Abstract
Purpose
We use semiautomated segmentation of fluorescein angiography (FA) to determine whether anti-vascular endothelial growth factor (VEGF) treatment for diabetic macular edema (DME) differentially affects microaneurysm (MA)–associated leakage, termed focal leakage, versus non-MA–associated leakage, termed diffuse leakage.
Methods
We performed a retrospective study of 29 subjects treated with at least three consecutive injections of anti-VEGF agents for DME (mean 4.6 injections; range, 3–10) who underwent Heidelberg FA before and after anti-VEGF therapy. Inclusion criteria were macula center involving DME and at least 3 consecutive anti-VEGF injections. Exclusion criteria were macular edema due to cause besides DME, anti-VEGF within 3 months of initial FA, concurrent treatment for DME besides anti-VEGF, and macular photocoagulation within 1 year. At each time point, total leakage was semiautomatically segmented using a modified version of our previously published software. Microaneurysms were identified by an expert grader and leakage within a 117 μm radius of each MA was classified as focal leakage. Remaining leakage was classified as diffuse leakage. The absolute and percent changes in total, diffuse, and focal leakage were calculated for each subject.
Results
Mean pretreatment total leakage was 8.2 mm2 and decreased by a mean of 40.1% (P < 0.0001; 95% confidence interval [CI], [−28.6, −52.5]) following treatment. Diffuse leakage decreased by a mean of 45.5% (P < 0.0001; 95% CI, [−31.3, −59.6]) while focal leakage decreased by 17.9% (P = 0.02; 95% CI, [−1.0, −34.8]). The difference in treatment response between focal and diffuse leakage was statistically significant (P = 0.01).
Conclusions
Anti-VEGF treatment for DME results in decreased diffuse leakage but had relatively little effect on focal leakage as assessed by FA. This suggests that diffuse leakage may be a marker of VEGF-mediated pathobiology. Patients with predominantly focal leakage may be less responsive to anti-VEGF therapy.
Translational Relevance
Fluorescein angiography can define focal and diffuse subtypes of diabetic macular edema and these may respond differently to anti-VEGF treatment.
Keywords: diabetic macular edema, fluorescein angiography, image segmentation, anti-VEGF
Introduction
The prevalence of diabetes is increasing throughout the world and it is estimated that over 400 million people will be affected by the year 2025.1 Diabetic retinopathy is considered the leading cause of vision loss in working age adults.2 The main causes of vision loss due to diabetes are proliferative diabetic retinopathy (PDR) and diabetic macular edema (DME).3 Diabetic macular edema, which affects over 21 million people worldwide, is a major cause of central vision loss and results from complex pathobiology, including breakdown of the blood retinal barrier and failure of Müller cell fluid pumping action.4–7 Currently, treatment options for DME include intravitreal anti-vascular endothelial growth factor agents (anti-VEGF), intravitreal corticosteroids, and thermal laser. While anti-VEGF agents are regarded by most as first line therapy for DME, they are not universally effective and corticosteroids and thermal laser also are effective therapies in a subset of patients with DME.8–13 Clinicians wishing to offer individualized treatment regimens for their patients are challenged to predict a priori which treatment is optimal. Thus, it is common practice to initiate treatment with anti-VEGF and then use alternate therapies for persistent or nonresponsive DME. This process of elimination approach is inefficient and increases the already substantial treatment burden for patients. In the RISE and RIDE studies, subjects treated with monthly ranibizumab for 24 months showed that complete resolution of leakage on FA occurred in only 17% to 30% of subjects.14 This suggests that some forms of leakage are not fully controlled by VEGF inhibition.
Numerous investigators have subtyped DME as diffuse or focal according to various criteria and using variable tools, which include biomicroscopy, fundus photography, fluorescein angiography (FA), and optical coherence tomography (OCT). The use of nonangiographic methods of classifying DME has been reviewed previously.15 With regard to the use of FA to subtype DME, focal leakage has been defined most frequently as leakage originating from microaneurysms (MAs), while diffuse leakage has been defined as leakage without a clear source.16–18 The utility of classifying DME based on FA has been limited by inherent subjectivity, multiple definitions, and challenges in reproducibility.15,19 Additionally, classifying an entire eye as focal or diffuse does not take into consideration the fact that both types of leakage frequently coexist in most patients. For this study, focal leakage was defined as originating from MAs, while diffuse leakage was defined as leakage without a clear source. We hypothesize that the pathobiology of focal leakage is distinct from that driving diffuse leakage. Seeking to explore this hypothesis, we have developed custom MATLAB-based software (MathWorks, Natick, MA) to semiautomatically segment total fluorescein leakage and MAs. This software permits quantification of the diffuse and focal components of leakage. To probe whether one subtype is more responsive to anti-VEGF treatment, we have performed a retrospective analysis of 29 subjects who received FA before and after anti-VEGF therapy, and quantified changes in total, focal, and diffuse leakage.
