Abstract
The Na/K-ATPase is an energy-transducing ion pump that converts the free energy of ATP into trans-membrane ion gradients. It also serves as a functional receptor for cardiotonic steroids such as ouabain and digoxin. Binding of ouabain to the Na/K-ATPase can activate calcium signaling in a cell-specific manner. The exquisite calcium modulation via the Na/K-ATPase is achieved by the ability of the pump to integrate signals from numerous protein and non-protein molecules including ion transporters, channels, protein kinases/phosphatases, as well as cellular Na+. This review focuses on the unique properties of the Na/K-ATPase and its role in the formation of different calcium signaling microdomains.
Introduction
The Na,K-ATPase, or sodium pump, is the molecular machine for ATP-dependent and -coupled transport of Na+ and K+ across the plasma membrane. The Na+ gradient produced by the Na/K-ATPase is the energy source for cellular uptake of many nutrients. It is also the major force for maintaining the balance of electrolytes and fluids at the whole body level (28). A functional Na/K-ATPase consists of two non-covalently linked α and β subunits (40, 75). An additional γ subunit also exists in some tissues as a regulator of Na/K-ATPase (76). Studies over the past decade have made several new advances in understanding the physiological functions of the Na/K-ATPase. These include: 1) identification of the molecular mechanism by which the Na/K-ATPase transduces ouabain binding to the activation of protein kinase cascades and the generation of second messengers such as inositol 1,4,5-trisphosphate (IP3) and calcium transients; 2) the demonstration of endogenous Na/K-ATPase ligands and their role in the development of hypertension and cardiac remodeling; 3) the involvement of Na/K-ATPase in control of cancer cell growth. These findings have been detailed in some recent reviews about Na/K-ATPase (5, 8, 68, 85). A noticeable fact in these studies is that involvement of protein-protein interactions rather than the pumping activity alone contributes to the regulatory effects of the Na/K-ATPase on cellular functions.
It is known that the plasma membrane is compartmentalized into structurally and functionally different microdomains. These microdomains may sequester specific proteins and lipids, while excluding others, to form a dynamic center for regulating cellular processes such as signal transduction, vesicular transport, and cargo delivery (50, 63, 71, 73). Although special lipid composition and protein markers have been used to classify different microdomains such as lipid rafts and caveolae (65), the formation of microdomains may be more functionally oriented. To this end, it is important to note that the calcium signaling microdomains have been the focus of investigation of many laboratories for decades. Interestingly, recent studies have identified several important protein interactions of the Na/K-ATPase and revealed that these protein interactions play a pivotal role in the formation of functionally distinct calcium signaling microdomains, which will be the focus of this mini-review.
Interaction between the Na/K-ATPase and the Na/Ca exchanger (NCX)
Calcium is a highly versatile intracellular signal and is responsible for regulating numerous cellular processes such as contraction, secretion, fertilization, proliferation and apoptosis. Consistently, cells have developed a series of mechanisms that exert an exquisite control of calcium signaling. This is achieved, at least partially, by the formation of different calcium signaling microdomains. For example, in cardiac muscle, T-tubular and sarcoplasmic reticulum (SR) membranes can form “diad junctions” that allow calcium to flow through the plasma membrane calcium channel, to bind and open the ryanodine receptor, and to trigger the subsequent larger calcium release from the SR and muscle contraction (27, 65). In neuronal cells, a high concentration of calcium was visualized in a size of ≈0.15 µm2 area at the presynaptic active zone (69). The sites in the cytoplasm for these compartmentalized high calcium concentration profiles are defined as “calcium concentration microdomains” (47). Biochemical studies reveal that the plasma membrane calcium channels are targeted to presynaptic sites by specific protein–protein interactions that involve both the intracellular and extracellular channel domains (12, 74). Disruption of the interaction between the synaptic protein interaction sites in the calcium channels with SNARE proteins reduces effectiveness of calcium release, providing evidence for the importance in the proximity of proteins involved in calcium movement in cells (12).
The Na/K-ATPase is known to be important in regulation of intracellular calcium. For example, binding of ouabain to the Na/K-ATPase raises intracellular calcium in cardiac myocytes, resulting in increases in myocardial contraction. This serves as a basis for using digitalis drugs to treat congestive heart failure. Mechanistically, the Na/K-ATPase w a s originally hypothesized to regulate calcium entry through the Na/Ca exchanger (NCX) by altering intracellular Na+ concentration in cardiac myocytes (2, 10, 42). Physical coupling among the Na/K-ATPase, NCX and SR calcium store was first demonstrated in smooth muscle cells (58). Blaustein and colleagues (39, 72) have recently provided further support to the notion that the Na/K-ATPase interacts with NCX to form a specific calcium signaling microdomain in many different cell types including smooth muscle cells and astrocytes. Specifically, they have proposed that the interaction between the NCX with either α2 or α3, but not α1, isoform of the Na/K-ATPase forms a signaling microdomain that is responsible for the ouabain-induced calcium increases in astrocytes and smooth muscle cells (39, 72). Moreover, they have identified that the N-termini of α2 and α3 isoforms contain a common structural motif that allows these isoforms to be targeted to the plasma membrane microdomains overlying the “junctional” SR/endoplasmic reticulum (ER) (72). The NCX activity in these microdomains is regulated by the local Na+ or Ca2+ concentrations, but the ion binding sites for the regulation are not the sites participating the ion transportation (19). Differential regulatory mechanisms were also found between the cardiac/neuronal specific NCX isoform and the kidney isoform (7, 22, 23).
The cardiac/neuronal specific NCX type-1 (NCX1) isoform consists of nine transmembrane (TM) helices with an extracellular N terminus and a cytosolic C terminus (49). An amphipathic sequence, located close by the intracellular end of TM5, is considered as an important site in regulation by Na+ and acidic phospholipids (19, 52). When ouabain binds to the Na/K-ATPase, an increase in Na+ near the calcium signaling microdomain may stimulate and then inactivate the NCX (33), thus regulating the local calcium concentration within or nearby the microdomain. Based on this hypothesis, Edwards and Pallone have recently formulated a mathematical model describing this microdomain-mediated Ca2+ signal transduction (24, 25). Remarkably, the mathematical simulation essentially recapitulates the experimental changes detected in response to ouabain binding to the α2 isoform (24, 25). It is important to note that this α2 and α3-specific interaction is most likely cell-specific because the α1 isoform was found to be equally capable of interacting with the NCX1 in cardiac myocytes (21). Moreover, this interaction was sufficient for ouabain to regulate calcium and then increase contractility in these cells.
