Abstract
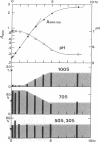
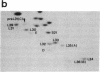
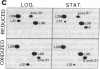
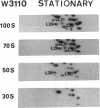
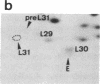
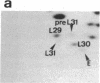
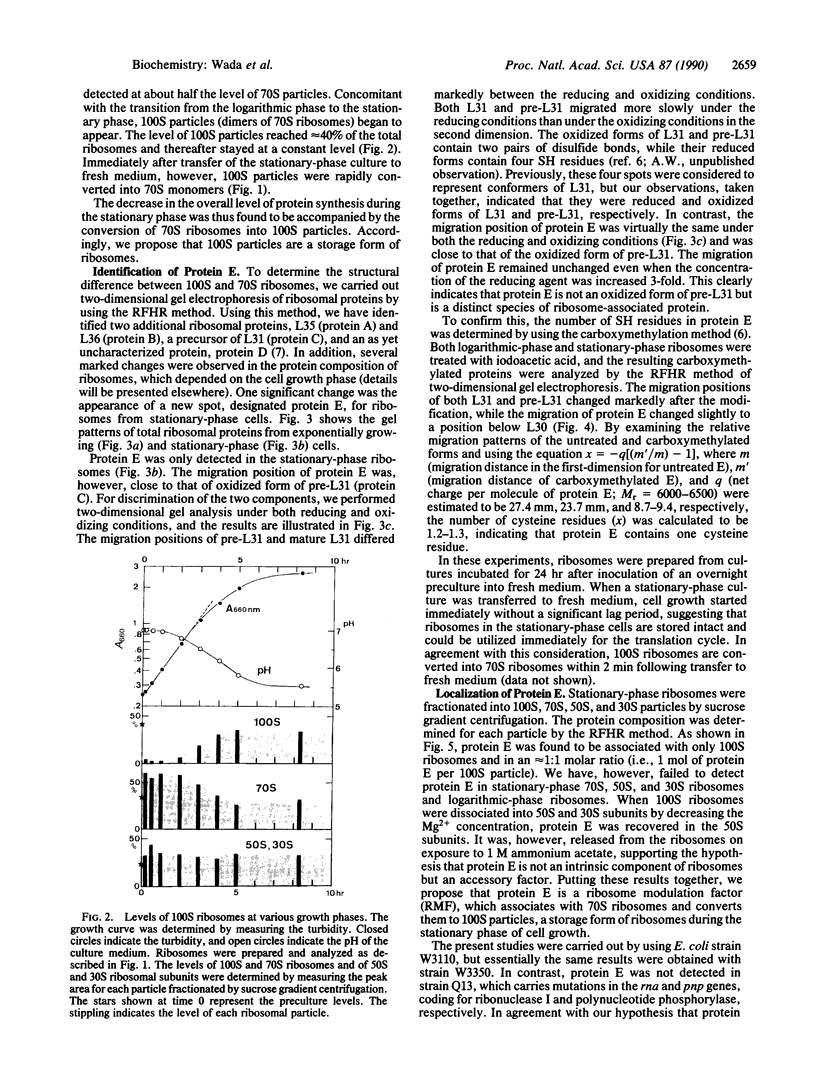
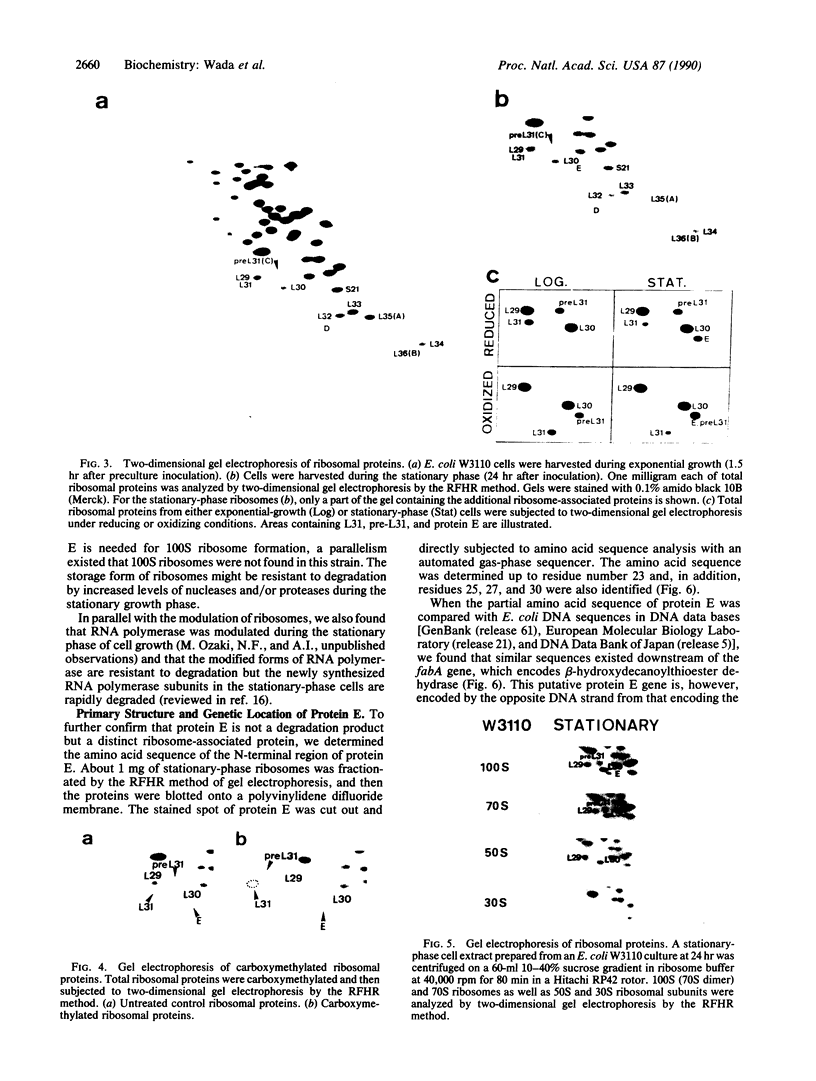
The decrease in overall translation activity occurring concomitantly with the transition from the exponential growth phase to the stationary phase of Escherichia coli cells was found to be accompanied by the appearance of 100S ribosomes (dimers of 70S ribosome monomers). Analysis of ribosomal proteins by the radical-free and highly reducing method of two-dimensional gel electrophoresis indicated that a protein, designated protein E, was exclusively associated with 100S ribosomes. From the results, we propose that protein E is a "ribosome modulation factor" (RMF), which associates with 70S ribosomes and converts them to a dimeric form. A homology search of the partial amino acid sequence of RMF using the DNA sequence data bases revealed that the rmf gene, which encodes RMF, is located next to the fabA gene at 21.8 min on the E. coli chromosome.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cerretti D. P., Dean D., Davis G. R., Bedwell D. M., Nomura M. The spc ribosomal protein operon of Escherichia coli: sequence and cotranscription of the ribosomal protein genes and a protein export gene. Nucleic Acids Res. 1983 May 11;11(9):2599–2616. doi: 10.1093/nar/11.9.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Jr, Li W. B., Coleman R., Narasimhan M., de Mendoza D., Schwab J. M. Derived amino acid sequence and identification of active site residues of Escherichia coli beta-hydroxydecanoyl thioester dehydrase. J Biol Chem. 1988 Apr 5;263(10):4641–4646. [PubMed] [Google Scholar]
- Hardy S. J., Kurland C. G., Voynow P., Mora G. The ribosomal proteins of Escherichia coli. I. Purification of the 30S ribosomal proteins. Biochemistry. 1969 Jul;8(7):2897–2905. doi: 10.1021/bi00835a031. [DOI] [PubMed] [Google Scholar]