Methods
Subjects
This study was approved by the Duke University Medical Center Institutional Review Board, was conducted in compliance with the Health Insurance Portability and Accountability Act (HIPAA), and adhered to the tenets of the Declaration of Helsinki. A total of 29 eyes of 29 subjects were included in the study. Subjects with visually significant DME who also received FA transiting the treated eye before and after anti-VEGF as part of their routine care between January 1, 2013 and November 3, 2016 were identified. Inclusion criteria were macula center involving DME and at least 3 consecutive anti-VEGF injections. Exclusion criteria were macular edema due to cause besides DME, anti-VEGF within 3 months of initial FA, concurrent treatment for DME besides anti-VEGF, and macular photocoagulation within 1 year. To test the use and generalizability of our image analysis software in a real world setting, image quality was not used as an exclusion criteria.
Image Acquisition
All images for this study were obtained using the Heidelberg Retina Angiograph 2 (HRA2; Heidelberg Engineering, Heidelberg, Germany) device and either 30° or 55° field of view. The transit phase of the study was captured in Movie mode using the high resolution setting (4.7 frames per second) and subsequent middle and late phase images were captured as single images in ART mode (averaging 9 images). Each grayscale image in the sequence was composed of 768 × 768 pixel images. Following acquisition, image files were deidentified and exported in E2E format for further analysis.
Image Analysis
To determine the amount of leakage attributable to MAs, we reviewed images from an independent dataset, which includes equal proportions of predominantly focal, predominantly diffuse, and mixed leakage patterns (as judged by expert clinicians MJA, PM, and SC), and was previously reported and is publicly available.20 An expert grader (MJA) identified MAs that were sufficiently isolated from other leaking structures as to allow measurement of a radius of leakage. Fifty MAs were identified from this dataset and the radius of leakage was manually measured using the Heidelberg measurement tool. The mean radius of leakage was normally distributed with a mean of 117 μm (standard deviation [SD] 30.8; 95% confidence interval [CI], [105, 128]). Based on these data, leakage within a radius of 117 μm surrounding the center point of each MA was attributed to that MA. For each FA image, MAs were segmented by an expert retina-trained clinician grader (MJA) using both individual frames of the movie capturing the transit phase as well as subsequent mid and late phase still images. Microaneurysms were identified as punctate hyperfluorescent lesions that increased in intensity after their initial appearance and persisted over the course of the study.
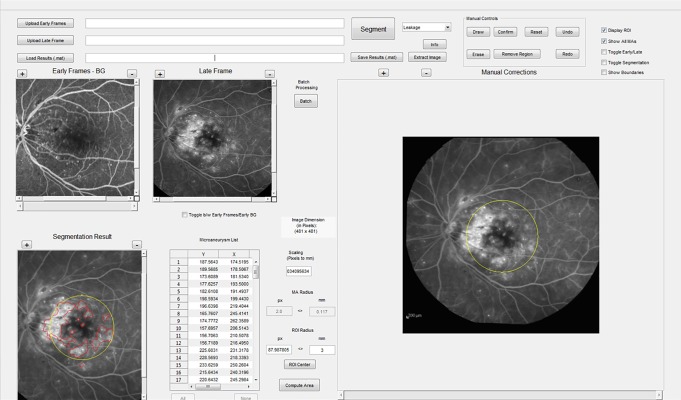
Quantification of total leakage used our previously published segmentation algorithm20 with changes and additions detailed below. To permit segmentation of MAs, grader correction of errors in leakage segmentation, and the quantification of focal and diffuse leakage, a graphical user interface (GUI) was developed (Fig. 1). The GUI was designed to allow: (1) uploading the transit stage video and the single late phase image, (2) registering early video frames and the late phase image, (3) manual correction of automated leakage segmentation, (4) manual marking of MAs, and (5) automatic quantification of focal and diffuse leakage. To reduce bias in manual segmentation, the GUI allowed segmentation of leakage and MAs to be performed independently; that is, the grader was masked to detected leakage while segmenting MAs and vice versa. The leakage in the 117 μm radius circle centered at each MA was classified as focal leakage. Remaining leakage was classified as diffuse leakage. After completion of segmentation the total, focal, and diffuse leakage areas were estimated automatically. Representative examples of clinical imaging and segmentation results are shown in Figure 2.
