Abstract
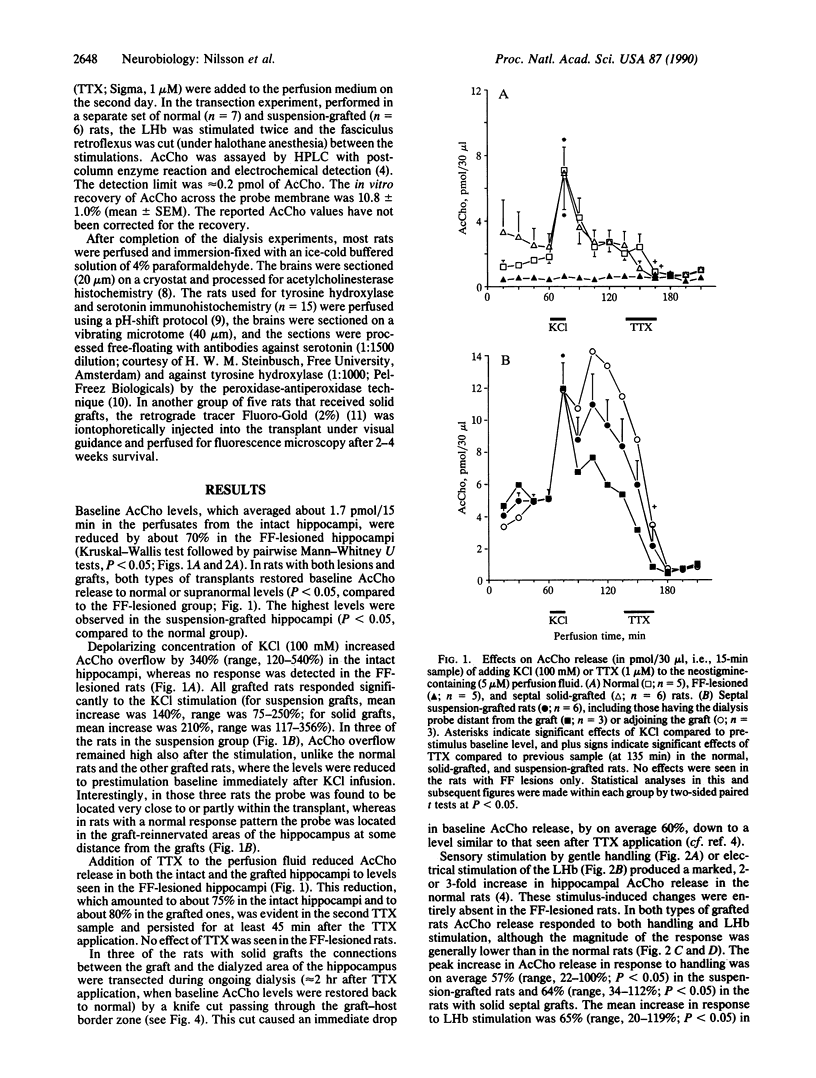
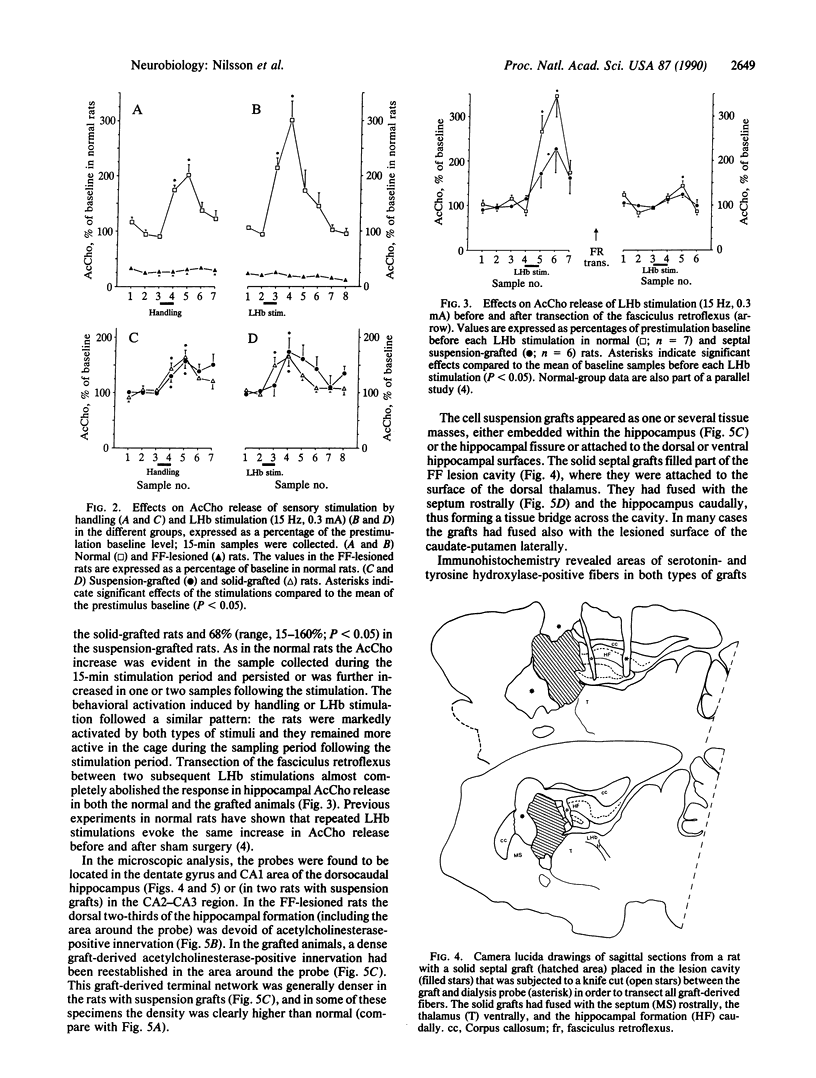
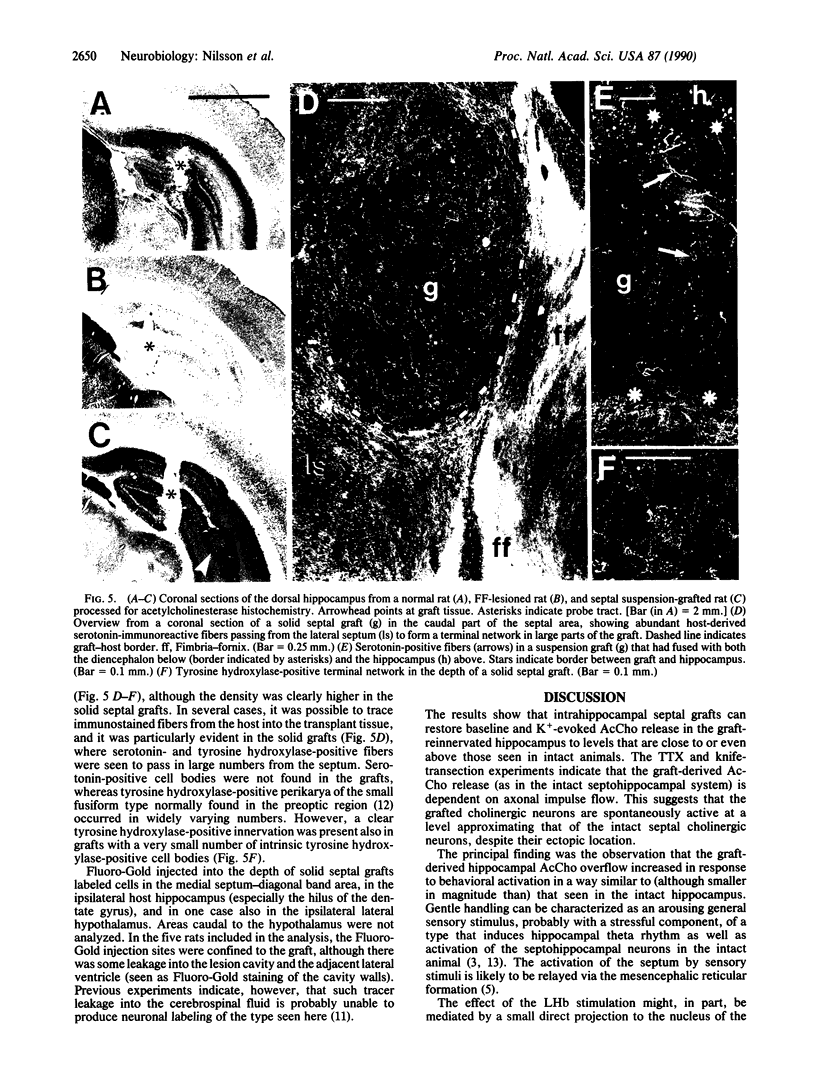
The activity of intrahippocampal transplants of cholinergic neurons was monitored by microdialysis in awake, freely moving rats. Fetal septal-diagonal band tissue was implanted into rats with a complete transection of the fimbria-fornix cholinergic pathway either as a cell suspension injected into the hippocampus or as a solid graft implanted in the lesion cavity. The grafts restored baseline acetylcholine release in the graft-reinnervated hippocampus to normal or supranormal levels. The graft-derived acetylcholine release was dependent on intact axonal impulse flow, and it was markedly increased during behavioral activation by sensory stimulation or by electrical stimulation of the lateral habenula. The results demonstrate that the septal grafts, despite their ectopic location, can become functionally integrated with the host brain and that the activity of the transplanted cholinergic neurons can be modulated from the host brain during ongoing behavior. Anatomical observations, using immunohistochemistry and retrograde tracing, indicate that direct or indirect brainstem afferents to the graft could mediate this functional integration. Host afferent control of the graft may thus play a role in the recovery of lesion-induced functional deficits seen with these types of transplants.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki M., McGeer P. L., Kimura H. The efferent projections of the rat lateral habenular nucleus revealed by the PHA-L anterograde tracing method. Brain Res. 1988 Feb 16;441(1-2):319–330. doi: 10.1016/0006-8993(88)91410-2. [DOI] [PubMed] [Google Scholar]
