Abstract
Trauma-induced coagulopathy (TIC) is a recently described condition which traditionally has been diagnosed by the common coagulation tests (CCTs) such as prothrombin time/international normalized ratio (PT/INR), activated partial thromboplastin time (aPTT), platelet count, and fibrinogen levels. The varying sensitivity and specificity of these CCTs have led trauma coagulation researchers and clinicians to use Viscoelastic Tests (VET) such as Thromboelastography (TEG) to provide Targeted Thromboelastographic Hemostatic and Adjunctive Therapy (TTHAT) in a goal directed fashion to those trauma patients in need of hemostatic resuscitation. This review describes the utility of VETs, in particular, TEG, to provide TTHAT in trauma and acquired non-trauma-induced coagulopathy.
Keywords: Thromboelastography, point-of-care, acquired coagulopathy, blood component therapy, systemic hemostatic agents, trauma-induced coagulopathy, hemostatic resuscitation, tranexamic acid, targeted pharmacologic therapy
1. Trauma Induced Coagulopathy and Acquired Coagulopathy
1.1. Introduction
Coagulopathy is found in approximately 25% of severely injured trauma patients on admission to the emergency department (ED). Patients with Trauma-Induced Coagulopathy (TIC) are at a higher risk for increased transfusion requirements and death compared to those without TIC [1-5]. The etiology of TIC has been a matter of speculation. Trauma induced disturbances of compensatory activation of activated protein C (APC), hypofibrinogenemia, Tissue Factor (TF) release, coagulation factor consumption and dilution, platelet dysfunction, and fibrinolysis have been cited as possible causes of TIC [1-9]. In addition, it has been argued by Gando and others that TIC is a variant of disseminated intravascular coagulation [10, 11]. Most recently, Dobson et al. have described the etiology of TIC in relation to four paradigms of hemostatic derangement which are: 1) the DIC/ consumption/ fibrinolysis hypothesis 2) the activated protein-C hypothesis 3) the glycocalyx hypothesis and 4) the “fibrinogen-centric” hypothesis. These hypotheses are not mutually exclusive. It is necessary to refer to this theoretical aspect of
TIC in order to understand the significance of VETs as an ex vivo manifestation of the fine balance between anti-coagulant, prothrombotic, fibrinolytic pathways, endothelium, and circulating platelets whose function is uniquely manifest with the VETs as opposed to the single point indications of clot potential as measured by the prothrombin time and activated partial thromboplastin time [12].
As an understanding of TIC increases, the limitations of conventional coagulation tests (CCTs) such as prothrombin time/international normalized ratio (PT/INR), activated partial thromboplastin time (aPTT), platelet counts, and fibrinogen levels have become more evident. These tests were not designed for assessment of hemostatic integrity in the preoperative period and they have been shown to lack accuracy in trauma settings. This has led to increased investigations into point-of-care (POC) viscoelastic tests (VETs) [13-18]. Recently, it has been suggested that Thromboelastography (TEG) may replace CCTs for the guidance of Blood Component Therapy (BCT) in the trauma population [19]. In addition, the interest of the POC VETs in TIC has led to their use to guide BCT in acquired coagulopathy [13-19].
CCTs are based on the coagulation cascade model of hemostasis and provide only a static evaluation of clot formation [18, 19]. The cell-based theory of hemostasis, unlike the coagulation cascade concept of hemostasis, describes the mechanism of thrombus formation as successive steps of initiation, amplification, propagation, and termination through fibrinolysis. These steps, which reflect the cell-based theory of hemostasis, are best delineated by POC testing, which includes VETs [18-20].
CCTs’ inconsistent sensitivities and specificities not only fail to identify deficiencies in coagulation factors, fibrinogen and fibrin, but also do not describe platelet function and fibrinolysis in the setting of TIC. CCTs also take a much longer time to perform and provide less pertinent data about coagulopathy when compared to VETs [14-19]. VETs provide a rapid and accurate assessment of clot formation, stability, and firmness, which allows for individualized treatment of patients with TIC [19-23].
Guidance of BCT and the administration of adjunct hemostatic agents (AHA) for trauma patients has taken on new importance given the large number of elderly trauma patients who are taking some form of anticoagulant prior to trauma. The increased use of warfarin and antiplatelet agents among the elderly will result in more patients involved in trauma requiring some form of Targeted Thromboelastographic Hemostatic and Adjunctive Therapy (TTHAT) for trauma resuscitation [24-30].
Finally, the emergence of new oral direct and indirect thrombin inhibitors (dabigatran, rivaroxaban, apixaban, edoxaban) is promising and more patients are now on these agents. However, the safety of these drugs in the trauma patient remains a matter of continued scrutiny [19, 31-33].
In this setting of novel oral anti-coagulants (NOAC) and insensitive and nonspecific CCTs, TEG is a unique tool that can assist in guiding reversal of anticoagulants and hemostatic agents for patients who have significant hemorrhage related to trauma while on anticoagulants [19, 22, 32, 34-36].
We conducted a review using computer database literature searches which was performed using the indexed online database MEDLINE/ PubMed. Lists of cited literature within relevant articles were also screened. The goal of the bibliographic methodology of this review was to identify prospective randomized controlled trials (RCTs) and non-RCTs, existing systematic reviews and guidelines. In addition, relevant, case-control studies, observational studies, and case reports were considered. This paper focuses on the TEG which is used primarily in the United States.
1.2. History
In 1948, Hellmut Hartert illustrated hemostatic function for whole blood samples using TEG [37, 38]. TEG was then used to guide BCT during the Vietnam War [39]. In the 1980s, BCT for liver transplant surgery was made more efficient by TEG [40].
Subsequently, a similar reduction in blood component use was described for cardiac surgery patients, which confirmed the TEG-guided blood product reduction of the liver transplant a decade earlier [41, 42]. By the late 1990s, TEG had also been used to provide targeted BCT in patients who require resuscitation in trauma [43].
Evidence from European and United States combat and civilian data demonstrated the utility of VETs in providing targeted BCT in a goal directed fashion in the military as well as the civilian population [14, 19, 44-48].
Subsequent studies have confirmed the value of VETs in targeting BCT for trauma patients who require blood component resuscitation as well as adjunct hemostatic agent resuscitation with Prothrombin Complex Concentrate (PCC), fibrinogen concentrate, activated recombinant Factor VIIa (rFVIIa), and the antifibrinolytic tranexamic acid (TXA) [49-59].
1.3. VETs’ Methodology and Interpretation of Results
TEG demonstrates hemostatic integrity and measures the ability of whole blood samples to form a clot. This is performed by placing an aliquot of 0.36 mL of whole blood into a cup that has been pre-warmed to 37°C. A pin, attached by a wire to a transducer, is suspended in the sample of whole blood. The cup rotates around the pin in the TEG autoanalyzer at an angle of 4.45 degrees every 10 seconds. As the clot forms, the pin and the cup are joined by the formation of the clot. This causes the pin and the cup to rotate together. The subsequent change in tension mediated by the pin attached to the cup is detected by a transducer. A graphical output is then plotted as change in strength, measured in millimeters, on the y-axis over time, measured in minutes, on the x-axis [22, 46, 47] (Fig. 1).
Fig. (1).
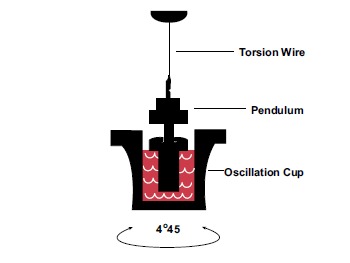
Schematic of TEG with 0.36 mL aliquot of whole blood in cup with pin attached to torsion wire measuring pin synchronization with the cup, reflecting the stages of clot formation.
TEG provides continuous monitoring of the clotting process from its steps of initiation, amplification, propagation, and termination through fibrinolysis, generating parameters and producing values related to each step [22, 44, 46, 47, 60].
The “R” value of TEG represents the first step of clot formation. The “R” is a measure of “reaction time” denoting the amount of time it takes the blood to begin forming a clot and to move the pin 2 mm on the “y” axis. It indicates the initiation phase of enzymatic clotting factor activation, also known as the fluid phase of coagulation, which correlates with INR and PTT. The “K” represents the time to move the pin to 20 mm on the y-axis. The slope of the curve caused by that movement to 20 mm on the y-axis is called the “alpha (α)-angle.” A software system calculates this angle between the slope and the aforementioned axis of time. It has been noted that the TEG system calculates, by default, the “α-angle” as the angle formed by the tangential line to the curve starting from the split point of the trace [61]. “K” and “α-angle” are interpreted correlatively and reflect clot kinetics or the rate of clot formation. Both the “K” and the “α-angle” denote the rate at which the clot strengthens and are most representative of thrombin cleaving available fibrinogen into fibrin. The maximum height of the curve is called the Maximum Amplitude (MA), representing maximum clot strength, which is the result of the maximal fibrin-platelet interaction. [44, 46]. The final stage, “termination,” begins with the fibrinolytic induced dissolution of the fibrin-platelet bond between the pin and the cup. This dissolution is manifested by the tracing returning to baseline. The percentage return to baseline of the total MA at 30 minutes after the MA was reached is described as the lysis at 30 minutes (LY30) [22, 46, 47, 60, 62]. Although this paper concerns itself with the application of TEG to guide blood component, pharmacologic, and hemostatic adjuncts in traumatic and acquired coagulopathy, it is important to note that the rotational thromboelastometry (ROTEM), which is more frequently used in Europe, possesses a similar ability to predict BCT use in patients with traumatic and acquired coagulopathy [63]. The principles of ROTEM are the same as those of the TEG except that the pin is supported by a ball-bearing and rotates through an angle 4.75 degrees. The subsequent curve is similar to the TEG curve, although the terminology is different [64-66]. The results of the TEG/ ROTEM systems vary depending on the activation mode or assay (Fig. 2; Table 1).
Fig. (2).
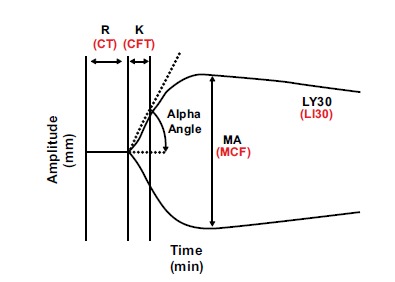
Basic TEG/ROTEM parameters at 30 minutes after MA/MCF. TEG in black and ROTEM in red.
Table 1.
Correlative normal parameters of the TEG and ROTEM with coagulation function measured for each parameter [67].
| Parameter | TEG® | ROTEM® | |
|---|---|---|---|
| Clotting time (2 mm amplitude) | R (reaction time) Normal (citrate/ kaolin) = 3-8 min |
CT (clotting time) Normal (EXTEM) = 42-74 s Normal (EXTEM) = 137-246 s |
|
|
Clot formation/ kinetics (20 mm amplitude) |
K (kinetics) Normal (citrate/kaolin) = 1-3 min |
CFT (clot formation time) Normal (EXTEM) = 46-148 s Normal (INTEM) 40-100 s |
|
|
Clot strengthening (angle of clot formation) |
Alpha angle (slope between R and K points) Normal (citrate/kaolin) = 55-78º |
Alpha angle (slope of tangent at 2 mm amplitude) Normal (EXTEM) = 63-81º Normal (INTEM) = 71-82º |
|
| Amplitude/ maximal firmness | MA (maximal amplitude) Normal (citrate/kaolin) = 51-69 mm |
MCF (maximum clot firmness) Normal (EXTEM) = 49-71 mm Normal (INTEM) = 52-72 mm Normal (FIBTEM) = 9-25 mm A5, A10, etc.-amplitudes at dedicated time points predicting the final clot firmness |
|
| Lysis | LY30, CL30, CL60, CL | LI30, LI60, ML | |
| Test | Activator/ Inhibitor | Description | |
| TEG® Tests | |||
| Kaolin TEG | Kaolin | Test of “intrinsic pathway” | |
| RapidTEG | Kaolin + tissue factor | Test of both “intrinsic and extrinsic pathways” | |
| Functional fibrinogen | Kaolin + GpIIb/ IIIa inhibition | Test of fibrin net polymerization after platelet inhibition | |
| ROTEM® Tests | |||
| EXTEM | Tissue factor | Test of “extrinsic pathway” - fastest clot analysis | |
| INTEM | Elegiac acid | Test of “intrinsic pathway” | |
| FIBTEM | Tissue factor + platelet inhibitor | Test of fibrin net polymerization after inactivation of platelets | |
| APTEM | Tissue factor + aprotinin | Test of fibrinolysis | |
The standard-kaolin TEG reflects partial platelet function when only 5% of thrombin is produced in the clot. Complete platelet function can be studied using TEG via the method of modified thromboelastography with platelet mapping (TEG/ PM), which measures the ability of the platelet agonists thromboxane A2 to activate arachidonic acid (AA) and adenosine diphosphate (ADP) to stimulate a fibrin-platelet clot independent of thrombin [68] (Fig. 3). The recent standard protocol for those who use the ROTEM is to add multiple (multiplate) electrode aggregometry (MEA) to test for platelet function [6].
Fig. (3).
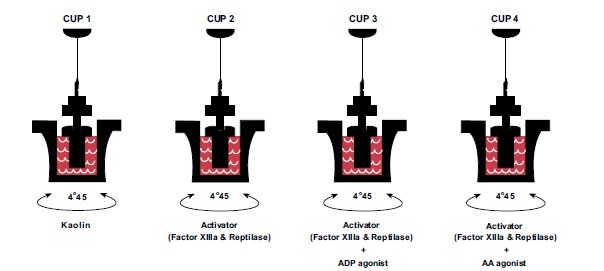
Cup 1 is a standard-kaolin TEG that represents baseline clotting. Heparin is added to cups 2-4 in order to neutralize thrombin, while factor XIIIa and reptilase stimulate isolated fibrinogen formation so that the ADP and AA receptors in cups 3 and 4 platelet can be activated by ADP and AA respectively to create an isolated fibrinogen-platelet clot.
Finally, functional fibrinogen can be measured using a glycoprotein IIb/IIIa inhibitor, abciximab. This allows assessment of clot strength under platelet inhibition and the calculation of the percentage contribution of fibrinogen and platelets to the clot in patients with traumatic coagulopathy [69].
The TEG Functional Fibrinogen assay measures the fibrinogen that contributes to the structure and strength of the clot. Platelet function is blocked by an inhibitor, abciximab, and the resulting maximal amplitude of functional fibrinogen (MAFF) reflects the fibrin component of the clot [69] (Fig. 4). The ROTEM has an equivalent test to MAFF called FIBTEM which uses cytochalsin D to inhibit platelets rather than abciximab [70, 71]. Darlington and colleagues showed correlation of platelet count, α angle and Clauss fibrinogen concentration [72]. Harr and colleagues showed that MA was correlated significantly to both platelet count and, Clauss fibrinogen concentration [69]. The Functional Fibrinogen assay is needed to identify whether treatment with fibrinogen or platelets is needed when a low value of MA is observed with standard kaolin TEG.