Figure 1.
Screen capture of the GUI used for image segmentation. The upper left shows the composite early frame and late images, and allow the user to zoom and scroll within each. Lower left shows the current segmentation result with leakage outlined in red. The right side of the GUI allows manual adjustment of leakage and segmentation of MAs. The user can toggle between early individual frames and the late image while maintaining registration of segmentation. The middle bottom portion allows input of scaling information allowing conversion of pixels to millimeters, altering region of interest, leakage radius attributed to individual MA, and selection/removal of individual MAs.
Figure 2.
Fluorescein angiograms from three representative subjects are shown. For each subject, the top row shows pretreatment imaging and the bottom row shows post-treatment imaging. The left column is the composite early frame image, the middle column is the late image, and the right column shows the segmentation result. In the segmentation image, the region of interest is outlined by the yellow circle, leakage is outlined in red and shaded, and MAs are marked with green dots with the radius off attributable leakage appearing as a yellow circle around each MA.
Statistical Analysis
Descriptive and comparative statistics were performed using JMP, Version 12 (SAS Institute, Inc., Cary, NC). Normality was tested using histogram plot and Shapiro-Wilks test. Wilcoxon signed rank testing was used to compare total, diffuse, and focal leakage before and after anti-VEGF treatment.
Results
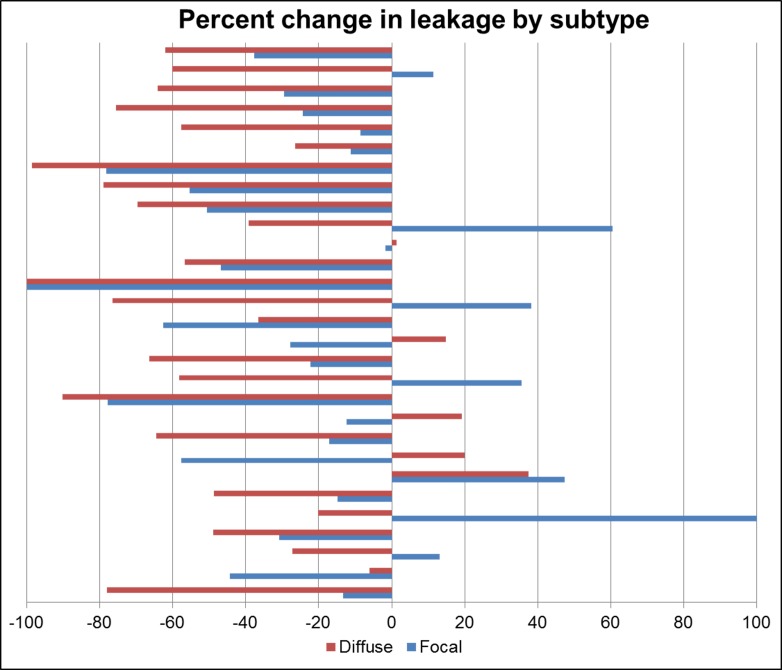
Subjects received an average of 4.6 injections between initial and follow up FAs. Ten subjects received bevacizumab, 6 received ranibizumab, 6 received aflibercept, and 7 received more than one anti-VEGF agent. Absolute total leakage decreased from a mean of 8.2 mm2 (median, 7.6 mm2) to a mean of 5.0 mm2 (median, 3.9 mm2) and this was statistically significant (P = 0.001). There also was a statistically significant reduction in absolute diffuse leakage after treatment; mean absolute diffuse leakage was 7.0 mm2 (median, 6.5 mm2) pretreatment and 4.0 mm2 (median, 2.9 mm2) post-treatment (P = 0.0006). Mean absolute focal leakage was 1.3 mm2 (median, 1.2 mm2) pretreatment and 1.0 mm2 (median, 0.90 mm2) post-treatment, but this change was not statistically significant (P = 0.14). Change in leakage also was analyzed by percent change in total, diffuse, and focal leakage. Total leakage decreased by a mean of 40.1% (P < 0.0001; 95% CI, [−28.6, −52.5]) following treatment. Diffuse leakage decreased by a mean of 45.5% (P < 0.0001; 95% CI, [−31.3, −59.6]). Focal leakage was 1.1 mm2 (median, 1.0 mm2) and decreased by a mean of 17.9% (P = 0.02; 95% CI, [−1.0, −34.8]). The difference in treatment response between focal and diffuse leakage was statistically significant for absolute (P < 0.0001) and percent (P = 0.01) change. The percent change in diffuse and focal leakage for each subject is displayed in Figure 3. As expected, there was heterogeneity in clinical response. However, most subjects have improvement in both forms of leakage. Notably diffuse leakage improved more than focal leakage in 22 of 29 subjects.