Interaction between the Na/K-ATPase and the IP3 receptor
In addition to its effect on calcium entry through NCX, early studies had suggested that ouabain and other cardiotonic steroids might activate L-type Ca2+ channels and stimulate Ca2+ release from SR and/or ER (53). Consistently, several laboratories in recent years have reported a direct interaction between the Na/K-ATPase α subunit and IP3 receptors (IP3Rs) in the ER (1). IP3Rs are IP3-gated Ca2+ channels (64). In response to stimulation of G protein-coupled receptors or receptor-tyrosine kinases, either phospholipase C (PLC)-β or PLC-γ is recruited to the membrane and activated (66). The activated PLC in turn catalyzes the metabolism of phosphatidylinositol bisphosphate (PIP2), producing the second messenger IP3, and thus the opening of IP3Rs. Structurally, the IP3R contains a small pore-forming C-terminus and a large regulatory N-terminus. In fact, the N-terminus contains more than 2000 amino acid residues and was found to interact with ion channels, protein kinase/phosphatases, and structural proteins. These interactions not only make it possible for the targeting and regulation of receptor function via various protein kinase cascades, but also for regulating the function of the interacting proteins. For example, IP3Rs interact and keep the inositol 1,4,5-trisphosphate receptor-binding protein (IRBIT) in an inactive state. Binding of IP3 to the IP3Rs changes the conformation of the receptor, resulting in the release of IRBIT from IP3R and the subsequent activation of the Na+/HCO3 − co-transporter (4).
The interaction between the Na/K-ATPase and IP3R was first reported by Aperia’s laboratory during the investigation of ouabain-induced calcium oscillations in renal epithelial cells (1). Independently, we came to the same conclusion that there might be a pool of the Na/K-ATPase that directly interacts with IP3Rs in renal epithelial cells because these proteins could be co-purified from the kidney outer medulla (88). Further biochemical studies revealed that the N-termini of all α subunits were capable of interacting with the IP3Rs directly. Interestingly, co-immunoprecipitation analysis showed that the formation of Na/K-ATPase/ IP3R complex might be isoform-specific (43). While the α1 isoform co-precipitated with the IP3R in renal epithelial cells, only the α2 and α3, but not the α1, isoform were able to form the signaling complex in neurons and astrocytes. Functional analysis demonstrated that this interaction is required for the formation of Na/K-ATPase/IP3R signaling microdomain that may provide at least three different ways for cells to modulate the calcium signaling in response to extracellular stimuli (43).
It has been demonstrated that the interaction between Na/K-ATPase and the IP3R is essential for ouabain to stimulate low-frequency calcium oscillations in renal epithelial cells (1). In general, calcium signals can occur transiently or in an oscillatory manner. Calcium oscillations are the most versatile cell signals, because the cell can decode the frequency of oscillations (14, 20). Consistently, ouabain-induced calcium oscillations with a periodicity of 4–5 min were sufficient to activate the NF-κB pathway (35) and then protect the renal cells from serum starvation-induced apoptosis (61). Mechanistically, ouabain was found to stimulate calcium oscillations at concentrations that did not change intracellular Na+. Moreover, inhibition of the Na/K-ATPase by lowering extracellular K+ failed to elicit the same change in intracellular calcium, although it increased intracellular Na+. Interestingly, inhibition of PLC or addition of IP3 “sponge” did not block ouabain-induced calcium oscillations in renal epithelial cells (54). These findings led the authors to propose that ouabain-induced changes in the interaction between the Na/K-ATPase and IP3Rs may be sufficient to stimulate calcium release from the calcium store (54). Alternatively, these interactions may alter the gating properties of IP3R. It is important to mention that ouabain-induced calcium oscillations have also been found in cells other than renal epithelial cells (46, 67).
The formation and regulation of this microdomain may also involve another important component of Na/K-ATPase signaling pathway, the Na/K-ATPase/ Src complex (44, 78). The Na/K-ATPase and Src are enriched in caveolae of renal epithelial cells (83), and they directly interact with each other (78). More importantly, the Na/K-ATPase/Src complex serves as a receptor for ouabain to increase protein tyrosine phosphorylation and consequently stimulate protein kinase cascades and phospholipase (78). For example, activation of the Na/K-ATPase/Src receptor complex by ouabain transactivates EGF receptor and subsequently results in the activation Ras/ERK cascade. Moreover, activation of this receptor has been found to stimulate tyrosine phosphorylation of PLC-γ and the generation of IP3 (88). Because activation of this latter pathway plays an important role in regulation of IP3Rs, we examined whether the Na/K-ATPase/ Src complex is involved in the formation of a calcium signaling microdomain. GST pull-down assays have revealed that the central loop of the Na/K-ATPase α1 subunit actually interacts with PLC-γ whereas the N-terminus binds IP3Rs. These findings suggest that the Na/K-ATPase may be able to tether PLC and IP3Rs into a calcium-regulatory microdomain to facilitate the ouabain-activated signal transduction. In accordance, both PLC-γ and IP3Rs co-immunoprecipitated with the Na/K-ATPase/Src complex and ouabain increased formation of this signaling microdomain in a Src-dependent manner. Moreover, we found that activation of the Na/K-ATPase/Src receptor complex by ouabain stimulated tyrosine phosphorylation of IP3Rs. Functionally, activation of this Na/K-ATPase/Src/PLC-γ complex by ouabain led to the opening of IP3Rs and a rise in intracellular calcium. Inhibition of either Src or PLC was sufficient to block ouabain-induced calcium transients. Taken together, these findings indicate that the Na/K-ATPase functions as an important scaffold capable of bringing IP3Rs to their effector PLC-γ to facilitate calcium release in response to ouabain-induced activation of receptor Na/K-ATPase/Src complex.
