Abstract
Patient: Female, 32
Final Diagnosis: Obstructive primary cardiac T-cell lymphoma
Symptoms: Right heart failure
Medication: —
Clinical Procedure: None
Specialty: Cardiology
Objective:
Rare disease
Background:
Cardiac lymphoma is a rare entity, defined by the non-extra cardiac location at diagnosis.
Case Report:
Our patient was a 32-year-old female with no particular medical history, who presented with right heart failure with recurrent ascites and pleural effusion. There was a progressive worsening exertional dyspnea. On admission, examination revealed an irregular tachycardia at 170 beats per minute (bpm) and congestive heart failure. The electrocardiogram scored full tachyarrhythmia by atrial fibrillation with an average ventricular rate of 179 cycles per minute. Doppler echocardiography showed dilatation and systolic dysfunction of the left ventricle. There were dilated atria. We noted a large mass in the right atrium, which was less mobile, heterogeneous, integral with the wall, and filling three quarters of the cavity. It clogged the tricuspid valve in diastole. CT scan showed a tissue process enhanced after contrast injection, occupying the predominant cavities in the right atrium and filling it. Its borders were irregular. The lesion was extended to the posterior mediastinum, in front of the vertebral axis. In addition, there was a thrombosis of the jugular vein and the inferior vena cava. There was no other tumor site noted.
The patient died after presenting with cardiovascular shock associated with refractory right heart failure. Pathology examination confirmed T-cell lymphoma.
Conclusions:
The primitive cardiac lymphoma is an entity of intra-cardiac masses. It is therefore to be considered even if the diagnosis is challenging.
MeSH Keywords: Echocardiography; Heart Failure; Heart Neoplasms; Lymphoma, T-Cell
Background
Cardiac tumors are not common. Non-neoplastic causes such as infections and thrombi account for most intra-cardiac masses [1]. 80% to 90% of cases are benign, and most of them are myxomas [2]. Cardiac lymphoma is often an extension of non-Hodgkin lymphoma and is noticed in 9% to 27% of disseminated lymphomas [3]. The primitive form is quite an exceptional entity, sometimes debated, corresponding to a lymphoma exclusively confined to the heart and/or pericardium, with no other extra cardiac localization at the time of diagnosis [4]. Spontaneous prognosis is constantly unfavorable, due to rapid tumor proliferation, thus the need for early diagnosis and management [5].
We report a case of a 32-year-old female patient who presented with cardiac lymphoma with refractory right heart failure and had a fatal outcome.
Case Report
Mrs. FD is a 32-year-old woman with no particular medical history. She was sent to us for right heart failure with recurrent ascites and pleurisy. This situation was evolving in a context of postpartum (four months) and was associated with progressive exertional dyspnea. The intake examination revealed a blood pressure at 100/70 mm Hg, irregular tachycardia at 170 beats per minute, tachypnea with a respiratory rate of 38 cycles per minute, and the oxygen saturation was 88% on room air. Her heart sounds were muffled; there was spontaneous jugular vein distension, a stasis hepatomegaly (hepatic long axis at 17 cm), gross ascites, and bilateral pleural effusion. The electrocardiogram scored a full tachyarrhythmia by atrial fibrillation with an average ventricular rate of 179 cycles per minute and right ventricular hypertrophy. Laboratory tests revealed azotemia (0.63 g/L), hypoproteinemia (61 g/L), and hypoalbuminemia (26 g/L). Hemoglobin was 12.7 g/dL. There was hyponatremia and hypochloremia (Na+=123 mEq/L, Cl–=79 mEq/L) together with elevated liver enzymes twice the normal range. Ascitic fluid analysis showed positive Rivalta reaction with 35 g/L of proteins, with no germ or malignant cells.
