Abstract
Purpose
Vascular endothelial growth factor (VEGF) regulates microvascular endothelial permeability, and the permeability of Schlemm's canal (SC) endothelium influences conventional aqueous humor outflow. We hypothesize that VEGF signaling regulates outflow facility.
Methods
We measured outflow facility (C) in enucleated mouse eyes perfused with VEGF-A164a, VEGF-A165b, VEGF-D, or inhibitors to VEGF receptor 2 (VEGFR-2). We monitored VEGF-A secretion from human trabecular meshwork (TM) cells by ELISA after 24 hours of static culture or cyclic stretch. We used immunofluorescence microscopy to localize VEGF-A protein within the TM of mice.
Results
VEGF-A164a increased C in enucleated mouse eyes. Cyclic stretch increased VEGF-A secretion by human TM cells, which corresponded to VEGF-A localization in the TM of mice. Blockade of VEGFR-2 decreased C, using either of the inhibitors SU5416 or Ki8751 or the inactive splice variant VEGF-A165b. VEGF-D increased C, which could be blocked by Ki8751.
Conclusions
VEGF is a paracrine regulator of conventional outflow facility that is secreted by TM cells in response to mechanical stress. VEGF affects facility via VEGFR-2 likely at the level of SC endothelium. Disruption of VEGF signaling in the TM may explain why anti-VEGF therapy is associated with decreased outflow facility and sustained ocular hypertension.
Keywords: vascular endothelial growth factor, Schlemm's canal, trabecular meshwork, outflow facility, mouse models
Intraocular pressure (IOP) is determined by the facility of aqueous humor outflow through the conventional outflow pathway. While decreased outflow facility causes IOP elevation in most forms of glaucoma,1 the factors controlling outflow facility remain largely unknown. Within the conventional outflow pathway, facility is predominately regulated within the outer trabecular meshwork (TM) and the underlying inner wall endothelium of Schlemm's canal (SC).2,3 Aqueous humor likely crosses SC endothelium through micrometer-sized pores,4–8 and SC pore density is reduced in glaucoma.9–11 As pores may influence outflow facility,12 the porosity or permeability of SC endothelium presumably is an important factor controlling outflow facility and, hence, IOP.
Vascular endothelial growth factor (VEGF) is a potent regulator of endothelial permeability.13 In vascular endothelia, VEGF induces formation of pore-like fenestrae14,15 and disassembly of intercellular junctions.16,17 VEGF is secreted by human TM cells in culture, and VEGF has been proposed to regulate the permeability of SC endothelium to affect outflow.18,19 The inner wall endothelium of SC expresses all three VEGF receptors (VEGFR), including VEGFR-1 and -2, common to vascular endothelia, and VEGFR-3, which is typical of lymphatic endothelia but absent from vascular endothelia.20–25 VEGF increases outflow facility in pigs,19 and pigment-epithelium derived factor (PEDF) that functionally antagonizes VEGF decreases outflow facility in mice.26 Furthermore, heterozygous deletion of VEGFR-1 and/or VEGFR-2 leads to ocular hypertension and buphthalmia in mice.22
Drugs that disrupt VEGF signaling are being used to treat a range of retinal diseases, including neovascular age-related macular degeneration (NVAMD), diabetic macular edema, and retinal vein occlusion. Despite the benefits of anti-VEGF therapy, a number of observational studies have reported sustained ocular hypertension lasting several months or longer in 3% to 11% of patients receiving repeated injections of anti-VEGF.27–36 In our companion study37 we show that prolonged anti-VEGF therapy is associated with reduced tonographic outflow facility in patients receiving unilateral treatment for NVAMD. These data suggest that disruption of endogenous VEGF signaling inhibits normal outflow function and IOP homeostasis.
We hypothesize that VEGF is a paracrine regulator of conventional outflow facility that is secreted in response to IOP-related mechanical cues. To test this hypothesis, we investigated the effect of different isoforms of VEGF and VEGFR inhibitors on aqueous humor outflow facility, and we localize VEGF protein within the TM of mice. Mice are a valuable animal model for studying outflow because, like primates, mice have a continuous SC and lamellated TM,38,39 and mice demonstrate a similar pharmacologic response to compounds that affect outflow facility in humans.26,40–43 To mimic the repetitive mechanical stress induced by IOP pulsations within the TM,44 we subjected TM cells in culture to cyclic stretch and measured VEGF secretion.
Materials and Methods
Ex Vivo Mouse Eye Perfusions
Perfusion of enucleated mouse eyes was used to assess the effect of VEGF or related compounds on pressure-dependent outflow facility. Outflow facility (C) was measured in paired contralateral eyes by multilevel constant pressure perfusion using iPerfusion.45 All mice were male C57BL/6 (Charles River UK Ltd, Margate, UK) aged between 9 and 13 weeks at the time of perfusion. Mice were fed ad libitum and maintained at 21°C with a 12-hour light/dark cycle. All animals were treated in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research under the authority of a UK Home Office Project License.
Eyes were enucleated within 10 minutes of death by cervical dislocation, affixed to a support platform using cyanoacrylate glue, and submerged in a bath of Dulbecco's PBS at 35°C. The perfusion needle and tubing were backfilled with approximately 200 μl of perfusion fluid; either Dulbecco's PBS including divalent cations and 5.5 mM glucose that was passed through a 0.22 μm filter (collectively referred to as “DBG”), or DBG containing the desired concentration of compound. If the compound was solubilized in vehicle, typically DMSO, then an equivalent concentration of vehicle was added to the DBG solution for the contralateral control eye. The anterior chamber was cannulated using a 33-gauge needle mounted on a micromanipulator while visualizing through a stereomicroscope. The eye was pressurized at 8 mm Hg for 45 minutes to allow the eye to equilibrate to the perfusion system and provide time for the drug to permeate through the anterior chamber and outflow pathway. The eye then was perfused over 9 increasing pressure steps from approximately 4 to 20 mm Hg using an actuated reservoir. Intraocular pressure was measured using a differential pressure transducer (PX409; Omegadyne, Sunbury, OH, USA), while the flow rate into the eye was measured using a thermal flow sensor (SLG64-0075; Sensirion, Staefa, Switzerland) as previously described.45
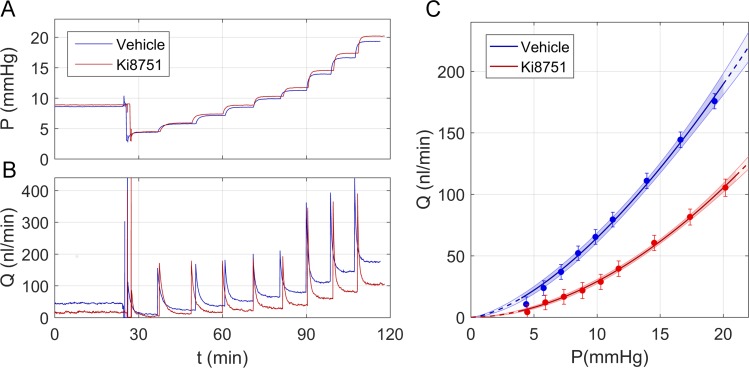
For each pressure step, the tracing was considered objectively to have reached steady state once the magnitude of the slope of the ratio of flow rate to pressure, calculated over a 5-minute window, remained consistently below 0.1 nl/min/mm Hg/min for 1 minute. Data were then averaged over the last 4 minutes to calculate the steady state flow rate and pressure values for that step, and the actuated reservoir was automatically elevated to initiate the next pressure step. Pressure steps that did not reach the stability condition defined above were excluded, along with all subsequent steps, and eye pairs with less than 4 stable pressure steps in either eye were excluded. Exclusion rates ranged between 10% and 50% across all experimental sets. For each eye, the stable flow rate (Q) and pressure (P) values for each step were fit by an empirical power law model of the form Q = Cr (P/Pr)β P, where Cr is the facility at a reference pressure Pr, chosen to be 8 mm Hg as the physiologic pressure drop across the outflow pathway,45 and β describes the nonlinearity in the flow-pressure relationship that represents the pressure dependence of C. The difference in facility between contralateral eyes, where one eye received the treatment and the other vehicle control, was the primary readout. The analysis followed the methodology described by Sherwood et al.45 to account for various uncertainties associated with the measurement and analysis. Representative perfusion tracings and flow-pressure relationships are provided in Figure 1.
Figure 1.