It has been proposed that formation of the junctional microdomains that force the proximity of IP3Rs to the plasma membrane receptors provides a mechanism for defining spatially and temporally specific Ca2+ signaling in cells other than cardiac myocytes (6, 9, 16, 17, 34, 77, 82, 84, 87). For instance, the forced coupling between B2 bradykinin receptors and IP3Rs ensures a robust Ca2+ signaling when the receptor is activated by the ligand in neuronal cells (17). Several candidate proteins have been identified over the years that may play an important role in the formation of junctional microdomains. These include Homer, junctophilins and stromal interaction molecule 1 (STIM1) as well as ankyrin (38, 59, 70, 79). For example, the interaction between IP3Rs and transient receptor potential canonical 1 or 3 (TRPC1 or TRPC3) appears to be mediated via adaptor proteins Homer (87) or Junctate (79). It has been reported that the conserved EVH1 domain of Homer interacts with a proline-rich motif that is present in both group 1 metabotropic glutamate receptors (mGluRs) and IP3Rs. Disruption of the complex by short form Homer 1a results in an alteration of mGluR-induced Ca2+ release (80). The ER-associated protein, Junctate, is also found to bind with IP3R and TRPC3, and forms the TRPC3-Junctate-IP3Rs complex. This complex plays an important role both in Ca2+ release after IP3R activation and in calcium entry induced by store-depletion, perhaps by facilitating and/or stabilizing connections between the ER and the plasma membrane (79).
It is well documented that many G-protein-coupled receptors and receptor tyrosine kinases are concentrated in caveolae (3, 13). Because the Na/K-ATPase represents a highly abundant caveolar membrane protein, the identified interaction between the caveolar Na/K-ATPase and ER IP3Rs could contribute to formation of junctional microdomains by forcing the proximity of ER IP3Rs to other plasma membrane receptors. Thus, the interaction between the Na/K-ATPase and IP3Rs may not only be important for ouabain-induced ER Ca2+ release, but also plays a role in other stimuli-induced Ca2+ signaling. To test this proposal, we determined whether the Na/K-ATPase is important for the subcellular distribution of IP3Rs and ATP-induced ER calcium release. These investigations revealed that graded knockdown of cellular Na/K-ATPase α1 caused a parallel attenuation of ATP-induced ER calcium release. This defect could be rescued by knocking in a rat α1. Mechanistically, this defect was neither due to the changes in the amount or the function of cellular IP3 and P2Y receptors nor the ER calcium contents. On the other hand, α1 knockdown changed cellular distribution of IP3Rs. Specifically, it abolished a pool of IP3Rs that resided close to the plasma membrane. When dose-dependent effects of ATP on PKC activation and ER calcium release were determined, we found that the α1 knockdown de-sensitized the ATP-induced Ca2+ release, but not PKC activation. Moreover, expression of the N-terminus of the Na/K-ATPase α1 subunit, as expected, disrupted formation of the Na/K-ATPase/IP3R complex and attenuated ER Ca2+ release provoked by ATP. Finally, the α1 knockdown also reduced both angiotensin II and EGF-induced ER Ca2+ release. Taken together, these findings support the notion that interaction between the Na/K-ATPase and IP3Rs is important for ER calcium signaling emanated from activation of the receptor Na/K-ATPase/Src complex as well as several other PLC-coupled receptors.
Structural feature of the Na/K-ATPase-mediated protein interaction
The recently resolved crystal structure showed that the pig Na/K-ATPase α1 subunit, similar to the SERCA calcium ATPase, contains 3 major domains (60). The A domain consists of the N-terminus and the second cytoplasmic domain (CD2) connected to transmembrane helices M2 and M3. The enzyme also has the highly conserved phosphorylation (P) domain that is close to the membrane and a nucleotide binding (N) domain. Among them, A and N domains are extruded and more exposed, which is consistent with the fact that most of the binding motifs found in the α1 subunit reside in these two domains. The N domain of the Na/K-ATPase α1 subunit interacts with many proteins including Src and PLC-γ. These interactions are important for the receptor function of the Na/K-ATPase. Moreover, they provide spatial proximity between the generation of IP3 and opening of IP3R because the Na/K-ATPase/Src/PLC-γ complex also interacts with IP3Rs. Interestingly, the same N domain also interacts with arrestin 2 and spinophilin as well as structural proteins such as ankyrin (18, 41). The interaction with arrestin and spinophilin is important for intracellular trafficking of the Na/K-ATPase (41).
The A domain of the Na/K-ATPase is another site of the molecule that is involved in protein-protein interaction. For example, a three-amino acid sequence (LKK) at the N-terminus of the Na/K-ATPase is essential for binding to IP3R (90). Interestingly, Blaustein and colleagues have identified that L and A, flanking the LKK sequence, are important for targeting the α2 and the α3 to the NCX signaling microdomain in astrocytes (72). In addition, the A domain also interacts with the SH2 domain of Src, caveolin-1, PI3 kinase and ankyrin. These interactions are important for the signaling function of Na/K-ATPase. It is interesting to note that the Na/K-ATPase α1 subunit often contains two binding sites for the same partner proteins, such as ankyrin, Src and caveolin-1 (18, 78, 83).
While interaction with membrane transporters, channels, receptors, protein kinases and phosphatases constitutes formation of the aforementioned calcium signaling microdomains, interaction with structural proteins such as ankyrin, adducin, cofilin, and 14-3-3 protein may play an important role in stabilizing the microdomain structure (26, 29, 51, 86). For instance, the actin cytoskeleton is involved in coordinating interactions among IP3Rs, the Na/K-ATPase (54), and other membrane receptors such as the B2 bradykinin receptor (17). Consistently, disruption of cytoskeletal structure abolished ouabain-induced calcium oscillations in renal epithelial cells (1). Of many structural proteins, ankyrin seems to be very important for formation of the calcium signaling microdomains because it may use the Na/K-ATPase as an anchor to bridge the ER IP3Rs to the plasma membrane receptors or channels/transporters. The interaction between ankyrin and IP3Rs has also been well documented (11, 37). The 11 amino acid-sequence (GGVGDVLRKPS) located at the C-terminus of IP3R serves as the ankyrin binding site. Functionally, this direct and high affinity interaction between IP3R and ankyrin-B is critical for IP3R post-translational stability and localization in cultures of neonatal cardiac myocytes. Reduced accumulation and abnormal localization of IP3R were observed in cardiac myocytes of ankyrin B knock-out mice (56). Moreover, recent studies have shown that ankyrin-B links the ER IP3Rs to the plasma membrane Na/K-ATPase and NCX to form a functional calcium signaling domain in cardiac myocytes (81). Significantly, a loss-of-function (E1425G) mutation in ankyrin-B (also known as ankyrin 2) causes dominantly inherited type 4 long-QT cardiac arrhythmia in humans (55, 57). Finally, a similar protein complex has also been detected in cells other than cardiac myocytes (72).
