Abstract
Glial cells play a critical role in neuronal support which includes the production and release of the neurotrophin brain-derived neurotrophic factor (BDNF). Activation of the sigma-1 receptor (S1R) has been shown to attenuate inflammatory stress-mediated brain injuries, and there is emerging evidence that this may involve a BDNF-dependent mechanism. In this report we studied S1R-mediated BDNF release from human astrocytic glial cells. Astrocytes express the S1R, which mediates BDNF release when stimulated with the prototypical S1R agonists 4-PPBP and (+)-SKF10047. This effect could be antagonized by a selective concentration of the S1R antagonist BD1063. Haloperidol is known to have high affinity interactions with the S1R, yet it was unable to facilitate BDNF release. Remarkably, however, two metabolites of haloperidol, haloperidol I and haloperidol II (reduced haloperidol), were discovered to facilitate BDNF secretion and this effect was antagonized by BD1063. Neither 4-PPBP, nor either of the haloperidol metabolites affected the level of BDNF mRNA as assessed by qPCR. These results demonstrate for the first time that haloperidol metabolites I and II facilitate the secretion of BDNF from astrocytes by acting as functionally selective S1R agonists.
Keywords: Astrocytes, Sigma receptor, BDNF, Neurotrophin, in situ ELISA
1. Introduction
The Sigma-1 receptor (S1R) is a stress and ligand-regulated, endoplasmic reticulum chaperone protein that shuttles lipids and proteins to the plasma membrane (Su et al., 2010). Its ability to modulate the actions of neurotransmitter receptors, ion channels and kinases helps to explain its involvement in the regulation of diverse processes such as neuroprotection, neurorestoration, neuroplasticity, and the release of neurotransmitters (Kourrich et al., 2012; Ruscher and Wieloch, 2015; Su et al., 2010; Zheng, 2009). The S1R is widely distributed throughout organs of the body and in the CNS (Walker et al., 1990). High densities are found in brain tissue, including the prefrontal and parietal cortex and various limbic structures such as the olfactory bulb, the hypothalamus, and the hippocampus (Alonso et al., 2000; Hashimoto et al., 1995). Within the tissues of the nervous system, the S1R is located predominantly in the gray matter, including neurons (Alonso et al., 2000; Klette et al., 1995; Peviani et al., 2014) and a variety of glial cell types: astrocytes, microglia, Müller cells, oligodendrocytes and Schwann cells (Gekker et al., 2006; Hayashi and Su, 2004; Jiang et al., 2006; Palacios et al., 2003; Palacios et al., 2004; Peviani et al., 2014; Robson et al., 2014).
Numerous primary culture studies have demonstrated that different types of glial cells are capable of releasing brain-derived neurotrophic factor (BDNF) in response to neurotransmitter stimulation or inflammatory stress. This includes oligodendrocytes (Bagayogo and Dreyfus, 2009), enteric glia cells (Hansebout et al., 2012), Müller cells (Taylor et al., 2003), astrocytes (Bejot et al., 2011; Chen et al., 2015a; Inoue et al., 1997; Jean et al., 2008; Miklic et al., 2004; Qu et al., 2010; Toyomoto et al., 2004; Wu et al., 2004; Zhang et al., 2012), and activated microglia (Nakajima et al., 2001; Sun et al., 2014; Trang et al., 2009; Yang et al., 2012). Increased levels of BDNF protein have been reported following chronic administration of S1R agonists, though these in vivo studies were not designed to evaluate cell types or secretion, but instead intracellular tissue levels of BDNF which likely reflects an increase in BDNF expression (Kikuchi-Utsumi and Nakaki, 2008; Peviani et al., 2014; Ring and Regan, 2013). Recently, a novel S1R agonist was shown to stimulate BDNF release from astrocytes derived from rat primary cortical cultures (Malik et al., 2015) suggesting that the S1R may facilitate secretion of BDNF. To probe this possibility further we sought to develop a robust, higher throughput cellular model for evaluating S1R-mediated BDNF secretion from human-derived astroglia. The human astrocytic CCF-STTG1 cell line (Barna et al., 1985) was investigated for this purpose because these cells are noted for their similarity to native astrocytes (Mentz et al., 1999), and, like primary astrocyte cultures, they had been reported to release BDNF when stimulated with prostaglandins thus providing us with a convenient positive assay control for BDNF secretion (Hutchinson et al., 2009; Toyomoto et al., 2004). An in situ ELISA approach (Balkowiec and Katz, 2000) was adopted in order to maximize the sensitivity and reproducibility of BDNF detection in a 96-well format.
Here we report for the first time that the human-derived astroglia express the S1R and facilitate BDNF secretion from these cells by activating this receptor. The S1R-mediated increase in extracellular mature BDNF occurred in the absence of any change in BDNF steady state mRNA levels. Remarkably, the two S1R-binding metabolites of haloperidol, known as reduced haloperidol and haloperidol metabolite I, were discovered to function as S1R agonists in the context of BDNF secretion, even though the parent compound haloperidol, which is a well-known S1R antagonist, does not.
2. Materials and Methods
2.1. Chemicals and Reagents
Compounds were purchased from the following sources: BD1063, 4-PPBP, and PF-04418948 from Tocris Biosciences (Minneapolis, MN); Haloperidol from Santa Cruz Biotechnologies, Inc. (Dallas, TX); Haloperidol metabolites I and II (4-(4-Chlorophenyl)-4-hydroxypiperidine and (±)-4-(4-Chlorophenyl)-α-(4-fluorophenyl)-4-hydroxy-1-piperidinebutanol, respectively) from Sigma-Aldrich (St. Louis, MO); Prostaglandin E2 (PGE2) from Cayman Chemical (Ann Arbor, MI). Radioligands were purchased from PerkinElmer: ([3H]-(+)-pentazocine ((+)-Pentazocine, [RING-1,3-3H], 33.9 Ci/mmol, NET1056), [3H]DTG (1,3-Di-o-tolylguanidine, [p-RING-3H]-, 50 Ci/mmol, NET986) (Saint Louis, MO). With the exception of PGE2, which was dissolved in ethanol:water (1:1 v/v), stock concentrations of all compounds were prepared in DMSO at concentrations ranging from 10–100 mM. Stocks were then diluted 1:1000 (v/v) in the final assay solution. ProBDNF was from Alomone Labs (Cat. No. B-257, Jerusalem, Israel) and the source of the mature BDNF was from the BDNF standard provided in the Emax ImmunoAssay kit (Cat. No. G7611, Promega, Madison, WI).
2.2. Cell culture
Human MCF-7 cells (American Type Cell Culture, Manassas, VA) were grown in Dulbecco’s Modified Eagle’s Medium (DMEM; Fisher Scientific, Pittsburgh, PA) supplemented with 10% Fetal Bovine Serum (FBS, Fisher Scientific, Pittsburgh, PA), 100 μg/ml nonessential amino acids (Hyclone, Logan, UT), 2 mM L-glutamine (Sigma Sigma-Aldrich, St. Louis, MO), and 10 μg/L Bovine Insulin (Sigma-Aldrich, Sigma-Aldrich, St. Louis, MO). MCF-7 cells served as the source of human Sigma-2 receptors (S2R) as they express the S2R but lack detectable levels of the S1R (Schetz et al., 2007; Vilner et al., 1995). MCF-7 cells stably expressing the human S1R (MCF-7-S1R) were prepared as described previously (Schetz et al., 2007) and kept under constant selective pressure with 100 μg/mL G-418 (Invivogen, San Diego, CA). The human astrocytic CCF-STTG1 and U87MG cell lines were purchased from the European Collection of Cell Cultures via Sigma-Aldrich (St. Louis, MO). CCF-STTG1 cells were cultured in RPMI 1640 supplemented with 10% (v/v) FBS (35), while U87MG cells were cultured in DMEM supplemented with 10% (v/v) FBS and non-essential amino acids (Corning, Manassas, VA). The microglial BV-2 cell line was provided as a gift from Elisabetta Blasi at the University of Perugia (Genoa, Italy) via Dr. Linda J. Van Eldik at the University of Kentucky (Lexington, KY) and were grown in DMEM:F12 (1:1) supplemented with 10% (v/v) FBS (Blasi et al., 1990). All culture media were additionally supplemented by 100 IU/mL of penicillin and streptomycin (Corning, Manassas, VA) and 1 mM sodium pyruvate (Sigma-Aldrich, St. Louis, MO), and grown at 37 °C under 95% air, 95% humidity, and 5% CO2.
2.3. Measurement of BDNF secretion via in situ ELISA
The amount of BDNF secreted from the glial cells was quantified using an in situ ELISA assay developed using the BDNF Emax ImmunoAssay kit (Cat. No. G7611, Promega, Madison, WI). Briefly, a Nunc MaxiSorp flat-bottom, polystyrene, 96-well immunoplate was coated for 48 hrs at 4°C with an anti-BDNF monoclonal antibody diluted 1:1000 v/v in carbonate buffer containing 25 mM sodium bicarbonate and 25 mM sodium carbonate, pH 9.7. Unbound antibody was removed by washing 5 times with 150 μL of TBST buffer (20 mM Tris-HCl, pH7.6, 150 mM NaCl, and 0.05% (v/v) Tween 20), before blocking non-specific sites first with blocking buffer for 1 hr and then with culture medium for 2 hrs. Glial cells were seeded at 10,000 cells per well and incubated overnight at 37°C in a humidified CO2 incubator. The following day, the wells were replaced with fresh culture media containing either experimental compounds or vehicle controls, then incubated for an additional 3 days. In addition, on the same plate, but in separate wells, BDNF standards were added ranging in concentration from 15.6–250 pg/mL. After three days incubation, the media was aspirated and 100 μL of Dulbecco’s phosphate saline (D-PBS without Ca2+ and Mg2+ supplemented with 5 mM EDTA) was added to each well and incubated for 15 min at 37°C to promote cell lifting. Cells were then detached from the bottom of wells by triturating in the center and around the edges of the well. After removing all cell debris, the wells were rinsed five times with 150 μL of TBST. The plate was then incubated with 1:500 v/v diluted polyclonal anti-human BDNF antibody for 2 hrs at room temperature. This antibody was removed and wells were washed five times with 150 μL of TBST, before incubating with 1:200 v/v diluted polyclonal Anti-IgY HRP conjugate for 2 hrs at room temperature. Wells were then washed five times with 150 μL of TBST and the remaining specifically bound polyclonal antibody was detected with the 50 μL colorimetric HRP substrate 3,3’,5,5’-Tetramethylbenzidine (TMB). The reaction was terminated with 50 μL of 1 M HCl and the color intensity was quantified by measuring the absorbance at 450 nm using a Flex Station 3 plate reader (Molecular Devices, Sunnyvale, CA). Measurements from multiple experiments were normalized to maximal BDNF responses achieved by stimulating the endogenous prostaglandin E2 receptors (EP2R) with a saturating concentration of PGE2 (10 μM). A one-way ANOVA with a Bonferroni multiple comparisons post-hoc analysis (P < 0.05) was applied to determine significant differences between groups. When converted to pg/mL averaged BDNF values ± SEM (n = 6 experiments) were: 75.4 ± 6.9 for baseline (vehicle control) and 128.6 ± 12.8 for maximal stimulation by 10 μM PGE2.
