Abstract
How does the developing brain support the transition from spoken language to print? Two spoken language abilities form the initial base of child literacy across languages: knowledge of language sounds (phonology) and knowledge of the smallest units that carry meaning (morphology). While phonology has received much attention from the field, the brain mechanisms that support morphological competence for learning to read remain largely unknown. In the present study, young English‐speaking children completed an auditory morphological awareness task behaviorally (n = 69, ages 6–12) and in fMRI (n = 16). The data revealed two findings: First, children with better morphological abilities showed greater activation in left temporoparietal regions previously thought to be important for supporting phonological reading skills, suggesting that this region supports multiple language abilities for successful reading acquisition. Second, children showed activation in left frontal regions previously found active in young Chinese readers, suggesting morphological processes for reading acquisition might be similar across languages. These findings offer new insights for developing a comprehensive model of how spoken language abilities support children's reading acquisition across languages. Hum Brain Mapp 36:2890–2900, 2015. © 2015 Wiley Periodicals, Inc.
Keywords: reading, fMRI, language, children, morphemes, literacy, auditory
INTRODUCTION
Acquisition of natural human languages typically precedes and predicts learning to read [Ziegler and Goswami, 2005]. Spoken words are comprised of sounds (phonemes) and the smallest units of grammar that carry meaning (morphemes); hence, children's phonological and morphological abilities are important for learning to read across languages [Carlisle and Goodwin, 2013; McBride‐Chang et al., 2013]. Phonological abilities help children map language sounds onto their orthographic representations. In contrast, morphological awareness supports children's ability to extract meaning in print through morphosyllabic units [e.g., emotional; Ehri, 2014]. Thus, an understanding of the brain‐based mechanisms for morphophonological competence, and how they emerge in the developing brain, is essential to understanding how children become literate [Frost, 2012]. Yet, little is known about the brain bases of morphological awareness [Aylward et al., 2003; Liu et al., 2013] and whether they overlap or are independent from the brain bases of phonological competence [Deacon, 2012]. This study offers the first functional magnetic resonance (fMRI) brain imaging investigation of young readers’ morphological abilities in the auditory modality and their relation to literacy in the brain.
Research with alphabetic languages, like English, has shown that phonological awareness contributes the most to children's early achievements in reading acquisition [Ziegler and Goswami, 2005]. However, the balance begins to shift away from phonology toward morphology around 3rd grade [Carlisle, 2000]. Originally, this trend was thought to occur because phonology is a precursor for learning to read, while morphology comes to support literacy predominantly as a consequence of children's phonological, vocabulary and orthographic experiences [e.g., Share, 1999]. Newly emerging theoretical frameworks suggest that morphological awareness is also an early‐emerging and significant contributor to reading acquisition [Deacon, 2012]. Specifically, research now shows that morphology makes a small but significant contribution to literacy in 1st–3rd grades, even after controlling for variables such as phonological awareness, vocabulary and IQ [Deacon, 2012]. Importantly, morphological awareness supports multiple reading skills such as single word and pseudoword reading, as well as text comprehension [cf. Carlisle and Goodwin, 2013; Marinova‐Todd, Siegel, and Mazabel, 2013]. This is likely the case because English has many instances of phonologically irregular but morphologically regular spellings (e.g, magic‐magician), and because morphology taps into both the meaning and grammatical representations of language [Deacon, 2012].
Finally, dyslexia research suggests that there might be different consequences to having deficits in phonology versus morphology. Specifically, children with phonological word reading deficits might benefit from learning morphological strategies as a compensatory mechanism for reading [Elbro and Arnbak, 1996]. In contrast, children with deficits in text comprehension, but not word reading, show selective impairments in morphology but not in phonology [Tong et al., 2011]. Despite the growing evidence suggesting that it is equally important to understand the cognitive underpinnings of morphological reading skill, little is known about the brain bases of morphological competence in young children [Aylward et al., 2003; Liu et al., 2013], especially in the auditory modality, which potentially precedes and predicts reading competence [Deacon, 2012].
Emerging perspectives on literacy aim to explain reading acquisition across languages, across development, and in the brain [Frost, 2012; Perfetti, et al., 2013; Pugh et al., 2013]. Recent research has found significant cross‐linguistic variation on how phonology and morphology contribute to reading acquisition: Alphabetic orthographies include relatively overt associations between phonology and individual letters, whereas Chinese orthography offers relatively more overt associations between morphemic units and their characters [McBride‐Chang et al., 2013]. Not surprisingly, neuroimaging studies of phonological reading abilities also yield significant cross‐linguistic differences: Alphabetic readers show robust activation in left temporal regions classically associated with phonological processing [near Wernike's area; Hoeft et al., 2006, 2007], whereas Chinese readers show robust activation in left frontal regions coupled with somewhat less activation in left temporal regions [Siok et al., 2004, 2008]. It remains unclear whether cross‐linguistic differences stem from highly salient lexico‐semantic and morphological features in Chinese [Liu et al., 2013; McBride‐Chang et al., 2013], higher mnemonic demands for characters, or greater efforts to compute phonology with Chinese characters [Cao et al., 2010; Siok et al., 2004]. The general paucity of knowledge on the brain bases of morphology in young children [Aylward et al., 2003; Liu et al., 2013] further complicates the interpretation of divergent cross‐linguistic findings and impedes the construction of a comprehensive model of how children's language and cognition support reading acquisition.
In particular, little is known about the brain bases of morphological awareness, and to our knowledge, no study has yet examined this ability in the auditory modality with children. Children's language abilities in the spoken modality typically precede and predict reading acquisition [Ziegler and Goswami, 2005]. While core language knowledge, including morphology, can be effectively accessed through both auditory and visual modalities in adult proficient readers [Bozic, et al., 2013], studies with children show that automaticity in accessing mental representations for language via print continues to develop into the adolescent years [Coch et al., 2002; Coch et al., 2005; Grossi et al., 2001]. Therefore, this study used an auditory paradigm to avoid confounding reading proficiency with children's underlying morphological ability.