In addition to ankyrin, caveolin-1 appears to be another important structural protein. It plays a role in targeting the Na/K-ATPase into caveolae (83). It is known that caveolae serve as an important calcium signaling microdomain (36). However, the plasma membrane delivery of the Na/K-ATPase does not require the presence of caveolin-1, since the cells from caveolin-1 knockout mice still exhibit surface Na/K-ATPase activity (our unpublished data). The mechanism of sorting Na/K-ATPase to the caveolae or microdomains remains unclear.
Besides protein-protein interaction, the lipids composition and their specific binding with membrane proteins are important for the formation of microdomains. Indeed, the Na/K-ATPase, as an ion pump, functions only when it resides in the membrane with proper composition of lipids. Removal of the phospholipids from the plasma membrane of cardiac cells led to almost complete inhibition of Na/K-ATPase activity (32). In addition to phospholipids, cholesterol was also found to regulate Na/K-ATPase activity. Na/K-ATPase activity in lens fibre cells was much lower than that in lens epithelial cells, which was closely correlated with cholesterol levels in these cells (15). Depletion of cholesterol from the cell membrane was found to induce biphasic response in Na/K-ATPase activity (30, 48). The mechanism of such regulation was considered as a result of increasing Na+ affinity by cholesterol (30). Finally, recent studies have shown that the signaling Na/K-ATPase resides in a cholesterol-enriched caveolae structure. Cholesterol depletion could disrupt the caveolae structure and diminish the signaling function of the Na/K-ATPase (45, 83).
Perspectives
Studies of the past ten years have identified many important protein interactions of the Na/K-ATPase. The interactions among the Na/K-ATPase, protein kinase, membrane transporters/channels and structural proteins ensure formation of dynamic and cell-specific calcium signaling microdomains. It is important to recognize that the aforementioned investigations only mark the beginning of a fascinating new field. Besides identification and functional characterization of new partners such as TRPCs, polycystin-1 and protein phosphatases (31, 62, 89), studies have to be conducted to understand the dynamics, regulation, isoform- and cell-specific aspects of these interactions among the Na/K-ATPase and its partners. Further efforts of many laboratories are clearly required. It is also important to recognize that we know little about how the ion transporting function of Na/K-ATPase is related with its signaling function in the regulation of these cell functions. However, the continual efforts will eventually provide insights into the newly appreciated functions of the Na/K-ATPase and their roles in cell biology and animal physiology.
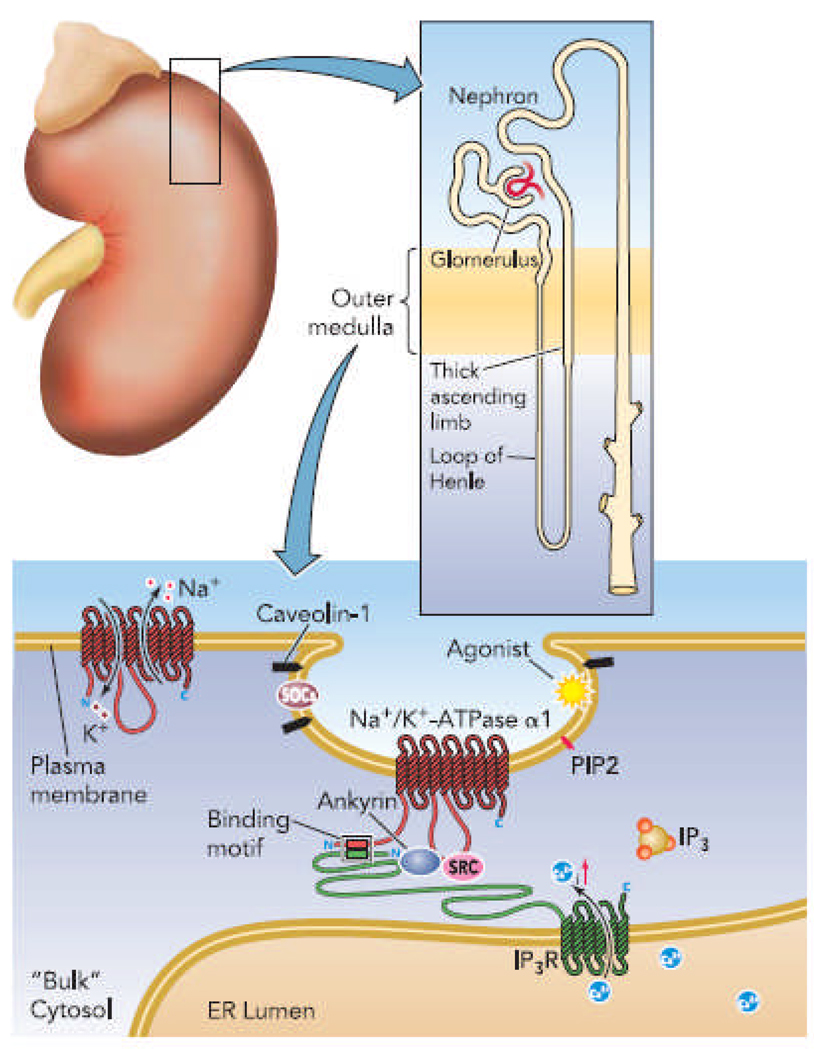
FIGURE 1.
The potential role of Na-K-ATPase in the formation of a junctional calcium-signaling microdomain in renal epithelial cells.
Acknowledgement
We thank Ms. Marta Heck for editing the manuscript. This work was supported by NIH grants HL-36573 and HL-67963 awarded by the National Heart, Lung and Blood Institute and NIH grant GM-78565 awarded by National Institute of General Medical Sciences.
