Abstract
The Na/K-ATPase as an essential ion pump was discovered more than 50 years ago (1,2). The signaling function of Na/K-ATPase has been gradually appreciated over the last 20 years, first from the studies of regulatory effects of ouabain on cardiac cell growth. Several reviews on this topic have been written during the last few years (3–8). This article will focus on the molecular mechanism of Na/KATPase-mediated signal transduction and its potential regulatory role in renal physiology and diseases.
Introduction
The Na/K-ATPase
The Na/K-ATPase (or sodium pump) was discovered by Skou in 1957 (1). It is a member of the P-type ATPase family and the most important ion pump in animal physiology. By hydrolyzing ATP, Na/K-ATPase is capable of transporting sodium and potassium ions across the cell membrane against their concentration gradients. The Na/K-ATPase consists of two noncovalently linked α and β subunits. A γ subunit (a member of the FXYD proteins) is associated with the Na/K-ATPase in a tissue-specific manner and regulates the functionality of the enzyme (9,10). The α subunit (about 112 kDa) contains the ATP and other ligand binding sites, and couples ATP hydrolysis with ion movement; meanwhile the β subunit is essential for the assembly of a fully functional enzyme. Four α isoforms are found in human tissues and they are expressed in a tissue-specific manner. The α1 isoform is found in all cells. The α2 and α3 isoforms are mainly expressed in skeletal muscle, neuronal tissue, and cardiac myocytes. The α4 isoform is in testis and regulates sperm motility (11–19). Na/KATPase alpha subunits consist of three functional domains: The Actuator (A) domain is composed of the N-terminus and the second cytosolic domain (CD2)1 connected to transmembrane helices M2 and M3 and which functions to regulate the sodium and potassium binding site. Next, the highly conserved phosphorylation (P) domain resides close to the membrane and finally, the nucleotide binding (N) domain. The Na/K-ATPase resides in two main conformational states, the E1 sodium bound state and the E2 potassium bound state. During the transport cycle, the A domain rotates while the N domain closes up, which opens and closes the A, N and P domains in the E1 and E2 conformations, respectively (20–23). Studies during the last 20 years have indicated that the Na/K-ATPase possess an important cell-signaling role as well through its interactions with endogenous cardiotonic steroids (CTS) and signaling proteins such as Src kinase (3–8).
Molecular mechanism of Na/K-ATPase in signal transduction
Regulation of Cell Growth by Cardiotonic Steroids (CTS)
CTS include plant-derived digitalis drugs such as digoxin, and vertebrate-derived aglycones such as bufalin (3, 5). Digoxin has long been used to treat congestive heart failure (CHF) and/or atria fibrillation while bufalin and its derivatives represent the major active components of a traditional Chinese medicine called Chan'su, which is prescribed as a cardiotonic, diuretic and anodyne agent (3, 25, 26). Further, ouabain and marinobufagenin (MBG) have both been found in animal and human blood, and have been considered as endogenous steroids. Their synthesis and release appears to be regulated (3, 5).
It has been well established that CTS bind and fix Na/K-ATPase (either phosphorylated or nonphosphorylated) in an E2-like conformation, resulting in the inhibition of the pumping cycle (27, 28). Moreover, studies utilizing purified pig and shark Na/K-ATPase indicate that ouabain-bound Na/K-ATPase is less prone to thermal denaturation than non-ouabain bound pump (28), suggesting a more compact conformation that may make it less likely to interact with its partner proteins.
Since the 1970s, many studies have documented a role of CTS in the regulation of cell growth (29,30). Recent studies have revealed some important pathways relevant to this regulation. Specifically, it has been demonstrated that CTS activate multiple protein/lipid kinases and stimulate either differentiation/apoptosis or hypertrophic/proliferative growth in a cell type–specific manner (30–53). Moreover, it appears that the signaling role that CTS play could occur at nano- and subnanomolar concentrations that would not affect the overall cellular Na/K-ATPase pumping activity. Therefore, it becomes evident that the Na/K-ATPase may have CTS-regulated non-pumping functions that are important to cell growth.
Interaction between the Na/K-ATPase and Src
Src family kinases are 52–62 kDa membrane associated non-receptor tyrosine kinases (54,55). They are present in a wide variety of cell types including kidney epithelial cells, and play a vital role in the regulation of signal transduction pathways. For example, activation of Src has been implicated in the generation of reactive oxygen species, the development of tissue fibrosis, and the growth and metastasis of cancer. Src contains a kinase domain, a N terminus that regulates the kinase association with the plasma membrane, and a C-terminal regulatory domain. The Src homology 2 (SH2) and Src homology 3 (SH3) domains represent the most crucial domains of Src regulation. The two most important phosphorylation sites are Y529 and Y418. Phosphorylation of Y529 keeps Src in an inactive state by facilitating the interaction between pY529 and the SH2 domain. Due to this, the SH3 domain then becomes bound to the kinase domain linker, preventing the formation of a salt bridge in the kinase domain between E310 and K295. Autophosphorylation of the second site, Y418, leads to an open conformation of Src, which stimulates Src activity. Detection of phosphorylation in this site is a marker for overall Src activity.
Many laboratories have investigated the role of Src in Na/K-ATPase-mediated signal transduction (37, 46, 56–85). Moreover, the following data indicate that the Na/K-ATPase and Src most likely interact directly to form a functional receptor complex (46, 58–64, 68–71). First, the Na/K-ATPase and Src are co-enriched in caveolar fractions in different cell lines and in tissues. Second, immunofluorescence imaging analyses show a co-localization of these two proteins in the plasma membrane. Third, both proteins could be co-immunoprecipitated by either anti-Na/K-ATPase or anti-Src antibodies. Fourth, fluorescence resonance energy transfer (FRET) analysis indicates that both proteins are in close proximity, providing further support of a direct interaction in live cells. Finally, in vitro GST pull-down assays demonstrate direct interactions between the α1 subunit of Na/K-ATPase and Src. Based on these assays, binding of the α1 and Src involves at least two contacting sites: one being the CD2 of α1 and Src SH2, and the other consisting of the N domain of α1 and the Src kinase domain (64). This N domain/kinase domain interaction is responsible for keeping Src in an inactive state (64). Further mapping of this interaction leads to the identification of 20 amino acid peptide (NaKtide) that binds and inhibits Src in an ATP-independent manner (86).
It should be noted that both Na/K-ATPase and Src are highly expressed in the plasma membrane, especially in epithelial cells. Moreover, it has been estimated that the pool of Na/K-ATPase is much larger than that of Src in renal epithelial cells. Therefore, it is likely that the Na/K-ATPase represents an important negative regulator of cellular Src signaling through its interaction with the SH2 and kinase domain of Src. Indeed, both in vitro and in vivo studies have revealed a Src-interacting pool of Na/KATPase (62,67,83). There is evidence that a reduction in cellular expression of Na/K-ATPase is sufficient to increase Src activity. Moreover, restoration of Na/K-ATPase expression, even by a nonfunctional mutant pump, is capable of reducing cellular Src activity in cell cultures (62). Although it remains to be further studied, the potential clinical relevance of these findings is self-evident. It is known that the expression of Na/K-ATPase is reduced under many clinical conditions such as chronic renal insufficiency, heart failure and in certain types of cancer. It is also known that Src activity is elevated and plays an important role in the pathogeneses of these diseases. Thus, this newly appreciated Na/K-ATPase/Src interaction may serve as a potential target for therapeutic intervention. To this end, recent studies have demonstrated the effectiveness of the Na/K-ATPase-derived pNaKtide in blocking Src-mediated signal transduction, and growth of human prostate cancer cells and tumor xenografts (87).
Despite the evidence of a direct interaction between the Na/K-ATPase and Src, it is important to point out other potential mechanisms of regulation. Biochemical assays using purified Na/K-ATPase demonstrate that ouabain and Na/K-ATPase may exert regulation over Src activity through the changing energetic state of the cell, in addition to a direct interaction with Src (88). Moreover, others have reported that ouabain was incapable of regulating the activity of Src that was co-purified with the Na/KATPase from pig kidney (134). Although these studies took place in a cell-free system, it is clear that more studies are required to further clarify the role of Na/K-ATPase in Src regulation.
