Abstract
Partner and localizer of breast cancer 2 (PALB2) was identified as a moderate-risk gene of breast and pancreas cancer. The present authors previously reported that no PALB2 germline mutations with a deleterious frameshift or stop codons were identified in 155 Japanese patients with breast and/or ovarian cancer who were estimated to be at risk of hereditary cancer, according to the National Comprehensive Cancer Network (NCCN) criteria. In the present study, one patient with a deleterious mutation of PALB2 (c. 2834+2 T>C) has been identified from a study of an additional 128 cases. Therefore, the prevalence of PALB2 among Japanese patients is now estimated to be 0.35% (1/283). The proband was a 63-year-old woman with bilateral breast cancer, although she had experienced no other cancers. The proband had two elder sisters, the eldest of whom died from pancreatic cancer at 60 years of age. The proband's 40-year-old daughter was affected, but did not show any malignancies. There are only a few reports concerning PALB2 mutations in Japan. To the best of our knowledge, this is the first case study to reveal the significance of DNA-repair genes in the development of malignancies in Japanese patients with breast cancer.
Keywords: PALB2, Japanese, breast cancer, pancreas cancer, case report
Introduction
The significance of the breast cancer 1 (BRCA1) and BRCA2 mutations in familial breast and ovarian cancer has been well established (1,2). However, the mutations of these genes are estimated to cause, at most, 20–30% of cases of hereditary breast cancer (3). The present authors studied the BRCA1/2 mutations in 191 patients in a previous study, but the prevalence was shown to be unexpectedly low (4,5). In fact, it was only 7% among the analyzed patients who had a family history of breast cancers.
Partner and localizer of BRCA2 (PALB2) was identified as a moderate-risk gene in breast and pancreas cancer (6). PALB2 is located on chromosome 16p12.2 containing 13 exons and 12 introns, and is involved in BRCA2-associated pathways (6). Recently, Antoniou et al (7) reported that PALB2 carriers have a high risk of developing breast cancer, and concluded that the cumulative risk of mutation carrier was 34% by the age of 70 in their prospective follow-up study on 154 families.
The prevalence of the PALB2 mutation was reported to be 1.2–3.4% in European countries, whereas it is very rare in Asian countries (8–18). To the best of our knowledge, no study has been performed that has identified the PALB2 deleterious mutation in Japanese patients with breast cancer. From our first cohort data, no deleterious PALB2 mutations were identified in 155 patients with breast and/or ovarian cancer who were estimated to be at risk of hereditary cancer according to the National Comprehensive Cancer Network (NCCN) criteria (19). In the present case study, an additional 128 cases having breast and/or ovarian cancer were studied, and the case of a patient with bilateral breast cancer is presented who harbors the deleterious mutation in PALB2. Factoring in the first cohort of 155 cases, the frequency of the PALB2 mutation is now estimated at 0.35 % (1/283) in the Japanese population.
Case report
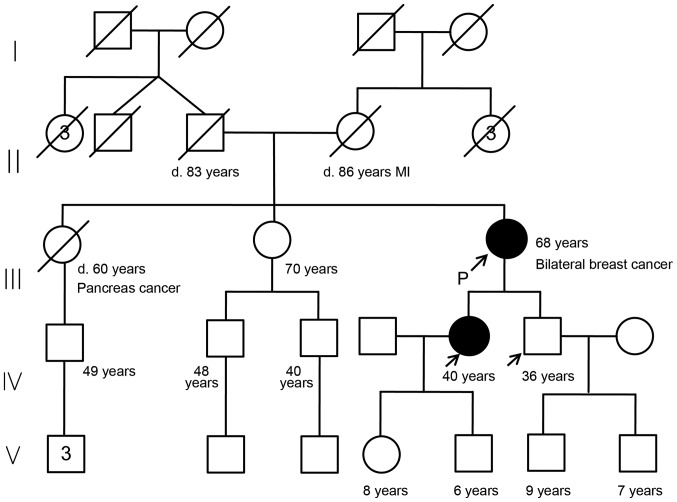
A 63-year-old female was referred to our hospital (Department of Breast Surgery, Yamanashi Prefectural Central Hospital, Kofu, Japan) due to the presence of a lump in her left breast and bloody discharge from the right-side nipple. The patient had no personal history of other cancers or diseases. Her family history is shown in the pedigree chart (Fig. 1). The patient had two gravidas and two parities.
