Abstract
Psychosocial stressors activate two distinct stress–response systems, a central, behavioral response, and a peripheral, endocrine response. Both behavioral and endocrine responses to stressors are subject to individual and developmental variables, but it is not known whether stressor induced behaviors are stable across development, and how they correspond with changes in the endocrine component of the stress response. We characterized the development and stability of behavioral responses to a mild psychosocial stressor in marmosets (Callithrix geoffroyi), and assessed the degree to which the behavioral and endocrine stress–response systems were co-activated. The behavioral response to stressors was stable within individuals, but only some stressor-induced behaviors changed as the monkeys developed. Overall, there was more variability in the development of behavioral responses compared to stress-induced endocrine profiles found previously [French et al., 2012. Horm Behav 61:196–03]. In young marmosets, only increased alarm calling was correlated with increased cortisol reactivity, and in older marmosets increased cage manipulations and motor activity were associated with poorer post-stressor cortisol regulation. Because these relationships were so few, we conclude that while the behavioral and endocrine systems follow a similar developmental trajectory, each system maintains a level of independence. Furthermore, the relationship between stressor-induced behaviors and HPA activity changes across development.
Keywords: stress, development, HPA, co-activation, marmoset, sex differences
INTRODUCTION
Responses to psychosocial stressors involve both a physiological component and behavioral components. However, the evidence regarding the degree to which physiological and behavioral responses to stressors co-develop and are co-activated is mixed [Coe et al., 1983; Gunnar et al., 1981, 1995; Levine et al., 1993; Norcross & Newman, 1999; Smith et al., 1998; Suomi, 1997]. Rhesus macaques (Macaca mulatta) classified early in life as highly behaviorally reactive demonstrate both behavioral and endocrine differences in response to stressful stimuli, such as elevated neophobia, reduced behavioral habituation to a stressor, increased heart rate, and elevated circulating ACTH and cortisol when compared to monkeys classified early as less-reactive [see Suomi, 1997 for review]. Similarly, marmosets (genus Callithrix) exposed to novel or isolated housing conditions show significant increases in urinary and plasma cortisol, as well as increases in locomotion and vocalizations. If paired with a heterosexual partner, both the endocrine and behavioral responses to the stressor are attenuated [Norcross & Newman, 1999; Smith etal., 1998]. However, individual rates of locomotion and cortisol were correlated only on the first, and not the second day of novel housing and no correlation was found between cortisol and vocalizations [Smith et al., 1998]. Both physiological behavioral responses to stress and are influenced by corticotropin-releasing hormone (CRH), as administration of a CRH receptor antagonist reduces both endocrine and behavioral responses to stressors in nonhuman primates [French et al., 2007; Habib et al., 2000]. However, this differential cortisol-behavior relationship for locomotion and vocalizations suggests that factors independent of CRH, such as development, experience, and context, may affect both behavioral and endocrine responses to stressors. Furthermore, this indicates that stressor induced “anxious” behavior, may not always serve as a good proxy for physiological reactivity.
In primates, social isolation from conspecifics produces increases in locomotion, vocalizations, and cortisol [Coe et al., 1983; Gunnar et al., 1981; Levine et al., 1993; Norcross & Newman, 1999; Smith et al., 1998]. In young primates, even a short separation from the caregivers can be a stressful experience [see Parker & Maestripieri, 2011 for review], and the specific context of the separation procedure differentially affects behavioral and HPA stressor responses. Infant squirrel monkeys (Saimiri sciureus) who are housed adjacent to their mothers (allowing olfactory and auditory, but not visual or tactile contact) exhibit more vocalizations than infants who are completely isolated, however, cortisol levels are only affected by isolation procedure at long (24 hr) intervals [Levine et al., 1993]. Similarly, repeated, complete isolation results in eventual attenuation of vocalization behavior, but locomotion and the cortisol response do not decline [Coe et al., 1983]. In infant rhesus macaques, total isolation results in almost no vocalizations, while monkeys isolated with auditory contact with the mother or peers will respond by calling and increased cage biting [Levine et al., 1985]. Similarly, housing with limited contact with peers results in slightly faster habituation of the cortisol response than either calling or locomotion [Gunnar et al., 1981]. Not surprisingly, partial contact with conspecifics, the mother in particular, results in infants behaving in order to establish or maintain contact with that individual, while the physiological response is either blunted or unaffected. However, this work highlights the fact that behavioral and physiological responses to stress do not always match, and are differentially affected by context.
Though many contextual factors influence HPA responses, there are also significant changes in normative and stress-induced HPA activity across development. Marmosets naturally exhibit very high levels of corticosteroids, even at a resting state [Yamamoto et al., 1977], and neonatal marmosets have even higher levels of ACTH and cortisol. ACTH levels drop to adult levels by 8 weeks of age, but the high levels of cortisol do not drop to adult levels until 7 months of age [Pryce et al., 2002]. Infant marmosets also do not exhibit an afternoon decline in cortisol, which is typical of adult diurnal cortisol variation, and this normal cortisol profile is not established until about 6 months of age [Pryce et al., 2002]. Young marmosets’ responses to stressors differ across development as well. While 2-, 6-, and 12-month-old infants had similar initial increases in plasma ACTH and cortisol in response to a brief stressor, 2-month-old marmosets exhibited elevated cortisol levels 2 hr after cessation of the stressor, significantly longer than 6- and 12-month-old marmosets [Pryce et al., 2002]. When subjected to an 8-hr social isolation stressor at 6, 12, and 18 months, peak cortisol reactivity and overall cortisol release declined with age, while post-stressor cortisol on the morning after the stressor (a measure of cortisol negative feedback and homeostatic regulation) did not [French et al., 2012]. This suggests that while long-term HPA regulatory mechanisms are mostly developed by 6 months of age [Pryce et al., 2002], short-term reactivity continues to develop (i.e., decrease to adult levels) as marmosets transition through juvenile, subadult, and young adult stages of life [French et al., 2012]. Furthermore, three of the five measures of HPA activity used (peak reactivity, AUCG, and AUCI) were highly correlated across ages within individuals, indicating that though overall cortisol reactivity changes across development, marmosets demonstrate individual stability in HPA responses to stressors [French et al., 2012]. Though previous research has elucidated the developmental trajectory of the stressor-induced HPA response in marmosets [French et al., 2012; Pryce et al., 2002], the longitudinal development of stressor-induced behavioral responses for juvenile, subadult, and young adult marmosets is not yet known.