The Na/K-ATPase/Src complex as a potential receptor mechanism of CTS-induced protein tyrosine phosphorylation
Studies utilizing Src inhibitors as well as Src knock out and knock in cells provided the first clue that the Na/K-ATPase/Src complex may function as a receptor mechanism of CTS-induced protein tyrosine phosphorylation (58–69). Subsequently, studies using recombinant Src kinase and other functional domains indicate that the binding of the kinase domain to the Na/K-ATPase is inhibited by ouabain, suggesting that the Na/K-ATPase/Src interaction may depend on the conformational state of Na/K-ATPase. Indeed, it has been demonstrated that chemical modifiers that stabilize the Na/K-ATPase in either E1- or E2-like conformation have opposite effects on Src activity (89). Moreover, expression of E1/E2 conformation transition-defective mutants is sufficient to alter cellular Src activity and inhibits ouabain-induced activation of protein kinases in live cells (90). Finally, mutation of the Src binding site at the N domain of Na/K-ATPase increases intracellular Src activity and inhibits ouabain-induced signal transduction (91). These new findings have the following two important implications. First, they reveal that the Na/K-ATPase/Src complex could serve as a receptor mechanism and that cardiotonic steroids such as ouabain are agonists of this newly appreciated receptor. Second, it suggests that ligands other than cardiotonic steroids may also use this receptor mechanism to transduce signals as long as they are capable of altering the conformational states of Na/K-ATPase. To this end, it has been demonstrated that changes in intracellular Na+ or extracellular K+ can affect cellular kinase activity through the expression of Na/K-ATPase (89).
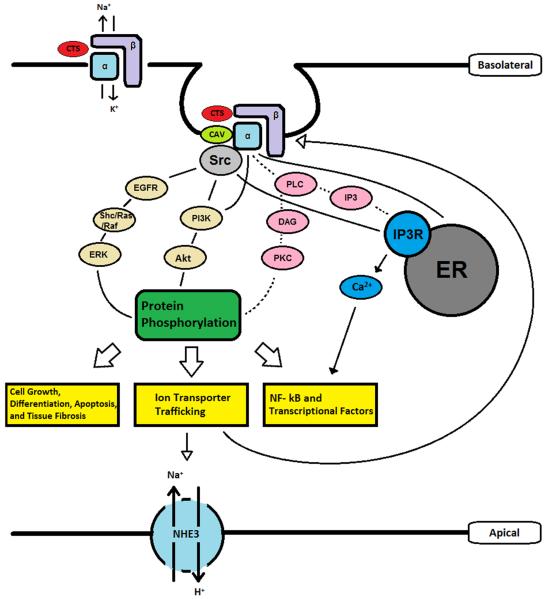
In short, recent studies suggest that the Na/K-ATPase/Src complex mimics the actions of a receptor tyrosine kinase. Ouabain and other CTS serve as the ligand of this receptor complex, leading to the generation and amplification of signaling cascades through the recruitment and assembly of a cell-specific Na/K-ATPase signalosome (Fig. 1). For example, in renal epithelial cells, CTS activation of the Na/K-ATPase/Src receptor complex has been shown to trans-activate the EGF receptor (EGFR) in a Src-dependent manner (37,59,68,71,72). The activation of EGFR in turn results in the assembly of several signaling platforms through recruitment of Shc, PLC-γ and PI3K to the receptor complex. This leads to the stimulation of the Ras/Raf/MEK/ERK1/2 and the activation of PLC-γ/PKC cascades, resulting in the production of the second messengers, DAG and IP3 (66,75,76). The termination of this signaling process has also been investigated and the data suggest the endocytosis of Na/K-ATPase signalosome as being important. Moreover, it appears that the endocytosis requires the activation of Src, EGFR, PI3K and PKCs (92–94). It is of interest to note that this endocytosis seems to involve both clathrin and caveolin-1. Because caveolin-1 is important for ouabain to activate Src and EGFR, it seems to be likely that the ouabain-induced endocytosis of Na/K-ATPase is mediated through the clathrin pathway (93).
Figure 1.
Schematic presentation of Na/K-ATPase-Mediated Signal Transduction in Renal Epithelial Cells. Src, proto-oncogene tyrosine-protein kinase Src (sarcoma); CTS, cardiotonic steroids; Cav, caveolin-1; EGF, epidermal growth factor; EGFR, EGF receptor; PLC, phospholipase C; DAG, diacyl glycerol; IP3, Inositol trisphosphate; IP3R, IP3 receptor; PI3K, Phosphoinositide 3-kinase; Akt, protein kinase B; ERK, extracellular signal-regulated kinase.
It is important to note that signaling proteins other than Src may also interact with the Na/K-ATPase in a conformation-dependent manner. For example, it has been shown that the Na/K-ATPase interacts with IP3 receptor (IP3R) directly (95,96). Ankyrin-B, an intracellular scaffolding protein, is thought to be essential in linking the IP3R with Na/K-ATPase as it has been shown to bind to the C-terminus of the IP3R as well as to the α1 subunit of Na/K-ATPase. This direct interaction between the IP3R and Na/K-ATPase allows ouabain to function in regulating low frequency calcium oscillations. Similarly, the Na/K-ATPase may directly interact and regulate PI3K through the N-terminal polyproline domain (97–100). However, it is equally important to point out that cross-talk and redundancy do exist among these different interactions. For example, both IP3R and PI3K are enriched with the Na/K-ATPase/Src receptor complex and caveolin-1 (75,76). Functionally, inhibition of Src blocked ouabain-induced calcium oscillations (7) and attenuated ouabain-induced activation of PI3K (98). Moreover, we have demonstrated recently that while expression of an E2 mutant of Na/K-ATPase blocked ouabain-induced activation of Src and ERK, it also produced a partial inhibition of ouabain-induced stimulation of PI3K/Akt pathway in LLC-PK1 cells (90).
The signaling Na/K-ATPase and the Kidney Structure and Function
CTS and renal salt handling
Guyton suggested that the kidney is the most important organ in the regulation of Na+ handling and thus blood pressure (101). This concept has now been well accepted (102). The Na/K-ATPase, and in particular, the α1 isoform, reside on the basolateral membrane of renal epithelial cells, and therefore plays a crucial role in renal salt handling (2–5). Because CTS inhibit the ionic pumping function of Na/K-ATPase, endogenous CTS have been considered as the natriuretic hormones that Dahl and others suggested many years ago (103–105). Hamlyn and others have provided supporting evidence as they found a correlation between levels of serum CTS and blood pressure in patients suffering from hypertension (106,107). Furthermore, the application of anti-digoxin antibodies was able to lower blood pressure (108). However, the most convincing evidence that CTS and Na/K-ATPase are involved in renal salt handling comes from Lingrel's laboratory. By utilizing transgenic mice, they were able to demonstrate greater natriuretic response in response to Na+ loading, in mice expressing a mutated form of Na/K-ATPase α1 isoform that is highly sensitive to ouabain, than in wild type mice (109). Finally, studies have shown that elevated levels of endogenous CTS are found in patients suffering from chronic renal disease as well as congestive heart failure (107,110–112).
Because CTS can also activate the Na/K-ATPase/Src receptor complex, it has been suggested that CTS may be able to regulate renal salt handling via pathways separate from its role in inhibiting the pumping function of Na/K-ATPase. There is evidence that CTS induce the endocytosis of Na/K-ATPase through the activation of Src/PLC/PKC and PI3K cascades in renal epithelial cells (92–94). Concomitantly, CTS also reduced surface expression of NHE3 through both short-term (i.e, endocytosis/exocytosis-mediated) and long-term (i.e., gene expression-related) mechanisms (113–116). The NHE3 belongs to a family of electroneutral mammalian Na+/H+ exchangers (117). In the renal proximal tubules, NHE3 resides in the apical membrane, and acts as a major Na+ transporter, responsible for Na+, HCO3−, and fluid reabsorption (117). As expected, up-regulation of NHE3 in the proximal tubular cells is associated with the development of hypertension (118). Thus, this coordinated regulation of basolateral Na/K-ATPase and apical NHE3 by the CTS may represent an important mechanism of salt handling by the kidney. Indeed, application of CTS to the basolateral but not apical side of renal epithelial cell monolayer in culture reduces the transcellular movement of Na+ (113,114). Ex vivo studies provide additional evidence of the importance of CTS and Na/K-ATPase-mediated signal transduction in the regulation of tubular Na+ movement (84). Finally, studies with Dahl salt-sensitive rats have revealed the inability of CTS in the activation of Na/K-ATPase-mediated signal transduction in renal proximal tubular cells, resulting in decreased ability of renal salt handling and consequently an increase in blood pressure in response to salt loading (119).