Representative data obtained using the iPerfusion system.45 Raw unfiltered data showing the pressure P (A) and flow rate Q (B) tracings for a pair of enucleated mouse eyes perfused with either antagonist to VEGFR-2 (1 nM Ki8751 in vehicle, red tracings) or vehicle alone (Dulbecco's PBS + 5.5 mM glucose, blue tracings). (C) The average Q versus P data from the last 4 minutes of each pressure step using the tracings shown in (A) and (B) with power law fittings to the data as described in the main text. Error bars: 95% CI on the measured value of Q for each pressure step accounting for sensor uncertainty. The shaded blue and red regions indicate the 95% CI on the power law fittings.
Perfusions included in this study examined the effect of recombinant murine VEGF-A164a (Sigma-Aldrich Company Ltd, Dorset, United Kingdom) and VEGF-D (Abcam, Cambridge, United Kingdom) and human VEGF-A165b (Abcam), which is an inactive splice variant that lacks the neuropilin binding domain and thereby acts as a competitive inhibitor for the VEGF receptor.46 Studies also examined the effect of the VEGFR-2 inhibitors SU5416 (Sigma-Aldrich Company Ltd) and Ki8751 (Selleckchem, Munich, Germany). We also examined the effect on C of ranibizumab, an Fab antibody fragment against human VEGF (Novartis, Basel, Switzerland) and a polyclonal antibody against mouse VEGF-A164 (AF-493; R&D Biosystems, Minneapolis, MN, USA). Perfusions typically were obtained using contralateral eyes perfused simultaneously using two identical iPerfusion systems, with cannulations occurring within 10 to 30 minutes of enucleation. Exceptions include perfusions with VEGF-A164a, VEGF-A165b, and SU5416, where contralateral eyes were perfused sequentially because only a single iPerfusion system was available. For these experiments, we randomized the order of perfusion (i.e., whether the treated eye was perfused first or second). During the first perfusion, the second eye was stored in PBS for a maximum of 4 hours at 4°C.
Human TM Cell Culture, Cell Stretching, and VEGF Secretion
Human TM cells were assayed for VEGF production under static conditions and in response to cyclic stretch. TM cells were isolated and characterized as described previously.47 This study included three cell strains aged 64 years (TM89), 72 years (TM121), and an adult donor of unknown age (TM94). TM cells were cultured in a humidified air incubator at 37°C and 5% CO2 using Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Atlanta Biologicals, Flowery Branch, GA, USA), 100 U/ml penicillin, 0.1 mg/ml streptomycin, and 0.29 mg/ml L-glutamine.
For stretching experiments, human TM cells were seeded onto flexible membranes mounted into a 6-well culture plate (BioFlex; Flexcell International Corp., Hillsborough, NC, USA) and grown to confluence under static conditions in DMEM containing 10% FBS. Media was replaced every 2 to 3 days until the cells became confluent, at which time the media was switched to DMEM containing 1% FBS for at least 1 week. Cyclic mechanical stretch (16% peak strain, 1 Hz) was then initiated and continuously applied for 24 hours using a commercial cellular stretching device (FX-5000; Flexcell International Corp.). The applied stretch approximates the predicted strain (7%–33%) if the pressure drop across the TM changes by 2 to 10 mm Hg as occurs during the ocular pulse or saccades,48 assuming an elastic modulus of 4 kPa.49 Unstretched controls were incubated simultaneously under zero mechanical strain alongside stretched samples. After 24 hours of stretch or static culture, conditioned media was collected and centrifuged at 4000g for 3 minutes to pellet cell debris. VEGF-A165 concentration was measured in the supernatant using ELISA (Human VEGF Quantikine Kit; R&D Biosystems) and quantified using a microplate reader (SpectraMax M3; Molecular Devices, Sunnyvale, CA, USA).
Immunofluorescence Labeling
Immunofluorescence was used to label and identify the distribution of VEGF-A within the outflow pathway and limbus of mice. We used two commercially available antibodies: polyclonal rabbit anti-human that cross-reacts with mouse VEGF-A (sc-507; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) and polyclonal goat anti-mouse VEGF-A164 (AF-493, R&D Systems). To identify vascular and SC endothelial cells, we dual-labeled with monoclonal rat anti-mouse CD31/PECAM1 (Clone MEC 13.3; BD Biosciences, San Jose, CA, USA). In total, these studies examined 13 eyes from 8 C57BL/6 mice aged 9 to 13 weeks.
The following describes methods used for the polyclonal rabbit antibody applied to 10 eyes from 5 mice. Immediately after enucleation, a pinhole was made through the central cornea, and the eyes were submerged in 4% paraformaldehyde in PBS for 2 hours at room temperature. Eyes were then washed 3 times in PBS for 30 minutes each and bisected at the equator. The lens was removed carefully, and the anterior segments were cut into 4 wedges. Each wedge was frozen in isopentane and embedded into tissue freezing medium (Leica Mikrosysteme Vertrieb GmbH, Wetzlar, Germany). Sagittal sections (10 μm thick) were cut through each wedge using a cryostat (CM3050 S, Leica Mikrosysteme Vertrieb GmbH) and mounted on adhesion slides. After blocking with 1% milk solution for 3 minutes at room temperature, sections were first incubated with rat anti-mouse CD31/PECAM1 diluted 1:100 overnight at 4°C. Following washing in PBS, sections were incubated with goat anti-rat IgG (Alexa Fluor 488; Abcam) diluted 1:1000 for 1 hour at room temperature. Sections were then washed and incubated with polyclonal rabbit anti-VEGF (sc-507, Santa Cruz Biotechnology, Inc.) diluted 1:10 overnight at 4°C. Sections then were washed and incubated with goat anti-rabbit IgG (Alexa Fluor 555; Invitrogen, Life Technologies, Darmstadt, Germany) diluted 1:2000 for 1 hour at room temperature. Sections then were washed and mounted in fluorescent mounting medium (Dako, Biozol, Eching, Germany), and imaged (BZ-9000; Keyence, Neu Isenburg, Germany). Negative controls were processed identically except omitting the primary antibodies. Approximately 144 sections were acquired per eye. Similar methods were used for the second antibody that examined 3 eyes from 3 mice with 10 to 15 sections per eye (See Supplementary Information).
Statistics
We report the geometric average percent change in facility between contralateral eyes, along with the 95% confidence interval (CI) on the average percent change. Statistical analysis for facility used log-transformed data, with the weighted t-test used to calculate significance, as described previously.45 A 1-way repeated measures ANOVA on log-transformed concentration values was used to compare the VEGF-A expression by TM cells.
Results
VEGF-A Increases Aqueous Humor Outflow Facility
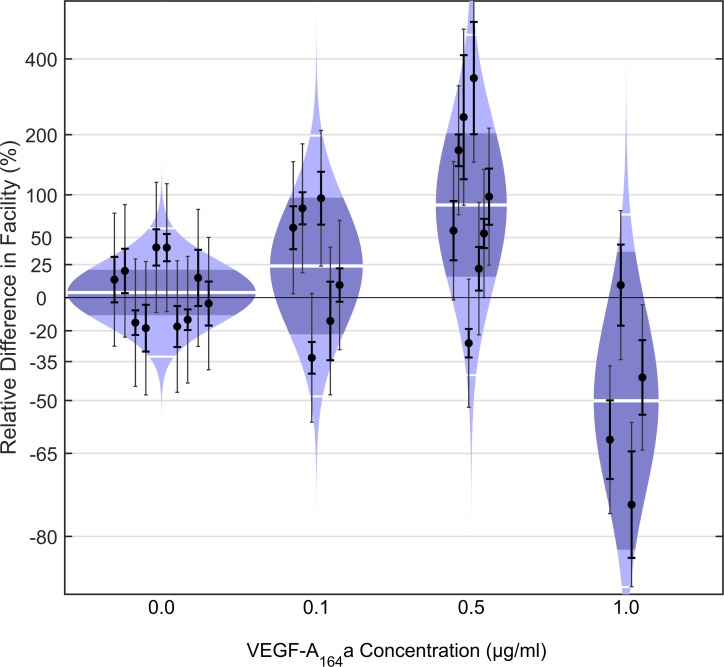
We first examined the effect of exogenous VEGF-A164a on outflow facility in enucleated eyes from C57BL/6 mice using the iPerfusion system.45 This isoform was chosen because it typically is the most prevalent isoform and retains binding affinity to VEGFR-2.50 We compared C between paired eyes perfused with murine VEGF-A164a, and their contralateral control eyes perfused with vehicle alone, over 4 different concentrations (0, 0.1, 0.5, 1.0 μg/ml, equivalent to 0, 3, 13, and 26 nM; Fig. 2). In control experiments where both eyes were perfused without VEGF-A164a, there was no observed difference in C between paired eyes (mean 4%; CI: −11, 21%; P = 0.62; n = 10 pairs; weighted t-test). At the lowest concentration of 0.1 μg/ml VEGF-A164a, the average difference in C was 24% (CI: −22, 96%), which was not significantly different from zero (P = 0.29, n = 6). At an intermediate concentration of 0.5 μg/ml, C was significantly increased on average by 87% (CI: 15, 203%; P = 0.02, n = 8). An increase in C was not observed, however, at a higher concentration of 1 μg/ml; the average difference being −50% (CI: −82, 36%; P = 0.11, n = 4). These data indicate that VEGF-A164a increases outflow facility at a concentration of approximately 0.5 μg/ml.
