Abstract
Several randomized controlled trials have shown a benefit of high-dose intensive statin treatment in reducing risk of death and second cardiovascular disease (CVD) events in patients previously diagnosed with an acute coronary syndrome (ACS). Non-randomized studies in clinical settings support these findings, but large, long-term, observational studies addressing CVD and non-CVD endpoints are lacking. In this retrospective longitudinal study, we followed ACS patients in Sweden during 2001–2012 using national health registry and medical record data. A total of 49,857 patients were identified, of whom 10,092 (20.2%) received high dose statins and 21,174 (42.7%) received no statins. Royston-Parmar parametric time-to-event models were implemented to model hazard for second CVD events and death, stratified by gender and diabetes diagnosis. We found that risk of a second CVD event developed similarly in both treatment groups, but was much higher in the no statin group. Risk of CVD-related death remained relatively constant for the high-statin group, while it increased over time for the no-statin group. Interestingly, males had higher mortality rates in the no-statin group, but not in the high-statin group. All-cause mortality and non-CVD-related death followed similar trends to those observed for CVD-related death. This work provides additional real-world evidence for effect of statins in CVD-related mortality. The hazard functions presented here can provide a basis for future survival modeling and health economic evaluation.
Abbreviations: ACS, Acute Coronary Syndrome; CVD, Cardiovascular Disease; EMR, Electronic Medical Records; HF, Heart Failure; ICD, International Classification of Diseases; IS, Ischemic Stroke; LDL, Low Density Lipoprotein; MI, Myocardial Infarction; PCI, Percutaneous Coronary Intervention; RCT, Randomized Controlled Trial; STEMI, ST Elevation Myocardial Infarction
Keywords: Epidemiology, Cardiovascular disease, Secondary prevention, Mortality, Acute coronary syndromes
Highlights
-
•
10,092 ACS patients on high dose and 21,174 on no statins were followed 2001–12.
-
•
Royston-Parmar models were implemented to model hazard for new CVD events and death.
-
•
Risk of a new event developed similarly, but was much higher for those not on statins.
-
•
Risk of death increased over time for the no-statin, but not the high statin, group.
-
•
High statin treatment reduced gender effects on risk of mortality and new events.
1. Introduction
Cardiovascular disease (CVD) is the leading cause of death world-wide, responsible for 17.3 million, about 30% of all, deaths each year (Mozaffarian et al., 2015), (World Health Organization, 2011), a figure expected to grow to > 23.6 million deaths per year by 2030 according to the World Health Organization. CVD encompasses many different diagnoses and conditions, including acute coronary syndrome (ACS), which alone was responsible for 1,141,000 unique hospitalizations in the USA in 2010 (Mozaffarian et al., 2015).
ACS is a group of conditions including unstable angina and myocardial infarctions (MI) with or without an observed ST elevation (Grech and Ramsdale, 2003, Kumar and Cannon, 2009). Risk factors for ACS are common to other CVD and include behavioural and non-behavioural factors such as smoking, physical inactivity, obesity, high blood pressure, high blood cholesterol, diabetes mellitus, metabolic syndrome and chronic kidney disease (Mozaffarian et al., 2015). In the acute phase ACS is treated with anti-thrombotic and anti-ischemic medication, and revascularization procedures (percutaneous coronary intervention (PCI) or coronary artery bypass grafting) (Hamm et al., 2011, Van de Werf et al., 2008). Secondary prevention according to current guidelines typically includes lifestyle changes, medical treatment to control risk factors, and continued anti-thrombotic therapy (Hamm et al., 2011, Van de Werf et al., 2008).
Statins, widely used to lower cholesterol levels in primary and secondary prevention of CVD (Zhou and Liao, 2010), have an anti-thrombotic effect and are also often a part of secondary prevention of ACS. While their short-term benefits are unclear (Vale et al., 2014), several randomized controlled trials (RCTs), clinical observational studies, and meta-analyses have shown that statin treatment, in particular high-dose intensive treatment, improves long-term (months to years) outcomes in post-ACS patients reducing risk for death and/or cardiovascular events compared to lower-intensity treatments (Bavry et al., 2007, Cannon et al., 2006, Cholesterol Treatment Trialists et al., 2010, Farnier, 2008, Hulten et al., 2006, Josan et al., 2008), (Kasai et al., 2007a, Kasai et al., 2007b, Tentzeris et al., 2014). However, large, long-term, observational studies addressing their effect on risk of cardiovascular and non-cardiovascular morbidity and mortality in a clinical setting over time are warranted.
For the purposes of risk estimation and health economic evaluation we derived and compared time-dependent hazard functions for post-ACS mortality and recurrence of CVD events. Risk estimates were based on up to 12 years of continuous follow-up health data for a large cohort of Swedish patients receiving no statins or on sustained intensive statin treatment after experiencing their first ACS event.
2. Materials and methods
2.1. Data sources
Retrospective longitudinal observational data from 1992 to 2012 was collected from electronic medical records (EMRs) of selected primary care centres in Sweden and supplemented with data from mandatory national health registers containing information reported by Swedish health care providers, some going back to 1967.
Individual patient data from EMRs was extracted using the Pygargus Customized eXtraction Program, CXP (Pygargus, Stockholm, Sweden). This data extraction method allows extraction of anonymized structured and non-structured data, and has been validated (Martinell et al., 2012) and used in a number of earlier studies (Bodegard et al., 2013, Janson et al., 2013, Kjeldsen et al., 2010, Lindgren et al., 2005, Pettersson et al., 2010, Ringborg et al., 2008). Data extracted from the EMRs included patient's age, gender, prescriptions (coded according to the Anatomical Therapeutic Chemical (ATC) Classification System), diagnoses, physical measures, lab tests, health care visits, referrals, and lifestyle factors. During the extraction process, a key code was automatically generated to allow linkage of patient-level data across different datasets.
Patient-level data from the National Patient Register (NPR), Cause of Death Register (CDR), and Prescription Drug Register (PDR) was linked to extracted EMR data by the Swedish National Board of Health and Welfare using the key code generated at the EMR extraction step. These registries are compulsory and have been found to have a high degree of completeness (Ludvigsson et al., 2016, Ludvigsson et al., 2011). Data extracted from the NPR included patient age, gender, records of hospital procedures, visits, hospital admissions and discharges, as well as underlying diagnoses. Data extracted from CDR included patient age, date of death and cause of death. Data extracted from the PDR included patient age, gender, and prescriptions (ATC code, dose, length).