The receptor Na/K-ATPase and tissue fibrosis
The first suggestion that chronic activation of receptor Na/K-ATPase by CTS could lead to tissue fibrosis came from studies of cardiac fibrosis in 5/6 nephrectomized rats (77). Subsequent studies showed that infusion of CTS was sufficient to increase tissue fibrosis and that neutralization of the increase in endogenous CTS by either passive or active immunization against MBG or by administration of a non-specific ouabain inhibitor spironolactone was effective in preventing or reversing fibrosis in 5/6 nephrectomized rats (78–80, 120, 121). Moreover, these studies demonstrated that CTS were potent stimulators of collagen production in dermal and cardiac fibroblasts as well as renal epithelial cells. These stimulatory effects were initiated by the activation of receptor Na/K-ATPase/Src complex and involved the increased production of ROS, activation of PKC and the down-regulation of transcriptional factor Fli-1 (80).
More recently, an interaction between the Na/K-ATPase and scavenger receptor CD36 in the formation of proinflammatory signaling loop has been demonstrated in the renal proximal tubular cells (122). In addition, the data suggest that ligands generated in hyperlipidemia could work in concert with endogenous CTS to activate the receptor Na/K-ATPase and CD36 signaling loop, which promotes the development of chronic inflammation, ROS stress and renal fibrosis underlying the renal dysfunction common to proatherogenic hyperlipidemic states. In short, we suggest that while the activation of receptor Na/K-ATPase/Src complex by endogenous CTS is required for the kidney to get rid of excess salt, the trade-off could be the development of tissue fibrosis especially under the stress conditions such as chronic renal insufficiency, high salt intake and hyperlipidimic states where the receptor function has been compromised (78, 119, 122).
CTS in relation to other renal conditions
Blanco's group has provided evidence that the CTS-activated Na/K-ATPase/Src receptor complex may plays a role in the development of Autosomal Dominant Polycystic Kidney Disease (ADPKD) by stimulating cell proliferation (8,67,123,124). ADPKD occurs in approximately 1 in 500 to 1000 births and is marked by the presence of fluid filled cysts that form within the kidney (125). ADPKD is considered one of the most prevalent causes of end stage renal disease and approximately half of all patients with ADPKD develop end-stage renal disease between the ages of 50 and 60. The cysts begin to form in utero and eventually, lead to the expansion of the kidney. In addition to the stimulation of human ADPKD cell proliferation, ouabain, working in concert with the increase in cellular cAMP, promotes the formation and growth of cysts (124). Interestingly, Blanco and others have demonstrated an increased sensitivity of human ADPKD cells to ouabain (68). This increase in ouabain sensitivity could be an important link between endogenous ouabain and the pathogenesis of cysts. Mechanistically, it appears that the increase in ouabain sensitivity is due to the interaction between the Na/K-ATPase and polycystin-1 (126). As discussed below, ouabain appears to also have anti-apoptotic effects in renal epithelial cells. This could provide further stimulation of ADPDK cell proliferation. In short, the new findings suggest a potential role of Na/K-ATPase signaling in the pathogenesis of ADPKD.
Cereijido and his colleagues have long used MDCK cells as a model to study the role of CTS-activated signal transduction in epithelial biology. They and others have demonstrated that ouabain exerts a complex regulation of the expression, trafficking and degradation of proteins that are important for the formation of tight junctions in MDCK cells (127–130, 133). These regulations are mediated by the activation of Src-dependent signaling pathways. While ouabain at lower, more physiologically relevant doses, promotes the formation of tight junction, it causes the disassembly of tight junction at higher doses. This disassembly pathway may be dependent on Src and ERK1/2 signaling pathways (133). Moreover, recent studies have shown that ouabain also regulates the assembly and growth of cilia in MDCK cells (130). In view of the role of cilia in the pathogenesis of ADPKD, the new findings also call for more mechanistic investigations of the Na/K-ATPase-mediated signal transduction in the development of ADPKD and in cilia biogenesis.
As discussed above, Aperia's group has reported the interaction between the Na/K-ATPase and IP3R in primary cultures of rat proximal tubular cells and COS-7 cells derived from embryonic monkey kidney. Moreover, they have demonstrated that ouabain stimulates this interaction, resulting in slow calcium oscillations (7, 95,96). We have subsequently confirmed the interaction between the Na/K-ATPase and IP3R, and provided evidence that this regulation involves the activation of receptor Na/K-ATPase/Src complex in LLC-PK1 cells (75,76). The importance of Src activation has been recently confirmed by Aperia's laboratory in COS-7 cells (7). Interestingly, several studies have indicated that CTS-induced calcium oscillations have anti-apoptotic effects in renal epithelial cells (131, 132). Functionally, the anti-apoptotic effects of CTS have been found to be protective in the developmental programming of kidney exposed to malnutrition and in kidneys exposed to shiga toxin.
A Look Ahead
The appreciation of the Na/K-ATPase in physiology and diseases has only grown in recent years as the mechanisms by which the Na/K-ATPase/Src receptor complex functions as a receptor have been elucidated. There is ample evidence for a role of this receptor complex in the formation of tight junction, kidney development and in the maintenance of renal function. Furthermore, a chronic stimulation of the receptor appears to be involved in renal fibrosis, inflammation and the pathogenesis of ADPKD. It has become clear that further studies still need to be conducted in order to better understand the intricacies and complexities of Na/K-ATPase-mediated signal transduction in the kidney. Moreover, there is a clear need to develop, and test novel specific receptor agonists and antagonists in animal models of renal diseases. Such work is crucial in the pursuit of new therapeutic strategies to remedy renal diseases related to CTS and the Na/K-ATPase/Src receptor complex.
Acknowledgments
This work was supported by National Institutes of Health under grants HL-109015 from NHLBI.
Footnotes
Conflict of Interests The authors have no conflicts of interest to declare.
CD2: second cytosolic domain, P: phosphorylation, N: nucleotide binding, CTS: cardiotonic steroids, CHF: congestive heart failure, MBG: marinobufagenin, SH2: Src homology 2, SH3: Src homology 3, FRET: fluorescence resonance energy transfer, EGFR: EGF receptor, ADPKD: autosomal dominant polycystic kidney disease.