Figure 2.
VEGF-A164a increases C in enucleated mouse eyes. Modified violin or “cello” plots showing the difference in facility for enucleated C57BL/6 mouse eyes perfused with varying concentrations of VEGF-A164a relative to contralateral eyes perfused with vehicle. Paired eyes perfused with vehicle in both eyes (0 μg/ml) indicate the expected range of variability between untreated contralateral eyes, which showed an average difference in facility of 4% that was not statistically significant (P = 0.62, n = 10 pairs, weighted t-test). At a low concentration of 0.1 μg/ml VEGF-A164a, the average difference in facility was 24% (CI: −22, 96%; P = 0.29, n = 6, weighted t-test). At an intermediate concentration of 0.5 μg/ml VEGF-A164a, facility was significantly increased on average by 87% (CI: 15, 203%; P = 0.02, n = 8). A higher concentration of 1.0 μg/ml VEGF-A164a decreased facility by 50% on average (CI: −82, 36%; P = 0.11, n = 4). Data points represent the measured relative facility difference of the treated eye with respect to the contralateral untreated eye for individual pairs. Thick error bars represent the 95% CI on the measured relative difference in facility, while the thin error bars represent the additional uncertainty due to the variability between untreated contralateral eyes measured from a previous study.45 The colored regions represent the log-normal distribution that best describes the data, while the thick white central lines indicate the geometric means. The dark shaded regions indicate the 95% CI on the geometric mean, and the outer thin white lines indicate the range that encompasses approximately 95% of the measured data.
VEGF-A Is Expressed Endogenously Within the Conventional Outflow Pathway
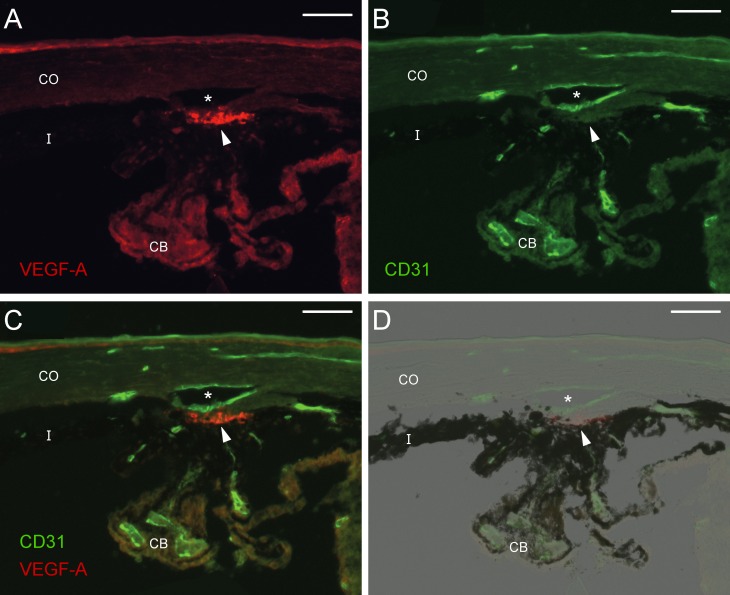
We immunolabeled the conventional outflow pathway of mice to determine whether VEGF-A is present within the TM in situ, where it may influence outflow facility. As visualized using two different antibodies, intense labeling was observed in the TM, particularly in the innermost lamellated region. Single cells were stained in some regions of the juxtacanicular tissue (JCT), whereas most of the JCT appeared unstained, indicating lower levels of VEGF-A expression in the JCT compared to the innermost TM (Fig. 3; Supplementary Fig. S1). VEGF-A labeling also was present in the corneal epithelium, with weaker labeling observed along the ciliary epithelium and surrounding the CD31-positive vessels in the ciliary processes. Similar weak labeling was present in the ciliary body. Control sections that were treated with secondary antibody alone, showed no staining (Supplementary Fig. S1).
Figure 3.
VEGF-A is present within the murine trabecular meshwork. Immunohistochemistry of the limbus in C57BL/6 mice showing the localization of VEGF-A (A, red, sc-507), CD31/PECAM-1 (B, green), the merged image (C) and merged with bright field (D). Intense VEGF-A labeling is present within the trabecular meshwork (arrowhead) near the inner wall endothelium of SC (*canal lumen) that expresses CD31. Weaker VEGF-A labeling is present in the corneal epithelium, ciliary epithelium and surrounding CD31-positive vessels in the ciliary body (CB). Scale bars: 50 μm. CO, cornea; I, iris.
VEGF-A Is Secreted by TM Cells in Response to Mechanical Stretch
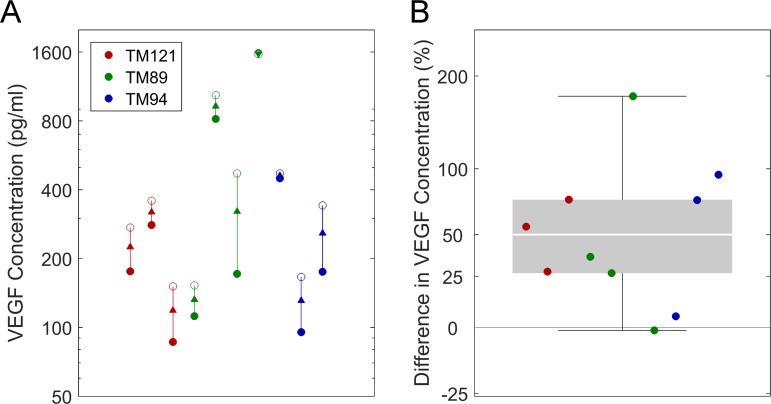
We then examined the production of VEGF-A by human TM cells in static culture and in response to cyclic mechanical stretch. The latter mimics the in vivo environment where mechanical stimulation is imposed on the TM from continuous pressure pulsations associated with the ocular pulse or saccades.48 After 24 hours of static culture, the average VEGF-A concentration in conditioned medium was 246 (CI: 124, 487) pg/ml (mean, 95% CI; n = 10, 3 cell strains, 3–4 samples per strain). The average VEGF-A concentration was significantly increased to 369 (CI: 210, 648) pg/ml after 24 hours of cyclic stretch (Fig. 4A), corresponding to a mean relative increase of 50% (P = 0.008, n = 10, 3 cell strains, 3–4 samples per strain, 1-way repeated measures ANOVA; Fig. 4B). No difference was observed in VEGF-A expression between cell strains (P = 0.99).
Figure 4.
Cyclic stretch promotes VEGF-A secretion from human TM cells. VEGF-A concentration in conditioned medium from human TM cells in static culture (A, filled symbols) versus 16% cyclic mechanical stretch at 1 cycle per second for 24 hours (open symbols). Data points show replicate experiments from 3 different human TM cell strains (TM121 in red n = 3, TM89 in green n = 4, TM94 in blue n = 3) with and without stretch. Arrowheads indicate the direction of change in response to stretch. (B) The relative increase in VEGF-A concentration in conditioned medium from stretched versus static TM cells based on the data shown in (A). Stretch increases VEGF-A production on average by 50% (P = 0.008, n = 3 cell strains, 1-way repeated measured ANOVA). Shaded box indicates the interquartile range. Error bars: indicate the range, and the thick white line indicates the median.