All diagnoses in EMRs, NPR, and CDR were coded according to ICD-9 until 1997 and ICD-10 from 1997 and onwards. Data from the EMRs and NPR was extracted from January 1992 (earliest EMR availability) to December 2012. Data from the CDR was extracted from January 2000 to December 2012. Data from the NPR was extracted from 2005 to 2012.
The study was approved and data access granted by the regional ethical review board in Stockholm.
2.2. Study population and outcome variables
The source population for the study was patients treated at any of the 43 participating Swedish primary care centres at any time during the inclusion period (January 2001 to extraction date). The chosen primary care centres, covering 14% of the Swedish population, were selected to reflect the full spectrum of Swedish primary care and varied in size (small, medium, or large centre), location (urban and rural), and type of practice (public and private). Patients from the source population who had a record of at least one ACS diagnosis during the inclusion period were included in the study, with ACS diagnosis date taken as the index date. Patients under 30 years of age at index or on a low-dose/intermittent statin regimen (see below), as well as patients with a diagnosis of malignancy other than skin, or a CVD diagnosis (CVD event [see below], chronic ischemic heart disease [ICD-10 I25.x] peripheral vascular disease [ICD-10 I70.x, I73.9, G45.0]) within the two years prior to index were excluded.
Included patients were followed until loss to follow-up (death, emigration) or 31st December 2012. During the study period, the time from first ACS event to next CVD event or death was monitored. Diagnosis codes were used to identify ACS events (unstable angina [ICD-10 I20.0] or myocardial infarction [ICD-10 I21.x]) and CVD events (ACS event, ischemic stroke [ICD-10 G45.9], heart failure [ICD-10 I50.x]). CVD death was defined as death where the recorded cause of death was an ACS or CVD event.
2.3. Statin therapy
Statin treatment regimens for all patients were analysed based on prescription data from EMRs and CDR and classified as no statin, low-dose/intermittent treatment, or high-dose continuous treatment. No statins regimen was defined as an average of < 0.1 statin prescriptions/year of follow-up (i.e., < 1 prescription during 10 years of follow-up). High-dose continuous treatment regimen was defined as any treatment regimen equivalent to an average of > 1.3 prescription/year of follow-up with each prescription being for at a daily intake of any of the following: 40 mg of atorvastatin, simvastatin or pravastatin; 40 mg of lovastatin; 20 mg of rosuvastatin and 80 mg of fluvastatin. Statin regimens not fulfilling either of the criteria above were classified as low-dose/intermittent.
The demographic characteristics of the two study groups, divided according to presence or absence of information on low density lipoprotein (LDL) cholesterol levels, is presented in Table 1. LDL was calculated according to the formula of Friedewald (Friedewald et al., 1972).
Table 1.
Characteristics (age, gender, prescriptions and co-morbidities before index date) of patients in the study population (Swedish patients, followed 2001–2012), stratified by statin usage and low density lipoprotein (LDL)-cholesterol measurement.
| Variable | No statin, No LDL value | No statin, With LDL value | High statin, No LDL value | High Statin, With LDL value | Total |
|---|---|---|---|---|---|
| N | 17,122 | 3923 | 5563 | 4496 | 31,104 |
| Age (y) at index date (mean) | 75.26 | 70.338 | 65.528 | 66.167 | 71.584 |
| Female (%) | 7855 (46) | 1578 (40) | 1674 (30) | 1440 (32) | 12,547 (40) |
| Male (%) | 9267 (54) | 2345 (60) | 3889 (70) | 3056 (68) | 18,557 (60) |
| HbA1C in % | 6.1868 | 5.8944 | 5.8477 | 5.6775 | 5.8987 |
| BMI, kg/m2 | 26.386 | 27.647 | 27.823 | 28.684 | 27.838 |
| LDL during study in mg/dL | |||||
| N | 0 | 3923 | 0 | 4496 | 8419 |
| Mean (SD) | 0 | 135.56 (37.20) | 0 | 117.98 (36.88) | 126.17 (38.05) |
| Median | 0 | 133.17 | 0 | 114.18 | 123.52 |
| Range | 0, 0 | 28.4, 294.6 | 0, 0 | 16.1, 281.8 | 16.1, 294.6 |
| Interquartile range | 110.0, 158.3 | 91.9, 140.8 | 99.2, 150.5 | ||
| Missing values | 17,122 | 0 | 5563 | 0 | 22,685 |
| HDL during study in mg/dL | |||||
| N | 118 | 3887 | 49 | 4483 | 8537 |
| Mean (SD) | 79.46 (66.35) | 51.82 (16.80) | 44.14 (26.29) | 50.12 (13.67) | 51.27 (17.40) |
| Median | 46.32 | 48.99 | 38.60 | 47.86 | 48.25 |
| Range | 21.2, 227.7 | 15.4, 227.7 | 19.3, 204.6 | 15.8, 135.1 | 15.4, 227.7 |
| Interquartile range | 34.9, 84.9 | 40.5, 59.4 | 34.6, 45.0 | 40.5, 57.9 | 40.5, 57.9 |
| Missing values | 17,004 | 36 | 5514 | 13 | 22,567 |
| Any diabetes before index date, Yes/No | |||||
| No | 14,994 (88) | 3461 (88) | 4855 (87) | 3968 (88) | 27,278 (88) |
| Yes | 2128 (12) | 462 (12) | 708 (13) | 528 (12) | 3826 (12) |
| % patients with diagnosis of any cardiovascular diseases (I0-I99) | |||||
| No | 4187 (24) | 955 (24) | 1334 (24) | 1066 (24) | 7542 (24) |