2.4. Measuring receptor density with radioligand binding
The density or maximum number of binding sites (Bmax) for the S1R in CCF-STTG1, U87MG and BV-2 cells were estimated employing 2–10 nM of [3H]-(+)-pentazocine as radioligand followed by calculation of the Bmax at saturation using a square hyperbola model: Bmax = (Y • (KD+X))/X, where Y is [specifically bound radioligand] and X = [radioligand concentration]. The affinity (KD) for [3H]-(+)-pentazocine at the cloned human S1R had been previously determined to be 3.7 nM (Schetz et al., 2007).
2.5. Measuring ligand affinities at S1R and S2R receptors by radioligand binding
The affinity (Ki) values of compounds interacting with the S1R and S2R were determined by displacement of 0.5 nM [3H]-(+)-pentazocine from MCF-7-S1R and 2.5 nM [3H]DTG from untransfected MCF-7 cells, respectively. Binding conditions were the same as described previously for the S1R (Lee et al., 2008): binding buffer (Tris 50 mM, pH = 8.1 at 37°C), ice-cold wash buffer (Tris 10 mM, pH = 8.1 at 0–2°C), and incubation time (3 hrs at 37°C) with shaking. Non-specific binding for the S1R and S2R was determined in the presence of 5 μM BD1063 and 15 μM haloperidol, respectively. Following incubation, receptors were collected via rapid filtration through GF/C filters (Brandel, Gaithersburg, MD) followed by washing three times with 3 mL of ice-cold wash buffer. Dried filters were transferred to vials filled with 3.5 ml of scintillation fluid, and the radioactivity was quantified on a liquid scintillation analyzer (Tri Carb 2800TR) from Perkin Elmer (Saint Louis, MO). Mean values from duplicate or triplicate determinations are reported along with their associated standard error of the mean (SEM). Ki values were calculated from IC50 values using the Cheng-Prusoff equation. The concentration of membrane protein was determined using a BCA Protein Assay kit (Life Technologies, Grand Island, NY) following the manufacturer’s protocol.
2.6. Measuring ligand affinities at dopamine D2 receptor by radioligand binding
Ligand affinities at the D2R were determined by competition binding as described by us previously (Ericksen et al., 2012). Briefly, [3H]methylspiperone (0.5 nM) was the radioligand and (+)-butaclamol (10 μM) was used to define nonspecific binding. Membranes purified from cloned rat D2LR stably expressed in HEK293 cells were incubated for 90 minutes in binding buffer consisting of Tris 50 mM, pH = 7.4 at 22°C and then rapidly filtered with a Tris 50 mM, pH = 7.4 at 0–2°C wash buffer. The remainder of the procedure and the calculation of affinity (Ki) values is as described above for the Sigma receptors.
2.7. qPCR to detect the BDNF gene expression
CCF-STTG1 cells were plated at density of approximately 1.5 million cells per T-25 flask. The following day, cells were either left untreated (No Additions) or treated for 3 hr with vehicle (1:1000 v/v DMSO), 100 μM IBMX, 100 μM IBMX + 100 μM forskolin, 100 μM haloperidol metabolite I, 10 μM haloperidol metabolite II or 10 μM 4-PPBP. Forskolin is a direct stimulator of adenylate cyclase and IBMX is a phosphodiesterase inhibitor. The 3 hr time point was selected because time course testing at 0.25, 1, 3 and 12 hrs revealed a maximal effect for the forskolin positive control at 3 hrs. Following treatment, cells were lysed in Tri-Reagent and total RNA was extracted as described above using Direct-zol RNA MiniPrep Kit. A total of 2 μg of RNA was used to synthesize cDNA using the High-Capacity RNA-to-cDNA kit (Cat. No. 4387406, Life Technologies) as described in the manufacturer’s protocol. A total of 100 ng of cDNA was used to perform each qPCR reaction using the TaqMan Assay and the TaqMan Gene Expression Master Mix (Cat. No. 4369016, Life Technologies) to prepare the multiplex reaction mixture per manufacturer’s protocol for BDNF (Assay ID: Hs02718934_s1 (Cat. No. 4331182)) and GAPDH (Assay ID: Hs03929097_g1 (Cat. No. 4448484)). The data was normalized to the vehicle using the ΔΔCt method.
2.8. Statistical analyses
Each data point for each experiment was sampled in triplicate and then each experiment was repeated three or more times. The data for all experiments were averaged and plotted as means ± SEM. To evaluate statistical significance, a one-way ANOVA, followed by Bonferroni’s post hoc analysis was performed, and a P-value less than 0.05 was considered significant.
3. Results
The focus of this study was on the S1R-mediated BDNF-secreting effects of haloperidol and its metabolites on astrocytes. Radioligand binding was utilized to measure affinities and an in situ ELISA approach was utilized to evaluate BDNF secretion. Reduced haloperidol had affinity interactions with the cloned human S1R and facilitated BDNF secretion from two different human astrocytic cell lines via the S1R.
3.1. The astrocytic CCF-STTG1 cell line as a model for evaluating BDNF secretion
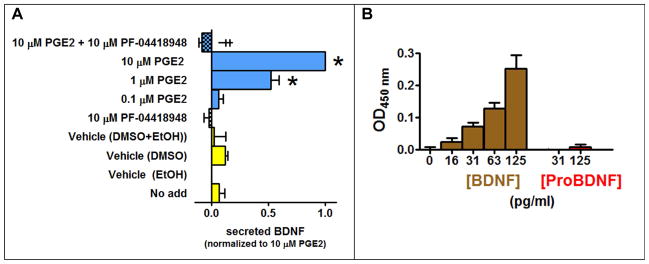
The human-derived astrocytic CCF-STTG1 cell line was selected for our studies due to noted similarities to native astrocytes (Mentz et al., 1999) and an endogenously expressed EP2 receptor (EP2R) which served as a convenient source of a positive control for BDNF release when stimulated by prostaglandin PGE2 (Hutchinson et al., 2009). Utilizing this cell line and an in situ design that has been shown to have improved sensitivity related to its rapid capture of secreted BDNF (Balkowiec and Katz, 2000), we developed and then validated an in situ ELISA for measuring secreted BDNF from glia in a 96-well format. A pilot time course study had revealed that 72 hrs stimulation provided the best balance of cell viability in the absence of media changes, the shortest assay time, and highest signal-to-noise ratio in the 96-well format (data not shown), thus all subsequent treatments were performed for 72 hrs. CCF-STTG1 cells treated with increasing concentrations of PGE2 secreted BDNF in a concentration-dependent manner (Figure 1A). No additional BDNF secretion was achieved at PGE2 concentrations beyond 10 μM (data not shown) indicating the effect was maximal at this concentration and for this reason this concentration of PGE2 was used as a positive control for subsequent experiments. Even though it had no effect by itself, the EP2-selective antagonist PF-04418948 blocked the stimulatory effect of PGE2 demonstrating that the response by this agonist was mediated via the EP2R (Figure 1A). The antagonist was tested at 10 μM to achieve maximum (99.9%) receptor occupancy (af Forselles et al., 2011).
Figure 1. The endogenous prostaglandin EP2R present in CCF-STTG1 cells is capable of mediating mature BDNF secretion.
A, The EP2R agonist PGE2 facilitates BDNF secretion in a concentration dependent manner and is blocked by the EP2R antagonist PF-04418948. Statistical significant at P < 0.05 was determined by one-way ANOVA followed by a Bonferroni post-hoc test. * Different from Vehicle (EtOH). ╪Different versus 10 μM PGE2. Levels of secreted BDNF were measured using in situ ELISA. Data is normalized to the response produced by endogenous EP2 receptor. B, Only mature BDNF is detected under our in situ ELISA assay conditions. Human BDNF and proBDNF peptides were tested at different concentrations in the in situ ELISA assay, within the concentration range that the glial cells secreted BDNF. All optical density values were background subtracted.
Because no published data accompanied literature claims of selectivity for proBDNF versus mature BDNF using a sandwich assay (Chen et al., 2006; Yoshida et al., 2012), we tested the ability of the BDNF Emax ImmunoAssay kit (Promega) to discriminate between proBDNF (precursor) and mature BDNF (processed) under our in situ ELISA conditions. Specifically, we tested high and low concentrations of purified human proBDNF and mature BDNF under identical assay conditions. While mature BDNF produced a concentration-dependent increase in optical density signal at 450 nm over the range 16–125 pg/mL, no significant signal was detected for proBDNF at the highest concentration tested (125 pg/mL) (Figure 1B). This demonstrates that the BDNF Emax ImmunoAssay kit detects only mature BDNF.
