Abstract
Treatment of alcohol use disorder (AUD) is complicated by the presence of psychiatric comorbidity including posttraumatic stress disorder (PTSD). This is a critical review of the literature to date on pharmacotherapy treatments of AUD and PTSD.
A systematic literature search using PubMed MESH terms for alcohol and substance use disorders, PTSD, and treatment was undertaken to identify relevant randomized clinical trials (RCTs). The studies were independently evaluated (IP and TS) and those that evaluated the efficacy of a pharmacotherapy for individuals diagnosed with AUD and PTSD and were randomized controlled trials (RCT) were selected. Studies were grouped in three categories: (1) those that evaluated first line treatments for PTSD, (2) those that evaluated medications to target AUD and (3) those that evaluated medications hypothesized to be effective in targeting alcohol consumption as well as PTSD symptoms.
Nine RCTs were identified; three focused on medications to treat PTSD, four focused on AUD, and three to target both. One study included both a medication to treat PTSD and one to treat AUD so was discussed twice. All but one of the studies found that PTSD symptoms and drinking outcomes improved significantly over time. There is not one agent with clear evidence of efficacy in this comorbid group. The results for medications to treat PTSD are inconclusive because of contradictory results. There was weak evidence to support the use of medications to treat AUD among those with comorbidity with PTSD. Findings for medications that were hypothesized to treat both disorders were also contradictory.
Most studies provided a combination of interventions to treat both disorders. Despite the contradictory results, this review suggests that individuals with AUD and comorbid PTSD can safely be prescribed medications used in non-comorbid populations and patients improve with treatment.
Introduction
Evidence-based pharmacological treatments for alcohol use disorders (AUDs) evaluated in well-designed clinical studies are not being adopted in clinical treatment settings as evidenced by the low uptake of the use of medications to treat AUD (Jonas et al. 2014). With new provisions for reimbursement for treatment for addiction under the Affordable Care Act, there may be new contingencies and motivations for agencies to adopt best-practices. However, if evidence-based treatments continue to be only narrowly disseminated and adopted, treatment organizations, some of which are motivated by profit only, may offer treatments that are at best not effective and at worst are harmful (Woodworth and McLellan 2016). One of the reasons for this low uptake may be a mismatch between “real world” clinical populations which have high rates of concurrent psychiatric comorbidity, and the participants in clinical trials in which patients with comorbidity are often systematically excluded (e.g. (Anton et al. 2003, Johnson et al. 2003, Johnson et al. 2007, Mason et al. 2014). Conducting studies in populations with “multi-morbidities” is increasingly recognized as an important area of study. This concept challenges the single disease framework used throughout medicine in education, reimbursement, and research (Barnett et al. 2012). Because efficacy may be different in those with comorbid conditions, treatments for multi-morbidities need to be tested empirically.
One important comorbid condition for individuals with AUD is posttraumatic stress disorder (PTSD). The symptoms which occur after the experience of a traumatic event, include: intrusive symptoms associated with the traumatic event, persistent avoidance of stimuli related to the event, negative changes in cognition and mood, and alterations in arousal and reactivity (American Psychiatric Association 2013). PTSD has a lifetime prevalence of approximately 6.8 % of the general population (Kessler et al. 2005) and a 12-month prevalence of 3.5%; both rates are higher among women than men (Kessler et al. 2005). Other populations also have higher incidence including military veterans, with lifetime rates as high as 30% in Vietnam era veterans (Kulka et al. 1990). Individuals with PTSD have high rates of comorbid alcohol and substance use disorders (Kessler et al. 1995); these rates range from 28% to as high as 75% of individuals (Baker et al. 2009, Kulka et al. 1990). Individuals with AUD are also much more likely than the general population to suffer from PTSD (Grant et al. 2015). Among veterans from the recent conflicts, of those seeking treatment at VA, 11% were diagnosed with a substance use disorder and 55–75% of those had comorbid PTSD (Seal et al. 2011). In a sample of veterans in the community, those with a lifetime history of alcohol use disorders had higher rates of both mood and anxiety disorders (Fuehrlein et al. 2016). Comorbidity is associated with a number of worse outcomes including higher rates of psychological problems, higher rates of relapse, hospitalizations, as well as medical and social complications such as unemployment and homelessness (Blanco et al. 2013, Drapkin et al. 2011, Ouimette et al. 2006).
A growing number of studies have attempted to systematically study potential pharmacologic treatments in individuals with comorbidity. These studies range from evaluations of FDA-approved interventions already in use in non-comorbid conditions in comorbid populations to agents that are thought to target the underlying neurobiology of both disorders. This makes sense in light of the evidence that common biological factors are involved in the underpinnings of both disorders. There is a well-documented link between the major neuroendocrine stress response system, the hypothalamic pituitary adrenal (HPA) axis, and the development and maintenance of both PTSD and AUD (Geracioti et al. 2001, Logrip et al. 2012, Pervanidou and Chrousos 2012). Stress responses mediated by corticotropin-releasing hormone (CRH) are associated with drug taking behavior in laboratory animals and craving and relapse in humans (Sinha 2008). The role of stress and negative emotional states, sometimes termed the “dark side of addiction”, is increasingly recognized as important in the development and maintenance of addiction (Koob 2014, Koob and Le Moal 2005). CRH is implicated in fear related behaviors and the development of PTSD (reviewed in (Jacobsen et al. 2001)). Release of CRH stimulates norepinephrine release and norepinephrine is important in attention, arousal, and emotional memories (Arnsten and Pliszka 2011, Krystal and Neumeister 2009). Norepinephrine levels have been found to be elevated in PTSD (Geracioti et al. 2008) and in alcohol withdrawal (Smith et al. 1990). Chronic stress has been hypothesized to create a “feed forward” system with an exaggerated stress response CRH mediated release of norepinephrine may explain some of the symptoms of PTSD including hyperarousal (Jacobsen et al. 2001, Koob 2008). It has been hypothesized that individuals with PTSD use substances, particularly those that acutely dampen the stress response, to reduce this response. Noradrenergic agents have been used to treat both PTSD (Boehnlein and Kinzie 2007, Raskind et al. 2013) and addictive disorders including AUD (Simpson et al. 2009).
Other neurotransmitter systems are also thought to be involved in the underlying neurobiology of both disorders, most notably dopamine, serotonin, glutamate, and gamma-aminobutyric acid (GABA). Dopamine’s role in reward and addictive disorders including AUD is well documented (Koob and Volkow 2016). Dopamine levels have been associated with alterations in the salience of reward and while not well understood, may influence the rewarding aspects of drugs of abuse including alcohol (Koob and Volkow 2016). It should be noted that excess dopamine has also been reported in PTSD and a correlation of dopamine levels and PTSD has been reported (Drury et al. 2009). This may be mediated through norepinephrine release which also leads to the release of dopamine and serotonin. Serotonin release is also implicated in alcohol use disorders, however the role of serotonergic medications in alcohol use disorders is complicated by the heterogeneity of AUD. SRI’s are not effective in treating AUD in non-comorbid populations (Torrens et al. 2005) and SRI’s can make drinking worse among those alcohol dependent subjects with early onset AUD (Kranzler et al. 1996, H. Pettinati et al. 2000). Serotonin release is also associated with stress in PTSD and has been implicated in depression and anxiety. It should be noted that the only medications approved by the Food and Drug Administration to treat PTSD are the serotonin reuptake inhibitors (SRI) sertraline and paroxetine, although their effect is modest with small effect sizes and some conflicting results (Friedman et al. 2007).