| Yes | 12,935 (76) | 2968 (76) | 4229 (76) | 3430 (76) | 23,562 (76) |
| Any angina before index date, Yes/No | |||||
| No | 14,931 (87) | 3405 (87) | 4833 (87) | 3914 (87) | 27,083 (87) |
| Yes | 2191 (13) | 518 (13) | 730 (13) | 582 (13) | 4021 (13) |
| Any depression before index date, Yes/No | |||||
| No | 16,356 (96) | 3754 (96) | 5329 (96) | 4325 (96) | 29,764 (96) |
| Yes | 766 (4.5) | 169 (4.3) | 234 (4.2) | 171 (3.8) | 1340 (4.3) |
| Any COPD before index date, Yes/No | |||||
| No | 16,525 (97) | 3766 (96) | 5369 (97) | 4315 (96) | 29,975 (96) |
| Yes | 597 (3.5) | 157 (4) | 194 (3.5) | 181 (4) | 1129 (3.6) |
| Any asthma before index date, Yes/No | |||||
| No | 16,601 (97) | 3790 (97) | 5384 (97) | 4338 (96) | 30,113 (97) |
| Yes | 521 (3) | 133 (3.4) | 179 (3.2) | 158 (3.5) | 991 (3.2) |
| Any cancer before index date, Yes/No | |||||
| No | 16,080 (94) | 3698 (94) | 5240 (94) | 4214 (94) | 29,232 (94) |
| Yes | 1042 (6.1) | 225 (5.7) | 323 (5.8) | 282 (6.3) | 1872 (6) |
| Any rheumatic disease before index date, Yes/No | |||||
| No | 16,790 (98) | 3852 (98) | 5474 (98) | 4406 (98) | 30,522 (98) |
| Yes | 332 (1.9) | 71 (1.8) | 89 (1.6) | 90 (2) | 582 (1.9) |
| Any heart failure before index date, Yes/No | |||||
| No | 17,121 (100) | 3923 (100) | 5563 (100) | 4496 (100) | 31,103 (100) |
| Yes | 1 (0.01) | 0 (0) | 0 (0) | 0 (0) | 1 (0) |
| Any hypertension before index date, Yes/No | |||||
| No | 12,433 (73) | 2859 (73) | 4013 (72) | 3268 (73) | 22,573 (73) |
| Yes | 4689 (27) | 1064 (27) | 1550 (28) | 1228 (27) | 8531 (27) |
2.4. Missing data
No imputation of missing data was performed. The number of subjects included in different analyses therefore varied according to availability of some data elements.
2.5. Statistical analyses
Data management and descriptive statistics were performed using SAS v 9.3 (Leavitt et al., 1990).
In order to analyse the hazard functions for a second CVD-event after the initial ACS-event over time, a Royston-Parmar (RP) model was used. Unlike the Cox proportional hazard model, which does not contain any assumptions on the hazard function, the RP model is a parametric model for analysing time-to-event data, relying on splines to model the baseline function (Royston and Parmar, 2002). The RP model, first published in 2002, offers a more flexible analysis than the classical parametric models (Reibnegger, 2012). The main drawback of parametric hazard models is the risk for arbitrary decisions regarding the baseline hazard rate (Box-Steffensmeier, 2004). At the time of analysis the RP models were not implemented in SAS, so input files were generated in SAS and the RP analyses performed using R version 3.0.2 (R Core Team, 2013) and the flexsurv package (Jackson, 2014).
The hazard functions for events of interest were estimated by modeling the Log cumulative Hazard (scale = hazard) as a spline function of log time, using two knots (k = 2). For each group/strata and outcome one crude analysis (presented as “crude rate” in results tables) and one adjusted analysis were run. In the adjusted analysis gender and diabetes were treated as categorical covariates (female/male, and absence/presence of diabetes, respectively) and age and LDL as continuous covariates (presented in results tables). In each analysis (every group/strata and outcome) age and LDL were centered around the same values. For age, 65 years was used, and for LDL the values were centered around 100 mg/dl, meaning that the difference between each data point for age and LDL and their global mean values were modeled in each case.
3. Results
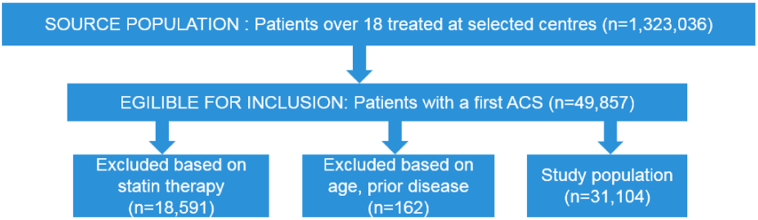
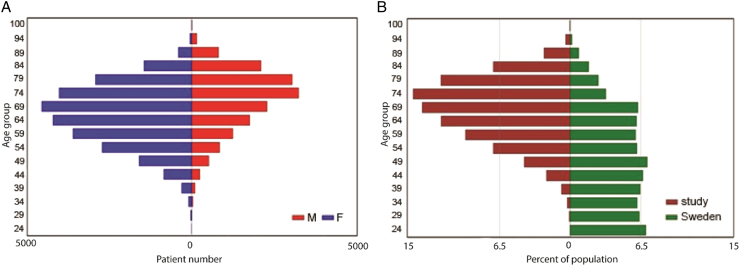
There were 49,857 patients with at least one ACS event identified in the source population, of whom 10,092 received high dose statins and 21,174 did not receive statins after the first event. After exclusion of patients on the low-dose/intermittent statin regimens (n = 18,591) and those fulfilling the other exclusion criteria (n = 162), a total of 31,104 patients (21,045 (67.7%) patients on no statins and 10,059 (32.3%) on high-dose continuous statin regimen) were included in the study (see Fig. 1). Basic demographic and clinical data on the two study populations, divided into those for whom data on LDL levels was available and those for whom it was lacking, is presented in Table 1. The high-statin group contained younger subjects than the no-statin group and a higher proportion of males (Table 1). From looking at age distribution by gender it was evident that males were younger than females at diagnosis (Fig. 2).
Fig. 1.
Flow-chart of Study population selection in Sweden, 2001–2012.
Patients with ACS diagnosis and without a previous diagnosis of ACS, IS, HF or malignancy were included in the study.
Fig. 2.
Age and gender pyramid of Swedish ACS patient cohort followed 2001–2012. Age pyramid of the ACS cohort comparing male and female patients (A) and comparing the patient population to the general population of Sweden (B).