- Assaf S. Y., Miller J. J. The role of a raphe serotonin system in the control of septal unit activity and hippocampal desynchronization. Neuroscience. 1978;3(6):539–550. doi: 10.1016/0306-4522(78)90018-0. [DOI] [PubMed] [Google Scholar]
- Björklund A., Stenevi U. Reformation of the severed septohippocampal cholinergic pathway in the adult rat by transplanted septal neurons. Cell Tissue Res. 1977 Dec 19;185(3):289–302. doi: 10.1007/BF00220290. [DOI] [PubMed] [Google Scholar]
- Björklund A., Stenevi U., Schmidt R. H., Dunnett S. B., Gage F. H. Intracerebral grafting of neuronal cell suspensions. I. Introduction and general methods of preparation. Acta Physiol Scand Suppl. 1983;522:1–7. [PubMed] [Google Scholar]
- Buzsáki G., Gage F. H., Czopf J., Björklund A. Restoration of rhythmic slow activity (theta) in the subcortically denervated hippocampus by fetal CNS transplants. Brain Res. 1987 Jan 6;400(2):334–347. doi: 10.1016/0006-8993(87)90632-9. [DOI] [PubMed] [Google Scholar]
- Buzsáki G., Gage F. H., Kellényi L., Björklund A. Behavioral dependence of the electrical activity of intracerebrally transplanted fetal hippocampus. Brain Res. 1987 Jan 6;400(2):321–333. doi: 10.1016/0006-8993(87)90631-7. [DOI] [PubMed] [Google Scholar]
- Costa E., Panula P., Thompson H. K., Cheney D. L. The transsynaptic regulation of the septal-hippocampal cholinergic neurons. Life Sci. 1983 Jan 17;32(3):165–179. doi: 10.1016/0024-3205(83)90028-0. [DOI] [PubMed] [Google Scholar]
- Dudar J. D., Whishaw I. Q., Szerb J. C. Release of acetylcholine from the hippocampus of freely moving rats during sensory stimulation and running. Neuropharmacology. 1979 Aug-Sep;18(8-9):673–678. doi: 10.1016/0028-3908(79)90034-0. [DOI] [PubMed] [Google Scholar]
- Geneser-Jensen F. A., Blackstad T. W. Distribution of acetyl cholinesterase in the hippocampal region of the guinea pig. I. Entorhinal area, parasubiculum, and presubiculum. Z Zellforsch Mikrosk Anat. 1971;114(4):460–481. doi: 10.1007/BF00325634. [DOI] [PubMed] [Google Scholar]
