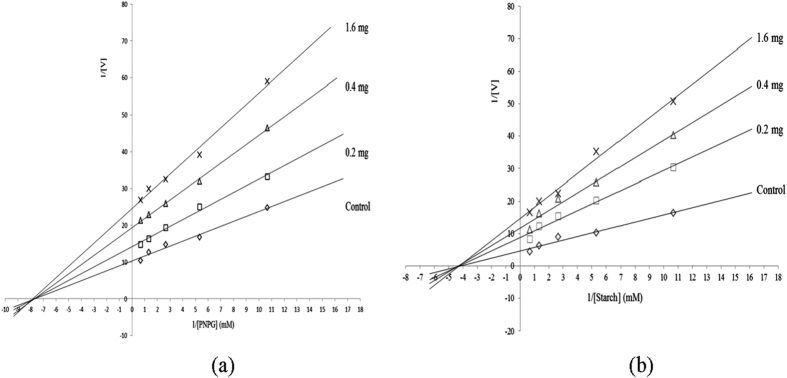
The initial velocity ‘v’ of the hydrolysis reactions catalyzed by α-amylase and α-glucosidase was measured using starch or p-nitrophenyl-α-D-glucopyranoside (PNPG) as the substrate in the presence and absence of the chloroform fraction (0.2-1.6 mg/mL) are presented in Fig. 1. The regression and extrapolation lines consist of a series of lines crossing on the horizontal and vertical axes. The intercept of the vertical axis (1/Vm) increased as the concentration of the chloroform fraction increased. However, the intercept of the horizontal axis (–1/Km) remained the same. The reaction velocity catalyzed by α-amylase and α-glucosidase slowed and were correlated with an increase in the concentration of the chloroform fraction. The Km values of α-amylase and α-glucosidase were not affected by the concentration of the chloroform fraction, typical of non-competitive inhibition. The results indicate that the chloroform fraction and the substrate did not bind to α-amylase and α-glucosidase at the same site. The data are similar to a prior description of the non-competitive inhibition of α-glucosidase and porcine pancreatic amylase by Rhus chinensis extract13.
Figure 1.
Lineweaver–Burk plot of the inhibition kinetics against α–glucosidase (a) and α–amylase (b) by the chloroform subfraction subdivided of the methanol extract of O. fragrans.
2,2′-Diphenyl-1-picrylhydrazl (DPPH) radical scavenging activities of O. fragrans methanol extract and its five fractions were determined (Table 1). The DPPH IC50 value of the methanol extract was 69.8 μg/mL. The IC50 values of the chloroform, hexane, ethyl acetate, butanol, and distilled water fractions were 60.7, 62.5, 75.2, 76.4, and 129.8 μg/mL, respectively. The inhibitory effects of O. fragrans extract and its five fractions against AGEs formation were evaluated by detecting fluorescence; fluorescence intensity of the bovine serum albumin-glucose complex increases with incubation time. The IC50 values of O. fragrans extract, chloroform, hexane and butanol fractions were 185.8, 110.5, 152.8, and 258.8 μg/mL, respectively. The strength of DPPH radical scavenging and inhibition of AGEs formation were butanol fraction <ethyl acetate fraction <hexane fraction <methanol extract <chloroform fraction. In earlier study, O. fragrans extract presented DPPH radical and hydroxyl anion scavenging activity with IC50 values of 9.99 and 11.19 μg/mL, respectively10. The same study documented the efficiency of O. fragrans extract on the ferric reducing/antioxidant power (FRAP) and inhibition of Fe2+ chelation with IC50 values of 7.74 and 0.23 μg/mL, respectively. The authors described that O. fragrans extract reacts with and neutralizes stable free radicals (DPPH and OH radical) independent of any enzymatic activity (FRAP and Fe2+ chelating ability). Furthermore, treatment with O. fragrans extract can reduce the lipid peroxidation induced by oxidative stress in rat tissues10. These prior and the present results indicate that the chloroform subfraction subdivided from the methanol extract of O. fragrans flowers may alleviate oxidative stress-related diabetic pathological conditions through the reduction of lipid peroxidation.