We examined how the hazard rates for experiencing a second ACS event or an ischemic stroke changed over time in the two treatment groups (see Table 2). Looking at the crude rates, the pattern for risk is similar in both treatment groups but differs depending on event observed. When looking at a second ACS, the patients are at a high risk early after the initial event, with risk declining rapidly and become more stable in the long term. In the case of a follow-up ischemic stroke event, the risk increases over time. As expected, the risk is generally lower in the high statin group regardless of event of interest, with the interesting exception of hazard for a subsequent ACS in the first year, when it is the no-statin group that has a lower risk. This is in line with high statins being prescribed to most at-risk patient and supports their effectiveness, as already 5 years after the initial event the high-statin patients had a lower risk of a subsequent event than the patients in the no-statin group, and continue having a lower risk in the long term. Stratification on gender and diabetes status did not change the patterns in hazard, and as expected, males were are at a higher risk of a subsequent CVD event, while somewhat surprisingly, diabetes did not have a substantial impact on hazard.
Table 2.
Hazard rates for a new ACS or ischemic stroke (IS) event among Swedish ACS patients followed 2001–2012, overall (crude rate) and stratified by gender and diabetes diagnosis (adjusted for age, gender, LDL, and diabetes status).
| Time point (years) | Crude rate |
Females |
Males |
With diabetes |
No diabetes |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| High statin | No statin | High statin | No statin | High statin | No statin | High statin | No statin | High statin | No statin | |
| Second ACS | ||||||||||
| 1 | 83.48 (78.71:88.08) | 63.70 (61.56:65.85) | 80.03 (72.15:87.83) | 46.26 (40.80:51.62) | 95.08 (85.72:104.35) | 61.18 (53.95:68.27) | 91.59 (82.58:100.52) | 57.45 (50.67:64.12) | 83.08 (74.90:91.18) | 49.26 (43.44:54.97) |
| 5 | 20.94 (19.37:22.44) | 37.82 (36.34:39.40) | 21.14 (18.83:23.72) | 28.30 (25.08:31.60) | 25.11 (22.37:28.18) | 37.42 (33.17:41.79) | 24.19 (21.55:27.15) | 35.14 (31.15:39.25) | 21.94 (19.55:24.63) | 30.13 (26.70:33.65) |
| 10 | 11.55 (10.60:12.46) | 27.51 (26.18:28.91) | 11.86 (10.48:13.43) | 20.42 (17.95:23.10) | 14.10 (12.45:15.96) | 27.01 (23.74:30.55) | 13.58 (11.99:15.37) | 25.37 (22.29:28.69) | 12.32 (10.88:13.94) | 21.75 (19.11:24.60) |
| IS event | ||||||||||
| 1 | 8.99 (7.66:10.27) | 17.67 (16.62:18.78) | 6.70 (4.90:8.75) | 8.30 (6.52:10.36) | 7.82 (5.71:10.21) | 9.86 (7.75:12.31) | 6.95 (5.08:9.07) | 8.68 (6.82:10.83) | 7.54 (5.51:9.85) | 9.43 (7.41:11.78) |
| 5 | 9.75 (8.79:10.79) | 13.35 (12.57:14.17) | 7.36 (5.95:9.11) | 6.94 (5.51:8.69) | 8.58 (6.93:10.63) | 8.25 (6.54:10.32) | 7.63 (6.16:9.45) | 7.26 (5.76:9.08) | 8.28 (6.69:10.25) | 7.89 (6.26:9.87) |
| 10 | 12.26 (10.43:14.36) | 17.37 (16.17:18.62) | 10.10 (7.64:13.11) | 10.94 (8.75:13.49) | 11.79 (8.91:15.29) | 13.00 (10.39:16.03) | 10.48 (7.92:13.59) | 11.44 (9.14:14.11) | 11.37 (8.59:14.75) | 12.44 (9.94:15.34) |
Next, we calculated hazard rates for CVD- and non-CVD-related death in the two study groups (see Table 3). For high statin users, the risk of both types of death changed little over the duration of follow-up, whereas the no statin users showed an increasing risk of death over time, especially for CVD-related death, with a nearly 50% increase in hazard rate from 1 year to 10 years after the event. Stratification on sex showed consistently higher hazard ratios for death for males compared to females in the no statin group, with males having a hazard ratio of 25.0 for CVD and 24.0 for non-CVD death 10 years after the initial event, compared to ratios of 16.0 and 14.6 for females. Interestingly, this gender difference was absent in the high statin group, where males actually tended to have a slightly lower hazard for death compared to females (ratios of 6.5 for CVD and 6.1 for non-CVD death 10 years after the initial event, compared to 7.7 and 6.4 among females). However, high-statin patients of both sexes saw a significant and major reduction in hazard for both types of death compared to those not on statins, which was clear already after the first year of follow-up and persisted throughout. Differences in hazard ratios for CVD-related death were minor between those with and without diabetes, with a slight trend towards lower hazard among those with the disease in the high statin group, and the reverse in the no statin group. Surprisingly, hazard for non-CVD related death tended to be lower among diabetics regardless of statin regimen, although both diabetics and non-diabetics on high statin treatment had significantly and substantially lower hazard ratios for death when compared to their non-statin treated counterparts.
Table 3.
Hazard rates for CVD- and non-CVD related death among Swedish ACS patients followed 2001–2012, overall (crude rate) and stratified by gender and diabetes diagnosis (adjusted for age, gender, LDL, and diabetes status).