Figure 1.
Chart showing the family pedigree of the case patient, showing that she had two elder sisters, one of whom (the eldest) died from pancreatic cancer at 60 years of age, while the other remained healthy at 70 years of age. The patient's parents died from causes unrelated to cancer. To the best of the patient's knowledge, no other family members (7 uncles or aunts, and 3 nephews and their descendants) have experienced cancer. The patient's 40-year-old daughter and 36-year-old son underwent genetic testing for the PALB2 mutation, revealing the daughter is affected, whereas the son is not. The black circle indicates the affected individual, and the arrows indicate tested individuals. MI, myocardial infarction.
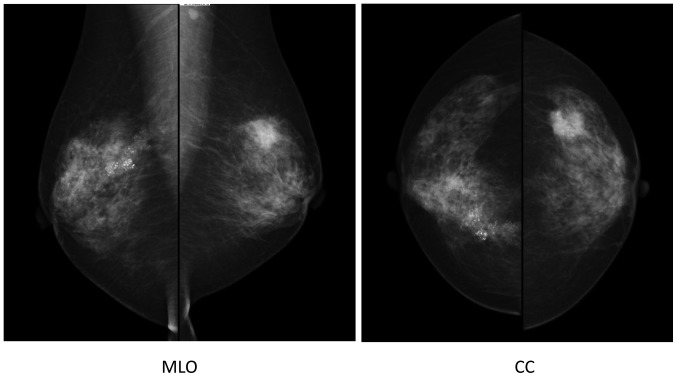
The cytology of nipple discharge was performed by the clinic, revealing the presence of malignant cells. Mammography indicated segmental pleomorphic calcification in the right breast, and a spiculated polygonal tumor measuring 2 cm in diameter with pleomorphic calcification in the left breast. Furthermore, an irregularly shaped axillar lymph node was observed on the left side (Fig. 2).
Figure 2.
Mammography, showing segmental pleomorphic calcification in the right breast and a spiculated polygonal tumor measuring 2 cm in diameter with pleomorphic calcification in the left breast. Furthermore, an irregularly shaped axillar lymph node was observed on the left side. MLO, mediolateral-oblique (view); CC, cranial-cadual (view).
Fine-needle aspiration cytology for the left-sided breast tumor also revealed the presence of malignant cells. The patient was diagnosed with bilateral breast cancer, and underwent a right-sided mastectomy and breast reconstruction, and left-sided breast-conserving therapy. Pathological findings revealed that the right-sided breast cancer was ductal carcinoma in situ (DCIS), with no lymph node metastasis, grade 2, estrogen receptor (ER) (7+) and progesterone receptor (PR) (3+) according to the Allred Score (20), and human epidermal growth factor 2 (HER2) (1+) according to the American Society of Clinical Oncology (ASCO)/College of American Pathologists (CAP) criteria (21). The left-sided breast cancer was invasive ductal carcinoma (non-specific type) with lymph node metastases (2/12), grade 2, ER (8+), PR (6+), and HER2 (1+). Epirubicin-cyclophosphamide (EC) adjuvant chemotherapy (epirubicin, 90 mg/m2, and cyclophosphamide, 600 mg/m2, 3 times a week for 4 cycles, followed by docetaxel, 75 mg/m2, 3 times a week for 4 cycles) was administered, and subsequently, radiation therapy (50 Gray) for the left-side breast was performed. The patient received oral hormone therapy with toremifen (40 mg/day) for 5 years.