The first goal of this study was to characterize the development and stability of the behavioral response of marmosets to separation stress during the first 18 months of life. If the developmental pattern of stressor-induced behaviors follows the concurrent developmental pattern of stressor-induced HPA activation [French et al., 2012] then overall behavioral responding should decrease across development, and relative individual rates of behavioral responding should remain consistent. The second goal was to examine the relationship between stressor-induced behavioral changes and cortisol reactivity. If psychosocial stressors co-activate the behavioral and HPA stress response systems, then increases in stressor-induced behaviors should be associated with increases in measures of stressor-related cortisol activity.
METHODS
Subjects
Fifty white-faced marmosets (C. geoffroyi) born to breeding pairs in the Callitrichid Research Center (CRC) at the University of Nebraska at Omaha were included in the study. Twenty-nine of the marmosets recruited into the study were male, and 22 were female. All subjects were housed in natal family groups with their mother, father, and, in some cases older and younger siblings. Full data on endocrine and behavioral measures were available for 44 marmosets at 6 months, 40 marmosets at 12 months, and 29 marmosets at 18 months. Marmosets were removed from analyses if behavioral or endocrine data were missing, or if the monkey was unable to complete the stressor procedure (6 months, 6 marmosets; 12 months, 10 marmosets; 18 months, 21 marmosets). Complete behavioral and endocrine data at all three time points were available for 27 marmosets. Family groups were housed in large enclosures (minimum of 0.8 m3 per individual) that contained natural branches, sleeping enclosures, and various enrichment and foraging devices. The marmosets were fed commercially prepared marmoset food (Zu-Preme) at approximately 0800 hr, and fresh fruit, eggs, and invertebrate protein at approximately 1400 hr. For additional details on husbandry, see Schaffner et al. [1995]. All procedures were approved by UNMC/UNO Institutional Animal Care and Use Committee (IACUC 07-033-05-FC), and the CRC is a USDA-licensed and AALAC-accredited facility. All procedures followed the ethical policies of the American Society of Primatologists.
Standardized Psychosocial Stressor Procedure
At 6, 12, and 18 months of age, each marmoset was exposed to a standardized psychosocial stressor. These ages represent distinct stages in development (juvenile, sub-adult, young adult; [Yamamoto, 1993], and are sufficiently separated in time to reduce the potential for habituation to the stress procedure. On the day of the stressor, the target marmoset was captured by net, removed from its family group, and transferred to a transport cage (50 cm × 50 cm × 50 cm) made of nylon-coated wire. The marmoset was placed in a novel room some distance from the family’s colony room, such that there was no visual and only limited auditory contact with the family group. The marmoset was given food and water, and remained isolated for 8hr from 0900 to 1700 hr.
Urine Collection
First-void urine samples were collected the day prior to, the morning of, and the day after the stressor procedure at approximately 0700 hr. We used a noninvasive collection procedure in which the animals were trained to urinate into handheld aluminum pans in exchange for a preferred food treat [see French et al., 1996]. During the separation procedure, plastic sheeting was placed under the transport cage, and urine samples were collected opportunistically once per hour. Because some monkeys did not provide samples at each collection point, urine was averaged into 2-hr blocks. Urine samples were centrifuged after collection to remove debris, and the supernatant was pipetted into a clean vial and then frozen at −20°C until time of assay. Urine provides a simple, noninvasive way to measure peripheral hormones, while disturbing the isolation procedure as little as possible. Furthermore, it allows for repeated measurements even from very young animals.
Hormone Measurement and Analysis
A cortisol enzyme immunoassay, developed and validated for marmosets, was used to determine urine cortisol concentrations [Smith & French, 1997]. Samples, standards (1,000–7.8 pg/well), and high and low quality controls created from pooled marmoset urine were added to coated well plates [see French et al., 2012 for a full description]. Intra-assay coefficients of variation (CV) for quality control pools were 8.94% and 9.78% for high and low pools, respectively, and inter-assay CVs were 12.28% and 14.90% for high and low pools, respectively. To control for variable fluid intake and output, cortisol concentrations were divided by creatinine concentration to give a value of μg cortisol/mg creatinine. Creatinine was measured using a standard Jaffé reaction colorimetric assay [French et al., 1996].
Several derived measures of HPA function were computed based on raw cortisol data. Area under the curve with respect to increase (AUCI) was calculated by plotting the data points for each time period and finding the area of the polygon with the baseline first-void samples serving as the base of the polygon. AUCI is a measure of overall cortisol exposure over a time period [Pruessner et al., 2003]. Post-stressor regulation was calculated by subtracting the pre-stressor baseline from the cortisol measured from the morning after the separation procedure. This provides a measure of the animal’s ability to maintain HPA homeostasis, and thus a high value indicates decreased ability to return to normative (pre-stressor) levels upon cessation of the stressor (poorer HPA regulation). Finally, peak reactivity was calculated as change from baseline to peak cortisol concentration [French et al., 2012]. This measure represents the maximal intensity of the glucocorticoid response at any point during this particular stressor, and functions as a measure of acute physiological reactivity.
