Abstract
Purpose
The newly designed unibody AFX endograft system for endovascular aortic aneurysm repair is the only graft with anatomical fixation to the aortic bifurcation in comparison to most other grafts that use the infrarenal neck as the main fixation point. The aim of this study was to assess the preliminary results of the AFX stent-graft system used with infrarenal aortic component and compare them with those obtained in patients treated with a well established endograft of the same material and pure infrarenal fixation as the Gore Excluder.
Materials and Methods
A retrospective analysis of prospectively collected data from March 2014 to December 2014 identified 10 elective abdominal aortic aneurysm patients treated with the AFX endograft, in comparison to a matched group of 20 patients treated with the Excluder stent-graft. Endpoints included technical and clinical success, freedom from any secondary intervention, any type of endoleak and aneurysm related death.
Results
Primary technical success was achieved in all patients and no 30-day device related complications or deaths were occurred. The two groups were similar in terms of radiation burden, contrast media, duration of the procedure, post implantation syndrome and in-hospital stay. During a median follow-up period of 23 months (range, 18–26 months) there were no differences in clinical success, freedom from reintervention and aneurysm related death. No type I endoleak was observed in either group. Five of the 6 type II endoleaks (1 in the AFX and 4 in the Excluder group) spontaneously resolved, while in only one patient (Excluder) the endoleak remained without however any change in aneurysm sac diameter (log rank=0.34).
Conclusion
The initial experience with the AFX stent graft system is promising, with successful aneurysm exclusion and good short-term results. Further and larger studies are needed to fully evaluate the sort as well as the long-term results.
Keywords: AFX graft, Endovascular aortic aneurysm repair, Endovascular, Aneurysm
INTRODUCTION
During the last decade, endovascular aneurysm repair (EVAR) has gained wide acceptance as the preferred method of treating suitable patients with abdominal aortic aneurysm (AAA). The technique is associated with lower 30-day mortality and morbidity, faster discharge and fewer complications, although there are more late interventions related to the stent graft in comparison with open surgical repair [1,2].
Graft-related adverse events such as migration and endoleak still occur consisting a challenge not only for the specialist’s technical abilities but also for the stent-grafts [3]. Endografts’ manufacturers are constantly designing new devices to eliminate these undesirable effects and reduce complications related to EVAR.
AFX (Endologix, Inc., Irvine, CA, USA) is a newly designed endograft which offers fixation and sealing into both landing zones [4]. It is the only graft with anatomical fixation to the aortic bifurcation in comparison to all other grafts that use the infrarenal neck as the main fixation point. Its proximal neck is made to affix firmly to the aorta and reduce the possibility of device migration and leakage [5–7]. The proximal aortic extension is permitted to focus only on sealing, can be selected with infrarenal or suprarenal fixation, while the new expanded polytetrafluoroethylene (ePTFE) graft material called STRATA conforms to irregular surfaces. In clinical trials, the AFX endograft is accompnied by a lower rate of complications, such as endoleak type I, behaving efficiently even in an aortic neck anatomy with an irregular shape [5].
The aim of this report is to assess the preliminary results of the AFX stent-graft system used with infrarenal aortic component and compare them with those obtained in patients treated with a well-established endograft of the same material and pure infrarenal fixation as the Gore Excluder (Gore & Associates, Flagstaff, AZ, USA).
MATERIALS AND METHODS
1) Study design
A case-control study was conducted to assess the efficacy and safety of EVAR using the Endologix AFX device. Between March 2014 and December 2014, 52 patients with an infrarenal AAA were treated with EVAR in our center, and 10 were electively treated with the AFX stent graft. A dedicated database was established to prospectively collect patients’ data, including demographics, preoperative risk factors, operative time, contrast media use, patient outcomes, length of stay, and complications.
None of the patients was considered at high risk or unsuitable for endovascular surgical repair. All patients signed the hospital’s informed consent form before the procedure, while ethical approval was obtained by the institutional ethics committee. Sizing and planning before EVAR were performed using a workstation with the Osirix (Pixmeo, Bernex, Switzerland) or 3Mensio (Medical Imaging BV, Bilthoven, The Netherlands) dedicated reconstruction software.