Previous neuroimaging studies on English morphology that used visual tasks have found activation in left ventral inferior (IFG BA 47) and middle frontal (MFG BA 9) regions in both adults and older children [Aylward et al., 2003; Bozic et al., 2007, 2013; Bick et al., 2008]. These regions are also typically active in young Chinese readers during phonological reading tasks [Bolger et al., 2008; Brennan et al., 2012; Cao et al., 2010, 2011; Siok et al., 2004, 2008], possibly due to children's greater morphological processing when reading in Chinese [McBride‐Chang et al., 2013]. We hypothesized that English‐speaking children (ages 6–12) should also exhibit significant activation in left ventral inferior (BA 47) and middle frontal (MFG BA 9) regions during an auditory morphology task. In this study, children completed language and literacy tasks, and a subset also completed an auditory morphology task during fMRI scanning. To explore the potentially common and distinct brain mechanisms that support morphology and other aspects of metalinguistic competence across languages, we conducted whole‐brain analyses for the morphological task used in this study, as well as region of interest analyses with left temporoparietal and frontal regions that were reported active during phonological reading tasks in English [Hoeft et al., 2007] and in Chinese [Siok et al., 2004].
MATERIALS AND METHOD
Participants
Sixty‐nine English‐monolingual children completed language and literacy tasks (33 females; age range = 6.1–12.8 years‐old, mean age [M] = 9.1 years, standard deviation [SD] = 1.8). A subset of 20 right‐handed children also completed a morphological awareness task in the fMRI scanner, however, four participants were excluded due to below 70% task accuracy. Consequently, a subset of 16 participants’ neuroimaging data was analyzed (8 females; age range = 6.6–12.5 years‐old, M = 9.3, SD = 1.6). The study was broadly advertised throughout the community in southeast Michigan (libraries, gyms, afterschool programs etc.). After an initial phone or e‐mail screening for age, handedness and neurodevelopment disabilities, the participants were invited for a mock‐scanner visit and behavioral assessments. All participants that fit the eligibility criteria (see below) and were still interested in fMRI imaging after visiting the mock scanner were then invited to participate in the fMRI scanning. All participants in this study met eligibility criteria, including: native English speaker, typical development with no history of cognitive/motor developmental difficulties and brain injury, no current regimen of medication affecting brain functioning, normal hearing, and a standard score above 85 (−1.5 SD) for IQ and reading ability as measured by the Kaufman Brief Intelligence Test of Verbal Knowledge [KBIT‐2; Kaufman and Kaufman, 2004] and Woodcock Reading Mastery Tests (WRMT) [Woodcock, 1998], respectively. The study was reviewed and approved by medical institutional review boards; parents and children completed informed consent/assent forms and were monetarily compensated for their time.
Procedure
All children completed assessments of morphological awareness (see details below), phonological awareness [Elision subtest, Comprehensive Test of Phonological Processing; Wagner, et al., 1999], and single word reading [Word ID subtest, WRMT; Woodcock, 1998] while their parents completed a detailed questionnaire about their child's development. See Table 1 for children's performance on all tasks. Participants were then invited to an fMRI session after visiting a mock scanner and agreeing to participate in the imaging part of the study. Before fMRI scanning, children completed a computer version of the task with a set of practice stimuli.
Table 1.
Mean and standard deviation task performances for participants taking part in behavioral‐only and neuroimaging sessions
| Variable | Behavioral‐only M ± SD | Neuroimaging M ± SD |
|---|---|---|
| 53 participants | 16 participants | |
| Age in years | 9.04 ± 1.84 | 9.28 ± 1.56 |
| Behavioral measures | ||
| Phonological Awareness Raw Score | 15.58 ± 4.04 | 15.63 ± 4.21 |
| Phonological Awareness Standard Scorea | 12.23 ± 2.62 | 11.75 ± 2.02 |
| Morphological Awareness Raw Scoreb | 27.06 ± 2.25 | 27.94 ± 1.24 |
| Reading Raw Score | 68.79 ± 17.98 | 72.13 ± 20.19 |
| Reading Standard Score | 115.12 ± 10.89 | 116.09 ± 11.31 |
| KBIT‐2 Standard Score | 113.2 ± 10.84 | 115.94 ± 12 |
| Morphology Condition (% correct)c | 82.38 ± 10.43 | 82.03 ± 11.78 |
| Morphology Condition RT (ms.)c | 2,642 ± 389 | 2,698 ± 353 |
| In‐scanner task performanced | ||
| Morphology Condition (% correct) | — | 76.67 ± 11.44 |
| Morphology Condition RT (ms) | — | 2,599 ± 205 |
| Control Match Condition (% correct) | — | 94.72 ± 16.02 |
| Control Match Condition RT (ms) | — | 2,406 ± 257 |
Mean performance scores are presented separately for those who completed behavioral‐only, and for those who completed neuroimaging and behavioral.
This subtest standard score is based on a mean of 10 rather than 100.
Two children in the behavioral‐only group did not complete this task due to failure to complete practice trials correctly. The morphology task included a total of 30 items; that is, 2 subtests of decomposition (18 items) and derivational (12 items) morphology.
Task performance for 7 children in the behavioral‐only group and 1 child in the neuroimaging group is missing due to technical error, experimenter error, and/or participant not passing practice trials.
In‐scanner task accuracy performance for 1 participant and response time for 2 participants are missing due to technical error.
Morphological awareness behavioral task
Children completed a modified version of the Test of Morphological Structure [Carlisle, 2000] that included subtests of decomposition and derivational morphology. In the decomposition subtest, participants were instructed to take away part of a given word to correctly complete a sentence, such as “Driver. Children are too young to… (‘drive’).” In the derivation subtest, participants were instructed to add a part to a given word to correctly complete a sentence, such as “Help. Mother says I am a good… (‘helper’).” A composite score for each participant included 18 decomposition and 12 derivation sentences. Each subtest also included 2 additional practice items. The experimenter presented the trials aurally (no reading was involved from the participant).
Imaging Experimental Design
Children who partook in the fMRI session completed a derivational morphology task during brain scanning. The task was based on English derivational morphology principles [Carlisle, 2000], and its methodology previously used for assessing morphological competence in young Chinese readers and prereaders [asking children to generate or judge novel morphological word items; McBride‐Chang et al., 2003]. The task included an experimental morphological awareness condition, a control word‐matching condition, and rest periods. During resting periods, a white fixation cross was displayed on a black background. During the morphological awareness condition, participants heard a high‐frequency real word (e.g., “jump”) and a morphologically derived new word (“re‐jump”), in which the new word either conformed to or violated morphological structures of English. Participants were asked to indicate with a button‐press whether the new word was a good (acceptable) or a bad (unacceptable) word. For example, “re‐jump” is acceptable because the prefix re‐ can be applied to verbs as to define that something can be done again; conversely, re‐apple is unacceptable because the prefix re‐ cannot be applied meaningfully to nouns. The morphology condition included verbs and nouns with lexical morphemes that were appropriate for testing at this age [Carlisle, 2000]. Other derivational morphemes used in the study were un‐, ‐er, ‐ness, ‐ly, and –ful. In half of the trials, the derivational rules of morphology were applied correctly (“jump” “re‐jump”), while the rules were applied incorrectly in the remaining trials (“apple” “re‐apple”). See Supporting Information Appendix A for stimuli.