Recent evidence has also suggested a role in the underlying neurobiology of both PTSD and AUD for glutamate and GABA, which are the most prevalent neurotransmitters in the brain. Glutamate is the most abundant excitatory neurotransmitter while GABA is the main inhibitory neurotransmitter. They work synergistically and are important in regulating the overall level of excitation, as well as in learning and in memory (Davis and Myers 2002). These processes are important for memory consolidation, fear learning, and involuntary activation of reward circuits in response to cues and in craving (Kalivas and O’Brien 2007). Several brain regions are thought to be particularly relevant for these processes and include the hippocampus, the site of memory formation, the amygdala and the prefrontal cortex. Circuits between these functions have been hypothesized to be important in the maintenance of addictive disorders (Koob and Volkow 2016) and PTSD (Sripada et al. 2012).
Comorbidity between PTSD and AUD represents a key area in alcohol research, made richer by developments in both basic and clinical science and one in which there is an urgent need to identify effective treatments. The purpose of this review is to provide a comprehensive summary of the pharmacological treatment literature that exists for AUD and comorbid PTSD specifically for the alcoholism field. Summarizing this literature can inform researchers and clinicians about effective treatments, future research directions, and may offer insight into underlying mechanisms that can be studied pre-clinically in a bench to bedside and back approach. While several previous reviews of pharmacologic management have been conducted (Norman et al. 2012, Ralevski et al. 2014, Shorter et al. 2015, Sofuoglu et al. 2014), this review represents a comprehensive critical review that also extends previous work by including several randomized clinical trials (RCTs) that have been very recently published (Batki et al. 2014, Hien et al. 2015, Petrakis et al. 2016, Simpson et al. 2015).
Methods
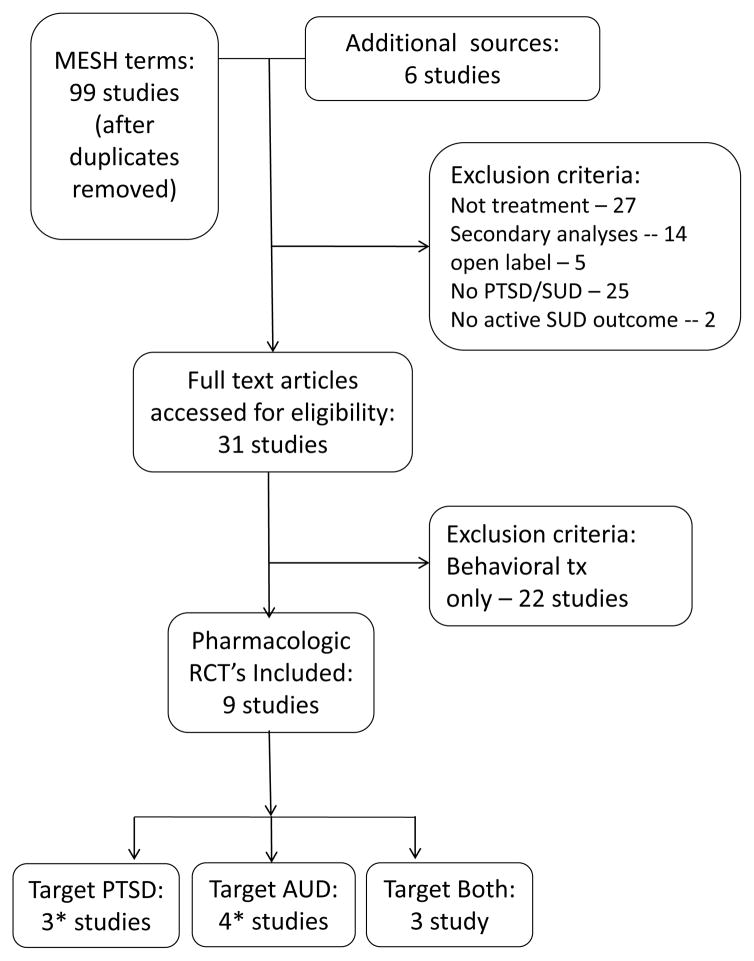
We conducted a comprehensive search on PsycINFO and MEDLINE/PUBMED databases using the following MESH terms in various combinations: “PTSD {or post traumatic stress disorder} AUD intervention”; “PTSD alcohol abuse intervention”; “PTSD AUD treatment” “PTSD alcohol abuse treatment”; “PTSD AUD pharmacotherapy”; PTSD AUD medication”; “PTSD alcohol abuse medication”; “PTSD alcoholism treatment”; “PTSD alcoholism medication”; “PTSD alcoholism pharmacotherapy”; PTSD alcoholism intervention”. The search resulted in 105 articles, the titles of which were independently evaluated and the abstracts or full text of 63 were further reviewed (IP & TS). Ultimately, 9 studies met our inclusion criteria (See Figure 1). The included studies were: 1) those that evaluated the efficacy of a pharmacotherapy with or without behavioral intervention; 2) the sample consisted of individuals diagnosed with AUD and PTSD and 3) were randomized controlled trials (RCT). Studies evaluating medications to treat alcohol use disorders (AUD) and PTSD were grouped into three categories: those that focused on first line treatments for PTSD, the serotonin reuptake inhibitors, those that focused on medications to target alcohol use disorders, and those that focused on medications that have evidence to suggest they may be effective in targeting alcohol consumption as well as PTSD symptoms. In this critical review of medication RCTs we provide an overview regarding within subject changes over time, between group similarities and differences in AUD and PTSD outcomes, and treatment dropout. The studies were independently evaluated for risk of bias (IP & TS) using elements of well-designed clinical trials and include: presence or absence of randomization, blinding, and use of intention to treat analysis; all 9 studies included all 3 elements. Effect size calculations were also conducted on the positive studies, and are included within Table 2.
Figure 1.
PRISM Diagram
*One study used both a medication for PTSD and a medication for AUD so is included twice
Table 2.
Summary of outcomes
| Study/Source | Study retention | Medication compliance | AUD Findings | Craving | PTSD Findings | Treatment Findings | Other Findings |
|---|---|---|---|---|---|---|---|
| Batki et al. 2014 | 27/30 (90%) completed trial (attending week 12) No significant difference between groups |
Average dose
Medication adherence rate
|
Significant time effect for drinking outcomes Trend for effect of treatment on drinks/week; drinks/drinking day |
Significant effect of time; significant main effect of treatment but no treatment by week interaction | Significant time effect on PTSD symptoms Trends for medication effect on PTSD symptoms (total PCL scores) |
d = 0.57% drinking days d = 0.41 PTSD |
Significant changes (worsening) in cognitive symptoms found with topiramate, with return to baseline at week 12 |
| Brady et al. 2005 | Treatment completion rates
|
Medication adherence rates based on riboflavin levels. No Significant difference |
No significant medication effects on drinking outcomes | Not reported for overall analysis | Trend toward lower PTSD symptoms at end of treatment for SER group (CAP total; intrusion, hyperarousal sub-scores) | Cluster analysis: based on characteristics of drinking history/craving/PTSD characteristics/functional impairment | |
| Foa et al. 2013 | Dropout rate was 32% No Significant difference |
Medication adherence was 85 % (defined as ≥80 % adherence) No Significant Difference |
Significant time effect for drinking outcomes At post treatment, a significant main effect of naltrexone for % drinking days |
Significant effect of time At post treatment a significant main effect of treatment |
Significant time effect of PTSD symptoms (PSSI) Significant difference in achievement of low level of PTSD defined as < 10 on PSSI at 6 months.