The active compounds of the chloroform fraction were isolated using various types of column chromatography and prep HPLC. The chemical structures were identified by EI-MS, 13C-NMR, 1H-NMR, DEPT-NMR, HMQC and 1H-1H COSY. EI-MS of OF12 resulted in a signal at m/z 280.43, indicating a molecular formula of C18H32O2. The NMR spectra of OF12 are shown in Table 2. In the 1H-NMR (600 MHz, CDCl3) spectra, 7 distinct peaks were observed. One singlet at δ 9.18 was assigned to the carboxyl (COOH) proton. The signal at δ 2.79–2.83 (t, J = 13.6 Hz) was assigned to the methyl (CH3) proton. Four signals with two integrated protons (2 H) at δ 3.22-3.28 (m, J = 24.4 Hz), δ 3.94-4.00 (q, J = 20.8 Hz), δ 4.25-4.29 (t, J = 15.2 Hz), and δ 4.68-4.71 (t, J = 12.8 Hz) were assigned to the protons attached at alkane (–CH2–) and one signal with the integration of one proton (H) at δ 7.28-7.29 (q, J = 6.8 Hz) was assigned to the proton attached at alkene (=CH). The 13C-NMR (150 MHz, CDCl3) spectra showed 18 distinct peaks. A peak at δ 16.39 revealed the methyl functional group (CH3). The signal at δ 182.56 was assigned to the acid (–C=O). The signals at δ 24.90, 26.99, 27.97, 29.51, 29.54, 31.36, 31.41, 31.47, 31.68, 31.92, 33.86, and 36.39 were attributed to alkane (–CH2–). Other signals at δ 130.24, 130.40, 132.35, and 132.54 were attributed to alkene (C=C). According to the EI-MS and NMR data, this constituent was identified as 9,12-octadecadienoic acid [white liquid, EI-MS, m/z 280.43; 1H-NMR δ (CDCl3) ppm (J in Hz): 2.79-2.83 (t, J = 13.6 Hz), 3.22-3.28 (m, J = 24.4 Hz), 3.94-4.00 (q, J = 20.8 Hz), 4.25-4.29 (t, J = 15.2 Hz), 4.68-4.71 (t, J = 12.8 Hz), 7.28-7.29 (q, J = 6.8 Hz), 9.18 (s); 13C-NMR δ (CDCl3) ppm: 16.39 (C-18), 24.90 (C-17), 26.99 (C-3), 27.97 (C-11), 29.51 (C-14), 29.54 (C-8), 31.36 (C-4), 31.41 (C-5), 31.47 (C-15), 31.68 (C-6), 31.92 (C-7), 33.86 (C-16), 36.39 (C-2), 130.24 (C-10), 130.40 (C-12), 132.35 (C-9), 132.54 (C-13), 182.56 (C-1)]. The NMR data were confirmed from the literature14. EI-MS of another fraction designated OF453 resulted in a signal at m/z 192.45, indicating a molecular formula of C13H20O. The NMR spectra of OF453 are shown in Table 3. In the 1H-NMR (600 MHz, CDCl3) spectra, even distinct peaks were observed. Three singlets at δ 1.26, 1.95, and 2.49 were assigned to the methyl (CH3) proton. The signals at δ 2.25–2.28 (t, J = 12.4 Hz) and δ 6.29-6.33 (d, J = 16.4 Hz) were assigned to the alkene (=CH) proton. The signals with two integrated protons (2H) at δ 1.66-1.68 (t, J = 9.2 Hz) and δ 1.79-1.83 (q, J = 18.4 Hz) were assigned to the protons attached at alkane (–CH2–) and one signal with the integration of one proton (H) at δ 7.45-7.48 (d, J = 15.6 Hz) was assigned to the proton attached at the aromatic ring. The 13C-NMR (150 MHz, CDCl3) spectra showed 12 distinct peaks. The peaks at δ 21.40, 24.25, and 29.68 revealed the methyl functional group (CH3). The signal at δ 201.24 was assigned to the ketone (–C=O). The signals at δ 31.32, 36.08, 36.59, and 42.26 were attributed to alkane. Other signals at δ 134.12, 138.46, 138.57, and 145.68 were attributed to alkene (C=C), respectively. According to the EI-MS and NMR data, this compound was identified as 4-(2,6,6-trimethyl-1-cyclohexenyl)-3-buten-2-one [white liquid, EI-MS, m/z 192.45; 1H-NMR δ (CDCl3) ppm (J in Hz): 1.26 (s), 1.66-1.68 (t, J = 9.2 Hz), 1.79-1.83 (q, J = 18.4 Hz), 1.95 (s), 2.25-2.28 (t, J = 12.4 Hz), 2.485 (s), 6.29-6.33 (d, J = 16.4 Hz), 7.45-7.48 (d, J = 15.6 Hz); 13C-NMR δ (CDCl3) ppm: 21.40 (C-2’), 24.25 (C-6’), 29.68 (C-1), 31.32 (C-4’), 36.08 (C-5’), 36.59 (C-6’), 42.26 (C-1’), 134.12 (C-3’), 138.46 (C-3), 138.57 (C-2’), 145.68 (C-4), 201.24 (C-2)]. The NMR data were confirmed with existing literature15.
Table 2. 1H- and 13C-NMR spectra of OF12.
| Carbon | Partial structure | δC (ppm) | δH (ppm) |
|---|---|---|---|
| 1 | COOH | 182.56 | 9.18 (s) |
| 2 | C-2H | 36.39 | 4.25-4.29 (t, J = 15.2 Hz) |
| 3 | C-2H | 26.99 | 3.22-3.28 (m, J = 24.4 Hz) |
| 4 | C-2H | 31.36 | 3.22-3.28 (m, J = 24.4 Hz) |
| 5 | C-2H | 31.41 | 3.22-3.28 (m, J = 24.4 Hz) |
| 6 | C-2H | 31.68 | 3.22-3.28 (m, J = 24.4 Hz) |
| 7 | C-2H | 31.92 | 3.22-3.28 (m, J = 24.4 Hz) |
| 8 | C-2H | 29.54 | 3.94-4.00 (q, J = 20.8 Hz) |
| 9 | C-H | 132.35 | 7.28-7.29 (q, J = 6.8 Hz) |
| 10 | C-H | 130.24 | 7.28-7.29 (q, J = 6.8 Hz) |
| 11 | C-2H | 27.97 | 4.68-4.71 (t, J = 12.8 Hz) |
| 12 | C-H | 130.40 | 7.28-7.29 (q, J = 6.8 Hz) |
| 13 | C-H | 132.54 | 7.28-7.29 (q, J = 6.8 Hz) |
| 14 | C-2H | 29.51 | 3.94-4.00 (q, J = 20.8 Hz) |
| 15 | C-2H | 31.47 | 3.22-3.28 (m, J = 24.4 Hz) |
| 16 | C-2H | 33.86 | 3.22-3.28 (m, J = 24.4 Hz) |
| 17 | C-2H | 24.90 | 3.22-3.28 (m, J = 24.4 Hz) |
| 18 | C-3H | 16.39 | 2.79-2.83 (t, J = 13.6 Hz) |
s, singlet; t, triplet; m, multilet; q, quartlet.