VEGFR-2 Mediates the Effects of Endogenous VEGF-A on Outflow Facility
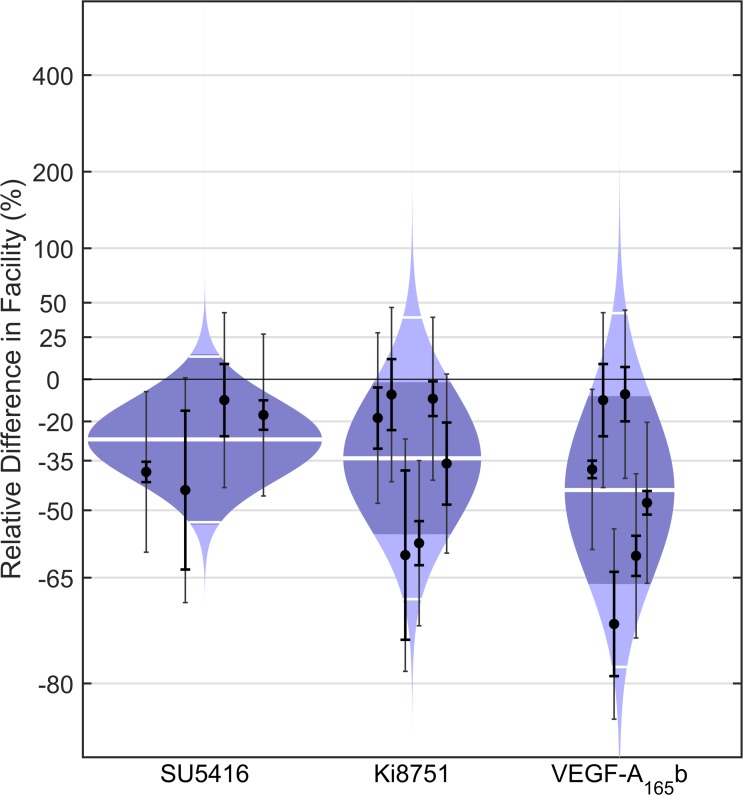
To determine whether VEGF-A produced by the TM influences outflow facility through VEGFR-2, we perfused enucleated mouse eyes with VEGFR antagonists SU5416 or Ki8751 in the absence of exogenous VEGF (Fig. 5). In initial studies with 3 μM SU5416, a moderately selective antagonist to VEGFR-2 with an IC50 of 1 μM,51–53 we observed a decrease in facility in all eyes with an average difference in C of −27% (CI: −54, 14%; P = 0.10, n = 4). We then investigated the response to a more selective antagonist of VEGFR-2, Ki8751, which has an IC50 of 0.9 nM.54 In response to 1 nM Ki8751, C decreased with an average difference of −34% (CI: −56, −2%; P = 0.04, n = 6). As an alternative approach to inhibit endogenous VEGF signaling, we perfused enucleated mouse eyes with human VEGF-A165b, an alternative splice variant of VEGF-A that acts as a competitive inhibitor of VEGF receptor activity.46 VEGF-A165b lacks the neuropilin-binding domain required for VEGF receptor activation, while retaining its ligand-binding domain to VEGFR-1 and -2.55–57 In the presence of 0.5 μg/ml VEGF-A165b, C was decreased with an average difference of −44% (CI: −66, −8%; P = 0.03; n = 6). These data demonstrated, using three different agents, that blockade of VEGFR-2 decreases C in mice, presumably by inhibiting endogenous VEGF signaling in the TM.
Figure 5.
Antagonists to VEGFR-2 decrease outflow facility in enucleated mouse eyes. Cello plots showing the relative difference in C between contralateral eyes of C57BL/6 mice perfused with or without 3 μM SU5416, 1 nM Ki8751, or 0.5 μg/ml human VEGF-A165b. Ki8751 (P = 0.04, n = 6, weighted t-test) and VEGF-A165b (P = 0.03, n = 6) reduced facility by 34% (CI: −56, −2%) and 44% (CI: −66, −8%) on average, respectively, while SU5416 reduced facility on average by 27% (CI: −54, 14%) but did not achieve significance (P = 0.10, n = 4). Data points represent the relative facility difference of a treated eye with respect to its contralateral untreated eye for individual pairs. The thick white lines represent the geometric mean of the relative difference for each group. The remaining symbols are as defined in Figure 2.
VEGFR-2 Mediates the Effects of VEGF-D on Outflow Facility
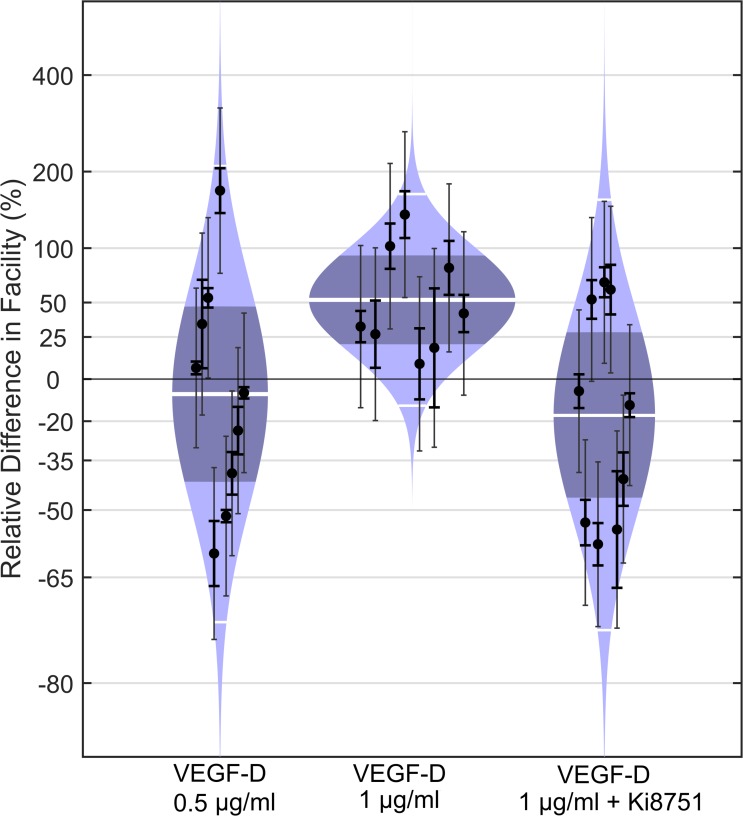
To determine whether the activation of VEGFR-2 is specific to VEGF-A, we measured outflow facility in mice following perfusion with VEGF-D. VEGF-D is an endogenous ligand for VEGFR-2 and -3, which typically are expressed by lymphatic endothelia58,59 as well as SC endothelium, along with VEGFR-1.20–25 Perfusion with 1 μg/ml murine VEGF-D significantly increased C in all eyes on average by 52% (CI: 20, 92%; P = 0.004, n = 8), while 0.5 μg/ml VEGF-D had no detectable effect on C with an average difference of −8% (CI: −42, 47%; P = 0.70, n = 9; Fig. 6). The selective antagonist to VEGFR-2, Ki8751 (1 nM), blocked the effect of 1 μg/ml VEGF-D compared to contralateral eyes perfused with 1 nM Ki8751 alone, with an average facility difference of −17% (CI: −47, 28; P = 0.35, n = 9). These data demonstrated that modulation of outflow facility via VEGFR-2 is not specific to VEGF-A.
Figure 6.
VEGF-D increases outflow facility via VEGFR-2 in enucleated mouse eyes. Cello plots showing the relative difference in C between contralateral eyes of C57BL/6 mice perfused with or without VEGF-D at 0.5 or 1.0 μg/ml. The average facility difference was 52% in response to 1.0 μg/ml VEGF-D (P = 0.004, n = 8, weighted t-test), while 0.5 μg/ml VEGF-D had no detectible effect on facility with an average difference of −8% (P = 0.70, n = 9). No facility difference was observed in response to 1.0 μg/ml VEGF-D in the presence of 1 nM Ki8751 compared to the contralateral eye perfused with Ki8751 alone (P = 0.35, n = 9), indicating that the facility-increasing effect of VEGF-D is mediated via VEGFR-2. Data points represent the relative facility difference of a treated eye with respect to its contralateral untreated eye for individual pairs. The thick white lines represent the geometric mean of the relative difference for each group. The remaining symbols are as defined in Figure 2.
