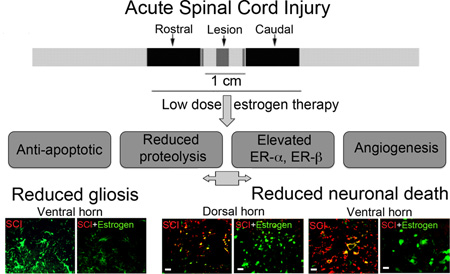
Abstract
Spinal cord injury (SCI) is a debilitating condition with neurological deficits and loss of motor function that, depending on the severity, may lead to paralysis. The only treatment currently available is methylprednisolone, which is widely used and renders limited efficacy in SCI. Therefore, other therapeutic agents must be developed. The neuroprotective efficacy of estrogen in SCI was studied with a pre-clinical and pro-translational perspective. Acute SCI was induced in rats that were treated with low doses of estrogen (1, 5, 10, or 100 µg/kg) and compared with vehicle-treated injured rats or laminectomy control (sham) rats at 48 hours post-SCI. Changes in gliosis and other pro-inflammatory responses, expression and activity of proteolytic enzymes (e.g., calpain, caspase-3), apoptosis of neurons in SCI, and cell death were monitored via Western blot and immunohistochemistry. Negligible pro-inflammatory responses or proteolytic events and very low levels of neuronal death were found in sham rats. In contrast, vehicle-treated SCI rats showed profound pro-inflammatory responses with reactive gliosis, elevated expression and activity of calpain and caspase-3, elevated Bax:Bcl2 ratio, and high levels of neuronal death in lesion and caudal regions of the injured spinal cord. Estrogen treatment at each dose reduced pro-inflammatory and proteolytic activities and protected neurons in the caudal penumbra in acute SCI. Estrogen treatment at 10 µg was found to be as effective as 100 µg in ameliorating the above parameters in injured animals. Results from this investigation indicated that estrogen at a low dose may be a promising therapeutic agent for treating acute SCI.
Keywords: Acute spinal cord injury, calpain, estrogen, estrogen receptors, gliosis, neuroprotection, VEGF
Graphical Abstract
Introduction
Spinal cord injury (SCI) is a devastating debilitating trauma that affects mostly young adults, causing neurological deficit, and loss of sphincter function. Also, depending on the severity of the injury, SCI often leads to paralysis and death. No effective pharmacotherapy is currently available except for methylprednisolone (MP), which is controversial due to limited efficacy and many unwanted side effects (Bracken et al. 1984, Hurlbert 2000, Dumont et al. 2001). Thus, development of new therapeutics with maximal capacity that can improve locomotor and sensory function after SCI is critical and essential.
Because of the multiple destructive mechanisms that are responsible for cellular and tissue damage in secondary injury, development of a single therapeutic agent or drug that can promote or improve function following SCI has been difficult. Alternatively, identification of a multi-active drug or combination therapy that will affect several pathways in secondary injury in order to protect cells and their processes and promote functional recovery is crucial. Such beneficial neuroprotective effects of the multi-active steroidal hormones estrogen and progesterone have been demonstrated in animal models of ischemia and stroke (Sakurai et al. 2003, Alkayed et al. 2001, Roof & Hall 2000, Dubal et al. 1999, Dubal et al. 2001). In addition, progesterone at higher dose (4 mg/kg body weight) has been found to be neuroprotective in rat following SCI (Thomas et al. 1999, Gonzalez et al. 2005, Gonzalez et al. 2009). Over the years, our laboratory and others have demonstrated that pharmacological doses of estrogen (4 mg/kg) ameliorated secondary injury progression, including glial activation, inflammation, lesion volume, and cell death in acute SCI (Yune et al. 2004, Sribnick et al. 2005, Sribnick et al. 2010, Kachadroka et al. 2010, Ritz & Hausmann 2008). Subsequently, administration of such doses of estrogen has been found to promote improvement in the locomotor function in chronic SCI in rats (Yune et al. 2004, Sribnick et al. 2010, Lee et al. 2012, Elkabes & Nicot 2014, Hu et al. 2012, Kwon et al. 2011).
Although a substantial number of pre-clinical studies on the estrogen effect in SCI have found administration of estrogen to be beneficial and neuroprotective, the potential for use in the treatment of SCI remains limited. Among the issues that make unlikely for estrogen to be taken into the clinic are factors such as very high non-physiological doses (100 µg – 4 mg/kg) of estrogen (17β-estradiol) and the route and time of drug delivery that have been used in almost all studies (Yune et al. 2004, Sribnick et al. 2005, Lee et al. 2012, Elkabes & Nicot 2014, Hu et al. 2012). Administration of high doses of estrogen by intravenous route will have many unwanted side effects, including deep vein thrombosis and cancer (Nelson et al. 2002, Manson et al. 2013). In order to circumvent these adverse effects, our laboratory has recently published pilot studies that estrogen treatment at significantly lower doses (1–10 µg/kg) that are closer to physiological levels are anti-inflammatory and neuroprotective in acute SCI in rats, irrespective of time or route of delivery (Samantaray et al. 2011, Cox et al. 2015). The encouraging results obtained earlier from the pilot acute studies have now been expanded to assess more parameters in acute injury. Determination of the effectiveness of the low dose of estrogen on several detrimental parameters is important for long-term studies following SCI. In support of estrogen as a potential therapy for SCI, we report that estrogen administration at low dose (1, 5, 10 or 100 µg/kg) attenuated inflammation and calpain and caspase activation; upregulated estrogen receptors and angiogenic factor vascular endothelial growth factor (VEGF); and protected neurons, which are important for restoring/improving locomotor function following SCI. These findings may help investigate whether administration of low doses of estrogen will help restore or improve locomotor function in chronic estrogen efficacy studies in spinal cord following injury. Such studies are in progress in our laboratory.
Materials and Methods
Induction of SCI and estrogen treatment
A moderately severe (40g•cm) injury model was used, as described earlier (Perot et al. 1987, Samantaray et al. 2011). This model of SCI is clinically relevant in that it more closely mimics the type of impact injury that occurs following automobile and other accidents. Male Sprague-Dawley rats (Harlan Laboratories, Dublin, VA) weighing 200–250 grams were used for the studies. Rats were anesthetized with ketamine (Ketaset 80 mg/kg, Pfizer, New York, NY) and xylazine (AnaSed 10 mg/kg, Lloyd laboratories, Shenadoah, IA), and a laminectomy was performed at T10. After immobilizing the spine with a spinal stereotactic device, SCI was induced by a modified method by Perot of dropping a constant weight (5 gm) from a height of 8 cm onto an impounder (0.3 cm in diameter) gently placed on the spinal cord (Perot et al. 1987). Sham animals received laminectomy only. Buprenorphine (0.01–0.05 mg/kg) was administered for post-operative pain. Water-soluble estrogen, in the form of 17β-estradiol (10 µg/kg, Sigma, St. Louis, MO), was dissolved in saline, and vehicle-treated animals received an equivalent volume of saline. All animals received a bolus tail vein injection of the respective low dose estrogen or vehicle at 15 min post-injury and a second at 24 h. Rats were sacrificed by decapitation under anesthesia at 48 h post-injury. A 1 cm section of spinal cord centered on the lesion site as well as a 1 cm rostral section (starting 0.5 cm rostral from the impact site) and a caudal penumbra section (starting 0.5 cm caudal to the impact site) were taken for all groups so that the location and size remain the same for quantitative analysis, i.e., to determine the effects of estrogen on pathophysiological changes. A total of 30 rats were utilized in this study with n = 3 for sham + vehicle or sham + estrogen groups and n = 6 for injury + vehicle or injury + respective estrogen treatment groups. ARRIVE experimental guidelines were followed along with institutional approval (AR#2079, Medical University of South Carolina, SC) for care and handling of experimental animals during the course of this study.