Fig. (4).
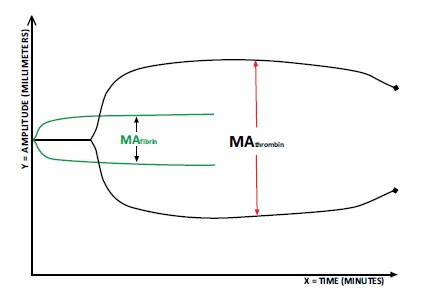
The standard-kaolin TEG provides the baseline maximum amplitude (MA) which is compared to the MA of a second cup that contains abciximab. This results in the assessment of clot strength under platelet inhibition, which is represented by the green MA.
1.4. Protocols for TEG Targeted Blood Component And Hemostatic Therapy in Multiple Trauma
On the TEG tracing, prolongation of “R” reflects coagulation factor deficiency, and it indicates the need for administration of fresh frozen plasma (FFP) and/or PCC. A long “K” and a low or flat α-angle correspond to a deficiency in fibrinogen and/or fibrin production or function. It indicates the need for treatment with cryoprecipitate or fibrinogen concentrate. A narrow MA reflects a lack of clot formation mediated by the fibrin-platelet mesh. It demonstrates the need for treatment with platelets. Finally, an increase in LY30% reflects fibrinolysis, which is treated with an antifibrinolytic agent [19, 22, 43-48, 54, 73, 74]. The relative contribution of fibrinogen and platelets as related to the α angle has been recently delineated by a number of publications. For example, Solomon et al. state that the α angle reflects the combined effects of platelets and fibrinogen to clot formation [61]. Ellis and colleagues described in 2007 that the α angle is a reflection of the speed with which clot strength is increasing and the MA is the combined contribution of platelets and fibrin to clot strength [75] (Fig. 5, Table 2). Similar algorithms with different nomenclature exist for ROTEM. The ROTEM is more commonly used in Europe and the TEG is more frequently utilized in the United States. In the United States as opposed to Europe, the TEG without platelet mapping and without functional fibrinogen is the most common VET used to determine POC clot integrity. As a result, published guidelines in the United States utilize the α angle as a temporary bedside surrogate for evaluating the need for fibrinogen replacement [19, 22, 46-48, 70, 71, 76-78]. Solomon et al. have most recently clarified the best test for assessing the need for fibrinogen and platelets. When available, the most accurate initial assessment for plasma fibrinogen contribution to clot strength would be the maximum amplitude functional fibrinogen (MAFF) on the TEG or the FIBTEM maximum clot firmness (MCF) on the ROTEM when combined with some form of platelet functionality [61, 79].
Fig. (5).
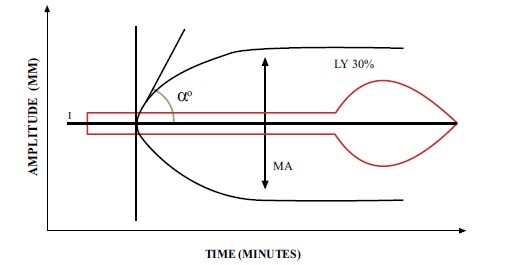
Normal TEG tracing (in black) resembles a wide flat (non-functional) shovel with a short handle. The superimposed “shovel” (in red) demonstrates a tracing with a prolonged R, flat α-angle, small MA and increased LY30%, indicative of a systemic coagulopathy with fibrinolysis. *Some recommend LY30% >3% as threshold for anti-fibrinolytic agent [19,74].
Table 2.
Fibrinogen concentrate may be substituted for cryoprecipitate and the use of rFVIIa is controversial and, is therefore, not included. *MAFF/FIBTEM MCF are more accurate guides for fibrinogen supplementation, however, the availability of MAFF/FIBTEM MCF is not universal [61, 79].
| Recommendations Based on Abnormal TEG Tracing 1,7 | |
|---|---|
| Potential Therapeutic Intervention | Significant Finding on “Standard” TEG Tracing |
| Plasma and/or prothrombin complex concentrate | Prolonged R-value (>7 minutes) |
| Cryoprecipitate/fibrinogen concentrate | Low or flat α angle (<45), MAFF* |
| Platelets+/Cryoprecipitate/fibrinogen concentrate | Narrow MA (<48 mm) |
| Anti-fibrinolytic agent | Increased LY30 (>7.5%)* |
Human clinical experience has shown that quality assurance and local standardization variability is a concern [73]. For example, clinical correlation with industry standardized values, such as a higher LY30% of 7.5% for kaolin TEG, is associated with more morbidity as well as in those patients with an LY30% of greater than 3% with RapidTEG [19, 74] (Fig. 5, Table 2).
2. Application of VETS in Trauma Patients
2.1. Primary TIC
Multiple studies have been conducted on VET-guided resuscitation of trauma patients using goal directed BCT [22, 47-54, 74]. VETs, including standard kaolin TEG and RapidTEG, can detect early changes in coagulation and may be used to predict which patients will progress to hemorrhagic shock or will need a massive transfusion (defined as 10 units of packed red blood cells [PRBC] within 6-24 hours) [47, 54, 80, 81]. This proves to be highly important when initiating early BCT on trauma patients in guiding the proper ratios of PRBC, FFP, platelets, cryoprecipitate, and AHA to these patients.
2.2. Damage Control Resuscitation (DCR) Guided By TEG For Multiple Trauma
2.1.1. Packed Red Blood Cells (PRBC)/Fresh Frozen Plasma
Experience from the Iraq War was published in May 2005 at an international expert conference at the United States Army Institute of Surgical Research. This research proposed the concept of Damage Control Resuscitation (DCR) for the management of patients with massive hemorrhage using a 1:1:1 ratio of PRBC to platelets to FFP [82-84]. This 1:1:1 ratio was chosen based on how closely this combination resembles whole blood [81, 83, 84]. However, the optimum ratio of blood components remains an object of continued study. Large clinical trials have been published and are underway to determine the optimal physiologic ratios of blood products that can be given to the trauma population [85, 86]. The Updated European Guidelines for the management of bleeding and coagulopathy in major trauma are quoted verbatim for clarity: “the initial administration of plasma (fresh frozen plasma (FFP) or pathogen-inactivated plasma) (Grade 1B) or fibrinogen (Grade 1C) in patients with massive bleeding. If further plasma is administered, we suggest an optimal plasma: red blood cell ratio of at least 1:2. (Grade 2C) We recommend that plasma transfusion be avoided in patients without substantial bleeding (Grade 1B).” [54].
2.2.2. VETs Guidance of Blood Component Therapy and Procoagulant Hemostatic Agents
VETs may be used to anticipate the need for BCT or procoagulant hemostatic agents such as PCC, rFVIIa, fibrinogen concentrate, and TXA in hypocoagulable patients with high Injury Severity Scores (ISS) and coagulopathic VET tracings. It has been proposed that the coagulopathy of trauma reflected by VETs finds its etiology in part due to progressive, catecholamine-induced endothelial activation, which is reflected by the severity of the trauma as manifested by the ISS [11]. In moderately traumatized patients, there is a subset of hypercoagulable patients that can be identified by TEG [11]. These patients, who may potentially benefit from deep vein thrombosis (DVT) prophylaxis, can be identified by TEG [87, 88].
Targeted Thromboelastographic Hemostatic and Adjunctive Therapy (TTHAT) can provide BCT and AHA in an individualized, adaptable, and protocol-driven fashion which responds to the immediate needs of the bleeding trauma patient. This protocolized therapy minimizes the overuse and waste of blood products and AHA, instead of a “blindly” fixed ratio therapy [19, 22, 44-54, 62, 81, 89, 90].
The limited use of blood components and AHA is important because excessive use of these products can lead to complications such as an venous thromboembolism (VTE), Transfusion Related Acute Lung Injury (TRALI), Transfusion Associated Cardiac Overload (TACO), acute respiratory distress syndrome (ARDS), infections, allergic reactions, and thrombotic complications [54, 91-99].
2.2.3. Platelets
2.2.3.1. Primary Platelet Dysfunction in Trauma As Determined By the Modified TEG With Platelet Mapping
The etiology of platelet dysfunction in TIC has recently been established [6, 8]. TIC has been found to be a consequence of severe hypotensive multiple traumas and/or isolated Traumatic Brain Injury (TBI), and is correlated with increased rates of injury severity. In hypotensive multiple trauma patients without TBI, base deficit and platelet dysfunction as represented by TEG/PM, predict the presence of coagulopathy and the need for blood component transfusion [8]. However, in the isolated TBI patient, TIC is not dependent on tissue hypoperfusion as in patients with multiple trauma without TBI [100]. In addition, the recently described thrombocytopathy of TBI correlates with a greater severity as measured by the Glasgow Coma Scale (GCS) [6, 8, 101-107].
Therefore, different types of injury such as hypotensive non-traumatic brain injury versus isolated non-hypotensive TBI may require different strategies of blood component and adjunctive hemostatic resuscitation. This requires POC testing devices such as TEG/PM that can provide information on the individual trauma patient’s platelet function [8].
Isolated TBI causes intracerebral, epidural, and subdural hemorrhage leading to morbidity. The progression of cerebral contusions reflects the severity of TBI. This progression is known as hemorrhagic progression of a contusion (HPC) after TBI and can be explained by two mechanisms. The first mechanism is the coagulopathy caused by immediate and delayed bleeding from fractured microvessels during the initial injury. The second mechanism proposes that the initial microvascular injury in the penumbra does not cause a fracture of vessels, but results in a series of maladaptive molecular events, causing further structural damage of the microvasculature. The differentiation between these mechanisms is important because the therapies counteract each other. For the coagulopathy associated with initial injury caused by microvasculature fracture, the goal should be to correct coagulopathy. For subsequent brain hemorrhage caused by maladaptive molecular events at the penumbra, the therapy should be aimed at reversing the maladaptive events in the microcirculation [107-110]. The coagulopathy could be a combination of both hypo- and hypercoagulative states, potentially regulated by the extent of injury, eventually resulting in life threatening ischemic and/or hemorrhagic events [106, 108-110].
Intracerebral hemorrhages, either spontaneous (sICH) or traumatic (tICH), often expand over time. An association between hemorrhage expansion and clinical outcomes has been described for sICH. The role of hemostatic agents in the treatment of patients with ICH, whether spontaneous or associated with trauma, is a matter of current basic scientific and clinical research [108, 109, 111, 112]. Reduced platelet activity correlates with early hemorrhage growth and reduced survival for patients with sICH. Increasing platelet activity through platelet transfusion reduces hemorrhage volume growth in those patients with sICH [111, 112]. Treatment with platelets improves mortality in this group of patients with sICH [112]. A similar primary platelet dysfunction has been described in the TBI animal model as well as in the human with TBI induced ICH and is an area of vigorous and ongoing research [6, 8, 102-107].
Guided platelet transfusion in patients with ICH due to TBI is of importance because the brain’s microcirculatory penumbra is more sensitive than the extra cerebral microcirculation to thrombosis associated with trauma. This further emphasizes the importance of a targeted approach to the administration of procoagulants to patients with TBI. Because of the competing interests of the TBI brain’s microcirculatory penumbra, it seems rational to guide platelet transfusion based on reliable and reproducible tests for platelet dysfunction. Since there is evidence as mentioned above of reduced platelet activity that correlates with early hemorrhage growth and reduced survival, and that increasing platelet activity through platelet transfusion reduces hemorrhage volume growth, it seems reasonable that future clinical research will confirm the utility of the TEG/PM to predict platelet dysfunction for those patients who have TBI with and without associated antiplatelet therapy [101, 103-107, 110].
2.2.4. Platelet Transfusion Guidelines Not Dependent On Platelet Function Testing
The updated 2013 European Guidelines recommend the administration of platelets for counts below 100,000 in patients with multiple trauma, massive bleeding, and TBI by treatment with a single donor apheresis pack (SDAP) [54]. Normal platelet counts are present in the majority of trauma patients. The platelet count does not identify platelet dysfunction caused by severe trauma or pre-trauma antiplatelet medication use and therefore is an insufficient marker for platelet transfusion requirements in trauma [8, 102-106]. Improved survival rate among resuscitated trauma patients receiving a high platelet to PRBC ratio has been reported in retrospective and observational studies. These studies may have been influenced by confounding factors such as survivor bias. The optimal ratio of platelet to PRBC transfusion remains elusive [113-121]. TEG/PM may help guide the traumatologist in determining those patients who need platelets in severe trauma [8, 107]. Recently, the Pragmatic, Randomized Optimal Platelet and Plasma Ratios (PROPPR) trial by Holcomb et al. proposed the concept of “platelet first” strategy after the second round of PRBC/platelets/FFP in a fixed ratio 1:1:1 damage control resuscitation for hemorrhagic shock. Forty-five percent of the PROPPR patients received massive transfusion using this strategy. The rationale for this “platelet first” strategy may be in part due to the recently recognized platelet dysfunction as an early manifestation of TIC and the observed increased platelet: RBC ratios associated with the reduction in death by exsanguination and the improved time to hemostasis in the 1:1:1 group in the PROPPR trial [6, 8, 86, 122].
In order to properly gauge the need for platelet administration there also should be an assessment of fibrinogen functionality that is a assayed by the MAFF or FIBTEM MCF as noted above in order to demonstrate the relative contributions of platelet and fibrin/fibrinogen to clot strength [61, 79].
2.2.5. Cryoprecipitate
Cryoprecipitate has been recommended for significant bleeding that is accompanied by thromboelastographic signs of functional fibrinogen deficit or a plasma fibrinogen level of less than 1.5-2.0 g/L. The dose of cryoprecipitate is 50 mg/kg or approximately 15-20 single donor units. Repeat doses may be guided by VETs monitoring and laboratory assessment of fibrinogen levels [49, 54, 64]. Such monitoring by VETs and Clauss of determining fibrinogen levels is a matter of intense clinical research. We have described above that, when available, the MAFF/FIBTEM MCF are the most accurate assays for determining the need of fibrinogen replacement. It has been shown that the administration of cryoprecipitate and TXA may improve the survival in seriously injured patients requiring transfusion. The effect of cryoprecipitate appears to be additive to that of TXA, suggesting that the repletion of fibrinogen may be as important as preventing its degradation in this setting [123].
2.3. Importance of Early Monitoring of TIC
Early monitoring of TIC by POC VETs and CCTs is necessary to identify specific abnormalities of initiation, amplification, propagation, and termination by fibrinolysis in this group of patients with severe hemorrhage [124, 125]. The early coagulopathy of trauma that occurs in the hypoperfused patient manifests with an arterial base deficit greater than 6 mEq/L [5, 7, 126]. Such early diagnosis of TIC leads to early intervention with improvement of CCTs with subsequent reduction in the need for PRBC, FFP, and platelets [14, 19, 22, 23, 44-54, 123-131]. Early diagnosis of TIC and the administration of goal directed or predefined ratios of blood products leads to more efficient use of blood components, AHA such as fibrinogen concentrate and PCC with less multi-organ failure [50, 81, 89, 132, 133].