Figure 3.
Graphical representation of the percent change in focal and diffuse leakage following anti-VEGF treatment for each subject. Focal leakage is represented by blue bars and diffuse leakage is represented by red bars. In 22 of 29 subjects, diffuse leakage improved more than focal leakage.
Discussion
We used semiautomated segmentation software to quantify total as well as the focal and diffuse components of leakage before and after anti-VEGF therapy. Anti-VEGF therapy effectively reduced total leakage as well as diffuse and focal leakage. However, the absolute and percent reduction in diffuse leakage was significantly greater than the reduction in focal leakage. These findings suggested that diffuse leakage is significantly more responsive to a moderate course of anti-VEGF therapy (approximately 5 injections on average). Numerous investigators have subtyped DME as diffuse or focal according to various criteria and using variable tools, which include biomicroscopy,21–29 fundus photography.23,24,27,28,30,31 FA,31–40 and OCT.41–44 To date, the utility of classifying DME based on FA has been limited by inherent subjectivity, multiple definitions and challenges in reproducibility.15,19 Fluorescein angiography has been used to distinguish diffuse and focal DME in numerous studies31,34,36,38,40 and in randomized clinical trials.18,35,39 Most of these studies examined various treatment modalities in the management of diffuse DME. The RESTORE study performed a subgroup analysis of subjects classified as having diffuse, focal, or mixed DME, and failed to identify differences in treatment response among groups.18 However, the methodology used in RESTORE differs from our study in several important ways, which may explain the differences in results. In RESTORE, trained graders categorized each study eye as “focal,” “diffuse,” or “mixed” based on the proportion of leakage in the central subfield associated with MAs (0%–33% of leakage from MAs classified as diffuse, 33%–66% of leakage from MAs classified as mixed, and 66%–100% of leakage from MAs classified as focal). Because most eyes have components of diffuse and focal leakage, using a global classification for the entire eye could obscure differential responses to treatment between focal and diffuse leakage types. By contrast, our study examined the diffuse and focal components of leakage in each eye independently and, therefore, may have been able to detect differences in response to treatment in focal and diffuse leakage types. In addition, using a quantitative, semiautomated segmentation approach to classify leakage may be more sensitive and less subjective than the qualitative assessment of expert graders.17,19,33 Finally, it must be stated that RESTORE is a randomized, prospective study, while ours is a small, retrospective case series. Clearly, our results must be confirmed in the context of a larger prospective study. This study currently is underway.
Our study suggested that focal and diffuse leakage are, indeed, differentially responsive to anti-VEGF therapy. From a mechanistic standpoint, preclinical studies characterizing the effects of VEGF on the retinal vasculature support the concept that diffuse leakage may be a sign of VEGF-driven pathobiology. Vascular endothelial growth factor has been well characterized as a mediator of vascular permeability in many diseases, including diabetic retinopathy.45–47 A single intravitreal injection of VEGF has been shown to cause vessel dilation, tortuosity, and extensive diffuse leakage in a primate model.46 By contrast, only repeated intravitreal injection of VEGF induced formation of MAs.46 While this finding suggests a role for VEGF in MA formation, it does not inform the effects of VEGF inhibition on the biology of MAs. Observations from large clinical trials of anti-VEGF agents do not clearly address this question either. For example, reduced progression or even regression of nonproliferative diabetic retinopathy, which includes MAs in its definition has been reported with long-term administration of anti-VEGF in the RISE and RIDE trials.48 However, the specific effects of anti-VEGF therapy on MA turnover or resolution have not been specifically explored. Our results suggested that focal leakage is slow to respond to anti-VEGF when compared to diffuse leakage, but it is unknown what the findings would be after a longer continuous regimen of anti-VEGF therapy. This is a topic worthy of future study.