Immunofluorescent labeling
Rat spinal cord samples embedded in Tissue-Tek OCT freezing media were warmed to −20°C, and 5 µm thick sections were sliced using a Leica CM1850 cryostat (Leica, Deerfield, IL, USA). Tissue sections were fixed in 95% ethanol, rinsed in phosphate-buffered saline (PBS, containing 137 mM NaCl, 2.7 mM KCl, 11.9 mM phosphates, pH 7.4), and stored in the same buffer at 4°C for further studies within a week. Sections were blocked in PBS containing 2% appropriate serum (horse or sheep) then incubated with respective primary IgG antibodies. Single immunofluorescent staining was performed to detect glial fibrillary acidic protein (GFAP, 1:400 dilution). Terminal deoxynucleotidyl transferase recombinant–mediated dUTP nick-end labeling (TUNEL) was performed following the manufacturer’s protocol (Promega, Madison, WI, USA). Sections prefixed in 95% ethanol were immersed in 4% methanol-free formaldehyde, washed in PBS, equilibrated in buffer, and incubated with digoxigenin labeled nucleotides (Roche, Indianapolis, IN, USA) and recombinant TdT enzyme (Promega) using a humidified Hybaid OmniSlide Thermal Cycler (Hybaid Ltd., Teddington, UK). TUNEL reaction was terminated with 2× NaCl/Na-citrate solution, and unincorporated nucleotides were removed by three rinses in PBS. Spinal cord sections were double stained with primary antibodies, to detect co-localization of TUNEL with neuronal nuclei marker (NeuN, 1:100 dilution) overnight at 4°C, and images were captured as described earlier (Samantaray et al. 2007). GFAP immunoreactive pixels and TUNEL positive neurons in dorsal and ventral horn were estimated following our previously poblished methods (Samantaray et al. 2007).
Western blotting
Approximately 50–100 mg samples were prepared as follows: spinal cords were homogenized in an ice-cold homogenizing buffer (50 mM Tris-HCl, pH 7.4; 5 mM EGTA) with phenylmethylsulfonyl fluoride (1 mM); protein concentration was estimated with Coomassie PlusTM Protein Assay Reagent (Pierce, Rockford, IL, USA) at 595 nm. Samples were equilibrated (1:1 v/v) in a sample buffer (62.5 mM Tris-HCl, pH 6.8, 2% sodium dodecyl sulfate, 5 mM β-mercaptoethanol, 10% glycerol), boiled, and briefly spun before sodium dodecyl sulfate soluble supernatant was collected. Samples were diluted to a final protein concentration of 1.5 mg/mL with mix (1:1 v/v) of homogenizing and sample buffers containing bromophenol blue dye (0.01%). Proteins were resolved in a 4–20% pre-cast gradient gel (Bio-Rad Laboratories, Hercules, CA, USA) at 100 V for 60 min and transferred to ImmobilonTM-P polyvinylidene fluoride microporous membranes (Millipore, Bedford, MA, USA). Western blotting was carried out according to Samantaray et al. (Samantaray et al. 2007). Calpain was detected with rabbit polyclonal antibody [1: 500 dilution; (Banik et al. 1983, Samantaray et al. 2007)]. Spectrin breakdown products (SBDP), caspase-3, Bax, Bcl2, ER-α, ER-β and VEGF were detected (antibody specifications in Table S1). Blots, except those for spectrin, were re-probed for β-actin, as a protein loading control. Quantification of the immunoblots were conducted following our previously published methods (Samantaray et al. 2007).
Statistical analysis
Optical density (OD) of protein immunoreactive bands obtained from Western blotting was analyzed with NIH Image J 1.45 software. Results were assessed in Stat View software (Abacus Concepts, Piscataway, NJ, USA) and compared by using one-way ANOVA with Fisher’s protected least significant difference post hoc test at 95% confidence interval. Data were expressed as mean ± SEM (n = 3–6). The difference in SCI or sham spinal cords with or without estrogen treatment was considered significant at p < 0.05.
Results
Estrogen attenuates inflammatory events following acute SCI
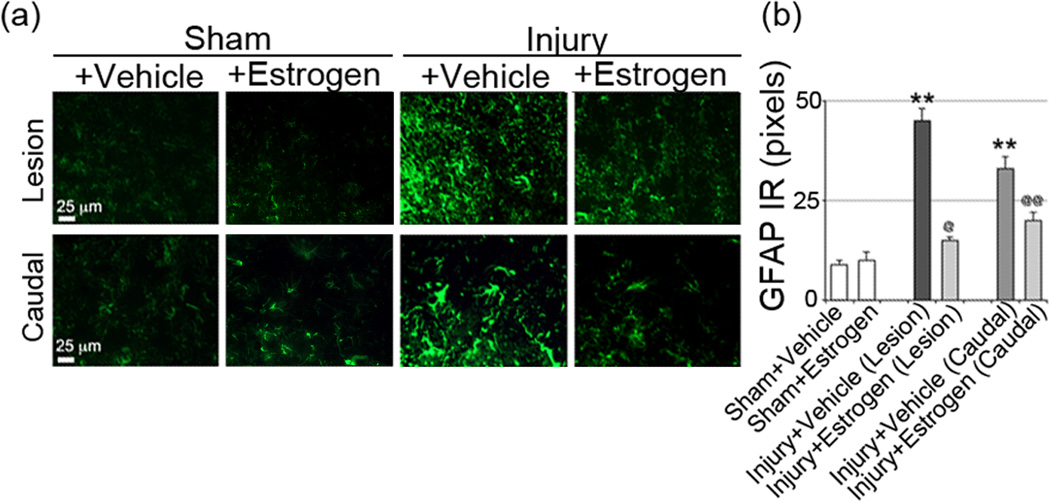
One of the aims of this investigation has been to pinpoint the lowest dose of estrogen required to reduce secondary pathophysiological events in spinal cord following acute injury. Since inflammatory events are a major source of secondary damage, activation of cells that respond to inflammation were examined in the injured spinal cord. Activation of macrophages and glial cells (microglia, astrocytes) is a common feature in the inflammatory process following SCI. Thus, the efficacy of low dose estrogen in reducing microgliosis was previously examined in our laboratory via immunostaining with OX-42 antibody, which was significantly attenuated, as shown earlier (Samantaray et al. 2011). In addition, astroglial reactivity was examined in spinal cord following acute SCI using GFAP-immunostaining. Increased astroglial reactivity was seen in the lesion ventral horn as well as in the white matter from SCI animals, as compared to the sham-vehicle group. Estrogen therapy (10–100 µg/kg) reduced astroglial GFAP reactivity in gray matter (ventral horn, Figure 1) and white matter of the lesion (data not shown). Astrogliosis was also increased in caudal penumbra SCI tissue from acute SCI rats, and 17β-estradiol also decreased astrogliosis at each of the doses employed, indicating that the glial reactivity was decreased with estrogen treatment. These data suggest an estrogen mediated anti-inflammatory effect following acute SCI.
Fig. 1.
Low dose estrogen attenuated astrogliosis following acute SCI. Immunofluoresecent labelings for astrogliosis were performed using lesion and caudal penumbra spinal cord sections from sham and SCI rats treated with vehicle or estrogen (10 µg/kg). (a) Astrogliosis were assessed using GFAP antibody (green), representative images captured at the ventral horn are shown. Acute SCI-induced pervading astrogliosis in the ventral horn were significantly attenuated with estrogen treatment (n = 3–6, 200× magnification). (b) Quantitative assessment of GFAP IR is represented. At P = 0.05, sham + vehicle and sham + estrogen were not significantly different. *P < 0.05 as compared to sham and @P < 0.05 or @@P < 0.01 as compared to injury + vehicle.
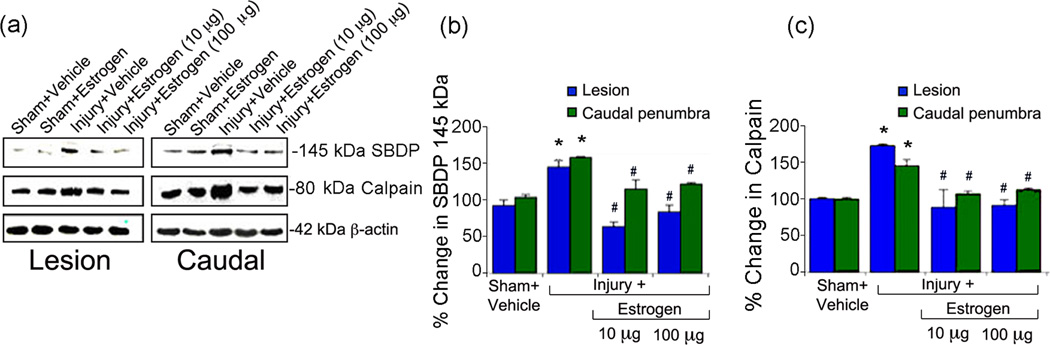
Estrogen treatment reduces calpain expression and activity following acute SCI
Since calpain is known to be a central mediator in various apoptotic pathways, the dose-dependent effects of estrogen treatment (10–100 µg/kg/day) on calpain activity and expression were examined following acute SCI in lesion and caudal penumbral tissue by Western blot analysis using mcalpain (80kD) antibody. Calpain activity was measured using an antibody against spectrin, which recognizes the 145kD calpain-specific spectrin breakdown product (SBDP). Figure 2 demonstrates representative immunoblots from the following groups: sham-vehicle, sham treated with estrogen (100 µg), injury-vehicle, or injury with estrogen (10 or 100 µg/kg). Calpain expression (Figure 2a, b) and activity (Figure 2a, c) were significantly increased (more than 50%) in lesion and caudal penumbra from animals treated with vehicle (saline) alone compared to sham controls. In contrast, treatment with estrogen significantly decreased calpain expression and activity at 10 and 100 µg/kg/day in the lesion and caudal spinal cord tissue.