3. The Utility of TEG in Guiding BCT for Patients on Anticoagulants
3.1. TEG in NSAIDS and Antiplatelet Drugs
Platelet dysfunction is a much more common phenomenon than originally believed in the trauma population [6, 8, 102, 103, 105, 107]. A significant percentage of patients with sICH have reduced platelet activity without a history of antiplatelet agent use [134]. Inhibition of platelet function has also been described in patients with multiple trauma and severe TBI not taking antiplatelet agents [6, 8, 103, 107]. This suggests that traumatologists cannot rely on history alone, but in addition they may need a test to determine platelet function in trauma patients because of the aforementioned association with platelet dysfunction in trauma. The standard kaolin TEG and RapidTEGs (rTEG) do not provide information regarding isolated platelets function. TEG/PM, however, does test for platelet function at the AA and ADP receptors, which have been shown to be abnormal in patients on AA and ADP antagonists such as aspirin, clopidogrel, and the newer anti-P2Y12 inhibitors [135-138]. It has been proposed that the early dysfunction of platelets associated with Multiple Trauma and Traumatic Brain Injury (TBI) may offer therapeutic opportunities similar to those for patients on platelet inhibitors who require cardiac surgery [138, 139]. One area of interest is the association of platelet dysfunction and ICH whether spontaneous or iatrogenic.
There are few data to guide platelet transfusion for those patients with platelet dysfunction associated with ICH while on antiplatelet agents. The potential benefit of platelet transfusions in patients with sICH or tICH who are on pre-injury antiplatelet agents remains an object of current clinical research. Nevertheless, published algorithms exist for the reversal of platelet dysfunction for those patients with TBI and ICH that use varying types of platelet function assays [140]. The benefit of the modified TEG/PM is that with one test, the patient’s entire coagulation status can be assayed, including platelet function. No other VET is able to reproduce this [6, 8, 68, 103, 107].
3.2. TEG Anticoagulants and Herbal Products
3.2.1. Warfarin, Heparin and TEG
Industry standard normal values for the R-value or “reaction time” of TEG correlate with significant changes in INR. Reversal of warfarin by rFVIIa causes normalization of the TEG tracing. Further research is needed to determine the TEG's ability to monitor warfarin [141, 142].1 However, routine monitoring of warfarin therapy by TEG is not indicated at this time, although it may be used as an adjunct to INR in the guidance of reversal of warfarin induced coagulopathy in patients with trauma who require immediate blood product resuscitation [19].
While the TEG does not correlate with the prothrombin time for patients on warfarin, it has been used with success in treating patients with unfractionated heparin [40, 41].
3.2.2. Direct and Indirect Thrombin Inhibitors
Although routine monitoring of the direct and indirect thrombin inhibitor agents are not currently routinely performed, a POC VET would give a better understanding and direction of treatment for trauma patients using these agents [143].
Dabigatran, rivaroxaban, apixaban, and edoxaban cause lower rates of severe hemorrhage and intracranial bleeding events than warfarin [33, 144, 145]. Many reversal strategies have been suggested which involve the use of PCC, rFVIIA, and TXA in varying sequences and doses; however, this has not yet been standardized in the literature [31, 143, 146-159]. Bleeding events involving these agents will continue to be a clinical challenge until satisfactory monitoring tests and reversal strategies are found [158]. For dabigatran-associated bleeding, preliminary clinical data reveals that kaolin TEG and RapidTEG with or without ecarin can be used as a method of measuring the anticoagulant activity of dabigatran and quantifying reversibility [34-36, 67, 159-162].
Anti-factor Xa levels have been recognized as the definitive test for determining the degree of factor Xa inhibition and to aid in the prediction of those patients who will require DVT prophylaxis. However, the anti-factor Xa assay is not immediately available at most trauma centers. In contrast, TEG is a functional POC assay that can be collected and completed within 30-50 minutes. For prediction of the hypercoagulable patient in need of DVT prophylaxis, on the other hand, TEG may be more useful than anti-factor Xa levels in assessing critically ill patients on anti-factor Xa inhibitors [88].
Very recently, the FDA has approved of the use of idarucizumab, a monoclonal antibody specific for the dabigatran molecule as an antidote. Similar antibodies are in process for requesting FDA approval for reversal of Xa inhibitors known as Andexanet Alpha (ANNEXA-A for Apixaban and ANNEXA-R for Rivaroxaban). It is probable that the TEG/ ROTEM will be utilized in determining efficacy of these antidotes since the thrombin time and the ecarin clotting time which can quantify coagulation with the NOACs are not universally available. The RapidTEG activated clotting time test and the kaolin test appear to be capable of detecting and monitoring NOACs. The kaolin TEG ecarin test may be used to differentiate between Xa inhibitors and direct thrombin inhibitors. Therefore, TEG may be a valuable tool to investigate hemostasis and the effectiveness of reversal strategies for patients receiving NOACs in the future [162-165].
3.2.3. Heparin and Low Molecular Weight Heparin
For many years, reversal of heparin with protamine in cardiac surgery has been guided by TEG. TEG is also useful in diagnosis and treatment of heparin induced coagulopathy [41, 42]. The TEG has been used to document anti-Xa efficacy of anti-Xa inhibitors in small studies and detect low molecular weight heparin activity [162].
3.2.4. Herbal Products That Cause Anticoagulation
Many herbal agents, such as Saw Palmetto, can magnify the effect of anticoagulation medications or even cause anticoagulation [166, 167]. For a bleeding trauma patient in the ED with an unexplained thrombocytopathy, the surreptitious use of herbal agents should be considered if platelet dysfunction is detected by TEG/PM [167].
4. Reversal of TIC with Hemostatic Agents: The Utility of TEG
4.1. Tranexamic Acid (TXA) and Fibrinolysis
The Clinical Randomisation of Anti-fibrinolytic in Severe Hemorrhage trial (CRASH-2), a 2010 study published in “The Lancet” by Shakur et al., found that the use of antifibrinolytic agent TXA was a safe treatment for trauma patients [168]. This is an important benchmark study for the future use of the procoagulant, hemostatic, antifibrinolytic agent, TXA, to treat TIC. However, this large randomized controlled trial treated a large number of patients who may not have required antifibrinolytic treatment. The lack of clinical criteria to define significant fibrinolysis in trauma patients was another limitation in this study [169].
The definition of clinically significant fibrinolysis in the trauma patient has varied [19, 21, 22, 169]. In the literature published by the manufacturer of TEG (Haemonetics), 7.5% fibrinolysis is thought be an “abnormal” amount of fibrinolysis by measure of VETs, and antifibrinolytics have been recommended for this population [18]. However, levels of fibrinolysis as defined by an LY30% of 3-15% have been proposed as pathologic in trauma [69, 170, 171]. To date, there is no clinical definition of pathologic fibrinolysis. There are ongoing studies searching for acceptable clinical surrogates for fibrinolysis that might guide the use of antifibrinolytics in the setting of trauma. Currently, there is experimental work regarding the ability of plasmin-antiplasmin (PAP) levels to predict clinically significant fibrinolysis [170].
The CRASH-2 trial was the first randomized, placebo-controlled trial to evaluate the effects of antifibrinolytic TXA in trauma patients [168]. A more selective population of patients requiring blood transfusions was evaluated in the Military Application of Tranexamic Acid in Trauma Emergency Resuscitation (MATTERs) Study trial. This trial evaluated 896 patients in southern Afghanistan to look at the effectiveness of TXA in patients needing massive transfusion. The MATTERs study showed the effectiveness of TXA in decreasing mortality and the numbers of necessary blood products in the massively transfused trauma patient [169, 172]. TXA seems to exert its best effects when administered within the first 3 hours. The MATTERs study showed that rates of Pulmonary Embolism (PE) and DVT among patients who received TXA were 9 and 12 times higher, respectively, than those who did not [92, 169, 173].
In MATTERs, VETs were also used to guide BCT. Since the effect of TXA in the trauma population may be associated with increased risk of DVT, as noted in the MATTERs trial, it seems reasonable to use the TEG definition of fibrinolysis when guiding the use of TXA. In fact, the following guidelines for the use of TXA in trauma resuscitation of adult trauma patients have been proposed: severe hemorrhagic shock (SBP < 75 mm Hg) or known predictors of fibrinolysis detected by TEG (LY30% > 3%). LY30% and Lysis Index at 30 minute values from 3% to 30% for the VETs have been noted in the literature as the threshold diagnosis for fibrinolysis for patients receiving BCT and AHA. Currently, there is no consensus regarding the level of fibrinolysis that indicates administration of TXA [62, 64, 169, 174-177].
Significant questions remain regarding the indications for the near ubiquitous use of TXA in trauma resuscitation as recommended by the CRASH-2 trial. For this reason, there has been a substantial delay in the implementation of TXA in trauma protocols [169, 173].
Professor Martin Schreiber MD, the military trauma surgeon, was the first to question the CRASH-2 trial and its findings at the 2010 NIH Conference section on the Current Practice of Medicine for Severe Bleeding stating, “I think the bottom line here is that we really haven’t found a way to beat that qualified trauma surgeon with the $0.50 silk suture. There are no magic bullets when it comes to drugs for stopping bleeding”.2
Building on his concern, further criticism of the CRASH-2 trial findings and methodology was initiated by the Department of Defense (DoD) Priority trial into TXA, coauthored by Dr. Ken Mattox among others, where the term “knowledge gap” was first introduced to describe the inconsistencies of the CRASH-2 trial [173]. Immediately following in June 2013, Napolitano et al.’s paper in the Journal of Trauma delineated several major problems of the CRASH-2 trial [169]. The major flaws pointed out were the “difficult to believe” 100% follow-up of 20,000 patients among 274 hospitals in 40 low-to-moderate income countries, the lack of looking for and reporting of complications, and the absence of TXA’s mechanism of action as the survival benefit was not associated with transfusion [169].
Four major Randomized Controlled Trial (RCT) studies were launched to address and investigate these shortcomings. The same year, 2013, the first RCT study, the Pre Hospital Antifibrinolytics for the Coagulopathy of Traumatic Hemorrhage or the PATCH trial, by Dr. Russell Gruen et al., highlighted the same problems of the CRASH-2 trial raised by Mattox and Napolitano while proposing the mechanistic possibility that TXA may have caused the increased rate of DVT of 12 times and PE of 9 times in the MATTERs trial [92, 172]. Subsequently, in 2014, three trials were launched to delve into the mechanism of TXA and directly address these knowledge gaps. The first is the Study of Tranexamic Acid during Air Medical Prehospital Transport (STAAMP) Trial from the University of Pittsburgh [178]. The second is the Tranexamic Acid Mechanisms and Pharmacokinetics In Traumatic Injury (TAMPITI) Trial from Washington University Saint Louis, which will look at the mechanistic analysis of pro-inflammatory markers for TXA in trauma.3Finally, the last is the newly NIH registered Study of TXA in acute orthopedic fractures from the University of Tennessee.4Since early 2013, these 5 RCT studies have been designed specifically to clarify the uncertainties surrounding TXA and to fill the “knowledge gaps” that originated from the CRASH-2 trial.
In addition, in June of 2014, Valle et al. published their experience in the Journal of Trauma at Jackson Memorial Hospital in Miami, Florida regarding more than 1,200 trauma patients, of whom 300 were evaluated for TXA in “shocked” patients [179]. They described propensity matched study populations in shock-one half of whom received TXA and the other half of whom did not. The surprising finding was that the TXA group was associated with greater mortality. There are two possible, contributing explanations. One possibility is that the bolus infusion of TXA required a larger dose of crystalloid, which is well known to worsen coagulopathy [179]. Another possibility is that TXA may have caused hypotension [179]. Every patient in the Valle et al. study was in severe shock and those who received TXA and hemostatic control with surgery and transfusion did so within a very short amount of time. In Valle et al.’s hands TXA was administered in the OR after the patient had already received a transfusion. Valle et al. therefore recommended that in mature trauma systems the ubiquitous use of TXA in the prehospital and hospital trauma systems needs to be further investigated [179].
Similar conclusions regarding the ubiquitous use of TXA in mature trauma systems with limitation to its use in “shocked patients” have been noted by the CRASH-2 authors in a non-randomized, controlled, prospective observational study. Cole et al. in this study “could not identify a clear outcome benefit to patients without shock,” and therefore, “the findings give a clear signal for using TXA in severely injured, shocked civilian patients.” They also found that “VTE was more common in patients who received TXA… in the more severely shocked population.” Their analysis states: “there was a fourfold increase in the thromboembolic events in the TXA group (No TXA: 2% vs TXA: 8%, P < 0.01).” They note that: “TXA was independently associated with a reduction in MOF… and was adjusted for all-cause mortality in shocked patients.” These findings of limitation to the benefit of TXA to severely injured and shocked patients with associated increased rates of VTE are not consistent with the initial CRASH-2 trial [180].
Finally, Holcomb and his group have recently studied the impact of TXA on mortality in injured patients with hyperfibrinolysis with LYS30> 3% as determined by a RapidTEG, which uses tissue factor (TF) as an initiator [181]. They found that the use of TXA was not associated with a reduction in mortality [181]. It was noted that the rTEG compared to kaolin-TEG is less accurate at identifying functional hyperfibrinolysis [181]. Therefore, when combined with the studies of Valle et al., they suggest that mandatory use of TXA as advocated by the CRASH-2 investigators may not be applicable to mature level 1 trauma centers where prompt blood product resuscitation can be initiated and prompt surgical control of hemorrhage can be obtained, reiterating Schreiber’s point concerning the administration of hemostatic adjuncts such as recombinant factor VIIa, PCC, and TXA, which may have a place in trauma protocols with or without viscoelastic-test guidance [179, 181].2
In summary, a solution to the above mentioned controversy regarding the indications for TXA in hemorrhage has led to suggestions that TXA be given to patients with TEG/ROTEM values indicative of hyperfibrinolysis [19, 22, 74, 89, 171].
