Abstract
We herein report a case of dramatic intracranial response to osimertinib in a poor performance status patient with lung adenocarcinoma harboring the epidermal growth factor receptor (EGFR) T790M mutation encoded in exon 20. The patient was a 59-year-old woman with EGFR exon 19 deletion-positive lung adenocarcinoma, who relapsed with multiple brain metastases. Computed tomography-guided biopsy of the left pleural tumor revealed adenocarcinoma harboring an EGFR exon 19 deletion and an EGFR T790M mutation encoded in exon 20. The patient was treated with osimertinib, a third-generation EGFR tyrosine kinase inhibitor. Two days after treatment initiation, the patient displayed profound disturbance of consciousness, possibly due to carcinomatous meningitis, and treatment had to be discontinued due to difficulty in taking osimertinib. However, the patient gradually started to recover consciousness and, after 3 days, she was again able to take osimertinib. One month after the initiation of osimertinib treatment, magnetic resonance imaging revealed an apparent reduction in brain metastases. The patient is currently under continued treatment with osimertinib. At the last follow-up (February, 2017) she exhibited partial response to the treatment.
Keywords: epidermal growth factor receptor-tyrosine kinase inhibitor, leptomeningeal carcinomatosis, poor performance status, osimertinib, brain metastasis
Introduction
There have been major advances in the medical treatment of advanced non-small-cell lung cancer (NSCLC) with the use of molecular-targeted therapies (1). The efficacy of epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs), such as gefitinib, erlotinib and afatinib, in the treatment of NSCLC has been proven, particularly in EGFR mutation-positive patients (2). Efficacy has been shown even in patients with poor Eastern Cooperative Oncology Group performance status (PS), particularly those who had previously been solely treated with best supportive care (3). However, EGFR mutation-positive patients eventually develop resistance to EGFR-TKIs. The most frequent reason for such resistance is a secondary EGFR T790M mutation encoded in exon 20 (4).
A third-generation EGFR-TKI, osimertinib, was recently approved for NSCLC patients harboring the EGFR T790M mutation (5). Since osimertinib is now used for patients who have been previously treated with an EGFR-TKI and/or chemotherapy, such patients include cases with poor PS. We herein report a case of dramatic intracranial response to osimertinib in a poor PS patient with lung adenocarcinoma harboring the EGFR T790M mutation.
Case report
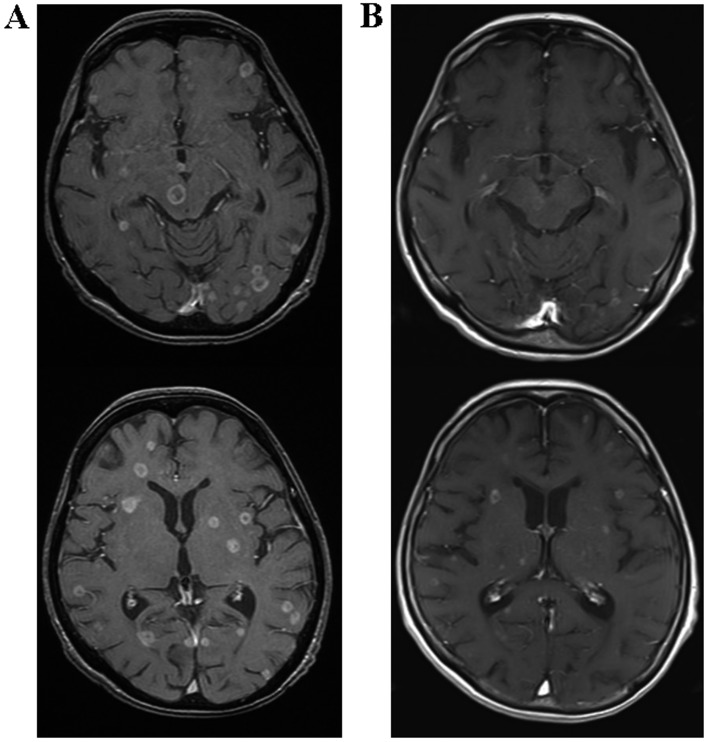
A 59-year-old woman with EGFR exon 19 deletion-positive lung adenocarcinoma was admitted to the Nagoya City University Hospital (Nagoya, Japan) due to relapse with multiple brain metastases in September, 2017. Brain metastases were already present at her diagnosis 4 years prior. At first, the patient received whole-brain radiation therapy. Subsequently, she was treated with carboplatin/pemetrexed/bevacizumab for ~6 months [achieving partial response (PR)], erlotinib for 3 months (PR), afatinib for 4 months [stable disease (SD)] and carboplatin/albumin-bound paclitaxel for 2 months (SD). Tumor tissue specimens were obtained by computed tomography (CT)-guided biopsy (CTGB) of the left pleural tumor, in which only EGFR exon 19 deletion was detected. Therefore, the brain metastases were treated with gamma knife radiosurgery and then re-challenged with erlotinib treatment. However, 2 months after this re-challenge, the brain metastases, multiple pulmonary nodules and pleural metastases all exhibited progression (Figs. 1A and 2A). CTGB of the left pleural tumor was again performed, and this time adenocarcinoma harboring both the EGFR exon 19 deletion and the EGFR T790M mutation encoded in EGFR exon 20 was detected. Although the patient's PS was 4, treatment with oral osimertinib was initiated at a dose of 80 mg per day. Two days after treatment initiation, the patient