| Time point (years) | Crude rate |
Females |
Males |
With diabetes |
No diabetes |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| High statin | No statin | High statin | No statin | High statin | No statin | High statin | No statin | High statin | No statin | |
| CVD-related death | ||||||||||
| 1 | 6.87 (5.81:7.99) | 39.13 (37.26:41.14) | 2.66 (1.81:3.68) | 6.10 (5.01:7.39) | 2.27 (1.54:3.14) | 9.53 (7.83:11.55) | 2.04 (1.38:2.82) | 7.78 (6.39:9.42) | 2.97 (2.01:4.10) | 7.48 (6.14:9.06) |
| 5 | 7.17 (6.33:8.06) | 46.99 (45.83:48.15) | 5.35 (4.12:6.96) | 7.41 (6.38:8.53) | 4.57 (3.52:5.94) | 11.57 (9.97:13.33) | 4.10 (3.16:5.33) | 9.44 (8.13:10.88) | 5.97 (4.60:7.76) | 9.08 (7.82:10.45) |
| 10 | 7.26 (6.04:8.68) | 60.21 (58.18:62.34) | 7.65 (5.70:10.27) | 16.02 (13.91:18.46) | 6.53 (4.86:8.77) | 25.03 (21.74:28.85) | 5.86 (4.36:7.87) | 20.43 (17.74:23.54) | 8.53 (6.35:11.45) | 19.63 (17.05:22.63) |
| Non-CVD-related death | ||||||||||
| 1 | 8.77 (7.73:9.95) | 39.46 (38.04:41.07) | 4.56 (3.21:6.26) | 8.61 (7.15:10.40) | 4.30 (3.03:5.90) | 14.11 (11.71:17.03) | 3.39 (2.39:4.66) | 9.35 (7.76:11.29) | 5.77 (4.06:7.92) | 13.00 (10.79:15.69) |
| 5 | 6.65 (5.88:7.49) | 29.26 (28.27:30.35) | 4.77 (3.58:6.27) | 6.68 (5.51:7.87) | 4.49 (3.37:5.92) | 10.94 (9.02:12.89) | 3.55 (2.66:4.67) | 7.25 (5.98:8.54) | 6.03 (4.53:7.94) | 10.08 (8.31:11.87) |
| 10 | 6.33 (4.92:7.83) | 39.77 (38.11:41.57) | 6.44 (4.50:8.92) | 14.62 (12.37:16.99) | 6.07 (4.25:8.41) | 23.96 (20.27:27.83) | 4.79 (3.35:6.64) | 15.87 (13.43:18.44) | 8.15 (5.70:11.29) | 22.07 (18.67:25.64) |
We also studied the risk for a third CVD-event, separately for any ACS event, heart failure (HF), and ischemic stroke (IS) (see Table 4). Overall, the hazard rate for all types of events declined over time for both high statin and no statin groups. This decline in hazard was most pronounced for ACS and IS events, where crude hazard ratios went down by up to six fold from year 1 to year 10 (for high statin and no statin patients ACS event hazard ratios: 84.9 and 59.8 at 1 year vs 15.3 and 12.1 at 10 years; IS event hazard ratios: 104.8 and 48.5 at 1 year vs 16.9 and 7.6 at 10 years). Surprisingly, unlike what was seen for second events, patients on high statin treatment did not have lower hazard ratios for ACS and IS events compared to their no statin counterparts, but did have constantly lower hazard for HF. Interestingly, the time trend for hazard for HF was also distinct from what was seen for ACS and IS, with a much less substantial decline over time (year 1 rates of 30.0 and 61.1 among high statin and no statin users, compared to 10 year rates of 22.3 and 42.9, representing a reduction by 26% and 30% respectively). This held true regardless of gender or diabetes history, while previously observed trends (higher hazard rates among men) persisted.
Table 4.
Hazard rates for a third CVD event (ACS, IS, or HF) among Swedish ACS patients followed 2001–2012, overall (crude rate) and stratified by gender and diabetes diagnosis (adjusted for age, gender, LDL, and diabetes status).
| Time point (years) | Crude rate |
Females |
Males |
With diabetes |
No diabetes |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| High statin | No statin | High statin | No statin | High statin | No statin | High statin | No statin | High statin | No statin | |
| ACS event | ||||||||||
| 1 | 84.9 (79.8:89.6) | 59.8 (56.9:62.9) | 98.7 (87.6:111.5) | 58.6 (49.5:69.0) | 93.2 (82.7:105.3) | 70.9 (59.9:83.5) | 87.9 (78.0:99.3) | 68.3 (57.7:80.5) | 104.7 (92.9:118.3) | 60.8 (51.4:71.6) |
| 5 | 25.8 (23.5:28.1) | 19.9 (18.7:21.2) | 35.2 (30.1:41.2) | 25.1 (21.1:29.5) | 33.3 (28.5:38.9) | 30.4 (25.5:35.7) | 31.4 (26.8:36.7) | 29.3 (24.6:34.4) | 37.4 (32.0:43.7) | 26.1 (21.9:30.6) |
| 10 | 15.3 (13.7:17.0) | 12.1 (11.2:13.0) | 22.0 (18.4:26.4) | 16.4 (13.6:19.7) | 20.8 (17.4:24.9) | 19.9 (16.5:23.8) | 19.6 (16.4:23.5) | 19.2 (15.9:23.0) | 23.3 (19.5:28.0) | 17.0 (14.1:20.4) |
| IS event | ||||||||||
| 1 | 104.8 (87.4:121.7) | 48.5 (43.4:52.8) | 80.7 (55.4:114.5) | 50.7 (36.2:69.9) | 118.2 (81.1:167.6) | 63.7 (45.4:87.7) | 104.5 (71.7:148.2) | 49.9 (35.6:68.7) | 91.3 (62.6:129.5) | 64.7 (46.2:89.1) |
| 5 | 29.1 (20.2:38.6) | 13.5 (11.5:15.2) | 25.3 (14.8:40.1) | 18.3 (12.3:26.3) | 37.0 (21.6:58.8) | 23.0 (15.4:33.0) | 32.7 (19.1:52.0) | 18.0 (12.1:25.9) | 28.6 (16.7:45.4) | 23.4 (15.7:33.6) |
| 10 | 16.9 (11.0:23.5) | 7.6 (6.4:8.8) | 15.1 (8.3:25.4) | 11.1 (7.3:16.5) | 22.2 (12.2:37.2) | 14.0 (9.1:20.7) | 19.6 (10.8:32.9) | 11.0 (7.1:16.2) | 17.1 (9.4:28.7) | 14.2 (9.3:21.0) |
| HF event | ||||||||||
| 1 | 30.0 (27.7:32.3) | 61.1 (58.3:63.9) | 24.4 (20.6:28.8) | 29.9 (25.8:34.4) | 25.4 (21.4:29.9) | 34.2 (29.5:39.4) | 23.8 (20.1:28.0) | 33.5 (28.9:38.6) | 26.0 (22.0:30.7) | 30.4 (26.2:35.0) |
| 5 | 24.7 (23.0:26.6) | 42.2 (40.9:43.6) | 25.6 (22.3:29.4) | 23.9 (21.1:26.9) | 26.7 (23.2:30.6) | 27.4 (24.2:30.7) | 25.0 (21.7:28.7) | 26.9 (23.7:30.2) | 27.4 (23.8:31.5) | 24.4 (21.5:27.4) |
| 10 | 22.3 (20.1:24.6) | 42.9 (40.9:45.0) | 26.9 (22.9:32.1) | 35.4 (31.2:40.4) | 28.0 (23.8:33.4) | 40.5 (35.7:46.3) | 26.2 (22.3:31.3) | 39.8 (35.0:45.4) | 28.7 (24.4:34.3) | 36.1 (31.8:41.2) |
Looking further at patients that survived two CVD events, we calculated hazard rates for all-cause mortality over time (see Table 5). As expected, hazard ratios for most groups increased during follow-up, going up between 2.4 and 3.2 fold, with a notable exception of the crude hazard ratios. The increase in crude hazard ratio for the no statin population was a lot more moderate than what was seen for other groups, increasing from 75.6 at 1 year to only 79.8 at 10 years, while for the high statin group the crude hazard decreased with time, dropping from 10.3 after 1 year to 7.7 after 10 years. Looking at the subgroups analysed, gender differences were, as previously observed, pronounced in the no statin group, with females having lower hazard, but absent in the high statin group. Interestingly, diabetes had a minor effect on the hazard rate, which was slightly more pronounced in the high statin group, where diabetic patients were at a slightly lower risk of death following a second CVD event.