Transthoracic echocardiography showed left ventricular systolic dilatation and dysfunction (ejection fraction=37% by Simpson method). There was bilateral atrial dilatation (31 cm2 and 34 cm2 respectively for right and left atrium). We noted a large mass in the right atrium, less mobile, incorporated to the atrial wall, and filling three quarters of the cavity. It was obstructing the tricuspid valve in diastole. This mass was heterogeneous, seemed to infiltrate the inter-atrial septum that was thickened and was also noted in the left atrium filling half of it. The pulmonary artery was free of thrombus and the systolic function of the right ventricle was normal. There was moderate pericardial effusion. There was a mobile mass, thrombotic in appearance, in the inferior vena cava (Figure 1).
Figure 1.
Transthoracic echocardiography images showing the tumor and its extension. (arrows). (A) 4 chamber view, (B) parasternal long axis view, (C) subcostal view, (D) inferior vena cava.
Thoracic CT scan showed a tissue process with contrast enhancement occupying atrial cavities predominantly in the right cavity and filling it. It had irregular contours. The lesion extended to the posterior mediastinum before vertebral axis (Figure 2). In addition, there was right jugular vein thrombosis just before its confluence with the subclavian vein as well as inferior vena cava thrombosis. A moderate pericardial effusion, right pleural effusion, and moderate ascites were noticed, as well as cardiac cirrhosis. Pancreas, spleen, kidneys, and adrenal glands appeared normal. No lymph nodes were noted.
Figure 2.

Thoracic CT-scan image showing the intra-cardiac tumor and its extension (arrow).
The patient died in a clinical picture of shock associated with refractory right heart failure.
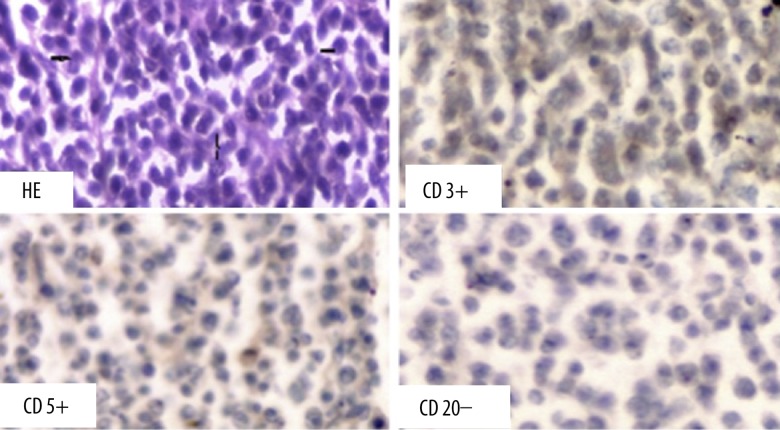
The postmortem analysis revealed, at the opening of the pericardial sac, a whitish tumor, friable at some points, infiltrating the two pericardial layers. This tumor involved the whole heart, sheathing the aorta and the pulmonary artery (Figure 3). Pathology analysis showed a monomorphic lymphoid proliferation with atypical lymphocytes. Histochemical marking were positive for CD3 and CD5, and negative for CD20, in favor of T-type (Figure 4).
Figure 3.
Anatomopathological examinations showing a white tumor around and in the heart.
Figure 4.
Histochemical examination: T-cells lymphoma.
Discussion
Primary cardiac tumors are rare. Their postmortem incidence is estimated between 0.0017% and 0.03%. Most of the time they are benign (75%); malignant forms appear in 25% of cases.
Myxomas are the most common primary heart tumors [6]. Primitive lymphomas represent only 1.3% of primitive cardiac tumors, and 0.5% of extra-nodal ones. They are often of type B. Only two cases of T-cell lymphomas have been described in the literature. Park reported a patient diagnosed with angioimmunoblastic T-cell lymphoma in whom Klinefelter syndrome was newly detected [7]. This was the first case. Burkitt lymphoma, as an intra-cardiac mass, is also a rare entity with 22 cases reported in the English literature, and is often associated with human immunodeficiency virus(HIV) [1]. Most of the time, cardiac tumors affect the right cavities, especially the atrium.