Our study is limited by its retrospective nature and small sample size. Because this is a retrospective study of subjects who received a second FA as part of their care, our cohort may be biased in favor of more severe or recalcitrant disease. A larger prospective observational trial with a standardized imaging protocol currently is under way and will allow us to confirm and extend the findings of the current study as well as examine the impact of other treatment modalities, such as thermal laser and corticosteroids.
In addition, the emergence of OCT angiography (OCT-A), raises the possibility that noninvasive, nondye based imaging will be capable of discriminating among different subtypes of DME. It also will be informative to evaluate whether there is correlation between FA subtypes and specific findings on traditional OCT. We have found in this study that a single eye may contain variable amounts of diffuse and focal leakage, which suggests the need for localized rather than global comparisons between FA and OCT or OCT-A. These investigations are part of our ongoing work.49
In conclusion, we have found that in eyes with a mixture of leakage subtypes, the diffuse component is more responsive to anti-VEGF therapy. It is possible that focal leakage is less responsive to anti-VEGF and that, therefore, patients with predominantly focal leakage may benefit from early or adjunctive focal laser therapy targeting leaking MAs or possibly steroid based treatments. From a more broad perspective, there may be differences in the pathobiology driving different leakage subtypes. As additional therapeutics become available to treat DME, robust subtyping using multimodal imaging will become increasingly important to guide individualized treatment planning.
Acknowledgments
MJA Supported by National Institute of Health (NIH; Bethesda, MD) K12 Grant 5K12EY016333-09 (MJA) and by NIH Grant RO1 EY022691 (SF).
Disclosure: M. J. Allingham, None; D. Mukherjee, None; E. B. Lally, None; H. Rabbani, None; P. S. Mettu, None; S. W. Cousins, None; S. Farsiu, None
References
- 1. Shaw JE,, Sicree RA,, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract. 2010. ; 87: 4–14. [DOI] [PubMed] [Google Scholar]
- 2. Klein BEK. Overview of epidemiologic studies of diabetic retinopathy. Ophthalmic Epidemiol. 14: 179–183. [DOI] [PubMed] [Google Scholar]
- 3. Stitt AW,, Curtis TM,, Chen M,, et al. The progress in understanding and treatment of diabetic retinopathy. Prog Retin Eye Res. 2016. ; 51: 156–186. [DOI] [PubMed] [Google Scholar]
- 4. Yau JWY,, Rogers SL,, Kawasaki R,, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012; 35: 556–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reichenbach A,, Wurm A,, Pannicke T,, Iandiev I,, Wiedemann P,, Bringmann A. Müller cells as players in retinal degeneration and edema. Graefes Arch Clin Exp Ophthalmol. 2007. ; 245: 627–636. [DOI] [PubMed] [Google Scholar]
- 6. Pannicke T,, Iandiev I,, Wurm A,, et al. Diabetes alters osmotic swelling characteristics and membrane conductance of glial cells in rat retina. Diabetes. 2006. ; 55: 633–639. [DOI] [PubMed] [Google Scholar]
- 7. Heier JS,, Campochiaro PA,, Yau L,, et al. Ranibizumab for macular edema due to retinal vein occlusions: long-term follow-up in the HORIZON trial. Ophthalmology. 2012. ; 119: 802–809. [DOI] [PubMed] [Google Scholar]
- 8. Elman MJ,, Aiello LP,, Beck RW,, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010. ; 117: 1064–1077.e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Korobelnik J,, Do D,, Schmidt-Erfurth U. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014; 121: 2247–2254. [DOI] [PubMed] [Google Scholar]
- 10. Boyer DS,, Yoon YH,, Belfort R,, et al. Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology. 2014; 121: 1904–1914. [DOI] [PubMed] [Google Scholar]
- 11. Campochiaro PA,, Brown DM,, Pearson A,, et al. Long-term benefit of sustained-delivery fluocinolone acetonide vitreous inserts for diabetic macular edema. Ophthalmology. 2011; 118: 626–635.e2. [DOI] [PubMed] [Google Scholar]