Fig. 2.
Estrogen reduced calpain expression and activity following acute SCI. (a) Representative Western blots using lesion and caudal penumbra spinal cord segments from sham and SCI rats treated with vehicle or estrogen (10 or 100 µg/kg). (b) Determination of OD of bands representing calpain activity in terms of 145 kDa specific spectrin breakdown products or SBDP. (c) Determination of OD of bands representing calpain expression. Data presented as percentage change in comparison with sham + vehicle set at 100% (n = 3–6). Sham + vehicle vs. injury + vehicle (*P < 0.05); and injury + vehicle vs. injury + estrogen (#P < 0.05)
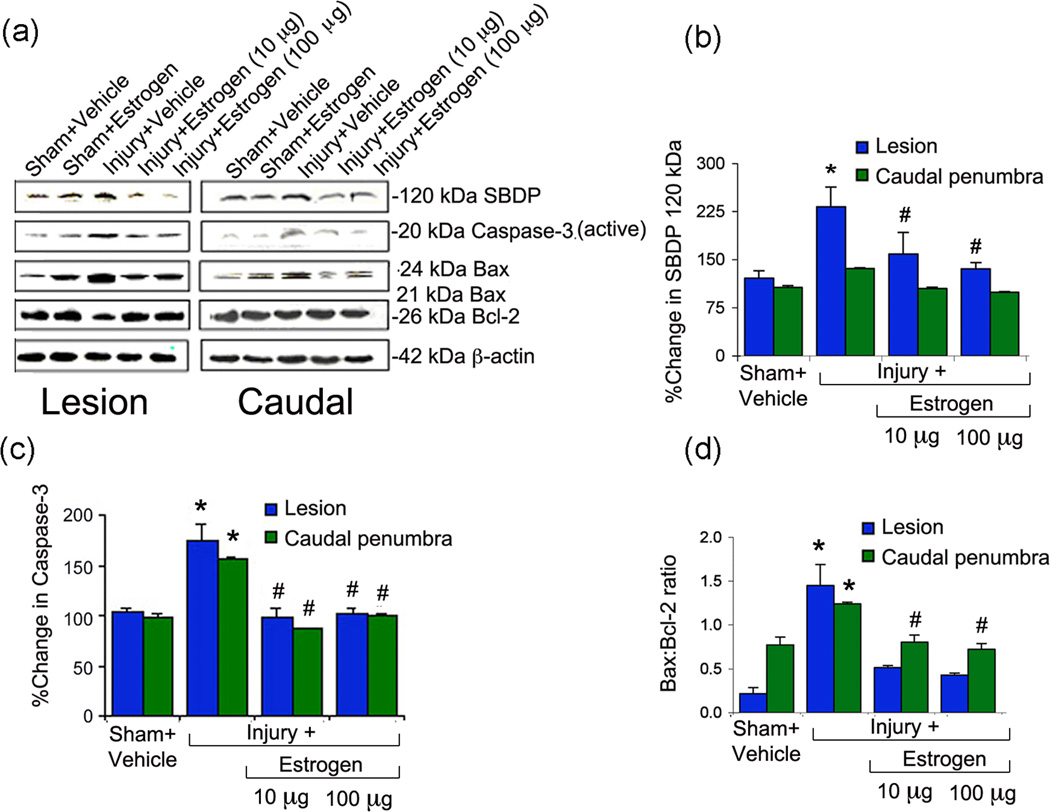
Estrogen treatment reduces caspase-3 expression and activity following acute SCI
Since pro-apoptotic caspase-3 is involved in the final pathway of apoptosis, the effects of estrogen treatment on its activity and expression were examined following SCI. Experiments were performed as described above and tissue was analyzed for expression of the active 20 kD caspase-3 protein (Figure 3) and determining the activity by production of caspase-3-specific 120 kD SBDP. As seen with calpain expression, active-caspase-3 expression (Figure 3a, c) and activity (Figure 3a, b) were increased in lesion and caudal penumbra of spinal cord tissue from injured rats, as compared to sham. Our data indicate that the expression of active caspase-3 was decreased by estrogen treatment at all concentrations in lesion and caudal penumbra. In addition, production of the 120 kD caspase-3-specific SBDP was significantly decreased at all concentrations in the lesion and penumbra following treatment with estrogen. The extent of decrease seen with 10 µg estrogen treatment was found to be as good as 100 µg estrogen. This finding suggested estrogen-mediated protection of cells by attenuating caspase-3 activity in SCI. The effects of low dose estrogen on other apoptotic parameters such as the Bax:Bcl2 ratio was also observed (Figure 3a, d), as in our previous study (Samantaray et al. 2011).
Fig. 3.
Estrogen reduced caspase-3 expression and activity and Bax:Bcl-2 ratio following acute SCI. (a) Representative Western blots using lesion and caudal penumbra spinal cord segments from sham and SCI rats treated with vehicle or estrogen (10 or 100 µg/kg). (b) Determination of OD of bands representing caspase-3 activity in terms of 120 kDa specific spectrin breakdown products or SBDP. (c) Determination of OD of bands representing caspase-3 active fragment. (d) Determination of OD of bands representing the ratio between Bax:Bcl-2. Data presented as percentage change in comparison with sham + vehicle set at 100% (n = 3–6). Sham + vehicle vs. injury + vehicle (*P < 0.05); and injury + vehicle vs. injury + estrogen (#P < 0.05)
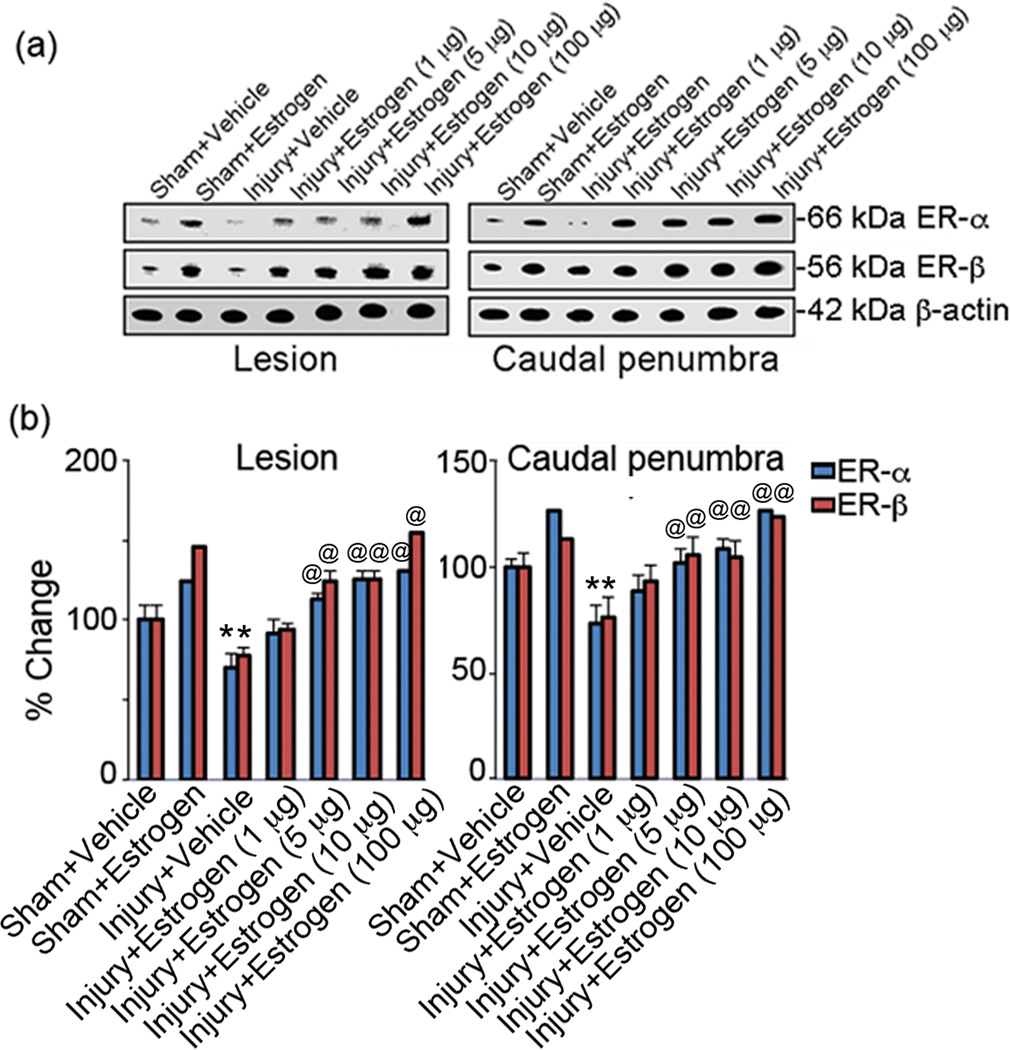
Estrogen therapy increases expression of the estrogen receptors ERα and ERβ following acute SCI
Since many of the effects of estrogen are both receptor-dependent, the effects of 17β-estradiol (1–100 µg/kg/daily) were examined in the lesion and caudal penumbra of sham and injury animals after 48 hours. The receptors were identified with marker antibodies that recognize ERα and ERβ and β-actin served as an internal control for loading. Western blot analysis indicates estrogen therapy increased expression of ERβ at all concentrations employed in the injured spinal cord and in the sham animal (Figure 4). ERα was also increased at all doses of estrogen compared to sham and vehicle treated SCI animals. A greater effect was found at the highest dose of estrogen used in the injured and sham groups. These data suggest the effects of 17β-estradiol may be in part due to signaling through increased ERs.