During the control condition, participants heard two words and made judgments using button‐presses on whether the words were identical or not (e.g., “car” and “car” are identical; “key” and “pen” are not). In designing the control condition, we had to take into account that morphology is a higher order linguistic process that involves access to phonology (word sounds), semantics (word meanings), and word structure (grammar). Therefore, we used a language‐based word‐matching control task in which participants heard two words and decided if the two words were the same or not. In this control task, children had to access the word sound, meaning, and structure as well as retain two words in phonological short‐term memory to make the judgment. We designed this control task to best match the processes required for completing the experimental morphology task, with the exception of the added effort for actively evaluating morphological structure. We have validated the utility of this control task in our prior published work [Kovelman et al., 2012], in which we used this task as a control for a phonological awareness rhyme judgment task. In our previous work on phonological awareness, this control method successfully yielded group differences in brain activation between typical and dyslexic readers during contrasts between the experimental phonological rhyme task and the control word matching task [see Kovelman et al., 2012].
The 7‐min blocked design task included six 24‐s randomized blocks for each condition (morphology, control and rest). Participants received an audio and visual prompt indicating whether the upcoming condition was a “word game” (morphology condition) or a “matching game” (control condition). Experimental (morphology and control conditions) blocks included four 6‐s trials, which totaled 24 trials per condition. During each trial, the first word was played and the second word followed 2‐s later with an average of 1.5‐s between the words. Children saw a fixation cross during presentation of the words and a question mark during the last 2‐s of the trial cuing for a button‐press response. The order of trials and blocks was randomized with an equal number of “yes” and “no” answers.
Stimuli
All words were child‐friendly, high‐frequency monosyllabic words matched within and across conditions (morphology and control) for concreteness, written and verbal frequency, number of sounds, syllables and letters (data from MRC Psycholinguistic database). Ad hoc t‐tests comparing the conditions within and across were nonsignificant (P > 0.05). All words were recorded by a female‐speaker who was native to the Midwest region in the United States (same locale as the participants). The words were then filtered and normalized to 80‐dB using Adobe Audition 1.5 software. The task was presented using Psychophysics Toolbox Version 3 (PTB‐3) in MATLAB (2010a, MathWorks). While in the fMRI, sounds were played using Pyle Home PCA1 30‐Watt Stereo Mini Power amplifier to moderate the volume, and children wore Sensimetrics insert earphones model S14 and MRI nonmagnetic earmuffs Ultra‐33 (NRR 33) to attenuate scanner noise and allow better quality of audio.
Imaging Data Acquisition
Image acquisition was collected using a 3 Tesla GE Signa scanner equipped with a quadrature head coil (General Electric, Milwaukee, WI). Participants used a button box to make responses. The task was projected onto a screen and participants wore goggles with built‐in mirrors (VisuaStim XGA, Resonance Technologies) to view the display. Foam padding and a cloth forehead restraint were used to prevent head movement. A T1 overlay with Fast Gradient Echo Sequence 15 was conducted to obtain an anatomical image (TR = 250 ms, TE = 5.7 ms, flip angle = 90°, field of view (FOV) = 24 cm, 43 slices). Automatic slice prescription, based on alignment of localizer scans to a multisubject atlas, was used to achieve a consistent head position across subjects. Functional T2* BOLD images were acquired with a spiral reverse only sequence. For each TR, 43 3 mm slices were captured (TR = 2,000 ms, TE = 30 ms, flip angle = 90°, FOV = 22 cm, voxel size = 3.44 mm × 3.44 mm × 3 mm).
Imaging Data Analysis
Imaging data was processed and analyzed using statistical parametric mapping software SPM8 (Wellcome Department of Cognitive Neurology, London, UK) using MATLAB (2011a, MathWorks). We performed the following steps in the following order: slice timing, realignment, normalisation, and smoothing. There were a total of 218 TRs (excluding 4 dummy scans). After image reconstruction, each subject's data was realigned to the first functional volume using SPM8's spline interpolation. Movement parameters calculated by SPM8 realignment were used to exclude volumes with potential artifacts. This procedure was implemented for each participant separately. Sessions were then normalized using the mean functional volume into a standard EPI anatomical space; these were then resampled to fit Montreal Neurological Institute (MNI) stereotactic space. Spatial smoothing was done using a 6‐mm full‐width half‐maximum Gaussian filter, which is a typical level for reducing noise that Hopfinger et al. [2000] have found to work best for examining data in the cortex. Smoothing was done after normalisation to increase the probability that the activity was reflected in the grand average accurately. Each subject's data was then high‐pass filtered at 128 s; we chose 128 s due to the length of our blocks. Poldrack et al. [2011] suggest that the high‐pass filter should be at least twice the period of a block, but given that we had two experimental conditions each lasting 24 s and our participants were children, we decided on the standard 128 s high‐pass filter.
Each subject's data was then analyzed using a fixed‐effects model that included morphology and control conditions as factors; rest served as the implicit baseline and was not included as a regressor in the model. For each participant, BOLD impulse response was then modeled using the dual‐gamma canonical hemodynamic response function. Statistical images for the following contrasts were generated: control > rest, morphology > rest, control > morphology, and morphology > control.
Second‐level analyses were performed to obtain group‐level contrast images, which were then examined using one‐sample t tests for whole‐brain activations. Analyses that included contrast images against rest (control > rest, morphology > rest) had a height threshold of P < 0.001 and extent threshold (ET) of > 35 voxels. Analyses that included both of the experimental conditions (control > morphology, morphology > control) had a height threshold of P < 0.005 and ET of > 30 voxels. All analyses were corrected for multiple comparisons at P < 0.05 (False Discovery Rate, [FDR]).
Brain‐behavior associations were examined using whole‐brain correlations between children's brain activity in the morphology > control contrast image to age, task accuracy on phonological awareness and behavioral morphological awareness task (overall score and each subtest [decomposition and derivational]), separately. Whole‐brain analyses were FDR corrected for multiple comparisons at P < 0.05, height threshold P < 0.005, ET > 15 voxels.