References
- 1.Skou JC. The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim Biophys Acta. 1989;1000:439–46. [PubMed] [Google Scholar]
- 2.Feraille E, Doucet A. Sodium-potassium-adenosinetriphosphatase-dependent sodium transport in the kidney: hormonal control. Physiol Rev. 2001;81(1):345–418. doi: 10.1152/physrev.2001.81.1.345. [DOI] [PubMed] [Google Scholar]
- 3.Schoner W, Scheiner-Bobis G. Endogenous and exogenous cardiac glycosides: their roles in hypertension, salt metabolism, and cell growth. Am J Physiol Cell Physiol. 2007;293(2):C509–36. doi: 10.1152/ajpcell.00098.2007. [DOI] [PubMed] [Google Scholar]
- 4.Xie Z, Cai T. Na+-K+--ATPase-mediated signal transduction: from protein interaction to cellular function. Mol Interv. 2003;3(3):157–68. doi: 10.1124/mi.3.3.157. [DOI] [PubMed] [Google Scholar]
- 5.Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacol Rev. 2009;61(1):9–38. doi: 10.1124/pr.108.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tian J, Xie Z. The Na-k-ATPase and calcium-signaling microdomains. Physiology (Bethesda) 2008 Aug;23:205–11. doi: 10.1152/physiol.00008.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fontana JM, Burlaka I, Khodus G, Brismar H, Aperia A. Calcium oscillations triggered by cardiotonic steroids. FEBS J. 2013 Nov;280(21):5450–5. doi: 10.1111/febs.12448. [DOI] [PubMed] [Google Scholar]
- 8.Blanco G, Wallace DP. Novel role of ouabain as a cystogenic factor in autosomal dominant polycystic kidney disease. Am J Physiol Renal Physiol. 2013 Sep 15;305(6):F797–812. doi: 10.1152/ajprenal.00248.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sweadner KJ. Isozymes of the Na+/K+-ATPase. Biochim Biophys Acta. 1989 May 9;988(2):185–220. doi: 10.1016/0304-4157(89)90019-1. [DOI] [PubMed] [Google Scholar]
- 10.Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol. 1998 Nov;275(5 Pt 2):F633–50. doi: 10.1152/ajprenal.1998.275.5.F633. [DOI] [PubMed] [Google Scholar]
- 11.Woo AL, James PF, Lingrel JB. Sperm motility is dependent on a unique isoform of the Na,K-ATPase. J Biol Chem. 2000 Jul 7;275(27):20693–9. doi: 10.1074/jbc.M002323200. [DOI] [PubMed] [Google Scholar]
- 12.Sanchez G, Nguyen AN, Timmerberg B, Tash JS, Blanco G. The Na,K-ATPase alpha4 isoform from humans has distinct enzymatic properties and is important for sperm motility. Mol Hum Reprod. 2006 Sep;12(9):565–76. doi: 10.1093/molehr/gal062. [DOI] [PubMed] [Google Scholar]
- 13.Shull GE, Schwartz A, Lingrel JB. Amino-acid sequence of the catalytic subunit of the (Na+ + K+)ATPase deduced from a complementary DNA. Nature. 1985 Aug 22–28;316(6030):691–5. doi: 10.1038/316691a0. [DOI] [PubMed] [Google Scholar]
- 14.Shull GE, Lane LK, Lingrel JB. Amino-acid sequence of the beta-subunit of the (Na+ + K+)ATPase deduced from a cDNA. Nature. 1986 May 22–28;321(6068):429–31. doi: 10.1038/321429a0. [DOI] [PubMed] [Google Scholar]
- 15.Shull GE, Greeb J, Lingrel JB. Molecular cloning of three distinct forms of the Na+,K+-ATPase alpha-subunit from rat brain. Biochemistry. 1986 Dec 16;25(25):8125–32. doi: 10.1021/bi00373a001. [DOI] [PubMed] [Google Scholar]
- 16.Young RM, Lingrel JB. Tissue distribution of mRNAs encoding the alpha isoforms and beta subunit of rat Na+,K+-ATPase. Biochem Biophys Res Commun. 1987 May 29;145(1):52–8. doi: 10.1016/0006-291x(87)91286-1. [DOI] [PubMed] [Google Scholar]
- 17.Shull MM, Lingrel JB. Multiple genes encode the human Na+,K+-ATPase catalytic subunit. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4039–43. doi: 10.1073/pnas.84.12.4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fambrough DM, Bayne EK. Multiple forms of (Na+ + K+)-ATPase in the chicken: selective detection of the major nerve, skeletal muscle, and kidney form by a monoclonal antibody. J Biol Chem. 1983;258:3926–3935. [PubMed] [Google Scholar]
- 19.Sweadner KJ. Two molecular forms of (Na+ + K+)-stimulated ATPase in brain: separation, and difference in affinity for strophanthidin. J Biol Chem. 1979 Jul 10;254(13):6060–7. [PubMed] [Google Scholar]
- 20.Toyoshima C, Nakasako M, Nomura H, Ogawa H. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 A resolution. Nature. 2000 Jun 8;405(6787):647–55. doi: 10.1038/35015017. [DOI] [PubMed] [Google Scholar]
- 21.Morth JP, Pedersen BP, Toustrup-Jensen MS, Sorensen TL, Petersen J, Andersen JP. Crystal structure of the sodium-potassium pump. Nature. 2007 Dec 13;450(7172):1043–9. doi: 10.1038/nature06419. [DOI] [PubMed] [Google Scholar]
- 22.Laursen M, Yatime L, Nissen P, Fedosova NU. Crystal structure of the high-affinity Na+,K+-ATPase-ouabain complex with Mg2+ bound in the cation binding site. Proc Natl Acad Sci U S A. 2013 Jul 2;110(27):10958–63. doi: 10.1073/pnas.1222308110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yatime L, Laursen M, Morth JP, Esmann M, Nissen P, Fedosova NU. Structural insights into the high affinity binding of cardiotonic steroids to the Na+,K+-ATPase. Journal of structural biology. 2011;174(2):296–306. doi: 10.1016/j.jsb.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Hamlyn JM, Blaustein MP, Bova S, DuCharme DW, Harris DW, Mandel F, Mathews WR, Ludens JH. Identification and characterization of a ouabain-like compound from human plasma. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6259–63. doi: 10.1073/pnas.88.14.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akera T, Brody TM. Inotropic action of digitalis and ion transport. Life Sci. 1976;18:135–44. doi: 10.1016/0024-3205(76)90017-5. [DOI] [PubMed] [Google Scholar]
- 26.Chen KK, Kovaríková A. Pharmacology and toxicology of toad venom. J Pharm Sci. 1967;56:1535–1541. doi: 10.1002/jps.2600561202. [DOI] [PubMed] [Google Scholar]
- 27.Post RL, Kume S, Tobin T, Orcutt B, Sen AK. J Gen Physiol. 1969;54:306–326. doi: 10.1085/jgp.54.1.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miles AJ, Fedosova NU, Hoffmann SV, Wallace BA, Esmann M. Stabilisation of Na,KATPase structure by the cardiotonic steroid ouabain. Biochem Biophy Res Comm. 2013;435:300–305. doi: 10.1016/j.bbrc.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 29.Cuff JM, Lichtman A. The early effects of ouabain on potassium metabolism and rate of proliferation of mouse lymphoblasts. J Cell Physiol. 1975;85:209–15. doi: 10.1002/jcp.1040850207. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan JG. Membrane cation transport and the control of proliferation of mammalian cells. Annu Rev Physiol. 1978;40:19–41. doi: 10.1146/annurev.ph.40.030178.000315. [DOI] [PubMed] [Google Scholar]
- 31.Pollack LR, Tate EH, Cook JS. Na+, K+-ATPase in HeLa cells after prolonged growth in low K+ or ouabain. J Cell Physiol. 1981;106:85–97. doi: 10.1002/jcp.1041060110. [DOI] [PubMed] [Google Scholar]