|
d = 0.42 % drinking days | Psychotherapy results reported elsewhere (Simpson et al, submitted) |
| Hien et al, 2015 | Study Retention Rates
Attendance at Seeking Safety
|
Medication adherence rates based on rates of riboflavin detection
|
Significant decrease in drinking outcomes from baseline to end-of-treatment No significant medication effects on drinking outcomes |
Not reported | PTSD symptoms (CAPS) decrease significantly from baseline to end of treatment, sustained at 6 & 12-month follow-up Trend for time by treatment interaction and a greater reduction in CAPS scores at end of treatment in SER vs. PLA Clinically significant change (15-point drop in CAPS) difference in 79 % SER vs 48% PLA) |
d = 0.19 for medication group differences at end of treatment | Subtype analysis (early vs. late onset AUD) not significant |
| Kwako et al. 2015 | 53/60 (88%) completed | 100% (medication administered) | Alcohol consumption not evaluated | No effect of treatment on craving in the laboratory | PTSD symptoms (CAPS) decreased over time No medication effect |
No effect of treatment on stress reactivity response in the laboratory | |
| Petrakis et al. 2006 | Secondary analysis: reported treatment retention between those with PTSD and those without No Significant Differences |
Not reported | Significant decrease in drinking over time Significant interaction between diagnosis and any medication on maximum consecutive days of abstinence and % heavy drinking days |
Significant decrease of craving over time PTSD diagnosis associated with higher craving Significant diagnosis x medication effect on craving |
Significant decrease in PTSD symptoms (CAPS) over time Significant finding: disulfiram associated with lower scores over time (CAPS total); naltrexone associated with lower scores over time on hyperarousal subscale. |
Secondary analysis of larger paper | |
| Petrakis et al. 2012 | 49/88 (56%) completed Significant difference in completers
|
Significant difference in medication adherence
|
Alcohol consumption decreases over time Significant medication effect (DMI vs. PAR) for several outcomes (# drinks/week, % drinking and heavy drinking days, drinks/drinking days) Significant medication (DMI vs. PLA) effect of GGT results No significant NLTX medication effect |
Significant medication effect on craving (NLTX vs. PLA) | PTSD (CAPS) symptoms decrease over time No significant medication effect (4-way interaction) |
d = 0.42% heavy drinking days d = 0.45 drinks per drinking day |
Four cell design, but results reported as DMI vs. PAR; NLTX vs. PLA |
| Petrakis et al. 2016 | 75/96 (78%) completed Significant difference in completion
|
Average dose of medication=14.5 ±3.14 Medication adherence 56% No significant difference in medication adherence |
Significant decrease over time in drinking variables No significant medication effect |
Significant decrease in craving over time | Significant time effect in PTSD symptoms (CAPS) and in measures of sleep disturbance No significant medication effect on any of the variables |
Site differences in drinking variables: Consecutive days of abstinence and drinking days | |
| Simpson et al. 2015 | 20/30 (67%) received medication through week 6 Significant differences in group: 12-week study
|
Medication compliance defined by riboflavin and pill counts No significant difference |
Greater reduction in drinking variables with PRA vs. PLA (% drinking days and heavy drinking days, drinks per week) | Craving decrease over time Trend toward significance: PRA greater decrease than PLA | PTSD symptoms did not improve over time or between groups | d = 1.59% drinking days d = 1.00 % heavy drinking days |
Originally 12-week trial (n=18) scaled back to 6-week study IVR completion rate was 77% |
AUD= Alcohol Use Disorder; CAP= Clinician Administered PTSD Symptom Scale; DMI=Desipramine; GGT= Gamma glutamyl transferase; IVR= Interactive Voice Response; NLTX=Naltrexone; PAR=Paroxetine; PCL=PTSD Checklist; PE=Prolonged Exposure; PLA=Placebo; PRA=Prazosin; PSSI=PTSD Symptom Scale-Interview; PTSD=Posttraumatic Stress Disorder; SC=Supportive Counseling; SER=Sertraline; SS=Seeking Safety; Top=Topiramate
Results
Overall, we found 9 relevant medication RCT studies. One of the studies reviewed was based on sub-group secondary analyses that were not the study’s original focus (Petrakis et al. 2006) and another was a 4-week inpatient study in which PTSD symptoms, but not alcohol consumption, were evaluated (Kwako et al. 2015). Given the paucity of studies we opted to include the latter two studies in this review (See Table 1). The results of these two studies do not significantly alter the conclusions/recommendations except to help suggest future research directions.
Table 1.
Study Characteristics
| Study/ Source |
Participants | Number Assessed or Screened |
Percent female |
Other Psychiatric Diagnosis |
Other Psychiatric medications |
Setting | Experimental Intervention(s) |
Control Condition |
Behavioral Platform |
|---|---|---|---|---|---|---|---|---|---|
| Batki et al. 2014 | 30 Participants PTSD & AD (DSM IV) All have “at risk or heavy” drinking during 4 weeks before randomization All have desire to quit |
137 assessed | 2/30 (7%) female | Excluded: Psychosis, bipolar and dementia | Excluded medications to treat AD | Outpatient VA setting | Topiramate (25–300 mg flexible dose) 12-week study |
Placebo | Medical Management |
| Brady et al. 2005 | 94 Participants PTSD & AD (DSM-III), Drinking in last 30 days | 360 screened | 43/94 (46%) female | Allowed: Depression/anxiety | Excluded | Outpatient substance abuse treatment Program | Sertraline 150 mg/day Placebo run-in: 1 week 12-week study |
Placebo | CBT for AD |
| Foa et al. 2013 | 165 Participants PTSD & AD (DSM-IV) PTSD severity (>15 on PSS-I*) Heavy drinking in last 30 days |
657 assessed | 57/165 (35%) female | Excluded: Psychotic disorder | Stable antidepressant medications allowed | Outpatient university clinic and VA | Naltrexone (100 mg per day) Prolonged Exposure leading to 4 groups: PE +NLTX, PE +PLA, SC +NLTX, SC +PLA 6-month study |
Placebo | Supportive Counseling (SC) |
| Hien et al, 2015 | 69 Participants PTSD (DSM IV) –full or sub-threshold* & AD Heavy drinking days in last 90 |
2207 assessed, 1179 screened | 56/69 (81%) female | Excluded: Bipolar and psychotic spectrum Allowed: drug dependence |
Excluded | Outpatient Mental Health Centers | Sertraline (up to 200 mg) 1-week single blind lead in phase 12-week study |
Placebo | Seeking Safety |
| Kwako et al. 2015 | 53 Participants PTSD and AD (DSM-IV) | 60 recruited | 24/53 (45%) female | Excluded: Complicated psychiatric problems | Excluded | Inpatient Setting | Aprepitant (125 mg) 4-week study |
Placebo | Inpatient Standard Behavioral Alcoholism treatment |
| Petrakis et al. 2006 | 93 Participants PTSD & AD (DSM-VI), Drinking in last 30 days Secondary analysis of parent study |
254 in parent study Parent study: Axis I Disorder +AD |
2/93 (2%) female | Allowed: all major Axis I disorders | Excluded medications to treat AD | Outpatients at 3 VA sites | Disulfiram 250 mg (open randomization) Naltrexone 50 mg 12-week study |
No Disulfiram Placebo |
Medication Management |
| Petrakis et al. 2012 | 88 Participants PTSD +Current AD (DSM-IV) Drinking in past 30 days |
775 assessed | 8/88 (9%) female | Excluded: psychotic spectrum disorders | Excluded | Outpatient 2 VA sites | Paroxetine (PAR) 40 mg Naltrexone (NLTX) 50 mg (4 groups; PAR + NLTX, PAR + PLA; DMI +NLTX, DMI + PLA) 12-week study |
Active control (Desipramine (DMI) 200 mg) Placebo |
Medication Management |
| Petrakis et al. 2016 | 96 Participants Current PTSD +AD Drinking in past 30 days |
2241 screened | 6/96 (6%) female | Excluded: psychotic spectrum disorders | Excluded medications to treat AD | Outpatient 2 VA sites | Prazosin (16mg) 12-week study |
Placebo | Medication Management |
| Simpson et al. 2015 | 30 Participants PTSD and AD (DSM-IV) Drinking in past 30 days |
354 screened | 11/30 (37%) female | Excluded: psychotic spectrum disorders | Excluded medications to treat AD | Outpatient Research Clinic | Prazosin (16 mg) 6-week study** |
Placebo | Medication Management |
PTSD Symptom Severity Interview
Originally 12-week study
Medications targeting PTSD
To date, three published studies have evaluated medications used to treat PTSD symptoms among individuals with co-occurring AUD and PTSD. All three evaluated one of the serotonin reuptake inhibitor antidepressants approved by the Food and Drug Administration to treat PTSD; two used sertraline and one evaluated paroxetine. Following a small open label study (Brady et al. 1995) using sertraline to treat both PTSD and alcohol consumption in a small comorbid group, these investigators (Brady et al. 2005) were the first to conduct a moderately large randomized clinical trial to evaluate whether this medication would be effective at reducing alcohol consumption and PTSD symptoms among individuals with current PTSD and alcohol dependence (AD). Subjects in this study were 94 outpatients; almost 50% were women. Randomized subjects had a 1-week placebo run in phase and were then treated with sertraline (150 mg) vs. placebo for 12 weeks; all subjects received cognitive behavioral therapy (CBT) focused on addiction rather than PTSD symptoms. Retention and compliance was reported as mean riboflavin levels and treatment completion rates; both indices were similar across groups and there were no reported medication discontinuations (See Table 2). The PTSD symptoms and the alcohol use outcomes which included percentage of drinking days, the number of drinks per day, the number of heavy drinking days, all significantly decreased over time-but there were no between groups medication effects. There were trends suggesting that PTSD symptom severity, based on the Clinician Administered PTSD Scale (CAPS)(Blake et al. 1990) total scores and the intrusion and hyperarousal subscales, were lower for the sertraline-treated groups. In a subgroup analysis based on age of onset of each disorder the authors found that less severe alcohol dependence was associated with better outcomes on sertraline while those with more severe alcohol dependence had worse outcomes. These results are consistent with results in AD that show that serotonin reuptake inhibitor response is dependent on alcohol use disorder subtype, such as early onset AD (Kranzler et al. 1996, H. M. Pettinati et al. 2000).
