Abstract
Here, we report the draft genome sequence and annotation of plant growth promoting rhizobacterium Enterobacter cloacae SBP-8 isolated from the rhizosphere of Sorghum bicolor L. growing in desert region of Rajasthan, India. From the genome sequences, we identified the genes encoding plant growth promoting properties such as 1-aminocyclopropane-1-carboxylate deaminase (AcdS), phosphate solubilisation, siderophore, and IAA (indole acetic acid) production. The genes encoding different functions required for colonization including motility, chemotaxis, adherence, and secretion system (I, II, IV, VI) were also identified. The complete genome sequence of this bacterium consists of one chromosome (48,54,065 bp) and one plasmid (85,398). The genome sequence of Enterobacter cloacae SBP-8 was deposited in the Genbank with the accession number CP016906 (chromosome) and CP017413 (plasmid).
| Specifications | |
|---|---|
| Organism/cell line/tissue | Enterobacter cloacae SBP-8 |
| Sequencer or array type | Illumina Paired-end sequencing |
| Data format | Analyzed |
| Experimental factors | Microbial strain |
| Experimental features | Draft genome sequence of Enterobacter cloacae SBP-8 |
| Consent | N/A |
| Sample source location | Desert region of Rajasthan, India (28°18′N, and 74°58′E) |
1. Direct link to deposited data
https://www.ncbi.nlm.nih.gov/nuccore/1057959579
2. Introduction
Enterobacter cloacae SBP-8 is a gram negative, facultative anaerobic rod shaped proteobacteria, a genus of Enterobacteriaceae widely found in all natural environments. Complete genome annotation and interpretation of a few Enterobacter cloacae is available, however, most analysis was focused on their endophytic as well as pathogenic behaviour [1], [2], [3]. Enterobacter cloacae have been reported to increase plant growth and crop yields [3]. Recently, draft genome sequence of a chromium-resistant bacterium, Enterobacter cloacae B2-DHA was analyzed with regard to search the genes potentially involved in bioremediation of chromium and other toxic metals in polluted environments [4]. The plant pathogenic effects of Enterobacter cloacae strains have been reported [5], [6]. In addition, a few Enterobacter species are also known to be human opportunistic pathogens responsible for nosocomial infections such as osteomyelitis, cholecystitis, and neonatal meningitis [2]. However, information about plant associative Enterobacter sp. effectively behaving as PGPR is scarce. Therefore, sequencing and genome information of PGPR Enterobacter cloacae SBP-8 would provide the valuable information related to mechanism of plant growth promotion, antagonistic activity and its pathogenecity. Ability of Enterobacter cloacae SBP-8 (previously identified as Klebsiella sp. based partial rRNA gene sequence) to promote growth of wheat plant under salt stress has been reported in our previous study [7].
3. Experimental design, materials and methods
3.1. Sequencing and assembly of Enterobacter cloacae SBP-8 genome
Genomic DNA of isolate Enterobacter cloacae SBP-8 was extracted by standard method using bacterial DNA extraction kit (Qiagen, USA). De-novo whole genome sequencing was done using Illumina Paired-end sequencing platform technology. The Illumina paired end raw reads were quality checked using FastQC. Illumina raw reads were processed by in-house perl script for adapters and low quality bases trimming towards 3′-end. De-novo assembly of Illumina PE data was performed using SPAdes assembler. SPAdes assembler is intended for de novo assembly after error-correction of sequenced reads. Assembled contigs were further scaffold using SSPACE program. Genes from assembled scaffolds were annotated using NCBI genomic tools and pathways were determined using online available program RAST server. The Enterobacter cloacae SBP-8 genome sequence was first annotated using web-based automated pipelines including Bacterial Annotation System (BASys) v1.0. The functional annotation of genome sequence was performed against the databases of KEGG (http://www.genome.jp/kegg/), COG (http://www.ncbi.nlm.nih.gov/COG/) and GO (http://www.geneontology.org/).