References
- 1.Aizman O, Uhlen P, Lal M, Brismar H, Aperia A. Ouabain, a steroid hormone that signals with slow calcium oscillations. Proc Natl Acad Sci U S A. 2001;98:13420–13424. doi: 10.1073/pnas.221315298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akera T, Larsen FS, Brody TM. Correlation of cardiac sodium- and potassium-activated adenosine triphosphatase activity with ouabain-induced inotropic stimulation. J Pharmacol Exp Ther. 1970;173:145–151. [PubMed] [Google Scholar]
- 3.Anderson RG. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 4.Ando H, Mizutani A, Matsu-ura T, Mikoshiba K. IRBIT, a novel inositol 1,4,5-trisphosphate (IP3) receptor-binding protein, is released from the IP3 receptor upon IP3 binding to the receptor. J Biol Chem. 2003;278:10602–10612. doi: 10.1074/jbc.M210119200. [DOI] [PubMed] [Google Scholar]
- 5.Aperia A. New roles for an old enzyme: Na,K-ATPase emerges as an interesting drug target. J Intern Med. 2007;261:44–52. doi: 10.1111/j.1365-2796.2006.01745.x. [DOI] [PubMed] [Google Scholar]
- 6.Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. doi: 10.1016/s0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 7.Besserer GM, Ottolia M, Nicoll DA, Chaptal V, Cascio D, Philipson KD, Abramson J. The second Ca2+-binding domain of the Na+ Ca2+ exchanger is essential for regulation: crystal structures and mutational analysis. Proc Natl Acad Sci U S A. 2007;104:18467–18472. doi: 10.1073/pnas.0707417104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blaustein MP, Charpentier TH, Weber DJ. Getting a grip on calcium regulation. Proc Natl Acad Sci U S A. 2007;104:18349–18350. doi: 10.1073/pnas.0709008104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blaustein MP, Golovina VA. Structural complexity and functional diversity of endoplasmic reticulum Ca(2+) stores. Trends Neurosci. 2001;24:602–608. doi: 10.1016/s0166-2236(00)01891-9. [DOI] [PubMed] [Google Scholar]
- 10.Blaustein MP, Juhaszova M, Golovina VA. The cellular mechanism of action of cardiotonic steroids: a new hypothesis. Clin Exp Hypertens. 1998;20:691–703. doi: 10.3109/10641969809053247. [DOI] [PubMed] [Google Scholar]
- 11.Bourguignon LY, Jin H, Iida N, Brandt NR, Zhang SH. The involvement of ankyrin in the regulation of inositol 1,4,5-trisphosphate receptor-mediated internal Ca2+ release from Ca2+ storage vesicles in mouse T-lymphoma cells. J Biol Chem. 1993;268:7290–7297. [PubMed] [Google Scholar]
- 12.Catterall WA. Interactions of presynaptic Ca2+ channels and snare proteins in neurotransmitter release. Ann N Y Acad Sci. 1999;868:144–159. doi: 10.1111/j.1749-6632.1999.tb11284.x. [DOI] [PubMed] [Google Scholar]
- 13.Chini B, Parenti M. G-protein coupled receptors in lipid rafts and caveolae: how, when and why do they go there? J Mol Endocrinol. 2004;32:325–338. doi: 10.1677/jme.0.0320325. [DOI] [PubMed] [Google Scholar]
- 14.De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 1998;279:227–230. doi: 10.1126/science.279.5348.227. [DOI] [PubMed] [Google Scholar]
- 15.Dean WL, Delamere NA, Borchman D, Moseley AE, Ahuja RP. Studies on lipids and the activity of Na,K-ATPase in lens fibre cells. Biochem J. 1996;314(Pt 3):961–967. doi: 10.1042/bj3140961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delmas P, Brown DA. Junctional signaling microdomains: bridging the gap between the neuronal cell surface and Ca2+ stores. Neuron. 2002;36:787–790. doi: 10.1016/s0896-6273(02)01097-8. [DOI] [PubMed] [Google Scholar]
- 17.Delmas P, Wanaverbecq N, Abogadie FC, Mistry M, Brown DA. Signaling microdomains define the specificity of receptor-mediated InsP(3) pathways in neurons. Neuron. 2002;34:209–220. doi: 10.1016/s0896-6273(02)00641-4. [DOI] [PubMed] [Google Scholar]
- 18.Devarajan P, Scaramuzzino DA, Morrow JS. Ankyrin binds to two distinct cytoplasmic domains of Na,K-ATPase alpha subunit. Proc Natl Acad Sci U S A. 1994;91:2965–2969. doi: 10.1073/pnas.91.8.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DiPolo R, Beauge L. Sodium/calcium exchanger: influence of metabolic regulation on ion carrier interactions. Physiol Rev. 2006;86:155–203. doi: 10.1152/physrev.00018.2005. [DOI] [PubMed] [Google Scholar]
- 20.Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 21.Dostanic I, Schultz Jel J, Lorenz JN, Lingrel JB. The alpha 1 isoform of Na,K-ATPase regulates cardiac contractility and functionally interacts and co-localizes with the Na/Ca exchanger in heart. J Biol Chem. 2004;279:54053–54061. doi: 10.1074/jbc.M410737200. [DOI] [PubMed] [Google Scholar]
- 22.Dunn J, Elias CL, Le HD, Omelchenko A, Hryshko LV, Lytton J. The molecular determinants of ionic regulatory differences between brain and kidney Na+/Ca2+ exchanger (NCX1) isoforms. J Biol Chem. 2002;277:33957–33962. doi: 10.1074/jbc.M206677200. [DOI] [PubMed] [Google Scholar]
- 23.Dyck C, Omelchenko A, Elias CL, Quednau BD, Philipson KD, Hnatowich M, Hryshko LV. Ionic regulatory properties of brain and kidney splice variants of the NCX1 Na(+)-Ca(2+) exchanger. J Gen Physiol. 1999;114:701–711. doi: 10.1085/jgp.114.5.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edwards A, Pallone TL. Modification of cytosolic calcium signaling by subplasmalemmal microdomains. Am J Physiol Renal Physiol. 2007;292:F1827–F1845. doi: 10.1152/ajprenal.00387.2006. [DOI] [PubMed] [Google Scholar]