- 32.Griffiths NM, Ogden PH, Cormack R, Lamb JF. Discrepancy between the short and long term effects of ouabain on the sodium pumps of human cells grown in culture. Br J Pharmacol. 1991;104:419–27. doi: 10.1111/j.1476-5381.1991.tb12445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakagawa Y, Rivera V, Larner AC. A role for the Na/K-ATPase in the control of human c-fos and c-jun transcription. J Biol Chem. 1992;267:8785–8. [PubMed] [Google Scholar]
- 34.Golomb E, Hill MR, Brown RG, Keiser HR. Ouabain enhances the mitogenic effect of serum in vascular smooth muscle cells. Am J Hypertens. 1994;7:69–74. doi: 10.1093/ajh/7.1.69. [DOI] [PubMed] [Google Scholar]
- 35.Peng M, Huang L, Xie Z, Huang WH, Askari A. Partial inhibition of Na+/K+-ATPase by ouabain induces the Ca2+-dependent expressions of early-response genes in cardiac myocytes. J Biol Chem. 1996;271:10372–8. doi: 10.1074/jbc.271.17.10372. [DOI] [PubMed] [Google Scholar]
- 36.Huang L, Li H, Xie Z. Ouabain-induced hypertrophy in cultured cardiac myocytes is accompanied by changes in expression of several late response genes. J Mol Cell Cardiol. 1997;29:429–37. doi: 10.1006/jmcc.1996.0320. [DOI] [PubMed] [Google Scholar]
- 37.Aydemir-Koksoy A, Abramowitz J, Allen JC. Ouabain-induced signaling and vascular smooth muscle cell proliferation. J Biol Chem. 2001;276:46605–11. doi: 10.1074/jbc.M106178200. [DOI] [PubMed] [Google Scholar]
- 38.Saunders R, Scheiner-Bobis G. Ouabain stimulates endothelin release and expression in human endothelial cells without inhibiting the sodium pump. Eur J Biochem. 2004;271:1054–62. doi: 10.1111/j.1432-1033.2004.04012.x. [DOI] [PubMed] [Google Scholar]
- 39.Watabe M, Ito K, Masuda Y, Nakajo S, Nakaya K. Activation of AP-1 is required for bufalin-induced apoptosis in human leukemia U937 cells. Oncogene. 1998;16:779–87. doi: 10.1038/sj.onc.1201592. [DOI] [PubMed] [Google Scholar]
- 40.Kawazoe N, Watabe M, Masuda Y, Nakajo S, Nakaya K. Tiam1 is involved in the regulation of bufalin-induced apoptosis in human leukemia cells. Oncogene. 1999;18:2413–21. doi: 10.1038/sj.onc.1202555. [DOI] [PubMed] [Google Scholar]
- 41.McConkey DJ, Lin Y, Nutt LK, Ozel HZ, Newman RA. Cardiac glycosides stimulate Ca2+ increases and apoptosis in androgen-independent, metastatic human prostate adenocarcinoma cells. Cancer Res. 2000;60:3807–12. [PubMed] [Google Scholar]
- 42.Kulikov A, Eva A, Kirch U, Boldyrev A, Scheiner-Bobis G. Ouabain activates signaling pathways associated with cell death in human neuroblastoma. Biochim Biophys Acta. 2007;1768:1691–702. doi: 10.1016/j.bbamem.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 43.Lopez-Lazaro M, Pastor N, Azrak SS, Ayuso MJ, Austin CA, Cortes F. Digitoxin inhibits the growth of cancer cell lines at concentrations commonly found in cardiac patients. J Nat Prod. 2005;68:1642–5. doi: 10.1021/np050226l. [DOI] [PubMed] [Google Scholar]
- 44.Bielawski K, Winnicka K, Bielawska A. Inhibition of DNA topoisomerases I and II, and growth inhibition of breast cancer MCF-7 cells by ouabain, digoxin and proscillaridin A. Biol Pharm Bull. 2006;29:1493–7. doi: 10.1248/bpb.29.1493. [DOI] [PubMed] [Google Scholar]
- 45.Ramirez-Ortega M, Maldonado-Lagunas V, Melendez-Zajgla J, Carrillo-Hernandez JF, Pastelin-Hernandez G, Picazo-Picazo O, Ceballos-Reyes G. Proliferation and apoptosis of HeLa cells induced by in vitro stimulation with digitalis. Eur J Pharmacol. 2006;534:71–6. doi: 10.1016/j.ejphar.2006.01.035. [DOI] [PubMed] [Google Scholar]
- 46.Kometiani P, Liu L, Askari A. Digitalis-induced signaling by Na+/K+-ATPase in human breast cancer cells. Mol Pharmacol. 2005;67:929–36. doi: 10.1124/mol.104.007302. [DOI] [PubMed] [Google Scholar]
- 47.Mijatovic T, Roland I, Van Quaquebeke E, Nilsson B, Mathieu A, Van Vynckt F, Darro F, Blanco G, Facchini V, Kiss R. The alpha1 subunit of the sodium pump could represent a novel target to combat non-small cell lung cancers. J Pathol. 2007;212:170–9. doi: 10.1002/path.2172. [DOI] [PubMed] [Google Scholar]
- 48.Wang Y, Qiu Q, Shen JJ, Li DD, Jiang XJ, Si SY, Shao RG, Wang Z. Cardiac glycosides induce autophagy in human non-small cell lung cancer cells through regulation of dual signaling pathways. Int J Biochem Cell Biol. 2012;44(11):1813–24. doi: 10.1016/j.biocel.2012.06.028. [DOI] [PubMed] [Google Scholar]
- 49.Wang Z, Zheng M, Li Z, Li R, Jia L, Xiong X, Southall N, Wang S, Xia M, Austin CP, Zheng W, Xie Z, Sun Y. Cardiac glycosides inhibit p53 synthesis by a mechanism relieved by Src or MAPK inhibition. Cancer Res. 2009;69(16):6556–64. doi: 10.1158/0008-5472.CAN-09-0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Golden WC, Martin LJ. Low-dose ouabain protects against excitotoxic apoptosis and up-regulates nuclear Bcl-2 in vivo. Neuroscience. 2006;137:133–44. doi: 10.1016/j.neuroscience.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 51.Li J, Zelenin S, Aperia A, Aizman O. Low Doses of Ouabain Protect from Serum Deprivation-Triggered Apoptosis and Stimulate Kidney Cell Proliferation via Activation of NF-{kappa}B. J Am Soc Nephrol. 2006;17:1848–1857. doi: 10.1681/ASN.2005080894. [DOI] [PubMed] [Google Scholar]
- 52.Khundmiri SJ, Metzler MA, Ameen M, Amin V, Rane MJ, Delamere NA. Ouabain induces cell proliferation through calcium-dependent phosphorylation of Akt (protein kinase B) in opossum kidney proximal tubule cells. Am J Physiol Cell Physiol. 2006;291:C1247–57. doi: 10.1152/ajpcell.00593.2005. [DOI] [PubMed] [Google Scholar]
- 53.Dvela M, Rosen H, Ben-Ami HC, Lichtstein D. Endogenous ouabain regulates cell viability. Am J Physiol Cell Physiol. 2012;302(2):C442–52. doi: 10.1152/ajpcell.00336.2011. [DOI] [PubMed] [Google Scholar]
- 54.Courtneidge SA, Smith AE. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature. 1983;303:435–9. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- 55.Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- 56.Xu W, Harrison SC, Eck MJ. Three-dimensional structure of the tyrosine kinase c-Src. Nature. 1997;385:595–602. doi: 10.1038/385595a0. [DOI] [PubMed] [Google Scholar]
- 57.Kometiani P, Li J, Gnudi L, Kahn BB, Askari A, Xie Z. Multiple signal transduction pathways link Na+/K+-ATPase to growth-related genes in cardiac myocytes. The roles of Ras and mitogen-activated protein kinases. J Biol Chem. 1998;273:15249–56. doi: 10.1074/jbc.273.24.15249. [DOI] [PubMed] [Google Scholar]
- 58.Haas M, Askari A, Xie Z. Involvement of Src and epidermal growth factor receptor in the signal-transducing function of Na+/K+-ATPase. J Biol Chem. 2000;275:27832–7. doi: 10.1074/jbc.M002951200. [DOI] [PubMed] [Google Scholar]
- 59.Haas M, Wang H, Tian J, Xie Z. Src-mediated inter-receptor cross-talk between the Na+/K+-ATPase and the epidermal growth factor receptor relays the signal from ouabain to mitogen-activated protein kinases. J Biol Chem. 2002;277:18694–70264. doi: 10.1074/jbc.M111357200. [DOI] [PubMed] [Google Scholar]