All procedures were performed with bilateral femoral artery exposure and the patients under general anesthesia in an adequately equipped operating room using a BV Pulsera 12” mobile fluoroscopic C-arm unit (Philips Healthcare, Best, The Netherlands) and a radiographic carbon table equipped with side-table shielding (Varay Laborix, Bourges, France). The postoperative surveillance protocol included physical examination and imaging control with a computed tomography angiography at 1, 6, 12, and 24 months postoperatively for all patients.
2) Technical aspects of the AFX stent graft system
The AFX device consists of a main bifurcated body and a proximal aortic extension, which affix firmly to the aorta and provides sealing, while reducing the possibility of stent’s migration at the same time. The skeleton of the device is made of a cobalt-chromium alloy in a multilinked self-expanding unibody. External to the stent, the fabric is made of multilayer ePTFE material (STRATA). The stent is attached only to the proximal and distal ends at the proximal aortic extension and allows ePTFE to move independently and conform to abnormal surfaces, facilitating sealing of the sac.
3) Comparison group
The comparison group was selected from the sample of elective infrarenal EVARs using the Excluder stent-graft during the same period. The Gore Excluder stent-graft is a third-generation modern device featuring an original ePTFE design with a flexible catheter-mounted introduction and active infrarenal attachment with barbs. This endograft is similar to the AFX endograft regarding the material and the proximal attachment system, but without anatomical fixation to the aortic bifurcation. All patients were treated according to the instructions for use (IFU) of each endograft. To include a control group with the least selection bias, after the original cohort was formed, one of the investigators (G.K.), blinded to patient data apart from age, sex, and AAA diameter, matched the 10 patients from the AFX group 1:2 with individuals from the cohort treated with the Excluder endograft (40 patients) for age (<2 years), sex, and AAA diameter (<1 cm). The final population of this analysis included 30 patients, 10 patients in the AFX and 20 patients in the Excluder group.
4) Outcome measurements
The reporting standards according to the guidelines from the Society for Vascular Surgery/American Association for Vascular Surgery were used [8]. Technical success for both groups was defined as successful deployment of the endograft and completion of the procedure with no type I or III endoleaks and without the need for a secondary intervention within the first 24 hours. Clinical success was defined as freedom from aneurysm expansion >5 mm, type I or III endoleaks, aneurysm rupture, conversion to open surgery, graft infection, migration, or thrombosis, and aneurysm-related death during follow-up periods.
5) Statistical analysis
Data are expressed as mean±standard deviation except for non-Gaussian parameters that are presented as median and interquartile range. Categorical data are presented by absolute values and percentages (%). Statistical significance between the groups for continuous variables used the independent t-test for normally distributed data or the Mann-Whitney U-test for nonparametric data. The Pearson ×2 test or the Fisher exact test was used for categorical variables, as appropriate. Midterm follow-up data were analyzed by Kaplan-Meier life-table analysis, and results were compared by the log-rank test. Statistical analyses used IBM SPSS Statistics ver. 20.0 software (IBM Co., Armonk, NY, USA). A P-value of <0.05 was considered statistically significant.
RESULTS
Patient demographic data and preoperative risk factors are presented in Table 1. Median age of the AFX group (all males) was 71.5 years (range, 59–78 years). No significant differences in comorbidities were noted between the two groups.
Table 1.
Baseline characteristics of the two groups (n=30)
| Baseline characteristic | AFX group (n=10) | Excluder group (n=20) | P-value |
|---|---|---|---|
| Demographics | |||
| Age (y) | 71.5 (59–78) | 67 (63–81) | 0.55a |
| Gender (male) | 10 (100) | 19 (95.0) | 1b |
| Preoperative risk factors | |||
| Hypertension | 6 (60.0) | 14 (70.0) | 0.69b |
| Coronary artery disease | 4 (40.0) | 7 (35.0) | 1b |
| COPD | 3 (30.0) | 5 (25.0) | 1b |
| Hyperlipidemia | 7 (70.0) | 12 (60.0) | 0.71b |
| Diabetes | 1 (10.0) | 2 (10.0) | 1b |
| Cardiac failure | 1 (10.0) | 2 (10.0) | 1b |
| Smoking | 3 (30.0) | 6 (30.0) | 0.69b |
| AAA anatomy | |||
| Neck length (mm) | 18.5 (16–22) | 18.0 (16–24) | 0.95a |
| Suprarenal angle (°) | 7.5 (5–10) | 9 (5–13) | 0.28a |
| Infrarenal angle (°) | 15 (10–40) | 15 (5–40) | 0.71a |
| Maximum aneurysm diameter (mm) | 58.0 (51–85) | 56.3 (50–63) | 0.15a |
| Thrombus >30% | 1 (10.0) | 2 (10.0) | 1b |
| Calcification >30% | 2 (20.0) | 3 (15.0) | 1b |
Values are presented as median (range) or number (%).