Behavioral Observations
Focal subject observations were conducted during the first hour of the separation from the family group. Behaviors were recorded continuously via Observer Software (Noldus Information Technology, Leesburg, VA). We recorded phee calls, alarm calls, cage manipulations and locomotion. Phee calls are high-pitched, long distance contact calls used most often when isolated in order to reestablish contact. Alarm calls are short, sharp calls used most often when in danger or distress. Cage manipulations were scored as attempts to grab, pull, or bite at the cage or fasteners. Locomotion was scored as number of moves from one plane (wall, ceiling or floor) of the cage to another (Supplementary Table SI). With the exception of locomotion, all of these measures are reduced following CRHR1 antagonist treatment, suggesting that they are mediated by central CRH release [French et al., 2007].
Data Analysis
Changes in behavioral responses across the three time periods were evaluated using 3 (age) × 2 (sex) mixed ANOVAs. Post hoc analyses were done using Fisher’s LSD. Individual behavioral stability was assessed using correlations between ages.
The relationship between stressor-induced behaviors and endocrine activity was assessed using correlational analyses. To examine the unique contribution of sex and behaviors when predicting cortisol, as well as any sex × behavior interactions, a multivariate analysis of covariance (MANCOVA) was done at each age. Peak reactivity, post-stressor cortisol, and AUCI were entered as dependent variables, sex was entered as a fixed factor, and phee calling, alarm calling, cage manipulation, and locomotion were mean-deviated and entered as covariates. Post hoc tests of interactions were done by regressing cortisol measures on all behaviors for each sex separately. Criterion for significance was set at P = 0.05 for all analyses. All analyses were two-tailed.
RESULTS
Stressor Response Across Development
Behavioral responding across development
Throughout development, mean rates of separation-induced behavioral responding in marmosets changed (Fig. 1). Rates of cage manipulations and alarm calling followed opposite developmental trajectories (cage manipulations, F(1,30) = 4.41, P = 0.044; alarm calling, F(1,30) = 6.58, P = 0.016). Cage manipulations increased from 6 to 18 months of age, while alarm calling decreased during the same time period. There were no age differences in rates of locomotion during social separation (F(1,30) = 2.93, P = 0.061). There were no overall sex differences for any of the behaviors measured, however there was a sex by age interaction for phee calling, in which phee calling rates decrease among females, while males had low calling rates at all ages (F(1,30) = 4.72, P = 0.038).
Fig. 1.
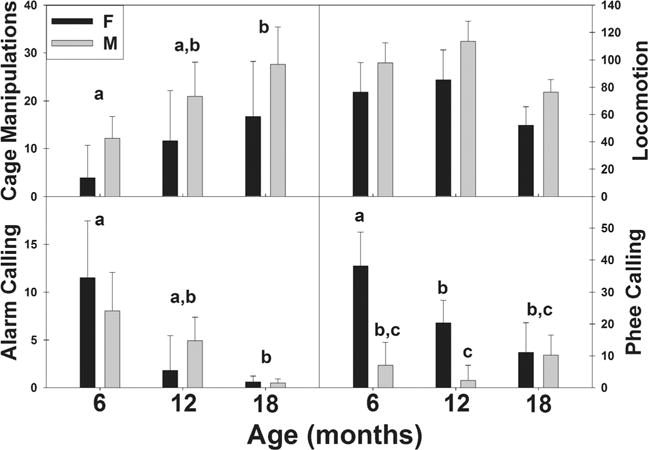
Behavioral responding (mean ± SE) during the first hour of a social isolation stressor at 6, 12, and 18 months. Differing letters between bars (Females, dark bars; Males, light bars) indicate a significant difference indicate significant differences between ages when sexes are combined, differing letters above bars indicate differences when sexes are compared (Fisher’s LSD, P < 0.05; N = 32).
Behavioral stability across development
Across development, relative rates of individual behavioral responding were stable (Table I). Individual rates of phee calling and cage manipulations at 6 months were predictive of individual rates of these behavioral measures at 12 and 18 months of age. However, individual rates of locomotion during separation were consistent only later in development (12–18 months). In contrast, alarm calling showed no stability across development.
TABLE I.
Stability in Behavioral Responding Across Ages
| Stress measure | 6–12 months (N = 47) | 12–18 months (N = 32) | 6–18 months (N = 32) |
|---|---|---|---|
| Phee calling | 0.67** | 0.42** | 0.31 |
| Alarm calling | 0.03 | −0.07 | 0.05 |
| Cage manipulations | 0.49** | 0.54** | 0.37* |
| Locomotion | 0.18 | 0.36* | 0.33 |
P<0.05.
P< 0.01.
Co-Activation of Cortisol and Behavioral Responses
Only a few behaviors and measures of cortisol during social separation were correlated, and these relationships changed over the course of development. At 6 months, higher rates of alarm calling were correlated with increased peak cortisol reactivity (r (47) = 0.32, P = 0.027). Due to the number of animals that failed to exhibit alarm calling during separation, this analysis was performed again only with individuals who exhibited at least one or more alarm calls. Even with this reduced sample, higher peak cortisol was still significantly correlated with alarm calling (r (16) = 0.64, P = 0.008). No other behaviors were significantly associated with any of the cortisol measures at 6 months. At 12 months, increased locomotion tended to be positively correlated with increased post-stressor cortisol, though not significantly. Finally, at 18 months, marmosets exhibiting higher rates of cage manipulations and locomotion had higher post-stressor cortisol (r(30) = 0.40, P = 0.030; r(30) = 0.49, P = 0.006). Overall, correlations between endocrine and behavioral responses to the social separation protocol were few, but the relationship between motor behavior and HPA activity strengthened across development (Supplementary Table SII).