The second serotonin reuptake inhibitor study used a 2 X 2 designed and evaluated paroxetine (40 mg) with an active control, the noradrenergic antidepressant desipramine (200 mg) (Petrakis et al. 2012). Subjects were also randomized to receive naltrexone (50 mg) or placebo, resulting in 4 cells. All subjects received Medication Management (MM) therapy in this 12-week trial. In this section we describe the paroxetine and desipramine results and in the following section on AUD medications we cover the naltrexone results. Subjects in this study were 88 outpatients, with PTSD and current AD; they were mostly male (90%) veterans with an average age in their mid-40’s. There was a significant difference in completion rate between medication groups, such that the desipramine-treated individuals had better retention than the paroxetine-treated participants (65.2% vs 36.5%) and there was significantly better medication compliance with desipramine compared to paroxetine. There was a significant decrease over time in PTSD symptoms for all subjects as a group (significant effect of time), but no medication effect between the paroxetine and desipramine treated subjects. In terms of alcohol use outcomes, there was a significant decrease in alcohol consumption for all subjects, and there was a medication effect, such that the desipramine group reported a significantly greater decrease in percent drinking and heavy drinking days, drinks per week and drinks per drinking days compared to the paroxetine treated groups; these results were confirmed by biological marker results using gamma-glutamyl transferase (GGT) levels.
The third RCT to use a serotonin re-uptake inhibitor also evaluated sertraline (200 mg) and compared it to placebo in outpatients (n=69) with AD and current full or sub threshold PTSD (Hien et al. 2015). This study included a majority of women (81%) and all subjects were offered 12 sessions of Seeking Safety in an individual setting. Subjects attended approximately half of the Seeking Safety sessions with no significant differences in attendance between groups (sertraline: 6.0 sessions vs. placebo: 6.7), and there was no difference in medication adherence between groups. Outcomes were assessed at end of treatment, 6 months and 12-month follow-up. The results of this study are complicated and limited by missing data at various endpoints. Overall, there was a significant decrease in PTSD symptoms over time at the end of treatment for both groups, which was sustained at 6 and 12 months. There was a trend toward a significant between-group difference in PTSD symptoms with greater decrease in symptoms among those on sertraline. Further, using an index of clinically meaningful change as an outcome, which was defined as 15-point drop in CAPS, there were significantly more participants with a clinically meaningful change in the sertraline group compared to the placebo group (79 vs 48%) at end of treatment. These results were sustained at post treatment with a non-significant difference at 6-month follow-up and a significant difference at 12-month follow-up in the sertraline compared to the placebo group (95% vs. 64%). Across both conditions, participants significantly decreased their alcohol use and there was no difference between the sertraline and the placebo groups.
Interim summary of pharmacologic interventions for PTSD
One of the three studies clearly found that sertraline was more effective in decreasing PTSD symptoms than placebo (Hien et al. 2015) while another found a trend-level advantage of sertraline over placebo on PTSD outcomes (Brady). The third study (Petrakis et al. 2012) used an active control (the antidepressant desipramine) and compared it to paroxetine; both antidepressants were equally effective in significantly decreasing PTSD symptoms over time but without a placebo comparison it is difficult to fully interpret these data. Neither of the sertraline studies found the serotonergic antidepressant medications more effective than placebo in decreasing alcohol use outcomes. One study (Petrakis et al. 2012) found that the active control, desipramine, was more effective than the serotonergic medication in terms of alcohol use outcomes. Desipramine (and the other tricylic antidepressants) are considered second line medications by the VA/DoD Clinical Practice Guidelines (The Management of Substance Abuse Use Disorders Working Group 2009).
Medications targeting Alcohol Use Outcomes
Four studies have evaluated medications targeting alcohol use in comorbid group of subjects. Three studies evaluated the Food and Drug Administration (FDA)-approved medication naltrexone; one of these studies also included disulfiram, which is also FDA approved for treating AUD. A fourth study evaluated topiramate; which although not FDA-approved is recommended as a second line treatment for alcohol use disorders (Johnson 2016) and therefore is included in this section. It should be noted that while these studies assessed PTSD symptoms, the main outcomes were alcohol use outcomes.
The first study is a secondary analysis of the subgroup of veterans with PTSD from a 12- week 2×2 clinical trial conducted to evaluate naltrexone (50 mg) and disulfiram (250 mg) in patients with AD and any Axis I disorder (Petrakis et al. 2005). The parent study involved 254 veterans and there were 93 in the PTSD subgroup. Disulfiram was randomized in an open fashion to medication or no medication; naltrexone was randomized in a double blind, placebo controlled fashion resulting in four groups: disulfiram + naltrexone; disulfiram + placebo; naltrexone, and placebo. The secondary analysis evaluated whether the presence of PTSD compared to the absence of PTSD (n=161) influenced alcohol use outcomes (Petrakis et al. 2006) and compared any medication vs. no medication; naltrexone vs. disulfiram, and the combination of naltrexone and disulfiram vs. each medication alone. Results suggested that individuals with PTSD had better alcohol use outcomes, specifically on the percent of heavy drinking days and consecutive days of abstinence in the presence of active medication compared to no medication. On the primary AUD outcomes of number of heavy drinking days and consecutive days of abstinence there were no significant differences between active medication groups and no significant effect of the combination of medications compared to either alone in the PTSD subgroup. PTSD symptoms were also evaluated and improved over time for all four groups in the PTSD subgroup; disulfiram treatment was associated with lower CAPS scores over time and naltrexone treatment was associated with lower scores over time on the hyperarousal subscales.