COPD, chronic obstructive pulmonary disease; AAA, abdominal aortic aneurysm.
Student t-test or Mann-Whitney U-test,
Chi-square test.
1) AAA measurements
The median aortic neck length was 18.5 mm in the AFX group and 18.0 mm in the Excluder group (P=0.95). The median maximum diameter of the aneurysm in the respective groups was 58.0 mm vs. 56.3 mm (P=0.15). Angulations of the proximal neck angulations were not significantly different between groups. Neck circumferential characteristics of >30% thrombus or >30% calcification also were statistically similar.
Perioperative data are summarized in Table 2. General anesthesia was used in all patients of both groups. The median operation times were 97.5 minutes and 82.5 minutes respectively (P=0.10). Radiation burden and contrast media used were not significantly different between the groups (P=0.25 and P=0.11 respectively).
Table 2.
Periprocedural data
| Perioperative characteristic | AFX group (n=10) | Excluder group (n=20) | P-value |
|---|---|---|---|
| Procedure duration (min) | 97.5 (80–145) | 82.5 (65–125) | 0.10a |
| Contrast media (ml) | 62.5 (45–110) | 55.5 (30–110) | 0.11a |
| Radiation burden (mGy) | 84.3 (51–143) | 102 (52.6–167) | 0.25a |
| Post implantation syndrome | 1 (10.0) | 3 (15.0) | 1b |
| Hospital length of stay (d) | 3 (3–4) | 3 (3–5) | 0.59a |
Values are presented as median (range) or number (%).
Student t-test or Mann-Whitney U-test,
Chi-square test.
Technical success was achieved in all patients. No intraoperative conversion, migration, type I or III endoleak at the completion angiogram or death were recorded at the end of the procedure. All renal arteries were patent at the completion angiography. No endoleak type IV was observed in either group. Two patients (20.0%) of the AFX group had complications during the perioperative period. One patient with significant common iliac artery tortuosity suffered from limb thrombosis during the second postoperative day and was treated with mechanical thrombectomy and relining of the affected limb by stenting. Another patient with severe atherosclerotic disease of the common femoral artery had limb ischemia at the first postoperative day and was treated with common femoral artery endarterectomy. Both re-interventions were successful. Post implantation syndrome was encountered in 20.0% of the patients of the whole cohort with no differences between the groups (P=1). Median hospital stay was 3 days for both groups (P=0.59).
During a median follow-up of 23 months (range, 18–26 months), no reinterventions were performed in either group (Fig. 1). No graft-related deaths were recorded, while no conversion to open repair or graft migration occurred. Clinical success was achieved in all patients during follow-up. Six type II endoleaks (1 in the AFX and 5 in the Excluder group) were discovered during the follow-up period. Five of these endoleaks spontaneously resolved, while in only one patient (Excluder) the endoleak remained without any change in aneurysm sac diameter (log rank=0.34).
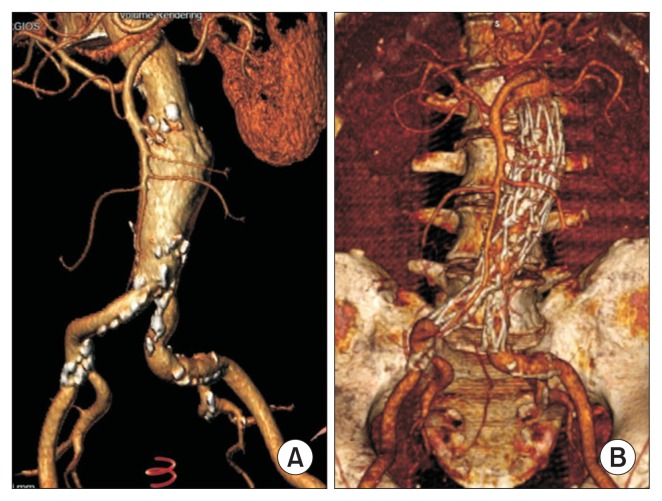
Fig. 1.