Table 3. 1H- and 13C-NMR spectra of OF453.
| Carbon | Partial structure | δC (ppm) | δH (ppm) |
|---|---|---|---|
| 1 | C-3H | 29.68 | 2.485 (s) |
| 2 | C | 201.24 | |
| 3 | C-H | 138.46 | 6.29-6.33 (d, J = 16.4 Hz) |
| 4 | C-H | 145.68 | 2.25-2.28 (t, J = 12.4 Hz) |
| 1’ | C-H | 42.26 | 7.45-7.48 (d, J = 15.6 Hz) |
| 2’ | C | 138.57 | |
| C-3H | 21.40 | 1.95 (s) | |
| 3’ | C-H | 134.12 | 2.25-2.28 (t, J = 12.4 Hz) |
| 4’ | C-2H | 31.32 | 1.79-1.83 (q, J = 18.4 Hz) |
| 5’ | C-2H | 36.08 | 1.66-1.68 (t, J = 9.2 Hz) |
| 6’ | C | 36.59 | |
| C-3H | 24.25 | 1.26 (s) |
s, singlet; d, doublet; t, triplet; q, quartlet.
The inhibitory activities of 4-(2,6,6-trimethyl-1-cyclohexenyl)-3-buten-2-one and 9,12-octadecadienoic acid isolated from O. fragrans flowers were measured against α-amylase and α-glucosidase, AGEs formation, and oxidative stress (Table 4). The IC50 values of 4-(2,6,6-trimethyl-1-cyclohexenyl)-3-buten-2-one against α-amylase and α-glucosidase were 84.4 and 31.5 μg/mL, respectively. The IC50 values of 9,12-octadecadienoic acid against α-amylase and α-glucosidase were 15.8 and 3.4 μg/mL, respectively. Compared with the IC50 value of acarbose, which served as a positive control, 4-(2,6,6-trimethyl-1-cyclohexenyl)-3-buten-2-one and 9,12-octadecadienoic acid was 2.40 and 22.21 times more active, respectively, than acarbose (IC50 value, 75.5 μg/mL) against α-glucosidase. Against α-amylase, 4-(2,6,6-trimethyl-1-cyclohexenyl)-3-buten-2-one and 9,12-octadecadienoic acid was 1.88 and 10.26 times, respectively, more effective than acarbose (IC50 value, 158.4 μg/mL). 4-(2,6,6-Trimethyl-1-cyclohexenyl)-3-buten-2-one and 9,12-octadecadienoic acid significantly inhibit α-glucosidases, which are membrane-bound enzymes secreted in the epithelia of the small intestine and which are crucial for carbohydrate digestion16. Inhibition of these enzyme results in a delayed and reduced rise in PBGLs. To confirm that 4-(2,6,6-trimethyl-1-cyclohexenyl)-3-buten-2-one and 9,12-octadecadienoic acid isolated from O. fragrans flowers had significant effects on blood glucose levels in vivo, a disaccharide loading test was done. Administration of sucrose to fasted mice resulted in a rapid increase in blood glucose concentrations from 146 ± 11.71 to a maximum of 241 ± 13.61 mg/dL after 30 min (Fig. 2). 9,12-Octadecadienoic acid exerted significant suppressive effect on blood glucose levels in mice at 15 and 30 mins compared to sucrose alone. In contrast, 4-(2,6,6-trimethyl-1-cyclohexenyl)-3-buten-2-one showed weak suppressive effect on blood glucose levels at 15 and 30 mins. The results were similar to prior experimental results17. These authors assumed that the major components of Matricaria chamomilla L., esculetin and quercetin, exerted significant suppressive effect on blood glucose levels in mice. In this regard, our results indicate that 9,12-octadecadienoic acid isolated from O. fragrans flowers may alleviate hyperglycemia resulting from high sucrose ingestion by lowering the blood glucose level.