- Gerfen C. R., Sawchenko P. E. An anterograde neuroanatomical tracing method that shows the detailed morphology of neurons, their axons and terminals: immunohistochemical localization of an axonally transported plant lectin, Phaseolus vulgaris leucoagglutinin (PHA-L). Brain Res. 1984 Jan 9;290(2):219–238. doi: 10.1016/0006-8993(84)90940-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs R. B., Harris E. W., Cotman C. W. Replacement of damaged cortical projections by homotypic transplants of entorhinal cortex. J Comp Neurol. 1985 Jul 1;237(1):47–64. doi: 10.1002/cne.902370104. [DOI] [PubMed] [Google Scholar]
- Kalén P., Strecker R. E., Rosengren E., Björklund A. Regulation of striatal serotonin release by the lateral habenula-dorsal raphe pathway in the rat as demonstrated by in vivo microdialysis: role of excitatory amino acids and GABA. Brain Res. 1989 Jul 17;492(1-2):187–202. doi: 10.1016/0006-8993(89)90901-3. [DOI] [PubMed] [Google Scholar]
- Kromer L. F., Björklund A., Stenevi U. Regeneration of the septohippocampal pathways in adult rats is promoted by utilizing embryonic hippocampal implants as bridges. Brain Res. 1981 Apr 6;210(1-2):173–200. doi: 10.1016/0006-8993(81)90893-3. [DOI] [PubMed] [Google Scholar]
- Lamour Y., Dutar P., Jobert A. Septo-hippocampal and other medial septum-diagonal band neurons: electrophysiological and pharmacological properties. Brain Res. 1984 Sep 10;309(2):227–239. doi: 10.1016/0006-8993(84)90588-2. [DOI] [PubMed] [Google Scholar]
- Nilsson O. G., Shapiro M. L., Gage F. H., Olton D. S., Björklund A. Spatial learning and memory following fimbria-fornix transection and grafting of fetal septal neurons to the hippocampus. Exp Brain Res. 1987;67(1):195–215. doi: 10.1007/BF00269466. [DOI] [PubMed] [Google Scholar]