Having established that our in situ ELISA assay system detects mature BDNF secreted from CCF-STTG1 cells, we next sought to determine whether the S1R, like the EP2R, was capable of mediating BDNF secretion from this astrocytic cell line. The presence of the S1R protein was established by measuring the density of the S1R protein in cell membranes by utilizing the [3H]-(+)-pentazocine radioligand binding assay, and the maximum number of binding sites (Bmax) was determined to be 0.41 ± 0.09 pmoles/mg membrane protein.
3.2. Evaluation of S1R mediated BDNF secretion
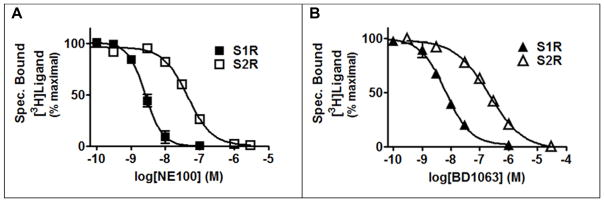
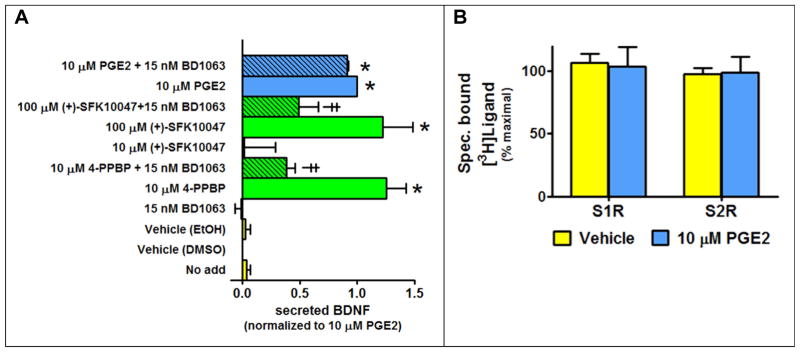
In order to evaluate the ability of the S1R to mediate BDNF secretion when activated, CCF-STTG1 cells were challenged with the prototypical reference agonists 4-PPBP and (+)-SKF10047, and the S1R reference antagonist BD1063. Both BD1063 and NE-100 are commonly employed S1R reference antagonists reported to have excellent selectivity over a number of GPCR off-targets, but also about a 50-fold selectivity over the S2R (Chaki et al., 1994; Matsumoto et al., 1995; Okuyama et al., 1993). We evaluated empirically the claims of S1R over S2R selectivity as this was an important parameter to know for our next set of experiments involving antagonist inhibition of agonist responses. The reference antagonist BD1063 was chosen over NE-100, because in a parallel comparison BD1063 displayed a superior S1R over S2R selectivity profile (Figure 2): Ki(S2R)/Ki(S1R) = 19-fold for NE100 versus Ki(S2R)/Ki(S1R) = 37-fold for BD1063. The reference agonists 4-PPBP and (+)-SKF10047, but not the reference antagonist BD1063, facilitated BDNF secretion from astrocytic cells. In the case of (+)-SKF10047, a higher concentration (at the limit of its solubility in this assay) was needed to observe an agonist effect consistent with the relatively low affinity of this compound for the S1R (Lee et al., 2008).
Figure 2. The antagonist BD1063 has greater selectivity for S1R over S2R than does the antagonist NE100.
Human MCF-7 cells were used as the source of the S2R because these cells express the endogenous S2R but lack detectable levels of the S1R (Schetz et al., 2007). [3H]-1,3-di-o-tolylguanidine ([3H]-DTG) was used as the radioligand to probe for the S2R. MCF-7 cells stably expressing the cloned human S1R were used as the source of S1R and the high affinity S1R selective radioligand [3H]-(+)-pentazocine was used to probe for the S1R (Schetz et al., 2007). A, Competition binding of NE100 with [3H]-(+)-pentazocine or [3H]-DTG at the human S1R and S2R, respectively. The corresponding IC50 and Ki values (nM) ± SEM are: 2.8 ± 0.67 and 2.3 ± 0.55 for S1R and 46 ± 6.4 and 43 ± 6.0 for S2R. B, Competition binding of BD1063 with [3H]-(+)-pentazocine or [3H]-DTG at human S1R and S2R, respectively. The corresponding IC50 and Ki values (nM) ± SEM are: 6.5 ±0.87 and 5.3 ± 0.71 for S1R and 208 ± 30 and 194 ± 28 for S2R.
While BD1063 had no effect on its own, it significantly reduced BDNF secretion produced by the S1R agonists, but not the EP2R agonist PGE2 (Figure 3A). BD1063 was applied at the S1R-selective concentration of 15 nM, which corresponds to a calculated S1R and S2R occupancy of 74% and 7%, respectively (Fractional Occupancy = [ligand]/([ligand]+Ki)). Though the extend of attenuation was less due to lower expected levels of S1R occupancy (27%), similar results were achieved using 2 nM BD1063, instead of 15 nM, to reduce S1R agonist responses (data not shown). PGE2 was unable to displace specifically bound radiolabeled agonists from the S1R or the S2R (Figure 3B), indicating a lack of detectable interaction of PGE2 with sigma receptors. These outcomes show that BDNF secretion from CFF-STTG1 glial cells can be mediated by activation of the S1R. For comparison, the BV-2 microglial cell line and the U87MG astrocytic cell line were evaluated for the presence of S1R, as well as the ability of the S1R to mediate BDNF secretion. Like the astrocytic CCF-SSTG1 cells, both the BV-2 and U87MG cell lines also express the S1R. In this report, using radioligand binding, we demonstrated that the S1R protein is also present in both the U87MG and the BV-2 cell lines. The maximum number of binding sites (Bmax) in the U87MG and the BV-2 cells were estimated to be 2.0 ± 0.14 and 0.6 ± 0.1 pmoles/mg membrane protein, respectively, based upon measurements of specifically bound [3H]-(+)-pentazocine followed by calculation of the Bmax at saturation using a square hyperbola model as describe in the methods. However, in contrast to the astrocytic cell lines (CCF-STTG1 and U87MG), the reference agonist 4-PPBP failed to significantly stimulate the secretion of detectable levels of BDNF in the microglial (BV-2) cell line (exposure of BV-2 cells to 10 μM 4-PPBP produced a signal that was only 1.1 ± 0.06 fold above DMSO vehicle, P = 0.4): no signal over vehicle control was observed, hence these cells were not evaluated further.
Figure 3. Prototypical S1R agonists facilitate secretion of BDNF from CCF-STTG1 cells.
Levels of secreted BDNF were measured using in situ ELISA. Data is normalized to the maximal response produced by stimulation of endogenous EP2 receptors. A, The S1R reference agonists 4-PPBP and (+)-SKF10047, exert a robust BDNF secretion response that is reduced by a S1R-selective dose of the S1R reference antagonist BD1063. However, BD1063 does not modify the PGE2 stimulated response mediated by EP2R. B, At a concentration that causes maximal secretion of BDNF via interactions with the EP2R, PGE2 (10 μM) is unable to displace specific bound radioligand from either S1R or S2R. [3H]-(+)-pentazocine was the radioligand used to probe the S1R in MCF-7 cells stably expressing the cloned human S1R, while [3H]-DTG was used as the radioligand to probe the S2R in untransfected MCF-7 cells.
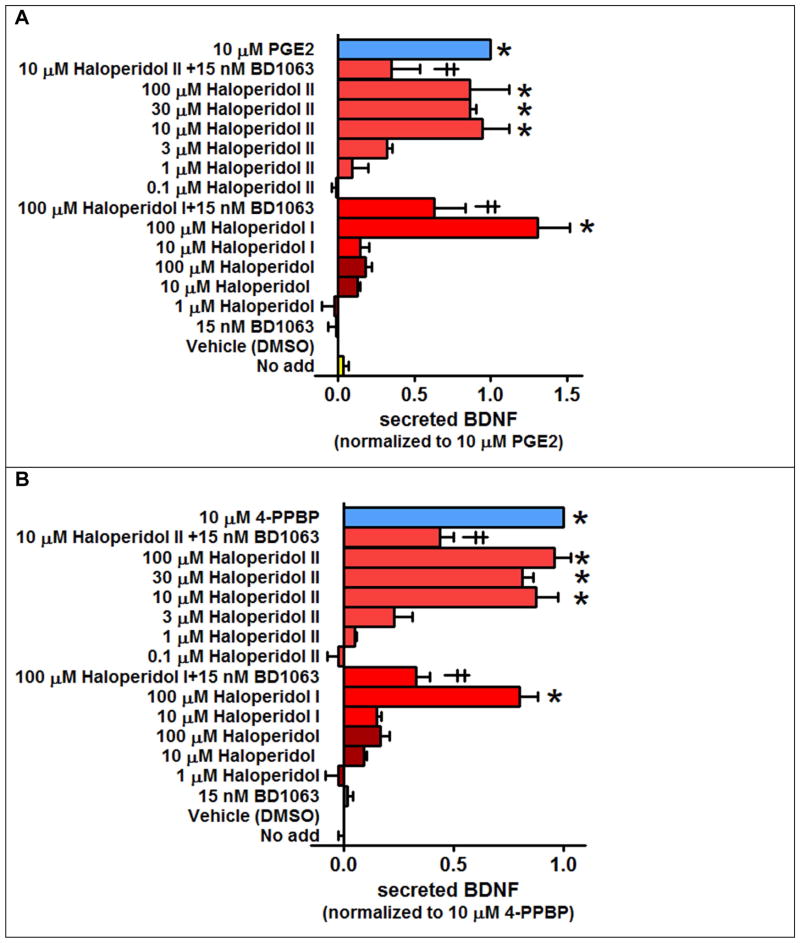
Elevation of brain BDNF has been shown to be beneficial in rodent models of ischemic stroke (Espinera et al., 2013; Kim et al., 2009). Thus, in an effort to gain insight into the possible mechanisms for the finding that a low dose of haloperidol (0.05 mg/kg) protected against acute ischemic stroke (Schetz et al., 2007), haloperidol and its major metabolites having measurable affinity for the S1R were evaluated for their ability to mediate BDNF secretion from two astrocytic cell lines. Haloperidol metabolite I and reduced haloperidol (metabolite II), but not haloperidol, facilitated BDNF secretion from both the CCF-STTG1 and U87MG cells (Figure 4A–B). A higher concentration of haloperidol metabolite I was used since it has >75-fold lower affinity for the S1R than haloperidol and reduced haloperidol (Lee et al., 2008): affinity (Ki) = 1.7, 1.5 and 128 nM for haloperidol, reduced haloperidol, and metabolite I, respectively. Since haloperidol metabolite III (3-(4-Fluorobenzoyl)propionic acid) has essentially no detectable affinity for the S1R (Lee et al., 2008), it was not tested here. While the S1R antagonist BD1063 had no effect on its own, it significantly diminished BDNF secretion produced by haloperidol metabolites I and II (Figure 4). These findings demonstrate that reduced haloperidol and to a lesser extent haloperidol metabolite I act as functionally-selective S1R agonists in the context of BDNF secretion.