The use of VETs to deliver TTHAT has been proposed in postpartum hemorrhage. Postpartum hemorrhage is a major cause of women’s death around the world. An international randomized placebo controlled trial is ongoing right now to provide valuable scientific evidence on whether the use of TXA can reduce postpartum hemorrhage [182, 183].5
4.2. Prothrombin Complex Concentrate and Fibrinogen Concentrate
In Europe, where PCCs and fibrinogen concentrates are more commonly used as a substitute for FFP and cryoprecipitate, traumatologists have presented data regarding the use of PCC and fibrinogen concentrate guided by POC VETs for the resuscitation of trauma patients [49, 52, 54, 64, 131, 175-177].2 When paired with the trauma ISS, a decrease in the mortality rate is observed when guiding administration of fibrinogen concentrate and PCC as first-line hemostatic therapy with ROTEM compared with the mortality rate of patients not guided by VETs. Patients treated in accordance with an algorithm for ROTEM-guided administration of fibrinogen concentrate and PCC had lower mortality than that predicted by The Trauma and Injury Severity Score (TRISS) revised or RISC. Fibrinogen concentrate was given as first-line hemostatic therapy when maximum clot firmness (MCF) measured by FIBTEM (fibrin-based test) was <10 mm. PCC was given in case of recent warfarin intake or clotting time measured by extrinsic activation test (EXTEM) >1.5 times normal [49]. However, the non-randomized controlled observational studies from Europe promoting the use of hemostatic adjunctive agents (for example fibrinogen concentrate, PCC and tranexamic acid) have not involved significant penetrating trauma and may not be relevant to a combat or urban United States population. For example, the incidence of penetrating trauma in Europe is lower than the incidence in the urban population of the United States [122, 179, 184]. Early treatment algorithms for VET guided hemostatic therapy with fibrinogen concentrate and PCC can help to minimize the risk of thrombosis in trauma patients by ensuring that excessive dosing is avoided [23].
There are no large randomized controlled trials to support the use of PCC in trauma other than in its use in hemophilia and for the rapid reversal of the effect of oral vitamin K antagonists. However, it is recommended to use PCC early for the emergency reversal of vitamin K-dependent oral anticoagulants. In the setting of trauma patients treated with pre-injury warfarin, a retrospective analysis shows that the use of PCC results in a more rapid time to reversal of the INR [54]. Four factor PCC has been used for NOAC reversal as well. There is limited literature on reversal of the new oral direct and indirect thrombin inhibitors, such as dabigatran, rivaroxaban, apixaban, and edoxaban with PCC. However, small case reports and series have demonstrated successful use of PCC to completely reverse the anticoagulant effects of rivaroxaban. Currently there is little literature for using TEG-monitored PCC reversal of patients with acquired coagulopathy [31, 143, 146-159].
4.3. Bypassing Agents
The optimal use of bypassing agents, rFVIIa and Factor VII Inhibitor Bypassing Agent (FEIBA) (a plasma-derived activated prothrombin complex concentrate [APCC]), needs a reliable laboratory method to predict and monitor their effects [185].
The initial use of rFVIIa for trauma resuscitation was greeted with great promise for patients with multiple traumas with and without TBI. However, subsequent studies have rendered its use problematic because of an increased incidence of thrombosis without survival benefit. Studies have shown no utility of rFVIIa in treating severely acidotic, coagulopathic trauma patients with high rates of bleeding (4 units of RBC/h) and, therefore, restriction has been set on its usage. However, rFVIIa has shown benefit in coagulopathic TBI patients, although this is controversial [110, 186-192].
For TBI, a prospective, randomized, placebo-controlled study regarding the preliminary effectiveness of rFVIIa to limit ICH progression has shown that rFVIIa was able to more quickly and less expensively correct the INR into normal ranges in preparation for surgery in coagulopathic TBI patients [193]. This study revealed that rVIIa allowed a more rapid neurosurgical intervention with less blood product transfusion and even a reduced need for cranial surgery [194].
rFVIIa has been shown to be effective for the reversal of dabigatran associated hemorrhage. rFVIIa corrected the lag time of a thrombin generation test [158]. Currently, rFVIIa is recommended for use in reversing Factor Xa inhibitors and antithrombin medications in some algorithms [146].5 Patients with autoimmune idiopathic thrombocytopenia undergoing a splenectomy, who require transfusion have benefited from TEG guided administration of rFVIIa administration with active bleeding [195]. Agreed upon guidelines regarding the use of rFVIIa for the bleeding trauma patient do not currently exist. Since VETs have been shown to be able to guide BCT in the trauma patient, they may be able to guide physicians to help determine which patients should receive rFVIIa [196]. However, the effectiveness of rFVIIa for reversal of dabigatran has been questioned. Also, rFVIIa is not universally recommended for reversing Factor Xa inhibitors [197, 198].
FEIBA has been shown to be effective for the reversal of warfarin-induced coagulopathy [199]. However, as noted above, four factor PCC is now indicated for emergency warfarin reversal for severe hemorrhage. And in the emergency setting, the TEG may be used for initial evaluation of successive reversal [19].
5. Future Uses of TEG
Recently, there has been a surge in the interest of using TEG for diagnosis and guidance of BCT for TIC and patients needing massive transfusions with particular emphasis on the use of TEG to guide TXA administration for hemorrhaging trauma patients. The CRASH-2 trial recommendations of near ubiquitous TXA use in trauma has prompted the search for objective markers of fibrinolysis such as LY30%, but this recommendation is controversial and needs further vetting [168].
Currently, there are no Evidence-Based Medicine (EBM) studies looking at the use of TEG in the orthopedic patient population to guide BCT. Another area in need of research is the use of serial platelet mapping with TEG in the orthopedic patient population. This could be used to reduce blood product wastage and simultaneously, to guide BCT with fluid resuscitation. Additional prospective randomized clinical trials are still needed on the use of VETs in providing goal-directed therapy with fibrinogen concentrate, PCC, and rFVIIa in trauma patients.
6. Limitations
Though VETs have multiple strengths compared to CCTs, there are nonetheless several weaknesses or limitations that cannot be overlooked. For example, VETs are limited by its varying sensitivity for diagnosing hyperfibrinolysis [170, 171]. VETs are performed at standard human body temperature, 37°C. For the hypothermic patient, the patient must be rewarmed. Also, it is hypothesized by Brooks et al. that the MA and α-angles are elevated due to stereotactic interference from the paucity of red cells in anemic patients, which is another limitation of VETs necessitating further research [200]. However, recent clinical observations regarding the effect of hematocrit on VETs reveals that the direct effects of anemia rather than an imbalance between thrombin and antithrombin may explain the findings of the elevation of the MA and α angles after hemodilution. Low hematocrit most likely worsens bleeding in vivo but improves the TEG variables in vitro, therefore, the TEG results should be interpreted with the knowledge of the severity of anemia, hemodilution, and other clinical parameters such as microvascular bleeding in patients after trauma [56, 201]. Finally, it has been noted that the effect of hematocrit is not reflected by plasma fibrinogen concentration, in contrast to FIBTEM MCF, which incorporates the contribution of the hematocrit to whole blood firmness. However, this effect appears to be negligible in hemodiluted patients [202]. Therefore, hematocrit does not represent a bias; rather, it is an integral part of the VET methodology.
The limitations of TEG also include lack of standardization in the following: use of kaolin and Tissue Factor as initiators, quality assurance, and consistency in interpretation of test results. In the United States, TEG is often performed by personnel trained in the nuances of VETs. Traditionally, perfusionists have operated TEG during cardiac surgery and therefore may be considered for performing this test in the trauma setting [62]. Another option is to have laboratory technicians perform the procedure while trauma physicians interpret the data [19]. In European trauma centers, VET laboratory technicians assist anesthesiologists specialized in trauma. These VET technicians and anesthesiologists assume a very prominent role in trauma resuscitation therapy. In addition, European trauma centers have greater access to products that require close monitoring, such as PCCs and fibrinogen concentrate [54, 64, 203].
A significant limitation to the TEG/ROTEM has been noted recently regarding the applicability of the results to trauma with specific concerns regarding the variation of definition of fibrinolysis as determined by the TEG/ROTEM and of the indirect nature of the measurements of fibrinogen and functional fibrinogen [204].
One final limitation regarding the TEG and the ROTEM is the lag time required to perform and read the test. During times of MT, the approach is to initially offer a “Foundation Ratio” of 6 PRBC to 3 FFP or to pursue a ratio of PRBC/platelets/FFP/Cryoprecipitate that approximates whole blood while the TEG is being prepared and run. In the PROPPR trial, the mean time to “anatomical hemostasis” an euphemism for local surgical and radiologic control of hemorrhage, was 100 minutes, meaning resuscitation resulted in local control of bleeding within two hours [86, 205, 206] (Fig. 6). For patients with severe coagulopathy, the parameters can be anticipated within approximately the first 35-40 minutes by looking at the initial TEG/PM tracing. Blood products, as well as hemostatic agents, can be given at this point and new samples drawn and inserted into another TEG channel which will allow downstream evaluation of the earlier treatment. While the rTEG saves approximately 10 minutes in reading, the previously mentioned inaccuracy for fibrinolysis is a concern as well as the lack of the accuracy of the ACT as a surrogate for the “R” [80]. For this reason, we recommend only the kaolin-TEG performed on multiple TEG channels that are sequentially overlapping during MT. Of concern is the delay in fibrinolysis determination which requires 30 minutes following the MA. However, it has been suggested that TXA be given to those hypotensive patients in shock regardless of the LY30% and therefore it would be reasonable to administer TXA to all shocked trauma patients [168, 169].
Fig. (6).
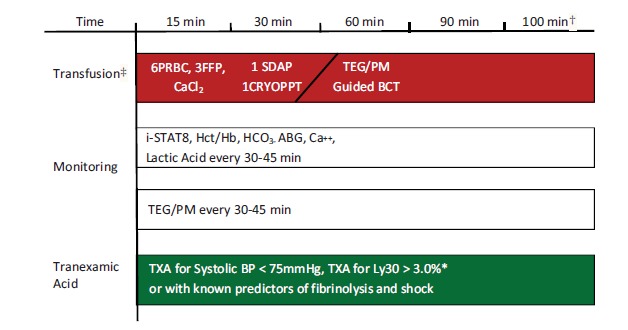
MTP TEG/PM-guided BCT Algorithm. TEG/PM is performed upon arrival and every 30-45 minutes thereafter depending on severity of shock. Note: Where available order fibrinogen concentrate, PCC and rarely rFVIIA.. *Some algorithms recommend TXA for LY30 > 7.5% [48]. †Time to “anatomic hemostasis” was 100 minutes according to the PROPPR trial. [86, 122, 169]. ‡ With central venous access foundation ratio of 6PRBC, 3FFP, 1 SDAP, 10 U Cryoprecipitate given in first 20 minutes with subsequent ratios determined by clinical, laboratory and TEG/PM parameters.
Conclusion
Viscoelastic tests utilize whole blood samples as opposed to plasma based coagulation tests. They provide a rapid, more accurate point of care assessment of the coagulation status in the coagulopathic trauma and non-trauma patient. The use of TEG and ROTEM may reduce the waste of blood products as well decrease mortality rates in the coagulopathic patient. However, before VETs and TEG can be accepted universally, their limitations need to be addressed, namely quality assurance and the availability of personnel to perform and interpret the test. Regardless, the use of targeted thromboelastographic blood component therapy and adjunctive hemostatic agents for damage control resuscitation (DCR) is becoming a more widely accepted strategy for traumatic patients. There will be an increasing need for emergency physicians, trauma surgeons, anesthesiologists, and other trauma related staff to be familiar with VETs. VETs, both TEG and ROTEM, provide rapid assessment of the immediate coagulation status of the patient, which is critical in trauma-induced coagulopathy and useful in guiding blood component therapy in the management of TIC and other types of acquired coagulopathy in the emergency setting [63, 65, 206, 207]. Very recent studies have shown the ability of the TEG/ROTEM to guide therapeutic decisions for patients on subcutaneous as well as oral Xa inhibitors and oral direct thrombin inhibitors [160-162].
The most recent development of new cartridge-based systems for VETs known as TEG6s and ROTEM Sigma systems are soon to be released. These easy to perform tests that can be done by the most basic of laboratories will allow for the expansion of these VETs to assess hemostatic competence in the trauma and non-trauma settings [208].6,7
Regardless of whether the ROTEM or TEG system is used to gauge hemostatic competence of those trauma and non-trauma patients in need of targeted BCT and AHA, the choice of modality is not as important as making the choice since the information gained from these tests has been shown to greatly assist in the guidance of targeted therapy. The lack of randomized controlled trials regarding the use of VET to provide targeted BCT and AHA for trauma patients has been a stumbling block for some clinical researchers. The best rebuttal to these critics was recently voiced most eloquently by Spahn: “This is by no means to say that we should stop doing outcomes research on coagulation management in severely injured patients, but that we should not dismiss existing evidence in favour of TEG/ROTEM-based goal-directed individualized coagulation algorithms on the basis that we lack the ultimate 'perfect' study. As a matter of fact, today all hospitals should have an individualized and goal-directed coagulation algorithm: don’t wait - act now!” [209]. It is not so important whether we choose ROTEM or TEG with the many iterations that come with these choices; rather, it is important that we begin to utilize VETs that are based on the cell-based theory of hemostasis and offer a much more physiologic and useful analysis of the hemostatic integrity of a bleeding patient [210].
ACKNOWLEDGEMENTS
Declared none.
Footnotes
A 2004 abstract by Lipski, I and Pivalizza, E titled “The thrombelastograph in patients taking coumadin.”
Professor Martin Schreiber MD, in a talk entitled “Current Practice of Medicine for Severe Bleeding. Product Development Program for Interventions in Severe Bleeding Due to Trauma or Other Causes.” In 2010 at Masur Auditorium, Bldg. 10, National Institutes of Health, 8800 Rockville Pike, Bethesda, MD, 20894: U.S. Food and Drug Administration.
Tranexamic Acid Mechanisms and Pharmacokinetics in Traumatic Injury (TAMPITI Trial); Study Investigators: Dr. Philip Spinella, MD, Dr. Grant Bochicchio, MD, MPH; Study is Currently Ongoing through Fall 2015-Spring 2017 http://www.tampiti.wustl.edu/
ClinicalTrials.gov. Tranexamic Acid in Orthopaedic Trauma Surger 2015. Available from: https://clinicaltrials.gov/ct2/show/NCT02080494?term=Tennessee+tranexamic+acid+orthopedics&rank=1.
ClinicalTrials.gov. Study in Healthy Volunteers of the Reversion by Haemostatic Drugs of the Anticoagulant Effect of New Anti-thrombotics. Available from: https://clinicaltrials.gov/show/NCT01210755
ClinicalTrials.gov. ROTEM® Sigma Performance Evaluation - Method Comparison With Predicate Device and Reference Intervals (ROSI-EVA). Available from: https://clinicaltrials.gov/ct2/show/NCT02379104
ClinicalTrials.gov. Trauma Equivalency Study of the CORA® and TEG® 5000 Systems 2015. Available from: https://clinicaltrials.gov/ct2/show/NCT02408029
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
AUTHORSHIP
MW, VP, FJC, MKB, AM, MJ, ALS, SB, JM, DH, MS, AC, SG wrote, reviewed, and prepared the manuscript. ALS, FS, SC, SF, BF, MJ, JM, SB, DH, MS, AC, CM prepared figures and tables. MW, SF, DH, MS, AC, MJ, SC, BF, AM, MKB, JM, SB, ALS, FS, VP, FJC, CM reviewed and prepared manuscript. The authors would like to recognize Cheyney McWilliams and Megan Maloney for editorial assistance and Marie Bourgoeis Davis MFA for graphic design consultation.