Table 4. IC50 values of isolated active constituents and their structural analogues.
| Chemicals | IC50 values (μg/ml, means ± SE) |
|||
|---|---|---|---|---|
| AGE | α–Amylase | DPPH | α–Glucosidase | |
| 4-(2,6,6-Trimethyl-2-cyclohexenyl)-3-buten-2-one | 50.5 ± 1.8 | 87.2 ± 1.3 | 23.2 ± 2.5 | 33.2 ± 1.8 |
| 4-(2,6,6-Trimethyl-1-cyclohexenyl)-3-buten-2-one | 48.4 ± 2.3 | 84.4 ± 0.8 | 20.5 ± 1.5 | 31.5 ± 2.2 |
| 9,12-Octadecadienoic acid | 15.4 ± 1.8 | 15.8 ± 2.3 | 6.8 ± 1.2 | 3.4 ± 1.5 |
| 9,12,15-Octadecatrienoic acid | 18.2 ± 2.3 | 21.2 ± 3.6 | 7.4 ± 0.9 | 4.5 ± 2.4 |
| 9-Octadecenoic acid | 18.8 ± 1.1 | 23.5 ± 1.8 | 7.6 ± 1.5 | 4.8 ± 0.9 |
| 1-Heptadecanecarboxylic acid | 34.7 ± 2.6 | 140.5 ± 2.3 | 15.6 ± 2.2 | 46.2 ± 2.5 |
| 1-Pentadecanecarboxylic acid | 40.6 ± 0.7 | 168.2 ± 1.4 | 20.8 ± 1.3 | 55.4 ± 3.2 |
| 1-Tridecanecarboxylic acid | 51.2 ± 1.8 | 225.2 ± 2.3 | 24.5 ± 1.8 | 60.4 ± 1.2 |
| Acarbose | 158.4 ± 1.4 | 75.5 ± 1.8 | ||
| Ascorbic acid | 25.5 ± 0.4 | |||
| Aminoguanidine | 54.5 ± 0.7 | |||
Acarbose served as the positive control for α–glucosidase and α–amylase. Ascorbic acid was the positive control for DPPH. Aminoguanidine was the positive control for the inhibition of AGEs formation.
Figure 2. Effects of 4-(2,6,6-trimethyl-1-cyclohexenyl)-3-buten-2-one and 9,12-octadecadienoic acid on blood glucose levels.
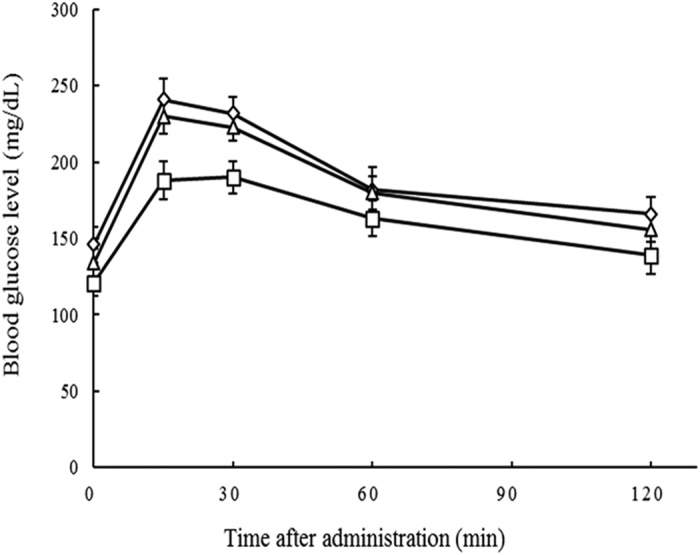
2.5 g/kg body weight, with 50 mg/kg with body weight 9,12-octadecadienoic acid (□), 4-(2,6,6-trimethyl-1-cyclohexenyl)-3-buten-2-one (△), and control (◇). Each point represents mean ± SD (n = 3).
The inhibitory activities of 4-(2,6,6-trimethyl-1-cyclohexenyl)-3-buten-2-one and 9,12-octadecadienoic acid were evaluated by DPPH radical scavenge activity and the inhibition of AGEs formation. These results were compared with those of ascorbic acid and aminoguanidine (Table 4). The DPPH IC50 values of 4-(2,6,6-trimethyl-1-cyclohexenyl)-3-buten-2-one and 9,12-octadecadienoic acid were 20.5 and 6.8 μg/mL, respectively. The IC50 values of 4-(2,6,6-trimethyl-1-cyclohexenyl)-3-buten-2-one and 9,12-octadecadienoic acid against AGEs formation were 48.4 and 15.4 μg/mL, respectively. Compared with the IC50 values of ascorbic acid, which served as a positive control for DPPH radical scavenging, 4-(2,6,6-trimethyl-1-cyclohexenyl)-3-buten-2-one and 9,12-octadecadienoic acid was 1.24 and 3.75 times more active, respectively, than ascorbic acid (IC50 value, 25.5 μg/mL). Concerning inhibition of AGEs formation, 4-(2,6,6-trimethyl-1-cyclohexenyl)-3-buten-2-one and 9,12-octadecadienoic acid were 1.13 and 3.54 times more effective, respectively, than aminoguanidine (IC50 value, 54.5 μg/mL). 4-(2,6,6-Trimethyl-1-cyclohexenyl)-3-buten-2-one reportedly has anti-proliferative activity and inhibits the cell proliferation of MCF-7 cells by inhibiting Cdk 2 activity18. 9,12-Octadecadienoic acid is the precursor contributing to the flavor of mushrooms19. A study involving five wild edible mushroom species (Clitocybe maxima, Catathelasma ventricosum, Stropharia rugoso-annulata, Craterellus cornucopioides, and Laccaria amethystea) comprised of 28.34-74.39% of 9,12-octadecadienoic acid exhibited anti-hyperglycemic and anti-oxidant activities by controlling DPPH radical, α-glucosidase, and hydroxyl free radicals20. Presently, 4-(2,6,6-trimethyl-1-cyclohexenyl)-3-buten-2-one and 9,12-octadecadienoic acid isolated from O. fragrans flowers significantly inhibited α-amylase and α-glucosidase activities, AGEs formation, and oxidative stress.