- PETSCHE H., STUMPF C., GOGOLAK G. [The significance of the rabbit's septum as a relay station between the midbrain and the hippocampus. I. The control of hippocampus arousal activity by the septum cells]. Electroencephalogr Clin Neurophysiol. 1962 Apr;14:202–211. doi: 10.1016/0013-4694(62)90030-5. [DOI] [PubMed] [Google Scholar]
- Robinson S. E., Malthe-Sørenssen D., Wood P. L., Commissiong J. Dopaminergic control of the septal-hippocampal cholinergic pathway. J Pharmacol Exp Ther. 1979 Mar;208(3):476–479. [PubMed] [Google Scholar]
- Schmued L. C., Fallon J. H. Fluoro-Gold: a new fluorescent retrograde axonal tracer with numerous unique properties. Brain Res. 1986 Jul 2;377(1):147–154. doi: 10.1016/0006-8993(86)91199-6. [DOI] [PubMed] [Google Scholar]
- Segal M. Interactions between grafted serotonin neurons and adult host rat hippocampus. Ann N Y Acad Sci. 1987;495:284–295. doi: 10.1111/j.1749-6632.1987.tb23681.x. [DOI] [PubMed] [Google Scholar]
- Shapiro M. L., Simon D. K., Olton D. S., Gage F. H., 3rd, Nilsson O., Björklund A. Intrahippocampal grafts of fetal basal forebrain tissue alter place fields in the hippocampus of rats with fimbria-fornix lesions. Neuroscience. 1989;32(1):1–18. doi: 10.1016/0306-4522(89)90103-6. [DOI] [PubMed] [Google Scholar]
- Tønder N., Sørensen T., Zimmer J. Enhanced host perforant path innervation of neonatal dentate tissue after grafting to axon sparing, ibotenic acid lesions in adult rats. Exp Brain Res. 1989;75(3):483–496. doi: 10.1007/BF00249900. [DOI] [PubMed] [Google Scholar]
- Vanderwolf C. H. Cerebral activity and behavior: control by central cholinergic and serotonergic systems. Int Rev Neurobiol. 1988;30:225–340. doi: 10.1016/s0074-7742(08)60050-1. [DOI] [PubMed] [Google Scholar]