Fig. 4.
Estrogen modulated expression of estrogen receptors ERα and ERβ following acute SCI. (a) Representative Western blots using lesion and caudal penumbra spinal cord segments from sham and SCI rats treated with vehicle or dose-dependent estrogen (1, 5, 10 or 100 µg/kg). (n = 3–6). (b) Quantitative assessment of ERα and ERβ is represented. At P = 0.05, sham + vehicle and sham + estrogen were not significantly different. *P < 0.05 as compared to sham and @P < 0.05 as compared to injury + vehicle.
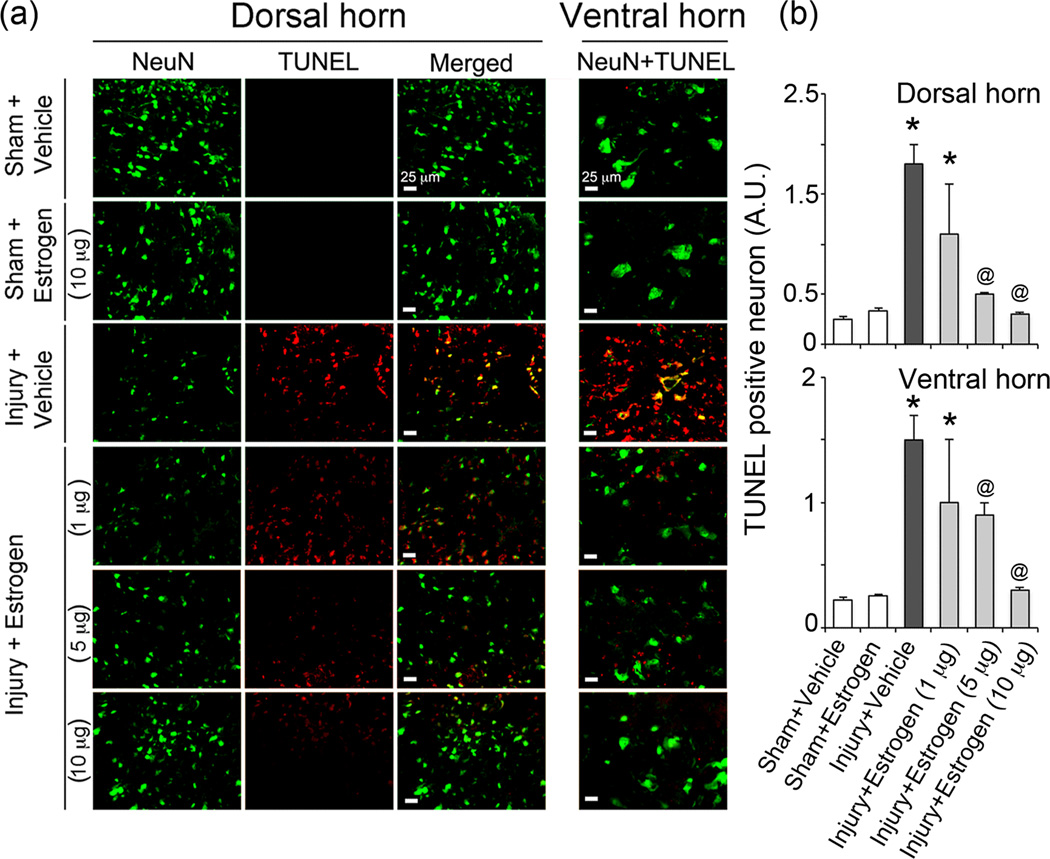
Estrogen attenuates neuronal death following SCI
The effects of estrogen therapy (1–10 µg/kg) on neuronal death were assessed following acute SCI. DNA fragmentation (marker for cell death) was examined in neurons of the caudal penumbra using the TUNEL assay. No TUNEL+ cells were found in the sham-vehicle or sham-estrogen animals while the injury vehicle animals showed extensive TUNEL+ cells in both dorsal and ventral regions of the spinal cord (Figure 5). Many of these cells co-stained for the neuronal marker NeuN. Estrogen therapy attenuated cell death in neurons in both dorsal and ventral regions of the injured cord at the 5 and 10 µg/kg doses compared to vehicle treated injured rats; however the 1 µg/kg dose of estrogen did not markedly alter neuronal death (Figure 5).
Fig. 5.
Estrogen attenuated neuronal death following acute SCI. (a) Double immunofluorescent labelings for detecting neuronal death were performed using caudal penumbra spinal cord sections from sham and SCI rats treated with vehicle or dose-dependent estrogen (1, 5, or 10 µg/kg). Images captured at the dorsal horn are represented as TUNEL staining (red), NeuN staining (green) and co-staining in the merge panel whereas images in ventral horn as represented as merge only. (n = 3–6, 200× magnification). (b) TUNEL-positive neurons (yellow, merge) were estimated in SCI tissue. At P = 0.05, sham + vehicle and sham + estrogen were not significantly different. *P < 0.05 as compared to sham and @P < 0.05 as compared to injury + vehicle.
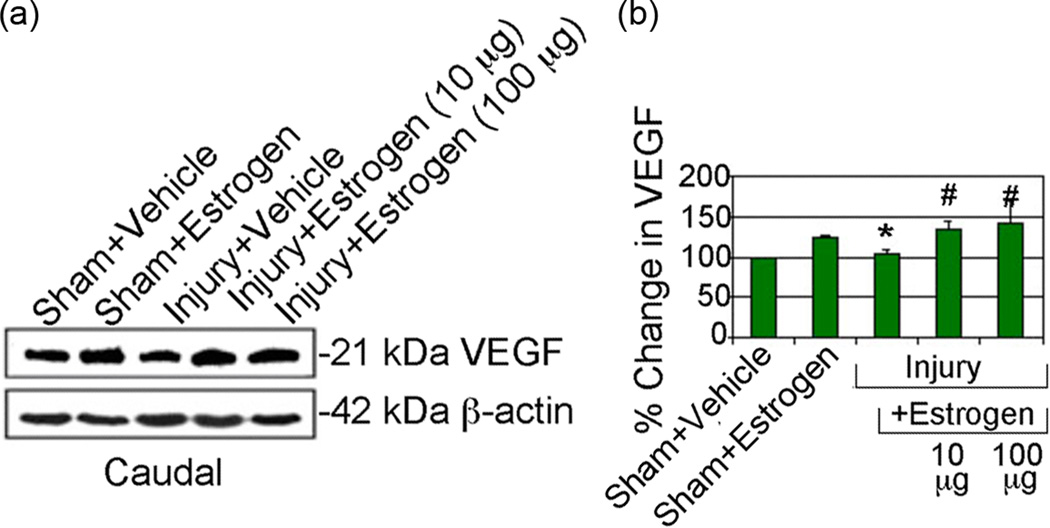
Estrogen treatment increases VEGF expression following acute SCI
Previous studies demonstrated that estrogen increases VEGF expression as well as other angiogenic markers (Duckles & Krause 2003b, Wise 2002, Yang et al. 2000). Since decreased blood supply is a serious problem and may lead to ischemic damage to cells following SCI, angiogenesis promote supply of blood for cell survival following estrogen treatment. Thus, the effects of estrogen treatment on expression of the angiogenic factor VEGF were examined following acute SCI (Figure 6). Results indicate a trend in increased VEGF expression in caudal penumbra from both sham and SCI animals following estrogen treatment with both doses of estrogen. These data suggest that estrogen therapy may expedite the return of blood flow and nutrients following injury.
