Abstract
Esmolol has been shown to improve postoperative pain and reduce opioid requirements. The aim of this systematic review was to evaluate the effect of perioperative esmolol as an adjunct on early postoperative pain intensity, recovery profile, and anesthetic requirement. Databases were searched for randomized placebo-controlled trials evaluating the effects of esmolol during general anesthesia. Primary outcomes were related to early postoperative pain whereas secondary outcomes were related to emergence time, postoperative nausea and vomiting, and intraoperative anesthetic requirement. Nineteen trials were identified involving 936 patients (esmolol = 470, placebo = 466). In esmolol group, numeric pain scores at rest in the immediate postoperative period were reduced by 1.16 (95% confidence interval [CI]: 1.97–0.35, I2 = 96.7%) out of 10. Opioid consumption was also decreased in the postanesthesia care unit compared with placebo, mean difference of 5.1 mg (95% CI: 7.0–3.2, I2 = 96.9%) morphine IV equivalents; a 69% reduction in opioid rescue dosing was noted (odds ratio [OR]: 0.31, 95% CI: 0.16–0.80, I2 = 0.0%). A 61% reduction in postoperative nausea and vomiting was also evident (OR: 0.39, 95% CI: 0.20–0.75, I2 = 60.7%). A reduction in propofol induction dose was noted in the esmolol group (mean difference: −0.53 mg/kg, 95% CI: −0.63–−0.44, I2 = 0.0%). A decrease in end-tidal desflurane equivalent (mean difference: 1.70%, 95% CI: −2.39–−1.02, I2 = 92.0%) and intraoperative opioid usage (fentanyl equivalent, mean difference: 440 μg, 95% CI: −637–−244, I2 = 99.6%) was observed in esmolol group. Esmolol had no effect on the emergence time. Perioperative esmolol as an adjunct may reduce postoperative pain intensity, opioid consumption, and postoperative nausea vomiting. Given the heterogeneity, larger clinical trials are warranted to confirm these findings.
Key words: Analgesia, esmolol, opioid sparing
Introduction
Esmolol is an ultra-short acting intravenous β-blocker having a rapid onset and offset of effect.1 It provides an unprecedented level of tolerability and safety in the perioperative setting.[2,3,4] The ability of esmolol to rapidly achieve steady-state β-blockade also makes it ideal to attenuate the adverse sympathetic hemodynamic effects of noxious stimuli such as endotracheal intubation, surgical incision, and extubation.[4,5] When used as an adjunct, it has been shown to improve the postoperative recovery by reducing postoperative pain intensity and intraoperative anesthetic and opioid requirements and preventing opioid-induced hyperalgesia.[6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25]
The mechanism of this synergistic effect is uncertain, but both pharmacokinetic and pharmacodynamics interactions with anesthetic drugs have been proposed.[20,26] A limited meta-analysis comparing perioperative esmolol to opioid showed a reduction in postoperative nausea and vomiting (PONV) and discharge time but no effect on postoperative opioid requirement.[3]
The primary aim of this systematic review and meta-analysis was to assess the efficacy of esmolol as an adjunct in reducing acute postoperative pain and opioid consumption. Our secondary aim was to assess its role on emergence from general anesthesia and intraoperative anesthetic requirements.
Material and Methods
Search strategy
We followed the recommendations of the PRISMA statement[27] in creating this review. We searched OVID MEDLINE (1980–February 2014), OVID EMBASE, EBSCO, CINAHL, and the Cochrane Register of Controlled Trials for randomized controlled trials (RCTs) that compared esmolol with placebo in adults undergoing general anesthesia. Databases were searched using the MeSH term “esmolol” used in conjunction with “pain scores,” “analgesia requirement,” “emergence time,” and “PONV.” The search further included a set of items using the esmolol set in conjunction with opioid drugs, including “morphine,” “fentanyl,” “remifentanil,” “oxycodone,” “alfentanil,” “pethidine,” and “sufentanil,” and a further set using the terms “propofol,” “isoflurane,” “desflurane,” “halothane,” and “sevoflurane.” No language restriction was used. Additional studies were searched using the bibliographies of relevant articles and related systematic reviews. The manufacturers of esmolol were contacted, but they reported no unpublished studies on file.
Study selection and validity scoring
RCTs comparing perioperative esmolol with placebo in all types of surgery, where at least one patient outcome or anesthetic variable such as pain scores, intraoperative and postoperative opioid consumption, emergence time, PONV, and anesthetic requirement was reported, were included in the meta-analysis. Trials investigating esmolol against opioids were excluded from the meta-analysis. Studies only reporting attenuation of hemodynamic responses to laryngoscopy or surgery[5] were excluded from the meta-analysis, but the reference lists were manually searched. Studies on the effect of esmolol on intraoperative arrhythmias, electroconvulsive therapy, intracranial pressure, bispectral index[28] attenuation, and cardiovascular morbidity and mortality were excluded, unless patient recovery or anesthetic data were also reported.