DISCLOSURES
MW, FJC, VP received research grants from Haemonetics Corporation, Niles, IL. MW has received educational honoraria from Boehringer Ingelheim, CSL Behring.
REFERENCES
- 1.Frith D., Goslings J., Gaarder C., et al. Definition and drivers of acute traumatic coagulopathy: clinical and experimental investigations. J. Thromb. Haemost. 2010;8(9):1919–1925. doi: 10.1111/j.1538-7836.2010.03945.x. [DOI] [PubMed] [Google Scholar]
- 2.Brohi K., Singh J., Heron M., Coats T. Acute traumatic coagulopathy. J. Trauma. 2003;54(6):1127–1130. doi: 10.1097/01.TA.0000069184.82147.06. [DOI] [PubMed] [Google Scholar]
- 3.Maegele M. Frequency, risk stratification and therapeutic management of acute post-traumatic coagulopathy. Vox Sang. 2009;97(1):39–49. doi: 10.1111/j.1423-0410.2009.01179.x. [DOI] [PubMed] [Google Scholar]
- 4.Maegele M., Lefering R., Yucel N., Tjardes T., Rixen D., Paffrath T. Early coagulopathy in multiple injury: an analysis from the german trauma registry on 8724 patients. Injury. 2007;38(3):298–304. doi: 10.1016/j.injury.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Brohi K., Cohen M., Ganter M., Matthay M., Mackersie R., Pittet J. Acute traumatic coagulopathy: initiated by hypoperfusion: modulated through the protein C pathway? Ann. Surg. 2007;245(5):298–304. doi: 10.1097/01.sla.0000256862.79374.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solomon C., Traintinger S., Ziegler B., et al. Platelet function following trauma: a multiple electrode aggregometry study. Thromb. Haemost. 2011;106(2):322–330. doi: 10.1160/TH11-03-0175. [DOI] [PubMed] [Google Scholar]
- 7.Hess J., Brohi K., Dutton R., et al. The coagulopathy of trauma: a review of mechanisms. J. Trauma. 2008;65(4):748–754. doi: 10.1097/TA.0b013e3181877a9c. [DOI] [PubMed] [Google Scholar]
- 8.Wohlauer M., Moore E., Thomas S., et al. Early platelet dysfunction: an unrecognized role in the acute coagulopathy of trauma. J. Am. Coll. Surg. 2012;214(5):739–746. doi: 10.1016/j.jamcollsurg.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cap A., Hunt B. The pathogenesis of traumatic coagulopathy. Anaesthesia. 2015;70(Suppl. 1):E32–E34. doi: 10.1111/anae.12914. [DOI] [PubMed] [Google Scholar]
- 10.Gando S. Acute coagulopathy of trauma shock and coagulopathy of trauma: a rebuttal. you are now going down the wrong path. J. Trauma. 2009;67(2):381–383. doi: 10.1097/TA.0b013e3181a84f63. [DOI] [PubMed] [Google Scholar]
- 11.Johansson P., Ostrowski S. Acute coagulopathy of trauma: balancing progressive catecholamine induced endothelial activation and damage by fluid phase anticoagulation. Med. Hypotheses. 2010;75(6):564–567. doi: 10.1016/j.mehy.2010.07.031. [DOI] [PubMed] [Google Scholar]
- 12.Dobson G., Letson H., Sharma R., Sheppard F., Cap A. Mechanisms of early trauma-induced coagulopathy: the clot thickens or not? J. Trauma Acute Care Surg. 2015;79(2):301–309. doi: 10.1097/TA.0000000000000729. [DOI] [PubMed] [Google Scholar]
- 13.Dzik W. Predicting hemorrhage using preoperative coagulation screening assays. Curr. Hematol. Malig. Rep. 2004;3(5):324–330. [PubMed] [Google Scholar]
- 14.Johansson P. Coagulation monitoring of the bleeding traumatized patient. Curr. Opin. Anaesthesiol. 2012;25(2):234–241. doi: 10.1097/ACO.0b013e32834fab76. [DOI] [PubMed] [Google Scholar]
- 15.Kitchens C. To bleed or not to bleed? Is that the question for the PTT? J. Thromb. Haemost. 2005;3(12):2607–2611. doi: 10.1111/j.1538-7836.2005.01552.x. [DOI] [PubMed] [Google Scholar]
- 16.Segal J., Dzik W. Paucity of studies to support that abnormal coagulation test results predict bleeding in the setting of invasive procedures: an evidence-based review. Transfusion. 2005;45(9):1413–1425. doi: 10.1111/j.1537-2995.2005.00546.x. [DOI] [PubMed] [Google Scholar]
- 17.Davenport R., Manson J., De'Ath H., et al. Functional definition and characterisation of acute traumatic coagulopathy. Crit. Care Med. 2011;39(12):2652–2658. doi: 10.1097/CCM.0b013e3182281af5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toulon P., Ozier Y., Ankri A., Fléron M-H., Leroux G., Samama C. Point-of-care versus central laboratory coagulation testing during haemorrhagic surgery. A multicenter study. Thromb. Haemost. 2009;101(2):394–401. [PubMed] [Google Scholar]
- 19.Holcomb J., Minei K., Scerbo M., et al. Admission rapid thrombelastography can replace conventional coagulation tests in the emergency department: experience with 1974 consecutive trauma patients. Ann. Surg. 2012;256(3):476–486. doi: 10.1097/SLA.0b013e3182658180. [DOI] [PubMed] [Google Scholar]
- 20.Hoffman M., Monroe D. Coagulation 2006: a modern view of hemostasis. Hematol. Oncol. Clin. North Am. 2007;21(1):1–11. doi: 10.1016/j.hoc.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Schöchl H., Solomon C., Voelckel W. Thromboelastometry in the perioperative setting. Netherlands J Crit Care. 2010;14(1):23–31. [Google Scholar]
- 22.Schöchl H., Voelckel W., Grassetto A., Schlimp C. Practical application of point-of-care coagulation testing to guide treatment decisions in trauma. J. Trauma Acute Care Surg. 2013;74(6):1587–1598. doi: 10.1097/TA.0b013e31828c3171. [DOI] [PubMed] [Google Scholar]
- 23.Schöchl H., Maegele M., Solomon C., Görlinger K., Voelckel W. Early and individualized goal-directed therapy for trauma-induced coagulopathy. Scand. J. Trauma Resusc. Emerg. Med. 2012;20(15) doi: 10.1186/1757-7241-20-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Siguret V., Gouin-Thibault I., Gaussem P., Pautas E. Optimizing the use of anticoagulants (heparins and oral anticoagulants) in the elderly. Drugs Aging. 2013;30(9):687–699. doi: 10.1007/s40266-013-0101-0. [DOI] [PubMed] [Google Scholar]
- 25.Garcia D., Regan S., Crowther M., Hughes R., Hylek E. Warfarin maintenance dosing patterns in clinical practice: implications for safer anticoagulation in the elderly population. Chest. 2005;127(6):2049–2056. doi: 10.1378/chest.127.6.2049. [DOI] [PubMed] [Google Scholar]
- 26.Faul M., Xu L., Wald M., Coronado V. Traumatic brain injury in the United States: emergency department visits, hospitalizations and deaths 2002-2006. Atlanta, GA: Centers for Disease Control and Prevention National Center for Injury Prevention and Control; 2010. [Google Scholar]
- 27.Day J. Population projections of the United States by age, sex, race, and hispanic origin: 1995 to 2050. US Bureau of the Census. Curr. Popul. Rep. [Spec Censuses] 1996:25–1130. [Google Scholar]
- 28.Susman M., Dirusso S., Sullivan T., Risucci D., Nealon P., Cuff S. Traumatic brain injury in the elderly: increased mortality and worse functional outcome at discharge despite lower injury severity. J. Trauma. 2002;53(2):219–224. doi: 10.1097/00005373-200208000-00004. [DOI] [PubMed] [Google Scholar]
- 29.Heron M., Hoyert D., Xu J., Scott C., Tejada-Vera B. Deaths: preliminary data for 2006. Natl. Vital Stat. Rep. 2008;56(16) [Google Scholar]
- 30.Sjögren H., Björnstig U. Injuries to the elderly in the traffic environment. Accid. Anal. Prev. 1991;23(1):77–86. doi: 10.1016/0001-4575(91)90037-6. [DOI] [PubMed] [Google Scholar]
- 31.Schulman S., Ritchie B., Goy J., Nahirniak S., Almutawa M., Ghanny S. Activated prothrombin complex concentrate for dabigatran-associated bleeding. Br. J. Haematol. 2013;164:296–310. doi: 10.1111/bjh.12620. [DOI] [PubMed] [Google Scholar]
- 32.Cotton B., McCarthy J., Holcomb J. Acutely injured patients on dabigatran. N. Engl. J. Med. 2011;365(21):2039–2040. doi: 10.1056/NEJMc1111095. [DOI] [PubMed] [Google Scholar]
- 33.Giugliano R., Ruff C., Braunwald E., et al. Edoxaban versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2013;369(22):2093–2104. doi: 10.1056/NEJMoa1310907. [DOI] [PubMed] [Google Scholar]
- 34.Solbeck S., Meyer M., Johansson P., et al. Monitoring of dabigatran anticoagulation and its reversal in vitro by thrombelastography. Int. J. Cardiol. 2014;176(3):794–799. doi: 10.1016/j.ijcard.2014.07.084. [DOI] [PubMed] [Google Scholar]
- 35.Neyens R., Bohm N., Cearley M., Andrews C., Chalela J. Dabigatran-associated subdural hemorrhage: using thromboelastography (TEG®) to guide decision-making. J. Thromb. Thrombolysis. 2013;(37):80–83. doi: 10.1007/s11239-013-0933-9. [DOI] [PubMed] [Google Scholar]
- 36.Davis P., Musunuru H., Walsh M., Mitra R., Ploplis V., Castellino F. The ex vivo reversibility of dabigatran-induced whole-blood coagulopathy as monitored by thromboelastography: mechanistic implications for clinical medicine. Thromb. Haemost. 2012;108(3):586–588. doi: 10.1160/TH12-04-0222. [DOI] [PubMed] [Google Scholar]
- 37.Hartert H. Blutherinnungsstudien nit der thromboelastographie, einem neuen untersuchungsverfarhren. Klin. Wochenschr. 1948;26(37/38):577–583. doi: 10.1007/BF01697545. [DOI] [PubMed] [Google Scholar]
- 38.Hartert H., Schaeder J. The physical and biological constants of thrombelastography. Biorheology. 1962;1:31–39. [Google Scholar]
- 39.Hardaway R., Bredenberg C. Monitoring hematology laboratory values.Care of Wounded in Vietnam. Manhatten, Kansas. 1988. [Google Scholar]
- 40.Kang Y., Martin D., Marquez J., et al. Intraoperative changes in blood coagulation and thrombelastographic monitoring in liver transplantation. Anesth. Analg. 1985;64(9) [PMC free article] [PubMed] [Google Scholar]
- 41.Shore-Lesserson L., Manspeizer H., DePerio M., Francis S., Vela-Cantos F., Ergin M. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth. Analg. 1999;88(2):312–319. doi: 10.1097/00000539-199902000-00016. [DOI] [PubMed] [Google Scholar]
- 42.Enriquez L., Shore-Lesserson L. Point-of-care coagulation testing and transfusion algorithms. Br. J. Anaesth. 2009;103(Suppl. I):il4–il22. doi: 10.1093/bja/aep318. [DOI] [PubMed] [Google Scholar]
- 43.Kaufmann C., Dwyer K., Crews J., Dols S., Trask A. Usefulness of thrombelastography in assessment of trauma patient coagulation. J. Trauma. 1997;42(4):716–722. doi: 10.1097/00005373-199704000-00023. [DOI] [PubMed] [Google Scholar]
- 44.Johansson P., Stissing T., Bochsen L., Ostrowski S. Thrombelastography and tromboelastometry in assessing coagulopathy in trauma. Scand. J. Trauma Resusc. Emerg. Med. 2009;17(45) doi: 10.1186/1757-7241-17-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plotkin A., Wade C., Jenkins D., et al. A reduction in clot formation rate and strength assessed by thrombelastography is indicative of transfusion requirements in patients with penetrating injuries. J. Trauma. 2008;64(2) Suppl.:S64–S68. doi: 10.1097/TA.0b013e318160772d. [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez E., Pieracci F., Moore E., Kashuk J. Coagulation abnormalities in the trauma patient: the role of point-of-care thromboelastography. Semin. Thromb. Hemost. 2010;36(7):723–737. doi: 10.1055/s-0030-1265289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kashuk J., Moore E., Sawyer M., et al. Postinjury coagulopathy management: goal directed resuscitation via POC thrombelastography. Ann. Surg. 2010;251(4):604–614. doi: 10.1097/SLA.0b013e3181d3599c. [DOI] [PubMed] [Google Scholar]
- 48.Tapia N., Chang A., Norman M., et al. TEG-guided resuscitation is superior to standardized MTP resuscitation in massively transfused penetrating trauma patients. J. Trauma Acute Care Surg. 2013;74(2):378–386. doi: 10.1097/TA.0b013e31827e20e0. [DOI] [PubMed] [Google Scholar]
- 49.Schöchl H., Nienaber U., Hofer G., et al. Goal-directed coagulation management of major trauma patients using thromboelastometry (ROTEM)-guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit. Care. 2010;14(2):R55. doi: 10.1186/cc8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Theusinger O., Felix C., Spahn D. Strategies to reduce the use of blood products: a European perspective. Curr. Opin. Anaesthesiol. 2012;25(1):59–65. doi: 10.1097/ACO.0b013e32834dec98. [DOI] [PubMed] [Google Scholar]
- 51.Woolley T., Midwinter M., Spencer P., Watts S., Doran C., Kirkman E. Utility of interim ROTEM® values of clot strength, A5 and A10, in predicting final assessment of coagulation status in severely injured battle patients. Injury. 2013;44(5):593–599. doi: 10.1016/j.injury.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 52.Ziegler B., Schimke C., Marchet P., Stögermüller B., Schöchl H., Solomon C. Severe pediatric blunt trauma-successful ROTEM-guided hemostatic therapy with fibrinogen concentrate and no administration of fresh frozen plasma or platelets. Clin. Appl. Thromb. Hemost. 2013;19(4):453–459. doi: 10.1177/1076029612458149. [DOI] [PubMed] [Google Scholar]
- 53.Hauser C., Boffard K., Dutton R., Bernard G., Croce M., Holcomb J. Results of the CONTROL trial: efficacy and safety of recombinant activated Factor VII in the management of refractory traumatic hemorrhage. J. Trauma. 2010;69(3):489–500. doi: 10.1097/TA.0b013e3181edf36e. [DOI] [PubMed] [Google Scholar]
- 54.Spahn D., Bouillon B., Cerny V., et al. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit. Care. 2013;17(2):R76. doi: 10.1186/cc12685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stensballe J., Ostrowski S., Johansson P. Viscoelastic guidance of resuscitation. Curr. Opin. Anaesthesiol. 2014;27(2):212–218. doi: 10.1097/ACO.0000000000000051. [DOI] [PubMed] [Google Scholar]
- 56.Bolliger D., Seeberger M., Tanaka K. Principles and practice of thromboelastography in clinical coagulation management and transfusion practice. Transfus. Med. Rev. 2012;26(1):1–13. doi: 10.1016/j.tmrv.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 57.Branco B., Inaba K., Ives C., et al. Thromboelastogram evaluation of the impact of hypercoagulability in trauma patients. Shock. 2014;41(3):200–207. doi: 10.1097/SHK.0000000000000109. [DOI] [PubMed] [Google Scholar]