Additionally, the relationship between behaviors and cortisol was dependent on the sex of the marmoset, but only in young monkeys (Figs. 2–4, Supplementary Tables SIII and SIV). At 6 months of age, there was a significant sex × locomotion interaction for both post-stressor cortisol and AUCI when all other behaviors were controlled (Wilk’s λ = 0.73, F(4, 31) = 2.88, P = 0.039; post-stressor cortisol, F(1,34)= 12.29, P = 0.001; AUCI, F(1,34)= 9.60, P = 0.004). There was also a nonsignificant trend for the interaction between sex and alarm calling, again for both post-stressor cortisol and AUCI (Wilk’s λ = 0.73, F (4,31) = 2.19, P = 0.093; post-stressor cortisol, F (1,34) = 8.67, P = 0.006), AUCI, F(1,34) = 7.46, P = 0.010). Post hoc regressions (separated by sex) showed that females who responded to the stressor with increased locomotion had increased AUCI and increased post-stressor cortisol. However, males who responded to the stressor with decreased locomotion and increased alarm calling had increased AUCI (Supplementary Tables SV and VI). At 12 and 18 months of age, relationships between behaviors and cortisol measures were not sex-dependent.
Fig. 2.
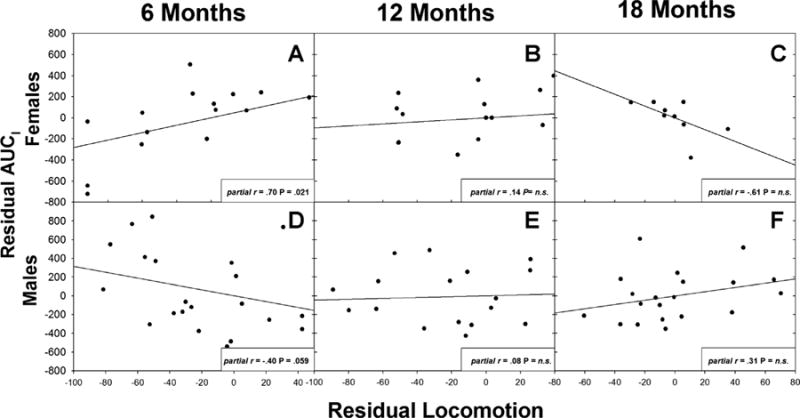
Residual scatterplots between locomotion and area under the curve (AUCI) when other stressor behaviors (alarm calling, phee calling, cage manipulations) are controlled. Locomotion predicted cortisol in 6-month-old females (a) and males (d), but not in older animals (b,c,e,f).
Fig. 4.
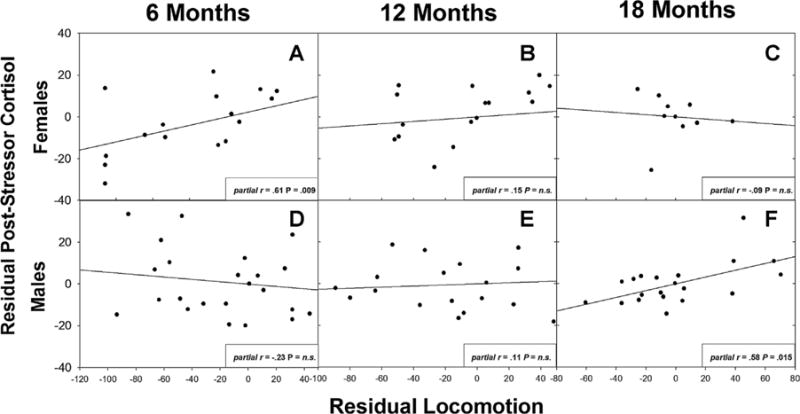
Residual scatterplots between locomotion and 24-hr post-stressor cortisol when other stressor behaviors (alarm calling, phee calling, cage manipulations) are controlled. Locomotion predicted cortisol in 6-month-old females (a), but not males (d–f) or older females (b,c). Though the partial correlation between post-stressor cortisol and locomotion for males at 18 months is significant, the multivariate omnibus test was not.
DISCUSSION
We demonstrated that while marmosets show age-related differences in some behavioral responses to stressors, individual marmosets exhibit longitudinal stability in specific behavioral responses. Additionally, the degree of co-activation between endocrine and behavioral stressor responses is subject to developmental change as well. Finally, we found sex-related differences in both the development of certain behavioral responses and the co-activation of behavioral responses with physiology. Across development, alarm calling decreased from 6 to 18 months, while phee calling followed this pattern only in females. Cage manipulations increased from 6 to 18 months, while rates of locomotion did not change. There was intra-individual consistency among all ages for cage manipulations and phee calling, but rates of locomotion were only consistent later in development. We also found that stressor-induced behaviors and cortisol were not consistently co-activated across ages, but rather the relationship between behaviors and cortisol changed across development. In juveniles, alarm calling alone was significantly higher in marmosets with higher peak cortisol reactivity, but in young adults, this relationship weakened, and locomotion and cage manipulations became associated with post-stressor cortisol. Moreover, the co-activation of the behavioral and cortisol responses differed by sex in juveniles only. Increased locomotion was associated with increased cortisol in females, while increased vocalizations and decreased locomotion were associated with increased cortisol in males.