- 12. Do DV,, Schmidt-Erfurth U,, Gonzalez VH,, et al. The DA VINCI study: phase 2 primary results of VEGF trap-eye in patients with diabetic macular edema. Ophthalmology. 2011. ; 118: 1819–1826. [DOI] [PubMed] [Google Scholar]
- 13. Wells JA,, Glassman AR,, Ayala AR,, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016. ; 123: 1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nguyen QD,, Brown DM,, Marcus DM,, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012. ; 119: 789–801. [DOI] [PubMed] [Google Scholar]
- 15. Browning DJ,, Altaweel MM,, Bressler NM,, Bressler SB,, Scott IU. Diabetic macular edema: what is focal and what is diffuse? Am J Ophthalmol. 2008. ; 146: 649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bresnick GH. Diabetic maculopathy. A critical review highlighting diffuse macular edema. Ophthalmology. 1983. ; 90: 1301–1317. [DOI] [PubMed] [Google Scholar]
- 17. Browning DJ,, Altaweel MM,, Bressler NM,, Bressler SB,, Scott IU. Diabetic macular edema: what is focal and what is diffuse? Am J Ophthalmol. 2008; 146: 649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mitchell P,, Bandello F,, Schmidt-Erfurth U,, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011. ; 118: 615–625. [DOI] [PubMed] [Google Scholar]
- 19. Browning DJ. Diabetic Retinopathy: Evidence-Based Management, 1st ed. New York: Springer Science & Business Media; 2010. [Google Scholar]
- 20. Rabbani H,, Allingham MJ,, Mettu PS,, Cousins SW,, Farsiu S. Fully automatic segmentation of fluorescein leakage in subjects with diabetic macular edema. Invest Ophthalmol Vis Sci. 2015. ; 56: 1482–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Otani T,, Kishi S. Tomographic assessment of vitreous surgery for diabetic macular edema. Am J Ophthalmol. 2000. ; 129: 487–494. [DOI] [PubMed] [Google Scholar]
- 22. Toda J,, Fukushima H,, Kato S. Injection of triamcinolone acetonide into the posterior sub-tenon capsule for treatment of diabetic macular edema. Retina. 27: 764–769. [DOI] [PubMed] [Google Scholar]
- 23. Catier A,, Tadayoni R,, Paques M,, et al. Characterization of macular edema from various etiologies by optical coherence tomography. Am J Ophthalmol. 2005. ; 140: 200.e1–200.e9. [DOI] [PubMed] [Google Scholar]
- 24. Ciardella AP,, Klancnik J,, Schiff W,, Barile G,, Langton K,, Chang S. Intravitreal triamcinolone for the treatment of refractory diabetic macular oedema with hard exudates: an optical coherence tomography study. Br J Ophthalmol. 2004. ; 88: 1131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Abu el Asrar AM, Morse PH. Laser photocoagulation control of diabetic macular oedema without fluorescein angiography. Br J Ophthalmol. 1991. ; 75: 97–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gibran SK,, Cullinane A,, Jungkim S,, Cleary PE. Intravitreal triamcinolone for diffuse diabetic macular oedema. Eye (Lond). 2006. ; 20: 720–724. [DOI] [PubMed] [Google Scholar]
- 27. Patz A. Cystoid maculopathy in diabetics. Arch Ophthalmol. 1976. ; 94: 761–768. [DOI] [PubMed] [Google Scholar]
- 28. Jensen DB,, Knudsen LL. Stereoscopic fluorescein angiography in diabetic maculopathy. Retina. 2006. ; 26: 153–158. [DOI] [PubMed] [Google Scholar]
- 29. Jeppesen P,, Bek T. Impaired retinal autoregulation in small retinal arterioles before and after focal laser treatment for diabetic maculopathy. Br J Ophthalmol. 2006. ; 90: 198–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Laursen ML,, Moeller F,, Sander B,, Sjoelie AK. Subthreshold micropulse diode laser treatment in diabetic macular oedema. Br J Ophthalmol. 2004. ; 88: 1173–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kang SW,, Park CY,, Ham D-I. The correlation between fluorescein angiographic and optical coherence tomographic features in clinically significant diabetic macular edema. Am J Ophthalmol. 2004. ; 137: 313–322. [DOI] [PubMed] [Google Scholar]