Fig. 6.
Estrogen modulated expression of VEGF following acute SCI. Representative Western blots using caudal penumbra spinal cord segments from sham and SCI rats treated with vehicle or estrogen (10 or 100 µg/kg). (n = 4–6). Determination of OD of bands representing VEGF expression showing data presented as percentage change in comparison with sham + vehicle set at 100% (n = 4–6). Sham + vehicle vs. injury + vehicle (*P < 0.05); and injury + vehicle vs. injury + estrogen (#P < 0.05)
Discussion
Spinal cord injury (SCI) is a devastating clinical problem with neurological deficits that, depending on the severity of the injury, can lead to paralysis. There is no effective therapy available for the treatment of SCI that can improve function. Understanding of secondary injury mechanisms involved following SCI has prompted investigations on the role of estrogen as a potential therapy. In our present study, the efficacy of estrogen therapy was examined using very low doses administered by IV injection in an acute rat model of SCI.
Attenuating a single damaging mechanism had not been proven to be beneficial for treatment of SCI; however, estrogen is a multi-action steroid hormone that attenuates multiple diverse destructive pathways involved in cell and tissue damage and is ideally suited for combating secondary injury mechanisms in spinal cord following injury (Sribnick et al. 2004a). A major factor involved in secondary damage to SCI and traumatic brain injury (TBI) is inflammation, which also plays a significant role in many neurodegenerative diseases. In addition to infiltration of inflammatory cells into CNS following injuries and in degenerative diseases, neural cells that respond to inflammation (astrocytes, microglia) are also activated (Popovich et al. 1997, Ritz & Hausmann 2008, Samantaray et al. 2011, Shields et al. 2000). Since inflammatory factors are known to cause cell death in vitro (Sribnick et al. 2004b, Das et al. 2011), one of the targeted therapies in SCI and other diseases has been to reduce inflammation by inhibiting activation of inflammatory cells and blocking production of inflammatory components in SCI following treatment with estrogen and other agents (Sribnick et al. 2005, Samantaray et al. 2008, Chaovipoch et al. 2006). The present study with low dose estrogen (ranging from 1–100 µg) confirmed earlier findings and supported the hypothesis that one of the functions of estrogen is as an anti-inflammatory agent, possibly mediated by estrogen receptors ERα and β (Tiwari-Woodruff et al. 2007). Also, activation of pro-inflammatory transcription factor NFκB, a major player in the development of inflammation, is increased in SCI (Bethea et al. 1998, Sribnick et al. 2010), and estrogen treatment has been found to block this activation in vivo in SCI (Sribnick et al. 2005, Sribnick et al. 2010) as well as in vitro in thymocytes (Xie et al. 2002) and neural cells (Das et al. 2011). The upregulation of ERα and ERβ in estrogen treated SCI has been suggested as a possible link to the inhibition of NFκB activation (Sribnick et al. 2006). Low dose estrogen treatment (1–10 µg/kg) was previously found to reduce inflammation (e.g., microgliosis, macrophage activation), decrease death genes, and protect neurons (Samantaray et al. 2011). In the present study, the same dose of estrogen markedly reduced astrogliosis and calpain/caspase activities in gray and white matter (Figures 1–3) and upregulated estrogen receptors α and β, which have been shown to mediate the anti-inflammatory effect rendered by estrogen in acute SCI. This confirms the findings of high dose estrogen studies reported earlier by our laboratory and others (Sribnick et al. 2003, Sribnick et al. 2005, Sribnick et al. 2006, Yune et al. 2004, Chaovipoch et al. 2006) in acute as well as chronic SCI with improvement of motor function (Sribnick et al. 2010, Cuzzocrea et al. 2008, Siriphorn et al. 2012). The neuroprotective effects of estrogen have been attributed to its anti-oxidative, anti-inflammatory, and other properties. Estrogen has been found to reduce calcium influx in vivo in SCI (Wingrave et al. 2003) as well as in vitro in cell undergoing excitatory or inflammatory damage (Nilsen et al. 2002, Sribnick et al. 2004b, Sur et al. 2005). We have recently shown that estrogen reduces calcium influx via modulating L-type Ca2+-channels and Na+-K+ exchanger in vitro (Sribnick et al. 2009).
While calpains are involved in signaling and synaptic plasticity at the physiological level, pathologic calpains are hyperactive and degenerative (Ray et al. 2003, Lynch & Baudry 1984). Since an increase in calcium activates calpain and this elevated activity degrades cytoskeletal protein, damaging mitochondria, neurofilaments, and microtubules in SCI (Sribnick et al. 2006, Sribnick et al. 2005, Wingrave et al. 2003) as well as in TBI (Posmantur et al. 1997, Ray & Banik 2003, Saatman et al. 2010), resulting in neurotoxicity and cell death. The present study investigated whether the increased calpain activity is inhibited by low dose estrogen, contributing to neuroprotection. The current findings on estrogen-mediated calpain inhibition and the resulting neuroprotection have confirmed our earlier report (Samantaray et al. 2011). Our data also showed significant inhibition of caspase-3 and calpain expression and activity both in the lesion and penumbra, indicating that estrogen may provide protection to cells which are mainly apoptotic, particularly in the penumbra. However, pathologic calpains have been associated with neurodegenerative diseases, including Alzheimer’s disease, multiple sclerosis, Parkinson’s disease, and others (Shields & Banik 1998a, Shields & Banik 1998b, Shields et al. 1999, Samantaray et al. 2007, Samantaray et al. 2013, Guyton et al. 2005, Bartus et al. 1994, Battaglia et al. 2003). Active caspase-3, the final executioner of apoptotic cell death mechanisms, is a substrate of calpain and becomes active following degradation, and therefore, upregulated calpain is expected to activate caspase-3 in injured tissue (Gao & Dou 2000, Blomgren et al. 2001, Wu et al. 2004).
Increased activity of caspase-3 in SCI is involved in apoptosis and is inhibited in lesion and penumbra of SCI following treatment with estrogen. Like our previous findings (Sribnick et al. 2010, Sribnick et al. 2006, Ray et al. 2003, Samantaray et al. 2011), in the present study estrogen treatment attenuated pro-apoptotic Bax and upregulated anti-apoptotic Bcl2, thus altering the Bax;Bcl2 ratio essential for cell survival. This protection of neurons, whether in dorsal or ventral horn, is confirmed by TUNEL staining of the caudal penumbra in SCI following treatment with low doses (5–10 µg) of estrogen. The attenuation of sensory and motor neuron death by estrogen indicates their pivotal roles for improvement of function in SCI (Samantaray et al. 2011, Sribnick et al. 2006, Sribnick et al. 2010, Samantaray et al. 2010, Siriphorn et al. 2012, Kachadroka et al. 2010). Estrogen treatment not only provided protection to neurons in acute SCI, it also attenuated axonal degeneration in the damaged spinal cord following injury. We previously suggested that cells in the lesion tissue undergo irreversible necrotic death while cells in the penumbra are partially damaged and apoptotic and that the apoptotic death may be reversible if treated with proper agents and time following injury. Our results with low estrogen treatment showed calpain-caspase inhibition and greater cell protection in penumbra, as previously demonstrated (Sribnick et al. 2006, Chaovipoch et al. 2006, Samantaray et al. 2011). Although increased caspase-3 activity is inhibited by estrogen treatment, it is downstream of calpain. Thus, since caspase is a substrate of calpain, inhibition of calpain by estrogen is very likely to also block caspase activation and provide neuroprotection. Therefore, the inhibition of calpain by estrogen is likely to be an important pathway for the neuroprotection rendered by estrogen.