- Horie K., Wada A., Fukutome H. Conformational studies of Escherichia coli ribosomes with the use of acridine orange as a probe. J Biochem. 1981 Aug;90(2):449–461. doi: 10.1093/oxfordjournals.jbchem.a133492. [DOI] [PubMed] [Google Scholar]
- Ishihama A. Subunit of assembly of Escherichia coli RNA polymerase. Adv Biophys. 1981;14:1–35. [PubMed] [Google Scholar]
- Kaltschmidt E., Wittmann H. G. Ribosomal proteins. VII. Two-dimensional polyacrylamide gel electrophoresis for fingerprinting of ribosomal proteins. Anal Biochem. 1970 Aug;36(2):401–412. doi: 10.1016/0003-2697(70)90376-3. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Noll M., Hapke B., Schreier M. H., Noll H. Structural dynamics of bacterial ribosomes. I. Characterization of vacant couples and their relation to complexed ribosomes. J Mol Biol. 1973 Apr 5;75(2):281–294. doi: 10.1016/0022-2836(73)90021-1. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Wada A. Analysis of Escherichia coli ribosomal proteins by an improved two dimensional gel electrophoresis. I. Detection of four new proteins. J Biochem. 1986 Dec;100(6):1583–1594. doi: 10.1093/oxfordjournals.jbchem.a121866. [DOI] [PubMed] [Google Scholar]
- Wada A. Analysis of Escherichia coli ribosomal proteins by an improved two dimensional gel electrophoresis. II. Characterization of four new proteins. J Biochem. 1986 Dec;100(6):1595–1605. doi: 10.1093/oxfordjournals.jbchem.a121867. [DOI] [PubMed] [Google Scholar]
- Wada A., Sako T. Primary structures of and genes for new ribosomal proteins A and B in Escherichia coli. J Biochem. 1987 Mar;101(3):817–820. doi: 10.1093/jb/101.3.817. [DOI] [PubMed] [Google Scholar]
- Wittmann H. G., Stöfflet G., Hindennach I., Kurland C. G., Birge E. A., Randall-Hazelbauer L., Nomura M., Kaltschmidt E., Mizushima S., Traut R. R. Correlation of 30S ribosomal proteins of Escherichia coli isolated in different laboratories. Mol Gen Genet. 1971;111(4):327–333. doi: 10.1007/BF00569784. [DOI] [PubMed] [Google Scholar]