- 58.Hunt H., Stanworth S., Curry N., et al. Thromboelastography (TEG) and rotational thromboelastometry (ROTEM) for trauma-induced coagulopathy in adult trauma patients with bleeding. Cochrane Libr. 2015 doi: 10.1002/14651858.CD010438.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Da Luz L., Nascimento B., Shankarakutty A., Rizoli S., Adhikari N. Effect of thromboelastography (TEG®) and rotational thromboelastometry (ROTEM®) on diagnosis of coagulopathy, transfusion guidance and mortality in trauma: descriptive systematic review. Crit. Care. 2014;18:518. doi: 10.1186/s13054-014-0518-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.MacDonald S., Luddington R. Critical factors contributing to the thromboelastography trace. Semin. Thromb. Hemost. 2010;36(7):712–722. doi: 10.1055/s-0030-1265288. [DOI] [PubMed] [Google Scholar]
- 61.Solomon C., Schöchl H., Ranucci M., Schlimp C. Can the viscoelastic parameter α-angle distinguish fibrinogen from platelet deficiency and guide fibrinogen supplementation? Anesth. Analg. 2015;121(2):289–301. doi: 10.1213/ANE.0000000000000738. [DOI] [PubMed] [Google Scholar]
- 62.Walsh M., Jbara M., Miller A., Lawson J. Thromboelastographic guided blood component therapy for severe hemorrhage. Blood Bulletin; 2014. [Google Scholar]
- 63.Sankarankutty A., Nascimento B., Da Luz L., Rizoli S. TEG and ROTEM in trauma: similar tests but different results? World J. Emerg. Surg. 2012;7(Suppl. 1):S3. doi: 10.1186/1749-7922-7-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schöchl H., Forster L., Woidke R., Solomon C., Voelckel W. Use of rotation thromboelastometry (ROTEM) to achieve successful treatment of polytrauma with fibrinogen concentrate and prothrombin complex concentrate. Anaesthesia. 2010;65(2):199–203. doi: 10.1111/j.1365-2044.2009.06188.x. [DOI] [PubMed] [Google Scholar]
- 65.Theusinger O., Baulig W., Seifert B., Müller S., Mariotti S., Spahn D. Changes in coagulation in standard laboratory tests and ROTEM in trauma patients between on-scene and arrival in the emergency department. Anesth. Analg. 2015;120(3):627–635. doi: 10.1213/ANE.0000000000000561. [DOI] [PubMed] [Google Scholar]
- 66.Fayed N., Mourad W., Yassen K., Gorlinger K. Preoperative thromboelastometry as a predictor of transfusion requirements during adult living donor liver transplantation. Transfus. Med. Hemother. 2015;42:99–108. doi: 10.1159/000381733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Benes J., Zatloukal J., Kletecka J. Viscoelastic methods of blood clotting assessment-a multidisciplinary review. Front. Med. 2015:2. doi: 10.3389/fmed.2015.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bochsen L., Wiinberg B., Kjelgaard-Hansen M. Steinbrüche lD, Johansson P. Evaluation of the TEG platelet mapping assay in blood donors. Thromb. J. 2007;5(3) doi: 10.1186/1477-9560-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Harr J., Moore E., Ghasabyan A., et al. Functional fibrinogen assay indicates that fibrinogen is critical in correcting abnormal clot strength following trauma. Shock. 2013;39(1):45–49. doi: 10.1097/SHK.0b013e3182787122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Theusinger O., Stein P., Spahn D. Transfusion strategy in multiple trauma patients. Curr. Opin. Crit. Care. 2014;20(6):646–655. doi: 10.1097/MCC.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 71.Schöchl H., Schlimp C. Trauma bleeding management: the concept of goal-directed primary care. Anesth. Analg. 2014;119(5):1064–1073. doi: 10.1213/ANE.0b013e318270a6f7. [DOI] [PubMed] [Google Scholar]
- 72.Darlington D., Delgado A., Kheirabadi B., et al. Effect of hemodilution on coagulation and recombinant factor VIIa efficacy in human blood in vitro. J. Trauma Acute Care Surg. 2011;71(5):1152–1163. doi: 10.1097/TA.0b013e318215178c. [DOI] [PubMed] [Google Scholar]
- 73.Walsh M., Thomas S., Howard J., Evans E., Guyer K., Medvecz A. Blood component therapy in trauma guided with the utilization of the perfusionist and thromboelastography. J. Extra Corpor. Technol. 2011;43(3):162–167. [PMC free article] [PubMed] [Google Scholar]
- 74.Chapman M., Moore E., Ramos C., et al. Fibrinolysis greater than 3% is the critical value for initiation of antifibrinolytic therapy. J. Trauma Acute Care Surg. 2013;75(6):961–967. doi: 10.1097/TA.0b013e3182aa9c9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ellis T., Nielsen V., Marques M., Kirklin J. Thrombelastographic measures of clot propagation: a comparison of alpha with the maximum rate of thrombus generation. Blood Coagul. Fibrinolysis. 2007;18(1):45–48. doi: 10.1097/MBC.0b013e3280111a8e. [DOI] [PubMed] [Google Scholar]
- 76.Theusinger O.M., Stein P., Levy J.H. Point of care and factor concentrate-based coagulation algorithms. Transfus. Med. Hemother. 2015;42(2):115–121. doi: 10.1159/000381320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schochl H., Maegele M., Solomon C., Gorlinger K., Voelckel W. Early and individualized goal-directed therapy for trauma-induced coagulopathy. Scand. J. Trauma Resusc. Emerg. Med. 2012;20(15):1–11. doi: 10.1186/1757-7241-20-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Theusinger O., Levy J. Point of care devices for assessing bleeding and coagulation in the trauma patient. Anesthesiol. Clin. 2013;31(1):55–65. doi: 10.1016/j.anclin.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 79.Solomon C., Ranucci M., Hochleitner G., Schöchl H., Schlimp C.J. Review article: assessing the methodology for calculating platelet contribution to clot strength (platelet component) in thromboelastometry and thrombelastography. Anesth. Analg. 2015;121(4):868. doi: 10.1213/ANE.0000000000000859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jeger V., Zimmermann H., Exadaktylos A. Can rapid TEG accelerate the search for coagulopathies in the patient with multiple injuries? J. Trauma. 2009;66(4):1253–1257. doi: 10.1097/TA.0b013e31819d3caf. [DOI] [PubMed] [Google Scholar]
- 81.Riskin D., Tsai T., Riskin L., et al. Massive transfusion protocols: the role of aggressive resuscitation versus product ratio in mortality reduction. J. Am. Coll. Surg. 2009;209(2):198–205. doi: 10.1016/j.jamcollsurg.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 82.Holcomb J., Jenkins D., Rhee P., et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. J. Trauma. 2007;62(2):307–310. doi: 10.1097/TA.0b013e3180324124. [DOI] [PubMed] [Google Scholar]
- 83.Duchesne J., Barbeau J., Islam T., Wahl G., Greiffenstein P., McSwain N. Damage control resuscitation: from emergency department to the operating room. Am. Surg. 2011;77(2):201–206. doi: 10.1177/000313481107700222. [DOI] [PubMed] [Google Scholar]
- 84.Duchesne J., McSwain N., Cotton B., et al. Damage control resuscitation: the new face of damage control. J. Trauma. 2010;69(4):976–990. doi: 10.1097/TA.0b013e3181f2abc9. [DOI] [PubMed] [Google Scholar]
- 85.Holcomb J., Del Junco D., Fox E., et al. The prospective, observational, multicenter, major trauma transfusion (PROMMTT) study: comparative effectiveness of a time-varying treatment with competing risks. JAMA Surg. 2013;148(2):127–136. doi: 10.1001/2013.jamasurg.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Holcomb J., Tilley B., Baraniuk S., et al. PROPPR. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA. 2015;313(5):471–482. doi: 10.1001/jama.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Massaro A., Doerfler S., Nawalinski K., et al. Thromboelastography defines late hypercoagulability after TBI: a pilot study. Neurocrit. Care. 2015;22:45–51. doi: 10.1007/s12028-014-0051-3. [DOI] [PubMed] [Google Scholar]
- 88.Van P., Cho S., Underwood S., Morris M., Watters J., Schreiber M. Thrombelastography versus antifactor Xa levels in the assessment of prophylactic-dose enoxaparin in critically ill patients. J. Trauma. 2009;66(6):1509–1517. doi: 10.1097/TA.0b013e3181a51e33. [DOI] [PubMed] [Google Scholar]
- 89.Kashuk J., Moore E., Sawyer M., et al. Primary fibrinolysis is integral in the pathogenesis of the acute coagulopathy of trauma. Ann. Surg. 2010;252(3):434–444. doi: 10.1097/SLA.0b013e3181f09191. [DOI] [PubMed] [Google Scholar]
- 90.Velik-Salchner C., Haas T., Innerhofer P., et al. The effect of fibrinogen concentrate on thrombocytopenia. J. Thromb. Haemost. 2007;5(5):1019–10285. doi: 10.1111/j.1538-7836.2007.02481.x. [DOI] [PubMed] [Google Scholar]
- 91.Mitra B., Cameron P., Gruen R. Aggressive fresh frozen plasma (FFP) with massive blood transfusion in the absence of acute traumatic coagulopathy. Injury. 2012;43(1):33–37. doi: 10.1016/j.injury.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 92.Gruen R., Jacobs I., Reade M. Trauma and tranexamic acid. Med. J. Aust. 2013;199(5):310–311. doi: 10.5694/mja13.10747. [DOI] [PubMed] [Google Scholar]
- 93.Johnson J., Moore E., Kashuk J., et al. Effect of blood products transfusion on the development of postinjury multiple organ failure. Arch. Surg. 2010;145(10):973–977. doi: 10.1001/archsurg.2010.216. [DOI] [PubMed] [Google Scholar]
- 94.Inaba K., Branco B., Rhee P., et al. Impact of plasma transfusion in trauma patients who do not require massive transfusion. J. Am. Coll. Surg. 2010;210(6):957–965. doi: 10.1016/j.jamcollsurg.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 95.Watson G., Sperry J., Rosengart M., et al. Fresh frozen plasma is independently associated with a higher risk of multiple organ failure and acute respiratory distress syndrome. J. Trauma. 2009;67(2):221–230. doi: 10.1097/TA.0b013e3181ad5957. [DOI] [PubMed] [Google Scholar]
- 96.Silliman C., Ambruso D., Boshkov L. Transfusion-related acute lung injury. Blood. 2005;105(6):2266–2273. doi: 10.1182/blood-2004-07-2929. [DOI] [PubMed] [Google Scholar]
- 97.Sarani B., Dunkman W., Dean L., Sonnad S., Rohrbach J., Gracias V. Transfusion of fresh frozen plasma in critically ill surgical patients is associated with an increased risk of infection. Crit. Care Med. 2008;36(4):1114–1118. doi: 10.1097/CCM.0b013e318168f89d. [DOI] [PubMed] [Google Scholar]
- 98.Toy P., Popovsky M., Abraham E., et al. Transfusion-related acute lung injury: definition and review. Crit. Care Med. 2005;33(4):721–726. doi: 10.1097/01.ccm.0000159849.94750.51. [DOI] [PubMed] [Google Scholar]
- 99.Holness L., Knippen M., Simmons L., Lachenbruch P. Fatalities caused by TRALI. Transfus. Med. Rev. 2004;18(3):184–188. doi: 10.1016/j.tmrv.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 100.Lustenberger T., Talving P., Kobayashi L., et al. Early coagulopathy after isolated severe traumatic brain injury: relationship with hypoperfusion challenged. J. Trauma. 2010;69(6):1410–1414. doi: 10.1097/TA.0b013e3181cdae81. [DOI] [PubMed] [Google Scholar]
- 101.Wafaisade A., Lefering R., Tjardes T., et al. Acute coagulopathy in isolated blunt traumatic brain injury. Neurocrit. Care. 2010;12(2):211–219. doi: 10.1007/s12028-009-9281-1. [DOI] [PubMed] [Google Scholar]
- 102.Jacoby R., Owings J., Holmes J., Battistella F., Gosselin R., Paglieroni T. Platelet activation and function after trauma. J. Trauma. 2001;51(4):639–647. doi: 10.1097/00005373-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 103.Davis P., Musunuru H., Walsh M., et al. Platelet dysfunction is an early marker for traumatic brain injury-induced coagulopathy. Neurocrit. Care. 2013;18(2):201–208. doi: 10.1007/s12028-012-9745-6. [DOI] [PubMed] [Google Scholar]
- 104.Maegele M. Coagulopathy after traumatic brain injury: incidence, pathogenesis, and treatment options. Transfusion. 2013;53(Suppl. 1):28S–37. doi: 10.1111/trf.12033. [DOI] [PubMed] [Google Scholar]
- 105.Kutcher M., Redick B., McCreery R., et al. Characterization of platelet dysfunction after trauma. J. Trauma Acute Care Surg. 2012;73(1):13–19. doi: 10.1097/TA.0b013e318256deab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Laroche M., Kutcher M., Huang M., Cohen M., Manley G. Coagulopathy after traumatic brain injury. Neurosurgery. 2012;70(6):1334–1345. doi: 10.1227/NEU.0b013e31824d179b. [DOI] [PubMed] [Google Scholar]
- 107.Castellino F., Chapman M., Donahue D., et al. Traumatic brain injury causes platelet adenosine diphosphate and arachidonic acid receptor inhibition independent of hemorrhagic shock in humans and rats. J. Trauma Acute Care Surg. 2014;76(5):1169–1176. doi: 10.1097/TA.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Narayan R., Maas A., Servadei F., Skolnick B., Tillinger M., Marshall L. Progression of traumatic intracerebral hemorrhage: a prospective observational study. J. Neurotrauma. 2008;25(6):629–639. doi: 10.1089/neu.2007.0385. [DOI] [PubMed] [Google Scholar]
- 109.Kurland D., Hong C., Aarabi B., Gerzanich V., Simard J. Hemorrhagic progression of a contusion after traumatic brain injury: a review. J. Neurotrauma. 2012;29(1):19–31. doi: 10.1089/neu.2011.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stein D., Dutton R., Kramer M., Scalea T. Reversal of coagulopathy in critically ill patients with traumatic brain injury: recombinant factor VIIa is more cost-effective than plasma. J. Trauma. 2009;66(1):63–75. doi: 10.1097/TA.0b013e318191bc8a. [DOI] [PubMed] [Google Scholar]
- 111.Naidech A., Jovanovic B., Liebling S., et al. Reduced platelet activity is associated with early clot growth and worse 3-month outcome after intracerebral hemorrhage. Stroke. 2009;40(7):2398–2401. doi: 10.1161/STROKEAHA.109.550939. [DOI] [PubMed] [Google Scholar]