Table 5.
Hazard rates for any cause death after two CVD events among Swedish ACS patients followed 2001–2012, overall (crude rate) and stratified by gender and diabetes diagnosis (adjusted for age, gender, LDL, and diabetes status).
| Time point (years) | Crude rate |
Females |
Males |
With diabetes |
No diabetes |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| High statin | No statin | High statin | No statin | High statin | No statin | High statin | No statin | High statin | No statin | |
| 1 | 10.3 (8.6:12.1) | 75.6 (72.1:79.3) | 3.8 (2.5:5.8) | 15.0 (12.5:17.9) | 3.9 (2.5:5.9) | 21.5 (17.9:25.8) | 2.9 (1.9:4.4) | 18.7 (15.6:22.4) | 5.2 (3.3:7.8) | 17.3 (14.4:20.7) |
| 5 | 8.3 (7.1:9.5) | 68.2 (66.2:70.2) | 5.8 (4.3:7.7) | 14.9 (12.7:17.4) | 5.9 (4.4:7.9) | 21.4 (18.3:25.1) | 4.4 (3.2:5.9) | 18.6 (15.9:21.7) | 7.8 (5.8:10.4) | 17.2 (14.7:20.1) |
| 10 | 7.7 (6.0:9.4) | 79.8 (76.4:83.1) | 7.7 (5.3:11.1) | 28.2 (24.2:33.1) | 7.8 (5.4:11.3) | 40.6 (34.8:47.6) | 5.8 (4.0:8.4) | 35.2 (30.2:41.3) | 10.3 (7.1:14.9) | 32.5 (27.9:38.2) |
4. Discussion
As the leading global cause of mortality, CVDs represent a major social and financial burden worldwide. Research into their primary and secondary prevention is essential to reduce this burden. Statins have become the cornerstone of both primary and secondary prevention following promising RCT results, however their use warrants continued evaluation and monitoring from clinical and health economy perspectives.
In this study > 30,000 persons with a history of ACS from 43 different primary care centres were followed for up for over 10 years to estimate the risk of experiencing a recurrent CVD-related events and death. Using observational data from a large number of patient medical records merged with information from national registers, we computed hazard functions for important first and recurrent outcomes in patient groups stratified according to statin treatment, gender, and diabetes status. Importantly, these hazard functions can provide a basis for survival modeling and health economical evaluation. We kept the number of covariates low (age, sex, LDL, and diabetes status) in order to focus on the respective treatment groups and strata rather than covariate effects. We have also not done any propensity score matching for the two treatment groups in the study, since the main aim was to estimate hazard functions in the real-world setting, rather than comparing outcome of different treatment groups.
Taking advantage of the systematic patient-level health care data gathering efforts implemented in Sweden, we carried out a retrospective study aimed at examining the effects of a popular preventative measure in a clinical setting. This study design allowed for fast study completion, long follow-up, broad geographical coverage, and a patient population and care setting which are more in line with real-world clinical practice than what is available through prospective studies. This study design does, however, have a number of limitations. For example, although the uneven distribution of age and gender across the high-statin and no-statin groups is reflective of clinical reality and has been explicitly adjusted for in our analyses, it should be kept in mind when interpreting differences in hazard functions. Also, we had to exclude a considerable proportion of subjects from our study due to inconsistent statin treatment patterns or low-dose statin treatment only. Inclusion of patients with inconsistent/changing treatment patterns would have required more complex modeling procedures and may be subject to confounding due to indication or mixed treatment effects. Beyond complications related to inconsistent treatment patterns, low-dose statin treated patients were excluded in order to achieve well-defined treatment groups. We have not specifically investigated patients' adherence to prescribed treatment. However, the definitions of the statin treatment groups, i.e. requirements on consistency of prescriptions throughout the duration of the study, should reduce misclassification problems related to low adherence, which would instead result in a smaller size of the high statin group.
Nevertheless, our results contain several findings worth attention from a clinical perspective. Interestingly, we observed differences in non-CVD related mortality rate patterns between the statin treatment groups, indicating reduced non-CVD mortality in the high statin group. These findings are in line with previous real-world data showing survival benefits of statin treatment in ACS patients. Tentzeris et al. (Tentzeris et al., 2014) showed a significant reduction in short-term (3 months) all-cause mortality in ACS patients receiving intensive statin treatment in (n = 1528). In a small study of PCI patients (n = 575), Kasai et al. (Kasai et al., 2007a, Kasai et al., 2007b) found reduced mortality of statin-treated patients during an 11-year follow-up, compared with non-statin treated patients. Rasmussen et al. showed that low adherence to statin treatment post-MI was associated with higher mortality in a large Canadian study (Rasmussen et al., 2007). Moreover, our results indicate that high statin treatment diminishes the impact of important risk factors such as gender and diabetes regarding both CVD-related and non-CVD related mortality. In particular, gender differences appeared greatly suppressed or even absent. The increased risk of death (non-CVD and CVD related) observed for diabetics of either gender could be due to the fact that diabetes itself is associated with an increased risk of death from both vascular diseases, and other malignancies such as cancer, infectious diseases and degenerative disorders (Seshasai et al., 2011). Surprisingly, we did not see any major difference between statin treatment groups regarding risk for three other CVD events (ACS, IS, HF). This may reflect a selection effect whereby patients that have experienced and survived two CVD unique characteristics or disease aetiology, and respond differently than others.