- 60.Bozulic LD, Dean WL, Delamere NA. The influence of Lyn kinase on Na,K-ATPase in porcine lens epithelium. Am J Physiol Cell Physiol. 2004;286:C90–6. doi: 10.1152/ajpcell.00174.2003. [DOI] [PubMed] [Google Scholar]
- 61.Bozulic LD, Dean WL, Delamere NA. The influence of SRC-family tyrosine kinases on Na,KATPase activity in lens epithelium. Invest Ophthalmol Vis Sci. 2005;46:618–22. doi: 10.1167/iovs.04-0809. [DOI] [PubMed] [Google Scholar]
- 62.Liang M, Cai T, Tian J, Qu W, Xie ZJ. Functional characterization of Src-interacting Na/K-ATPase using RNA interference assay. J Biol Chem. 2006;281:19709–19. doi: 10.1074/jbc.M512240200. [DOI] [PubMed] [Google Scholar]
- 63.Wang H, Haas M, Liang M, Cai T, Tian J, Li S, Xie Z. Ouabain assembles signaling cascades through the caveolar Na+/K+-ATPase. J Biol Chem. 2004;279:17250–9. doi: 10.1074/jbc.M313239200. [DOI] [PubMed] [Google Scholar]
- 64.Tian J, Cai T, Yuan Z, Wang H, Liu L, Haas M, Maksimova E, Huang XY, Xie ZJ. Binding of Src to Na+/K+-ATPase forms a functional signaling complex. Mol Biol Cell. 2006;17:317–26. doi: 10.1091/mbc.E05-08-0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tian J, Gong X, Xie Z. Signal transducing function of Na+/K+-ATPase is essential for ouabain's effect on intracellular Ca2+ in rat cardiac myocytes. Am J Physiol. 2001;281:1899–1907. doi: 10.1152/ajpheart.2001.281.5.H1899. [DOI] [PubMed] [Google Scholar]
- 66.Pierre SV, Yang C, Yuan Z, Seminerio J, Mouas K, Dos-Santos P, Xie Z-J. Ouabain triggers preconditioning through activation of the Na+,K+-ATPase signaling cascade in rat hearts. Cardiovasc Res. 2007;73:488–496. doi: 10.1016/j.cardiores.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liang M, Tian J, Liu L, Pierre S, Shapiro J, Xie Z-J. Identification of a pool of non-pumping Na/K-ATPase. J Biol Chem. 2007;282:10585–10593. doi: 10.1074/jbc.M609181200. [DOI] [PubMed] [Google Scholar]
- 68.Nguyen AN, Wallace DP, Blanco G. Ouabain binds with high affinity to the Na,K-ATPase in human polycystic kidney cells and induces extracellular signal-regulated kinase activation and cell proliferation. J Am Soc Nephrol. 2007;18:46–57. doi: 10.1681/ASN.2006010086. [DOI] [PubMed] [Google Scholar]
- 69.Ferrandi M, Molinari I, Barassi P, Minotti E, Bianchi G, Ferrari P. Organ hypertrophic signaling within caveolae membrane subdomains triggered by ouabain and antagonized by PST 2238. J Biol Chem. 2004;279:33306–14. doi: 10.1074/jbc.M402187200. [DOI] [PubMed] [Google Scholar]
- 70.Jung J, Kim M, Choi S, Kim MJ, Suh JK, Choi EC, Lee K. Molecular mechanism of cofilin dephosphorylation by ouabain. Cell Signal. 2006;18:2033–40. doi: 10.1016/j.cellsig.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 71.Kotova O, Al-Khalili L, Talia S, Hooke C, Fedorova OV, Bagrov AY, Chibalin AV. Cardiotonic steroids stimulate glycogen synthesis in human skeletal muscle cells via a Src- and ERK1/2-dependent mechanism. J Biol Chem. 2006;281:20085–94. doi: 10.1074/jbc.M601577200. [DOI] [PubMed] [Google Scholar]
- 72.Thundathil JC, Anzar M, Buhr MM. Na+/K+ATPase as a signaling molecule during bovine sperm capacitation. Biol Reprod. 2006;75:308–17. doi: 10.1095/biolreprod.105.047852. [DOI] [PubMed] [Google Scholar]
- 73.Valente RC, Capella LS, Monteiro RQ, Rumjanek VM, Lopes AG, Capella MA. Mechanisms of ouabain toxicity. Faseb J. 2003;17:1700–2. doi: 10.1096/fj.02-0937fje. [DOI] [PubMed] [Google Scholar]
- 74.Arnaud-Batista FJ, Costa GT, Oliveira IM, Costa PP, Santos CF, Fonteles MC, Uchôa DE, Silveira ER, Cardi BA, Carvalho KM, Amaral LS, Pôças ES, Quintas LE, Noël F, Nascimento NR. Natriuretic effect of bufalin in isolated rat kidneys involves activation of the Na+-K+-ATPase-Src kinase pathway. Am J Physiol Renal Physiol. 2012;302:F959–66. doi: 10.1152/ajprenal.00130.2011. [DOI] [PubMed] [Google Scholar]
- 75.Chen Y, Cai T, Yang C, Turner DA, Giovannucci DR, Xie Z. Regulation of inositol 1,4,5-trisphosphate receptor-mediated calcium release by the Na/K-ATPase in cultured renal epithelial cells. J Biol Chem. 2008;283(2):1128–36. doi: 10.1074/jbc.M708025200. [DOI] [PubMed] [Google Scholar]
- 76.Yuan Z, Cai T, Tian J, Ivanov AV, Giovannucci DR, Xie Z. Na/K-ATPase tethers phospholipase C and IP3 receptor into a calcium-regulatory complex. Mol Biol Cell. 2005;16(9):4034–45. doi: 10.1091/mbc.E05-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kennedy DJ, Vetteth S, Periyasamy SM, Kanj M, Fedorova L, Khouri S, Kahaleh MB, Xie Z, Malhotra D, Kolodkin NI, Lakatta EG, Fedorova OV, Bagrov AY, Shapiro JI. Central role for the cardiotonic steroid marinobufagenin in the pathogenesis of experimental uremic cardiomyopathy. Hypertension. 2006;47:488–95. doi: 10.1161/01.HYP.0000202594.82271.92. [DOI] [PubMed] [Google Scholar]
- 78.Elkareh J, Kennedy DJ, Yashaswi B, Vetteth S, Shidyak A, Kim EG, Smaili S, Periyasamy SM, Hariri IM, Fedorova L, Liu J, Wu L, Kahaleh MB, Xie Z, Malhotra D, Fedorova OV, Kashkin VA, Bagrov AY, Shapiro JI. Marinobufagenin stimulates fibroblast collagen production and causes fibrosis in experimental uremic cardiomyopathy. Hypertension. 2007;49:215–24. doi: 10.1161/01.HYP.0000252409.36927.05. [DOI] [PubMed] [Google Scholar]
- 79.El-Okdi N, Smaili S, lkareh J, Periyasamy SM, Shapiro AP, Kahaleh MB, Malhotra D, Xie Z, Chin KV, Shapiro JI. The effects of cardiotonic steroids on dermal collagen synthesis and wound healing. J Appl Physiol. 2008;105:30–36. doi: 10.1152/japplphysiol.00119.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Elkareh J, Periyasamy SM, Shidyak A, Vetteth S, Schroder J, Raju V, Hariri I, El-Okdi N, Gupta S, Fedorova L, Liu J, Fedorova O, Kahaleh M, Xie Z, Malhotra D, Watson D, Bagrov A, Shapiro JI. Marinobufagenin induces increases in procollagen expression in a process involving protein kinase C and Fli-1: Implications for uremic cardiomyopathy. Am J Physiol. Renal Physiol. 2009;296:F1219–1226. doi: 10.1152/ajprenal.90710.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cai T, Wang H, Chen Y, Quintas LEM, Liu L, Gunning WT, Xie ZJ. Regulation of caveolin-1 membrane traffic by the Na/K-ATPase. J Cell Biol. 2008;182:1153–1169. doi: 10.1083/jcb.200712022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chen Y, Cai T, Wang H, Li Z, Loreaux E, Lingrel JB, Xie Z. Regulation of intracellular cholesterol distribution by the Na/K-ATPase. J Biol Chem. 2009;284:14881–14890. doi: 10.1074/jbc.M109.003574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen Y, Li X, Ye Q, Tian J, Jin R, Xie Z. Regulation of α1 expression by cholesterol. J Biol Chem. 2011;286:15517–15524. doi: 10.1074/jbc.M110.204396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Quintas LE, Pierre SV, Liu L, Bai Y, Liu X, Xie ZJ. Alterations of Na+/K+-ATPase function in caveolin-1 knockout cardiac fibroblasts. J Mol Cell Cardiol. 