- 25.Edwards A, Pallone TL. Ouabain modulation of cellular calcium stores and signaling. Am J Physiol Renal Physiol. 2007;293:F1518–F1532. doi: 10.1152/ajprenal.00251.2007. [DOI] [PubMed] [Google Scholar]
- 26.Efendiev R, Chen Z, Krmar RT, Uhles S, Katz AI, Pedemonte CH, Bertorello AM. The 14-3-3 protein translates the NA+,K+-ATPase {alpha}1-subunit phosphorylation signal into binding and activation of phosphoinositide 3-kinase during endocytosis. J Biol Chem. 2005;280:16272–16277. doi: 10.1074/jbc.M500486200. [DOI] [PubMed] [Google Scholar]
- 27.Endo M, Tanaka M, Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970;228:34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- 28.Feraille E, Doucet A. Sodium-potassium-adenosinetriphosphatase-dependent sodium transport in the kidney: hormonal control. Physiol Rev. 2001;81:345–418. doi: 10.1152/physrev.2001.81.1.345. [DOI] [PubMed] [Google Scholar]
- 29.Ferrandi M, Salardi S, Tripodi G, Barassi P, Rivera R, Manunta P, Goldshleger R, Ferrari P, Bianchi G, Karlish SJ. Evidence for an interaction between adducin and Na(+)-K(+)-ATPase: relation to genetic hypertension. Am J Physiol. 1999;277:H1338–H1349. doi: 10.1152/ajpheart.1999.277.4.H1338. [DOI] [PubMed] [Google Scholar]
- 30.Giraud F, Claret M, Garay R. Interactions of cholesterol with the Na pump in red blood cells. Nature. 1976;264:646–648. doi: 10.1038/264646a0. [DOI] [PubMed] [Google Scholar]
- 31.Goel M, Sinkins W, Keightley A, Kinter M, Schilling WP. Proteomic analysis of TRPC5- and TRPC6-binding partners reveals interaction with the plasmalemmal Na(+)/K(+)-ATPase. Pflugers Arch. 2005;451:87–98. doi: 10.1007/s00424-005-1454-y. [DOI] [PubMed] [Google Scholar]
- 32.Hegyvary C, Chigurupati R, Kang K, Mahoney D. Reversible alterations in the kinetics of cardiac sodium- and potassium-activated adenosine triphosphatase after partial removal of membrane lipids. J Biol Chem. 1980;255:3068–3074. [PubMed] [Google Scholar]
- 33.Hilgemann DW, Matsuoka S, Nagel GA, Collins A. Steady-state and dynamic properties of cardiac sodium-calcium exchange. Sodium-dependent inactivation. J Gen Physiol. 1992;100:905–932. doi: 10.1085/jgp.100.6.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hisatsune C, Mikoshiba K. Novel compartment implicated in calcium signaling--is it an "induced coupling domain"? Sci STKE. 2005;2005:pe53. doi: 10.1126/stke.3132005pe53. [DOI] [PubMed] [Google Scholar]
- 35.Hu Q, Deshpande S, Irani K, Ziegelstein RC. [Ca(2+)](i) oscillation frequency regulates agonist-stimulated NF-kappaB transcriptional activity. J Biol Chem. 1999;274:33995–33998. doi: 10.1074/jbc.274.48.33995. [DOI] [PubMed] [Google Scholar]
- 36.Isshiki M, Anderson RG. Calcium signal transduction from caveolae. Cell Calcium. 1999;26:201–208. doi: 10.1054/ceca.1999.0073. [DOI] [PubMed] [Google Scholar]
- 37.Joseph SK, Samanta S. Detergent solubility of the inositol trisphosphate receptor in rat brain membranes. Evidence for association of the receptor with ankyrin. J Biol Chem. 1993;268:6477–6486. [PubMed] [Google Scholar]
- 38.Jousset H, Frieden M, Demaurex N. STIM1 knockdown reveals that store-operated Ca2+ channels located close to sarco/endoplasmic Ca2+ ATPases (SERCA) pumps silently refill the endoplasmic reticulum. J Biol Chem. 2007;282:11456–11464. doi: 10.1074/jbc.M609551200. [DOI] [PubMed] [Google Scholar]
- 39.Juhaszova M, Blaustein MP. Na+ pump low and high ouabain affinity alpha subunit isoforms are differently distributed in cells. Proc Natl Acad Sci U S A. 1997;94:1800–1805. doi: 10.1073/pnas.94.5.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaplan JH. Biochemistry of Na,K-ATPase. Annu Rev Biochem. 2002;71:511–535. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- 41.Kimura T, Allen PB, Nairn AC, Caplan MJ. Arrestins and spinophilin competitively regulate Na+,K+-ATPase trafficking through association with a large cytoplasmic loop of the Na+,K+-ATPase. Mol Biol Cell. 2007;18:4508–4518. doi: 10.1091/mbc.E06-08-0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Langer GA. Effects of digitalis on myocardial ionic exchange. Circulation. 1972;46:180–187. doi: 10.1161/01.cir.46.1.180. [DOI] [PubMed] [Google Scholar]
- 43.Lencesova L, O'Neill A, Resneck WG, Bloch RJ, Blaustein MP. Plasma membrane-cytoskeleton-endoplasmic reticulum complexes in neurons and astrocytes. J Biol Chem. 2004;279:2885–2893. doi: 10.1074/jbc.M310365200. [DOI] [PubMed] [Google Scholar]
- 44.Li Z, Xie Z. The Na/K-ATPase/Src complex and cardiotonic steroid-activated protein kinase cascades. Pflugers Arch. 2008 doi: 10.1007/s00424-008-0470-0. [DOI] [PubMed] [Google Scholar]
- 45.Liu L, Mohammadi K, Aynafshar B, Wang H, Li D, Liu J, Ivanov AV, Xie Z, Askari A. Role of caveolae in signal-transducing function of cardiac Na+/K+-ATPase. Am J Physiol Cell Physiol. 2003;284:C1550–C1560. doi: 10.1152/ajpcell.00555.2002. [DOI] [PubMed] [Google Scholar]