Acute Sequestration of VEGF-A Does Not Significantly Affect Outflow Facility
To examine whether sequestration of VEGF-A affects outflow facility over acute time scales, we perfused mouse eyes with 0.14 mg/ml ranibizumab, a dose that is equivalent to that used to treat patients with NVAMD, or vehicle. Ranibizumab had no measurable effect on C with an average difference of −5% (CI: −25, 21%; P = 0.58, n = 5; Supplementary Fig. S2). We reasoned that the absence of an observable facility effect might be attributable to the reported low binding affinity of ranibizumab to the murine homolog of VEGF-A.60,61 To address this possibility, we perfused eyes with 0.14 mg/ml of a polyclonal anti-VEGF antibody that is selective and reportedly neutralizing for murine VEGF-A164, although it may not distinguish between VEGF-A164a and VEGF-A164b. These data also showed no apparent effect on C with an average facility difference of 3% (CI: −40, 64%; P = 0.85, n = 4; Supplementary Fig. S2). Therefore, acute exposure to anti-VEGF compounds may be insufficient to affect outflow facility over relatively short time scales (corresponding to the ∼2-hour duration of the perfusion), suggesting that prolonged exposure to VEGF-sequestering compounds may be necessary to significantly affect outflow function, possibly due to homeostatic compensation or to a sufficiently large intraocular reservoir of endogenous VEGF-A.
Discussion
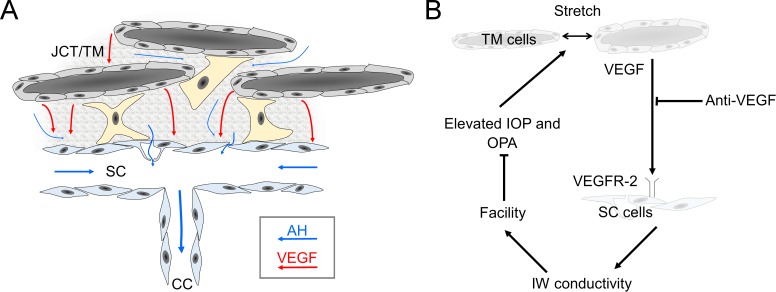
In this study, we demonstrate that VEGF modulates outflow facility in mice. We showed that human TM cells secrete VEGF-A in culture, consistent with earlier observations,18 and that VEGF-A is present within the murine TM. Pharmacologic blockade of VEGFR-2, which is expressed by SC endothelium,21,22,24,25 decreases outflow facility in mice, presumably by suppressing endogenous VEGF signaling within the TM/SC. These studies suggested a mechanism by which VEGF production within the TM acts as a paracrine signal to modulate outflow facility, presumably by altering the permeability of SC endothelium (Fig. 7).
Figure 7.
VEGF is a paracrine regulator of outflow facility that may be involved in IOP homeostasis. (A) VEGF secretion by TM cells may reach the inner wall (IW) of SC via either diffusion or advection due to aqueous humor (AH) outflow. (B) VEGF release in the TM increases in response to cyclic stretch that depends on ocular pulse amplitude (OPA) that increases with IOP. VEGF modulates facility via VEGFR-2 presumably at the level of SC endothelium to increase IW conductivity and outflow facility. The facility increase of VEGF opposes IOP and OPA elevation, and may thereby contribute to IOP homeostasis. Anti-VEGF therapy may disrupt VEGF signaling in the TM to cause ocular hypertension, as examined in our companion study.37
VEGF is a potent regulator of endothelial permeability.13 VEGF widens paracellular spaces and induces formation of small transcellular pores known as fenestrae in otherwise continuous endothelia of some vascular beds.62 Fenestrated endothelia typically are supported by paracrine VEGF expression from neighboring cells,63 and disruption of local VEGF expression often leads to loss of endothelial fenestrations.64 Like fenestrated endothelia, the inner wall of SC contains micrometer-sized pores as well as smaller “mini-pores” that have a similar ultrastructure to diaphragmed fenestrae.5,65 VEGF secreted by TM cells likely reaches and acts on the inner wall, which uniquely expresses all three VEGFRs,21,22,24,25 to potentially regulate pore formation.18 This provides a putative mechanism by which VEGF may modulate the hydraulic conductivity of SC endothelium to influence outflow facility.
The influence of VEGF on outflow facility mimics the VEGF response on vascular endothelia, where permeability effects are mediated via VEGFR-2.66–68 Likewise, in the outflow pathway, ligands for VEGFR-2, such as VEGF-A and VEGF-D, increase outflow facility while antagonists to VEGFR-2 decrease facility or block the effect of exogenous VEGF-D. Hence, as for vascular endothelia, VEGFR-2 appears to mediate the effects of VEGF on outflow facility, presumably by regulating the hydraulic conductivity of SC inner wall. This is consistent with recent data showing that VEGFR-2 mediates the effect of VEGF-A121 on the barrier function of cultured SC cells from nonhuman primates.19 However, despite its vascular origins,24,69 SC inner wall exhibits characteristics of lymphatic endothelia,20 namely expression of VEGFR-3.25 The unique lymphatic/vascular dual nature of SC differentiates it from other vasculature within the eye. Therefore, exploiting VEGFR-3 to target SC inner wall is an appealing strategy to affect outflow in glaucoma, as this could potentially minimize off-target effects on other endothelia. However, there are no known isoforms of VEGF that are selective for VEGFR-3, and VEGF-C and VEGF-D bind VEGFR-2 as well as VEGFR-3 (for review on VEGFRs activation and signaling see the report of Simons et al.70). In this study, we chose to examine VEGF-D because, in addition to signaling via VEGFR-3, it also inhibits 15-hydroxyprostaglandin dehydrogenase,71 resulting in higher prostaglandin availability that may improve TM outflow.72,73 However, despite VEGF-D increasing facility, its effects appeared to be entirely mediated via VEGFR-2. Although this observation may be attributable to the relatively short experimental exposure time or to differential sensitivity for VEGF concentration between receptors, these data suggested that signaling via VEGFR-3 is unlikely to affect outflow facility. VEGF-Rs also can be activated in a VEGF-independent manner, for example in response to oxidative stress74 or upon interaction with integrins,75 which would contribute to additional receptor activity. Further work is necessary to clarify the exact role of the different VEGF-Rs, their coreceptors, such as neuropilin, and other VEGF isoforms on outflow facility.
The increase in VEGF-A production by TM cells in response to cyclic stretch suggests that VEGF-A expression may be regulated in part by mechanical forces associated with IOP. IOP is not static in a living eye, but experiences continuous oscillations of 2 to 3 mm Hg due to the ocular pulse.48 Larger IOP changes of up to 10 mm Hg are expected due to blinking or saccades.48 The ocular pulse imposes cyclic stretch within the TM44 and the magnitude of the ocular pulse increases with IOP.76 Thus, cyclic stretch is a physiologic stressor, the magnitude of which depends upon IOP, that acts continuously on TM cells in vivo. Contraction of the ciliary muscle may apply additional mechanical stimulation to TM cells via anterior ciliary muscle tendons that insert into the TM.39,43,77 We thereby propose that stretch-induced VEGF production may provide a mechanosensitive feedback signal within the TM to modulate outflow facility for IOP homeostasis. Similar mechanisms have been proposed for metalloproteinases78 and ATP.79 Any stretch-induced VEGF production would be superimposed on background levels already present within aqueous humor (reported to be between 15 and 533 pg/ml80–88). VEGF levels also are subject to binding to heparin sulfate proteoglycans and may be affected by several factors, including TGF-β2,18 BMP-7,18 hypoxia,89 as well as additional VEGF secretion by any macrophages90 that may be present within the TM.91,92
Clinical Implications
Antiangiogenic antibodies or antibody fragments that bind and sequester VEGF are used to treat a variety of retinal vascular disorders, including NVAMD, diabetic macular edema and retinal vein occlusion. A small but significant portion of patients (3%–11%) receiving intravitreal anti-VEGF experience sustained ocular hypertension.28,29,32,93 Proposed mechanisms for ocular hypertension include obstruction of the TM by protein aggregates94 or foreign particles35 or by damage to the outflow pathway cells.34 However, following intravitreal injection, anti-VEGF antibodies permeate the anterior chamber,86 reduce the levels of VEGF-A within aqueous humor,86,88 and enter the TM and SC by bulk outflow.95 Within the TM, anti-VEGF antibodies would presumably interfere with VEGF signaling to disrupt outflow, similar to facility decrease observed in response to pharmacological blockade of VEGFR-2. Our perfusion studies with anti-VEGF antibodies suggest that acute antibody exposure is insufficient to affect outflow function, but this does not exclude the possibility for outflow disruption following long-term exposure. Based on these data, we hypothesize that prolonged exposure to anti-VEGF therapy induces sustained ocular hypertension by disrupting endogenous VEGF signaling in the TM/SC that is involved in IOP homeostasis.