- 32. Focal photocoagulation treatment of diabetic macular edema. Relationship of treatment effect to fluorescein angiographic and other retinal characteristics at baseline: ETDRS report no. 19. Early Treatment Diabetic Retinopathy Study Research Group. Arch Ophthalmol. 1995. ; 113: 1144–1155. [PubMed] [Google Scholar]
- 33. Sander B,, Larsen M,, Engler C,, et al. Diabetic macular oedema: a comparison of vitreous fluorometry, angiography, and retinopathy. Br J Ophthalmol. 2002. ; 86: 316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McDonald HR,, Schatz H. Grid photocoagulation for diffuse macular edema. Retina. 5: 65–72. [DOI] [PubMed] [Google Scholar]
- 35. Kumar A,, Sinha S,, Azad R,, Sharma YR,, Vohra R. Comparative evaluation of vitrectomy and dye-enhanced ILM peel with grid laser in diffuse diabetic macular edema. Graefe's Arch Clin Exp Ophthalmol. 2007. ; 245: 360–368. [DOI] [PubMed] [Google Scholar]
- 36. Massin P,, Duguid G,, Erginay A,, Haouchine B,, Gaudric A. Optical coherence tomography for evaluating diabetic macular edema before and after vitrectomy. Am J Ophthalmol. 2003. ; 135: 169–177. [DOI] [PubMed] [Google Scholar]
- 37. Watanabe M,, Oshima Y,, Emi K. Optical cross-sectional observation of resolved diabetic macular edema associated with vitreomacular separation. Am J Ophthalmol. 2000. ; 129: 264–267. [DOI] [PubMed] [Google Scholar]
- 38. Chieh JJ,, Roth DB,, Liu M,, et al. Intravitreal triamcinolone acetonide for diabetic macular edema. Retina. 25: 828–834. [DOI] [PubMed] [Google Scholar]
- 39. Yanyali A,, Nohutcu AF,, Horozoglu F,, Celik E. Modified grid laser photocoagulation versus pars plana vitrectomy with internal limiting membrane removal in diabetic macular edema. Am J Ophthalmol. 2005. ; 139: 795–801. [DOI] [PubMed] [Google Scholar]
- 40. Gandorfer A,, Messmer EM,, Ulbig MW,, Kampik A. Resolution of diabetic macular edema after surgical removal of the posterior hyaloid and the inner limiting membrane. Retina. 2000. ; 20: 126–133. [PubMed] [Google Scholar]
- 41. Browning DJ,, Fraser CM. Regional patterns of sight-threatening diabetic macular edema. Am J Ophthalmol. 2005. ; 140: 117.e1–117.e10. [DOI] [PubMed] [Google Scholar]
- 42. Kim BY,, Smith SD,, Kaiser PK. Optical coherence tomographic patterns of diabetic macular edema. Am J Ophthalmol. 2006. ; 142: 405–412.e1. [DOI] [PubMed] [Google Scholar]
- 43. Brasil OFM,, Smith SD,, Galor A,, Lowder CY,, Sears JE,, Kaiser PK. Predictive factors for short-term visual outcome after intravitreal triamcinolone acetonide injection for diabetic macular oedema: an optical coherence tomography study. Br J Ophthalmol. 2007. ; 91: 761–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Browning DJ,, McOwen MD,, Bowen RM,, O'Marah TL. Comparison of the clinical diagnosis of diabetic macular edema with diagnosis by optical coherence tomography. Ophthalmology. 2004. ; 111: 712–715. [DOI] [PubMed] [Google Scholar]
- 45. Senger DR,, Galli SJ,, Dvorak AM,, Perruzzi CA,, Harvey VS,, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983. ; 219: 983–985. [DOI] [PubMed] [Google Scholar]
- 46. Tolentino MJ, Miller JW, Gragoudas MJ, et al. Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology. 1996: 1820–1828. [DOI] [PubMed] [Google Scholar]
- 47. Antonetti DA,, Barber AJ,, Khin S,, Lieth E,, Tarbell JM,, Gardner TW. Vascular permeability in experimental diabetes is associated with reduced endothelial occludin content: vascular endothelial growth factor decreases occludin in retinal endothelial cells. Penn State Retina Research Group. Diabetes. 1998. ; 47: 1953–1959. [DOI] [PubMed] [Google Scholar]
- 48. Ip MS,, Domalpally A,, Hopkins JJ,, Wong P,, Ehrlich JS. Long-term effects of ranibizumab on diabetic retinopathy severity and progression. Arch Ophthalmol. 2012. ; 130: 1145–1152. [DOI] [PubMed] [Google Scholar]
- 49. Polans J,, Cunefare D,, Cole E,, et al. Enhanced visualization of peripheral retinal vasculature with wavefront sensorless adaptive optics optical coherence tomography angiography in diabetic patients. Opt Lett. 2017. ; 42: 17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]