Figure 4. Haloperidol metabolites I and II, but not haloperidol, stimulate S1R-mediated BDNF secretion from the astrocytic glial cell lines CCF-STTG1 and U87MG.
Levels of secreted BDNF were measured using in situ ELISA. A, S1R-mediated BDNF secretion in the CCF-STTG1 cell line. B, S1R-mediated BDNF secretion in the U87MG cell line. Data is normalized to the maximum response produced by PGE2 (for CCF-STTG1) or 4-PPBP (for U87MG). Statistical significance at P < 0.05 was determined by ANOVA followed by a Bonferroni post-hoc test. * Different from Vehicle (DMSO). ╪ Different versus corresponding treatment in the absence of the antagonists BD1063.
3.3. Comparison of the affinity of haloperidol and its metabolites at the S1R and D2R
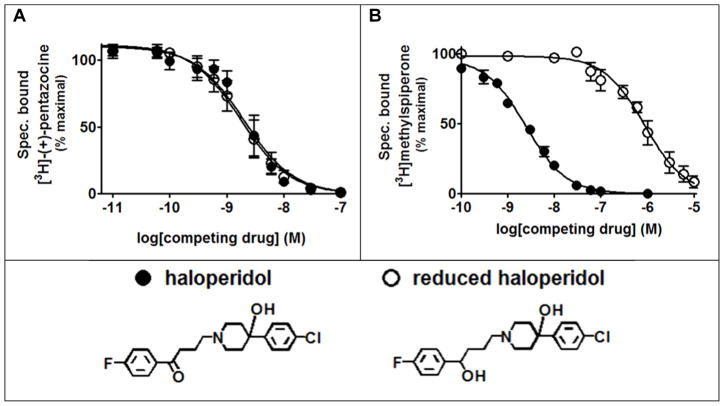
Previous studies investigating differences in the binding of haloperidol and its primary metabolite, reduced haloperidol, to the S1R and the D2 dopamine receptor (D2R) were conducted with low levels of receptors obtained from tissue sources (Korpi and Wyatt, 1984). Thus, we re-investigated the reported differences using cloned receptor systems (Figure 5). The measured affinities for both haloperidol and its metabolite reduced haloperidol for the S1R were indistinguishable (Figure 5A). In contrast, reduced haloperidol had a >350-fold lower affinity for the D2R than the parent compound haloperidol (Figure 5B). These results indicate that reduced haloperidol readily discriminates between S1R and D2R, which is consistent with previous reports.
Figure 5. Haloperidol and reduced haloperidol have the same affinities for the cloned S1R but drastically different affinities for the cloned D2 dopamine receptor (D2R).
Cloned receptors for S1R and D2R were stably expressed in MCF-7 and HEK293 cells lines, respectively, as the untransfected cells lack the receptor of interest. A, Competition binding with [3H]-(+)-pentazocine at the cloned S1R. The corresponding Ki values (nM) ± SEM are: 1.6 ± 0.25 for haloperidol and 1.4 ± 0.21 for reduced haloperidol. B, Competition binding with [3H]methylspiperone at the cloned D2 dopamine receptor. The corresponding Ki values (nM) ± SEM are: 0.087 ± 0.0055 for haloperidol and 31 ± 3.8 for reduced haloperidol.
3.4. Effect of haloperidol metabolites on the BDNF steady-state mRNA level
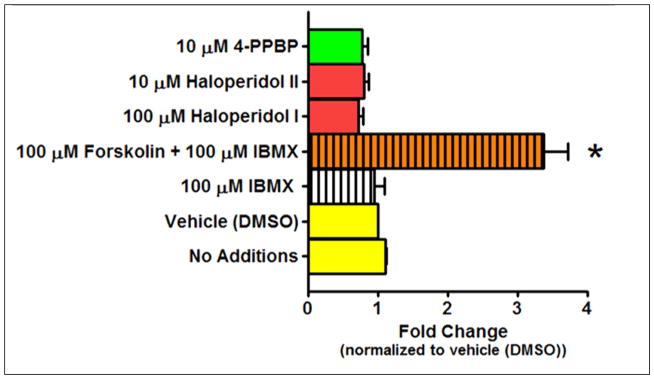
Since some S1R ligands have been reported to increase BDNF mRNA levels (Moriguchi et al., 2015; Peviani et al., 2014; Ring and Regan, 2013), qPCR was utilized to assess the ability of haloperidol metabolites to increase BDNF mRNA levels in CCF-STTG1 cells (Figure 6). Forskolin was used as a positive control, because it has been shown previously to increase BDNF expression (He et al., 2010). A 12 hr pilot time course study using forskolin had revealed that a 3 hr treatment resulted in the maximal stimulation of BDNF expression (data not shown), and thus all subsequent treatments were performed at this time point. The phosphodiesterase inhibitor, IBMX, was used to keep the forskolin-stimulated levels of intracellular cAMP high, though by itself it had no effect on BDNF expression. Forskolin produced a >3-fold increase in BDNF expression demonstrating that our assay conditions were appropriate for detecting changes in the expression of this neurotrophin. At concentrations that produced a robust S1R-mediated secretion of BDNF (Figures 3 and 4), neither the S1R reference agonist 4-PPBP nor the haloperidol metabolites changed BDNF mRNA levels (Figure 6). These findings indicate that S1R agonists do not change the steady state levels of BDNF mRNA in the CCF-STTG1 cells within the time frame tested.
Figure 6. Forskolin, but neither haloperidol metabolites nor the S1R reference agonist 4-PPBP, increase steady state BDNF mRNA levels as determined by qPCR.
CCF-STTG1 cells were treated with vehicle, IBMX, forskolin + IBMX, haloperidol metabolite I and II, and 4-PPBP for 3 hours. Three hours was selected because a time course study revealed that 3 hrs stimulation resulted in the maximum response for forskolin. Forskolin was used as a positive control because it was previously shown to increase BDNF mRNA levels (He et al., 2010). IBMX was utilized to prevent cAMP degradation and as expected, it had no effect on BDNF mRNA levels in the absence of forskolin. Haloperidol metabolite I and II and 4-PPBP also had no effect on BDNF mRNA levels. Only forskolin was able to induce a > 3 fold increase in BDNF mRNA levels. Statistical significance at P < 0.05 was determined by ANOVA followed by Bonferroni post-hoc test. * Different from vehicle (DMSO).
4. Discussion
A number of new findings are presented here. First, we report on the implementation of an in situ ELISA for measuring secreted BDNF from human glial cell lines in a 96-well assay format. Second, we demonstrate that activation of the S1R mediates BDNF secretion from astrocytic CCF-STTG1 and U87MG cells. Third, haloperidol metabolites I and II are shown to mediate BDNF secretion via their functionally-selective interactions with the S1R. The relevance to the current knowledge and practice of each of these findings are detailed below.
By modifying the approach originally described by Balkowiec and Katz, 2000, an in situ ELISA for measuring secreted mature BDNF in a 96-well format was developed and validated in CCF-STTG1 cells, because this astrocytic glial cell line closely resembles native astrocytes (Mentz et al., 1999) and was reported to contain an endogenous EP2R that mediates the secretion of BDNF when stimulated (Hutchinson et al., 2009). Relying on the endogenous EP2R-BDNF system in CCF-STTG1 cells allowed us to focus on miniaturization of the assay format. The in situ ELISA improves on traditional ELISA by seeding BDNF-secreting cells directly into the wells pre-coated with an anti-BDNF primary antibody. The BDNF secreted by these cells is immediately captured by the primary antibody, drastically increasing the sensitivity and reproducibility of the ELISA assay. Intact cells are removed subsequent to the addition of the secondary and tertiary antibodies. While the previous report by Hutchinson et al., 2009 had used standard ELISA and a 6-well plate format and a 24–48 hr treatment, we were able to achieve similar results using a 96-well plate format, a 72 hr treatment, and an in situ ELISA approach. Validation of the new assay system included demonstrating that the BDNF secreted from CCF-STTG1 cells was mediated in a concentration-dependent and saturable manner by the EP2R agonist PGE2, and that this stimulatory effect could be blocked by the EP2R-selective antagonist PF-04418948. A number of commercially-available assays for measuring BDNF exist and during our initial survey of cost, convenience and performance in initial test runs of sample kits, we selected the BDNF Emax Immuno Assay kit (Promega). However, while studies of specificity for BDNF over other neurotropic factors have been established, the manufacturer makes no claims as to which form(s) of BDNF is detected. i.e., mature versus proBDNF. The only published reports that we could find with such claims presented no supporting data (Chen et al., 2006; Yoshida et al., 2012). Further, a recent report concerning claims of selectivity used dot blotting, instead of an ELISA approach, for the evaluation (Polacchini et al., 2015). For these reasons, we tested the ability of the BDNF Emax ImmunoAssay kit (Promega) to detect proBDNF and mature BDNF using purified human proBDNF and mature BDNF, and, under identical assay conditions, we found that only mature BDNF was being detected. While one might assume that because BDNF is derived from cleavage of proBDNF, antibodies which detect BDNF must also detect proBDNF, however, there are rational reasons that this does not necessarily have to be the case. For instance, only mature BDNF has an N-terminus preceding the amino acids that mark the beginning of the mature BDNF sequence, proBDNF contains a large region capable of masking parts of the BDNF sequence (Tettamanti et al., 2010), and there may also be subtle conformational differences between the two forms as well. Such differences could account for our observation that the BDNF Emax Immuno Assay kit detects mature BDNF over proBDNF under our in situ ELISA conditions.