In the second naltrexone study (also described above), the antidepressants paroxetine and desipramine were each paired with either naltrexone 50 mg or matched placebo and were compared in a group of Veterans (n=88) in a 12-week trial (Petrakis, 2012). As noted above, alcohol consumption decreased significantly over time for all four groups with results favoring desipramine over paroxetine for most alcohol consumption outcomes. Naltrexone did not, however, outperform placebo with regard to consumption outcomes, though there was a significant difference in craving such that those assigned to naltrexone reported significantly greater decreases in alcohol craving than those assigned to placebo. This suggests that there may be some effect of naltrexone, but not enough to change drinking behavior in a clinically meaningful way. None of the medications were associated with significant between group differences on PTSD outcomes. The third study evaluated both a behavioral treatment for PTSD (Prolonged Exposure or PE) with and without alcohol-oriented supportive treatment along with naltrexone 100 mg versus placebo for 6 months (n=165; PE/SC + NLX; PE/SC + PLA; SC + NLX; SC + PLA)(Foa et al. 2013). The behavioral treatment results are described elsewhere (Simpson et al, submitted). Alcohol use outcomes improved significantly over time for all groups and there was a significant effect of naltrexone for percent drinking days at end of treatment; this finding was sustained at follow-up. Similarly, there was a significant effect of medication on alcohol craving over time. There was, however, not a main effect of medication on PTSD outcomes at any time point, though post hoc analyses indicated that people assigned to receive both naltrexone and PE were more likely to have clinically significant improvements in PTSD outcomes at the 6-month follow-up than those in the other three conditions.
One study has evaluated topiramate (300 mg) treatment compared to placebo in veterans (n=30) on both alcohol use outcomes and PTSD symptoms (Batki et al. 2014). Because there is a small literature evaluating topiramate as both augmentation and monotherapy (for review see Watts et al. 2013) to treat PTSD, the investigators hypothesized topiramate may improve PTSD symptoms as well reduce drinking. In this study subjects in both groups significantly decreased alcohol use over time. Although there were no significant main effects of medication by time for the primary alcohol use outcomes, there was a main effect of medication favoring topiramate for drinking days and a trend for a medication effect by time on drinks per week and drinks per drinking day. Similarly, there was a significant effect of time for PTSD symptoms and a trend regarding overall PTSD symptom severity and the hyperarousal symptom cluster indicating an effect of topiramate over placebo.
Interim summary of pharmacologic interventions for Alcohol Use Disorders
Two of the three studies using naltrexone found some evidence of an advantage of medication on alcohol use outcomes (Foa et al. 2013, Petrakis et al. 2006), though it should be noted that the Petrakis study evaluated the combination of two medications; disulfiram and naltrexone and so those results were not specific to naltrexone. The other was a more straightforward comparison (Foa et al. 2013), and naltrexone treatment was associated with better alcohol outcomes. Foa et al. (Foa et al. 2013) also found an indication that naltrexone in combination with PE was associated with better PTSD outcomes at the final assessment. The third study by Petrakis et al. (Petrakis et al. 2012) found an advantage of naltrexone for craving, as did the Foa study, but there were no differences found on any alcohol use outcomes. It should be noted that two of the three studies used a naltrexone dose of 50 mg while the third used a dose of 100 mg (Foa et al. 2013). In the only study evaluating topiramate, the active medication was associated with better outcomes for some alcohol use indices and showed a trend toward greater PTSD symptom reductions. Taken together, these studies suggest that naltrexone may have an advantage over placebo and that topiramate treatment is promising, but the small sample in a single site study is not definitive.
Medications with Novel Mechanisms of Action that target both Alcohol Use Outcomes and PTSD
Three studies have evaluated medications that were hypothesized to treat both disorders. Two of these studies used the alpha-adrenergic medication prazosin and one study used the neurokinin-1 receptor antagonist aprepitant in a proof of concept laboratory study. The first prazosin study involved veterans and civilians with PTSD and AD (Simpson et al. 2015) was originally designed as a 12-week study, but because of higher than expected dropout the study was scaled back to 6-weeks. Most (6/10) of the drop-outs left the study because of practical reasons (e.g. time commitment of the study, reimbursement, transportation). The titration was accomplished in 2 weeks, so a 6-week trial should be adequate to evaluate medication response. In this study 30 subjects, including 37% women, were randomized to receive 16 mg of prazosin vs. placebo; 18 subjects were included in the 12-week study before it was re-designed. There are differences in retention rates both across conditions and study time frames; those in the 12-week study duration had better retention on placebo but the opposite was found in the 6-week study duration. Medication compliance was slightly higher in the placebo group. Results from this study suggested an advantage of prazosin over placebo with greater reductions in percent drinking days and heavy drinking days for the prazosin group compared to the placebo group. In this study, there was no significant improvement in PTSD symptoms over time and no medication effect. Sleep outcomes were also assessed but there was no change over time and no medication effect. The second prazosin study was conducted in mostly male veterans from two VA outpatient sites (Petrakis et al. 2016). Veterans with PTSD and AD were randomized to 16 mg of prazosin vs. placebo for 12 weeks; Medication Management was the behavioral platform. Retention in this study was high, with no differences between groups. Subjects as a group decreased their drinking significantly over time, but there were no significant group differences. In this study, the drinking outcomes were confounded by a site difference such that they were better at the site in which a majority of subjects were also in sober housing. PTSD symptoms also decreased significantly over time, but there were no group differences. Sleep disturbances and nightmares were also assessed; these significantly improved over time but there was no effect of medication.
The final RCT was a 4-week inpatient study conducted with 53 individuals with PTSD and AD (Kwako et al. 2015). This was a proof of concept study evaluating the neurokinin-1 receptor antagonist aprepitant. Neurokinin-1 receptors are found in the amygdala and hippocampus and are thought to be involved in stress-response circuitry; antagonism of neurokinin-1 receptors blocks stress responses in laboratory animals (Schank et al. 2011). In this double-blind, placebo controlled study the main outcomes were PTSD symptoms, response to stress reactivity, and alcohol craving in the laboratory. Alcohol consumption data were not collected or relevant. There was no effect of aprepitant on PTSD symptoms, alcohol craving, nor on subjective physiologic response during the laboratory sessions.
Interim summary of pharmacologic interventions for Alcohol Use Disorders
Of the two studies evaluating prazosin, one suggested that prazosin was effective in decreasing alcohol use (Simpson et al. 2015) and the other did not (Petrakis et al. 2016); however, the latter was limited by a potential confound of sober housing which may have overwhelmed any medication effect. In both studies, prazosin was not effective in decreasing PTSD symptoms. In the only study with aprepitant, the active medication did not influence PTSD symptoms or alcohol craving in the laboratory in response to either stress reactivity or cue reactivity.
Discussion
There is a small but growing literature of pharmacotherapies to treat AUD with comorbid PTSD. The conclusions from this review suggest that there is not one agent that has clear evidence of efficacy in this comorbid group. There was at best weak evidence to support the use of medications to treat AUD among those with comorbidity with PTSD. Specifically, across the three studies evaluating naltrexone, one found modest efficacy in treatment of AUD-and it should be noted that this was the only study to use 100 mg (Foa et al. 2013), one found no effect (Petrakis et al. 2012), and one found some suggestion of a medication effect, though because naltrexone was paired with disulfiram the medication effect cannot be attributed solely to naltrexone (Petrakis et al. 2006). Naltrexone was effective in decreasing craving in those studies that evaluated it (Foa et al. 2013, Petrakis et al. 2012). Topiramate was promising as it was effective in decreasing alcohol use, but thus far has only been evaluated for comorbidity in one small study.