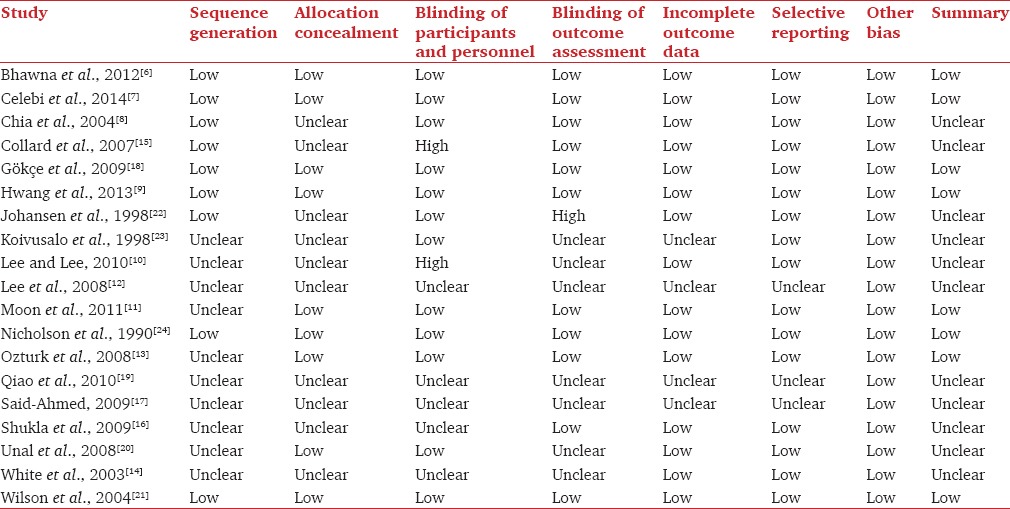
Three reviewers (Richard Watts, Venkatesan Thiruvenkatarajan, and Marni Calvert) assessed the methodological quality of the included studies using the Cochrane collaboration's tool for assessing the risk of bias in randomized trials.[29] Risk of bias was assessed under the following domains: random sequence generation, allocation concealment, blinding of participants, personnel and outcome measures, incomplete outcome data, selective reporting, and other biases.
Data extraction and definition of outcome parameters
Using a standardized form, data were extracted by three authors (Richard Watts, Marni Calvert, and Venkatesan Thiruvenkatarajan). The following demographic data were collected: mean age, mean weight, American Society of Anesthesiologists (ASA) status, type of surgery, and the total number of patients involved. Study characteristics documented included esmolol-loading dose, infusion rate and total dose, use of bispectral index scale (BIS), and use of nitrous oxide and antiemetic prophylaxis. Postoperative opioid usage, requirement of rescue opioid and anesthetics, and use of adjuvant analgesic agents were also recorded. Additional end points evaluated were esmolol-related adverse events, including hypotension and bradycardia requiring intervention. Data were originally extracted from text or tables. For data not available in table, figures, or graphs, the authors were contacted for clarification. If they did not respond, the data were extracted from graphs. Disagreements were resolved by consensus within the whole group.
Our primary outcomes were early (0–6 h) acute postoperative pain scores at rest, cumulative opioid consumption, and rescue analgesic administration. Secondary outcomes were emergence time, PONV, intraoperative anesthetic requirement, and adverse events such as bradycardia and hypotension. Trials reporting one or more of the primary or secondary outcomes were included in the study.
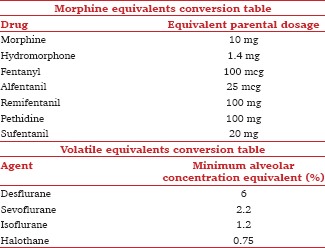
For pain intensity, visual analog score (VAS) or numeric rating scale (NRS) of pain at rest was converted to a 0–10 NRS. A single-value mean pain score reported earliest in the first 6 h after surgery was used. When there was no information available as to whether the pain scores were assessed at rest or movement, it was assumed that the scores were assessed at rest. Postoperative cumulative opioid consumption as reported by trials was converted to equianalgesic dose of intravenous morphine [Appendix 1].[30] Postoperative opioid rescue requirement was expressed as the number of patients requiring rescue opioid analgesics in the early postoperative (0–6 h) period.
Data on recovery profile included emergence time and PONV. Emergence time was recorded in minutes as the time taken from discontinuation of the anesthetic agent to the time at which the patient was oriented in time, person, and place, was awake and talking, or was providing an appropriate cognitive response. Definition of PONV was taken as reported in the original studies.
Propofol induction dose was recorded as mg/kg, and the end point was defined as either lack of response to command or loss of eyelash reflex as reported in the trials. Volatile usage was defined as the minimal alveolar concentration (MAC) required for anesthetic maintenance and converted to desflurane equivalence using a MAC equivalent conversion chart [Appendix 1].[31]
The loading dose of esmolol was recorded as actual (reported) or calculated to mean body weight, while the intraoperative infusion rate was actual or the dose range used (µg/kg/min). All titrated intraoperative opioids used were converted to equivalent doses of intravenous fentanyl [Appendix 1].
Statistical analysis
A meta-analysis was performed when two or more studies reported the end point of interest. The analyses were done with the statistical package R (The R Foundation for Statistical Computing, c/o Institute for Statistics and Mathematics, Wirtschaftsuniversitaet Wien, Welthandelsplatz 1, 1020 Vienna, Austria) and Metafor (Meta-Analysis Package for R).[32]
For continuous outcomes, means, standard deviations, and sample sizes were extracted for each of the randomized groups. Where studies reported medians and ranges, the mean was assumed to be equal to the median and the standard deviation was assumed to be equal to the range divided by four. In combining results across separate groups within a study, weighted means and pooled standard deviations were calculated. For binary outcomes, numerators and denominators were extracted for each of the randomized groups. The differences between randomized groups in continuous and binary outcomes were pooled across studies using random effects meta-analysis models.[33] The differences in means between groups were chosen as the effect measure of interest for continuous outcomes, while for binary outcomes, the odds ratio (OR) was used. Heterogeneity in mean differences and ORs was assessed using the I2 test[34] and Chi-square test goodness of fit tests. Evidence of publication bias was assessed visually using funnel plots. Fisher's exact test was used to compare the incidence of esmolol-related hypotension and bradycardia in placebo-controlled trials. P < 0.05 was considered statistically significant.
A meta-regression analysis was used in an attempt to explain heterogeneity observed in the studies reporting end-tidal volatile, intraoperative opioid, and postoperative opioid use by attributing the heterogeneity to a moderator variable or covariate. A mixed effects meta-regression analysis was used to determine whether the moderator variable, or the rate of esmolol infusion (fixed effect) influenced sparing of anesthetic. The adjusted R2 was calculated by fitting a meta-regression and a meta-analysis, and then the estimated τ2 values were compared.