The S1R-preferring agonist cutamesine (SA4503) (Lever et al., 2006) has been shown to facilitate the trafficking, processing and secretion of mature BDNF from a rat neuronal B104 cell line, and this BDNF secretion was blocked by a high concentration (1 μM) of the S1R-preferring antagonist NE-100 (Moon et al., 2014). Here we demonstrate for the first time that the S1R is present in human astrocytic CCF-STTG1 glial cells, its activation mediates the secretion of BDNF, and this agonist-stimulated secretion is attenuated by a selective concentration (15 nM) of the S1R-preferring antagonist BD1063. Our finding is in line with a recent report where BDNF release from astrocytes derived from a rat primary cortical culture was induced by the novel S1R agonist, LS-1-137 (Malik et al., 2015). Other studies investigating pathological conditions that lead to activation of astrocytes have indicated a role for S1R ligands in reducing astrocytosis (Moon et al., 2014; Peviani et al., 2014; Robson et al., 2014). While only the study using the agonist PRE-084 suggested a BDNF mechanism, all of these studies suggest the presence of the S1R in astrocytes, which is consistent with our finding of its existence in the astrocytic CCF-STTG1 cell line. Both S1R and BDNF were present in BV-2 microglial cells, yet treating these cells with an S1R agonist did not facilitate BDNF secretion. Although activated microglia (Nakajima et al., 2001; Sun et al., 2014), including a murine N9 microglial cell line (Gomes et al., 2013), are capable of secreting BDNF, our finding is in agreement with numerous studies indicating that the S1R is present in microglia and can modulate injury or toxicant-induced microgliosis by various mechanisms not involving BDNF (Behensky et al., 2013; Cuevas et al., 2011; Dong et al., 2015; Gekker et al., 2006; Hall et al., 2009; Mancuso et al., 2012; Moritz et al., 2015; Robson et al., 2013; Wegleiter et al., 2014; Wu et al., 2015; Zhao et al., 2014). Alternatively, the BV-2 cell line may lack the necessary machinery for investigating S1R-BDNF interactions in microglia or the microglia may need to be activated in order to study the effect.
Multiple recent studies support the idea that drugs which increase brain BDNF would be beneficial for the treatment of ischemic stroke at delayed time points. Daily dosing of the selective serotonin reuptake inhibitor (SSRI) citalopram for seven days after transient middle cerebral artery occlusion increases brain BDNF levels, improved functional recovery and correlated with BDNF-mediated increases in neurogenesis and angiogenesis in the peri-infarct region (Espinera et al., 2013). This preclinical result showed that a CNS-penetrating drug improves functional recovery from ischemic stroke via a BDNF mechanism. Other drugs that elevate brain BDNF, such as the histone deacetylase (HDAC) inhibitor sodium butyrate, promote functional recovery and neurogenesis in a transient ischemic stroke rat model (Kim et al., 2009). This effect was prevented by inhibiting the TrkB (BDNF) receptor. In a previous study, haloperidol, reduced haloperidol and a number of differentially halogenated analogues of haloperidol were shown to have high affinity for the S1R that correlated well with their in vitro neuroprotective potencies against an oxidative stress trigger, and further, an acute low dose of haloperidol (0.05 mg/kg, s.c.) protected rats against an ischemic stroke (Schetz et al., 2007). While it was clear that haloperidol interacts with the S1R, less clear was the mechanism by which haloperidol exerted its protective effects in the stroke model. In this report we demonstrate that two of haloperidol’s metabolites stimulate the secretion of BDNF from a glial cell line with reduced haloperidol being more potent than metabolite I, which is consistent with the latter’s lower affinity for the S1R. This new finding for haloperidol metabolites may help explain the beneficial effect in the stroke model observed for haloperidol. Our studies cannot, however, rule out that haloperidol or its metabolites might have other functionally selective actions that mediate its acute protective effect. These might include, as has been shown for S1R agonists, a reduction in pro-inflammatory cytokines and enhancement of anti-inflammatory cytokines (Allahtavakoli and Jarrott, 2011; Shen et al., 2008) or a reduction in inflammatory levels of nitrosative stress (Goyagi et al., 2001).
Haloperidol and reduced haloperidol have nanomolar affinity for the S1R but a micromolar concentration was needed to observe facilitation of BDNF release in vitro. This discrepancy between the measured affinity and in vitro potency values is consistent with the reports of others who have observed the same phenomena for other S1R ligands that facilitate BDNF secretion (Fujimoto et al., 2012; Malik et al., 2015), or other S1R drugs that produce other S1R-mediated functional outputs such as neurite sprouting or changes in intracellular calcium (Brimson et al., 2011; Ishima et al., 2014; Ishima and Hashimoto, 2012; Ishima et al., 2008; Nishimura et al., 2008; Wu and Bowen, 2008). Though reason for the discrepancy between the affinity and potency is not entirely clear, the findings of this study nevertheless provide new insight into the structure-activity relationship and functional selectivity of haloperidol metabolites at the S1R.
The in situ ELISA results measuring secreted BDNF and the qPCR measuring changes in BDNF mRNA indicate that while the S1R agonist 4-PPBP and haloperidol metabolites I and II each facilitate BDNF secretion, none of them influence the message level. While this finding is consistent with the report that the S1R ligand E-5842 failed to increase the expression of BDNF mRNA in rat brain (Ovalle et al., 2002), E-5842 has not been assigned functional properties as an agonist or antagonist of the S1R making a direct comparison with our studies difficult (Ulrich et al., 1999). The reported antipsychotic properties of E-5842 are due to its ability to block D2R (Ovalle et al., 2002). This is also the case for haloperidol which has similar affinity for S1R and D2R, because reduced haloperidol retains high affinity for S1R, has drastically reduced affinity for D2R, and it lacks the D2R-mediated antipsychotic activity and side effects of the parent compound haloperidol (Ulrich et al., 1999). Atypical antipsychotic drugs like clozapine, olanzapine, quetiapine, and aripiprazole have been reported to increase BDNF mRNA and/or protein levels in vivo (Chen et al., 2015b; Dong et al., 2016; Fumagalli et al., 2003; Murphy et al., 2014; Park et al., 2006; Seo et al., 2015; Shioda et al., 2015). Though in the case of these atypical antipsychotic drugs, the effects on BDNF are likely not mediated via the S1R, since, for example, clozapine has essentially no affinity for the S1R (Lee et al., 2008). Both haloperidol and its metabolite II have similarly strong high affinity interactions with the cloned S1R, while its metabolite I has moderate affinity and metabolite III has essentially no detectable affinity for S1R (Lee et al., 2008). However, in contrast to atypical antipsychotics, the typical antipsychotic haloperidol has been reported to either have no effect on BDNF (Dong et al., 2016; Seo et al., 2015) or to exacerbate the reduction in BDNF levels mediated by the NMDA receptor antagonist MK-801 (Fumagalli et al., 2003). Thus, while previous studies have shown that haloperidol does not affect BDNF message or protein levels, none of those studies tested the effects of haloperidol or its metabolites on BDNF secretion. The present study demonstrates that haloperidol metabolites I and II, but not haloperidol, facilitate the secretion of mature BDNF from astrocytic cells via their functionally selective interactions with the S1R. While the mechanism by which S1R facilitates BDNF secretion is not well understood and is not the focus of this study, we offer the following speculation. It has been reported that BDNF trafficking is modulated by sortilin and Huntingtin-associated protein 1 (HAP1) (Evans et al., 2011; Yang et al., 2011). Sortilin has been shown to colocalize with BDNF in secretory granules of neurons and influence activity-dependent BDNF secretion (Chen et al., 2005). Knockdown of sortilin reduced activity-dependent secretion of BDNF and enhanced constitutive secretion (Chen et al., 2005). Similarly, HAP1 also colocalizes with BDNF, and enhances activity-dependent BDNF secretion in neurons (Wu et al., 2010; Yang et al., 2011). HAP1 knockdown reduced activity-dependent BDNF secretion, however, in contrast to sortilin knockdown, HAP1 knockdown did not enhance constitutive secretion (Wu et al., 2010). Though we did not find any link between the S1R, and sortilin and HAP1 in the literature, it is possible that S1R could suppress the activity of sortilin or a sortilin-like protein and thereby enhance constitutive BDNF secretion. Though it appears that HAP1 is not involved in constitutive BDNF secretion, it is not known how a potential interaction with the S1R might influence HAP1-mediated constitutive BDNF secretion. The in vivo mechanism is likely going to be more involved due to the complex interplay between neurons and glia, which may play an important role in modulating BDNF secretion.
5. Conclusion
Amongst the key finding of these studies is that human-derived CCF-STTG1 cells appear to serve as a viable immortalized cellular system for studying S1R-mediated facilitation of BDNF secretion from astrocytes. In contrast, S1Rs present in BV-2 microglial cells do not seem to mediate BDNF secretion when tested under the same conditions. The measured increase in extracellular mature BDNF upon stimulation of the S1R in astroglia is consistent with the reported enhanced processing and secretion observed in a B104 neuronal cell line (Fujimoto et al., 2012), as it does not involve an increase in BDNF steady state mRNA levels. Reduced haloperidol is a primary metabolite of the antipsychotic drug haloperidol and is a high affinity S1R ligand whose BDNF functional selectivity profile is consistent with a S1R agonist; however, haloperidol does not share the same functional properties. Given the importance of the surrounding glial environment to neuronal processes including resilience to inflammatory insults and neurogenesis, our finding may help explain the previously reported observation that low dose haloperidol reduces ischemic lesion volume following transient middle cerebral artery occlusion (Schetz et al., 2007).
Highlights.