(A) Preoperative three-dimensional volume-rendered computed tomography reconstruction of an abdominal aortic aneurysm 6.2 cm in diameter. (B) Computed tomography angiography reconstruction 2 years after the procedure.
DISCUSSION
In EVAR successful proximal endograft fixation and sealing is critical to avoid both migration and endoleak type I. Several endografts have used different modalities to acquire proximal fixation, such as high radial force, suprarenal fixation, columnar rigidity, barbs, hooks or anchors. The AFX device has been shown to prevent the risk of migration as the endograft is anatomically fixed directly on the aortic bifurcation. Moreover, the use of STRATA conformable ePTFE material located external to the stent may provide greater graft-wall contact over the long-term, providing advantage in challenging proximal neck anatomy [5–7]. In the present preliminary report we compared the behavior of the AFX endograft with an infrarenally fixated proximal component with the behavior of the Excluder endograft with an infrarenally fixated graft of the same material. During a follow-up period of nearly 2 years, we found no significant differences in type I endoleak or migration occurrence between the two endografts.
The Endologix AFX endograft is a quite new device, designed to overcome some of the current EVAR limitations. The device can be deployed through a 17 French (F) ipsilateral and 9F contralateral introducer sheath, for aortic necks up to 32 mm in diameter; this is an important feature when treating patients with calcified or small-caliber iliac arteries [7]. Both endografts used in the current study are made from high-density, low permeability ePTFE material, which might be responsible for the absence of intraprocedural type IV endoleaks. Welborn et al. reported also no incidence of type IV endoleak in 108 patients treated with the AFX device. Endografts made from polyester, as the Endurant (Medtronic Inc., Santa Rosa, CA, USA) and the Incraft (Cordis Corp., Inc., Bridgewater, NJ, USA), have been related to relatively higher rates of intraoperative type IV endoleaks, 12% and 7% respectively, without any clinical sequlae in the follow-up [9,10].
Endoleak type III due to junctional endograft component disconnection has been a concern in modular EVAR platforms. From investigating 701 EVAR patients treated with the Endologix Powerlink or AFX endograft, Skibba et al. [6] recently reported a type IIIa endoleak in 17 patients (2.4%). Welborn et al. [7] reported a similar incidence (2.3%) in 108 patients treated with the AFX system. None of the patients in the present study had an endoleak type III. Since the beginning of 2013 revised IFU, requirement of a minimum component overlap of at least 30 mm to 40 mm have been introduced, and these were carefully followed in the present study. Similarly, in the Skibba et al. [6] series, no new type III endoleaks have been observed in patients treated with the revised IFU. Meticulous preoperative planning and proper selection of endograft components for a sufficient overlap are of utmost importance for endograft uncoupling and type III endoleak prevention.
The rate of type II endoleak was similar between the two endografts. Welborn et al. [7] reported an incidence of type II endoleak in only 6.7% of patients through 12 months, while Melas et al. [5] found only 1 endoleak in 21 patients treated with an AFX device. High rate of type II endoleak in the present study should be acknowledge, even though no reinterventions were needed during the follow-up.
Limb thrombosis occurred in one patient treated with the AFX device. Modern commercially available stent grafts each have important variations, both in graft material (polyester or PTFE), stent material (stainless steel or nitinol), and stent configuration (“Z-M” or helical shaped). These variations may result in different adaptations of the graft limb to the iliac artery anatomy, especially in cases of severe angulation or nonuniform-diameter landing zones. The Excluder limbs are made from ePTFE with an outer self-expanding nitinol support structure. These limbs are thin and highly flexible flexible, adapting well to complex iliac anatomy. Clinical reports have demonstrated promising results in clinical performance, with limb occlusion rates below 2% [11]. The unibody design of the AFX system with its ability to reline and stent the native aortic bifurcation provides a unique approach to iliac covering. Clinical reports confirm low limb occlusion rates. Melas et al. [5] and Welborn et al. [7] reported no limb occlusion in a total of 129 patients. Skibba et al. [6] by including 701 patients treated with the Endologix Powerlink and AFX platforms during a 8-year period, reported only 3 graft thrombosis that needed reintervention (0.4%). Moreover Carpenter et al. [12] by reporting results from 3 Investigational Device Exemption studies found a 1.9% limb occlusion rate. More data are needed to confirm the exact behavior of AFX iliac limbs in the long term as well as in hostile iliac anatomy.