The benefits and disadvantages of knowing the results of genetic testing were explained to the patient. Added to the explanation was the possibility that there could be uncertain results that would need to be clarified in future investigations. The patient and her family (40-year-old daughter and 36-year-old son) were referred to genetic counseling (S.N. and T.K.). Written informed consent was obtained from the patient and from her daughter and son.
Germline mutations for BRCA1/2 and PALB2 were analyzed using targeted sequencing, as previously reported (4,19,22). Briefly, the Ion AmpliSeq™ BRCA1 and BRCA2 and the Ion AmpliSeq™ BRCA Reflex Hereditary Cancer Research panels (Thermo Fisher Scientific, Inc., Waltham, MA, USA) were used, targeting the whole exons of the BRCA1/2 genes and an additional 25 hereditary cancer-associated genes (22,23). Buffy coat DNA was used as a template, and the sequencing library was generated using an AmpliSeq Library kit 2.0 (Thermo Fisher Scientific, Inc.) (24–31). Next-generation sequencing analysis was subsequently performed on an Ion PGM or Ion Proton platform (Thermo Fisher Scientific, Inc.) (24–31).
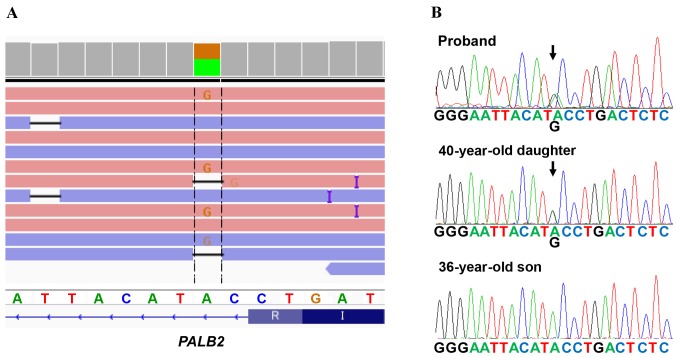
A deleterious mutation of PALB2 (chr16: 23635328, c. 2834+2 T>C) was identified (Fig. 3A), which is the first case in 283 analyzed patients in our hospital during the period between 2013 and 2016, i.e., 0.35% or 1/283 of Japanese patients were revealed to have the PALB2 deleterious mutation. Furthermore the splice-site mutation in PALB2 was not identified in the Exome Aggregation Consortium (ExAC), the Human Genetic Variation Database (HGVD), the Integrative Japanese Genome Variation (iJGVD) or the Catalogue Of Somatic Mutations In Cancer (COSMIC) databases. To the best of our knowledge, this variant has therefore not been reported previously, suggesting that our identified variant is novel one.
Figure 3.
Genetic analysis of PALB2 mutations. (A) Next-generation sequencing analysis for PALB2 mutations. The patient had a deleterious mutation of PALB2 (c. 2834+2 T>C). (B) Sanger sequencing revealed that the proband's 40-year-old daughter harbored the PALB2 mutation, whereas the 36-year-old son did not.
The pedigree chart of the patient is shown in Fig. 1. The patient had two elder sisters, the eldest of whom succumbed to pancreatic cancer at 60 years of age, whereas the other sister is alive and well at 70 years of age. The patients' parents died from causes unrelated to cancer. To the best of the patient's knowledge, no other family members (7 uncles or aunts, and 3 nephews and their descendants) have experienced cancer. The patient's 40-year-old daughter and 36-year-old son underwent gene informed consent to have genetic testing for the PALB2 mutation. It was revealed that the daughter was affected, whereas the son was not (Fig. 3B). The mutation was also confirmed using Sanger sequencing (Fig. 3B). The 40-year-old daughter is now receiving regular check-ups for malignancies, including those of the breast and pancreas.
Discussion
PALB2 serves a crucial role in the localization and stabilization of BRCA2 in nuclear chromatin, which is essential for BRCA2 to function in double-strand-break DNA repair by homologous recombination. PALB2 mono-allelic mutations result in cancer development, and bi-allelic mutations lead to a type of Fanconi anemia (6).