Results
Description of included studies
Of the 338 studies identified, 19 RCTs were included in this review with a total of 936 participants, 470 receiving esmolol and 466 placebo [Figure 1]. The range of trial sample sizes was 28–97 participants [Table 1]. Among the included studies, 17 were available in English, and one each in Chinese and Korean.
Figure 1.
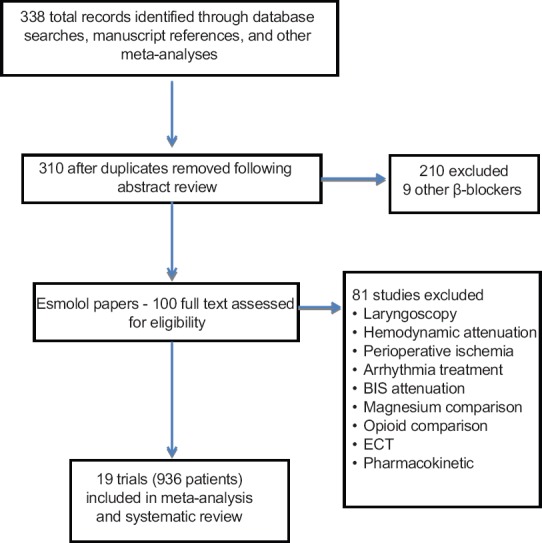
PRISMA flow chart detailing retrieved excluded assessed and included trials
Table 1.
Risk of bias assessment of the included studies
The ASA status of most patients in the includes studies was Class I or II, with the exception of two trials where ASA class III patients were included. Most of the studies recruited fit young or middle-aged patients with normal body weights. There were seven laparoscopic procedures and one cardiac procedure, and the rest included nonlaparoscopic abdominal, gynecological, and ear–nose–throat procedures.
A loading dose of esmolol followed by an infusion was used in 17 studies while the remaining two only used an infusion. The most common loading doses of esmolol were 0.5 or 1 mg/kg, given just prior to induction, while infusion rates varied between 5 and 500 µg/kg/min. The total esmolol dose ranged between 76 and 280 mg. Nitrous oxide use was reported in six studies and prophylactic antiemetics were administered in six trials [Table 1].
Risk of bias assessment
Only eight trials (42%) had a low risk of bias according to the Cochrane Risk of Bias Assessment tool [Table 2]. Methodological qualities were incompletely reported in many studies, making it difficult to assess the risk of bias within trials. Random sequence generation and allocation concealment were described in only nine trials. Similarly, participants and personnel were unblinded in ten studies and outcome assessment was unblinded in nine trials. Incomplete outcome data were not adequately addressed in four studies, and selective outcome reporting was noted in three studies [Table 2].
Table 2.
Trial characteristics
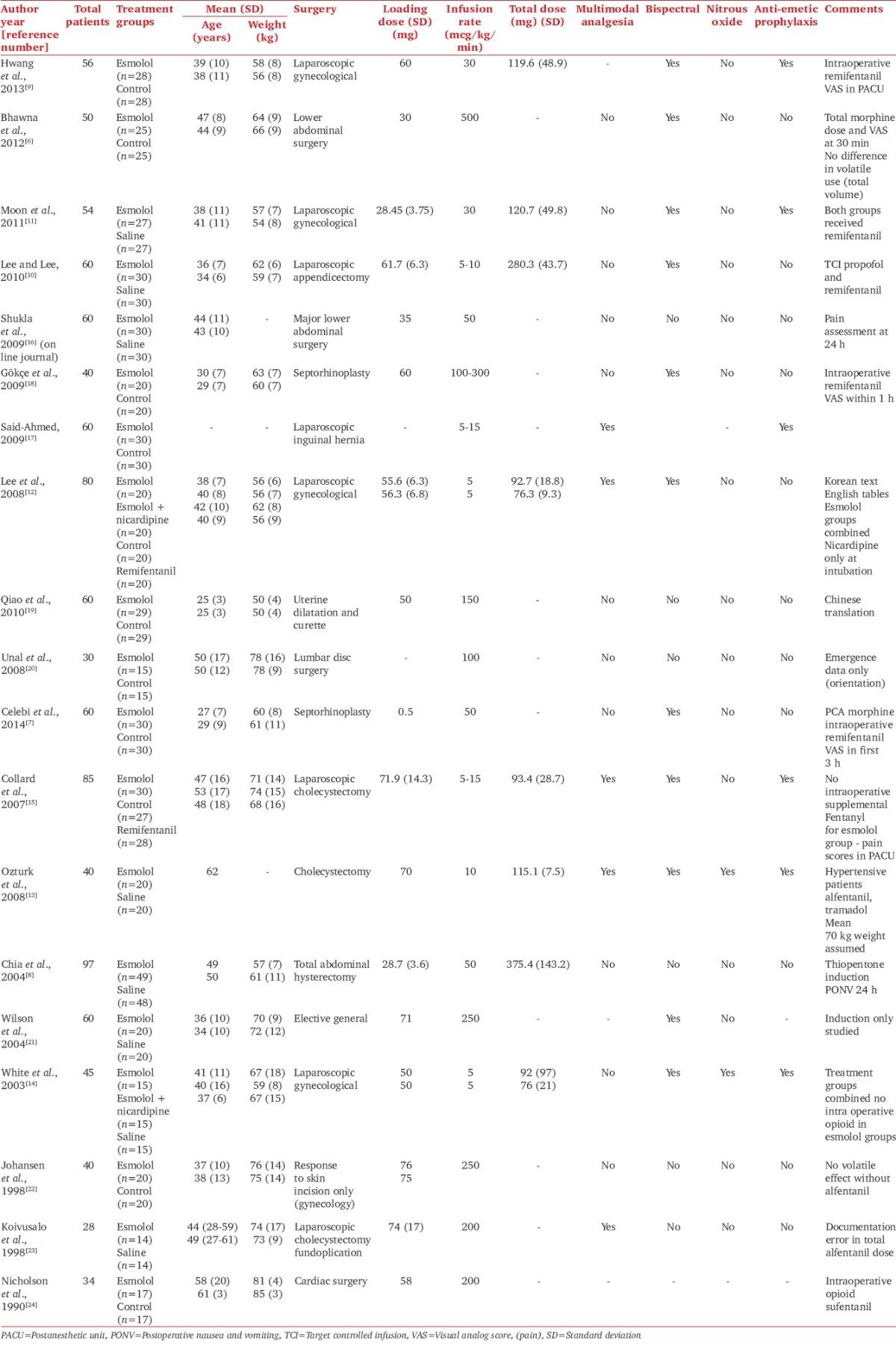
Primary outcomes
Early postoperative pain intensity
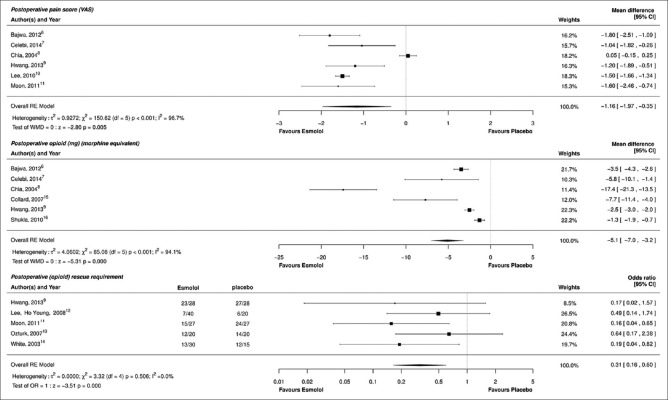