Region of interest analyses
To further examine morphophonological segmentations during reading acquisition, we correlated children's behavioral performance in the phonology, morphology, and reading tasks to brain regions considered to support English and Chinese phonological reading processes. We applied several principled criteria for selecting regions of interest (ROI) from previous research to use in our study: First, given that our study aimed to understand morphological relative to phonological awareness processes in young children, we chose developmental studies of phonological awareness with child participants’ age ranges similar to this study. Second, as we hoped for the results to generalize across typical development and dyslexia, we chose studies that included both typically developing children and children with dyslexia. Finally, the English language study [Hoeft et al., 2007] that was chosen was the only study matching the criteria that also included children who were both age‐ and reading ability‐matched to children with dyslexia. The Chinese language study chosen [Siok et al., 2004] was the first to demonstrate cross‐cultural differences in the brain bases for phonological awareness and developmental dyslexia in Chinese.
To examine English morphophonological competence, we extracted participants’ activation in the morphology > control contrast image from the selected left supramarginal gyrus region [MNI coordinates: x = −52, y = −42, z = 40; Hoeft et al., 2007]. To examine morphological competence compared to previous research with Chinese readers on phonological competence, we extracted participants’ activation in the morphology > control contrast image from the selected left middle frontal gyrus (MFG) region [MNI coordinates: x = −50, y = 10, z = 38; Siok et al., 2004]. MarsBaR toolbox [Brett, et al., 2002] in SPM8 was used to create spheres of 8‐mm radius and extract these regions’ beta values. During ROI extraction, the data was normalized using a hemodynamic response function and the temporal derivate to extract the percent signal change of contrast images. For details of the method see http://marsbar.sourceforge.net/
RESULTS
Behavioral Results for Phonology, Morphology and Reading Acquisition
Partial correlations controlling for age revealed that children with better phonological and morphological abilities also performed better in reading, r(64) = 0.63, P < 0.001 and r(64) = 0.42, P < 0.001 respectively. To investigate whether phonology and morphology were independent predictors of English literacy and whether their relative contribution changed over time, we conducted standard multiple regression analyses across younger (ages 6–9) and older (ages 9–12) readers, splitting at the mean age of the sample (9‐years‐old). The results indicated that phonological and morphological awareness explained 57.1% (R 2 = 0.60, F(2,32) = 22.26, P < 0.001) of the variance for younger readers and 17.3% (R 2 = 0.22, F(2,33) = 4.45, P = 0.02) for older readers. Analyses revealed that both variables made significant and independent contributions to younger children's literacy, but only morphology was a significant contributor for older children's reading ability (see Table 2).
Table 2.
Standard multiple regression analyses predicting reading in younger (6‐ to 9‐years‐old; R 2 = 0.60) and older (9‐ to 12‐years‐old; R 2 = 0.22) learners of English literacy
| Phonological Awareness | Morphological Awareness | |||
|---|---|---|---|---|
| Predictors | Standardized Beta | t | Standardized Beta | t |
| Younger Readers (n = 34) | 0.54c | 4.23 | 0.37b | 2.89 |
| Older Readers (n = 35) | 0.27 | 1.6 | 0.34a | 2.56 |
p ≤ 0.05.
p ≤ 0.01.
p ≤ 0.001.
Brain Bases of Morphological Language Ability
See Table 3 for brain imaging results, including Brodmann areas (BA). In the control condition (word‐matching), whole brain analyses for the control > rest contrast revealed that children showed significant activation in bilateral occipital, temporal and parietal regions (see Fig. 1a). In the morphology condition, whole‐brain analyses for morphology > rest contrast revealed that children showed significant activation in bilateral regions including occipital, temporal, inferior frontal and parietal regions, as well as left brain regions including middle/superior/medial frontal, cingulate gyrus, and superior motor areas.
Table 3.
Children's brain activation during morphological awareness processing (control > rest, morphology > rest, and morphology > control contrasts)
| Brain Regions | Brodmann Areas | Hemisphere | ET | x | y | z | t |
|---|---|---|---|---|---|---|---|
| Control > Resta | |||||||
| Inferior/middle occipital gyri | 18 | L | 676 | −26 | −94 | −12 | 9.73 |
| Superior/middle temporal gyri and (inferior) parietal lobe | 22, 13, 41, 42, 21,40 | L | 3,072 | −48 | −28 | 10 | 8.87 |
| Superior/middle temporal gyri and parietal lobe | 22, 41, 21, 42, 13 | R | 2,628 | 68 | −24 | 0 | 8.28 |
| Inferior/middle occipital gyri | 18 | R | 713 | 32 | −78 | −8 | 7.08 |
| Morphology > Resta | |||||||
| Superior/middle temporal gyri and (inferior) parietal lobe | 22, 13, 41, 21, 42, 40 | L | 2,969 | −52 | −38 | 16 | 10.42 |
| Inferior/middle frontal gyri | 6, 9, 45, 47, 13, 46, 44 | L | 2,515 | −36 | 22 | −2 | 9.48 |
| Superior motor areas; superior/medial frontal and cingulate gyri | 8, 6, 32 | L | 1,266 | −2 | 16 | 52 | 9.19 |
| Superior/middle temporal, inferior frontal gyri, and parietal areas | 22, 41, 21, 42, 47, 13 | R | 2,054 | 58 | −22 | 4 | 8.7 |
| Inferior/middle occipital gyri | 18 | L | 579 | −14 | −104 | −2 | 8.01 |
| Inferior/middle occipital gyri | 18 | R | 771 | 20 | −98 | −10 | 7.24 |
| Morphology > Controlb | |||||||
| Superior frontal gyrus | 6 | B | 141 | −4 | 4 | 70 | 7.89 |
| Anterior superior temporal gyrus | 38 | L | 16 | −42 | 18 | −26 | 7.12 |
| Inferior frontal gyrus | 45, 47, 13 | L | 543 | −52 | 22 | −2 | 6.97 |
| Middle/inferior frontal gyri | 46, 6, 9 | L | 667 | −46 | 12 | 40 | 6.90 |
| Whole‐Brain Correlation: Morphological Awarenessc | |||||||
| Superior temporal gyrus and inferior parietal lobe | 42, 40, 22 | L | 112 | −64 | −34 | 14 | 5.85 |
| Whole‐Brain Correlation: Morphological Awareness—Derivational Morphologyc | |||||||
| Superior temporal gyrus and inferior parietal lobe | 42, 40, 22 | L | 132 | −64 | −34 | 16 | 5.76 |
Brain activations for Control > Rest, Morphology > Rest, Morphology > Control, and whole‐brain correlation with morphological awareness (entire task and derivational morphology). Montreal Neurological Institute (MNI) coordinates.