Developmental Changes in Behavioral Responding
In comparison with the decrease in cortisol measures described in a subset of this sample by French et al. [2012], only one of the four behaviors measured here decreased as the marmosets developed (alarm calling). In contrast, cage manipulations during social separations increased throughout the three ages we tested. This change in behavioral responding may be part of a maturation process whereby young adult marmosets exhibit more independent behaviors in response to the stressor (i.e., manipulating their environment) and become less dependent on caregivers (i.e., vocalizing with contact calls). Phee calling exhibited developmental change only in females, indicating that while young females engage in calling as a strategy, young males and older marmosets of both sexes do not. Furthermore, phee calling was unrelated to the cortisol response, suggesting that it is not a particularly good indicator of physiological stress in marmosets (especially males) at any age. As a whole, when compared with the concurrent development of the endocrine stressor-response system described by French et al. [2012], these findings show that though some stressor-induced behavioral responses follow a similar developmental time course to stressor-induced endocrine activity, there is more variability in how stress behavior expression develops.
On an individual basis, only select behavioral stress responses were consistent across ages. That is, individuals who exhibited high behavioral responses at 6 months also exhibited high behavioral responses at 12 and 18 months. This suggests, in part, that marmosets show a “trait-like” behavioral reactivity to stressful stimuli, at least for phee calling and cage manipulations, and to a lesser extent, locomotion. This is similar to the “trait-like” cortisol response to stressful stimuli reported previously [French et al., 2012]. This behavioral stability is in line with other research demonstrating within-individual consistency in stressor-induced behaviors across development in other animals [macaques, Suomi, 1997; pigs, Ruis et al., 2000], and human children [Bates et al., 1985; Belsky & Rovine, 1987; Matheny et al., 1984]. So, while the expression of specific behavioral response patterns to stress change significantly from one age to the next (i.e., there are important developmental changes in overall responding), there does appear to be individual-level stability in the overall behavioral response to the social separation stressor as the marmosets mature into adolescence.
Relationships Between Behavior and HPA Measures
Increased behavioral responding to the psychosocial stressor was not generally associated with increased HPA activity. This suggests that despite the dual effect of CRH on behavior and endocrine activity [French et al., 2007; Habib et al., 2000], these two systems maintain a degree of independence in response to stressful stimuli. The sex-based differences in the strengths of these relationships found here also suggest that sex steroids (or other sexually dimorphic processes) play a role in mediating the behavioral, endocrine, or joint stressor response, even in young, prepubescent monkeys. Though sex steroids do have an acute effect (whether via injection or estrus cycle) on anxiety [see Toufexis et al., 2006 for review], we found sex differences only in young (prepubescent) monkeys, so the differences we found may be from the organizational effects of sex steroids during early development, or perhaps from differential treatment by the caregivers. It is also possible that there may be other behavioral patterns that better express the animal’s physiological state, that were not measured here. Increased tail–hair piloerection [Dettling et al., 2002] and scratching [Ayala, 2004; French et al., 2007; Maestripieri, 2000; Polizzi di Sorrentino et al., 2012] have been suggested as indicators of increased stress in nonhuman primates in response to a variety of stressors. However, French et al. [2007] found that CRH antagonist treatment reduced vocalizations and cage manipulations during a separation stressor, suggesting that these behaviors are controlled by CRH release. It is also possible that metabolic processes involved during corticosteroid excretion introduces additional variance which behavior cannot account for. Salivary sampling methods may provide a balance between temporal resolution and limited invasiveness, provided the animals can be reliably trained to the procedure [Cross et al., 2004]. Finally, given the fact that cortisol and stressor-induced behavior each represent two effects of central CRH release, we cannot exclude the possibility that cortisol and stressor-induced behaviors are not completely co-activated, and are related, but distinct systems.
Despite the general disconnect between behaviors and HPA response, an interesting finding that did emerge was the change in the behavior–cortisol relationship from vocalization to cage manipulations and locomotion. At 6 months, high rates of alarm calling were co-activated with increased cortisol reactivity. At 12 months, a positive relationship between locomotion and cortisol regulation was beginning to develop, and finally, at 18 months, stressor-induced alarm calling had dropped to very low levels, and locomotion and cage manipulations were associated with poorer cortisol regulation. It is worth noting that though behavioral reactivity was related to cortisol reactivity during the stressor in young monkeys, in older monkeys behavioral reactivity was instead related to long-term homeostatic regulation. This suggests that those monkeys who were most behaviorally disturbed during the stressor continued to respond physiologically for upwards of 24 hr. It is possible that poor familial reunion strategies after the stressor contribute to poorer physiological regulation [see Dettling et al., 2002]. Coupled with the overall changes in responding, with alarm calling rates decreasing from 6 to 18 months, and cage manipulations increasing during the same time period, this may reflect young marmosets’ emerging independence from the caregivers as they reach adolescence [Nunes et al., 2001; Yamamoto, 1993; Yamamoto et al., 1996]. Thus the difficulty in establishing a consistent cross-age behavior–cortisol relationship may be in part due to changing behavioral response styles as marmosets reach and progress from adolescence through adulthood, and thus we suggest that the behavioral–endocrine relationships are fluid across development as marmosets maintain some and alter other behavioral strategies to adjust to a social separation stressor.