Six trials reported data on early postoperative pain intensity at different time points up to 6 h,[6,7,8,9,10,11] VAS was reported in four,[6,7,8,10] and NRS was reported in two studies.[9,11] In five studies, it was unclear as to whether the pain intensity was reported at rest or with movement.[6,7,9,10,11] Esmolol reduced postanesthesia care unit (PACU) pain scores by a mean of 1.16 (95% CI: −1.97–−0.35, P < 0.005). Heterogeneity was very high [I2 = 96.7%, [Figure 2]. None of the studies reported concomitant intraoperative administration of nonsteroidal anti-inflammatory agents.
Figure 2.
Forest plots for primary postoperative pain outcomes: Pain intensity, cumulative opioid consumption, and rescue analgesic requirement
Postoperative opioid consumption
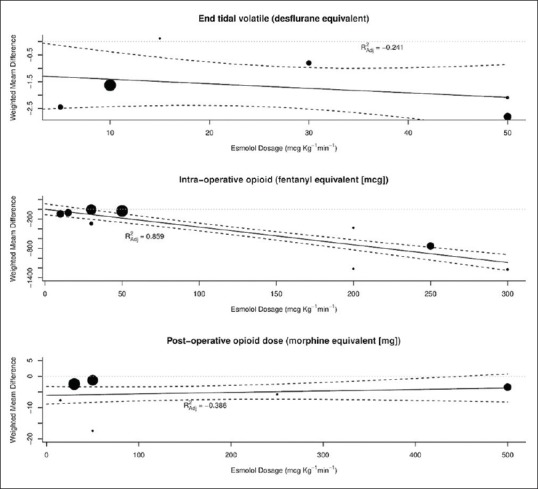
Six studies reported the cumulative consumption of a variety of opioids in the postoperative period from PACU until 3 days following the surgery.[6,7,8,9,15,16] The postoperative opioid dose in morphine equivalent was reduced by a mean of 5.1 mg (95% CI: −7.0–−3.2, P < 0.001). The studies were highly heterogeneous [I2 =96.9%, [Figure 2]. A meta-regression analysis revealed that the test of the moderator was not significant (P = 0.42), with residual heterogeneity remaining at 94.8%, suggesting that the esmolol infusion rate did not contribute to the heterogeneity observed for postoperative morphine dosing [Figure 3]. Similar amounts of opioids were used among the esmolol and the control groups in five studies;[6,7,8,9,16] multimodal analgesic technique was employed in only one study.[15]
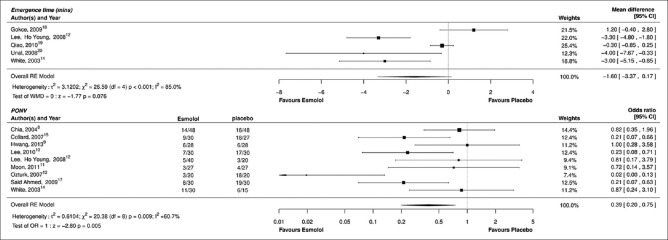
Figure 3.
Forest plot for secondary outcome: Postoperative nausea and vomiting and emergence time
Postoperative rescue opioid analgesic requirement
Five studies reported on the use of rescue analgesics in the PACU.[9,11,12,13,14] Intravenous fentanyl was used as the rescue analgesic in four studies and intravenous infusion of tramadol along with intramuscular diclofenac was administered in one study.[13] Overall, esmolol reduced the requirement for rescue opioid by 69% [OR: 0.31, 95% CI: 0.16–0.80, P = 0.0001, I2 = 0.0%, Figure 2]. Heterogeneity was low. All the included studies had equal usage of opioids in the esmolol and the control groups.