- 46.Liu XL, Miyakawa A, Aperia A, Krieger P. Na,K-ATPase generates calcium oscillations in hippocampal astrocytes. Neuroreport. 2007;18:597–600. doi: 10.1097/WNR.0b013e3280b07bc9. [DOI] [PubMed] [Google Scholar]
- 47.Llinas R, Sugimori M, Silver RB. Microdomains of high calcium concentration in a presynaptic terminal. Science. 1992;256:677–679. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- 48.Lucio FJ, Hendry BM, Ellory JC. The effects of cholesterol depletion on the sodium pump in human red cells. Exp Physiol. 1991;76:437–443. doi: 10.1113/expphysiol.1991.sp003510. [DOI] [PubMed] [Google Scholar]
- 49.Lytton J. Na+/Ca2+ exchangers: three mammalian gene families control Ca2+ transport. Biochem J. 2007;406:365–382. doi: 10.1042/BJ20070619. [DOI] [PubMed] [Google Scholar]
- 50.Manes S, del Real G, Martinez AC. Pathogens: raft hijackers. Nat Rev Immunol. 2003;3:557–568. doi: 10.1038/nri1129. [DOI] [PubMed] [Google Scholar]
- 51.Manunta P, Ferrandi M. Different effects of marinobufagenin and endogenous ouabain. J Hypertens. 2004;22:257–259. doi: 10.1097/00004872-200402000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Matsuoka S, Nicoll DA, He Z, Philipson KD. Regulation of cardiac Na(+)-Ca2+ exchanger by the endogenous XIP region. J Gen Physiol. 1997;109:273–286. doi: 10.1085/jgp.109.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGarry SJ, Williams AJ. Digoxin activates sarcoplasmic reticulum Ca(2+)-release channels: a possible role in cardiac inotropy. Br J Pharmacol. 1993;108:1043–1050. doi: 10.1111/j.1476-5381.1993.tb13503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Miyakawa-Naito A, Uhlen P, Lal M, Aizman O, Mikoshiba K, Brismar H, Zelenin S, Aperia A. Cell signaling microdomain with Na,K-ATPase and inositol 1,4,5-trisphosphate receptor generates calcium oscillations. J Biol Chem. 2003;278:50355–50361. doi: 10.1074/jbc.M305378200. [DOI] [PubMed] [Google Scholar]
- 55.Mohler PJ, Davis JQ, Bennett V. Ankyrin-B coordinates the Na/K ATPase, Na/Ca exchanger, and InsP3 receptor in a cardiac T-tubule/SR microdomain. PLoS Biol. 2005;3:e423. doi: 10.1371/journal.pbio.0030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mohler PJ, Davis JQ, Davis LH, Hoffman JA, Michaely P, Bennett V. Inositol 1,4,5-trisphosphate receptor localization and stability in neonatal cardiomyocytes requires interaction with ankyrin-B. J Biol Chem. 2004;279:12980–12987. doi: 10.1074/jbc.M313979200. [DOI] [PubMed] [Google Scholar]
- 57.Mohler PJ, Schott JJ, Gramolini AO, Dilly KW, Guatimosim S, duBell WH, Song LS, Haurogne K, Kyndt F, Ali ME, Rogers TB, Lederer WJ, Escande D, Le Marec H, Bennett V. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003;421:634–639. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 58.Moore ED, Etter EF, Philipson KD, Carrington WA, Fogarty KE, Lifshitz LM, Fay FS. Coupling of the Na+/Ca2+ exchanger, Na+/K+ pump and sarcoplasmic reticulum in smooth muscle. Nature. 1993;365:657–660. doi: 10.1038/365657a0. [DOI] [PubMed] [Google Scholar]
- 59.Moriguchi S, Nishi M, Komazaki S, Sakagami H, Miyazaki T, Masumiya H, Saito SY, Watanabe M, Kondo H, Yawo H, Fukunaga K, Takeshima H. Functional uncoupling between Ca2+ release and afterhyperpolarization in mutant hippocampal neurons lacking junctophilins. Proc Natl Acad Sci U S Aq. 2006;103:10811–10816. doi: 10.1073/pnas.0509863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morth JP, Pedersen BP, Toustrup-Jensen MS, Sorensen TL, Petersen J, Andersen JP, Vilsen B, Nissen P. Crystal structure of the sodium-potassium pump. Nature. 2007;450:1043–1049. doi: 10.1038/nature06419. [DOI] [PubMed] [Google Scholar]
- 61.Nakanishi C, Toi M. Nuclear factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat Rev Cancer. 2005;5:297–309. doi: 10.1038/nrc1588. [DOI] [PubMed] [Google Scholar]
- 62.Pagel P, Zatti A, Kimura T, Duffield A, Chauvet V, Rajendran V, Caplan MJ. Ion pump-interacting proteins: promising new partners. Ann N Y Acad Sci. 2003;986:360–368. doi: 10.1111/j.1749-6632.2003.tb07215.x. [DOI] [PubMed] [Google Scholar]
- 63.Parton RG. Caveolae--from ultrastructure to molecular mechanisms. Nat Rev Mol Cell Biol. 2003;4:162–167. doi: 10.1038/nrm1017. [DOI] [PubMed] [Google Scholar]
- 64.Patterson RL, Boehning D, Snyder SH. Inositol 1,4,5-trisphosphate receptors as signal integrators. Annu Rev Biochem. 2004;73:437–465. doi: 10.1146/annurev.biochem.73.071403.161303. [DOI] [PubMed] [Google Scholar]
- 65.Rayns DG, Simpson FO, Bertaud WS. Surface features of striated muscle. I. Guinea-pig cardiac muscle. J Cell Sci. 1968;3:467–474. doi: 10.1242/jcs.3.4.467. [DOI] [PubMed] [Google Scholar]
- 66.Rebecchi MJ, Pentyala SN. Structure, function, and control of phosphoinositide-specific phospholipase C. Physiol Rev. 2000;80:1291–1335. doi: 10.1152/physrev.2000.80.4.1291. [DOI] [PubMed] [Google Scholar]
- 67.Saunders R, Scheiner-Bobis G. Ouabain stimulates endothelin release and expression in human endothelial cells without inhibiting the sodium pump. Eur J Biochem. 2004;271:1054–1062. doi: 10.1111/j.1432-1033.2004.04012.x. [DOI] [PubMed] [Google Scholar]
- 68.Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides: their roles in hypertension, salt metabolism, and cell growth. Am J Physiol Cell Physiol. 2007;293:C509–C536. doi: 10.1152/ajpcell.00098.2007. [DOI] [PubMed] [Google Scholar]