displayed profound disturbance of consciousness with neck stiffness, and treatment could not be continued. The clinical diagnosis was carcinomatous meningitis caused by progression of the brain metastases. Although treatment had been discontinued, the patient gradually recovered consciousness over the next 3 days and was again able to take osimertinib. The PS improved from 4 to 2. One month after osimertinib treatment initiation, magnetic resonance imaging revealed regression of the brain metastases (Fig. 1B). The chest CT images also revealed reduction of the multiple pulmonary nodules and pleural metastases (Fig. 2B). The serum level of carcinoembryonic antigen also decreased from 72.1 to 22.7 ng/ml (upper limit of normal value, 5.0 ng/ml). One adverse event, namely grade 3 leukopenia, as determined by the National Cancer Institute Common Terminology Criteria, version 4.0 (https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdf), was observed, which recovered after discontinuation of osimertinib treatment for 1 week and one-time subcutaneous administration of 100 μg lenograstim. The patient is currently under continued treatment with daily osimertinib at a decreased dose of 40 mg per day; at the last follow-up (February, 2017) she exhibited PR to the treatment.
Figure 1.
T1-weighted magnetic resonance images of the brain with gadolinium enhancement (A) prior to and (B) after treatment with osimertinib.
Figure 2.

Chest computed tomography (A) prior to and (B) after treatment with osimertinib.
Discussion
In the present case, an NSCLC patient with a poor PS due to brain metastases, who harbored the EGFR T790M mutation, was successfully treated with osimertinib.
It has been demonstrated that individual NSCLC patients with oncogenic drivers who receive a matched targeted agent exhibit improved survival (1). EGFR mutation is an oncogenic driver mutation, and treatment with an EGFR-TKI is recommended as first-line therapy for EGFR mutation-positive NSCLC patients (2), even for those with a poor PS or for elderly patients (3). However, EGFR mutation-positive patients eventually develop resistance to these EGFR-TKIs.
A third-generation EGFR-TKI, osimertinib, was recently found to be of clinical use for NSCLC patients who have a secondary EGFR T790M mutation, which is the most frequent reason for resistance to the first-line treatment with EGFR-TKIs (4,5). Osimertinib exhibited a high activity against NSCLC tumors harboring this EGFR T790M mutation, showing a response rate (RR) of 61%. Therefore, osimertinib is currently recommended for such patients who have had disease progression during prior therapy with EGFR-TKIs (6). Young et al reported that never-smoker female patients with adenocarcinoma harboring EGFR mutations and a poor PS who were treated with first-line gefitinib exhibited a RR of 50.0%, a median progression-free survival (PFS) of 130 days (95% CI: 51–209 days), and a median overall survival (OS) of 236 days (95% CI: 150–322 days) (7). However, the efficacy of osimertinib treatment for patients with a poor PS remains uncertain. As only few patients in the osimertinib group reported grade ≥3 adverse events in the AURA3 clinical trial (8), the administration of osimertinib was considered to be a viable option for our patient, despite her poor PS. The disturbance of consciousness gradually improved, despite only 2 days of treatment with 80 mg osimertinib. The 20-mg osimertinib dose (RR=52%; 95% CI: 30–57) was found to be as effective in lung cancer patients with the EGFR T790M mutation as the 80-mg dose (RR=52%; 95% CI: 40–63) (5). As the Cmax of osimertinib after a single administration of the 80-mg capsule [247.2±173.6 nM (https://ec.europa.eu/health/documents/community-register/2016/20160202133956/anx_133956_en.pdf)] is higher compared with the Cmax of osimertinib after 22 days of multiple once-daily dosing with the 20-mg capsule (106.3 nM; 95% CI: 45.4-280), even 2 days of treatment with the 80-mg capsule would be expected to achieve an effective blood concentration.