Secondary outcome
Postoperative nausea and vomiting
Dichotomous data on PONV could be extracted from nine studies.[8,9,10,11,12,13,14,15,17] Pooled results showed that there was a 61% reduction in the incidence of PONV in patients who received esmolol compared with placebo (OR: 0.39, 95% CI: 0.20–0.75; P = 0.005, I2 = 60.7%), however heterogeneity was high [Figure 4]. A single antiemetic was administered prophylactically in four studies (three at the end and one at induction)[9,11,15,17] and dual antiemetics were administered in two studies (one at the end and one as a premedication).[13,14] In three trials, antiemetics were used only as a rescue medication.[8,10,12]
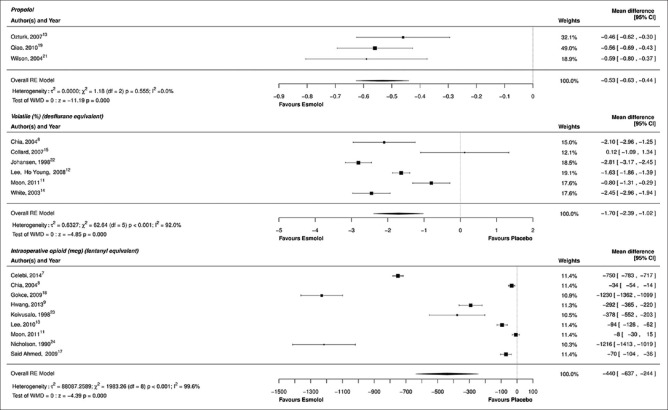
Figure 4.
Forest plot for secondary outcomes: Intraoperative propofol, volatile and opioid requirement
Emergence time
Five studies reported the emergence time, and most of them were based on spontaneous eye opening, ability to follow simple commands, and orientation to person and place.[12,14,18,19,20] The mean difference in emergence time between esmolol and placebo was 1.60 min (95% CI: −3.37–0.07, P = 0.07, I2 = 89.9%); heterogeneity was noted to be high [Figure 4].
Propofol requirement at induction
Three studies reported the effect of prior administration of esmolol on the induction doses of propofol.[13,19,21] When compared with placebo, esmolol reduced the propofol induction dose by a mean of 0.53 mg/kg [95% CI: −0.63–−0.44, P = 0.0001, I2 = 0.0%, Figure 5]. Heterogeneity was low. All the three studies used a preinduction dose of esmolol 1 mg/kg followed by an infusion with propofol titrated to the loss of eyelash reflex. While two studies used a bolus dose manually, one study[21] utilized a target controlled infusion.
Figure 5.
Meta-regression: Esmolol dose and opioid requirement
Volatile anesthetic requirement
Data on volatile anesthetic requirement were available in six studies.[8,11,12,14,15,22] Two studies provided data on sevoflurane maintenance as volume percentage,[11,12] desflurane usage was described in two studies as end-tidal concentrations and MAC,[14,15] and isoflurane concentrations were provided by two studies.[8,22] A loading dose followed by an infusion of esmolol was employed in all these studies. Overall, esmolol administration reduced the end-tidal desflurane equivalent by a mean of 1.70% [95% CI: −2.39–−1.02, P < 0.0001, I2 = 92.0%, Figure 5]. A meta-regression analysis failed to identify an association between the esmolol dosage and volatile anesthetic requirement in desflurane equivalents (P = 0.41); residual heterogeneity was 91.6% [Figure 3].
Intraoperative opioid requirement
Nine studies reported data on intraoperative opioid consumption.[7,8,9,10,11,17,18,23,24] Remifentanil was used in five studies,[7,9,10,11,18] fentanyl in two,[8,17] and alfentanil[23] and sufentanil[24] in one each. All studies except one[17] reported equal opioid use in all the patients. The majority of the studies (eight) did not report the use of concomitant analgesic adjuvants and nonsteroidal analgesic medications. In the esmolol group, intraoperative opioid use was reduced by a mean of 440 µg (fentanyl equivalents) [95% CI: −637–−244, P < 0.0001, I2 = 99.6%, Figure 5]. The larger dose effect could be attributed to two studies producing mean differences around −1200 µg, a septorhinoplasty trial and a cardiac study. A meta-regression analysis showed that the test of the moderator was significant (P < 0.001), suggesting that there is a linear relationship between the esmolol infusion dose rate and fentanyl-sparing effect (R2 = 0.859), and in part, this accounts for some heterogeneity [Figure 3]. However, residual heterogeneity remained high at 97.1%.
Adverse events
There were no documented esmolol-related serious adverse events (including awareness) in the studies reviewed. Varying definitions of bradycardia (heart rate [HR]: 40–60/min) and hypotension (mean arterial pressure 50–75 mmHg and systolic blood pressure <80 mmHg) were used in the studies in defining hemodynamic instability. Very few studies reported on the incidence of perioperative hypotension and bradycardia.[16,24] Adverse effects were not consistently reported across studies. In two studies, patients were excluded if they developed significant hypotension or bradycardia.[9,11] In patients receiving esmolol,[6,8,9,10,11,12,13,14,15,16,17,18,19,21,22,23,24,35] the incidence of bradycardia requiring intervention was higher (3%, 95% CI: 0.6–5.4%, P = 0.03), while the risk of hypotension requiring intervention was not increased (1%, 95% CI: 0.4–2.4%, P = 0.5).
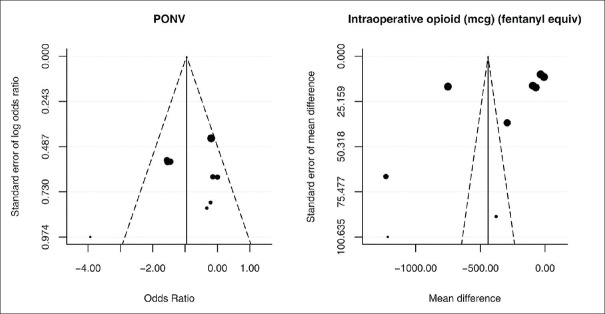
Assessment of publication bias
Funnel plots were used to assess the quality of the trials. However, because they are based on small number of studies, tests for asymmetry are not reported, but the plots can be visually inspected. Figure 6 is an example of a symmetrical plot for the effect of esmolol on PONV (I2 = 60.7%) and an asymmetrical plot for intraoperative opioid sparing (I2 = 99.6%). In the latter example, heterogeneity is a likely cause of asymmetry, rather than publication bias.