Human-derived astroglia express a stress and ligand-regulated chaperone protein
Functionally selective ligands of this protein facilitate the release of BDNF
Haloperidol metabolites act as functionally selective ligands of this protein
These same metabolites do not increase mRNA levels of BDNF
Acknowledgments
We thank Elisabetta Blasi at the University of Perugia for providing the BV-2 cell line as a generous gift.
Funding Sources:
National Institutes of Health [Grant R41AG043243 (JAS)]
ADDF grant 20121209 and 20140803 (JAS)
William and Ella Owens Medical Research Foundation (JAS)
Footnotes
7. Conflict of Interest:
Authors declare no competing financial interest.
Author Contribution:
Dhwanil A. Dalwadi – performed experiments, and helped with writing of the manuscript
Seongcheol Kim – performed experiments
John A. Schetz – conceived of the project, designed experiments, interpreted data, and wrote the manuscript
Chemical compounds studied in this article:
4-PPBP: (PubChem CID: 3035672); (+)-SKF10047 (PubChem CID: 13286169); BD1063 (PubChem CID: 574780); NE-100 (PubChem CID: 9841596); Haloperidol (PubChem CID: 3559); Reduced haloperidol (PubChem CID: 119265); Haloperidol metabolite I (PubChem CID: 38282); Haloperidol metabolite III (PubChem CID: 101359); PGE2 (PubChem CID: 5280360); PF-04418948 (PubChem CID: 25114442)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Thugs get high on stolen Aids drugs - South Africa | IOL News. Vol. 2015.
- af Forselles KJ, Root J, Clarke T, Davey D, Aughton K, Dack K, Pullen N. In vitro and in vivo characterization of PF-04418948, a novel, potent and selective prostaglandin EP(2) receptor antagonist. British journal of pharmacology. 2011;164:1847–1856. doi: 10.1111/j.1476-5381.2011.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allahtavakoli M, Jarrott B. Sigma-1 receptor ligand PRE-084 reduced infarct volume, neurological deficits, pro-inflammatory cytokines and enhanced anti-inflammatory cytokines after embolic stroke in rats. Brain research bulletin. 2011;85:219–224. doi: 10.1016/j.brainresbull.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Alonso G, Phan V, Guillemain I, Saunier M, Legrand A, Anoal M, Maurice T. Immunocytochemical localization of the sigma(1) receptor in the adult rat central nervous system. Neuroscience. 2000;97:155–170. doi: 10.1016/s0306-4522(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Bagayogo IP, Dreyfus CF. Regulated release of BDNF by cortical oligodendrocytes is mediated through metabotropic glutamate receptors and the PLC pathway. ASN neuro. 2009;1:10. doi: 10.1042/AN20090006. 1042/AN20090006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkowiec A, Katz DM. Activity-dependent release of endogenous brain-derived neurotrophic factor from primary sensory neurons detected by ELISA in situ. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:7417–7423. doi: 10.1523/JNEUROSCI.20-19-07417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barna BP, Chou SM, Jacobs B, Ransohoff RM, Hahn JF, Bay JW. Enhanced DNA synthesis of human glial cells exposed to human leukocyte products. Journal of neuroimmunology. 1985;10:151–158. doi: 10.1016/0165-5728(85)90005-0. [DOI] [PubMed] [Google Scholar]
- Behensky AA, Yasny IE, Shuster AM, Seredenin SB, Petrov AV, Cuevas J. Stimulation of sigma receptors with afobazole blocks activation of microglia and reduces toxicity caused by amyloid-beta25–35. The Journal of pharmacology and experimental therapeutics. 2013;347:458–467. doi: 10.1124/jpet.113.208348. [DOI] [PubMed] [Google Scholar]
- Bejot Y, Prigent-Tessier A, Cachia C, Giroud M, Mossiat C, Bertrand N, Garnier P, Marie C. Time-dependent contribution of non neuronal cells to BDNF production after ischemic stroke in rats. Neurochemistry international. 2011;58:102–111. doi: 10.1016/j.neuint.2010.10.019. [DOI] [PubMed] [Google Scholar]
- Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. Journal of neuroimmunology. 1990;27:229–237. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- Brimson JM, Brown CA, Safrany ST. Antagonists show GTP-sensitive high-affinity binding to the sigma-1 receptor. British journal of pharmacology. 2011;164:772–780. doi: 10.1111/j.1476-5381.2011.01417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaki S, Tanaka M, Muramatsu M, Otomo S. NE-100, a novel potent sigma ligand, preferentially binds to sigma 1 binding sites in guinea pig brain. European journal of pharmacology. 1994;251:R1–2. doi: 10.1016/0014-2999(94)90453-7. [DOI] [PubMed] [Google Scholar]
- Chen Y, Zhang J, Deng M. Furin mediates brain-derived neurotrophic factor upregulation in cultured rat astrocytes exposed to oxygen-glucose deprivation. Journal of neuroscience research. 2015a;93:189–194. doi: 10.1002/jnr.23455. [DOI] [PubMed] [Google Scholar]
- Chen YH, Zhang RG, Xue F, Wang HN, Chen YC, Hu GT, Peng Y, Peng ZW, Tan QR. Quetiapine and repetitive transcranial magnetic stimulation ameliorate depression-like behaviors and up-regulate the proliferation of hippocampal-derived neural stem cells in a rat model of depression: The involvement of the BDNF/ERK signal pathway. Pharmacology, biochemistry, and behavior. 2015b;136:39–46. doi: 10.1016/j.pbb.2015.07.005. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Ieraci A, Teng H, Dall H, Meng CX, Herrera DG, Nykjaer A, Hempstead BL, Lee FS. Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:6156–6166. doi: 10.1523/JNEUROSCI.1017-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Jing D, Bath KG, Ieraci A, Khan T, Siao CJ, Herrera DG, Toth M, Yang C, McEwen BS, Hempstead BL, Lee FS. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science (New York, NY) 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas J, Rodriguez A, Behensky A, Katnik C. Afobazole modulates microglial function via activation of both sigma-1 and sigma-2 receptors. The Journal of pharmacology and experimental therapeutics. 2011;339:161–172. doi: 10.1124/jpet.111.182816. [DOI] [PubMed] [Google Scholar]
- Dong E, Tueting P, Matrisciano F, Grayson DR, Guidotti A. Behavioral and molecular neuroepigenetic alterations in prenatally stressed mice: relevance for the study of chromatin remodeling properties of antipsychotic drugs. Translational psychiatry. 2016;6:e711. doi: 10.1038/tp.2015.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Ma Y, Ren Z, Xu B, Zhang Y, Chen J, Yang B. Sigma-1 Receptor Modulates Neuroinflammation After Traumatic Brain Injury. Cellular and molecular neurobiology. 2015 doi: 10.1007/s10571-015-0244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericksen SS, Cummings DF, Teer ME, Amdani S, Schetz JA. Ring substituents on substituted benzamide ligands indirectly mediate interactions with position 7.39 of transmembrane helix 7 of the D4 dopamine receptor. The Journal of pharmacology and experimental therapeutics. 2012;342:472–485. doi: 10.1124/jpet.112.193979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinera AR, Ogle ME, Gu X, Wei L. Citalopram enhances neurovascular regeneration and sensorimotor functional recovery after ischemic stroke in mice. Neuroscience. 2013;247:1–11. doi: 10.1016/j.neuroscience.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SF, Irmady K, Ostrow K, Kim T, Nykjaer A, Saftig P, Blobel C, Hempstead BL. Neuronal brain-derived neurotrophic factor is synthesized in excess, with levels regulated by sortilin-mediated trafficking and lysosomal degradation. The Journal of biological chemistry. 2011;286:29556–29567. doi: 10.1074/jbc.M111.219675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M, Hayashi T, Urfer R, Mita S, Su TP. Sigma-1 receptor chaperones regulate the secretion of brain-derived neurotrophic factor. Synapse (New York, NY) 2012;66:630–639. doi: 10.1002/syn.21549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli F, Molteni R, Roceri M, Bedogni F, Santero R, Fossati C, Gennarelli M, Racagni G, Riva MA. Effect of antipsychotic drugs on brain-derived neurotrophic factor expression under reduced N-methyl-D-aspartate receptor activity. Journal of neuroscience research. 2003;72:622–628. doi: 10.1002/jnr.10609. [DOI] [PubMed] [Google Scholar]
- Gekker G, Hu S, Sheng WS, Rock RB, Lokensgard JR, Peterson PK. Cocaine-induced HIV-1 expression in microglia involves sigma-1 receptors and transforming growth factor-beta1. International immunopharmacology. 2006;6:1029–1033. doi: 10.1016/j.intimp.2005.12.005. [DOI] [PubMed] [Google Scholar]
- Gomes C, Ferreira R, George J, Sanches R, Rodrigues DI, Goncalves N, Cunha RA. Activation of microglial cells triggers a release of brain-derived neurotrophic factor (BDNF) inducing their proliferation in an adenosine A2A receptor-dependent manner: A2A receptor blockade prevents BDNF release and proliferation of microglia. Journal of neuroinflammation. 2013;10 doi: 10.1186/1742-2094-10-16. 16-2094-2010-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyagi T, Goto S, Bhardwaj A, Dawson VL, Hurn PD, Kirsch JR. Neuroprotective effect of sigma(1)-receptor ligand 4-phenyl-1-(4-phenylbutyl) piperidine (PPBP) is linked to reduced neuronal nitric oxide production. Stroke; a journal of cerebral circulation. 2001;32:1613–1620. doi: 10.1161/01.str.32.7.1613. [DOI] [PubMed] [Google Scholar]