FDR corrected for multiple comparisons at P < 0.05, height threshold P < 0.001, ET > 35 voxels.
FDR corrected for multiple comparisons at P < 0.05, height threshold P < 0.005, ET > 30 voxels.
FDR corrected for multiple comparisons at P < 0.05, height threshold P < 0.005, ET > 15 voxels.
Figure 1.
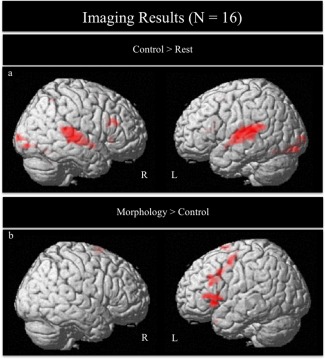
Brain activation for (a) Control > Rest, FDR corrected for multiple comparisons at P < 0.05, height threshold P < 0.001, ET > 35 voxels. (b) Morphology > Control contrasts, FDR corrected for multiple comparisons at P < 0.05, height threshold P < 0.005, ET > 30 voxels. [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
In the whole‐brain contrast for morphology > control, analyses revealed that children showed significant activation in bilateral superior frontal gyri (SFG), left MFG and inferior frontal gyri (IFG), as well as anterior superior temporal gyrus (aSTG; see Fig. 1 and Table 3). The whole brain analysis for control > morphology contrasts did not reveal any significant activation that passed the FDR threshold.
To examine the relationships between the morphological processing regions, whole‐brain correlations were performed between the morphology > control image contrasts and age, phonological awareness, behavioral morphology (overall score, as well as decomposition and derivational subtest scores), and reading performance. The whole‐brain correlation on the overall score of the behavioral morphology task revealed that children who performed better had stronger activation in left superior temporal and inferior parietal regions (see Table 3 and Fig. 2). This finding was followed with separate whole‐brain correlation analyses for derivational and decomposition morphology subtests, in which we found that the derivational subtest reached significance thresholds for positive correlations in the same regions as reported for the overall score, see Table 3. There were no significant correlations that passed the FDR threshold with participants’ age, phonological awareness, decomposition morphology subtest, and single word reading scores.
Figure 2.
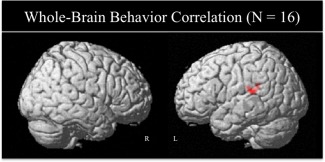
Whole‐brain correlations revealed greater activation in left temporo‐parietal regions in children with better morphological competence (Morphology > Control contrast; FDR corrected for multiple comparisons at P < 0.05, height threshold P < 0.005, ET > 15 voxels). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
Second, ROI analyses revealed that children who performed better in morphological competence also showed greater activation in regions previously associated with phonological abilities in English (left supramarginal gyrus: r(14) = 0.58, P = 0.02) and in Chinese (left MFG: r(14) = 0.53, P = 0.03); see Figure 3 for visualization of these correlations. We also correlated these regions to participants’ age, phonological awareness and reading ability scores, but these results were nonsignificant. Additionally, children's brain activation between these ROIs correlated, r(14) = 0.67, P = 0.005.
Figure 3.
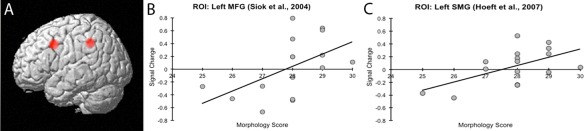
(a) Figure showing location of ROI 8‐mm spheres. Following are ROI scatterplots for correlations between morphology scores to signal change in Morphology > Control contrast, (b) left MFG (Siok et al., 2004), and (c) left SMG (Hoeft et al., 2007). [Color figure can be viewed in the online issue, which is available at http://wileyonlinelibrary.com.]
DISCUSSION
Phonological and morphological language abilities support children's reading acquisition, yet little is known about the brain bases of morphological development in young readers [Frost, 2012; Perfetti, et al., 2013; Pugh et al., 2013]. We used behavioral tasks of language and literacy to confirm that phonological and morphological abilities independently contribute to children's development of literacy, and that morphological competence continues to contribute to reading success in older readers [see prior cross‐linguistic work for English: Berninger, et al., 2010; Spanish: Ramirez, et al., 2011; Chinese: Zhang et al., 2013]. During the morphological brain‐imaging task, children showed activation in left IFG and anterior STG regions, as previously reported in studies of word structure and meaning [Booth et al., 2006; Bozic et al., 2013; Friederici, 2002]. Moreover, the children showed significant correlations between their morphological competence and brain activation in regions previously reported active during phonological awareness reading tasks in English [Hoeft et al., 2007] and in Chinese [Siok et al., 2004]. In sum, the convergent behavioral and brain imaging findings highlight the relevance of morphological processes for learning to read and that, for young readers, morphological tasks engage brain regions associated with processing word meaning and word structure.
Theoretical perspectives specific to alphabetic literacy suggest that a key characteristic of advanced reading ability is being able to progress beyond sound‐to‐letter mapping and to learn to rapidly recognize entire morphosyllabic units on a printed page [Ehri, 2014]. Consistent with this idea, the behavioral results of this study showed that both phonology and morphology explained a significant amount of variance in young children's (ages 6–9) reading ability, each being a separate and significant predictor of literacy. In contrast, for older children (ages 9–12), only morphological competence explained a significant amount of variance in children's reading ability. This developmental effect has been found in “deep” alphabetic orthographies like English [Carlisle, 2000; Roman, et al., 2009] and in “shallow” alphabetic orthographies like Spanish [Ramirez et al., 2011]. Importantly, while this analysis only included tasks of phonology and morphology, the validity of the present finding is supported by studies that have shown similar results using a broader range of language and literacy measures, including vocabulary, rapid automated naming and working memory tasks [Deacon, 2012; Tong et al., 2011, Deacon and Kirby, 2004; McBride‐Chang et al., 2005; Wolter, et al., 2009].