Consistent with this hypothesis, in our companion study37 we show that intravitreal anti-VEGF therapy reduces tonographic outflow facility in some patients. Interestingly, patients with the largest facility reduction were those who exhibited ocular hypertension independent of anti-VEGF therapy. These data suggested that patients with compromised aqueous humor dynamics, in many cases due to reduced outflow facility, are more susceptible to outflow disruption induced by anti-VEGF therapy. In other words, patients with a dysfunctional TM are at greater risk for further TM damage, IOP elevation, and vision loss caused by glaucomatous optic nerve damage. Patients who are most at risk are those who are ocular hypertensive (> = 21 mm Hg) at the start of anti-VEGF therapy or in whom ocular hypertension develops during therapy. As elevated IOP is a major risk factor for glaucoma, screening patients for elevated IOP before initiating anti-VEGF therapy can help identify patients who should be monitored more closely or who may require glaucoma therapy. This is particularly important since patients receiving anti-VEGF therapy, who have lost vision due to retinal disease, are at risk of additional vision loss caused by optic nerve damage.
In conclusion, VEGF is a paracrine regulator of outflow facility that is likely involved in IOP homeostasis as summarized by the model shown in Figure 7. VEGF is secreted by TM cells in response to IOP-dependent mechanical cues to affect outflow facility, presumably by modulating the hydraulic conductivity of SC inner wall. Differential isoforms of VEGF provoke bidirectional changes in outflow facility via VEGFR-2, consistent with effects of VEGF on microvascular endothelial permeability and the vascular origins of SC. Disrupting VEGF signaling in the TM, as may occur during intravitreal anti-VEGF therapy, may contribute to TM outflow dysfunction and sustained ocular hypertension. These data reinforced the notion that the hydraulic conductivity of SC inner wall is a key regulator of outflow facility and identify VEGF as a potential regulator of IOP homeostasis.
Supplementary Material
Acknowledgments
The authors thank Jacques Bertrand, Anabela Cepa Areias (Imperial College London), and Hong Nguyen (University of Erlangen-Nürnberg) for their technical assistance, Cristoforo Silvestri, PhD (Imperial College London) for critical discussion of the data, and Simon John, PhD, and Krishnakumar Kizhatil, PhD (The Jackson Laboratory, Bar Harbor, ME, USA) for helpful discussions regarding the VEGFR antagonist experiments.
Supported by a PhD studentship from Fight for Sight (UK), grants from the National Glaucoma Research Program of the BrightFocus Foundation, the Engineering and Physical Sciences Research Council (UK), the National Eye Institute (EY022359), and an International Research Scholar Award from the Research to Prevent Blindness Foundation.
Disclosure: E. Reina-Torres, None; J.C. Wen, None; K.C. Liu, None; G. Li, None; J.M. Sherwood, None; J.Y.H. Chang, None; P. Challa, None; C.M. Flügel-Koch, None; W.D. Stamer, None; R.R. Allingham, None; D.R. Overby, None
References
- 1. Grant WM. Clinical measurements of aqueous outflow. Am J Ophthalmol. 1951; 34: 1603–1605. [PubMed] [Google Scholar]
- 2. Lütjen-Drecoll E. Structural factors influencing outflow facility and its changeability under drugs. A study in Macaca arctoides. Invest Ophthalmol Vis Sci. 1973; 12: 280–294. [PubMed] [Google Scholar]
- 3. Mäepea O,, Bill A. Pressures in the juxtacanalicular tissue and Schlemm's canal in monkeys. Exp Eye Res. 1992; 54: 879–883. [DOI] [PubMed] [Google Scholar]
- 4. Holmberg A. The fine structure of the inner wall of Schlemm's canal. AMA Arch Ophthalmol. 1959; 62: 956–958. [Google Scholar]
- 5. Inomata H,, Smelser GK,, Bill A. Aqueous humor pathways through trabecular meshwork and into Schlemm's canal in cynomolgus monkey (Macaca Irus). Am J Ophthalmol. 1972; 73: 760–790. [DOI] [PubMed] [Google Scholar]
- 6. Grierson I,, Lee WR,, Moseley H,, Abraham S. The trabecular wall of Schlemm's canal: a study of the effects of pilocarpine by scanning electron microscopy. Br J Ophthalmol. 1979; 63: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ethier CR,, Coloma FM,, Sit AJ,, Johnson M. Two pore types in the inner-wall endothelium of Schlemm's canal. Invest Ophthalmol Vis Sci. 1998; 39: 2041–2048. [PubMed] [Google Scholar]
- 8. Braakman ST,, Read AT,, Chan DW,, Ethier CR,, Overby DR. Colocalization of outflow segmentation and pores along the inner wall of Schlemm's canal. Exp Eye Res. 2015; 130: 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Allingham RR,, Dekater AW,, Ethier CR,, et al. The relationship between pore density and outflow facility in human eyes. Invest Ophthalmol Vis Sci. 1992; 33: 1661–1669. [PubMed] [Google Scholar]
- 10. Johnson M,, Chan D,, Reed AT,, et al. The pore density in the inner wall endothelium of Schlemm's canal of glaucomatous eyes. Invest Ophthalmol Vis Sci. 2002; 43: 2950–2955. [PubMed] [Google Scholar]
- 11. Overby DR,, Zhou EH,, Vargas-Pinto R,, et al. Altered mechanobiology of Schlemm's canal endothelial cells in glaucoma. Proc Natl Acad Sci U S A. 2014; 111: 13876–13881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnson M,, Shapiro A,, Ethier CR,, Kamm RD. Modulation of outflow resistance by the pores of the inner wall endothelium. Invest Ophthalmol Vis Sci. 1992; 33: 1670–1675. [PubMed] [Google Scholar]
- 13. Senger DR,, Connolly DT,, Van de Water L,, Feder J,, Dvorak HF. Purification and NH2-terminal amino acid sequence of guinea pig tumor-secreted vascular permeability factor. Cancer Res. 1990; 50: 1774–1778. [PubMed] [Google Scholar]
- 14. Esser S,, Wolburg K,, Wolburg H,, et al. Vascular endothelial growth factor induces endothelial fenestrations in vitro. J Cell Biol. 1998; 140: 947–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Satchell SC,, Braet F. Glomerular endothelial cell fenestrations: an integral component of the glomerular filtration barrier. Am J Physiol Renal Physiol. 2009; 296: F947–F956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gavard J,, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006; 8: 1223–1234. [DOI] [PubMed] [Google Scholar]
- 17. Monaghan-Benson E,, Burridge K. The regulation of vascular endothelial growth factor-induced microvascular permeability requires Rac and reactive oxygen species. J Biol Chem. 2009; 284: 25602–25611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fuchshofer R,, Stephan DA,, Russell P,, Tamm ER. Gene expression profiling of TGFbeta2- and/or BMP7-treated trabecular meshwork cells: identification of Smad7 as a critical inhibitor of TGF-beta2 signaling. Exp Eye Res. 2009; 88: 1020–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fujimoto T,, Inoue T,, Maki K,, Inoue-Mochita M,, Tanihara H. Vascular endothelial growth factor-A increases the aqueous humor outflow facility. PLoS One. 2016; 11: e0161332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ramos RF,, Hoying JB,, Witte MH,, Stamer WD. Schlemm's canal endothelia, lymphatic, or blood vasculature? J Glaucoma. 2007; 16: 391–405. [DOI] [PubMed] [Google Scholar]
- 21. Perkumas KM,, Stamer WD. Protein markers and differentiation in culture for Schlemm's canal endothelial cells. Exp Eye Res. 2012; 96: 82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sano K,, Katsuta O,, Shirae S,, et al. Flt1 and Flk1 mediate regulation of intraocular pressure and their double heterozygosity causes the buphthalmia in mice. Biochem Biophys Res Commun. 2012; 420: 422–427. [DOI] [PubMed] [Google Scholar]