Recently, Antoniou et al (7) reported that PALB2 carriers have a high risk of developing breast cancer, and determined that the cumulative risk of mutation carrier was 34% by the age of 70 in their prospective follow-up study on 154 families. In the USA, Canada, and Europe, the frequency of PALB2 deleterious mutations was revealed to vary from 1.1 to 3.4% (8–15). A total of 4 previous studies have arisen from Asia. One study by Cao et al (16) from China revealed 3 cases out of 360 (0.8%) with the deleterious mutations, although there were none from Korea (300 cases) or from Malaysia (122 cases) (17,18). The previous study by the present authors on Japanese patients (n=155) revealed that none of them had the deleterious mutation (19).
The PALB2 mutation has been reported to be associated with the development of pancreatic cancer. The prevalence of the PALB2 mutation among familial pancreatic cancer was reported to be ~3–4% in the USA and European countries (32,33). In Japan, Takai et al (34) recently reported that two deleterious PALB2 mutations were detected in 54 familial pancreas cancer families, as well as three BRCA2 and two ATM deleterious mutations. However, the association between PALB2 mutations and the risk of pancreatic cancer has yet to be fully elucidated among the Japanese population.
In the present case study, a 60-year-old elder sister was known to have had pancreatic cancer. However, it was impossible to examine the PALB2 germline mutations, since a DNA sample was not available from the sister. To reveal whether the identified PALB2 splice-site mutation has affected tumor development, it will be better to perform segregation analysis in this family. As a minimum at the present time, the proband's daughter, who has the PALB2 mutation, should continue to have regular check-ups assessing the risk of developing pancreatic cancer, as well as breast cancer.
Compared with the USA and European countries, analysis of BRCA1/2 for the detection of hereditary breast and/or ovarian cancer has not been widely accepted in Japan. Reports originating from Japan remain few in number (5,35,36). Further investigations are required to reveal the genetic features of Japanese patients with breast and/or other cancers (ovary, pancreas, prostate, and so forth).
It is important to understand the association between carcinogenesis and the dysfunction of DNA-repair genes in Japanese patients due to the up-and-coming therapeutic strategies that employ poly(ADP-ribose) polymerase (PARP) inhibitors, such as Orapalib (37,38). Recently, multi-gene assays for hereditary cancer have been developed (23,39), and other genes associated with double-strand DNA repair, such as PALB2, ATM, BARD1, and RAD51, will be analyzed for patients with hereditary cancer. These analyses are expected to reveal the association between DNA-repair genes and carcinogenesis with various types of cancer.
In conclusion, to the best of our knowledge, this is the first identified case of PALB2 mutations in a Japanese patient with breast cancer. The present study therefore suggests that the PALB2 mutation is associated with the development of breast and pancreas cancer, even in Japanese patients. At present, the frequency of the germline mutation in PALB2 is 0.35% (1/283 cases).
Acknowledgements
We thank Takuro Uchida, Yumi Kubota and Shino Kirito for their assistance. This study was approved by the institutional review board at Yamanashi Prefectural Central Hospital, and funded by a Grant-in-aid for Genome Research program from Yamanashi Prefecture.