A further problem is that it is necessary to prove the presence of the EGFR T790M mutation in lung cancer biopsy specimens prior to the administration of osimertinib. In the present case, we were only able to confirm the presence of this mutation in tumor tissue obtained in the second CTGB. Spatiotemporal heterogeneity of the EGFR T790M mutation has been previously reported in individual patients, and the presence of this mutation may be proven by repeat biopsies (9). These results indicate that repeat biopsies should be performed in order not to miss the opportunity to administer osimertinib therapy to patients following development of resistance to first-line EGFR-TKIs. Although the effectiveness of EGFR-TKI re-challenge remains unknown (10), erlotinib was again selected in the present case. Hata et al have reported that the emergence of EGFR T790M in the central nervous system (CNS) is rare compared with other lesions following EGFR-TKI failure (11). As microscopic brain metastases harboring only the EGFR exon 19 deletion and not the EGFR T790M mutation were detected in the cerebrospinal fluid following gamma knife radiosurgery, erlotinib was administered due to its good penetration into the cerebrospinal fluid (12).
Another concern is that it is difficult to obtain tumor samples when patients develop recurrence, such as brain metastasis or carcinomatous meningitis. As lumbar puncture could not be performed in this patient due to her poor PS, carcinomatous meningitis was diagnosed clinically. In this case, re-challenge with erlotinib was not effective, but osimertinib was effective; thus, the status of EGFR T790M in the CNS was likely to be positive.
Osimertinib has exhibited good penetration through the blood-brain barrier in mice, delaying the development of leptomeningeal carcinomatosis in an EGFR mutation mouse model (13,14). Furthermore, Pareek et al also reported CNS disease improvement by administration of 80 mg osimertinib for 6 weeks in a case report (15). The efficacy and safety of osimertinib treatment for patients with emergence of EGFR T790M in the CNS remain uncertain and further investigation is required.
A previous study reported a high incidence of disease recurrence in the brain and the leptomeninges in NSCLC patients following response to gefitinib (16). Moreover, absence of brain metastasis has been shown to be associated with prolonged OS in treatment with EGFR-TKIs (17). Recently, a non-invasive approach to the detection of gene mutations using cell-free DNA extracted from the plasma has been developed (18). Novel methods for detecting gene mutations that develop during treatment with EGFR-TKIs are an important aspect of the optimization of personalized therapy.
We herein report a case of an NSCLC patient with a poor PS who was successfully treated with osimertinib. Therefore, osimertinib may represent a viable therapeutic option for EGFR T790M mutation-positive NSCLC patients with a poor PS. However, further prospective studies are required to establish the safety and efficacy of osimertinib for patients with a poor PS or brain metastasis.
References
- 1.Kris MG, Johnson BE, Berry LD, Kwiatkowski DJ, Iafrate AJ, Wistuba II, Varella-Garcia M, Franklin WA, Aronson SL, Su PF, et al. Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs. JAMA. 2014;311:1998–2006. doi: 10.1001/jama.2014.3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grigoriu B, Berghmans T, Meert AP. Management of EGFR mutated nonsmall cell lung carcinoma patients. Eur Respir J. 2015;45:1132–1141. doi: 10.1183/09031936.00156614. [DOI] [PubMed] [Google Scholar]
- 3.Inoue A, Kobayashi K, Usui K, Maemondo M, Okinaga S, Mikami I, Ando M, Yamazaki K, Saijo Y, Gemma A, et al. North East Japan Gefitinib Study Group First-line gefitinib for patients with advanced non-small-cell lung cancer harboring epidermal growth factor receptor mutations without indication for chemotherapy. J Clin Oncol. 2009;27:1394–1400. doi: 10.1200/JCO.2008.18.7658. [DOI] [PubMed] [Google Scholar]
- 4.Yu HA, Arcila ME, Rekhtman N, Sima CS, Zakowski MF, Pao W, Kris MG, Miller VA, Ladanyi M, Riely GJ. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19:2240–2247. doi: 10.1158/1078-0432.CCR-12-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jänne PA, Yang JC, Kim DW, Planchard D, Ohe Y, Ramalingam SS, Ahn MJ, Kim SW, Su WC, Horn L, et al. AZD9291 in EGFR inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372:1689–1699. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]