- 112.Naidech A., Liebling S., Rosenberg N., Lindholm P., Bernstein R., Batjer H. Early platelet transfusion improves platelet activity and may improve outcomes after intracerebral hemorrhage. Neurocrit. Care. 2012;16(1):82–87. doi: 10.1007/s12028-011-9619-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.British Committee for Standards in Haematology and Blood Tranfusion Task Force Guidelines for the use of platelet transfusions. Br. J. Haematol. 2003;122(1):10–23. doi: 10.1046/j.1365-2141.2003.04468.x. [DOI] [PubMed] [Google Scholar]
- 114.Stainsby D., MacLennan S., Thomas D., Isaac J., Hamilton P. Guidelines on the management of massive blood loss. Br. J. Haematol. 2006;135(5):634–641. doi: 10.1111/j.1365-2141.2006.06355.x. [DOI] [PubMed] [Google Scholar]
- 115.Gunter O., Au B., Isbell J., Mowery N., Young P., Cotton B. Optimizing outcomes in damage control resuscitation: identifying blood product ratios associated with improved survival. J. Trauma. 2008;65(3):527–534. doi: 10.1097/TA.0b013e3181826ddf. [DOI] [PubMed] [Google Scholar]
- 116.Holcomb J., Wade C., Michalek J., et al. Increased plasma and platelet to red blood cell ratios improves outcome in 466 massively transfused civilian trauma patients. Ann. Surg. 2008;248(3):447–458. doi: 10.1097/SLA.0b013e318185a9ad. [DOI] [PubMed] [Google Scholar]
- 117.Zink K., Sambasivan C., Holcomb J., Chisholm G., Schreiber M. A high ratio of plasma and platelets to packed red blood cells in the first 6 hours of massive transfusion improves outcomes in a large multicenter study. Am. J. Surg. 2009;197(5):565–570. doi: 10.1016/j.amjsurg.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 118.Perkins J., Cap A., Andrew C., et al. An evaluation of the impact of apheresis platelets used in the setting of massively transfused trauma patients. J. Trauma. 2009;66(4) Suppl.:S77–S84. doi: 10.1097/TA.0b013e31819d8936. [DOI] [PubMed] [Google Scholar]
- 119.Inaba K., Lustenberger T., Rhee P., et al. The impact of platelet transfusion in massively transfused trauma patients. J. Am. Coll. Surg. 2010;211(5):573–579. doi: 10.1016/j.jamcollsurg.2010.06.392. [DOI] [PubMed] [Google Scholar]
- 120.Holcomb J., Zarzabal L., Michalek J., Kozar R., Spinella P., Perkins J. Increased platelet: RBC ratios are associated with improved survival after massive transfusion. J. Trauma. 2011;71(2) Suppl. 3:S318–S328. doi: 10.1097/TA.0b013e318227edbb. [DOI] [PubMed] [Google Scholar]
- 121.Johansson P., Oliveri R., Ostrowski S. Hemostatic resuscitation with plasma and platelets in trauma. J. Emerg. Trauma Shock. 2012;5(2):120–125. doi: 10.4103/0974-2700.96479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.McCurdy M., Liew-Spilger A., Walsh M. Mortality and ratio of blood products used in patients with severe trauma. JAMA. 2015;313(20):2077–2079. doi: 10.1001/jama.2015.4421. [DOI] [PubMed] [Google Scholar]
- 123.Morrison J., Ross J., Dubose J., Jansen J., Midwinter M., Rasmussen T. Association of cryoprecipitate and tranexamic acid with improved survival following wartime injury: findings from the MATTERs II Study. JAMA Surg. 2013;148(3):218–225. doi: 10.1001/jamasurg.2013.764. [DOI] [PubMed] [Google Scholar]
- 124.Rugeri L., Levrat A., David J., et al. Diagnosis of early coagulation abnormalities in trauma patients by rotation thrombelastography. J. Thromb. Haemost. 2007;5(2):289–295. doi: 10.1111/j.1538-7836.2007.02319.x. [DOI] [PubMed] [Google Scholar]
- 125.Levrat A., Gros A., Rugeri L., et al. Evaluation of rotation thrombelastography for the diagnosis of hyperfibrinolysis in trauma patients. Br. J. Anaesth. 2008;100(6):792–797. doi: 10.1093/bja/aen083. [DOI] [PubMed] [Google Scholar]
- 126.Brohi K., Cohen M., Davenport R. Acute coagulopathy of trauma: mechanism, identification and effect. Curr. Opin. Crit. Care. 2007;13(6):680–685. doi: 10.1097/MCC.0b013e3282f1e78f. [DOI] [PubMed] [Google Scholar]
- 127.Schöchl H., Frietsch T., Pavelka M., Jámbor C. Hyperfibrinolysis after major trauma: differential diagnosis of lysis patterns and prognostic value of thrombelastometry. J. Trauma. 2009;67(1):125–131. doi: 10.1097/TA.0b013e31818b2483. [DOI] [PubMed] [Google Scholar]
- 128.Theusinger O., Wanner G., Emmert M., et al. Hyperfibrinolysis diagnosed by rotational thromboelastometry (ROTEM) is associated with higher mortality in patients with severe trauma. Anesth. Analg. 2011;113(5):1003–1012. doi: 10.1213/ANE.0b013e31822e183f. [DOI] [PubMed] [Google Scholar]
- 129.Brenni M., Worn M., Brüesch M., Spahn D., Ganter M. Successful rotational thromboelastometry-guided treatment of traumatic haemorrhage, hyperfibrinolysis and coagulopathy. Acta Anaesthesiol. Scand. 2010;54(1):111–117. doi: 10.1111/j.1399-6576.2009.02132.x. [DOI] [PubMed] [Google Scholar]
- 130.Carroll R., Craft R., Langdon R., et al. Early evaluation of acute traumatic coagulopathy by thrombelastography. Transl. Res. 2009;154(1):34–39. doi: 10.1016/j.trsl.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 131.Schöchl H., Nienaber U., Maegele M., et al. Transfusion in trauma: thromboelastometry-guided coagulation factor concentrate-based therapy versus standard fresh frozen plasma-based therapy. Crit. Care. 2011;15(2):34–39. doi: 10.1186/cc10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nienaber U., Innerhofer P., Westermann I., et al. The impact of fresh frozen plasma vs coagulation factor concentrates on morbidity and mortality in trauma-associated haemorrhage and massive transfusion. Injury. 2011;42(7):697–701. doi: 10.1016/j.injury.2010.12.015. [DOI] [PubMed] [Google Scholar]
- 133.Cotton B., Au B., Nunez T., Gunter O., Robertson A., Young P. Predefined massive transfusion protocols are associated with a reduction in organ failure and postinjury complications. J. Trauma. 2009;66(1):41–49. doi: 10.1097/TA.0b013e31819313bb. [DOI] [PubMed] [Google Scholar]
- 134.Naidech A., Bassin S., Bernstein R., et al. Reduced platelet activity is more common than reported anti-platelet medication use in patients with intracerebral hemorrhage. Neurocrit. Care. 2009;11(3):307–310. doi: 10.1007/s12028-009-9219-7. [DOI] [PubMed] [Google Scholar]
- 135.Collyer T., Gray D., Sandhu R., Berridge J., Lyons G. Assessment of platelet inhibition secondary to clopidogrel and aspirin therapy in preoperative acute surgical patients measured by thromboelastography platelet mapping. Br. J. Anaesth. 2009;102(4):492–498. doi: 10.1093/bja/aep039. [DOI] [PubMed] [Google Scholar]
- 136.Madsen E., Saw J., Kristensen S., Schmidt E., Pittendreigh C., Maurer-Spurej E. Long-term aspirin and clopidogrel response evaluated by light transmission aggregometry, verifynow, and thromboelastography in patients undergoing percutaneous coronary intervention. Clin. Chem. 2010;56(5):839–847. doi: 10.1373/clinchem.2009.137471. [DOI] [PubMed] [Google Scholar]
- 137.Simon D., Pairkh S. Hunting for the “sweet spot” in P2Y12 receptor blockade. J. Am. Coll. Cardiol. 2009;54(15):1447–1449. doi: 10.1016/j.jacc.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 138.Gurbel P., Mahla E., Tantry U. Peri-operative platelet function testing: the potential for reducing ischaemic and bleeding risks. Thromb. Haemost. 2011;106(2):248–252. doi: 10.1160/TH11-02-0063. [DOI] [PubMed] [Google Scholar]
- 139.Chen L., Bracey A., Radovancevic R., et al. Clopidogrel and bleeding in patients undergoing elective coronary artery bypass grafting. J. Thorac. Cardiovasc. Surg. 2004;128(3):425–431. doi: 10.1016/j.jtcvs.2004.02.019. [DOI] [PubMed] [Google Scholar]
- 140.Batchelor J., Grayson A. A meta-analysis to determine the effect on survival of platelet transfusions in patients with either spontaneous or traumatic antiplatelet medication-associated intracranial haemorrhage. BMJ Open. 2012;2:e000588. doi: 10.1136/bmjopen-2011-000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hepner D., Concepcion M., Bhavani-shankar K. Coagulation status using thromboelastography in patients receiving warfarin prophylaxis and epidural analgesia. J. Clin. Anesth. 2002;14(6):405–410. doi: 10.1016/s0952-8180(02)00373-2. [DOI] [PubMed] [Google Scholar]
- 142.Omert L. Thromboelastograph (TEG) Overview. Haemonetics. [Google Scholar]
- 143.Schiele F., van Ryn J., Canada K., et al. A specific antidote for dabigatran: functional and structural characterization. Blood. 2013;121(18):3554–3562. doi: 10.1182/blood-2012-11-468207. [DOI] [PubMed] [Google Scholar]
- 144.Pearson S., Troughton R., Richards A. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N. Engl. J. Med. 2011;365(24):2334–2335. doi: 10.1056/NEJMc1112233. [DOI] [PubMed] [Google Scholar]
- 145.Connolly S., Ezekowitz M., Yusuf S., et al. Dabigatran versus warfarin in patients with atrial fibrillation. N. Engl. J. Med. 2009;361(12):1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 146.Warkentin T., Margetts P., Connolly S., Lamy A., Ricci C., Eikelboom J. Recombinant factor VIIa (rFVIIa) and hemodialysis to manage massive dabigatran-associated postcardiac surgery bleeding. Blood. 2012;119(9):2172–2174. doi: 10.1182/blood-2011-11-393587. [DOI] [PubMed] [Google Scholar]
- 147.Garber S., Sivakumar W., Schmidt R. Neurosurgical complications of direct thrombin inhibitors-catastrophic hemorrhage after mild traumatic brain injury in a patient receiving dabigatran. J. Neurosurg. 2012;116(5):1093–1096. doi: 10.3171/2012.2.JNS112132. [DOI] [PubMed] [Google Scholar]
- 148.Truumees E., Gaudu T., Dieterichs C., Geck M., Stokes J. Epidural hematoma and intraoperative hemorrhage in a spine trauma patient on pradaxa (dabigatran). Spine. 2012;37(14):E863–E865. doi: 10.1097/BRS.0b013e31824ee320. [DOI] [PubMed] [Google Scholar]
- 149.Dager W., Gosselin R., Roberts A. Reversing dabigatran in life-threatening bleeding occurring during cardiac ablation with factor eight inhibitor bypassing activity. Crit. Care Med. 2013;41(5):e42–e46. doi: 10.1097/CCM.0b013e31827caaa3. [DOI] [PubMed] [Google Scholar]
- 150.Wychowski M., Kouides P. Dabigatran-induced gastrointestinal bleeding in an elderly patient with moderate renal impairment. Ann. Pharmacother. 2012;46(4):e10. doi: 10.1345/aph.1Q747. [DOI] [PubMed] [Google Scholar]
- 151.Dumkow L., Voss J., Peters M., Jennings D. Reversal of dabigatran-induced bleeding with a prothrombin complex concentrate and fresh frozen plasma. Am. J. Health Syst. Pharm. 2012;69(16):1646–1650. doi: 10.2146/ajhp120055. [DOI] [PubMed] [Google Scholar]
- 152.Southworth M., Reichman M., Unger E. Dabigatran and postmarketing reports of bleeding. N. Engl. J. Med. 2013;368(14):1275–4. doi: 10.1056/NEJMp1302834. [DOI] [PubMed] [Google Scholar]
- 153.Schulman S., Crowther M. How I treat with anticoagulants in 2012: new and old anticoagulants, and when and how to switch. Blood. 2012;119(13):3016–3023. doi: 10.1182/blood-2011-10-378950. [DOI] [PubMed] [Google Scholar]
- 154.Spahn D., Korte W. Novel oral anticoagulants: new challenges for anesthesiologists in bleeding patients. Anesthesiology. 2012;116(1):9–11. doi: 10.1097/ALN.0b013e318238c070. [DOI] [PubMed] [Google Scholar]
- 155.Godier A., Miclot A., Le Bonniec B., et al. Evaluation of prothrombin complex concentrate and recombinant activated factor VII to reverse rivaroxaban in a rabbit model. Anesthesiology. 2012;116(1):94–102. doi: 10.1097/ALN.0b013e318238c036. [DOI] [PubMed] [Google Scholar]
- 156.Eerenberg E., Kamphuisen P., Sijpkens M., Meijers J., Buller H., Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124(14):1573–1579. doi: 10.1161/CIRCULATIONAHA.111.029017. [DOI] [PubMed] [Google Scholar]
- 157.Kaatz S., Kouides P., Garcia D., et al. Guidance on the emergent reversal of oral thrombin and factor Xa inhibitors. Am. J. Hematol. 2012;87(Suppl. 1):S141–S145. doi: 10.1002/ajh.23202. [DOI] [PubMed] [Google Scholar]
- 158.Marlu R., Hodaj E., Paris A., Albaladejo P., Cracowski J., Pernod G. Effect of non-specific reversal agents on anticoagulant activity of dabigatran and rivaroxaban: a randomised crossover ex vivo study in healthy volunteers. Thromb. Haemost. 2012;108(2):217–224. doi: 10.1160/TH12-03-0179. [DOI] [PubMed] [Google Scholar]
- 159.Sarma A., Rossi J., Connors J., Giugliano R. Dabigatran excess: case report and review of the literature. Cardiol. Ther. 2013;2(1):111–124. doi: 10.1007/s40119-013-0016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schaden E., Schober A., Hacker S., Kozek-Langenecker S. Ecarin modified rotational thrombelastometry: a point-of-care applicable alternative to monitor the direct thrombin inhibitor argatroban. Wien. Klin. Wochenschr. 2013;125(5-6):156–159. doi: 10.1007/s00508-013-0327-1. [DOI] [PubMed] [Google Scholar]
- 161.Schaden E., Schober A., Hacker S., Spiss C., Chiari A., Kozek-Langenecker S. Determination of enoxaparin with rotational thrombelastometry using the prothrombinase-induced clotting time reagent. Blood Coagul. Fibrinolysis. 2010;21(3):256–261. doi: 10.1097/MBC.0b013e328337014c. [DOI] [PubMed] [Google Scholar]
- 162.Dias J., Norem K., Doorneweerd D., Thurer R., Popovsky M., Omert L. Use of thromboelastography (TEG) for detection of new oral anticoagulants. Arch. Pathol. Lab. Med. 2015;139:665–673. doi: 10.5858/arpa.2014-0170-OA. [DOI] [PubMed] [Google Scholar]
- 163.Connors J. Antidote for factor Xa anticoagulants. N. Engl. J. Med. 2015 doi: 10.1056/NEJMe1513258. [DOI] [PubMed] [Google Scholar]
- 164.Bauer K. Targeted anti-anticoagulants. N. Engl. J. Med. 2015;373:569–571. doi: 10.1056/NEJMe1506600. [DOI] [PubMed] [Google Scholar]
- 165.Pollack C., Jr, Reilly P., Eikelboom J., et al. Idarucizumab for dabigatran reversal. N. Engl. J. Med. 2015;373(6):511–520. doi: 10.1056/NEJMoa1502000. [DOI] [PubMed] [Google Scholar]
- 166.Konkle B. Acquired disorders of platelet function. Hematology Am Soc Mematol Educ Program; 2011. pp. 391–396. [DOI] [PubMed] [Google Scholar]
- 167. Saw palmetto indications and side effects. Available from: http://www.drugs.com/sfx/saw-palmetto-side-effects.html .