Figure 6.
Funnel plot assessing publication bias for outcome measures
Discussion
The main findings of this review and meta-analysis are that patients treated with an adjunct perioperative esmolol infusion had lower pain scores, reduced opioid rescue requirement, and less PONV. Esmolol also reduced the propofol induction dose, volatile anesthetic requirement, and intraoperative opioid dosing. There was no impact on the emergence time. The data were pooled from few, small low-powered studies.
Although a beneficial effect on postoperative pain intensity was perceived with esmolol, these observations can only be confirmed by larger trials, given the heterogeneity and high risk of bias of the included studies. A low heterogeneity was observed for esmolol in reducing the propofol requirement at induction. It would be interesting to investigate further whether a similar effect translates with propofol maintenance. A linear relationship was evident between the esmolol infusion and intraoperative fentanyl requirement. It has to be noted that the meta-regression analysis included data from two trials employing high-dose opioids.[7,24] Although our review indicated an anesthetic-sparing effect, esmolol did not reduce the emergence time. Similar results were produced by a recent systematic review that evaluated the safety of perioperative esmolol.[2]
The effect on PONV should be interpreted cautiously as antiemetic administration was inconsistent among the included studies. A similar effect on PONV was reported from an earlier systematic review on the safety of esmolol when esmolol was compared with opioids in attenuating the hemodynamic response to intubation and extubation.[3] The reduction in PONV that we observed is probably an indirect consequence of opioid and volatile-sparing effect, although there is some evidence that β-blockade may have a direct antiemetic effect.[36]
There are a number of theories as to how perioperative esmolol may have anti-nociceptive and anesthetic-sparing effects. Theoretically, esmolol has the potential to block noxious sensory response at various sites in the pathway, thus accounting for anesthetic-sparing effects and antinociception. Besides, a peripheral anti-inflammatory action-related antinociception has also been proposed.[37] Another theory of anesthetic-sparing effects relates to the reduction in cardiac output and hepatic blood flow associated with esmolol influencing the distribution of pharmacokinetics, metabolism, and clearance of propofol or volatile anesthetic agents.[38] Despite the experimental and clinical data relating to a possible antinociceptive effect, the current evidence favoring an inherent analgesic property of esmolol is very weak.[37,39,40]
The blunting of arousal and nociceptive transmission is suggested as a probable basis for anesthetic- and opioid-sparing effects.[41,42] This concept has raised concerns pertaining to BIS attenuation and “masking” of hemodynamic parameters resulting in “light anesthesia” and hence awareness with intraoperative esmolol administration.[26] Nonetheless, BIS was utilized in the studies evaluating the anesthetic-sparing effects of esmolol, and no changes in the anesthetic depth were reported.[37] BIS was not uniformly employed across the included trials.
Our review did not find any serious adverse events or detrimental hemodynamic consequences with perioperative esmolol use; the number of patients in this meta-analysis was low for this outcome. An increased risk of bradycardia without hypotension was evident with perioperative esmolol administration. A large systematic review of the safety of perioperative esmolol demonstrated no significant bradycardia. However, the combined incidence of unplanned hypotension after an esmolol bolus (0.5–4 mg/kg) and infusion (5–500 µg/kg/min) was significantly increased.[2] The authors showed that hypotension was dose-related and associated with a fixed esmolol-dosing schedule rather than titrating to HR and blood pressure. Interestingly, another smaller systematic review of the safety of perioperative esmolol (bolus 0.5–1.0 mg/kg followed by an infusion 100–300 µg/kg/min) demonstrated no significant increase in bradycardia or hypotension in noncardiac surgeries.[3] The authors commented that being an ultra-short-acting agent, esmolol causes reversible episodes of hypotension and bradycardia, thereby negating the concerns of a possible negative inotropic effect.[3]
A major limitation of our review is related to the wide variability of methodological qualities of included studies accounting for significant heterogeneity for all outcome measures except for pain scores and propofol requirement. Restrictions were not applied on the timing as well as the dosage of esmolol. A huge dose range was used across studies, and we could not find any studies describing a dose–response pilot study conducted prior to the RCT. Heterogeneity across studies including different surgical models and different dosage regimes may be inevitable.
Another significant limitation pertains to the high risk of bias with most of the trials. The smaller number of included studies and insufficient data precluded us in doing a sensitivity or subgroup analysis. Other limiting factors of this analysis include the lack of accurate anesthetic and opioid drug equivalence data and other potential confounders such as age, sex, monitoring with BIS and the use of nitrous oxide, multimodal pain therapy, and prophylactic antiemetics. The majority of patients in this evaluation were young and healthy, undergoing ambulatory laparoscopic surgery. This limits the wider applicability of the findings to other patient groups. Only few studies had reported the intraoperative use of nonopioid medications such as nonsteroidal anti-inflammatory agents[12,13,23] and paracetamol.[15] Hence, our results may have limitations when applied to the current anesthetic practice where multimodal agents are routinely employed.
Overall, adverse effects were poorly reported in the included studies. Failure to register our review protocol on a registry database of systematic reviews is a further limitation. There was a single cardiac study included in the review; however, only one outcome datum was extracted and analyzed (intraoperative opioid usage). In our view, the likelihood of this small size cardiac study influencing the overall outcome of the meta-analysis would be minimal.
Our review has several strengths. First, our search was systematic and extensive without language restriction including manual search. Our review presents the scope of our present understanding on the role of esmolol on perioperative pain, serving a rationale for future research.
Further studies are needed to establish the role of esmolol as an analgesic adjunct in patients receiving general anesthesia. There is a need to obtain more safety data about titrated perioperative esmolol dosing, particularly in patients at a higher risk of perioperative events. Trials in the future may explore this hypothesis in specific population groups such as bariatric surgery, morbid obesity, sleep apnea, and chronic pain.