Clinical presentation is less specific and depends on the localization of the tumor. Symptoms are mostly related to cavity obstruction. In our case, the important volume of the mass explained the clinical picture of refractory right heart failure with relapsing ascites.
Electrocardiogram may reveal non-specific abnormalities. These can be arrhythmias like atrial fibrillation or conduction defects like complete atrioventricular block [8].
Doppler echocardiography is a first-line exam for cardiac tumors diagnosis [9].
The use of different views and angles is fundamental in the description of the intra cavity mass. For instance, one element that was fundamental in a thrombus differential diagnosis, in our case was the infiltration of the right atrium wall and the inter-atrial septum which was better visualized in sub costal angle.
In a postpartum context, peripartum cardiomyopathy is one of the first diagnostic hypotheses in cases of heart failure in our tropical region. This is a frightening disease because of its many thromboembolic complications; especially when it is associated with atrial fibrillation, like in our case. Myxomas are also closely associated with embolic events [6].
Pericardial effusion is often found in primitive intra-cardiac tumors.
Transesophageal echography gives better results than transthoracic echography, especially for right-side located tumors [10]. Ultrasonographic examinations are needed in follow-up of a patient’s treatment. They allow on-time detection of complications associated with the treatment and its adequate management [11].
Computed tomography and magnetic resonance imaging are important complementary exams for the diagnosis of primitive cardiac tumors. They allow accurate localization thanks to their excellent spatial resolution [12].
Treatment consists of surgery and chemotherapy in most cases of primitive cardiac lymphomas. Complete resection is often difficult due to the infiltrative nature of this tumor and the risk of wall rupture. The priority is to reduce the tumor and remove the hemodynamic obstacle [13].
Chemotherapy uses various molecules and protocols; the most frequent one is CHOP (cyclophosphamide, doxorubicin, vincristine and prednisone) [14–16]. Despite the arrival of numerous chemotherapy protocols and especially the introduction of rituximab, the prognosis of cardiac lymphoma remains poor [17]. This is in part due to the increased risk of pulmonary embolism and myocardial necrosis when the cardiac muscle is infiltrated [18].
Conclusions
Lymphoma is one of the causes of intra-cardiac masses. It is not common but it is a possibility to be considered if there is refractory heart failure and a mass in the cardiac cavities, even in this case, the diagnosis will be challenging. Pregnancy accelerates the evolution of this tumor. In our context, treatment by surgery was impossible because of hemodynamic instability and inadequate available technical platform. Evolution was fatal.
Acknowledgments
Drs Mouhamadou Bamba Ndiaye, Adama Kane, Maboury Diao, and Serigne Abdou Ba are members of the team of cardiologists who took care of the patient after she was hospitalized, and performed para-clinical exams.