Brain imaging results revealed that during the control word‐matching brain imaging task, children showed activation in bilateral superior and middle temporal regions typically associated with auditory word recognition [Zatorre et al., 1996]. The morphology task also engaged these regions, as well as bilateral frontal regions (Table 3). The direct comparison between the morphology and the word‐matching control task (morphology > control contrast) revealed significant activation in left inferior and middle frontal regions, including a ventral aspect of IFG (BA 47), IFG (BA 45), MFG (BA 46/9), and anterior STG (BA 38). Researchers typically find that left ventral IFG (BA 47) is active during tasks of lexicosemantic access, while anterior STG has been linked to morphosyntactic processes [cf. Friederici and Gierhan, 2013]. Activation in left IFG BA 45 is frequently found during a broad range of language tasks spanning phonology, syntax and semantics [e.g., Friederici and Gierhan, 2013; Bozic et al., 2013]. Yet, children did not show significantly greater activation during the morphology task relative to the control word‐matching task in regions typically associated with phonology‐specific analyses, that is dorsal IFG (BA 44) and posterior STG regions [Booth et al., 2006; Katzev et al., 2013; Petitto et al., 2000]. The findings converge with adult research that contrasts lexical, morphological and phonological processes: greater activation in ventral IFG (BA 47) during morphological and lexical decision tasks [Bozic et al., 2013], greater activation in dorsal IFG (BA 44) during phonological tasks [Katzev et al., 2013], and shared activation in left IFG (BA 45) region across lexico‐semantic, lexical and verbal morphology, as well as phonological tasks [Bozic et al., 2013; Friederici and Gierhan, 2013; Tan et al., 2005; Zatorre et al., 1996]. These convergent developmental and adult findings hint at the possibility that at least in English, morphology tasks may engage cognitive processes (and associated brain regions) important for lexico‐semantic and syntax‐based tasks (ventral IFG, aSTG), as well as those that integrate various levels of language analyses, possibly localized around the general area of left IFG (BA 45).
During the morphology task, children showed significant activation in left MFG (BA 46/9), a region also found active during a broad variety of language tasks that include a verbal working memory component [cf. Smith, et al., 1998]. Importantly, activation in both ventral IFG and MFG regions are consistently found across studies that use phonological [Cao et al., 2009; Siok et al., 2004, 2008] and/or morphological [Liu et al., 2013] reading tasks in Chinese. We suggest that these two regions might be important for retrieving and evaluating the meaning and structure of suprasegmental or morphosyllabic language units [Bozic et al., 2013], which is an important aspect of learning to read across languages, especially during both early and later stages of learning to read in Chinese [McBride‐Chang et al., 2011].
Whole‐brain correlation analyses revealed that children with better morphological abilities also showed greater activation in left superior temporal and inferior parietal regions (Fig. 2); similar results are found during tasks of phonological awareness [e.g., Frost et al., 2009]. Based on prior behavioral findings, at least two interpretations are possible: Research shows that improvement in phonological awareness and experiences with sound‐to‐letter mapping contribute to children's growing morphological competence [cf. Ehri, 2014]. Thus, one possibility is that morphological competence builds on phonological competence and the functioning of brain regions that support phonological awareness. Another related possibility is that shared cognitive processes underlie children's improvement in a broad range of metalinguistic abilities, including phonological and morphological awareness abilities [Carlisle and Goodwin, 2013], and hence are supported by the functioning of similar temporal and parietal brain regions.
Finally, in an attempt to provide a first‐time bridge on cross‐linguistic cognitive differences between alphabetic and nonalphabetic literacy at the level of morphological competence, we correlated children's brain activity in the left supramarginal gyrus, which is associated with typical reading acquisition and dyslexia in English [Hoeft et al., 2007], as well as left middle frontal regions associated with typical reading and dyslexia in Chinese [Siok et al., 2004]. Remarkably, we found that children's activation in both of these regions was positively related to their morphological ability, which in turn was related to their reading ability. There were no significant correlations between the children's activation (whole‐brain and ROIs) and their age or to other language or reading abilities, possibly due to loss of power by our low sample size. In sum, it is possible that the significant correlations found between children's morphological competence and brain activation in left middle frontal and supramarginal regions previously found active during phonological awareness tasks in Chinese and in English, respectively, suggest that the functioning of these regions might support multiple types of metalinguistic abilities necessary for learning to read across languages.
The innovation of this study is the investigation of the brain bases of morphological awareness in the auditory modality in young alphabetic readers using fMRI imaging. The study developed a new, fMRI‐compatible morphological awareness task and contrasted children's brain activation during this task against their brain activity during a word‐matching control task. Akin to the morphology task, the control task also engaged phonological, morphological and semantic processes necessary to access word form and meaning. A similar control task was previously used to show differences in brain activation for phonological processing between typical and dyslexic young readers [Kovelman et al., 2012; Raschle, et al., 2012], suggesting that left inferior/middle frontal and left superior temporal activation stemmed from a combined difference in brain activity between control and experimental measures of phonology. Yet, some of the limitations of this study include a limited sample size along with a wide age range. The study also does not include children who speak languages other than English. Nevertheless, the convergence between present and past behavioral, neuroimaging and cross‐linguistic findings reinforce the idea that shared morphophonological and metalinguistic processes support children's emergent literacy across languages [Carlisle and Goodwyn, 2013; Frost, 2012; Pugh et al., 2013]. Another significant limitation is that this study only included tasks of morphological competence. Thus, while we find significant activation in regions thought to be specific to lexico‐semantic (ventral IFG) and not phonology‐specific [dorsal IFG or posterior STG; Katzev et al., 2013], further investigations that directly contrast experimental measures of morphology and phonology are necessary to adjudicate the specificity of shared and unique cognitive bases for morphology relative to phonology.
CONCLUSION
New theoretical perspectives suggest that by understanding morphological processes in addition to the typically considered phonological processes, we can better understand developmental mechanisms that give rise to reading acquisition across languages [Frost, 2012; Geva and Wang, 2001; Zhang et al., 2013]. This study aimed to shed light on the brain bases of morphological awareness in English‐speaking children. During the morphological awareness task, relative to control word matching task, children showed significant activation in brain regions typically reported as active during imaging tasks of word structure (aSTG) and word meaning (ventral IFG). These findings suggest that additional lexico‐semantic and grammatical processes are necessary to complete a morphological computation task, as compared to a simpler word matching control task. Moreover, the finding of significant correlations between children's morphological competence and brain activation in left middle frontal and supramarginal regions previously found active during phonological awareness tasks in Chinese and in English, respectively, suggests that the functioning of these regions might support multiple types of metalinguistic abilities necessary for learning to read across languages. Taken together, these findings pave the way for new insights for developing a comprehensive model of how spoken language abilities support children's reading acquisition across languages.