The neuroprotective mechanisms of estrogen have been suggested to be mediated by estrogen receptors ERα and ERβ since both are localized in mitochondria and both inhibit breakdown of calpain substrates (Chen et al. 2004, Morelli et al. 2003). ERs also upregulate the expression of anti-apoptotic proteins Akt and Bcl2 via phosphorylation of transcription factor cAMP response element binding protein (CREB) (Alkayed et al. 2001, Honda et al. 2001, Pugazhenthi et al. 2000). Calpain inhibition by estrogen at the lowest level significantly increased levels of both ERα and ERβ in SCI, confirming our previous findings (Sribnick et al. 2006). Estrogen receptor alpha (ERα) may be involved in the estrogen-mediated angiogenesis in tissue after insult (Stirone et al. 2003a), and more specifically SCI (Hu et al. 2012).
The lack of blood supply will induce ischemia due to changes in vasculature following injury may deprive tissue of beneficial factors or nutrients needed for cell survival. Thus, estrogen treatment may improve blood supply and play an important role in improving the neurovasculature, as evidenced with increased expression of angiogenic factor VEGF (Duckles & Krause 2003a, Stirone et al. 2003a, Stirone et al. 2003b). Estrogen has been found to promote angiogenesis by upregulating angiogenic factors Tie-1 and Tie-2 in cerebral ischemia (Davis et al. 1996, Mustonen & Alitalo 1995, Wei et al. 2005). This suggests that estrogen may play a significant role in promoting angiogenesis, which would provide beneficial factors to apoptotic and/or ischemia-induced damaged cells for their survival for functional recovery.
Conclusion
Tissue destruction in spinal cord following injury is mediated by overlapping multi-destructive pathways. Therefore, attenuating the tissue damage by single pharmacotherapy is problematic and will not have any beneficial effect. Estrogen has been shown to be a multi-active steroid that has been found to have anti-inflammatory, anti-oxidant, and anti-apoptotic effects, inhibits calpain activation, and is neuroprotective (Samantaray et al. 2011, Elkabes & Nicot 2014, Kwon et al. 2011). Although angiogenesis is important for cell survival, the limitation in this study is that restoration of blood supply has not been thoroughly investigated in acute injury. Such thorough studies in chronic SCI are currently being investigated in our laboratory. For clinical relevance, the current study provides evidence that treatment with low doses of estrogen (5–10µg of 17β-estradiol), as we have demonstrated in acute (48 hour) SCI is neuroprotective and attenuated many destructive pathways. Therefore, the importance of these findings is that low dose estrogen treatment may have significant potential for recovery of locomotor function in SCI.
Supplementary Material
Acknowledgements
This work was supported in part by funding from NIH (R01-NS3166); Veterans Administration (1IOBX00126; 1IOBX002349); South Carolina State Spinal Cord Research Fund (SCIRF-2015P-01, SCIRF-2015P-04); Department of Neurosurgery, MUSC; and MUSC-CTSA program. We also thank Dr. Kelly Guyton for her valuable contribution to this work.
Abbreviations
- ER α
Estrogen receptor alpha
- ER β
Estrogen receptor beta
- GFAP
glial fibrillary acidic protein
- MP
methylprednisolone
- OCT
optimum cutting temperature
- SBDP
spectrin breakdown product
- SCI
spinal cord injury
- VEGF
vascular endothelial growth factor
- T10
Thoracic 10
Footnotes
All experiments were conducted in compliance with the ARRIVE guidelines.
The authors have no conflict of interest to declare.
References
- Alkayed NJ, Goto S, Sugo N, Joh HD, Klaus J, Crain BJ, Bernard O, Traystman RJ, Hurn PD. Estrogen and Bcl-2: gene induction and effect of transgene in experimental stroke. J Neurosci. 2001;21:7543–7550. doi: 10.1523/JNEUROSCI.21-19-07543.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banik NL, Hogan EL, Jenkins MG, McDonald JK, McAlhaney WW, Sostek MB. Purification of a calcium-activated neutral proteinase from bovine brain. Neurochem Res. 1983;8:1389–1405. doi: 10.1007/BF00964996. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Hayward NJ, Elliott PJ, et al. Calpain inhibitor AK295 protects neurons from focal brain ischemia. Effects of postocclusion intra-arterial administration. Stroke. 1994;25:2265–2270. doi: 10.1161/01.str.25.11.2265. [DOI] [PubMed] [Google Scholar]
- Battaglia F, Trinchese F, Liu S, Walter S, Nixon RA, Arancio O. Calpain inhibitors, a treatment for Alzheimer's disease: position paper. J Molec Neurosci. 2003;20:357–362. doi: 10.1385/JMN:20:3:357. [DOI] [PubMed] [Google Scholar]
- Bethea JR, Castro M, Keane RW, Lee TT, Dietrich WD, Yezierski RP. Traumatic spinal cord injury induces nuclear factor-kappaB activation. J Neurosci. 1998;18:3251–3260. doi: 10.1523/JNEUROSCI.18-09-03251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomgren K, Zhu C, Wang X, Karlsson JO, Leverin AL, Bahr BA, Mallard C, Hagberg H. Synergistic activation of caspase-3 by m-calpain after neonatal hypoxia-ischemia: a mechanism of "pathological apoptosis"? J Biol Chem. 2001;276:10191–10198. doi: 10.1074/jbc.M007807200. [DOI] [PubMed] [Google Scholar]
- Bracken MB, Collins WF, Freeman DF, et al. Efficacy of methylprednisolone in acute spinal cord injury. JAMA. 1984;251:45–52. [PubMed] [Google Scholar]
- Chaovipoch P, Jelks KA, Gerhold LM, West EJ, Chongthammakun S, Floyd CL. 17beta-estradiol is protective in spinal cord injury in post- and pre-menopausal rats. J Neurotrauma. 2006;23:830–852. doi: 10.1089/neu.2006.23.830. [DOI] [PubMed] [Google Scholar]
- Chen JQ, Delannoy M, Cooke C, Yager JD. Mitochondrial localization of ERalpha and ERbeta in human MCF7 cells. Am J Physiol-Endoc Metabolism. 2004;286:E1011–E1022. doi: 10.1152/ajpendo.00508.2003. [DOI] [PubMed] [Google Scholar]
- Cox A, Varma A, Barry J, Vertegel A, Banik N. Nanoparticle Estrogen in Rat Spinal Cord Injury Elicits Rapid Anti-Inflammatory Effects in Plasma, Cerebrospinal Fluid, and Tissue. J Neurotrauma. 2015 doi: 10.1089/neu.2014.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzzocrea S, Genovese T, Mazzon E, et al. Effect of 17beta-estradiol on signal transduction pathways and secondary damage in experimental spinal cord trauma. Shock. 2008;29:362–371. doi: 10.1097/shk.0b013e31814545dc. [DOI] [PubMed] [Google Scholar]
- Das A, Smith JA, Gibson C, Varma AK, Ray SK, Banik NL. Estrogen receptor agonists and estrogen attenuate TNF-alpha-induced apoptosis in VSC4.1 motoneurons. J Endocrin. 2011;208:171–182. doi: 10.1677/JOE-10-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis S, Aldrich TH, Jones PF, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Shughrue PJ, Wilson ME, Merchenthaler I, Wise PM. Estradiol modulates bcl-2 in cerebral ischemia: a potential role for estrogen receptors. J Neurosci. 1999;19:6385–6393. doi: 10.1523/JNEUROSCI.19-15-06385.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci U S A. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckles SP, Krause DN. Vascular and endothelial function: Role of gonadal steroids in neuronal and vaascular plasticity. In: Maiese K, editor. Neuronal and Vascular Plasticity : Elucidating Basic Cellular Mechanisms for Future Therapeutic Discovery. New York: Kluwer Press; 2003a. pp. 95–115. [Google Scholar]
- Duckles SP, Krause DN. Vascular and endothelial function: Role of gonadal steroids in neuronal and vascular plasticity. In: Maiese K, editor. Neuronal and Vascular Plasticity : Elucidating Basic Cellular Mechanisms for Future Therapeutic Discovery. New York: Kluwer Press; 2003b. pp. 95–115. [Google Scholar]
- Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB, Dumont AS. Acute spinal cord injury, part I: Pathophysiologic mechanisms. Clin Neuropharmacol. 2001;24:254–264. doi: 10.1097/00002826-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Elkabes S, Nicot AB. Sex steroids and neuroprotection in spinal cord injury: a review of preclinical investigations. Exp Neurol. 2014;259:28–37. doi: 10.1016/j.expneurol.2014.01.008. [DOI] [PubMed] [Google Scholar]
- Gao G, Dou QP. N-terminal cleavage of bax by calpain generates a potent proapoptotic 18-kDa fragment that promotes bcl-2-independent cytochrome C release and apoptotic cell death. J Cell Biochem. 2000;80:53–72. doi: 10.1002/1097-4644(20010101)80:1<53::aid-jcb60>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Gonzalez SL, Labombarda F, Gonzalez Deniselle MC, Mougel A, Guennoun R, Schumacher M, De Nicola AF. Progesterone neuroprotection in spinal cord trauma involves up-regulation of brain-derived neurotrophic factor in motoneurons. J Steroid Biochem Mol Biol. 2005;94:143–149. doi: 10.1016/j.jsbmb.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Gonzalez SL, Lopez-Costa JJ, Labombarda F, Gonzalez Deniselle MC, Guennoun R, Schumacher M, De Nicola AF. Progesterone effects on neuronal ultrastructure and expression of microtubule-associated protein 2 (MAP2) in rats with acute spinal cord injury. Cell Molec Neurobiol. 2009;29:27–39. doi: 10.1007/s10571-008-9291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton MK, Sribnick EA, Ray SK, Banik NL. A role for calpain in optic neuritis. Ann N Y Acad Sci. 2005;1053:48–54. doi: 10.1196/annals.1344.005. [DOI] [PubMed] [Google Scholar]
- Honda K, Shimohama S, Sawada H, Kihara T, Nakamizo T, Shibasaki H, Akaike A. Nongenomic antiapoptotic signal transduction by estrogen in cultured cortical neurons. J Neurosci Res. 2001;64:466–475. doi: 10.1002/jnr.1098. [DOI] [PubMed] [Google Scholar]
- Hu R, Sun H, Zhang Q, et al. G-protein coupled estrogen receptor 1 mediated estrogenic neuroprotection against spinal cord injury. Critical care medicine. 2012;40:3230–3237. doi: 10.1097/CCM.0b013e3182657560. [DOI] [PubMed] [Google Scholar]
- Hurlbert RJ. Methylprednisolone for acute spinal cord injury: an inappropriate standard of care. J Neurosurg. 2000;93:1–7. doi: 10.3171/spi.2000.93.1.0001. [DOI] [PubMed] [Google Scholar]
- Kachadroka S, Hall AM, Niedzielko TL, Chongthammakun S, Floyd CL. Effect of endogenous androgens on 17beta-estradiol-mediated protection after spinal cord injury in male rats. J Neurotrauma. 2010;27:611–626. doi: 10.1089/neu.2009.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon BK, Okon E, Hillyer J, Mann C, Baptiste D, Weaver LC, Fehlings MG, Tetzlaff W. A systematic review of non-invasive pharmacologic neuroprotective treatments for acute spinal cord injury. J Neurotrauma. 2011;28:1545–1588. doi: 10.1089/neu.2009.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Choi SY, Oh TH, Yune TY. 17beta-Estradiol inhibits apoptotic cell death of oligodendrocytes by inhibiting RhoA-JNK3 activation after spinal cord injury. Endocrinology. 2012;153:3815–3827. doi: 10.1210/en.2012-1068. [DOI] [PubMed] [Google Scholar]
- Lynch G, Baudry M. The biochemistry of memory: a new and specific hypothesis. Science. 1984;224:1057–1063. doi: 10.1126/science.6144182. [DOI] [PubMed] [Google Scholar]
- Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women's Health Initiative randomized trials. JAMA. 2013;310:1353–1368. doi: 10.1001/jama.2013.278040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli C, Garofalo C, Bartucci M, Surmacz E. Estrogen receptor-alpha regulates the degradation of insulin receptor substrates 1 and 2 in breast cancer cells. Oncogene. 2003;22:4007–4016. doi: 10.1038/sj.onc.1206436. [DOI] [PubMed] [Google Scholar]
- Mustonen T, Alitalo K. Endothelial receptor tyrosine kinases involved in angiogenesis. J Cell Biol. 1995;129:895–898. doi: 10.1083/jcb.129.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson HD, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy: scientific review. JAMA. 2002;288:872–881. doi: 10.1001/jama.288.7.872. [DOI] [PubMed] [Google Scholar]
- Nilsen J, Chen S, Brinton RD. Dual action of estrogen on glutamate-induced calcium signaling: mechanisms requiring interaction between estrogen receptors and src/mitogen activated protein kinase pathway. Brain Res. 2002;930:216–234. doi: 10.1016/s0006-8993(02)02254-0. [DOI] [PubMed] [Google Scholar]
- Perot PL, Jr, Lee WA, Hsu CY, Hogan EL, Cox RD, Gross AJ. Therapeutic model for experimental spinal cord injury in the rat: I. Mortality and motor deficit. Central Nervous System Trauma : Journal of the American Paralysis Association. 1987;4:149–159. doi: 10.1089/cns.1987.4.149. [DOI] [PubMed] [Google Scholar]
- Popovich PG, Wei P, Stokes BT. Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats. J Comp Neurol. 1997;377:443–464. doi: 10.1002/(sici)1096-9861(19970120)377:3<443::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Posmantur R, Kampfl A, Siman R, Liu J, Zhao X, Clifton GL, Hayes RL. A calpain inhibitor attenuates cortical cytoskeletal protein loss after experimental traumatic brain injury in the rat. Neuroscience. 1997;77:875–888. doi: 10.1016/s0306-4522(96)00483-6. [DOI] [PubMed] [Google Scholar]
- Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasley LE, Reusch JE. Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem. 2000;275:10761–10766. doi: 10.1074/jbc.275.15.10761. [DOI] [PubMed] [Google Scholar]
- Ray SK, Banik NL. Calpain and its involvement in the pathophysiology of CNS injuries and diseases: therapeutic potential of calpain inhibitors for prevention of neurodegeneration. Curr Drug Targets CNS Neurol Disord. 2003;2:173–189. doi: 10.2174/1568007033482887. [DOI] [PubMed] [Google Scholar]
- Ray SK, Hogan EL, Banik NL. Calpain in the pathophysiology of spinal cord injury: neuroprotection with calpain inhibitors. Brain Res Brain Res Rev. 2003;42:169–185. doi: 10.1016/s0165-0173(03)00152-8. [DOI] [PubMed] [Google Scholar]
- Ritz MF, Hausmann ON. Effect of 17beta-estradiol on functional outcome, release of cytokines, astrocyte reactivity and inflammatory spreading after spinal cord injury in male rats. Brain Res. 2008;1203:177–188. doi: 10.1016/j.brainres.2008.01.091. [DOI] [PubMed] [Google Scholar]
- Roof RL, Hall ED. Estrogen-related gender difference in survival rate and cortical blood flow after impact-acceleration head injury in rats. J Neurotrauma. 2000;17:1155–1169. doi: 10.1089/neu.2000.17.1155. [DOI] [PubMed] [Google Scholar]
- Saatman KE, Creed J, Raghupathi R. Calpain as a therapeutic target in traumatic brain injury. Neurotherapeutics. 2010;7:31–42. doi: 10.1016/j.nurt.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai M, Nagata T, Abe K, Horinouchi T, Itoyama Y, Tabayashi K. Oxidative damage and reduction of redox factor-1 expression after transient spinal cord ischemia in rabbits. J Vasc Surg. 2003;37:446–452. doi: 10.1067/mva.2003.100. [DOI] [PubMed] [Google Scholar]