- 168.Shakur H., Roberts I., Bautista R., et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant hemorrhage (CRASH-2): a randomized, placebo-controlled trial. Lancet. 2010;376(9734):23–32. doi: 10.1016/S0140-6736(10)60835-5. [DOI] [PubMed] [Google Scholar]
- 169.Napolitano L., Cohen M., Cotton B., Schreiber M., Moore E. Tranexamic acid in trauma: how should we use it? J. Trauma Acute Care Surg. 2013;74(6):1575–1586. doi: 10.1097/TA.0b013e318292cc54. [DOI] [PubMed] [Google Scholar]
- 170.Raza I., Davenport R., Rourke C., Platton S., Manson J., Spoors C. The incidence and magnitude of fibrinolytic activation in trauma patients. J. Thromb. Haemost. 2013;11(2):307–314. doi: 10.1111/jth.12078. [DOI] [PubMed] [Google Scholar]
- 171.Schochl H., Voelckel W., Maegele M., Solomon C. Trauma-associated hyperfibrinolysis. Hamostaseologie. 2012;32(1):22–27. doi: 10.5482/ha-1178. [DOI] [PubMed] [Google Scholar]
- 172.Morrison J., Dubose J., Rasmussen T., Midwinter M. Military application of tranexamic acid in trauma emergency resuscitation (MATTERs) study. Arch. Surg. 2012;147(2):113–119. doi: 10.1001/archsurg.2011.287. [DOI] [PubMed] [Google Scholar]
- 173.Pusateri A., Weiskopf R., Bebarta V., et al. Tranexamic acid and trauma: current status and knowledge gaps with recommended research priorities. Shock. 2013;39(2):121–126. doi: 10.1097/SHK.0b013e318280409a. [DOI] [PubMed] [Google Scholar]
- 174.Grassetto A., De Nardin M., Ganzerla B., et al. ROTEM®-guided coagulation factor concentrate therapy in trauma: 2-year experience in Venice, Italy. Crit. Care. 2012;16(3):428. doi: 10.1186/cc11322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Grassetto A., Saggioro D., Caputo P., et al. Rotational thromboelastometry analysis and management of life-threatening haemorrhage in isolated craniofacial injury. Blood Coagul. Fibrinolysis. 2012;23(6):551–555. doi: 10.1097/MBC.0b013e32835553c0. [DOI] [PubMed] [Google Scholar]
- 176.Schöchl H., Posch A., Hanke A., Voelckel W., Solomon C. High-dose fibrinogen concentrate for haemostatic therapy of a major trauma patient with recent clopidogrel and aspirin intake. Scand. J. Clin. Lab. Invest. 2010;70(6):453–457. doi: 10.3109/00365513.2010.500396. [DOI] [PubMed] [Google Scholar]
- 177.Schöchl H., Schlimp C., Voelckel W. Potential value of pharmacological protocols in trauma. Curr. Opin. Anaesthesiol. 2013;26(2):221–229. doi: 10.1097/ACO.0b013e32835cca92. [DOI] [PubMed] [Google Scholar]
- 178.Brown J., Neal M., Guyette F., et al. Design of the study of tranexamic acid during air medical prehospital transport (STAAMP) trial: Addressing the knowledge gaps. Prehosp. Emerg. Care. 2014;19(1):79–86. doi: 10.3109/10903127.2014.936635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Valle E., Allen C., Van Haren R., et al. Do all trauma patients benefit from tranexamic acid? J. Trauma Acute Care Surg. 2014;76(6):1373–1378. doi: 10.1097/TA.0000000000000242. [DOI] [PubMed] [Google Scholar]
- 180.Cole E., Davenport R., Willett K., Brohi K. Tranexamic acid use and the effects on outcomes. Ann. Surg. 2014;261(2):390–394. doi: 10.1097/SLA.0000000000000717. [DOI] [PubMed] [Google Scholar]
- 181.Harvin J, Peirce C, Mims MH. The impact of tranexamic acid on mortality in injured patients with hyperfibrinolysis. J. Trauma Acute Care Surg. 2015;78(5):905–911. doi: 10.1097/TA.0000000000000612. [DOI] [PubMed] [Google Scholar]
- 182.Shakur H. The WOMAN trial (world maternal antifibrinolytic trial): tranexamic acid for the treatment of postpartum haemorrhage: an international randomised, double blind placebo controlled trial. Trials. 2010;11(40) doi: 10.1186/1745-6215-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.de Lange N., Lance M., de Groot R., Beckers E., Henskens H., Scheepers H. Obstetric hemorrhage and coagulation: an update. Thromboelastography, thromboelastometry, and conventional coagulation tests in the diagnosis and prediction of postpartum hemorrhage. Obstet. Gynecol. Surv. 2012;67(7):426–435. doi: 10.1097/OGX.0b013e3182605861. [DOI] [PubMed] [Google Scholar]
- 184.Soreide K. Epidemiology of major trauma. Br J Surg Soc Ltd. 2009;96:697–698. doi: 10.1002/bjs.6643. [DOI] [PubMed] [Google Scholar]
- 185.Hoffman M., Dargaud Y. Mechanisms and monitoring of bypassing agent therapy. J. Thromb. Haemost. 2012;10:1478–1485. doi: 10.1111/j.1538-7836.2012.04793.x. [DOI] [PubMed] [Google Scholar]
- 186.Spinella P., Perkins J., McLaughlin D., et al. The effect of recombinant activated factor VII on mortality in combat-related casualties with severe trauma and massive transfusion. J. Trauma. 2008;64(2):286–294. doi: 10.1097/TA.0b013e318162759f. [DOI] [PubMed] [Google Scholar]
- 187.Dutton R., McCunn M., Hyder M., et al. Factor VIIa for correction of traumatic coagulopathy. J. Trauma. 2004;57:709–719. doi: 10.1097/01.ta.0000140646.66852.ab. [DOI] [PubMed] [Google Scholar]
- 188.Clark A., Gordon W., Walker I., Tait R. Last-ditch use of recombinant factor VIIa in patients with massive haemorrhage is ineffective. Vox Sang. 2004;86(2):120–124. doi: 10.1111/j.0042-9007.2004.00393.x. [DOI] [PubMed] [Google Scholar]
- 189.Kenet G., Walden R., Eldad A., Martinowitz U. Treatment of traumatic bleeding with recombinant factor VIIa. Lancet. 1999;354(9193):1879. doi: 10.1016/S0140-6736(99)05155-7. [DOI] [PubMed] [Google Scholar]
- 190.Mitra B., Cameron P., Parr M., Phillips L. Recombinant factor VIIa in trauma patients with the “triad of death”. Injury. 2012;43(9):1409–1414. doi: 10.1016/j.injury.2011.01.033. [DOI] [PubMed] [Google Scholar]
- 191.Boffard K., Riou B., Warren B., Choong P., Rizoli S., Rossaint R. Recombinant factor VIIa as adjunctive therapy for bleeding control in severely injured trauma patients: two parallel randomized, placebo-controlled, double-blind clinical trials. J. Trauma Inj. Infect. Crit. Care. 2005;59(1):8–18. doi: 10.1097/01.ta.0000171453.37949.b7. [DOI] [PubMed] [Google Scholar]
- 192.Mamtani R., Nascimento B., Rizoli S., Pinto R., Lin Y., Tien H. The utility of recombinant factor VIIa as a last resort in trauma. World J. Emerg. Surg. 2012;22(1) Suppl.:S7. doi: 10.1186/1749-7922-7-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Narayan R., Maas A., Marshall L., Servadei F., Skolnick B., Tillinger M. Recombinant factor VIIA in traumatic intracerebral hemorrhage: results of a dose-escalation clinical trial. Neurosurgery. 2008;62(4):786–788. doi: 10.1227/01.neu.0000316898.78371.74. [DOI] [PubMed] [Google Scholar]
- 194.Bartal C., Freedman J., Bowman K., Cusimano M. Coagulopathic patients with traumatic intracranial bleeding: defining the role of recombinant factor VIIa. J. Trauma. 2007;63(4):725–732. doi: 10.1097/TA.0b013e318031ccca. [DOI] [PubMed] [Google Scholar]
- 195.James K., Melikian C., Chowdary P., Mallett S. Thromboelastography-guided recombinant factor VIIa administration in a patient with refractory autoimmune idiopathic thrombocytopenia. Anesth. Analg. 2008;107(2):402–405. doi: 10.1213/ane.0b013e3181770b87. [DOI] [PubMed] [Google Scholar]
- 196.Bartal C., Yitzhak A. The role of thromboelastometry and recombinant factor VIIa in trauma. Curr. Opin. Anaesthesiol. 2009;22(2):281–288. doi: 10.1097/ACO.0b013e328325a6be. [DOI] [PubMed] [Google Scholar]
- 197.Grottke O., van Ryn J., Spronk H.M., Rossaint R. Prothrombin complex concentrates and a specific antidote to dabigatran are effective ex-vivo in reversing the effects of dabigatran in an anticoagulation/liver trauma experimental model. Crit. Care. 2014;18(1):R27. doi: 10.1186/cc13717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Labrador J., González-Porras J. The balance between thrombosis and bleeding in allogeneic hematopoietic stem cell transplant recipients. J Hematol Thromb Dis. 2014;2:e105. [Google Scholar]
- 199.Wójcik C., Schymik M., Cure E. Activated prothrombin complex concentrate factor VIII inhibitor bypassing activity (FEIBA) for the reversal of warfarin-induced coagulopathy. Int. J. Emerg. Med. 2009;2(4):217–225. doi: 10.1007/s12245-009-0125-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Brooks A., Guillaumin J., Cooper E., Couto C. Effects of hematocrit and red blood cell-independent viscosity on canine thromboelastographic tracings. Transfusion. 2012;54:727–734. doi: 10.1111/trf.12354. [DOI] [PubMed] [Google Scholar]
- 201.Spiezia L., Radu C., Marchioro P., et al. Peculiar whole blood rotation thromboelastometry (Rotem) profile in 40 sideropenic anaemia patients. Thromb. Haemost. 2008;100(6):1106–1110. [PubMed] [Google Scholar]
- 202.Solomon C., Rahe-Meyer N., Schöchl H., Ranucci M., Görlinger K. Effect of haematocrit on fibrin-based clot firmness in the FIBTEM test. Blood Transfus. 2013;11(3):412. doi: 10.2450/2012.0043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Schöchl H., Cotton B., Inaba K., et al. FIBTEM provides early prediction of massive transfusion in trauma. Crit. Care. 2011;15(6):R265. doi: 10.1186/cc10539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Levi M., Hunt B. A critical appraisal of point‐of‐care coagulation testing in critically ill patients. J. Thromb. Haemost. 2015;13(11):1960–1967. doi: 10.1111/jth.13126. [DOI] [PubMed] [Google Scholar]
- 205.Dzik W., Blajchman M., Fergusson D., et al. Clinical review: Canadian national advisory committee on blood and blood products – massive transfusion consensus conference 2011: report of the panel. Crit. Care. 2011;15(242):1–12. doi: 10.1186/cc10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Johansson P., Stensballe J., Oliveri R., Wade C., Ostrowski S., Holcomb J. How I treat patients with massive hemorrhage. Blood. 2014;124(20):3052–3057. doi: 10.1182/blood-2014-05-575340. [DOI] [PubMed] [Google Scholar]
- 207.Kane I., Ong A., Orozco F., Post Z., Austin L., Radcliff K. Thromboelastography predictive of death in trauma patients. Orthop. Surg. 2015;7(1):26–30. doi: 10.1111/os.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Grassetto A., Paniccia R., Biancofiore G. General aspects of viscoelastic tests. In: Ranucci M., Simioni P., editors. Point-of-Care Tests for Severe Hemorrhage: A Manual for Diagnosis and Treatment. 1st ed. Springer; 2015. [Google Scholar]
- 209.Spahn D. TEG®-or ROTEM®-based individualized goal-directed coagulation algorithms: don’t wait-act now! Crit. Care. 2014;18(6):637. doi: 10.1186/s13054-014-0637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gonzalez E., Moore E., Moore H., et al. Goal-directed hemostatic resuscitation of trauma-induced coagulopathy: a pragmatic randomized clinical trial comparing a viscoelastic assay to conventional coagulation assays. Ann. Surg. 2015;262(6) doi: 10.1097/SLA.0000000000001608. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]