ACKNOWLEDGMENTS
The authors thank the University of Michigan Department of Psychology and Functional MRI Laboratory (NIH Grant No. 1S10OD012240‐01A1), participating families, and research assistants at the Language and Literacy Lab for their assistance with data collection. The first author thanks the National Science Foundation (NSF) Graduate Research Fellowship (Grant No. DGE 1256260); any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of NSF.
Conflict of interest: The authors confirm that this research complied with the University of Michigan ethical standards, and did not receive compensation from financial agreements or affiliations with any product or services.
REFERENCES
- Aylward EH, Richards TL, Berninger VW, Nagy WE, Field KM, Grimme AC, Richards AL, Thomson JB, Cramer SC (2003): Instructional treatment associated with changes in brain activation in children with dyslexia. Neurology 61:212–219. [DOI] [PubMed] [Google Scholar]
- Berninger VW, Abbott RD, Nagy W, Carlisle JF (2010): Growth in phonological, orthographic, and morphological awareness in grades 1 to 6. J Psycholinguist Res 39:141–163. [DOI] [PubMed] [Google Scholar]
- Bick A, Goelman G, Frost R (2008): Neural correlates of morphological processes in hebrew. J Cogn Neurosci 20:406–420. [DOI] [PubMed] [Google Scholar]
- Bolger DJ, Mina J, Burman DD, Booth JR (2008): Differential effects of orthographic and phonological consistency in cortex for children with and without reading impairment. Neuropsychologia 46:3210–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth JR, Lu D, Burman DD, Chou TL, Jin Z, Peng DL, Zhang L, Ding GS, Deng Y, Liu L (2006): Specialization of phonological and semantic processing in Chinese word reading. Brain Res 1071:197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozic M, Marslen‐Wilson WD, Stamatakis EA, Davis MH, Tyler LK (2007): Differentiating morphology, form, and meaning: neural correlates of morphological complexity. J Cogn Neurosci 19:1464–1475. [DOI] [PubMed] [Google Scholar]
- Bozic M, Tyler LK, Su L, Wingfield C, Marslen‐Wilson WD (2013): Neurobiological systems for lexical representation and analysis in english. J Cogn Neurosci 25:1678–1691. [DOI] [PubMed] [Google Scholar]
- Brennan C, Cao F, Pedroarena‐Leal N, McNorgan C, Booth JR (2012): Reading acquisition reorganizes the phonological awareness network only in alphabetic writing systems. Hum Brain Mapp 34:3354–3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R, Poline J (2002): Region of interest analysis using an SPM toolbox International Conference on Functional Mapping of the Human Brain, Sendai, Japan. [Google Scholar]
- Carlisle JF (2000): Awareness of the structure and meaning of morphologically complex words: Impact on reading. Read Writ 12:169–190. [Google Scholar]
- Carlisle JF, Goodwin AP (2013): Morphemes matter: How morphological knowledge contributes to reading and writing In: Stone CA, Silliman ER, Ehren BJ, Wallach GP, editors. Handbook of language and literacy: Development and disorders. New York: Guilford Press; pp 265–282. [Google Scholar]
- Cao F, Lee R, Shu H, Yang YH, Xu GQ, Li KC, Booth JR (2010): Cultural constraints on brain development: Evidence from a developmental study of visual word processing in mandarin chinese. Cereb Cortex 20:1223–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F, Khalid K, Lee R, Brennan C, Yang Y, Li K, Bolger DJ, Booth JR (2011): Development of brain networks involved in spoken word processing of mandarin chinese. NeuroImage 57:750–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coch G, Grossi G, Coffey‐Corina S, Holcomb PJ, Neville HJ (2002): A developmental investigation of the ERP auditory rhyming effect. Dev Sci 5:467–489. [Google Scholar]
- Coch G, Grossi G, Skendzel W, Neville HJ (2005): ERP nonword rhyming effects in children and adults. J Cogn Neurosci 17:168–182. [DOI] [PubMed] [Google Scholar]
- Deacon SH (2012): Sounds, letters and meanings: the independent influences of phonological, morphological and orthographic skills on early word reading accuracy. J Res Read 35:456–475. [Google Scholar]
- Deacon SH, Kirby JR (2004): Morphological awareness: just “more phonological”? the roles of morphological and phonological awareness in reading development. Appl Psycholinguist 25:223–238. [Google Scholar]
- Ehri LC (2014): Orthographic mapping in the acquisition of sight word reading, spelling memory, and vocabulary learning. Sci Stud Read 18:5–21. [Google Scholar]
- Elbro C, Arnbak E (1996): The role of morpheme recognition and morphological awareness in dyslexia. Ann Dyslexia 46:209–240. [DOI] [PubMed] [Google Scholar]
- Friederici AD (2002): Towards a neural basis of auditory sentence processing. Trends Cogn Sci 6:78–84. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Gierhan SME (2013): The language network. Curr Opin Neurobiol 23:250–254. [DOI] [PubMed] [Google Scholar]
- Frost R (2012): Towards a universal model of reading. Behav Brain Sci 35:263–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost R, Landi N, Mencl WE, Sandak R, Fulbright RK, Tejada ET, Jacobsen L, Grigorenko EL, Constable RT, Pugh KR (2009): Phonological awareness predicts activation patterns for print and speech. Ann Dyslexia 59:78–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geva E, Wang M (2001): The development of basic reading skills in children: a cross‐language perspective. Annu Rev Appl Linguist 21:182–204. [Google Scholar]
- Grossi G, Coch D, Coffey‐Corina S, Holcomb PJ, Neville HJ (2001): Phonological processing in a rhyming task: a developmental ERP study. J Cogn Neurosci 13:610–625. [DOI] [PubMed] [Google Scholar]
- Hoeft F, Hernandez A, McMillon G, Taylor‐Hill H, Martindale JL, Meyler A, Keller TA, Siok WT, Deutsch GK, Just MA, et al. (2006): Neural basis of dyslexia: a comparison between dyslexic and nondyslexic children equated for reading ability. J Neurosci 26:10700–10708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeft F, Meyler A, Hernandez A, Juel C, Taylor‐Hill H, Martindale JL, McMillon G, Kolchugina G, Black JM, Faizi A, GK Deutsch, WT Siok, AL Reiss, S Whitfield‐Gabrieli, JDE Gabrieli (2007): Functional and morphometric brain dissociation between dyslexia and reading ability. Proc Natl Acad Sci USA 104:4234–4239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfinger JB, Büchel C, Holmes AP, Friston KJ (2000): A study of analysis parameters that influence the sensitivity of event‐related fMRI analyses. Neuroimage 11:326–333. [DOI] [PubMed] [Google Scholar]