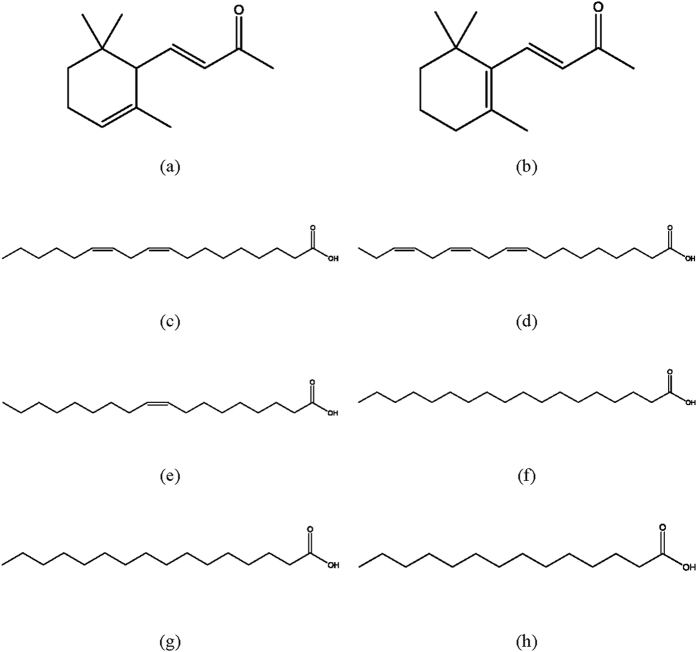
To elucidate the structure-activity relationships between the analogues of the isolated constituents and their inhibitory activities, 4-(2,6,6-trimethyl-2-cyclohexenyl)-3-buten-2-one, 9,12,15-octadecatrienoic acid, 9-octadecenoic acid, 1-heptadecanecarboxylic acid, 1-pentadecanecarboxylic acid, and 1-tridecanecarboxylic acid were selected (Fig. 3), and their inhibitory activities were evaluated against α-amylase and α-glucosidase (Table 4). 9,12,15-Octadecatrienoic acid (IC50 value, 4.5 μg/mL) was 16.78 times more active than acarbose (75.5 μg/mL) against α-glucosidase, followed by 9-octadecenoic acid (4.8 μg/mL), 4-(2,6,6-trimethyl-2-cyclohexenyl)-3-buten-2-one (33.2 μg/mL), 1-heptadecanecarboxylic acid (46.2 μg/mL), 1-pentadecanecarboxylic acid (55.4 μg/mL), and 1-tridecanecarboxylic acid (60.4 μg/mL). Against α-amylase, 9,12,15-octadecatrienoic acid (21.2 μg/mL) was 7.47 times more active than acarbose (158.4 μg/mL), followed by 9-octadecenoic acid (23.5 μg/mL), 4-(2,6,6-trimethyl-2-cyclohexenyl)-3-buten-2-one (87.2 μg/mL), and 1-heptadecanecarboxylic acid (140.5 μg/mL). 9,12,15-octadecatrienoic acid (IC50 value, 7.4 μg/mL) was 3.45 times more active in DPPH radical scavenging than ascorbic acid (25.5 μg/mL), followed by 9-octadecenoic acid (7.6 μg/mL), 1-heptadecanecarboxylic acid (15.6 μg/mL), 1-pentadecanecarboxylic acid (20.8 μg/mL), 4-(2,6,6-trimethyl-2-cyclohexenyl)-3-buten-2-one (23.2 μg/mL), and 1-tridecanecarboxylic acid (24.5 μg/mL). Concerning the inhibition of AGE formation, 9,12,15-octadecatrienoic acid (IC50 value, 18.5 μg/mL) was 2.95 times more active than aminoguanidine (54.5 μg/mL), followed by 9-octadecenoic acid (18.8 μg/mL), 1-heptadecanecarboxylic acid (34.7 μg/mL), 1-pentadecanecarboxylic acid (40.6 μg/mL), 4-(2,6,6-trimethyl-2-cyclohexenyl)-3-buten-2-one (50.5 μg/mL), and 1-tridecanecarboxylic acid (51.2 μg/mL). It was previously reported that the inhibitory effects of six pentacyclic triterpenes (corosolic acid, oleanolic acid, maslinic acid, arjunolic acid, asiatic acid and hydroxyursolic acid) against α-glucosidase are affected by the hydroxy group in their carbon 221. In case of flavonoids, the hydroxyl groups at carbons 5 and 7, and the double bond between carbon 2 and carbon 3 have been shown to be important for the strong inhibitory activity on xanthine oxidase22. In our study, the inhibitory activities against α-amylase and α-glucosidase activities, AGEs formation, and oxidative stress were affected by the number of carbon chains on the chemical structure, but not by the position and the number of double bonds.
Figure 3. Chemical structures of 4-(2,6,6-trimethyl-1-cyclohexenyl)-3-buten-2-one and 9,12-octadecadienoic acid analogues.