3.2. Features of Enterobacter cloacae SBP-8 genome
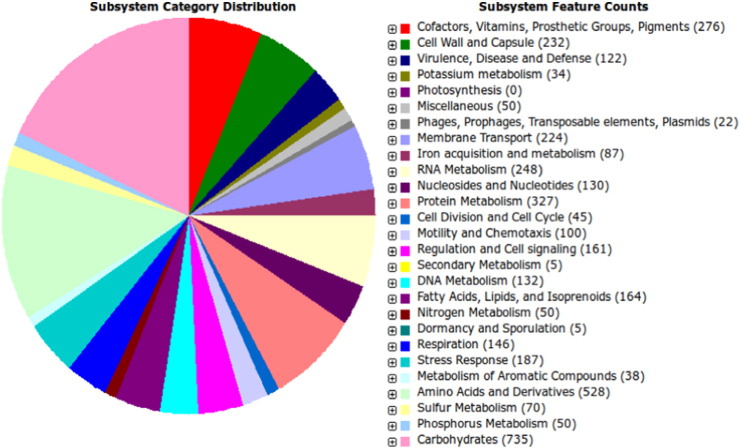
The complete genome of Enterobacter cloacae SBP-8 contains a circular chromosome (4,854,065) and a plasmid (85,398). A total of 4192 genes were identified, which encodes 4052 proteins. The genome consists of 73 genes for tRNA and 14 for rRNAs. The average G + C content was found to be 55%. The genomic analysis identified the presence of pseudogenes (44) and non-coding rRNA (9). The average contig length of the WGS library turned out to be 6241 bp with median contig length of 782 bp. The protein-coding genes have an average length of 915 bp and account for 87.5% of the chromosome. Twenty three putative GIs were identified by Island Viewer, which integrates two prediction methods Island Path (DNA composition comparison) and SIGI-HMM (codon usage). The size of putative islands ranged from 4176 bp to 54,345 bp. Several genes encoding flagellar proteins and chemotaxis were detected in the genome of SBP-8. It includes genes encoding flagellar structural proteins (FliL, FliP, FliR, FliC, FlhB, FliZ, FliS, FlgN, FlhA, FliQ, FliT, FliM, FliN), chemotaxis proteins (CheV, CheR, CheW, Che Z, Che Y, Che A,). RAST server based annotation of the whole genome describes the subsystem distribution of Enterobacter cloacae SBP-8. Genes responsible for carbohydrate metabolism (735 ORFs), amino acids and derivatives (528 ORFs), cofactors and vitamin sysnthesis (276) and cell wall and capsule formation (232 ORFs) were abundant among the subsystem categories. It also possessed several genes for heavy metals (Zn, As, and Cu) resistance (187), protein metabolism (327), membrane transport (224) and RNA metabolism (248) (Fig. 1). The presence of higher number of motility and chemotaxis genes (100) in the test isolate might be involved in the mechanisms of colonization. The test isolate showed the salinity and alkali tolerant behaviour and it contains a large number of stress-responsive genes.
Fig. 1.
Subsystems distribution statistic of Enterobacter cloacae SBP-8 (based on RAST annotation server).
Genomic analysis indicated presence of secretion systems, I, II, IV and VI which are known to play important role in infection process. Presence of curli and other genes required for biofilm formation suggest that the bacterial isolate SBP-8 can form biofilm on or in plants for its maintenance. The biofilm formation can be mediated by quorum sensing mechanism which supported from the presence of genes encoding AI-2 mediated quourm-sensing in the genome of SBP-8. Its function in Enterobacter cloacae SBP-8 illustrates the presence of pgaABCD operon, all of which are necessary for biofilm formation. PgaA mediates the protein-protein interactions, implying that it forms the outer membrane secretin for PGA synthesis. PgaB contains carbohydrate binding and polysaccharide N-deacetylase domains. The over-expression of pgaB increased the primary amine content (glucosamine) of PGA. PgaC is an apparent glycosyltransferase that is required for PGA synthesis. Consistent with this observation, several siderophore biosynthesis pathways were found, such as those for siderophore aerobactin (FhuA, FhuB, IucB, IucD, IucA, FhuC, FhuD), siderophore enterobactin (FepE, FepG, FepD, FepC, FepB, EntS, EntB), iron transporter (EfeB, EfeU), and iron transport regulator protein (FuR, PiuB, FeoC). For phytohormone IAA production, gene related to Tryptophan synthase (alpha and beta chain) catalyzes the last step in the biosynthesis of tryptophan was found. The gene related to biosynthesis of tryptophan (phosphoribosylanthranilate isomerase) was found as a single copy. Similarly, anthranilate phosphoribosyltransferase, that catalyze the most fundamental biochemical reactions in the aromatic amino acid biosynthesis pathway specifically the tryptophan synthesis were present. The strain SBP-8 is salt (NaCl) tolerant and have the ability to promote the plant growth in saline soil. Following these observation, a large number of genes related to osmoprotectant production were identified by genome sequencing. The genes involved in the biosynthesis and accumulation of osmoprotectants were