- Hall AA, Herrera Y, Ajmo CT, Jr, Cuevas J, Pennypacker KR. Sigma receptors suppress multiple aspects of microglial activation. Glia. 2009;57:744–754. doi: 10.1002/glia.20802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansebout CR, Su C, Reddy K, Zhang D, Jiang C, Rathbone MP, Jiang S. Enteric glia mediate neuronal outgrowth through release of neurotrophic factors. Neural regeneration research. 2012;7:2165–2175. doi: 10.3969/j.issn.1673-5374.2012.028.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Scheffel U, London ED. In vivo labeling of sigma receptors in mouse brain with [3H]4-phenyl-1-(4-phenylbutyl)piperidine. Synapse (New York, NY) 1995;20:85–90. doi: 10.1002/syn.890200112. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP. Sigma-1 receptors at galactosylceramide-enriched lipid microdomains regulate oligodendrocyte differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:14949–14954. doi: 10.1073/pnas.0402890101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He DY, Neasta J, Ron D. Epigenetic regulation of BDNF expression via the scaffolding protein RACK1. The Journal of biological chemistry. 2010;285:19043–19050. doi: 10.1074/jbc.M110.100693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson AJ, Chou CL, Israel DD, Xu W, Regan JW. Activation of EP2 prostanoid receptors in human glial cell lines stimulates the secretion of BDNF. Neurochemistry international. 2009;54:439–446. doi: 10.1016/j.neuint.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S, Susukida M, Ikeda K, Murase K, Hayashi K. Dopaminergic transmitter up-regulation of brain-derived neurotrophic factor (BDNF) and nerve growth factor (NGF) synthesis in mouse astrocytes in culture. Biochemical and biophysical research communications. 1997;238:468–472. doi: 10.1006/bbrc.1997.7324. [DOI] [PubMed] [Google Scholar]
- Ishima T, Fujita Y, Hashimoto K. Interaction of new antidepressants with sigma-1 receptor chaperones and their potentiation of neurite outgrowth in PC12 cells. European journal of pharmacology. 2014;727:167–173. doi: 10.1016/j.ejphar.2014.01.064. [DOI] [PubMed] [Google Scholar]
- Ishima T, Hashimoto K. Potentiation of nerve growth factor-induced neurite outgrowth in PC12 cells by ifenprodil: the role of sigma-1 and IP3 receptors. PloS one. 2012;7:e37989. doi: 10.1371/journal.pone.0037989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishima T, Nishimura T, Iyo M, Hashimoto K. Potentiation of nerve growth factor-induced neurite outgrowth in PC12 cells by donepezil: role of sigma-1 receptors and IP3 receptors. Progress in neuro-psychopharmacology & biological psychiatry. 2008;32:1656–1659. doi: 10.1016/j.pnpbp.2008.06.011. [DOI] [PubMed] [Google Scholar]
- Jean YY, Lercher LD, Dreyfus CF. Glutamate elicits release of BDNF from basal forebrain astrocytes in a process dependent on metabotropic receptors and the PLC pathway. Neuron glia biology. 2008;4:35–42. doi: 10.1017/S1740925X09000052. [DOI] [PubMed] [Google Scholar]
- Jiang G, Mysona B, Dun Y, Gnana-Prakasam JP, Pabla N, Li W, Dong Z, Ganapathy V, Smith SB. Expression, subcellular localization, and regulation of sigma receptor in retinal muller cells. Investigative ophthalmology & visual science. 2006;47:5576–5582. doi: 10.1167/iovs.06-0608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi-Utsumi K, Nakaki T. Chronic treatment with a selective ligand for the sigma-1 receptor chaperone, SA4503, up-regulates BDNF protein levels in the rat hippocampus. Neuroscience letters. 2008;440:19–22. doi: 10.1016/j.neulet.2008.05.055. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Leeds P, Chuang DM. The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. Journal of neurochemistry. 2009;110:1226–1240. doi: 10.1111/j.1471-4159.2009.06212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klette KL, DeCoster MA, Moreton JE, Tortella FC. Role of calcium in sigma-mediated neuroprotection in rat primary cortical neurons. Brain research. 1995;704:31–41. doi: 10.1016/0006-8993(95)01103-x. [DOI] [PubMed] [Google Scholar]
- Korpi ER, Wyatt RJ. Reduced haloperidol: effects on striatal dopamine metabolism and conversion to haloperidol in the rat. Psychopharmacology. 1984;83:34–37. doi: 10.1007/BF00427418. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Su TP, Fujimoto M, Bonci A. The sigma-1 receptor: roles in neuronal plasticity and disease. Trends in neurosciences. 2012;35:762–771. doi: 10.1016/j.tins.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee IT, Chen S, Schetz JA. An unambiguous assay for the cloned human sigma1 receptor reveals high affinity interactions with dopamine D4 receptor selective compounds and a distinct structure-affinity relationship for butyrophenones. European journal of pharmacology. 2008;578:123–136. doi: 10.1016/j.ejphar.2007.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever JR, Gustafson JL, Xu R, Allmon RL, Lever SZ. Sigma1 and sigma2 receptor binding affinity and selectivity of SA4503 and fluoroethyl SA4503. Synapse (New York, NY) 2006;59:350–358. doi: 10.1002/syn.20253. [DOI] [PubMed] [Google Scholar]
- Malik M, Rangel-Barajas C, Sumien N, Su C, Singh M, Chen Z, Huang RQ, Meunier J, Maurice T, Mach RH, Luedtke RR. The effects of sigma (sigma1) receptor-selective ligands on muscarinic receptor antagonist-induced cognitive deficits in mice. British journal of pharmacology. 2015;172:2519–2531. doi: 10.1111/bph.13076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso R, Olivan S, Rando A, Casas C, Osta R, Navarro X. Sigma-1R agonist improves motor function and motoneuron survival in ALS mice. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics. 2012;9:814–826. doi: 10.1007/s13311-012-0140-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto RR, Bowen WD, Tom MA, Vo VN, Truong DD, De Costa BR. Characterization of two novel sigma receptor ligands: antidystonic effects in rats suggest sigma receptor antagonism. European journal of pharmacology. 1995;280:301–310. doi: 10.1016/0014-2999(95)00208-3. [DOI] [PubMed] [Google Scholar]
- Mentz S, de Lacalle S, Baerga-Ortiz A, Knauer MF, Knauer DJ, Komives EA. Mechanism of thrombin clearance by human astrocytoma cells. Journal of neurochemistry. 1999;72:980–987. doi: 10.1046/j.1471-4159.1999.0720980.x. [DOI] [PubMed] [Google Scholar]
- Miklic S, Juric DM, Carman-Krzan M. Differences in the regulation of BDNF and NGF synthesis in cultured neonatal rat astrocytes. International journal of developmental neuroscience : the official journal of the International Society for Developmental Neuroscience. 2004;22:119–130. doi: 10.1016/j.ijdevneu.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Moon JY, Roh DH, Yoon SY, Choi SR, Kwon SG, Choi HS, Kang SY, Han HJ, Beitz AJ, Oh SB, Lee JH. sigma1 receptors activate astrocytes via p38 MAPK phosphorylation leading to the development of mechanical allodynia in a mouse model of neuropathic pain. British journal of pharmacology. 2014;171:5881–5897. doi: 10.1111/bph.12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriguchi S, Sakagami H, Yabuki Y, Sasaki Y, Izumi H, Zhang C, Han F, Fukunaga K. Stimulation of Sigma-1 Receptor Ameliorates Depressive-like Behaviors in CaMKIV Null Mice. Molecular neurobiology. 2015;52:1210–1222. doi: 10.1007/s12035-014-8923-2. [DOI] [PubMed] [Google Scholar]
- Moritz C, Berardi F, Abate C, Peri F. Live imaging reveals a new role for the sigma-1 (sigma1) receptor in allowing microglia to leave brain injuries. Neuroscience letters. 2015;591:13–18. doi: 10.1016/j.neulet.2015.02.004. [DOI] [PubMed] [Google Scholar]
- Murphy BP, Pang TY, Hannan AJ, Proffitt TM, McConchie M, Kerr M, Markulev C, O’Donnell C, McGorry PD, Berger GE. Vascular endothelial growth factor and brain-derived neurotrophic factor in quetiapine treated first-episode psychosis. Schizophrenia research and treatment. 2014;2014:719395. doi: 10.1155/2014/719395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Honda S, Tohyama Y, Imai Y, Kohsaka S, Kurihara T. Neurotrophin secretion from cultured microglia. Journal of neuroscience research. 2001;65:322–331. doi: 10.1002/jnr.1157. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Ishima T, Iyo M, Hashimoto K. Potentiation of nerve growth factor-induced neurite outgrowth by fluvoxamine: role of sigma-1 receptors, IP3 receptors and cellular signaling pathways. PloS one. 2008;3:e2558. doi: 10.1371/journal.pone.0002558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuyama S, Imagawa Y, Ogawa S, Araki H, Ajima A, Tanaka M, Muramatsu M, Nakazato A, Yamaguchi K, Yoshida M. NE-100, a novel sigma receptor ligand: in vivo tests. Life Sciences. 1993;53:PL285–290. doi: 10.1016/0024-3205(93)90588-t. [DOI] [PubMed] [Google Scholar]
- Ovalle S, Andreu F, Perez MP, Zamanillo D, Guitart X. Effect of the novel sigma1 receptor ligand and putative atypical antipsychotic E-5842 on BDNF mRNA expression in the rat brain. Neuroreport. 2002;13:2345–2348. doi: 10.1097/00001756-200212030-00035. [DOI] [PubMed] [Google Scholar]
- Palacios G, Muro A, Vela JM, Molina-Holgado E, Guitart X, Ovalle S, Zamanillo D. Immunohistochemical localization of the sigma1-receptor in oligodendrocytes in the rat central nervous system. Brain research. 2003;961:92–99. doi: 10.1016/s0006-8993(02)03892-1. [DOI] [PubMed] [Google Scholar]