References:
- 1.Chan O, Igwe M, Breburda CS, et al. Burkitt Lymphoma Presenting as an Intracardiac Mass: Case Report and Review of Literature. Am J Case Rep. 2016;17:553–58. doi: 10.12659/AJCR.899022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thiene G, Cristina Basso M, Rizzo S, et al. Cardiac tumors: Classification and epidemiology. In: Basso C, Valente M, Thiene G, editors. Cardiac tumor pathology. Heidelberg: Springer; 2013. pp. 23–30. [Google Scholar]
- 3.Ban-Hoefen M, Bernstein SH, Bisognano JD, et al. Symptomatic intracardiac diffuse large B-cell lymphoma. Am J Hematol. 2009;84:683–85. doi: 10.1002/ajh.21329. [DOI] [PubMed] [Google Scholar]
- 4.Bagwan IN, Desai S, Wotherspoon A, et al. Unusual presentation of primary cardiac lymphoma. Interact Cardiovasc Thorac Surg. 2009;9:127–93. doi: 10.1510/icvts.2009.204628. [DOI] [PubMed] [Google Scholar]
- 5.Roubille F, Massin F, Cayla G, et al. [Lymphome intracardiaque avec insuffisance cardiaque droite: à propos de deux cas] Arch Mal Cœur Vaiss. 2007;100(12):1025–29. [in French] [PubMed] [Google Scholar]
- 6.He D-k, Zhang Y-f, Liang Y, et al. Risk factors for embolism in cardiac myxoma: A retrospective analysis. Med Sci Monit. 2015;21:1146–54. doi: 10.12659/MSM.893855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park YT, Park C-H, Bae MA, et al. Angioimmunoblastic T-cell lymphoma in a patient with Klinefelter syndrome. Am J Case Rep. 2016;17:529–34. doi: 10.12659/AJCR.897572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deepti AN, Noone ML, Sahnkar SK. Primary cardiac cytotoxic T-cell lymphoma presenting with neurological deficits: A case report. Cardiovasc Pathol. 2008;17(5):334–38. doi: 10.1016/j.carpath.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Jeudy J, Kirsch J, Tavora F, et al. From the radiologic pathology archives: Cardiac lymphoma: Radiologic-pathologic correlation. Radiographics. 2012;32:1369–80. doi: 10.1148/rg.325115126. [DOI] [PubMed] [Google Scholar]
- 10.Mügge A, Daniel WG, Haverich A, Lichtlen PR. Diagnosis of non-infective cardiac mass lesions by two-dimensional echocardiography. Comparison of the transthoracic and transesophageal approaches. Circulation. 1991;83(1):70–78. doi: 10.1161/01.cir.83.1.70. [DOI] [PubMed] [Google Scholar]
- 11.Gaspar A, Salomé N, et al. Nabais S, et al. Echocardiographic assessment of a cardiac lymphoma: beyond two-dimensional imaging. Eur J Echocardiogr. 2009;10:975–78. doi: 10.1093/ejechocard/jep092. [DOI] [PubMed] [Google Scholar]
- 12.Miguel CE, Bestetti RB. Primary cardiac lymphoma. Int J Cardiol. 2011;149:358–63. doi: 10.1016/j.ijcard.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 13.Filali T, Lahidheb D, Ghodbene W, et al. [Lymphome cardiaque primitif obstructif] Hematologie. 2012;18(4):250–52. [in French] [Google Scholar]
- 14.Bertero MT, Pastena G, Tartagli N, et al. Primary lymphoma of the heart. A case report e review of literature. Leuk Res. 2002;26:117–20. doi: 10.1016/s0145-2126(01)00092-3. [DOI] [PubMed] [Google Scholar]
- 15.Kang SM, Rim SJ, Chung N. Primary cardiac lymphoma diagnosed by transvenous biopsy under transesophageal echocardiographic guidance andtreated with systemic chemotherapy. Echocardiography. 2003;20:101–2. doi: 10.1046/j.1540-8175.2003.00017.x. [DOI] [PubMed] [Google Scholar]
- 16.Kosugi M, Ono T, Yamaguchi H, et al. Successful treatment of primary cardiac lymphoma and pulmonary tumor embolism with chemotherapy. Int J Cardiol. 2006;111:172–73. doi: 10.1016/j.ijcard.2005.06.054. [DOI] [PubMed] [Google Scholar]
- 17.Takaya T, Takeuchi Y, Nakajima H, et al. The usefulness of tran-sesophageal echocardiographic observation during chemotherapy for cardiac metastasis of non-hodgkin lymphoma complicated with left diastolic collapse. J Cardiol. 2009;53:447–52. doi: 10.1016/j.jjcc.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Ceseroli GL, Bucci E, Villa E. Primary cardiac lymphoma in immunocompetent patients. Cancer. 1997;80(8):1497–506. doi: 10.1002/(sici)1097-0142(19971015)80:8<1497::aid-cncr18>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]