- 23. Karpinich NO,, Caron KM. Schlemm's canal: more than meets the eye, lymphatics in disguise. J Clin Invest. 2014; 124: 3701–3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kizhatil K,, Ryan M,, Marchant JK,, Henrich S,, John SW. Schlemm's canal is a unique vessel with a combination of blood vascular and lymphatic phenotypes that forms by a novel developmental process. PLoS Biol. 2014; 12: e1001912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Aspelund A,, Tammela T,, Antila S,, et al. The Schlemm's canal is a VEGF-C/VEGFR-3-responsive lymphatic-like vessel. J Clin Invest. 2014; 124: 3975–3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rogers ME,, Navarro ID,, Perkumas KM,, et al. Pigment epithelium-derived factor decreases outflow facility. Invest Ophthalmol Vis Sci. 2013; 54: 6655–6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sniegowski M,, Mandava N,, Kahook MY. Sustained intraocular pressure elevation after intravitreal injection of bevacizumab and ranibizumab associated with trabeculitis. Open Ophthalmol J. 2010; 4: 28–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Good TJ,, Kimura AE,, Mandava N,, Kahook MY. Sustained elevation of intraocular pressure after intravitreal injections of anti-VEGF agents. Br J Ophthalmol. 2011; 95: 1111–1114. [DOI] [PubMed] [Google Scholar]
- 29. Hoang QV,, Mendonca LS,, Della Torre KE,, et al. Effect on intraocular pressure in patients receiving unilateral intravitreal anti-vascular endothelial growth factor injections. Ophthalmology. 2012; 119: 321–326. [DOI] [PubMed] [Google Scholar]
- 30. Hoang QV,, Tsuang AJ,, Gelman R,, et al. Clinical predictors of sustained intraocular pressure elevation due to intravitreal anti-vascular endothelial growth factor therapy. Retina. 2013; 33: 179–187. [DOI] [PubMed] [Google Scholar]
- 31. Loukianou E,, Brouzas D,, Apostolopoulos M. Sustained ocular hypertension following intravitreal injections of 0.5 mg/0.05 ml ranibizumab. Int Ophthalmol. 2011; 31: 211–213. [DOI] [PubMed] [Google Scholar]
- 32. Mathalone N,, Arodi-Golan A,, Sar S,, et al. Sustained elevation of intraocular pressure after intravitreal injections of bevacizumab in eyes with neovascular age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol. 2012; 250: 1435–1440. [DOI] [PubMed] [Google Scholar]
- 33. Segal O,, Ferencz JR,, Cohen P,, Nemet AY,, Nesher R. Persistent elevation of intraocular pressure following intravitreal injection of bevacizumab. Isr Med Assoc J. 2013; 15: 352–355. [PubMed] [Google Scholar]
- 34. Kahook MY,, Ammar DA. In vitro effects of antivascular endothelial growth factors on cultured human trabecular meshwork cells. J Glaucoma. 2010; 19: 437–441. [DOI] [PubMed] [Google Scholar]
- 35. Liu L,, Ammar DA,, Ross LA,, et al. Silicone oil microdroplets and protein aggregates in repackaged bevacizumab and ranibizumab: effects of long-term storage and product mishandling. Invest Ophthalmol Vis Sci. 2011; 52: 1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yannuzzi NA,, Patel SN,, Bhavsar KV,, Sugiguchi F,, Freund KB. Predictors of sustained intraocular pressure elevation in eyes receiving intravitreal anti-vascular endothelial growth factor therapy. Am J Ophthalmol. 2014; 158: 319–327. [DOI] [PubMed] [Google Scholar]
- 37. Wen JC,, Reina-Torres E,, Sherwood JM,, et al. Intravitreal anti-VEGF injections reduce aqueous outflow facility in patients with neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017; 58: 1893–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Smith RS,, Zabaleta A,, Savinova OV,, John SW. The mouse anterior chamber angle and trabecular meshwork develop without cell death. BMC Dev Biol. 2001; 1: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Overby DR,, Bertrand J,, Schicht M,, et al. The structure of the trabecular meshwork, its connections to the ciliary muscle, and the effect of pilocarpine on outflow facility in mice. Invest Ophthalmol Vis Sci. 2014; 55: 3727–3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Millar JC,, Clark AF,, Pang IH. Assessment of aqueous humor dynamics in the mouse by a novel method of constant-flow infusion. Invest Ophthalmol Vis Sci. 2011; 52: 685–694. [DOI] [PubMed] [Google Scholar]
- 41. Boussommier-Calleja A,, Bertrand J,, Woodward DF,, et al. Pharmacologic manipulation of conventional outflow facility in ex vivo mouse eyes. Invest Ophthalmol Vis Sci. 2012; 53: 5838–5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Overby DR,, Bertrand J,, Tektas OY,, et al. Ultrastructural changes associated with dexamethasone-induced ocular hypertension in mice. Invest Ophthalmol Vis Sci. 2014; 55: 4922–4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li G,, Farsiu S,, Chiu SJ,, et al. Pilocarpine-induced dilation of Schlemm's canal and prevention of lumen collapse at elevated intraocular pressures in living mice visualized by OCT. Invest Ophthalmol Vis Sci. 2014; 55: 3737–3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li P,, Shen TT,, Johnstone M,, Wang RK. Pulsatile motion of the trabecular meshwork in healthy human subjects quantified by phase-sensitive optical coherence tomography. Biomed Opt Express. 2013; 4: 2051–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sherwood JM,, Reina-Torres E,, Bertrand JA,, Rowe B,, Overby DR. Measurement of outflow facility using iPerfusion. PLoS One. 2016; 11: e0150694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bates DO,, Cui TG,, Doughty JM,, et al. VEGF165b, an inhibitory splice variant of vascular endothelial growth factor, is down-regulated in renal cell carcinoma. Cancer Res. 2002; 62: 4123–4131. [PubMed] [Google Scholar]
- 47. Stamer WD,, Seftor RE,, Williams SK,, Samaha HA,, Snyder RW. Isolation and culture of human trabecular meshwork cells by extracellular matrix digestion. Curr Eye Res. 1995; 14: 611–617. [DOI] [PubMed] [Google Scholar]
- 48. Coleman DJ,, Trokel S. Direct-recorded intraocular pressure variations in a human subject. Arch Ophthalmol. 1969; 82: 637–640. [DOI] [PubMed] [Google Scholar]
- 49. Last JA,, Pan T,, Ding Y,, et al. Elastic modulus determination of normal and glaucomatous human trabecular meshwork. Invest Ophthalmol Vis Sci. 2011; 52: 2147–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ferrara N,, Gerber HP,, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003; 9: 669–676. [DOI] [PubMed] [Google Scholar]
- 51. Fong TA,, Shawver LK,, Sun L,, et al. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 1999; 59: 99–106. [PubMed] [Google Scholar]
- 52. Itokawa T,, Nokihara H,, Nishioka Y,, et al. Antiangiogenic effect by SU5416 is partly attributable to inhibition of Flt-1 receptor signaling. Mol Cancer Ther. 2002; 1: 295–302. [PubMed] [Google Scholar]
- 53. Tille JC,, Wang X,, Lipson KE,, et al. Vascular endothelial growth factor (VEGF) receptor-2 signaling mediates VEGF-C(deltaNdeltaC)- and VEGF-A-induced angiogenesis in vitro. Exp Cell Res. 2003; 285: 286–298. [DOI] [PubMed] [Google Scholar]
- 54. Kubo K,, Shimizu T,, Ohyama S,, et al. Novel potent orally active selective VEGFR-2 tyrosine kinase inhibitors: synthesis, structure-activity relationships, and antitumor activities of N-phenyl-N′-{4-(4-quinolyloxy)phenyl}ureas. J MedChem. 2005; 48: 1359–1366. [DOI] [PubMed] [Google Scholar]
- 55. Woolard J,, Wang WY,, Bevan HS,, et al. VEGF165b, an inhibitory vascular endothelial growth factor splice variant: mechanism of action, in vivo effect on angiogenesis and endogenous protein expression. Cancer Res. 2004; 64: 7822–7835. [DOI] [PubMed] [Google Scholar]
- 56. Qiu Y,, Ferguson J,, Oltean S,, et al. Overexpression of VEGF165b in podocytes reduces glomerular permeability. J Am Soc Nephrol. 2010; 21: 1498–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Peiris-Pagès M. The role of VEGF165b in pathophysiology. Cell Adh Migr. 2012; 6: 561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Achen MG,, Jeltsch M,, Kukk E,, et al. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc Natl Acad Sci U S A. 1998; 95: 548–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Achen MG,, Stacker SA. The vascular endothelial growth factor family; proteins which guide the development of the vasculature. Int J Exp Pathol. 1998; 79: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yu L,, Wu X,, Cheng Z,, et al. Interaction between bevacizumab and murine VEGF-A: a reassessment. Invest Ophthalmol Vis Sci. 2008; 49: 522–527. [DOI] [PubMed] [Google Scholar]