- Katzev M, Tüscher O, Hennig J, Weiller C, Kaller CP (2013): Revisiting the functional specialization of left inferior frontal gyrus in phonological and semantic fluency: The crucial role of task demands and individual ability. J Neurosci 33:7837–7845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman A, Kaufman N (2004): Kaufman Brief Intelligence Test, 2nd ed. Minneapolis, MN: NCS Pearson, Inc. [Google Scholar]
- Kovelman I, Norton ES, Christodoulou JA, Gaab N, Lieberman DA, Triantafyllou C, Wolf M, Whitfield‐Gabrieli S, Gabrieli JDE (2012): Brain basis of phonological awareness for spoken language in children and its disruption in dyslexia. Cerebral Cortex 22:754–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Tao R, Wang WJ, You WP, Peng DL, Booth JR (2013): Chinese dyslexics show neural differences in morphological processing. Dev Cogn Neurosci 6:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinova‐Todd SH, Siegel LS, Mazabel S (2013): The association between morphological awareness and literacy in English language learners from different language backgrounds. Top Lang Disord 22:93–107. [Google Scholar]
- McBride‐Chang C, Shu H, Zhou AB, Wat CP, Wagner RK (2003): Morphological awareness uniquely predicts young children's chinese character recognition. J Educ Psychol 95:743–751. [Google Scholar]
- McBride‐Chang C, Wagner RK, Muse A, Chow BWY, Shu H (2005): The role of morphological awareness in English. App Psycholingusit 26:415–435. [Google Scholar]
- McBride‐Chang C, Shu H, Chan W, Wong T, Wong AMY, Zhang YP, Pan J, Chan P (2013): Poor readers of Chinese and English: Overlap, stability, and longitudinal correlates. Sci Stud Read 17:57–70. [Google Scholar]
- Petitto LA, Zatorre RJ, Gauna K, Nikelski EJ, Dostie D, Evans AC (2000): Speech‐like cerebral activity in profoundly deaf people processing signed languages: Implications for the neural basis of human language. Proc Natl Acad Sci USA 97:13961–13966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfetti C, Cao F, Booth J (2013): Specialization and universals in the development of reading skill: How chinese research informs a universal science of reading. Sci Stud Read 17:5–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Mumford JA, Nichols TE (2011): Handbook of Functional MRI Data Analysis. New York, NY: Cambridge University Press. [Google Scholar]
- Pugh KR, Landi N, Preston JL, Mencl WE, Austin AC, Sibley D, Fulbright RK, Seidenberg MS, Grigorenko EL, Constable RT, P Molfese, SJ Frost (2013): The relationship between phonological and auditory processing and brain organization in beginning readers. Brain Lang 125:173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez G, Chen X, Geva E, Luo Y (2011): Morphological awareness and word reading in English language learners: evidence from Spanish‐ and Chinese‐speaking children. App Psycholingusit 32:601–618. [Google Scholar]
- Raschle NM, Zuk J, Gaab N (2012): Functional characteristics of developmental dyslexia in left‐hemispheric posterior brain regions predate reading onset. Proc Natl Acad Sci USA 109:2156–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman AA, Kirby JR, Parrila RK, Wade‐Woolley L, Deacon SH (2009): Towards a comprehensive view of the skills involved in word reading in grades 4, 6, and 8. J Exp Child Psychol 102:96–113. [DOI] [PubMed] [Google Scholar]
- Share DL (1999): Phonological recoding and orthographic learning: A direct test of the self‐teaching hypothesis. J Exp Child Psychol 72:95–129. [DOI] [PubMed] [Google Scholar]
- Siok WT, Perfetti CA, Jin Z, Tan LH (2004): Biological abnormality of impaired reading is constrained by culture. Nature 431:71–76. [DOI] [PubMed] [Google Scholar]
- Siok WT, Niu ZD, Jin Z, Perfetti CA, Tan LH (2008): A structural‐functional basis for dyslexia in the cortex of chinese readers. Proc Natl Acad Sci USA 105:5561–5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EE, Jonides J, Marshuetz C, Koeppe RA (1998): Components of verbal working memory: Evidence from neuroimaging. Proc Natl Acad Sci USA 95:876–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LH, Laird AR, Li K, Fox PT (2005): Neuroanatomical correlates of phonological processing of Chinese characters and alphabetic words: a meta‐analysis. Hum Brain Mapp 25:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Deacon SH, Kirby JR, Cain K, Parrila R (2011): Morphological awareness: A key to understanding poor reading comprehension in english. J Educ Psychol 103:523–234. [Google Scholar]
- Wagner RK, Torgesen JK, Rashotte CA (1999): Comprehensive Test of Phonological Processing. Austin, TX: PRO‐ED, Inc. [Google Scholar]
- Wolter JA, Wood A, D'zatko KW (2009): The influence of morphological awareness on the literacy development on first‐grade children. Lang Speech Hear Serv Sch 40:286–298. [DOI] [PubMed] [Google Scholar]
- Woodcock RW (1998): Woodcock Reading Mastery Tests – Revised/Normative Update. Minnesota: American Guidance Service. [Google Scholar]
- Zatorre RJ, Halpern AR, Perry DW, Meyer E, Evans AC (1996): Hearing in the mind's ear: A PET investigation of musical imagery and perception. J Cogn Neurosci 8:29–46. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Tardif TZ, Shu H, Li H, Liu H, McBride‐Chang C, Liang W, Zhang Z (2013): Phonological skills and vocabulary knowledge mediate socioeconomic status effects in predicting reading outcomes for Chinese children. Dev Psychol 49:665 [DOI] [PubMed] [Google Scholar]
- Ziegler JC, Goswami U (2005): Reading acquisition, developmental dyslexia, and skilled reading across languages: A psycholinguistic grain size theory. Psychol Bull 131:3–29. [DOI] [PubMed] [Google Scholar]