References
- 1.Collaborative Group on Hormonal Factors in Breast Cancer, corp-author. Familial breast cancer: Collaborative reanalysis of individual data from 52 epidemiological studies including 58,209 women with breast cancer and 101,986 women without the disease. Lancet. 2001;358:1389–1399. doi: 10.1016/S0140-6736(01)06524-2. [DOI] [PubMed] [Google Scholar]
- 2.Kim H, Choi DH. Distribution of BRCA1 and BRCA2 mutations in Asian patients with breast cancer. J Breast Cancer. 2013;16:357–365. doi: 10.4048/jbc.2013.16.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melchor L, Benítez J. The complex genetic landscape of familial breast cancer. Hum Genet. 2013;132:845–863. doi: 10.1007/s00439-013-1299-y. [DOI] [PubMed] [Google Scholar]
- 4.Hirotsu Y, Nakagomi H, Sakamoto I, Amemiya K, Mochizuki H, Omata M. Detection of BRCA1 and BRCA2 germline mutations in Japanese population using next-generation sequencing. Mol Genet Genomic Med. 2015;3:121–129. doi: 10.1002/mgg3.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakamoto I, Hirotsu Y, Nakagomi H, Ouchi H, Ikegami A, Teramoto K, Amemiya K, Mochizuki H, Omata M. BRCA1 and BRCA2 mutations in Japanese patients with ovarian, fallopian tube, and primary peritoneal cancer. Cancer. 2016;122:84–90. doi: 10.1002/cncr.29707. [DOI] [PubMed] [Google Scholar]
- 6.Tischkowitz M, Xia B. PALB2/FANCN: Recombining cancer and Fanconi anemia. Cancer Res. 2010;70:7353–7359. doi: 10.1158/0008-5472.CAN-10-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antoniou AC, Casadei S, Heikkinen T, Barrowdale D, Pylkäs K, Roberts J, Lee A, Subramanian D, De Leeneer K, Fostira F, et al. Breast-cancer risk in families with mutations in PALB2. N Engl J Med. 2014;371:497–506. doi: 10.1056/NEJMoa1400382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casadei S, Norquist BM, Walsh T, Stray S, Mandell JB, Lee MK, Stamatoyannopoulos JA, King MC. Contribution of inherited mutations in the BRCA2-interacting protein PALB2 to familial breast cancer. Cancer Res. 2011;71:2222–2229. doi: 10.1158/0008-5472.CAN-10-3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zheng Y, Zhang J, Niu Q, Huo D, Olopade OI. Novel germline PALB2 truncating mutations in African American breast cancer patients. Cancer. 2012;118:1362–1370. doi: 10.1002/cncr.26388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartley T, Cavallone L, Sabbaghian N, Silva-Smith R, Hamel N, Aleynikova O, Smith E, Hastings V, Pinto P, Tischkowitz M, et al. Mutation analysis of PALB2 in BRCA1 and BRCA2-negative breast and/or ovarian cancer families from Eastern Ontario, Canada. Hered Cancer Clin Pract. 2014;12:19. doi: 10.1186/1897-4287-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahman N, Seal S, Thompson D, Kelly P, Renwick A, Elliott A, Reid S, Spanova K, Barfoot R, Chagtai T, et al. PALB2, which encodes a BRCA2-interacting protein, is a breast cancer susceptibility gene. Nat Genet. 2007;39:165–167. doi: 10.1038/ng1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogdanova N, Sokolenko AP, Iyevleva AG, Abysheva SN, Blaut M, Bremer M, Christiansen H, Rave-Fränk M, Dörk T, Imyanitov EN. PALB2 mutations in German and Russian patients with bilateral breast cancer. Breast Cancer Res Treat. 2011;126:545–550. doi: 10.1007/s10549-010-1290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Catucci I, Peterlongo P, Ciceri S, Colombo M, Pasquini G, Barile M, Bonanni B, Verderio P, Pizzamiglio S, Foglia C, et al. PALB2 sequencing in Italian familial breast cancer cases reveals a high-risk mutation recurrent in the province of Bergamo. Genet Med. 2014;16:688–694. doi: 10.1038/gim.2014.13. [DOI] [PubMed] [Google Scholar]