The co-activation of locomotion and alarm calling with cortisol also varied by the sex of the marmoset, with females showing correlated increases in locomotion and cortisol, and males showing correlated increases in vocalizations and cortisol. It may be that that the locomotion–cortisol relationship develops earlier in females than it does in males. Also, young, but not older females engage in more long distance (phee) calling than males. While the reasons for these sex differences are unclear, it may be explained by sex-differential group emigration patterns. Not only are subordinate females more likely to participate in extragroup interactions [marmoset, Lazaro-Perea, 2001], but they are also more likely to be the target of aggression, especially as group size increases [marmoset, De Filippis et al., 2009; Snowdon & Pickhard, 1999]. Furthermore, group fission is more likely to occur when resource competition and environmental stress are high [toque macaque, Dittus, 1988]. Given that maternal stressors affect infant CRH, cortisol, and behavior in marmosets and other nonhuman primates [macaque, see Meaney, 2001 for review; Bardi & Huffman, 2006; Coplan et al., 2001; marmoset, Mustoe et al., unpublished data], increased locomotion and phee (long distance) calling in response to stress may be more advantageous for young females than young males. Specifically, when the group environment is particularly stressful, it may be more important for females than males to develop a stressor response style that may promote increased likelihood of contact with other groups and/or natal group dispersal at a young age. In contrast, stressed male infants behave in ways that re-establish contact (i.e., alarm calling) with the caregiver. Thus stressed young males behave in a way that is more dependent upon the caregivers when compared to stressed young females. It is worth noting that under the relatively low stress conditions in captivity, responses to isolation became more homogenous between the sexes as the marmosets developed.
General Discussion
This study is the first to use a longitudinal design to document the developmental changes in behavioral responding to an isolation stressor in this unique primate model, and adds to the existing literature describing marmoset endocrine development [French et al., 2012; Pryce et al., 2002]. We chose to focus not only on separation stress responses during early infancy, but instead focused on those changes which occur from infancy into adulthood, at distinct developmental time points. Additionally, we documented the developmental changes in the relationship between behavioral and endocrine measures of stress. Finally, we found sex-related differences in the development of the stressor response systems, particularly in young (prepubescent) marmosets. The findings here can be used to inform husbandry and animal welfare practices, as developmental stage and sex should be considered when using behavior as a heuristic proxy for physiological disturbance. In a related vein, developmental stage and sex should be taken into account when using behavior as a measure of stress in experimental settings.
The study of stressor responses raises questions about the effects of stress physiology on behavior, and the effects of behavior on stress physiology. For example, locomotion, cage directed behavior, or calling, all controlled by largely voluntary central processes, may promote sympathetic responses such as cortisol release, vasodilation, and pulse quickening. However, sympathetic responses themselves may promote behavior instead. Cortisol mobilizes energy resources, making increased locomotion possible, and increased blood flow to the skin may induce scratching. Relatedly, there is also the issue of the timing of each of these responses. Certain physiological responses to stress are likely to be less rapidly mobilized than behavioral responses (e. g., a captured animal is capable of distress calling almost instantly, but even plasma glucocorticoids would not be detectable for several minutes). However other physiological responses (e.g., pulse, vasodilation) can be expressed on a similar time scale as behavior. Finally, there is the issue of behaviors as stress coping mechanisms. Certainly escape serves as a coping behavior as it removes the individual from the stressful situation, and calling serves a similar purpose if the individual is retrieved. However, given that all of the associations found in the current study were positive, it is unlikely that the behaviors measured here function as stress-reducing coping mechanisms.
The complexity found in the behavior–cortisol relationship is perhaps best described in terms of maximizing stressor adaptability. A variety of environmental influences have been shown to affect the HPA axis and the stressor response, including gestational stressors [Glover et al., 2010; Mustoe et al., unpublished data], early parental deprivation [Dettling et al., 2002; Higley et al., 1992; Pryce et al., 2004], maternal and paternal rejection [Birnie, 2013], and milk quality and milk cortisol [Hinde & Capitanio, 2010; Karg, 2011; Sullivan et al., 2011]. Life events also interact with genes to produce varying stressor phenotypes [Barr et al., 2004; Karg, 2011; McCormack et al., 2009]. From an evolutionary standpoint, these various influences allow an individual to tailor its stress response to its early environment. Stressor-induced behaviors habituate faster and more completely than cortisol responses after repeated [Coe et al., 1983] or extended [Levine et al., 1993] social stressors, and are related differentially to the endocrine response across development (current study). Thus by having some independence in behavioral and biological stressor responses, individuals can maximize adaptation across the lifespan.
Supplementary Material
Fig. 3.
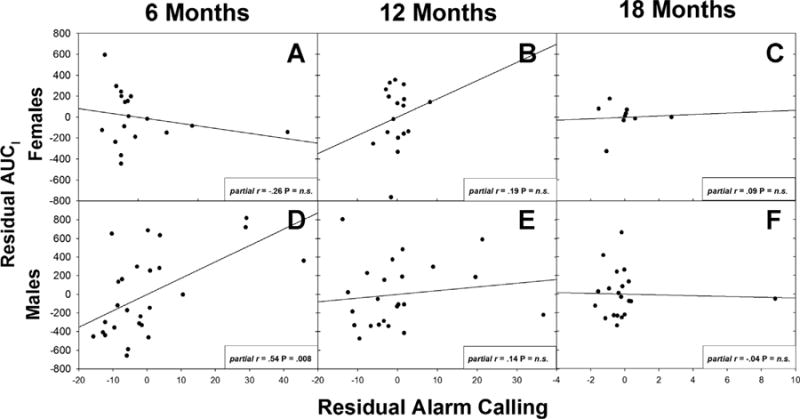
Residual scatterplots between alarm calling and area under the curve (AUCI) when other stressor behaviors (locomotion, phee calling, cage manipulations) are controlled. Alarm calling predicted cortisol in 6-month-old males (d), but not females (a–c) or older males (e,f).
Acknowledgments
The authors would like to thank Heather Jensen and her student staff and volunteers who work to provide excellent care to our marmosets. We would also like to thank Drew Birnie and Jon-Ryan Cavanaugh for their helpful comments on previous versions of this manuscript. We thank the University of Nebraska at Omaha for partial support of the marmoset colony, and the NIH (HD 042882), awarded to J.A.F., for support of the research program.