- 61. Miki K,, Miki A,, Matsuoka M,, et al. Effects of intraocular ranibizumab and bevacizumab in transgenic mice expressing human vascular endothelial growth factor. Ophthalmology. 2009; 116: 1748–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Roberts WG,, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995; 108: 2369–2379. [DOI] [PubMed] [Google Scholar]
- 63. Breier G,, Albrecht U,, Sterrer S,, Risau W. Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development. 1992; 114: 521–532. [DOI] [PubMed] [Google Scholar]
- 64. Ford KM,, Saint-Geniez M,, Walshe TE,, D'Amore PA. Expression and role of VEGF-A in the ciliary body. Invest Ophthalmol Vis Sci. 2012; 53: 7520–7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tamm ER. The trabecular meshwork outflow pathways: structural and functional aspects. Exp Eye Res. 2009; 88: 648–655. [DOI] [PubMed] [Google Scholar]
- 66. Hillman NJ,, Whittles CE,, Pocock TM,, Williams B,, Bates DO. Differential effects of vascular endothelial growth factor-C and placental growth factor-1 on the hydraulic conductivity of frog mesenteric capillaries. J Vasc Res. 2001; 38: 176–186. [DOI] [PubMed] [Google Scholar]
- 67. Whittles CE,, Pocock TM,, Wedge SR,, et al. ZM323881, a novel inhibitor of vascular endothelial growth factor-receptor-2 tyrosine kinase activity. Microcirculation. 2002; 9: 513–522. [DOI] [PubMed] [Google Scholar]
- 68. Olofsson B,, Jeltsch M,, Eriksson U,, Alitalo K. Current biology of VEGF-B and VEGF-C. Curr Opin Biotechnol. 1999; 10: 528–535. [DOI] [PubMed] [Google Scholar]
- 69. Hamanaka T,, Bill A,, Ichinohasama R,, Ishida T. Aspects of the development of Schlemm's canal. Exp Eye Res. 1992; 55: 479–488. [DOI] [PubMed] [Google Scholar]
- 70. Simons M,, Gordon E,, Claesson-Welsh L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat Rev Mol Cell Biol. 2016; 17: 611–625. [DOI] [PubMed] [Google Scholar]
- 71. Karnezis T,, Shayan R,, Caesar C,, et al. VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell. 2012; 21: 181–195. [DOI] [PubMed] [Google Scholar]
- 72. Winkler NS,, Fautsch MP. Effects of prostaglandin analogues on aqueous humor outflow pathways. J Ocul Pharmacol Ther. 2014; 30: 102–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Millard LH,, Woodward DF,, Stamer WD. The role of the prostaglandin EP4 receptor in the regulation of human outflow facility. Invest Ophthalmol Vis Sci. 2011; 52: 3506–3513. [DOI] [PubMed] [Google Scholar]
- 74. Kim YW,, Byzova TV. Oxidative stress in angiogenesis and vascular disease. Blood. 2014; 123: 625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Galvagni F,, Pennacchini S,, Salameh A,, et al. Endothelial cell adhesion to the extracellular matrix induces c-Src-dependent VEGFR-3 phosphorylation without the activation of the receptor intrinsic kinase activity. Circ Res. 2010; 106: 1839–1848. [DOI] [PubMed] [Google Scholar]
- 76. Downs JC,, Burgoyne CF,, Seigfreid WP,, et al. 24-hour IOP telemetry in the nonhuman primate: implant system performance and initial characterization of IOP at multiple timescales. Invest Ophthalmol Vis Sci. 2011; 52: 7365–7375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rohen JW,, Futa R,, Lütjen-Drecoll E. The fine-structure of the cribriform meshwork in normal and glaucomatous eyes as seen in tangential sections. Invest Ophthalmol Vis Sci. 1981; 21: 574–585. [PubMed] [Google Scholar]
- 78. Bradley JM,, Kelley MJ,, Zhu X,, et al. Effects of mechanical stretching on trabecular matrix metalloproteinases. Invest Ophthalmol Vis Sci. 2001; 42: 1505–1513. [PubMed] [Google Scholar]
- 79. Li A,, Leung CT,, Peterson-Yantorno K,, Stamer WD,, Civan MM. Cytoskeletal dependence of adenosine triphosphate release by human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011; 52: 7996–8005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tripathi RC,, Li JP,, Tripathi BJ,, Chalam KV,, Adamis AP. Increased level of vascular endothelial growth factor in aqueous humor of patients with neovascular glaucoma. Ophthalmology. 1998; 105: 232–237. [DOI] [PubMed] [Google Scholar]
- 81. Hu DN,, Ritch R,, Liebmann J,, et al. Vascular endothelial growth factor is increased in aqueous humor of glaucomatous eyes. J Glaucoma. 2002; 11: 406–410. [DOI] [PubMed] [Google Scholar]
- 82. Tong JP,, Chan WM,, Liu DT,, et al. Aqueous humor levels of vascular endothelial growth factor and pigment epithelium-derived factor in polypoidal choroidal vasculopathy and choroidal neovascularization. Am J Ophthalmol. 2006; 141: 456–462. [DOI] [PubMed] [Google Scholar]
- 83. Sawada O,, Kawamura H,, Kakinoki M,, Sawada T,, Ohji M. Vascular endothelial growth factor in aqueous humor before and after intravitreal injection of bevacizumab in eyes with diabetic retinopathy. Arch Ophthalmol. 2007; 125: 1363–1366. [DOI] [PubMed] [Google Scholar]
- 84. Roh MI,, Kim HS,, Song JH,, et al. Concentration of cytokines in the aqueous humor of patients with naive, recurrent and regressed CNV associated with amd after bevacizumab treatment. Retina. 2009; 29: 523–529. [DOI] [PubMed] [Google Scholar]
- 85. Takai Y,, Tanito M,, Ohira A. Multiplex cytokine analysis of aqueous humor in eyes with primary open-angle glaucoma, exfoliation glaucoma, and cataract. Invest Ophthalmol Vis Sci. 2012; 53: 241–247. [DOI] [PubMed] [Google Scholar]
- 86. Costagliola C,, Daniele A, dell'Omo R, et al. Aqueous humor levels of vascular endothelial growth factor and adiponectin in patients with type 2 diabetes before and after intravitreal bevacizumab injection. Exp Eye Res. 2013; 110: 50–54. [DOI] [PubMed] [Google Scholar]
- 87. Hsu MY,, Yang CY,, Hsu WH,, et al. Monitoring the VEGF level in aqueous humor of patients with ophthalmologically relevant diseases via ultrahigh sensitive paper-based ELISA. Biomaterials. 2014; 35: 3729–3735. [DOI] [PubMed] [Google Scholar]
- 88. Wang JW,, Zhou MW,, Zhang X,, et al. Short-term effect of intravitreal ranibizumab on intraocular concentrations of vascular endothelial growth factor-A and pigment epithelium-derived factor in neovascular glaucoma. Clin Experiment Ophthalmol. 2015; 43: 415–421. [DOI] [PubMed] [Google Scholar]
- 89. Liu XH,, Kirschenbaum A,, Lu M,, et al. Prostaglandin E2 induces hypoxia-inducible factor-1alpha stabilization and nuclear localization in a human prostate cancer cell line. J Biol Chem. 2002; 277: 50081–50086. [DOI] [PubMed] [Google Scholar]
- 90. Wu WK,, Llewellyn OP,, Bates DO,, Nicholson LB,, Dick AD. IL-10 regulation of macrophage VEGF production is dependent on macrophage polarisation and hypoxia. Immunobiology. 2010; 215: 796–803. [DOI] [PubMed] [Google Scholar]
- 91. McMenamin PG,, Holthouse I. Immunohistochemical characterization of dendritic cells and macrophages in the aqueous outflow pathways of the rat eye. Exp Eye Res. 1992; 55: 315–324. [DOI] [PubMed] [Google Scholar]
- 92. Flügel C,, Kinne RW,, Streilein JW,, Lütjen-Drecoll E. Distinctive distribution of HLA class II presenting and bone marrow derived cells in the anterior segment of human eyes. Curr Eye Res. 1992; 11: 1173–1183. [DOI] [PubMed] [Google Scholar]
- 93. Choi DY,, Ortube MC,, McCannel CA,, et al. Sustained elevated intraocular pressures after intravitreal injection of bevacizumab, ranibizumab, and pegaptanib. Retina. 2011; 31: 1028–1035. [DOI] [PubMed] [Google Scholar]
- 94. Kahook MY,, Liu L,, Ruzycki P,, et al. High-molecular-weight aggregates in repackaged bevacizumab. Retina. 2010; 30: 887–892. [DOI] [PubMed] [Google Scholar]
- 95. Gal-Or O,, Dotan A,, Dachbash M,, et al. Bevacizumab clearance through the iridocorneal angle following intravitreal injection in a rat model. Exp Eye Res. 2016; 145: 412–416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.