(a) 4-(2,6,6-trimethyl-2-cyclohexenyl)-3-buten-2-one; (b) 4-(2,6,6-trimethyl-1-cyclohexenyl)-3-buten-2-one; (c) 9,12-octadecadienoic acid; (d) 9,12,15-octadecatrienoic acid; (e) 9-octadecenoic acid; (f) 1-heptadecanecarboxylic acid; (g) 1-pentadecanecarboxylic acid; (h) 1-tridecanecarboxylic acid.
In conclusion, 4-(2,6,6-trimethyl-1-cyclohexenyl)-3-buten-2-one and 9,12-octadecadienoic acid isolated from the methanol extract of O. fragrans flowers showed potential inhibitory activities against α-amylase and α-glucosidase activities, AGEs formation, and oxidative stress. These results indicate that 4-(2,6,6-trimethyl-1-cyclohexenyl)-3-buten-2-one and 9,12-octadecadienoic acid exert comprehensive inhibitory effects for preventing related to diabetes and its complications, and have potential in the development of natural preventive or therapeutic agents.
Materials and Methods
Chemicals and sample preparation
Acarbose (95%), aminoguanidine hydrochloride (98%), ascorbic acid (98%), bovine serum albumin (98%), DPPH (97%), 1-heptadecanecarboxylic acid (98.5%), PNPG (99%), α-glucosidase (EC 3.2.1.20), 9-octadecenoic acid (99%), 9,12,15-octadecatrienoic acid (99%), porcine pancreatic α-amylase (EC 3.2.1.1, type VI), 1-pentadecanecarboxylic acid (99%), 1-tridecanecarboxylic acid (99%), and 4-(2,6,6-trimethyl-2-cyclohexenyl)-3-buten-2-one (90%) were obtained from Sigma-Aldrich (St. Louis, MO, USA). O. fragrans flowers (5 kg) were obtained from a domestic plant store in Jeonju, Korea. A voucher specimen was authenticated by Prof. Kim, Jeongmoon and deposited in Chonbuk National University. O. fragrans flowers were extracted with methanol by shaking at 150 rpm at 27 °C for 46 h. Extracts were concentrated with an evaporator at 45 °C23. The methanol extract was subdivided based on the polarity of hexane, chloroform, ethyl acetate, butanol, and distilled water. Five fractions were evaporator concentrated, except for the distilled water fraction, which was freeze-dried.
Isolation and identification of bioactive constituents
To isolate the active constituents from the chloroform subfraction of the methanol extract of O. fragrans flowers, column chromatography (4 cm i.d.×60 cm L.) was conducted with silica gel. Ethyl acetate and methanol were the elution solvents. The flow rate was 4.9 ml/min. The chromatographic analysis yielded five fractions (OF1-OF5) were obtained. OF1 and OF4 were subjected to chromatography using a Sephadex LH-20 column (GE Healthcare, Schenectady, NY, USA). From OF1, five fractions (OF11-OF15) were obtained. OF12 was isolated by prep HPLC as a single peak. From OF4, six fractions (OF41-OF46) were obtained. OF45 was isolated using a Jaigel-W253 column (2 cm i.d.×50 cm L., JAI Co., Tokyo, Japan) with 100% methanol at a flow rate of 2.9 mL/min, resulting in the five fractions (OF451-OF455). OF453 was examined further.
Mass spectra (m/z) were obtained by mass spectrometry using a QP-2010 quadrupole device (Shimadzu Co., Kyoto, Japan) equipped with an electrospray source, operating in electron ionization (EI) mode at 70 Ev16. Mass spectrometry (MS) parameters were as follows: negative ionization mode, capillary, 2.89 kV; column temperature, 51 °C and gradually increased to 209 °C; source temperature, 250 °C; and mass range, 10-300 eV. The chemical structures of the isolated active compounds were analyzed in CDCl3 on a JNM EX-600 spectrometer (JEOL Co., Tokyo, Japan). 1H (600 MHz), 13C (150 MHz), and DEPT (100 MHz) NMR were measured. 2D NMR (1H–1H COSY and HMQC) was performed to study the relationships between protons and carbons. Tetramethylsilane was used as a standard. The chemical shift was described as δ (ppm).
α-Glucosidase inhibition
The inhibitory activity of each sample against α-glucosidase was measured as previously described24. Sample (25 μL) and phosphate buffer (25 μL; PB, 100 mM and pH 6.8) containing α-glucosidase (0.2 U/mL) was preincubated in a 96 well plate at 37 °C for 10 min. After 10 min, 5 mM PNPG (50 μL) in 100 mM PB was added and incubated at 37 °C for 15 min. The reaction was stopped by adding 150 μL of 200 mM NaCO3. Absorbance at 405 nm was recorded with a SpectraMax® microplate reader (Molecular Devices, Sunnyvale, CA, USA) and compared with the control contained 25 μL of 100 mM PB in place of the sample.