- 14.Erkko H, Xia B, Nikkilä J, Schleutker J, Syrjäkoski K, Mannermaa A, Kallioniemi A, Pylkäs K, Karppinen SM, Rapakko K, et al. A recurrent mutation in PALB2 in Finnish cancer families. Nature. 2007;446:316–319. doi: 10.1038/nature05609. [DOI] [PubMed] [Google Scholar]
- 15.Blanco A, de la Hoya M, Osorio A, Diez O, Miramar MD, Infante M, Martinez-Bouzas C, Torres A, Lasa A, Llort G, et al. Analysis of PALB2 gene in BRCA1/BRCA2 negative Spanish hereditary breast/ovarian cancer families with pancreatic cancer cases. PLoS One. 2013;8:e67538. doi: 10.1371/journal.pone.0067538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao W, Wang X, Li JC. Hereditary breast cancer in the Han Chinese population. J Epidemiol. 2013;23:75–84. doi: 10.2188/jea.JE20120043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JH, Choi DH, Cho DY, Ahn SH, Son BH, Haffty BG. PALB2 mutations 1592delT and 229delT are not present in Korean breast cancer patients negative for BRCA1 and BRCA2 mutations. Breast Cancer Res Treat. 2010;122:303–306. doi: 10.1007/s10549-010-0806-2. [DOI] [PubMed] [Google Scholar]
- 18.Phuah SY, Lee SY, Kang P, Kang IN, Yoon SY, Thong MK, Hartman M, Sng JH, Yip CH, Taib NA, Teo SH. Prevalence of PALB2 mutations in breast cancer patients in multi-ethnic Asian population in Malaysia and Singapore. PLoS One. 2013;8:e73638. doi: 10.1371/journal.pone.0073638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakagomi H, Sakamoto I, Hirotsu Y, Amemiya K, Mochiduki H, Omata M. Analysis of PALB2 mutations in 155 Japanese patients with breast and/or ovarian cancer. Int J Clin Oncol. 2016;21:270–275. doi: 10.1007/s10147-015-0906-4. [DOI] [PubMed] [Google Scholar]
- 20.Daltoé RD, Madeira KP, de Carvalho AA, de Rezende LC, Silva IV, Rangel LB. Evaluation of the progesterone receptor status in breast cancer using three different antibodies: A comparison by Allred score system. Int J Clin Exp Pathol. 2014;7:331–339. [PMC free article] [PubMed] [Google Scholar]
- 21.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25:118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 22.Hirotsu Y, Nakagomi H, Sakamoto I, Amemiya K, Oyama T, Mochizuki H, Omata M. Multigene panel analysis identified germline mutations of DNA repair genes in breast and ovarian cancer. Mol Genet Genomic Med. 2015;3:459–466. doi: 10.1002/mgg3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kean S. Breast cancer. The ‘other’ breast cancer genes. Science. 2014;343:1457–1459. doi: 10.1126/science.343.6178.1457. [DOI] [PubMed] [Google Scholar]
- 24.Hirotsu Y, Zheng TH, Amemiya K, Mochizuki H, Guleng B, Omata M. Targeted and exome sequencing identified somatic mutations in hepatocellular carcinoma. Hepatol Res. 2016;46:1145–1151. doi: 10.1111/hepr.12663. [DOI] [PubMed] [Google Scholar]
- 25.Goto T, Hirotsu Y, Oyama T, Amemiya K, Omata M. Analysis of tumor-derived DNA in plasma and bone marrow fluid in lung cancer patients. Med Oncol. 2016;33:29. doi: 10.1007/s12032-016-0744-x. [DOI] [PubMed] [Google Scholar]