2010;49:525–531. doi: 10.1016/j.yjmcc.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang Z, Li Z, Tian J, Jiang W, Wang Y, Zhang X, Li Z, You Q, Shapiro JI, Si S, Xie Z. Identification of Hydroxyxanthones as Na/K-ATPase ligands. Mol Pharmacol. 2010;77:961–967. doi: 10.1124/mol.110.063974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Li Z, Cai T, Tian J, Xie JX, Zhao X, Liu L, Shapiro JI, Xie Z. NaKtide, a Na/K-ATPase-derived peptide Src inhibitor, antagonizes ouabain-activated signal transduction in cultured cells. J Biol Chem. 2009;284:21066–21076. doi: 10.1074/jbc.M109.013821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Li Z, Zhang Z, Xie JX, Li X, Tian J, Cai T, Cui H, Ding H, Shapiro JI, Xie Z. Na/K-ATPase mimetic pNaKtide peptide inhibits the growth of human cancer cells. J Biol Chem. 2011;286:32394–32403. doi: 10.1074/jbc.M110.207597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Weigand KM, Swarts HG, Fedosova NU, Russel FG, Koenderink JB. Na,K-ATPase activity modulates Src activation: a role for ATP/ADP ratio. Biochim Biophys Acta. 2012 May;1818(5):1269–73. doi: 10.1016/j.bbamem.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 89.Ye Q, Li Z, Tian J, Xie XJ, Liu L, Xie Z. Identification of a potential receptor that couples ion transport to protein kinase activity. J Biol Chem. 2011;286:6225–6232. doi: 10.1074/jbc.M110.202051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ye Q, Lai F, Banerjee M, Duan Q, Li Z, Si S, Xie Z. Expression of Mutant 1 Na/K-ATPase Defective in Conformational Transition Attenuates Src-mediated Signal Transduction. J Biol Chem. 2013;288:5803–5814. doi: 10.1074/jbc.M112.442608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lai F, Madan N, Ye Q, Duan Q, Li Z, Wang S, Si S, Xie Z. Identification of a Mutant α1 Na/K-ATPase That Pumps but is Defective in Signal Transduction. J Biol Chem. 2013;288(19):13295–304. doi: 10.1074/jbc.M113.467381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu J, Periyasamy S, Gunning W, Fedorova O, Bagrov AY, Malhotra D, Xie Z, Shapiro JI. Effects of cardiac glycosides on sodium pump expression and function in LLC-PK1 and MDCK cells. Kidney Int. 2002;62:2118–2125. doi: 10.1046/j.1523-1755.2002.00672.x. [DOI] [PubMed] [Google Scholar]
- 93.Liu J, Kesiry R, Periyasamy SM, Malhotra D, Xie Z, Shapiro JI. Ouabain induces endocytosis of plasmalemmal Na/K-ATPase in LLC-PK1 cells by a clathrin-dependent mechanism. Kidney Int. 2004;66:227–41. doi: 10.1111/j.1523-1755.2004.00723.x. [DOI] [PubMed] [Google Scholar]
- 94.Liu J, Liang M, Liu L, Malhotra D, Xie Z, Shapiro JI. Ouabin induced endocytosis of the plasmalemmal Na/K-ATPase in LLC-PK1 cells requires caveolin-1. Kidney Int. 2005;67:1844–1854. doi: 10.1111/j.1523-1755.2005.00283.x. [DOI] [PubMed] [Google Scholar]
- 95.Miyakawa-Naito A, Uhlen P, Lal M, Aizman O, Mikoshiba K, Brismar H. Cell signaling microdomain with Na,K-ATPase and inositol 1,4,5-trisphosphate receptor generates calcium oscillations. J Biol Chem. 2013;278(50):50355–61. doi: 10.1074/jbc.M305378200. [DOI] [PubMed] [Google Scholar]
- 96.Zhang S, Malmersjo S, Li J, Ando H, Aizman O, Uhlen P, Mikoshiba K, Aperia A. Distinct role of the N-terminal tail of the Na,K-ATPase catalytic subunit as a signal transducer. J Biol Chem. 2006;281:21954–62. doi: 10.1074/jbc.M601578200. [DOI] [PubMed] [Google Scholar]
- 97.Yudowski GA, Efendiev R, Pedemonte CH, Katz AI, Berggren PO, Bertorello AM. Phosphoinositide-3 kinase binds to a proline-rich motif in the Na+, K+-ATPase alpha subunit and regulates its trafficking. Proc Natl Acad Sci U S A. 2000;97:6556–61. doi: 10.1073/pnas.100128297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu L, Zhao X, Pierre SV, Askari A. Association of PI3K/Akt Signaling Pathway with Digitalis-Induced Hypertrophy of Cardiac Myocytes. Am J Physiol Cell Physiol. 2007 Nov;293(5):C1489–97. doi: 10.1152/ajpcell.00158.2007. 2007. [DOI] [PubMed] [Google Scholar]
- 99.Khundmiri SJ, Amin V, Henson J, Lewis J, Ameen M, Rane MJ, Delamere NA. Ouabain stimulates protein kinase B (Akt) phosphorylation in opossum kidney proximal tubule cells through an ERK-dependent pathway. Am J Physiol Cell Physiol. 2007;293:C1171–80. doi: 10.1152/ajpcell.00535.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Barwe SP, Anilkumar G, Moon SY, Zheng Y, Whitelegge JP, Rajasekaran SA, Rajasekaran AK. Novel role for Na,K-ATPase in phosphatidylinositol 3-kinase signaling and suppression of cell motility. Mol Biol Cell. 2005;16:1082–94. doi: 10.1091/mbc.E04-05-0427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guyton AC. Blood pressure control--special role of the kidneys and body fluids. Science. 1991;252(5014):1813–6. doi: 10.1126/science.2063193. [DOI] [PubMed] [Google Scholar]
- 102.Johnson RJ. Pathogenesis of essential hypertension: historical paradigms and modern insights. J Hypertens. 2008;26(3):381–91. doi: 10.1097/HJH.0b013e3282f29876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dahl LK, Knudsen KD, Iwai J. Humoral transmission of hypertension: evidence from parabiosis. Circ Res. 1969;24(Suppl):21–33. [PubMed] [Google Scholar]
- 104.de Wardener HE, Clarkson EM. Concept of natriuretic hormone. Physiol Rev. 1985;65:658–759. doi: 10.1152/physrev.1985.65.3.658. [DOI] [PubMed] [Google Scholar]
- 105.Bricker NS, Schmidt RW, Favre H, Fine L, Bourgoignie JJ. On the biology of sodium excretion: the search for natriuretic hormone. Yale J Biol Med. 1975;48:293–303. [PMC free article] [PubMed] [Google Scholar]
- 106.Hamlyn JM, Ringel R, Schaeffer J, Levinson PD, Hamilton BP, Kowarski AA, Blaustein MP. A circulating inhibitor of (Na__K_)ATPase associated with essential hypertension. Nature. 1982;300:650–652. doi: 10.1038/300650a0. [DOI] [PubMed] [Google Scholar]
- 107.Manunta P, Ferrandi M, Bianchi G, Hamlyn JM. Endogenous ouabain in cardiovascular function and disease. J Hypertens. 2009;27:9–18. doi: 10.1097/HJH.0b013e32831cf2c6. [DOI] [PubMed] [Google Scholar]
- 108.Kojima I, Yoshihara S, Ogata E. Involvement of endogenous digitalis-like substance in genesis of deoxycorticosterone-salt hypertension. Life Sci. 1982;30:1775–1781. doi: 10.1016/0024-3205(82)90313-7. [DOI] [PubMed] [Google Scholar]
- 109.Loreaux EL, Kaul B, Lorenz JN, Lingrel JB. Ouabain-Sensitive alpha1 Na,K-ATPase enhances natriuretic response to saline load. J Am Soc Nephrol. 2008;19(10):1947–54. doi: 10.1681/ASN.2008020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu ZQ, Ma AQ, Zhang L, Yang DY. Intra-cellular electrolyte changes and levels of endogenous digoxin-like substance within the plasma in patients with congestive heart failure. Int J Cardiol. 1990;27:47–53. doi: 10.1016/0167-5273(90)90190-g. [DOI] [PubMed] [Google Scholar]