found, such as those for carnitine (OpuAB, opuCB), choline (ChoV, choW), glycine betaine (GbsA), and proline (ProP, ProV, ProW, ProX, ProY). The draft genome sequence of Enterobacter cloacae SBP-8 identified the various heavy metal resistance genes. Copper translocating P-type ATPase (CopA) is involved in the copper resistance of bacteria. However, its presence in Enterobacter cloacae is still unknown. The gene encoding CopC a periplasmic protein involved in copper resistance, CopD trans-membrane protein and CueO protein involved in copper oxidizing were identified. Similarly, Copper-sensing two-component system response regulator (CusR) is the plasmid-borne system that is involved in metal-responsive gene regulation. Besides copper, SBP-8 also possesses resistance to other heavy metals, such as zinc and cobalt. Among the zinc resistance, genes for zinc efflux, and zinc transporter (ZitB) were noted. Similarly, multiple metal (cobalt/zinc/cadmium) resistance transporter proteins belonging to CzcB family was also identified in the genome of SBP-8. Arsenic resistance determinants were found in the genome of Enterobacter cloacae SBP-8, including operon ArsRBCH and ArsB, ArsC genes. Multiple antibiotic resistance genes (MarA, MarB, MarC, MarR) were identified in the genome of Enteroabcter cloacae SBP-8. Besides, six copy multidrug transporters (Mdt ABCD) capable of protecting cells against a wide variety of environmental toxins by active extrusion of noxious compounds were identified. In addition to this, multiple copies of multidrug-resistance efflux pumps including AcrAB operon were observed in the genome of SBP-8. Thus, the genome sequence of E. cloacae SBP-8 provides an opportunity to gain insights into the differences between the diverse group of E. cloacae involving endophytic-plant and human-pathogenic strains through comparative genome analysis. Moreover, the strain SBP-8 harbours several important genes whose functionality and pathogenic behaviour of strain has yet to be revealed.
Conflict of interest
The authors declare no conflicts of interest in this study.
Acknowledgement
The present work was financially supported by Department of Biotechnology (File No. BT/PR14527/AGR/21/326/2010), Govt. of India, New Delhi to PNJ.
References
- 1.Liu W.Y., Chung K.M.K., Wong C.F., Jiang J.W., Hui R.K.H., Leung F.C.C. Complete genome sequence of the endophytic Enterobacter cloacae subsp. cloacae strain ENHKU01. J. Bacteriol. 2012;194(21) doi: 10.1128/JB.01394-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ren Y., Zhou Z., Guo X., Li Y., Feng L., Wang L. Complete genome sequence of Enterobacter cloacae subsp. cloacae Type strain ATCC 13047. J. Bacteriol. 2010;192:2463–2464. doi: 10.1128/JB.00067-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taghavi S., Van der Lelie D., Hoffman D.A., Zhang Y.B., Walla M.D., Vangronsveld J., Newman L.J., Monchy S. Genome sequence of the plant growth promoting endophytic bacterium Enterobacter sp. PLoS Genet. 2010;638(6) doi: 10.1371/journal.pgen.1000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahman A., Nahar N., Olsson B., Mandal A. Complete genome sequence of Enterobacter cloacae B2-DHA, a chromium-resistant bacterium. Genome Announc. 2016;4(3) doi: 10.1128/genomeA.00483-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nishijima K.A., Alvarez A.M., Hepperly P.R., Shinta-ku M.H., Keith L.M., Sato D.M., Bushe B.C., Armstrong J.W., Zee F.T. Association of Enterobacter cloacae with rhizome rot of edible ginger in Hawaii. Plant Dis. 2004;88:1318–1327. doi: 10.1094/PDIS.2004.88.12.1318. [DOI] [PubMed] [Google Scholar]
- 6.Nishijima K.A., Wall M.M., Siderhurst M.S. Demonstrating pathogenicity of Enterobacter cloacae on macadamia and identifying associated volatiles of gray kernel of macadamia in Hawaii. Plant Dis. 2007;91:1221–1228. doi: 10.1094/PDIS-91-10-1221. [DOI] [PubMed] [Google Scholar]
- 7.Singh R.P., Jha P., Jha P.N. The plant growth promoting bacterium Klebsiella sp. SBP-8 confers induced systemic tolerance in wheat (Triticum aestivum) under salt stress. J. Plant Physiol. 2015;184:57–67. doi: 10.1016/j.jplph.2015.07.002. [DOI] [PubMed] [Google Scholar]