- Samantaray S, Knaryan VH, Guyton MK, Matzelle DD, Ray SK, Banik NL. The parkinsonian neurotoxin rotenone activates calpain and caspase-3 leading to motoneuron degeneration in spinal cord of Lewis rats. Neuroscience. 2007;146:741–755. doi: 10.1016/j.neuroscience.2007.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samantaray S, Knaryan VH, Shields DC, Banik NL. Critical role of calpain in spinal cord degeneration in Parkinson's disease. J Neurochem. 2013;127:880–890. doi: 10.1111/jnc.12374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samantaray S, Smith JA, Das A, Matzelle DD, Varma AK, Ray SK, Banik NL. Low dose estrogen prevents neuronal degeneration and microglial reactivity in an acute model of spinal cord injury: effect of dosing, route of administration, and therapy delay. Neurochem Res. 2011;36:1809–1816. doi: 10.1007/s11064-011-0498-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samantaray S, Sribnick EA, Das A, Knaryan VH, Matzelle DD, Yallapragada AV, Reiter RJ, Ray SK, Banik NL. Melatonin attenuates calpain upregulation, axonal damage and neuronal death in spinal cord injury in rats. J Pineal Res. 2008;44:348–357. doi: 10.1111/j.1600-079X.2007.00534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samantaray S, Sribnick EA, Das A, Thakore NP, Matzelle D, Yu SP, Ray SK, Wei L, Banik NL. Neuroprotective efficacy of estrogen in experimental spinal cord injury in rats. Ann N Y Acad Sci. 2010;1199:90–94. doi: 10.1111/j.1749-6632.2009.05357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields DC, Banik NL. Putative role of calpain in the pathophysiology of experimental optic neuritis. Exp Eye Res. 1998a;67:403–410. doi: 10.1006/exer.1998.0537. [DOI] [PubMed] [Google Scholar]
- Shields DC, Banik NL. Upregulation of calpain activity and expression in experimental allergic encephalomyelitis: a putative role for calpain in demyelination. Brain Res. 1998b;794:68–74. doi: 10.1016/s0006-8993(98)00193-0. [DOI] [PubMed] [Google Scholar]
- Shields DC, Schaecher KE, Hogan EL, Banik NL. Calpain activity and expression increased in activated glial and inflammatory cells in penumbra of spinal cord injury lesion. J Neurosci Res. 2000;61:146–150. doi: 10.1002/1097-4547(20000715)61:2<146::AID-JNR5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Shields DC, Schaecher KE, Saido TC, Banik NL. A putative mechanism of demyelination in multiple sclerosis by a proteolytic enzyme, calpain. Proc Natl Acad Sci U S A. 1999;96:11486–11491. doi: 10.1073/pnas.96.20.11486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siriphorn A, Dunham KA, Chompoopong S, Floyd CL. Postinjury administration of 17beta-estradiol induces protection in the gray and white matter with associated functional recovery after cervical spinal cord injury in male rats. J Comp Neurol. 2012;520:2630–2646. doi: 10.1002/cne.23056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sribnick EA, Del Re AM, Ray SK, Woodward JJ, Banik NL. Estrogen attenuates glutamate-induced cell death by inhibiting Ca2+ influx through L-type voltage-gated Ca2+ channels. Brain Res. 2009;1276:159–170. doi: 10.1016/j.brainres.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sribnick EA, Matzelle DD, Ray SK, Banik NL. Estrogen treatment of spinal cord injury attenuates calpain activation and apoptosis. J Neurosc Res. 2006;84:1064–1075. doi: 10.1002/jnr.21016. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Ray SK, Banik NL. Estrogen as a multi-active neuroprotective agent in traumatic injuries. Neurochem Res. 2004a;29:2007–2014. doi: 10.1007/s11064-004-6874-0. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Ray SK, Nowak MW, Li L, Banik NL. 17beta-estradiol attenuates glutamate-induced apoptosis and preserves electrophysiologic function in primary cortical neurons. J Neurosci Res. 2004b;76:688–696. doi: 10.1002/jnr.20124. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Samantaray S, Das A, Smith J, Matzelle DD, Ray SK, Banik NL. Postinjury estrogen treatment of chronic spinal cord injury improves locomotor function in rats. J Neurosci Res. 2010;88:1738–1750. doi: 10.1002/jnr.22337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sribnick EA, Wingrave JM, Matzelle DD, Ray SK, Banik NL. Estrogen as a neuroprotective agent in the treatment of spinal cord injury. Ann N Y Acad Sci. 2003;993:125–133. doi: 10.1111/j.1749-6632.2003.tb07521.x. discussion 159–160. [DOI] [PubMed] [Google Scholar]
- Sribnick EA, Wingrave JM, Matzelle DD, Wilford GG, Ray SK, Banik NL. Estrogen attenuated markers of inflammation and decreased lesion volume in acute spinal cord injury in rats. J Neurosc Res. 2005;82:283–293. doi: 10.1002/jnr.20622. [DOI] [PubMed] [Google Scholar]
- Stirone C, Chu Y, Sunday L, Duckles SP, Krause DN. 17 Beta-estradiol increases endothelial nitric oxide synthase mRNA copy number in cerebral blood vessels: quantification by real-time polymerase chain reaction. Eur J Pharmacol. 2003a;478:35–38. doi: 10.1016/j.ejphar.2003.08.037. [DOI] [PubMed] [Google Scholar]
- Stirone C, Duckles SP, Krause DN. Multiple forms of estrogen receptor-alpha in cerebral blood vessels: regulation by estrogen. Am J Phys-Endocrin M. 2003b;284:E184–E192. doi: 10.1152/ajpendo.00165.2002. [DOI] [PubMed] [Google Scholar]
- Sur P, Sribnick EA, Patel SJ, Ray SK, Banik NL. Dexamethasone decreases temozolomide-induced apoptosis in human gliobastoma T98G cells. Glia. 2005;50:160–167. doi: 10.1002/glia.20168. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Nockels RP, Pan HQ, Shaffrey CI, Chopp M. Progesterone is neuroprotective after acute experimental spinal cord trauma in rats. Spine. 1999;24:2134–2138. doi: 10.1097/00007632-199910150-00013. [DOI] [PubMed] [Google Scholar]
- Tiwari-Woodruff S, Morales LB, Lee R, Voskuhl RR. Differential neuroprotective and antiinflammatory effects of estrogen receptor (ER)alpha and ERbeta ligand treatment. Proc Natl Acad Sci U S A. 2007;104:14813–14818. doi: 10.1073/pnas.0703783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Keogh CL, Whitaker VR, Theus M, Hand Yu SP. Angiogenesis and stem cell transplantation as potential treatments of cerebral ischemic stroke. Pathophysiology. 2005;12:47–62. doi: 10.1016/j.pathophys.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Wingrave JM, Schaecher KE, Sribnick EA, Wilford GG, Ray SK, Hazen-Martin DJ, Hogan EL, Banik NL. Early induction of secondary injury factors causing activation of calpain and mitochondria-mediated neuronal apoptosis following spinal cord injury in rats. J Neurosci Res. 2003;73:95–104. doi: 10.1002/jnr.10607. [DOI] [PubMed] [Google Scholar]
- Wise PM. Estrogens and neuroprotection. Trends in endocrinology and metabolism: TEM. 2002;13:229–230. doi: 10.1016/s1043-2760(02)00611-2. [DOI] [PubMed] [Google Scholar]
- Wu HY, Tomizawa K, Oda Y, Wei FY, Lu YF, Matsushita M, Li ST, Moriwaki A, Matsui H. Critical role of calpain-mediated cleavage of calcineurin in excitotoxic neurodegeneration. J Biol Chem. 2004;279:4929–4940. doi: 10.1074/jbc.M309767200. [DOI] [PubMed] [Google Scholar]
- Xie LP, Fu WX, Jin C, Dong XY, Chen WF. Negative regulation of monocyte chemoattractant protein-1 gene expression by a mouse estrogen-enhanced transcript. Eur J Immunol. 2002;32:2837–2846. doi: 10.1002/1521-4141(2002010)32:10<2837::AID-IMMU2837>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Yang SH, Shi J, Day AL, Simpkins JW. Estradiol exerts neuroprotective effects when administered after ischemic insult. Stroke. 2000;31:745–749. doi: 10.1161/01.str.31.3.745. discussion 749–750. [DOI] [PubMed] [Google Scholar]
- Yune TY, Kim SJ, Lee SM, Lee YK, Oh YJ, Kim YC, Markelonis GJ, Oh TH. Systemic administration of 17beta-estradiol reduces apoptotic cell death and improves functional recovery following traumatic spinal cord injury in rats. J Neurotrauma. 2004;21:293–306. doi: 10.1089/089771504322972086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.