Only a minority of high-risk patients are believed to receive (high intensity) statin treatment and, of those who do receive treatment, a majority do not reach the therapeutic goal of total cholesterol level < 5.0 mmol/l (Lindgren et al., 2005, Primatesta and Poulter, 2000). There are a number of explanations for the relatively low use of statin: statins may not be prescribed by primary doctor (for example, due to previous side effects) or statins may be prescribed but not picked up, which has been recently shown to be a major phenomenon among Swedish ACS patients with poor kidney function (Khedri et al., 2017).
The mechanisms behind lower mortality rates, considering both CVD-related and non-CVD related in the high statin dose group can be several, as statins have effects on many systems and functions in the human body. Statins lower LDL by inhibiting HMG-CoA reductase, an enzyme in the cholesterol producing pathway, thus increasing cholesterol up-take from the blood. The lowered level of LDL in the bloodstream has an anti-atherosclerotic effect. Statins also have pleiotropic effects such as improving endothelial function, stabilizing atherosclerotic plaques and reducing oxidative stress and inflammation (Zhou and Liao, 2010). On the other hand, some association between statin use and diabetes onset has previously been shown in patients with cardiovascular disease (Shah and Goldfine, 2012), and possibly worsening insulin resistance, secretion and metabolic control (Muscogiuri et al., 2014). However, the general consensus is that the positive effects of statins on LDL outweighs the negative effects on metabolic control (Rocco, 2012, Shah and Goldfine, 2012). Furthermore, evidence indicates that statins have pro-apoptotic, anti-angiogenetic and immunomodulatory effects that make them potential research targets for cancer therapy and may contribute to their effect on risk for all-cause death (Vallianou et al., 2014).
5. Conclusion
Our results add real-world evidence to the already existing evidence that statin treatment has a beneficial effect on mortality rates among patients with a history of cardiovascular disease. The increased mortality among those with diabetes and among men not on any statin treatment highlights the need for extra careful treatment and monitoring in these risk groups. Correctly and promptly identifying patients with a high risk for secondary events will help save lives and reduce medical costs.
Disclosures
Funding sources
This study was funded by Amgen Inc.
Conflict of interest
B. Taylor was an employee and potential stockholder of Amgen Inc. when this work was carried out. At that time J. Rockberg, L. Jörgensen, and P. Sobocki were employees of IMS Health Sweden AB and consulting for Amgen Inc. G. Johansson was an advisor for Amgen Inc.
References
- Bavry A.A., Mood G.R., Kumbhani D.J., Borek P.P., Askari A.T., Bhatt D.L. Long-term benefit of statin therapy initiated during hospitalization for an acute coronary syndrome: a systematic review of randomized trials. Am. J. Cardiovasc. Drugs. 2007;7:135–141. doi: 10.2165/00129784-200707020-00005. [DOI] [PubMed] [Google Scholar]
- Bodegard J., Sundstrom J., Svennblad B., Ostgren C.J., Nilsson P.M., Johansson G. Changes in body mass index following newly diagnosed type 2 diabetes and risk of cardiovascular mortality: a cohort study of 8486 primary-care patients. Diabete Metab. 2013;39:306–313. doi: 10.1016/j.diabet.2013.05.004. [DOI] [PubMed] [Google Scholar]
- Box-Steffensmeier J.M. Cambridge University Press; 2004. Event History Modeling: A Guide for Social Scientists. [Google Scholar]
- Cannon C.P., Steinberg B.A., Murphy S.A., Mega J.L., Braunwald E. Meta-analysis of cardiovascular outcomes trials comparing intensive versus moderate statin therapy. J. Am. Coll. Cardiol. 2006;48:438–445. doi: 10.1016/j.jacc.2006.04.070. [DOI] [PubMed] [Google Scholar]
- Cholesterol Treatment Trialists C., Baigent C., Blackwell L. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Core Team R. R Foundation for Statistical Computing. Austria. URL; Vienna: 2013. R: A language and environment for statistical computing.http://www.R-project.org/ [Google Scholar]
- Farnier M. Update on the clinical utility of fenofibrate in mixed dyslipidemias: mechanisms of action and rational prescribing. Vasc. Health Risk Manag. 2008;4:991–1000. doi: 10.2147/vhrm.s3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Grech E.D., Ramsdale D.R. Acute coronary syndrome: unstable angina and non-ST segment elevation myocardial infarction. BMJ. 2003;326:1259–1261. doi: 10.1136/bmj.326.7401.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm C.W., Bassand J.P., Agewall S. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: the task force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC) Eur. Heart J. 2011;32:2999–3054. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- Hulten E., Jackson J.L., Douglas K., George S., Villines T.C. The effect of early, intensive statin therapy on acute coronary syndrome: a meta-analysis of randomized controlled trials. Arch. Intern. Med. 2006;166:1814–1821. doi: 10.1001/archinte.166.17.1814. [DOI] [PubMed] [Google Scholar]
- Jackson C. 2014. flexsurv: Flexible Parametric Survival and Mult-state Models. R Package Version 0.5. [Google Scholar]
- Janson C., Larsson K., Lisspers K.H. Pneumonia and pneumonia related mortality in patients with COPD treated with fixed combinations of inhaled corticosteroid and long acting beta2 agonist: observational matched cohort study (PATHOS) BMJ. 2013;346:f3306. doi: 10.1136/bmj.f3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josan K., Majumdar S.R., McAlister F.A. The efficacy and safety of intensive statin therapy: a meta-analysis of randomized trials. CMAJ. 2008;178:576–584. doi: 10.1503/cmaj.070675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai T., Miyauchi K., Kurata T. Long-term (11-year) statin therapy following percutaneous coronary intervention improves clinical outcome and is not associated with increased malignancy. Int. J. Cardiol. 2007;114:210–217. doi: 10.1016/j.ijcard.2006.01.028. [DOI] [PubMed] [Google Scholar]