- 111.Komiyama Y, Dong XH, Nishimura N, Masaki H, Yoshika M, Masuda M, Takahashi H. A novel endogenous digitalis, telocinobufagin, exhibits elevated plasma levels in patients with terminal renal failure. Clin Biochem. 2005;38:36–45. doi: 10.1016/j.clinbiochem.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 112.Tian J, Haller S, Periyasamy S, Brewster P, Zhang H, Adlakha S, Fedorova OV, Xie Z, Bagrov AY, Shapiro JI, Cooper CJ. Renal ischemia regulates marinobufagenin release in humans. Hypertension. 2010;56:914–919. doi: 10.1161/HYPERTENSIONAHA.110.155564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Oweis S. Cardiac glycoside downregulates NHE3 activity and expression in LLC-PK1 cells. Am J Physiol Renal Physiol. 2006;290(5):F997–1008. doi: 10.1152/ajprenal.00322.2005. [DOI] [PubMed] [Google Scholar]
- 114.Cai H, Wu L, Qu W, Malhotra D, Xie Z, Shapiro J, Liu J. Regulation of Apical NHE3 Trafficking by Ouabain-Induced Activation of Basolateral Na/K-ATPase Receptor Complex. Am J Physiol Cell Physiol. 2008;294:C555–563. doi: 10.1152/ajpcell.00475.2007. [DOI] [PubMed] [Google Scholar]
- 115.Gomes P, Soares-da-Silva P. Upregulation of apical NHE3 in renal OK cells overexpressing the rodent alpha(1)-subunit of the Na(+) pump. Am J Physiol Regul Integr Comp Physiol. 2006;290(4):R1142–50. doi: 10.1152/ajpregu.00102.2005. [DOI] [PubMed] [Google Scholar]
- 116.Zhang Y, Magyar CE, Norian JM, Holstein-Rathlou NH, Mircheff AK, McDonough AA. Reversible effects of acute hypertension on proximal tubule sodium transporters. Am J Physiol. 1998;274(4 Pt 1):C1090–100. doi: 10.1152/ajpcell.1998.274.4.C1090. [DOI] [PubMed] [Google Scholar]
- 117.Bobulescu IA, Moe OW. Luminal Na(+)/H (+) exchange in the proximal tubule. Pflugers Arch. 2009;458(1):5–21. doi: 10.1007/s00424-008-0595-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hayashi M. Na+/H+-exchanger 3 activity and its gene in the spontaneously hypertensive rat kidney. J Hypertens. 1997;15(1):43–8. [PubMed] [Google Scholar]
- 119.Liu J, Yan Y, Liu L, Xie Z, Malhotra D, Joe B, Shapiro JI. Impairment of Na/K-ATPase signaling in renal proximal tubule contributes to Dahl salt-sensitive hypertension. J Biol Chem. 2011;286:22806–22813. doi: 10.1074/jbc.M111.246249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tian J, Shidyak A, Periyasamy SM, Haller S, Taleb M, El-Okdi N, Elkareh J, Gohara S, Fedorova OV, Cooper CJ, Xie Z, Malhotra D, Bagrov AY, Shapiro JI. Spironolactone attenuates experimental uremic cardiomyopathy by antagonizing marinobufagenin. Hypertension. 2009;54:1313–1320. doi: 10.1161/HYPERTENSIONAHA.109.140038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Haller ST, Drummond CA, Yan Y, Liu J, Tian J, Malhotra D, Shapiro JI. Passive Immunization Against Marinobufagenin Attenuates Renal Fibrosis and Improves Renal Function in Experimental Renal Disease. Am J Hypertens. 2013 Sep 6; doi: 10.1093/ajh/hpt169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kennedy DJ, Chen Y, Huang W, Viterna J, Liu J, Westfall K, Tian J, Bartlett DJ, Tang WH, Xie Z, Shapiro JI, Silverstein RL. CD36 and Na/K-ATPase-α1 form a proinflammatory signaling loop in kidney. Hypertension. 2013;61:216–224. doi: 10.1161/HYPERTENSIONAHA.112.198770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nguyen AN, Jansson K, Sanchez G, Sharma M, Reif GA, Wallace DP, Blanco G. Ouabain activates the Na-K-ATPase signalosome to induce autosomal dominant polycystic kidney disease cell proliferation. Am j Physiol Renal physiology. 2011;301:F897–906. doi: 10.1152/ajprenal.00095.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jansson K, Nguyen AN, Magenheimer BS, Reif GA, Aramadhaka LR, Bello-Reuss E, Wallace DP, Calvet JP, Blanco G. Endogenous concentrations of ouabain act as a cofactor to stimulate fluid secretion and cyst growth of in vitro ADPKD models via cAMP and EGFR-Src-MEK pathways. Am J Physiol Renal Physiol. 2012;303(7):F982–90. doi: 10.1152/ajprenal.00677.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Grantham JJ. Fluid secretion, cellular proliferation, and the pathogenesis of renal epithelial cysts. J Am Soc Nephrol. 1993;3:1841–1857. doi: 10.1681/ASN.V3121841. [DOI] [PubMed] [Google Scholar]
- 126.Jansson K, Magenheimer BS, Maser RL, Calvet JP, Blanco G. Overexpression of the polycystin-1 C-tail enhances sensitivity of M-1 cells to ouabain. J Membr Biol. 2013;246(7):581–90. doi: 10.1007/s00232-013-9573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Contreras RG, Shoshani L, Flores-Maldonado C, Lazaro A, Cereijido M. Relationship between Na(+),K(+)-ATPase and cell attachment. J Cell Sci. 1999;112(Pt 23):4223–32. doi: 10.1242/jcs.112.23.4223. [DOI] [PubMed] [Google Scholar]
- 128.Larre I, Lazaro A, Contreras RG, Balda MS, Matter K, Flores-Maldonado C, Ponce A, Flores-Benitez D, Rincon-Heredia R, Padilla-Benavides T, Castillo A, Shoshani L, Cereijido M. Ouabain modulates epithelial cell tight junction. Proc Natl Acad Sci U S A. 2010;107(25):11387–92. doi: 10.1073/pnas.1000500107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Contreras RG, Flores-Beni Tez D, Flores-Maldonado C, Larre I, Shoshani L, Cereijido M. Na+,K+-ATPase and hormone ouabain:new roles for an old enzyme and an old inhibitor. Cell Mol Biol (Noisy-le-grand) 2006;52(8):31–40. [PubMed] [Google Scholar]
- 130.Larre I, Castillo A, Flores-Maldonado C, Contreras RG, Galvan I, Muñoz-Estrada J, Cereijido M. Ouabain modulates ciliogenesis in epithelial cells. Proc Natl Acad Sci U S A. 2011;108(51):20591–6. doi: 10.1073/pnas.1102617108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li J, Khodus GR, Kruusmägi M, Kamali-Zare P, Liu XL, Eklöf AC, Zelenin S, Brismar H, Aperia A. Ouabain protects against adverse developmental programming of the kidney. Nat Commun. 2010;1:42. doi: 10.1038/ncomms1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Burlaka I, Liu XL, Rebetz J, Arvidsson I, Yang L, Brismar H, Karpman D, Aperia A. Ouabain Protects against Shiga Toxin-Triggered Apoptosis by Reversing the Imbalance between Bax and BclxL. J Am Soc Nephrol. 2013;24(9):1413–23. doi: 10.1681/ASN.2012101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Rincon-Heredia R, Flores-Benitez D, Flores-Maldonado C, Bonilla-Delgado J, García-Hernández V, Verdejo-Torres O, Castillo AM, Larré I, Poot-Hernández AC, Franco M, Gariglio P, Reyes JL, Contreras RG. Ouabain induces endocytosis and degradation of tight junction proteins through ERK1/2-dependentpathways. ExpCellRes. 2013 Oct 17; doi: 10.1016/j.yexcr.2013.10.008. pii: S0014-4827(13)00425-4. [DOI] [PubMed] [Google Scholar]
- 134.Liu L, Ivanov AV, Gable ME, Jolivel F, Morrill GA, Askari A. Comparative Properties of Caveolar and Noncaveolar Preparations of Kidney Na+/K+-ATPase. Biochemistry. 2011;50:8664–8673. doi: 10.1021/bi2009008. [DOI] [PMC free article] [PubMed] [Google Scholar]