Contract grant sponsor: University of Nebraska at Omaha; contract grant sponsor: NIH (HD 042882)
Footnotes
Conflicts of interest: None.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
References
- Ayala AR. Behavioral, adrenal, and sympathetic responses to long-term administration of an oral corticotropin-releasing hormone receptor antagonist in a primate stress paradigm. J Clin Endocrinol Metab. 2004;89:5729–5737. doi: 10.1210/jc.2003-032170. [DOI] [PubMed] [Google Scholar]
- Bardi M, Huffman MA. Maternal behavior and maternal stress are associated with infant behavioral development in macaques. Dev Psychobiol. 2006;48:1–9. doi: 10.1002/dev.20111. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Schwandt M, et al. Sexual dichotomy of an interaction between early adversity and the serotonin transporter gene promoter variant in rhesus macaques. Proc Natl Acad Sci USA. 2004;101:12358–12363. doi: 10.1073/pnas.0403763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates JE, Maslin CA, Frankel KA. Attachment security, mother-child interaction, and temperament as predictors of behavior-problem ratings at age three years. Monogr Soc Res Child Dev. 1985;50:167–193. [PubMed] [Google Scholar]
- Belsky J, Rovine M. Temperament and attachment security in the strange situation: an empirical rapprochement. Child Dev. 1987;58:787–795. [PubMed] [Google Scholar]
- Birnie AK, Taylor JH, Cavanaugh J, French JA. Quality of maternal and paternal care predicts later stress reactivity in the cooperatively-breeding marmoset (Callithrix geoffroyi) Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2013.08.011. Available online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe CL, Glass JC, Wiener SG, Levine S. Behavioral, but not physiological, adaptation to repeated separation in mother and infant primates. Psychoneuroendocrinology. 1983;8:401–409. doi: 10.1016/0306-4530(83)90019-7. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Smith ELP, Altemus M, et al. Variable foraging demand rearing: sustained elevations in cisternal cerebrospinal fluid corticotropin-releasing factor concentrations in adult primates. Biol Psychiatry. 2001;50:200–204. doi: 10.1016/s0006-3223(01)01175-1. [DOI] [PubMed] [Google Scholar]
- Cross N, Pines MK, Rogers LJ. Saliva sampling to assess cortisol levels in unrestrained common marmosets and the effect of behavioral stress. Am J Primatol. 2004;62:107–114. doi: 10.1002/ajp.20005. [DOI] [PubMed] [Google Scholar]
- De Filippis B, Chiarotti F, Vitale A. Severe intragroup aggressions in captive common marmosets (Callithrix jacchus) J Appl Anim Welfare Sci. 2009;12:214–222. doi: 10.1080/10888700902955963. [DOI] [PubMed] [Google Scholar]
- Dettling AC, Feldon J, Pryce CR. Early deprivation and behavioral and physiological responses to social separation/novelty in the marmoset. Pharmacol Biochem Behav. 2002;73:259–269. doi: 10.1016/s0091-3057(02)00785-2. [DOI] [PubMed] [Google Scholar]
- Dittus WP. Group fission among wild toque macaques as a consequence of female resource competition and environmental stress. Anim Behav. 1988;36:1626–1645. [Google Scholar]
- French JA, Brewer KJ, Schaffner CM, et al. Urinary steroid and gonadotropin excretion across the reproductive cycle in female wied’s black tufted-ear marmosets (Callithrix kuhli) Am J Primatol. 1996;40:231–245. doi: 10.1002/(SICI)1098-2345(1996)40:3<231::AID-AJP2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- French JA, Fite JE, Jensen H, et al. Treatment with CRH-1 antagonist antalarmin reduces behavioral and endocrine responses to social stressors in marmosets (Callithrix kuhlii) Am J Primatol. 2007;69:877–889. doi: 10.1002/ajp.20385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French JA, Smith AS, Gleason AM, et al. Stress reactivity in young marmosets (Callithrix geoffroyi): ontogeny, stability, and lack of concordance among co-twins. Horm Behav. 2012;61:196–203. doi: 10.1016/j.yhbeh.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover V, O’Connor TG, O’Donnell K. Prenatal stress and the programming of the HPA axis. Neurosci Biobehav Rev. 2010;35:17–22. doi: 10.1016/j.neubiorev.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Gonzalez CA, Goodlin BL, Levine S. Behavioral and pituitary-adrenal responses during a prolonged separation period in infant rhesus macaques. Psychoneuroendocrinology. 1981;6:65–75. doi: 10.1016/0306-4530(81)90049-4. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Porter FL, Wolf CM, Rigatuso J, Larson MC. Neonatal stress reactivity: predictions to later emotional temperament. Child Dev. 1995;66:1–13. doi: 10.1111/j.1467-8624.1995.tb00851.x. [DOI] [PubMed] [Google Scholar]
- Habib KE, Weld KP, Rice KC, et al. Oral administration of a corticotropin-releasing hormone receptor antagonist significantly attenuates behavioral, neuroendocrine, and autonomic responses to stress in primates. Proc Natl Acad Sci. 2000;97:6079–6084. doi: 10.1073/pnas.97.11.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biol Psychiatry. 1992;32:127–145. doi: 10.1016/0006-3223(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Hinde K, Capitanio JP. Lactational programming? Mother’s milk energy predicts infant behavior and temperament in rhesus macaques (Macaca mulatta) Am J Primatol. 2010;72:522–529. doi: 10.1002/ajp.20806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karg K. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch Gen Psychiatry. 2011;68:444–454. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazaro-Perea C. Intergroup interactions in wild common marmosets, Callithrix jacchus: territorial defence and assessment of neighbours. Anim Behav. 2001;62:11–21. [Google Scholar]