- 6.Tan DS, Yom SS, Tsao MS, Pass HI, Kelly K, Peled N, Yung RC, Wistuba II, Yatabe Y, Unger M, et al. The International Association for the Study of Lung Cancer Consensus Statement on Optimizing Management of EGFR Mutation-Positive Non-Small Cell Lung Cancer: Status in 2016. J Thorac Oncol. 2016;11:946–963. doi: 10.1016/j.jtho.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Lee YJ, Kim HT, Han JY, Yun T, Lee GK, Kim HY, Sung JH, Lee JS. First-line gefitinib treatment for patients with advanced non-small cell lung cancer with poor performance status. J Thorac Oncol. 2010;5:361–368. doi: 10.1097/JTO.0b013e3181cee1ea. [DOI] [PubMed] [Google Scholar]
- 8.Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, et al. AURA3 Investigators. Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N Engl J Med. doi: 10.1056/NEJMoa1612674. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hata A, Katakami N, Yoshioka H, Kaji R, Masago K, Fujita S, Imai Y, Nishiyama A, Ishida T, Nishimura Y, et al. Spatiotemporal T790M Heterogeneity in Individual Patients with EGFR-Mutant Non-Small-Cell Lung Cancer after Acquired Resistance to EGFR-TKI. J Thorac Oncol. 2015;10:1553–1559. doi: 10.1097/JTO.0000000000000647. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Q, Ke E, Niu F, Deng W, Chen Z, Xu C, Zhang X, Zhao N, Su J, Yang J, et al. The role of T790M mutation in EGFR-TKI re-challenge for patients with EGFR-mutant advanced lung adenocarcinoma. Oncotarget. 2017;8:4994–5002. doi: 10.18632/oncotarget.14007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hata A, Katakami N, Yoshioka H, Takeshita J, Tanaka K, Nanjo S, Fujita S, Kaji R, Imai Y, Monden K, et al. Rebiopsy of non-small cell lung cancer patients with acquired resistance to epidermal growth factor receptor-tyrosine kinase inhibitor: Comparison between T790M mutation-positive and mutation-negative populations. Cancer. 2013;119:4325–4332. doi: 10.1002/cncr.28364. [DOI] [PubMed] [Google Scholar]
- 12.Togashi Y, Masago K, Masuda S, Mizuno T, Fukudo M, Ikemi Y, Sakamori Y, Nagai H, Kim YH, Katsura T, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol. 2012;70:399–405. doi: 10.1007/s00280-012-1929-4. [DOI] [PubMed] [Google Scholar]
- 13.Nanjo S, Ebi H, Arai S, Takeuchi S, Yamada T, Mochizuki S, Okada Y, Nakada M, Murakami T, Yano S. High efficacy of third generation EGFR inhibitor AZD9291 in a leptomeningeal carcinomatosis model with EGFR-mutant lung cancer cells. Oncotarget. 2016;7:3847–3856. doi: 10.18632/oncotarget.6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ballard P, Yates JW, Yang Z, Kim DW, Yang JC, Cantarini M, Pickup K, Jordan A, Hickey M, Grist M, et al. Preclinical Comparison of Osimertinib with Other EGFR-TKIs in EGFR-Mutant NSCLC Brain Metastases Models, and Early Evidence of Clinical Brain Metastases Activity. Clin Cancer Res. 2016;22:5130–5140. doi: 10.1158/1078-0432.CCR-16-0399. [DOI] [PubMed] [Google Scholar]
- 15.Pareek V, Welch M, Ravera E, Zampolin RL, Sequist LV, Halmos B. Marked Differences in CNS Activity among EGFR Inhibitors: Case Report and Mini-Review. J Thorac Oncol. 2016;11:e135–e139. doi: 10.1016/j.jtho.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Omuro AM, Kris MG, Miller VA, Franceschi E, Shah N, Milton DT, Abrey LE. High incidence of disease recurrence in the brain and leptomeninges in patients with nonsmall cell lung carcinoma after response to gefitinib. Cancer. 2005;103:2344–2348. doi: 10.1002/cncr.21033. [DOI] [PubMed] [Google Scholar]
- 17.Lin JJ, Cardarella S, Lydon CA, Dahlberg SE, Jackman DM, Jänne PA, Johnson BE. Five-Year Survival in EGFR-Mutant Metastatic Lung Adenocarcinoma Treated with EGFR-TKIs. J Thorac Oncol. 2016;11:556–565. doi: 10.1016/j.jtho.2015.12.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahama T, Sakai K, Takeda M, Azuma K, Hida T, Hirabayashi M, Oguri T, Tanaka H, Ebi N, Sawa T, et al. Detection of the T790M mutation of EGFR in plasma of advanced non-small cell lung cancer patients with acquired resistance to tyrosine kinase inhibitors (West Japan oncology group 8014LTR study) Oncotarget. 2016;7:58492–58499. doi: 10.18632/oncotarget.11303. [DOI] [PMC free article] [PubMed] [Google Scholar]