α-Amylase inhibition
The inhibitory activity of each sample against α-amylase was measured as described previously8. Sample (40 μL) dissolved in sodium phosphate buffer (SPB, 20 mM and pH 6.9) with 6 mM NaCl was added to 1.0 U/ml α-amylase (200 μL) in SPB and incubated at 30 °C for 10 min. After 10 min, 400 μL of 0.3% starch solution in SPB was added to each tube. This reaction was carried out at 37 °C for 10 min and stopped with the treatment of reagent (100 μL) consisting of 1% 3,5-dinitrosalicylic acid, 12% sodium potassium tartrate, and 400 mM NaOH. Each tube was incubated in boiling water for 20 min and cooled to 27 °C. The reaction was diluted by adding 10 ml distilled water and absorbance at 540 nm was measured using a UV-Vis spectrophotometer. The control contained 20 mM SPB (pH 6.9, 200 μL) instead of α-amylase.
Inhibitory kinetics against α-glucosidase and α-amylase
The inhibition mode of the chloroform subfraction from the methanol extract of O. fragrans flowers against α-glucosidase and α-amylase was evaluated with increasing concentrations of PNPG or starch as the substrate in the absence or presence of the chloroform fraction at a concentration of 0.2, 0.4, or 1.6 mg. The inhibition type was determined by Lineweaver-Burk plot analysis of the data, calculated from the results using Michaelis-Menten kinetics.
DPPH radical scavenging activity
DPPH radical scavenging activity of each sample for the inhibition of diabetic complications was evaluated as described previously25,26. Methanol solution (100 μL) containing some concentrations of the sample or ascorbic acid (0.2 mg/mL) as a positive control was mixed with 0.2 mM DPPH (100 μL) in wells of a 96-well microplate. Each reaction volume was mixed and allowed to stand in the dark at 28 °C for 30 min. After the reaction time, the absorbance change of the resulting solution was measured at 517 nm with the aforementioned SpectraMax® microplate reader. The DPPH solution was freshly prepared daily, and was covered and kept in the dark at 4 °C until the measurements were made. Measurements were performed at least in triplicate.
Inhibition of AGEs formation
The inhibitory activity against AGEs formation was measured as described previously27. To prepare the AGEs reaction solution, bovine serum albumin (10 mg/mL) dissolved in 50 mM SPB (pH 7.4) was added to 200 mM fructose, 200 mM glucose, and 0.02% NaN3 to prevent the growth of bacteria. The reaction solution (950 μL) was mixed with various concentrations of the samples (50 μL) in 10% (CH3)2SO. After incubation at 37 °C for 7 days, the fluorescence intensity of the reaction solution was analyzed using a spectrofluorometric detector (Bio-Tek Inc., Winooski, VT, USA) with an excitation wavelength of 350 nm and an emission wavelength of 450 nm. Aminoguanidine hydrochloride served as a positive control.
Disaccharide loading test
After an overnight fast, male mice (31-35 g) were used for acute disaccharide loading tests (Fig. 2). Disaccharide (sucrose, 2.5 g/kg body weight) as well as the test samples (50 mg/kg of body weight for 4-(2,6,6-trimethyl-1-cyclohexenyl)-3-buten-2-one and 9,12-octadecadienoic acid) were dissolved in 0.9% NaCl solution and administered to the mice via a stomach tube. A control group was loaded with sucrose only. The blood glucose levels were measured using an Accu-Check Performa portable kit, (Roche Diagnostics Korea Co., Ltd., Seoul, Korea).
Statistical analysis
The IC50 value of each sample is expressed as mean ± standard error (SE). Variance analysis for each sample was performed using one-way ANOVA. The significance of differences was obtained at a level of p < 0.05 using SAS 9.2 version software (SAS Institute Inc., Cary, NC, USA).
Additional Information
How to cite this article: Yang, J.-Y. et al. Inhibitory Potential of Constituents from Osmanthus fragrans and Structural Analogues Against Advanced Glycation End Products, α-Amylase, α-Glucosidase, and Oxidative Stress. Sci. Rep. 7, 45746; doi: 10.1038/srep45746 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and future Planning (2016R1A2A2A05918651).
Footnotes
The authors declare no competing financial interests.
Author Contributions J.Y.Y. and J.H.P. designed and carried out the experiments, prepared most of the data and wrote the paper; N.C. redesigned, carried out more experiments and rewrote the paper; H.S.L. proposed the key idea of this paper, designed the experiments, managed the research process and wrote the paper; All authors reviewed and approved the final manuscript.