- Kasai T., Miyauchi K., Kurata T. Long-term (11-year) statin therapy following percutaneous coronary intervention improves clinical outcome and is not associated with increased malignancy. Int. J. Cardiol. 2007;114:210–217. doi: 10.1016/j.ijcard.2006.01.028. [DOI] [PubMed] [Google Scholar]
- Khedri M., Szummer K., Carrero J.J. Systematic underutilisation of secondary preventive drugs in patients with acute coronary syndrome and reduced renal function. Eur. J. Prev. Cardiol. 2017 doi: 10.1177/2047487317693950. (epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Kjeldsen S.E., Stalhammar J., Hasvold P., Bodegard J., Olsson U., Russell D. Effects of losartan vs candesartan in reducing cardiovascular events in the primary treatment of hypertension. J. Hum. Hypertens. 2010;24:263–273. doi: 10.1038/jhh.2009.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A., Cannon C.P. Acute coronary syndromes: diagnosis and management, part I. Mayo Clin. Proc. 2009;84:917–938. doi: 10.4065/84.10.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavitt R.Y., Fauci A.S., Bloch D.A. The American College of Rheumatology 1990 criteria for the classification of Wegener's granulomatosis. Arthritis Rheum. 1990;33 doi: 10.1002/art.1780330807. [DOI] [PubMed] [Google Scholar]
- Lindgren P., Borgstrom F., Stalhammar J., Alemao E., Yin D.D., Jonsson L. Association between achieving treatment goals for lipid-lowering and cardiovascular events in real clinical practice. Eur. J. Cardiovasc. Prev. Rehabil. 2005;12:530–534. doi: 10.1097/01.hjr.0000160724.05165.dc. Working Groups on Epidemiology & Prevention and Cardiac Rehabilitation and Exercise Physiology. [DOI] [PubMed] [Google Scholar]
- Ludvigsson J.F., Andersson E., Ekbom A. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludvigsson J.F., Almqvist C., Bonamy A.K. Registers of the Swedish total population and their use in medical research. Eur. J. Epidemiol. 2016;31:125–136. doi: 10.1007/s10654-016-0117-y. [DOI] [PubMed] [Google Scholar]
- Martinell M., Stalhammar J., Hallqvist J. Automated data extraction—a feasible way to construct patient registers of primary care utilization. Ups. J. Med. Sci. 2012;117:52–56. doi: 10.3109/03009734.2011.653015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mozaffarian D., Benjamin E.J., Go A.S. Heart disease and stroke statistics-2015 update: a report from the American heart association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- Muscogiuri G., Sarno G., Gastaldelli A. The good and bad effects of statins on insulin sensitivity and secretion. Endocr. Res. 2014;39:137–143. doi: 10.3109/07435800.2014.952018. [DOI] [PubMed] [Google Scholar]
- Pettersson B., Ambegaonkar B., Sazonov V., Martinell M., Stalhammar J., Wandell P. Prevalence of lipid abnormalities before and after introduction of lipid modifying therapy among Swedish patients with dyslipidemia (PRIMULA) BMC Public Health. 2010;10:737. doi: 10.1186/1471-2458-10-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primatesta P., Poulter N. Lipid concentrations and the use of lipid lowering drugs: evidence from a national cross sectional survey. BMJ. 2000;321:1322–1325. doi: 10.1136/bmj.321.7272.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen J.N., Chong A., Alter D.A. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297:177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- Reibnegger G. Vol. 3. 2012. Modeling Time in Medical Education Research: The Potential of New Flexible Parametric Methods of Survival Analysis. Scientific Research. [Google Scholar]
- Ringborg A., Martinell M., Stalhammar J., Yin D.D., Lindgren P. Resource use and costs of type 2 diabetes in Sweden - estimates from population-based register data. Int. J. Clin. Pract. 2008;62:708–716. doi: 10.1111/j.1742-1241.2008.01716.x. [DOI] [PubMed] [Google Scholar]
- Rocco M.B. Statins and diabetes risk: fact, fiction, and clinical implications. Cleve. Clin. J. Med. 2012;79:883–893. doi: 10.3949/ccjm.79a.12091. [DOI] [PubMed] [Google Scholar]
- Royston P., Parmar M.K. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat. Med. 2002;21:2175–2197. doi: 10.1002/sim.1203. [DOI] [PubMed] [Google Scholar]
- Seshasai S.R., Kaptoge S., Thompson A. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N. Engl. J. Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah R.V., Goldfine A.B. Statins and risk of new-onset diabetes mellitus. Circulation. 2012;126:e282–e284. doi: 10.1161/CIRCULATIONAHA.112.122135. [DOI] [PubMed] [Google Scholar]
- Tentzeris I., Rohla M., Jarai R. Influence of high-dose highly efficient statins on short-term mortality in patients undergoing percutaneous coronary intervention with stenting for acute coronary syndromes. Am. J. Cardiol. 2014;113:1099–1104. doi: 10.1016/j.amjcard.2013.12.012. [DOI] [PubMed] [Google Scholar]
- Vale N., Nordmann A.J., Schwartz G.G. Cochrane Database of Systematic Reviews. 2014. Statins for acute coronary syndrome. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallianou N.G., Kostantinou A., Kougias M., Kazazis C. Statins and cancer. Anti Cancer Agents Med. Chem. 2014;14:706–712. doi: 10.2174/1871520613666131129105035. [DOI] [PubMed] [Google Scholar]
- Van de Werf F., Bax J., Betriu A. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the task force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology. Eur. Heart J. 2008;29:2909–2945. doi: 10.1093/eurheartj/ehn416. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Switzerland; Geneva: 2011. Global Status Report on Noncommunicable Diseases 2010. [Google Scholar]
- Zhou Q., Liao J.K. Pleiotropic effects of statins. - basic research and clinical perspectives. Circ. J. 2010;74:818–826. doi: 10.1253/circj.cj-10-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]