- 69.Serulle Y, Sugimori M, Llinas RR. Imaging synaptosomal calcium concentration microdomains and vesicle fusion by using total internal reflection fluorescent microscopy. Proc Natl Acad Sci U S A. 2007;104:1697–1702. doi: 10.1073/pnas.0610741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sgambato-Faure V, Xiong Y, Berke JD, Hyman SE, Strehler EE. The Homer-1 protein Ania-3 interacts with the plasma membrane calcium pump. Biochem Biophys Res Commun. 2006;343:630–637. doi: 10.1016/j.bbrc.2006.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 72.Song H, Lee MY, Kinsey SP, Weber DJ, Blaustein MP. An N-terminal sequence targets and tethers Na+ pump alpha2 subunits to specialized plasma membrane microdomains. J Biol Chem. 2006;281:12929–12940. doi: 10.1074/jbc.M507450200. [DOI] [PubMed] [Google Scholar]
- 73.Sprenger RR, Fontijn RD, van Marle J, Pannekoek H, Horrevoets AJ. Spatial segregation of transport and signalling functions between human endothelial caveolae and lipid raft proteomes. Biochem J. 2006;400:401–410. doi: 10.1042/BJ20060355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sunderland WJ, Son YJ, Miner JH, Sanes JR, Carlson SS. The presynaptic calcium channel is part of a transmembrane complex linking a synaptic laminin (alpha4beta2gamma1) with non-erythroid spectrin. J Neurosci. 2000;20:1009–1019. doi: 10.1523/JNEUROSCI.20-03-01009.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sweadner KJ. Isozymes of the Na+/K+-ATPase. Biochim Biophys Acta. 1989;988:185–220. doi: 10.1016/0304-4157(89)90019-1. [DOI] [PubMed] [Google Scholar]
- 76.Sweadner KJ, Arystarkhova E, Donnet C, Wetzel RK. FXYD proteins as regulators of the Na,K-ATPase in the kidney. Ann N Y Acad Sci. 2003;986:382–387. doi: 10.1111/j.1749-6632.2003.tb07218.x. [DOI] [PubMed] [Google Scholar]
- 77.Takeshima H, Komazaki S, Nishi M, Iino M, Kangawa K. Junctophilins: a novel family of junctional membrane complex proteins. Mol Cell. 2000;6:11–22. doi: 10.1016/s1097-2765(00)00003-4. [DOI] [PubMed] [Google Scholar]
- 78.Tian J, Cai T, Yuan Z, Wang H, Liu L, Haas M, Maksimova E, Huang XY, Xie ZJ. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol Biol Cell. 2006;17:317–326. doi: 10.1091/mbc.E05-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Treves S, Franzini-Armstrong C, Moccagatta L, Arnoult C, Grasso C, Schrum A, Ducreux S, Zhu MX, Mikoshiba K, Girard T, Smida-Rezgui S, Ronjat M, Zorzato F. Junctate is a key element in calcium entry induced by activation of InsP3 receptors and/or calcium store depletion. J Cell Biol. 2004;166:537–548. doi: 10.1083/jcb.200404079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–726. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- 81.Tuvia S, Buhusi M, Davis L, Reedy M, Bennett V. Ankyrin-B is required for intracellular sorting of structurally diverse Ca2+ homeostasis proteins. J Cell Biol. 1999;147:995–1008. doi: 10.1083/jcb.147.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vermassen E, Parys JB, Mauger JP. Subcellular distribution of the inositol 1,4,5-trisphosphate receptors: functional relevance and molecular determinants. Biol Cell. 2004;96:3–17. doi: 10.1016/j.biolcel.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 83.Wang H, Haas M, Liang M, Cai T, Tian J, Li S, Xie Z. Ouabain assembles signaling cascades through the caveolar Na+/K+-ATPase. J Biol Chem. 2004;279:17250–17259. doi: 10.1074/jbc.M313239200. [DOI] [PubMed] [Google Scholar]
- 84.Wu MM, Buchanan J, Luik RM, Lewis RS. Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol. 2006;174:803–813. doi: 10.1083/jcb.200604014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xie Z, Xie J. The Na/K-ATPase-mediated signal transduction as a target for new drug development. Front Biosci. 2005;10:3100–3109. doi: 10.2741/1766. [DOI] [PubMed] [Google Scholar]
- 86.Yonezawa N, Nishida E, Sakai H. pH control of actin polymerization by cofilin. J Biol Chem. 1985;260:14410–14412. [PubMed] [Google Scholar]
- 87.Yuan JP, Kiselyov K, Shin DM, Chen J, Shcheynikov N, Kang SH, Dehoff MH, Schwarz MK, Seeburg PH, Muallem S, Worley PF. Homer binds TRPC family channels and is required for gating of TRPC1 by IP3 receptors. Cell. 2003;114:777–789. doi: 10.1016/s0092-8674(03)00716-5. [DOI] [PubMed] [Google Scholar]
- 88.Yuan Z, Cai T, Tian J, Ivanov AV, Giovannucci DR, Xie Z. Na/K-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex. Mol Biol Cell. 2005;16:4034–4045. doi: 10.1091/mbc.E05-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zatti A, Chauvet V, Rajendran V, Kimura T, Pagel P, Caplan MJ. The C-terminal tail of the polycystin-1 protein interacts with the Na,K-ATPase alpha-subunit. Mol Biol Cell. 2005;16:5087–5093. doi: 10.1091/mbc.E05-03-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang S, Malmersjo S, Li J, Ando H, Aizman O, Uhlen P, Mikoshiba K, Aperia A. Distinct role of the N-terminal tail of the Na,K-ATPase catalytic subunit as a signal transducer. J Biol Chem. 2006;281:21954–21962. doi: 10.1074/jbc.M601578200. [DOI] [PubMed] [Google Scholar]