There is some promising evidence for the use of the SRI, sertraline to treat PTSD in comorbidity such that this medication was effective in treating PTSD in one (Hien et al. 2015) study and was found to outperform placebo at the trend level in another (Brady et al. 2005). However, neither of these studies found an advantage for sertraline over placebo for alcohol use outcomes. Interestingly the noradrenergic antidepressant desipramine was as effective as the serotonergic paroxetine for PTSD and desipramine had other advantages in alcohol use outcomes. Prazosin was effective in decreasing alcohol use in one study (Simpson et al. 2015) but not in the other larger trial (Petrakis et al. 2016); prazosin was not effective in treating PTSD symptoms in either study evaluating its efficacy. The neurokinin-1 receptor antagonist aprepitant had no effect on PTSD symptoms or alcohol craving (Kwako et al. 2015).
The randomized clinical trials treating AUD and comorbid PTSD were mostly well-designed studies that used similar inclusion/exclusion criteria, notably current DSM-IV diagnosis of alcohol dependence and PTSD, with current drinking requirements for entry. A few differences were noted for example, the Hein study included subjects with sub-threshold PTSD and only one study included PTSD severity as a criterion for entry into the study (Foa et al. 2013). Similarly, the outcome measures were mostly comparable; reporting on alcohol consumption based on the Time Line Followback Method and PTSD symptoms using Clinician Administered PTSD (CAPS) or its derivative, the PTSD Checklist (PCL). Only two studies reported on a “clinically meaningful change” (Foa et al. 2013, Hien et al. 2015) and one study characterized subjects based on onset of PTSD and onset of alcohol dependence (Brady et al. 2005) but the validity of these subgroups is not well established. Because the studies used similar inclusion/exclusion criteria and similar outcomes, making overall conclusions based on these studies seems reasonable.
Nevertheless, there are contradictory findings in every category. The reasons for these differences are likely not due to significant methodologic differences as outlined above. However, some issues should be noted. First, four of the nine studies were conducted in primarily male veteran subjects; the rest had significant numbers of women. There is evidence of gender differences in medication response for both the antidepressants (Keers and Aitchison 2010) and naltrexone (Garbutt et al. 2014, Roche and King 2015). Other potential confounds include severity and chronicity of illness, type of trauma experienced, other comorbid diagnoses, concomitant psychotropic medications, and whether additional treatment resources were available (e.g., sober housing, robust addiction counseling services, etc.). Most of the studies allowed comorbid depressive disorders, drug use disorders, and subjects who were prescribed other medications.
Several comments about methodologic challenges in conducting these studies should be highlighted. The first issue is how to handle providing treatment of multiple psychiatric disorders in a safe and ethical manner. Most of the studies provided treatment for both disorders using either a combination of medications (Petrakis 2012) or a medication plus a psychosocial intervention (Brady et al. 2005, Foa et al. 2013, Hien et al. 2015). In the Brady study, the psychosocial intervention was provided to all participants to treat addiction and the Hien study provided all participants an integrated treatment to address both PTSD and AUD. In contrast, the Foa study used a base behavioral treatment to address AD for all participants and randomized to either receive or not receive an additional behavioral treatment for PTSD (Foa et al. 2013). Some of the studies providing only one medication hypothesized that the medication would target both disorders (Batki et al. 2014, Kwako et al. 2015, Petrakis et al. 2016, Simpson et al. 2015) but in most of these studies, subjects were allowed concomitant psychotropic medications outside of the context of the study to treat PTSD. The one study that did not allow concomitant medication was conducted in a safe and controlled inpatient unit (Kwako et al. 2015).
Other methodological challenges include difficulty with recruitment. Generally, studies were conducted over many years and screened large numbers of subjects to reach target samples. Difficulty with recruitment may be another reason investigators have included subjects who are taking other psychotropic medications even though this complicates the interpretation of results. It should be noted, however, that to exclude patients with comorbid PTSD and AD who are taking psychotropic medications would not only make recruitment more challenging, it would also decrease the generalizability of the findings. Other issues that may have extra-medication bearing on findings include the different treatment settings noted across studies. As mentioned above, studies have been conducted at VA settings with male patients who have experienced combat, while others are in predominately female civilian populations, limiting the ability to compare findings across studies.
Despite these issues there are some positive conclusions. Overall, clinicians can be reassured that medications approved to treat one disorder can be used safely and with some efficacy in this comorbidity. Addressing both disorders, whether by using a combination of medications to treat each disorder or by combining medication with behavioral treatments seem most likely to be effective. Participants in these trials for the most part improved over time regardless of the interventions. Nevertheless, the results are disappointing from a research standpoint in that the effects of the target medication interventions were modest at best and no category of medication had consistent positive results across alcohol and PTSD outcomes.
Where does one go from here? Although there were 9 RCT, with over 700 subjects, there was not much depth in evaluating a particular medication and several trials were very small.
Additional large clinical trials with sample sizes that can account for gender differences as well as veteran/civilian status are needed. It is noteworthy that the studies involving a medication with a robust behavioral platform seem to have had the best results. Given the research to date, it seems unlikely that one medication will be effective in treatment of both disorders given the complexity of comorbidity. As medications emerge that appear to be effective at treating one of the disorders without comorbidity (e.g., gabapentin for alcohol), testing them in comorbidity, while not especially “innovative”, is important before disseminating in “real world” populations. Innovative studies evaluating medications based on neurobiology, such as other noradrenergic agents such as doxazosin, glutamatergic medications, such as the anticonvulsants and others that target the stress reactivity circuitry, such as the neurosteroids, should also be explored, but might need to be tested first in “proof of concept” studies, such as the Kwako et al study. Because inpatient studies are expensive, other innovative strategies such as laboratory studies using stress reactivity or cue induced craving may be more efficient and cost-effective for testing novel therapies. This is an exciting field of study, which has important ramifications both for research and clinical treatment settings and hopefully investigators will be encouraged to conduct studies that can move this field forward.
Acknowledgments
We gratefully acknowledge the contributions of Jessica Dascher, Erin Gandleman, and Diana Limoncelli. This work was funded in part by a grant from NIH/NIAAA (R01AA020252-01-Simpson). Dr. Petrakis has served as a consultant to Alkermes. Dr. Tracy Simpson declares no conflict of interest.