- 26.Nakada H, Nakagomi H, Hirotsu Y, Amemiya K, Mochizuki H, Inoue M, Oyama T, Omata M. A study of tumor heterogeneity in a case with breast cancer. Breast Cancer. 2016 Sep 29; doi: 10.1007/s12282-016-0733-0. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 27.Amemiya K, Hirotsu Y, Goto T, Nakagomi H, Mochizuki H, Oyama T, Omata M. Touch imprint cytology with massively parallel sequencing (TIC-seq): A simple and rapid method to snapshot genetic alterations in tumors. Cancer Med. 2016;5:3426–3436. doi: 10.1002/cam4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirotsu Y, Kojima Y, Okimoto K, Amemiya K, Mochizuki H, Omata M. Comparison between two amplicon-based sequencing panels of different scales in the detection of somatic mutations associated with gastric cancer. BMC Genomics. 2016;17:833. doi: 10.1186/s12864-016-3166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirotsu Y, Nakagomi H, Amemiya K, Oyama T, Inoue M, Mochizuki H, Omata M. Intrinsic HER2 V777L mutation mediates resistance to trastuzumab in a breast cancer patient. Med Oncol. 2017;34:3. doi: 10.1007/s12032-016-0857-2. [DOI] [PubMed] [Google Scholar]
- 30.Nakagomi H, Hirotsu Y, Amemiya K, Nakada H, Inoue M, Mochizuki H, Oyama T, Omata M. Rapid changes in circulating tumor DNA in serially sampled plasma during treatment of breast cancer: A case report. Am J Case Rep. 2017;18:26–32. doi: 10.12659/AJCR.901295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goto T, Hirotsu Y, Mochizuki H, Nakagomi T, Oyama T, Amemiya K, Omata M. Stepwise addition of genetic changes correlated with histological change from ‘well-differentiated’ to ‘sarcomatoid’ phenotypes: A case report. BMC Cancer. 2017;17:65. doi: 10.1186/s12885-017-3059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Slater EP, Langer P, Niemczyk E, Strauch K, Butler J, Habbe N, Neoptolemos JP, Greenhalf W, Bartsch DK. PALB2 mutations in European familial pancreatic cancer families. Clin Genet. 2010;78:490–494. doi: 10.1111/j.1399-0004.2010.01425.x. [DOI] [PubMed] [Google Scholar]
- 33.Hofstatter EW, Domchek SM, Miron A, Garber J, Wang M, Componeschi K, Boghossian L, Miron PL, Nathanson KL, Tung N. PALB2 mutations in familial breast and pancreatic cancer. Fam Cancer. 2011;10:225–231. doi: 10.1007/s10689-011-9426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takai E, Yachida S, Shimizu K, Furuse J, Kubo E, Ohmoto A, Suzuki M, Hruban RH, Okusaka T, Morizane C, Furukawa T. Germline mutations in Japanese familial pancreatic cancer patients. Oncotarget. 2016;7:74227–74235. doi: 10.18632/oncotarget.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sugano K, Nakamura S, Ando J, Takayama S, Kamata H, Sekiguchi I, Ubukata M, Kodama T, Arai M, Kasumi F, et al. Cross-sectional analysis of germline BRCA1 and BRCA2 mutations in Japanese patients suspected to have hereditary breast/ovarian cancer. Cancer Sci. 2008;99:1967–1976. doi: 10.1111/j.1349-7006.2008.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nakamura S, Takahashi M, Tozaki M, Nakayama T, Nomizu T, Miki Y, Murakami Y, Aoki D, Iwase T, Nishimura S, et al. Prevalence and differentiation of hereditary breast and ovarian cancers in Japan. Breast Cancer. 2015;22:462–468. doi: 10.1007/s12282-013-0503-1. [DOI] [PubMed] [Google Scholar]
- 37.Lee JM, Ledermann JA, Kohn EC. PARP Inhibitors for BRCA1/2 mutation-associated and BRCA-like malignancies. Ann Oncol. 2014;25:32–40. doi: 10.1093/annonc/mdt384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ledermann J, Harter P, Gourley C, Friedlander M, Vergote I, Rustin G, Scott CL, Meier W, Shapira-Frommer R, Safra T, et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: A preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014;15:852–861. doi: 10.1016/S1470-2045(14)70228-1. [DOI] [PubMed] [Google Scholar]
- 39.Wooster R, Bignell G, Lancaster J, Swift S, Seal S, Mangion J, Collins N, Gregory S, Gumbs C, Micklem G. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378:789–792. doi: 10.1038/378789a0. [DOI] [PubMed] [Google Scholar]