Conclusion
This systematic review presents the evidence that perioperative administration of esmolol decreases early postoperative pain intensity, opioid requirement, the requirement of rescue analgesics, and PONV. In addition, there is evidence that esmolol can reduce the induction doses of propofol, volatile anesthetic maintenance, and intraoperative opioid requirement. Esmolol has no effect on the emergence time. Yet, these findings have to be interpreted with caution, as the included studies were largely heterogeneous apart from having a significant risk of bias. Further research through well-designed studies is needed to confirm our promising findings and to determine a safe and efficacious regimen.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Appendix 1: Opioid and volatile conversion
References
- 1.Wiest D. Esmolol. A review of its therapeutic efficacy and pharmacokinetic characteristics. Clin Pharmacokinet. 1995;28:190–202. doi: 10.2165/00003088-199528030-00002. [DOI] [PubMed] [Google Scholar]
- 2.Yu SK, Tait G, Karkouti K, Wijeysundera D, McCluskey S, Beattie WS. The safety of perioperative esmolol: A systematic review and meta-analysis of randomized controlled trials. Anesth Analg. 2011;112:267–81. doi: 10.1213/ANE.0b013e3182025af7. [DOI] [PubMed] [Google Scholar]
- 3.Landoni G, Turi S, Biondi-Zoccai G, Bignami E, Testa V, Belloni I, et al. Esmolol reduces perioperative ischemia in noncardiac surgery: A meta-analysis of randomized controlled studies. J Cardiothorac Vasc Anesth. 2010;24:219–29. doi: 10.1053/j.jvca.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Reilly CS, Wood M, Koshakji RP, Wood AJ. Ultra-short-acting beta-blockade: A comparison with conventional beta-blockade. Clin Pharmacol Ther. 1985;38:579–85. doi: 10.1038/clpt.1985.227. [DOI] [PubMed] [Google Scholar]
- 5.Figueredo E, Garcia-Fuentes EM. Assessment of the efficacy of esmolol on the haemodynamic changes induced by laryngoscopy and tracheal intubation: A meta-analysis. Acta Anaesthesiol Scand. 2001;45:1011–22. doi: 10.1034/j.1399-6576.2001.450815.x. [DOI] [PubMed] [Google Scholar]
- 6.Bhawna, Bajwa SJ, Lalitha K, Dhar P, Kumar V. Influence of esmolol on requirement of inhalational agent using entropy and assessment of its effect on immediate postoperative pain score. Indian J Anaesth. 2012;56:535–41. doi: 10.4103/0019-5049.104570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celebi N, Cizmeci EA, Canbay O. Intraoperative esmolol infusion reduces postoperative analgesic consumption and anaesthetic use during septorhinoplasty: A randomized trial. Rev Bras Anestesiol. 2014;64:343–9. doi: 10.1016/j.bjan.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 8.Chia YY, Chan MH, Ko NH, Liu K. Role of beta-blockade in anaesthesia and postoperative pain management after hysterectomy. Br J Anaesth. 2004;93:799–805. doi: 10.1093/bja/aeh268. [DOI] [PubMed] [Google Scholar]
- 9.Hwang WJ, Moon YE, Cho SJ, Lee J. The effect of a continuous infusion of low-dose esmolol on the requirement for remifentanil during laparoscopic gynecologic surgery. J Clin Anesth. 2013;25:36–41. doi: 10.1016/j.jclinane.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 10.Lee SJ, Lee JN. The effect of perioperative esmolol infusion on the postoperative nausea, vomiting and pain after laparoscopic appendectomy. Korean J Anesthesiol. 2010;59:179–84. doi: 10.4097/kjae.2010.59.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moon YE, Hwang WJ, Koh HJ, Min JY, Lee J. The sparing effect of low-dose esmolol on sevoflurane during laparoscopic gynaecological surgery. J Int Med Res. 2011;39:1861–9. doi: 10.1177/147323001103900529. [DOI] [PubMed] [Google Scholar]
- 12.Lee H, Kwon W, Lee J. The effects of esmolol, esmolol and nicardipine or remifentanil on mean blood pressure, heart rate and recovery in gynaecologic laparoscopic surgery. Korean J Anesthesiol. 2008;55:709–15. [Google Scholar]
- 13.Ozturk T, Kaya H, Aran G, Aksun M, Savaci S. Postoperative beneficial effects of esmolol in treated hypertensive patients undergoing laparoscopic cholecystectomy. Br J Anaesth. 2008;100:211–4. doi: 10.1093/bja/aem333. [DOI] [PubMed] [Google Scholar]
- 14.White PF, Wang B, Tang J, Wender RH, Naruse R, Sloninsky A. The effect of intraoperative use of esmolol and nicardipine on recovery after ambulatory surgery. Anesth Analg. 2003;97:1633–8. doi: 10.1213/01.ANE.0000085296.07006.BA. [DOI] [PubMed] [Google Scholar]
- 15.Collard V, Mistraletti G, Taqi A, Asenjo JF, Feldman LS, Fried GM, et al. Intraoperative esmolol infusion in the absence of opioids spares postoperative fentanyl in patients undergoing ambulatory laparoscopic cholecystectomy. Anesth Analg. 2007;105:1255–62. doi: 10.1213/01.ane.0000282822.07437.02. [DOI] [PubMed] [Google Scholar]
- 16.Shukla S, Gupta K, Gurha P, Sharma M, Sanjay R, Shukla R, et al. Role of beta blockade in anaesthesia and postoperative pain management after major lower abdominal surgery. Internet J Anesthesiol. 2009;25:1. [Google Scholar]