In conclusion, the initial experience with the AFX stent graft system is promising, with successful aneurysm exclusion and good mid-term results with a low rate of device related complications. Further larger studies are needed to fully evaluate the long-term results.
Footnotes
Conflict of interest: None.
REFERENCES
- 1.Park BD, Azefor NM, Huang CC, Ricotta JJ. Elective endovascular aneurysm repair in the elderly: trends and outcomes from the nationwide inpatient sample. Ann Vasc Surg. 2014;28:798–807. doi: 10.1016/j.avsg.2013.07.029. [DOI] [PubMed] [Google Scholar]
- 2.United Kingdom EVAR Trial Investigators. Greenhalgh RM, Brown LC, Powell JT, Thompson SG, Epstein D, et al. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med. 2010;362:1863–1871. doi: 10.1056/NEJMoa0909305. [DOI] [PubMed] [Google Scholar]
- 3.Buckley CJ, Buckley SD. Deficiencies with current aortic endografts. J Cardiovasc Surg (Torino) 2015;56:369–373. [PubMed] [Google Scholar]
- 4.Diethrich EB. Novel sealing concept in the Endologix AFX unibody stent-graft. J Cardiovasc Surg (Torino) 2014;55:93–102. [PubMed] [Google Scholar]
- 5.Melas N, Stavridis K, Saratzis A, Lazarides J, Gitas C, Saratzis N. Active proximal sealing in the endovascular repair of abdominal aortic aneurysms: early results with a new stent-graft. J Endovasc Ther. 2015;22:174–178. doi: 10.1177/1526602815573232. [DOI] [PubMed] [Google Scholar]
- 6.Skibba AA, Evans JR, Greenfield DT, Yoon HR, Katras T, Ouriel K, et al. Management of late main-body aortic endograft component uncoupling and type IIIa endoleak encountered with the endologix powerlink and AFX platforms. J Vasc Surg. 2015;62:868–875. doi: 10.1016/j.jvs.2015.04.454. [DOI] [PubMed] [Google Scholar]
- 7.Welborn MB, 3rd, McDaniel HB, Johnson RC, Kennedy RE, Knott A, Mundinger GH, et al. Clinical outcome of an extended proximal seal zone with the AFX endovascular aortic aneurysm system. J Vasc Surg. 2014;60:876–883. doi: 10.1016/j.jvs.2014.04.017. discussion 884. [DOI] [PubMed] [Google Scholar]
- 8.Chaikof EL, Blankensteijn JD, Harris PL, White GH, Zarins CK, Bernhard VM, et al. Ad Hoc Committee for Standardized Reporting Practices in Vascular Surgery of The Society for Vascular Surgery/American Association for Vascular Surgery. Reporting standards for endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1048–1060. doi: 10.1067/mva.2002.123763. [DOI] [PubMed] [Google Scholar]
- 9.Makaroun MS, Tuchek M, Massop D, Henretta J, Rhee R, Buckley C, et al. One year outcomes of the United States regulatory trial of the endurant stent graft system. J Vasc Surg. 2011;54:601–608. doi: 10.1016/j.jvs.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Scheinert D, Pratesi C, Chiesa R, Coppi G, Brunkwall JS, Klarenbeek G, et al. First-in-human study of the INCRAFT endograft in patients with infrarenal abdominal aortic aneurysms in the INNOVATION trial. J Vasc Surg. 2013;57:906–914. doi: 10.1016/j.jvs.2012.09.079. [DOI] [PubMed] [Google Scholar]
- 11.Verhoeven EL, Katsargyris A, Bachoo P, Larzon T, Fisher R, Ettles D, et al. GREAT European C3 Module Investigators. Real-world performance of the new C3 gore excluder stent-graft: 1-year results from the European C3 module of the Global Registry for Endovascular Aortic Treatment (GREAT) Eur J Vasc Endovasc Surg. 2014;48:131–137. doi: 10.1016/j.ejvs.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Carpenter JP, Garcia MJ, Harlin SA, Jordan WD, Jung MT, Krajcer Z, et al. Contemporary results of endovascular repair of abdominal aortic aneurysms: effect of anatomical fixation on outcomes. J Endovasc Ther. 2010;17:153–162. doi: 10.1583/09-2977.1. [DOI] [PubMed] [Google Scholar]