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Washington, D.C: American Psychiatric Association; 2013. [Google Scholar]
- Anton R, Randall C, Latham P, Ciraulo D, LoCastro J, Donovan D, Kivlahan D, Saxon A, Johnson B, Roache J, Tiouririne NAD, Mason B, Salvato F, Williams L, Mattson M, Miller W, Westerberg V, Tonigan JS, O’Malley S, Petrakis I, Krystal J, Pettinati H, Flannery B, Swift R, Longabaugh R, Weiss R, Gastfriend D, Greenfield S, Zweben A, Cisler R, Fleming M, Hosking J, Garbutt J, Couper D, Grp CSR. Testing combined pharmacotherapies and behavioral interventions for alcohol dependence (The COMBINE study): A pilot feasibility study. Alcoholism-Clinical and Experimental Research. 2003;27(7):1123–1131. doi: 10.1097/01.ALC.0000078020.92938.0B. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Pliszka SR. Catecholamine influences on prefrontal cortical function: relevance to treatment of attention deficit/hyperactivity disorder and related disorders. Pharmacol Biochem Behav. 2011;99(2):211–6. doi: 10.1016/j.pbb.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DG, Heppner P, Afari N, Nunnink S, Kilmer M, Simmons A, Harder L, Bosse B. Trauma exposure, branch of service, and physical injury in relation to mental health among U.S. veterans returning from Iraq and Afghanistan. Military Medicine. 2009;174(8):773–778. [PubMed] [Google Scholar]
- Barnett K, Mercer SW, Norbury M, Watt G, Wyke S, Guthrie B. Epidemiology of multimorbidity and implications for health care, research, and medical education: a cross-sectional study. Lancet. 2012;380(9836):37–43. doi: 10.1016/S0140-6736(12)60240-2. [DOI] [PubMed] [Google Scholar]
- Batki SL, Pennington DL, Lasher B, Neylan TC, Metzler T, Waldrop A, Delucchi K, Herbst E. Topiramate treatment of alcohol use disorder in veterans with posttraumatic stress disorder: a randomized controlled pilot trial. Alcohol Clin Exp Res. 2014;38(8):2169–77. doi: 10.1111/acer.12496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Klauminzer G, Charney DS, Keane TM. A clinician rating scale for assessing current and lifetime PTSD: The CAPS-1. Behav Ther. 1990;13:187–188. [Google Scholar]
- Blanco C, Xu Y, Brady K, Perez-Fuentes G, Okuda M, Wang S. Comorbidity of posttraumatic stress disorder with alcohol dependence among US adults: results from National Epidemiological Survey on Alcohol and Related Conditions. Drug Alcohol Depend. 2013;132(3):630–8. doi: 10.1016/j.drugalcdep.2013.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehnlein JK, Kinzie JD. Pharmacologic reduction of CNS noradrenergic activity in PTSD: the case for clonidine and prazosin. Journal of Psychiatric Practice. 2007;13(2):72–8. doi: 10.1097/01.pra.0000265763.79753.c1. [DOI] [PubMed] [Google Scholar]
- Brady K, Sonne S, Roberts J. Sertraline treatment of comorbid post traumatic stress disorder and alcohol dependence. Journal of Clinical Psychiatry. 1995;56:502–505. [PubMed] [Google Scholar]
- Brady KT, Sonne S, Anton RF, Randall CL, Back SE, Simpson K. Sertraline in the treatment of co-occurring alcohol dependence and posttraumatic stress disorder. Alcoholism: Clinical & Experimental Research. 2005;29(3):395–401. doi: 10.1097/01.alc.0000156129.98265.57. [DOI] [PubMed] [Google Scholar]
- Davis M, Myers KM. The role of glutamate and gamma-aminobutyric acid in fear extinction: clinical implications for exposure therapy. Biol Psychiatry. 2002;52(10):998–1007. doi: 10.1016/s0006-3223(02)01507-x. [DOI] [PubMed] [Google Scholar]
- Drapkin ML, Yusko D, Yasinski C, Oslin D, Hembree EA, Foa EB. Baseline functioning among individuals with posttraumatic stress disorder and alcohol dependence. J Subst Abuse Treat. 2011;41(2):186–92. doi: 10.1016/j.jsat.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury SS, Theall KP, Keats BJ, Scheeringa M. The role of the dopamine transporter (DAT) in the development of PTSD in preschool children. J Trauma Stress. 2009;22(6):534–9. doi: 10.1002/jts.20475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Yusko DA, McLean CP, Suvak MK, Bux DA, Jr, Oslin D, O’Brien CP, Imms P, Riggs DS, Volpicelli J. Concurrent naltrexone and prolonged exposure therapy for patients with comorbid alcohol dependence and PTSD: a randomized clinical trial. Jama. 2013;310(5):488–95. doi: 10.1001/jama.2013.8268. [DOI] [PubMed] [Google Scholar]
- Friedman MJ, Marmar CR, Baker DG, Sikes CR, Farfel GM. Randomized, double-blind comparison of sertraline and placebo for posttraumatic stress disorder in a Department of Veterans Affairs setting. Journal of Clinical Psychiatry. 2007;68(5):711–20. doi: 10.4088/jcp.v68n0508. [DOI] [PubMed] [Google Scholar]
- Fuehrlein BS, Mota N, Arias AJ, Trevisan LA, Kachadourian LK, Krystal JH, Southwick SM, Pietrzak RH. The burden of alcohol use disorders in US military veterans: results from the National Health and Resilience in Veterans Study. Addiction. 2016;111(10):1786–94. doi: 10.1111/add.13423. [DOI] [PubMed] [Google Scholar]
- Garbutt JC, Greenblatt AM, West SL, Morgan LC, Kampov-Polevoy A, Jordan HS, Bobashev GV. Clinical and biological moderators of response to naltrexone in alcohol dependence: a systematic review of the evidence. Addiction. 2014;109(8):1274–84. doi: 10.1111/add.12557. [DOI] [PubMed] [Google Scholar]
- Geracioti TD, Jr, Baker DG, Ekhator NN, West SA, Hill KK, Bruce AB, Schmidt D, Rounds-Kugler B, Yehuda R, Keck PE, Jr, Kasckow JW. CSF norepinephrine concentrations in posttraumatic stress disorder. Am J Psychiatry. 2001;158(8):1227–30. doi: 10.1176/appi.ajp.158.8.1227. [DOI] [PubMed] [Google Scholar]
- Geracioti TD, Jr, Baker DG, Kasckow JW, Strawn JR, Jeffrey Mulchahey J, Dashevsky BA, Horn PS, Ekhator NN. Effects of trauma-related audiovisual stimulation on cerebrospinal fluid norepinephrine and corticotropin-releasing hormone concentrations in post-traumatic stress disorder. Psychoneuroendocrinology. 2008;33(4):416–24. doi: 10.1016/j.psyneuen.2007.12.012. [DOI] [PubMed] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, Pickering RP, Ruan WJ, Smith SM, Huang B, Hasin DS. Epidemiology of DSM-5 Alcohol Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757–66. doi: 10.1001/jamapsychiatry.2015.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hien DA, Levin FR, Ruglass LM, Lopez-Castro T, Papini S, Hu MC, Cohen LR, Herron A. Combining seeking safety with sertraline for PTSD and alcohol use disorders: A randomized controlled trial. J Consult Clin Psychol. 2015;83(2):359–69. doi: 10.1037/a0038719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen LK, Southwick SM, Kosten TR. Substance use disorders in patients with posttraumatic stress disorder: a review of the literature. American Journal of Psychiatry. 2001;158(8):1184–90. doi: 10.1176/appi.ajp.158.8.1184. [DOI] [PubMed] [Google Scholar]
- Johnson B. Pharmacotherapy for alcohol use disorder. [Accessed August 1 2016];UpToDate. 2016 Available. [Google Scholar]