- Levine S, Johnson DF, Gonzalez CA. Behavioral and hormonal responses to separation in infant rhesus monkeys and mothers. Behav Neurosci. 1985;99:399–410. doi: 10.1037//0735-7044.99.3.399. [DOI] [PubMed] [Google Scholar]
- Levine S, Wiener SG, Coe CL. Temporal and social factors influencing behavioral and hormonal responses to separation in mother and infant squirrel monkeys. Psycho-neuroendocrinology. 1993;18:297–306. doi: 10.1016/0306-4530(93)90026-h. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Measuring temperament in rhesus macaques: consistency and change in emotionality over time. Behav Processes. 2000;49:167–171. doi: 10.1016/s0376-6357(00)00083-8. [DOI] [PubMed] [Google Scholar]
- Matheny AP, Wilson RS, Nuss SM. Toddler temperament: stability across settings and over ages. Child Dev. 1984;55:1200–1211. [Google Scholar]
- McCormack K, Newman TK, Higley JD, Maestripieri D, Sanchez MM. Serotonin transporter gene variation, infant abuse, and responsiveness to stress in rhesus macaque mothers and infants. Horm Behav. 2009;55:538–547. doi: 10.1016/j.yhbeh.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Norcross JL, Newman JD. Effects of separation and novelty on distress vocalizations and cortisol in the common marmoset (Callithrix jacchus) Am J Primatol. 1999;47:209–222. doi: 10.1002/(SICI)1098-2345(1999)47:3<209::AID-AJP3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Nunes S, Fite JE, Patera KJ, French JA. Interactions among paternal behavior, steroid hormones, and parental experience in male marmosets (Callithrix kuhlii) Horm Behav. 2001;39:70–82. doi: 10.1006/hbeh.2000.1631. [DOI] [PubMed] [Google Scholar]
- Parker KJ, Maestripieri D. Identifying key features of early stressful experiences that produce stress vulnerability and resilience in primates. Neurosci Biobehav Rev. 2011;35:1466–1483. doi: 10.1016/j.neubiorev.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polizzi di Sorrentino E, Schino G, Tiddi B, Aureli F. Scratching as a window into the emotional responses of wild tufted capuchin monkeys. Ethology. 2012;118:1072–1084. [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Palme R, Feldon J. Development of pituitary-adrenal endocrine function in the marmoset monkey: infant hypercortisolism is the norm. J Clin Endocrinol Metab. 2002;87:691–699. doi: 10.1210/jcem.87.2.8244. [DOI] [PubMed] [Google Scholar]
- Pryce CR, Dettling A, Spengler M, Spaete C, Feldon J. Evidence for altered monoamine activity and emotional and cognitive disturbance in marmoset monkeys exposed to early life stress. Ann N Y Acad Sci. 2004;1032:245–249. doi: 10.1196/annals.1314.030. [DOI] [PubMed] [Google Scholar]
- Ruis MA, te Brake JH, van de Burgwal JA, et al. Personalities in female domesticated pigs: behavioural and physiological indications. Appl Anim Behav Sci. 2000;66:31–47. [Google Scholar]
- Schaffner CM, Shepherd RE, Santos CV, French JA. Development of heterosexual relationships in wied’s black tufted-ear marmosets (Callithrix kuhli) Am J Primatol. 1995;36:185–200. doi: 10.1002/ajp.1350360303. [DOI] [PubMed] [Google Scholar]
- Smith TE, French JA. Psychosocial stress and urinary cortisol excretion in marmoset monkeys. Physiol Behav. 1997;62:225–232. doi: 10.1016/s0031-9384(97)00103-0. [DOI] [PubMed] [Google Scholar]
- Smith TE, McGreer-Whitworth B, French JA. Close proximity of the heterosexual partner reduces the physiological and behavioral consequences of novel-cage housing in black tufted-ear marmosets (Callithrix kuhli) Horm Behav. 1998;34:211–222. doi: 10.1006/hbeh.1998.1469. [DOI] [PubMed] [Google Scholar]
- Snowdon CT, Pickhard JJ. Family feuds: severe aggression among cooperatively breeding cotton-top tamarins. Int J Primatol. 1999;20:651–663. [Google Scholar]
- Sullivan EC, Hinde K, Mendoza SP, Capitanio JP. Cortisol concentrations in the milk of rhesus monkey mothers are associated with confident temperament in sons, but not daughters. Dev Psychobiol. 2011;53:96–104. doi: 10.1002/dev.20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomi SJ. Early determinants of behaviour: evidence from primate studies. Br Med Bull. 1997;53:170–184. doi: 10.1093/oxfordjournals.bmb.a011598. [DOI] [PubMed] [Google Scholar]
- Toufexis DJ, Myers KM, Davis M. The effect of gonadal hormones and gender on anxiety and emotional learning. Horm Behav. 2006;50:539–549. doi: 10.1016/j.yhbeh.2006.06.020. [DOI] [PubMed] [Google Scholar]
- Yamamoto ME. From dependence to sexual maturity: the behavioural ontogeny of Callitrichidae Marmosets and tamarins: systematics, behaviour and ecology. Oxford: Oxford University Press; 1993. pp. 235–254. [Google Scholar]
- Yamamoto S, Utsu S, Tanioka Y, Ohsawa N. Extremely high levels of corticosteroids and low levels of corticosteroid binding macromolecule in plasma of marmoset monkeys. Acta Endocrinol. 1977;85:398–405. doi: 10.1530/acta.0.0850398. [DOI] [PubMed] [Google Scholar]
- Yamamoto ME, Box HO, Albuquerque FS, de Fátima Arruda M. Carrying behaviour in captive and wild marmosets (Callithrix jacchus): a comparison between two colonies and a field site. Primates. 1996;37:297–304. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