References
- Krentz A. J., Clough G. & Byrne C. D. Interaction between microvascular and macrovascular disease in diabetes: Pathophysiology and therapeutic implications. Diabetes Obes. Metab. 9, 781–791 (2007). [DOI] [PubMed] [Google Scholar]
- Wild S., Roglic G., Green A., Sicree R. & King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 27, 1047–1053 (2004). [DOI] [PubMed] [Google Scholar]
- Meng X. et al. Effects of Lactobacillus plantarum SCS2 on blood glucose level in hyperglycemia mice model. Appl Biol Chem. 59, 143–150 (2016). [Google Scholar]
- Peyrou J. & Sternberg M. Advanced glycation endproducts (AGEs): pharmacological inhibition in diabetes. Pathol. Biol. 54, 405–419 (2006). [DOI] [PubMed] [Google Scholar]
- Giacco F. & Brownlee M. Oxidative stress and diabetic complications. Cric Res. 29, 1058–1070 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriello O. Acute hyperglycaemia and oxidative stress generation. Diabet Med. 14, 45–49 (1997). [DOI] [PubMed] [Google Scholar]
- Nampoothiri S. V. et al. In vitro antioxidant and inhibitory potential of Terminalia bellerica and Emblica officinalis fruits against LDL oxidation and key enzymes linked to type 2 diabetes. Food Chem. Toxicol. 49, 125–131 (2011). [DOI] [PubMed] [Google Scholar]
- Dong H. Q., Li M., Zhu R., Liu F. L. & Huang J. B. Inhibitory potential of trilobatin from Lithocarpus polstachyus Rehd against α–glucosidase and α–amylase linked to type 2 diabetes. Food Chem. 130, 261–266 (2012). [Google Scholar]
- Jin H. X., Zheng H., Jin Y. J., Chen J. Y. & Wang Y. Research on major volatile components of 4 Osmanthus fragrance cultivars in Hangzhou Maolong Guiyu Park. Forest Res. 19, 612–615 (2006). [Google Scholar]
- Lee H. H., Lin C. T. & Yang L. L. Neuroprotection and free radical scavenging effects of Osmanthus fragrans. J. Biomed. Sci. 14, 819–827 (2007). [DOI] [PubMed] [Google Scholar]
- Lin C. H., Li K. Y., Hung T. J., Huang F. L. & Hung C. Y. The safety of Osmanthus fragrans ethanol extract treatment in BALB/c mice. Int. J. pharmacol. Toxicol. 2, 8–12 (2014). [Google Scholar]
- Kang W., Song Y. & Gu X. α-Glucosidase inhibitory in vitro and antidiabetic activity in vivo of Osmanthus fragrans. J. Med. Plants Res. 6, 2850–2856 (2012). [Google Scholar]
- Shim Y. J. et al. inhibitory effect of aqueous extract from the gall of Rhus chinensis on alpha-glucosidase activity and postprandial blood glucose. J. Ethnopharmacol. 85, 283–287 (2003). [DOI] [PubMed] [Google Scholar]
- Davis A. L., McNeill G. P. & Caswell D. C. Analysis of conjugated linoleic acid isomers by 13C NMR spectroscopy. Chem. Phys. Lipids 97, 155–165 (1999). [Google Scholar]
- Polyakov N. E., Leshina T. V., Hand E. O., Petrenko A. & Kispert L. D. β-Ionone cylodextrins inclusion complexes 1H NMR study and photolysis. J. Photochem. Photobio. A: Chem. 161, 261–267 (2004). [Google Scholar]
- Deshpande M. C., Venkateswarlu V., Babu R. K. & Trivedi R. K. Design and evaluation of oral bioadhesive controlled release formulations of miglitol, intended for prolonged inhibition of intestinal alpha-glucosidases and enhancement of plasma glycogen like peptide-1 levels. Int. J. Pharm. 380, 16–24 (2009). [DOI] [PubMed] [Google Scholar]
- Kato A. et al. Protective effects of dietary chamomile tea on diabetic complications. J. Agric. Food chem. 56, 8206–8211 (2008). [DOI] [PubMed] [Google Scholar]
- Liu J. R. et al. β-Ionone suppresses mammary carcinogenesis, proliferative activity and induces apoptosis in the mammary gland of the Sprague-Dawley rat. Int. J. Cancer. 122, 2689–2698 (2008). [DOI] [PubMed] [Google Scholar]
- Ribeiro B., Guedes de Pino P., Andrade P. B., Baptista P. & Valentão P. Fatty acid composition of wild edible mushrooms species: a comparative study. Microchem. J. 93, 29–35 (2009). [Google Scholar]
- Liu Y. T. et al. Chemical composition of five wild edible mushrooms collected from Southwest China and their antihyperglycemic and antioxidant activity. Food Chem. Toxicol. 50, 1238–1244 (2012). [DOI] [PubMed] [Google Scholar]
- Hou W. et al. Triterpene acids isolated from Lagerstroemia speciosa Leaves as α-glucosidase inhibitors. Phytother. Res. 23, 614–618 (2009). [DOI] [PubMed] [Google Scholar]
- Cos P. et al. Structure-activity relationship and classification of flavonoid as inhibitors of xanthin oxidase and superoxide scavengers. J. Nat. Prod. 61, 71–76 (1998). [DOI] [PubMed] [Google Scholar]
- Lee H. K. & Lee H. S. Toxicities of active constituent isolated from Thymus vulgaris flowers and its structural derivatives against Tribolium castaneum (Herbst). Appl Biol Chem. 59, 821–826 (2016). [Google Scholar]
- Kim Y., Jeon Y., Wang M., Lee W. & Rhee H. Inhibitory effect of pine extract on α–glucosidase activity and postprandial hyperglycemia. Nutrition 21, 756–761 (2005). [DOI] [PubMed] [Google Scholar]
- Chen F., Wu A., Shieh P., Kuo D. & Hsieh C. Evaluation of the antioxidant activity of Ruellia tuberosa. Food Chem. 94, 14–18 (2006). [Google Scholar]
- Yun J. H., Kim Y. J. & Koh K. H. Investigation into factors influencing antioxidant capacity of vinegars. Appl Biol Chem. 59, 495–509 (2016). [Google Scholar]
- Vinson J. A. & Howard T. B. Inhibition of protein glycation and advanced glycation end products by ascorbic acid and other vitamins and nutrients. J. Nutr. Biochem. 7, 659–663 (1996). [Google Scholar]