- Palacios G, Muro A, Verdu E, Pumarola M, Vela JM. Immunohistochemical localization of the sigma1 receptor in Schwann cells of rat sciatic nerve. Brain research. 2004;1007:65–70. doi: 10.1016/j.brainres.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Park SW, Lee SK, Kim JM, Yoon JS, Kim YH. Effects of quetiapine on the brain-derived neurotrophic factor expression in the hippocampus and neocortex of rats. Neuroscience letters. 2006;402:25–29. doi: 10.1016/j.neulet.2006.03.028. [DOI] [PubMed] [Google Scholar]
- Peviani M, Salvaneschi E, Bontempi L, Petese A, Manzo A, Rossi D, Salmona M, Collina S, Bigini P, Curti D. Neuroprotective effects of the Sigma-1 receptor (S1R) agonist PRE-084, in a mouse model of motor neuron disease not linked to SOD1 mutation. Neurobiology of disease. 2014;62:218–232. doi: 10.1016/j.nbd.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Polacchini A, Metelli G, Francavilla R, Baj G, Florean M, Mascaretti LG, Tongiorgi E. A method for reproducible measurements of serum BDNF: comparison of the performance of six commercial assays. Scientific reports. 2015;5:17989. doi: 10.1038/srep17989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu WS, Wang YH, Wang JP, Tang YX, Zhang Q, Tian DS, Yu ZY, Xie MJ, Wang W. Galectin-1 enhances astrocytic BDNF production and improves functional outcome in rats following ischemia. Neurochemical research. 2010;35:1716–1724. doi: 10.1007/s11064-010-0234-z. [DOI] [PubMed] [Google Scholar]
- Ring RM, Regan CM. Captodiamine, a putative antidepressant, enhances hypothalamic BDNF expression in vivo by synergistic 5-HT2c receptor antagonism and sigma-1 receptor agonism. Journal of psychopharmacology (Oxford, England) 2013;27:930–939. doi: 10.1177/0269881113497614. [DOI] [PubMed] [Google Scholar]
- Robson MJ, Turner RC, Naser ZJ, McCurdy CR, Huber JD, Matsumoto RR. SN79, a sigma receptor ligand, blocks methamphetamine-induced microglial activation and cytokine upregulation. Experimental neurology. 2013;247:134–142. doi: 10.1016/j.expneurol.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson MJ, Turner RC, Naser ZJ, McCurdy CR, O’Callaghan JP, Huber JD, Matsumoto RR. SN79, a sigma receptor antagonist, attenuates methamphetamine-induced astrogliosis through a blockade of OSMR/gp130 signaling and STAT3 phosphorylation. Experimental neurology. 2014;254:180–189. doi: 10.1016/j.expneurol.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscher K, Wieloch T. The involvement of the sigma-1 receptor in neurodegeneration and neurorestoration. Journal of pharmacological sciences. 2015;127:30–35. doi: 10.1016/j.jphs.2014.11.011. [DOI] [PubMed] [Google Scholar]
- Schetz JA, Perez E, Liu R, Chen S, Lee I, Simpkins JW. A prototypical Sigma-1 receptor antagonist protects against brain ischemia. Brain research. 2007;1181:1–9. doi: 10.1016/j.brainres.2007.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo MK, Lee CH, Cho HY, You YS, Lee BJ, Lee JG, Park SW, Kim YH. Effects of antipsychotic drugs on the expression of synapse-associated proteins in the frontal cortex of rats subjected to immobilization stress. Psychiatry research. 2015;229:968–974. doi: 10.1016/j.psychres.2015.05.098. [DOI] [PubMed] [Google Scholar]
- Shen YC, Wang YH, Chou YC, Liou KT, Yen JC, Wang WY, Liao JF. Dimemorfan protects rats against ischemic stroke through activation of sigma-1 receptor-mediated mechanisms by decreasing glutamate accumulation. Journal of neurochemistry. 2008;104:558–572. doi: 10.1111/j.1471-4159.2007.05058.x. [DOI] [PubMed] [Google Scholar]
- Shioda N, Sawai M, Ishizuka Y, Shirao T, Fukunaga K. Nuclear Translocation of Calcium/Calmodulin-dependent Protein Kinase IIdelta3 Promoted by Protein Phosphatase-1 Enhances Brain-derived Neurotrophic Factor Expression in Dopaminergic Neurons. The Journal of biological chemistry. 2015;290:21663–21675. doi: 10.1074/jbc.M115.664920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su TP, Hayashi T, Maurice T, Buch S, Ruoho AE. The sigma-1 receptor chaperone as an inter-organelle signaling modulator. Trends in pharmacological sciences. 2010;31:557–566. doi: 10.1016/j.tips.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun P, Li X, Chen C, Chen Q, Ouyang Q, Liu F, Xiang Z, Yuan H. Activating transcription factor 4 modulates BDNF release from microglial cells. Journal of molecular neuroscience : MN. 2014;52:225–230. doi: 10.1007/s12031-013-0126-1. [DOI] [PubMed] [Google Scholar]
- Taylor S, Srinivasan B, Wordinger RJ, Roque RS. Glutamate stimulates neurotrophin expression in cultured Muller cells. Brain research Molecular brain research. 2003;111:189–197. doi: 10.1016/s0169-328x(03)00030-5. [DOI] [PubMed] [Google Scholar]
- Tettamanti G, Cattaneo AG, Gornati R, de Eguileor M, Bernardini G, Binelli G. Phylogenesis of brain-derived neurotrophic factor (BDNF) in vertebrates. Gene. 2010;450:85–93. doi: 10.1016/j.gene.2009.07.023. [DOI] [PubMed] [Google Scholar]
- Toyomoto M, Ohta M, Okumura K, Yano H, Matsumoto K, Inoue S, Hayashi K, Ikeda K. Prostaglandins are powerful inducers of NGF and BDNF production in mouse astrocyte cultures. FEBS letters. 2004;562:211–215. doi: 10.1016/S0014-5793(04)00246-7. [DOI] [PubMed] [Google Scholar]
- Trang T, Beggs S, Wan X, Salter MW. P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:3518–3528. doi: 10.1523/JNEUROSCI.5714-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich S, Neuhof S, Braun V, Meyer FP. Reduced haloperidol does not interfere with the antipsychotic activity of haloperidol in the treatment of acute schizophrenia. International clinical psychopharmacology. 1999;14:219–228. doi: 10.1097/00004850-199907000-00003. [DOI] [PubMed] [Google Scholar]
- Vilner BJ, John CS, Bowen WD. Sigma-1 and sigma-2 receptors are expressed in a wide variety of human and rodent tumor cell lines. Cancer research. 1995;55:408–413. [PubMed] [Google Scholar]
- Walker JM, Bowen WD, Walker FO, Matsumoto RR, De Costa B, Rice KC. Sigma receptors: biology and function. Pharmacological reviews. 1990;42:355–402. [PubMed] [Google Scholar]
- Wegleiter K, Hermann M, Posod A, Wechselberger K, Stanika RI, Obermair GJ, Kiechl-Kohlendorfer U, Urbanek M, Griesmaier E. The sigma-1 receptor agonist 4-phenyl-1-(4-phenylbutyl) piperidine (PPBP) protects against newborn excitotoxic brain injury by stabilizing the mitochondrial membrane potential in vitro and inhibiting microglial activation in vivo. Experimental neurology. 2014;261:501–509. doi: 10.1016/j.expneurol.2014.07.022. [DOI] [PubMed] [Google Scholar]
- Wu H, Friedman WJ, Dreyfus CF. Differential regulation of neurotrophin expression in basal forebrain astrocytes by neuronal signals. Journal of neuroscience research. 2004;76:76–85. doi: 10.1002/jnr.20060. [DOI] [PubMed] [Google Scholar]
- Wu LL, Fan Y, Li S, Li XJ, Zhou XF. Huntingtin-associated protein-1 interacts with pro-brain-derived neurotrophic factor and mediates its transport and release. The Journal of biological chemistry. 2010;285:5614–5623. doi: 10.1074/jbc.M109.073197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Bowen WD. Role of sigma-1 receptor C-terminal segment in inositol 1,4,5-trisphosphate receptor activation: constitutive enhancement of calcium signaling in MCF-7 tumor cells. The Journal of biological chemistry. 2008;283:28198–28215. doi: 10.1074/jbc.M802099200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z, Li L, Zheng LT, Xu Z, Guo L, Zhen X. Allosteric modulation of sigma-1 receptors by SKF83959 inhibits microglia-mediated inflammation. Journal of neurochemistry. 2015;134:904–914. doi: 10.1111/jnc.13182. [DOI] [PubMed] [Google Scholar]
- Yang H, Feng GD, Liang Z, Vitale A, Jiao XY, Ju G, You SW. In vitro beneficial activation of microglial cells by mechanically-injured astrocytes enhances the synthesis and secretion of BDNF through p38MAPK. Neurochemistry international. 2012;61:175–186. doi: 10.1016/j.neuint.2012.04.020. [DOI] [PubMed] [Google Scholar]
- Yang M, Lim Y, Li X, Zhong JH, Zhou XF. Precursor of brain-derived neurotrophic factor (proBDNF) forms a complex with Huntingtin-associated protein-1 (HAP1) and sortilin that modulates proBDNF trafficking, degradation, and processing. The Journal of biological chemistry. 2011;286:16272–16284. doi: 10.1074/jbc.M110.195347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Ishikawa M, Iyo M, Hashimoto K. Serum Levels of Mature Brain-Derived Neurotrophic Factor (BDNF) and Its Precursor proBDNF in Healthy Subjects. The Open Clinical Chemistry Journal. 2012;5:7–12. [Google Scholar]
- Zhang F, Lu YF, Wu Q, Liu J, Shi JS. Resveratrol promotes neurotrophic factor release from astroglia. Experimental biology and medicine (Maywood, NJ) 2012;237:943–948. doi: 10.1258/ebm.2012.012044. [DOI] [PubMed] [Google Scholar]
- Zhao J, Ha Y, Liou GI, Gonsalvez GB, Smith SB, Bollinger KE. Sigma receptor ligand, (+)-pentazocine, suppresses inflammatory responses of retinal microglia. Investigative ophthalmology & visual science. 2014;55:3375–3384. doi: 10.1167/iovs.13-12823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P. Neuroactive steroid regulation of neurotransmitter release in the CNS: action, mechanism and possible significance. Progress in neurobiology. 2009;89:134–152. doi: 10.1016/j.pneurobio.2009.07.001. [DOI] [PubMed] [Google Scholar]