- 17.Said-Ahmed HA. The effect of intra-operative esmolol infusion on post-operative opioid consumption and incidence of shivering in patients undergoing ambulatory laparoscopic inguinal hernia repair. Acta Anaesth Ital. 2009;60:31–4. [Google Scholar]
- 18.Gökçe BM, Karabiyik L, Karadenizli Y. Hypotensive anesthesia with esmolol. Assessment of hemodynamics, consumption of anesthetic drugs, and recovery. Saudi Med J. 2009;30:771–7. [PubMed] [Google Scholar]
- 19.Qiao Q, Gu XJ, Xu J, Zhong TD. Effect of intravenous esmolol on BIS index and anesthesia emergence during sedation anesthesia for ambulatory surgery. Chin Med J. 2010;90:1631–4. [PubMed] [Google Scholar]
- 20.Unal G, Ozsoylar O, Sariguney D, Arslan M, Yardim RS. The efficacy of esmolol to blunt the haemodynamic response to endotracheal extubation in lumbar disc surgery. Res J Med Sci. 2008;2:99–104. [Google Scholar]
- 21.Wilson ES, McKinlay S, Crawford JM, Robb HM. The influence of esmolol on the dose of propofol required for induction of anaesthesia. Anaesthesia. 2004;59:122–6. doi: 10.1111/j.1365-2044.2004.03460.x. [DOI] [PubMed] [Google Scholar]
- 22.Johansen JW, Schneider G, Windsor AM, Sebel PS. Esmolol potentiates reduction of minimum alveolar isoflurane concentration by alfentanil. Anesth Analg. 1998;87:671–6. doi: 10.1097/00000539-199809000-00034. [DOI] [PubMed] [Google Scholar]
- 23.Koivusalo AM, Scheinin M, Tikkanen I, Yli-Suomu T, Ristkari S, Laakso J, et al. Effects of esmolol on haemodynamic response to CO2 pneumoperitoneum for laparoscopic surgery. Acta Anaesthesiol Scand. 1998;42:510–7. doi: 10.1111/j.1399-6576.1998.tb05159.x. [DOI] [PubMed] [Google Scholar]
- 24.Nicholson S, Jobes D, Quinlan J. Cardiovascular effects of esmolol in patients anaesthetized with sufentanil – Pancuronium for myocardial revascularization. J Cardiothorac Anesth. 1990;4:55–8. [Google Scholar]
- 25.Kim YH. The antinociceptive effect of esmolol. Korean J Anesthesiol. 2010;59:141–3. doi: 10.4097/kjae.2010.59.3.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang H, Fayad A. Are beta-blockers anesthetics? Can J Anaesth. 2003;50:627–30. doi: 10.1007/BF03018700. [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Berkenstadt H, Loebstein R, Faibishenko I, Halkin H, Keidan I, Perel A. Effect of a single dose of esmolol on the bispectral index scale (BIS) during propofol/fentanyl anaesthesia. Br J Anaesth. 2002;89:509–11. [PubMed] [Google Scholar]
- 29.Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gammaitoni AR, Fine P, Alvarez N, McPherson ML, Bergmark S. Clinical application of opioid equianalgesic data. Clin J Pain. 2003;19:286–97. doi: 10.1097/00002508-200309000-00002. [DOI] [PubMed] [Google Scholar]
- 31.Nickalls RW, Mapleson WW. Age-related iso-MAC charts for isoflurane, sevoflurane and desflurane in man. Br J Anaesth. 2003;91:170–4. doi: 10.1093/bja/aeg132. [DOI] [PubMed] [Google Scholar]
- 32.Viechtbauer W. Conducting meta-analysis in R with the metafor package. J Stat Softw. 2010;36:1–43. [Google Scholar]
- 33.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 34.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 35.Celebi N, Artukoglu F, Dal D, Saricaoglu F, Celiker V, Aypar U. Effect of hypotensive anesthesia on cognitive functions. A comparison of esmolol and remifentanil during tympanoplasty. Saudi Med J. 2007;28:1357–61. [PubMed] [Google Scholar]
- 36.Gupta YK, Chaudhary G. Effect of antiemetic drugs on decrease in gastric emptying in experimental model of motion sickness in rats. Acta Pharmacol Sin. 2003;24:296–300. [PubMed] [Google Scholar]
- 37.Kadoi Y, Saito S. Possible indications of beta-blockers in the perioperative period other than prevention of cardiac ischemia. J Anesth. 2010;24:81–95. doi: 10.1007/s00540-009-0865-x. [DOI] [PubMed] [Google Scholar]
- 38.Takeda S, Masuda R, Kanazawa T, Tomaru T. Esmolol attenuates hepatic blood flow responses during sodium nitroprusside-induced hypotension in dogs. Can J Anaesth. 2004;51:348–53. doi: 10.1007/BF03018238. [DOI] [PubMed] [Google Scholar]
- 39.Johansen JW, Flaishon R, Sebel PS. Esmolol reduces anesthetic requirement for skin incision during propofol/nitrous oxide/morphine anesthesia. Anesthesiology. 1997;86:364–71. doi: 10.1097/00000542-199702000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Oda Y, Nishikawa K, Hase I, Asada A. The short-acting beta1-adrenoceptor antagonists esmolol and landiolol suppress the bispectral index response to tracheal intubation during sevoflurane anesthesia. Anesth Analg. 2005;100:733–7. doi: 10.1213/01.ANE.0000154441.22654.11. [DOI] [PubMed] [Google Scholar]
- 41.Menigaux C, Guignard B, Adam F, Sessler DI, Joly V, Chauvin M. Esmolol prevents movement and attenuates the BIS response to orotracheal intubation. Br J Anaesth. 2002;89:857–62. doi: 10.1093/bja/aef275. [DOI] [PubMed] [Google Scholar]
- 42.Johansen JW. Esmolol promotes electroencephalographic burst suppression during propofol/alfentanil anesthesia. Anesth Analg. 2001;93:1526–31. doi: 10.1097/00000539-200112000-00039. [DOI] [PubMed] [Google Scholar]