- Johnson B, Ait-Daoud N, Bowden C, DiClemente C, Roache J, Lawson K, Javors M, Ma J. Oral Topiramate for Treatment of Alcohol Dependence: A randomised controlled trial. The Lancet. 2003;361:1677–85. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, Ait-Daoud N, Anton RF, Ciraulo DA, Kranzler HR, Mann K, O’Malley SS, Swift RM Topiramate for Alcoholism Advisory B, Topiramate for Alcoholism Study G. Topiramate for treating alcohol dependence: a randomized controlled trial. Jama. 2007;298(14):1641–51. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, Kim MM, Shanahan E, Gass CE, Rowe CJ, Garbutt JC. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. Jama. 2014;311(18):1889–900. doi: 10.1001/jama.2014.3628. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug Addiction as a Pathology of Staged Neuroplasticity. Neuropsychopharmacology. 2007;33(1):166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Keers R, Aitchison KJ. Gender differences in antidepressant drug response. Int Rev Psychiatry. 2010;22(5):485–500. doi: 10.3109/09540261.2010.496448. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry. 1995;52(12):1048–60. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59(1):11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neurocircuitry of alcohol addiction: synthesis from animal models. Handb Clin Neurol. 2014;125:33–54. doi: 10.1016/B978-0-444-62619-6.00003-3. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2005;8(11):1442–4. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760–73. doi: 10.1016/S2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler H, Burleson J, Brown J, Babor T. Fluoxetine treatment seems to reduce the beneficial effects of cognitive-behavioral therapy in type B alcoholics. Alcohol Clin Exp Res. 1996;20:1534–1541. doi: 10.1111/j.1530-0277.1996.tb01696.x. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Neumeister A. Noradrenergic and serotonergic mechanisms in the neurobiology of posttraumatic stress disorder and resilience. Brain Res. 2009;1293:13–23. doi: 10.1016/j.brainres.2009.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulka RA, Schlenger WE, Fairbank JA, Hough RL, Jordan BK, Marmar CR, Weiss DS. Brunner/Mazel, editor. Trauma and the Vietnam war generation: Report of findings from the national vietnam veterans readjustment study. New York: 1990. [Google Scholar]
- Kwako LE, George DT, Schwandt ML, Spagnolo PA, Momenan R, Hommer DW, Diamond CA, Sinha R, Shaham Y, Heilig M. The neurokinin-1 receptor antagonist aprepitant in co-morbid alcohol dependence and posttraumatic stress disorder: a human experimental study. Psychopharmacology (Berl) 2015;232(1):295–304. doi: 10.1007/s00213-014-3665-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logrip ML, Zorrilla EP, Koob GF. Stress modulation of drug self-administration: implications for addiction comorbidity with post-traumatic stress disorder. Neuropharmacology. 2012;62(2):552–64. doi: 10.1016/j.neuropharm.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason BJ, Quello S, Goodell V, Shadan F, Kyle M, Begovic A. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70–7. doi: 10.1001/jamainternmed.2013.11950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman SB, Myers US, Wilkins KC, Goldsmith AA, Hristova V, Huang Z, McCullough KC, Robinson SK. Review of biological mechanisms and pharmacological treatments of comorbid PTSD and substance use disorder. Neuropharmacology. 2012;62(2):542–51. doi: 10.1016/j.neuropharm.2011.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouimette P, Goodwin E, Brown PJ. Health and well being of substance use disorder patients with and without posttraumatic stress disorder. Addict Behav. 2006;31(8):1415–23. doi: 10.1016/j.addbeh.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Pervanidou P, Chrousos GP. Posttraumatic stress disorder in children and adolescents: neuroendocrine perspectives. Sci Signal. 2012;5(245):pt6. doi: 10.1126/scisignal.2003327. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Desai N, Gueorguieva R, Arias A, O’Brien E, Jane JS, Sevarino K, Southwick S, Ralevski E. Prazosin for Veterans with Posttraumatic Stress Disorder and Comorbid Alcohol Dependence: A Clinical Trial. Alcohol Clin Exp Res. 2016;40(1):178–86. doi: 10.1111/acer.12926. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Poling J, Levinson C, Nich C, Carroll K, Ralevski E, Rounsaville B. Naltrexone and disulfiram in patients with alcohol dependence and comorbid post-traumatic stress disorder. Biol Psychiatry. 2006;60(7):777–83. doi: 10.1016/j.biopsych.2006.03.074. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Poling J, Levinson C, Nich C, Carroll K, Rounsaville B, VNEVIMS G. Naltrexone and disulfiram in patients with alcohol dependence and comorbid psychiatric disorders. Biological Psychiatry. 2005;57(10):1128–1137. doi: 10.1016/j.biopsych.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Petrakis IL, Ralevski E, Desai N, Trevisan L, Gueorguieva R, Rounsaville B, Krystal JH. Noradrenergic vs Serotonergic Antidepressant with or without Naltrexone for Veterans with PTSD and Comorbid Alcohol Dependence. Neuropsychopharmacology. 2012;37(4):996–1004. doi: 10.1038/npp.2011.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinati H, Oslin D, Decker K. Role of Serotonin and Serotonin-Selective Pharmacotherapy in Alcohol Dependence. The International Journal of Neuropsychiatric Medicine. 2000;5(2):33–46. doi: 10.1017/s1092852900012803. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Volpicelli JR, Kranzler HR, Luck G, Rukstalis MR, Cnaan A. Sertraline treatment for alcohol dependence: interactive effects of medication and alcoholic subtype. Alcoholism: Clinical & Experimental Research. 2000;24(7):1041–9. [PubMed] [Google Scholar]
- Ralevski E, Olivera-Figueroa LA, Petrakis I. PTSD and comorbid AUD: a review of pharmacological and alternative treatment options. Subst Abuse Rehabil. 2014;5:25–36. doi: 10.2147/SAR.S37399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskind MA, Peterson K, Williams T, Hoff DJ, Hart K, Holmes H, Homas D, Hill J, Daniels C, Calohan J, Millard SP, Rohde K, O’Connell J, Pritzl D, Feiszli K, Petrie EC, Gross C, Mayer CL, Freed MC, Engel C, Peskind ER. A trial of prazosin for combat trauma PTSD with nightmares in active-duty soldiers returned from Iraq and Afghanistan. Am J Psychiatry. 2013;170(9):1003–10. doi: 10.1176/appi.ajp.2013.12081133. [DOI] [PubMed] [Google Scholar]
- Roche DJ, King AC. Sex differences in acute hormonal and subjective response to naltrexone: The impact of menstrual cycle phase. Psychoneuroendocrinology. 2015;52:59–71. doi: 10.1016/j.psyneuen.2014.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank JR, Pickens CL, Rowe KE, Cheng K, Thorsell A, Rice KC, Shaham Y, Heilig M. Stress-induced reinstatement of alcohol-seeking in rats is selectively suppressed by the neurokinin-1 (NK1) antagonist L822429. Psychopharmacology (Berl) 2011;218(1):111–9. doi: 10.1007/s00213-011-2201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal KH, Cohen G, Waldrop A, Cohen BE, Maguen S, Ren L. Substance use disorders in Iraq and Afghanistan veterans in VA healthcare, 2001–2010: Implications for screening, diagnosis and treatment. Drug Alcohol Depend. 2011;116(1–3):93–101. doi: 10.1016/j.drugalcdep.2010.11.027. [DOI] [PubMed] [Google Scholar]
- Shorter D, Hsieh J, Kosten TR. Pharmacologic management of comorbid post-traumatic stress disorder and addictions. Am J Addict. 2015;24(8):705–12. doi: 10.1111/ajad.12306. [DOI] [PubMed] [Google Scholar]
- Simpson TL, Malte CA, Dietel B, Tell D, Pocock I, Lyons R, Varon D, Raskind M, Saxon AJ. A pilot trial of prazosin, an alpha-1 adrenergic antagonist, for comorbid alcohol dependence and posttraumatic stress disorder. Alcohol Clin Exp Res. 2015;39(5):808–17. doi: 10.1111/acer.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson TL, Saxon AJ, Meredith CW, Malte CA, McBride B, Ferguson LC, Gross CA, Hart KL, Raskind M. A pilot trial of the alpha-1 adrenergic antagonist, prazosin for alcohol dependence. Alcoholism: Clinical & Experimental Research. 2009;33(2):255–263. doi: 10.1111/j.1530-0277.2008.00807.x. [DOI] [PubMed] [Google Scholar]
- Sinha R. Chronic stress, drug use, and vulnerability to addiction. Annals of the New York Academy of Sciences. 2008;1141:105–130. doi: 10.1196/annals.1441.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AJ, Brent PJ, Henry DA, Foy A. Plasma noradrenaline, platelet alpha 2-adrenoceptors, and functional scores during ethanol withdrawal. Alcohol Clin Exp Res. 1990;14(4):497–502. doi: 10.1111/j.1530-0277.1990.tb01187.x. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Rosenheck R, Petrakis I. Pharmacological treatment of comorbid PTSD and substance use disorder: recent progress. Addict Behav. 2014;39(2):428–33. doi: 10.1016/j.addbeh.2013.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sripada RK, King AP, Garfinkel SN, Wang X, Sripada CS, Welsh RC, Liberzon I. Altered resting-state amygdala functional connectivity in men with posttraumatic stress disorder. J Psychiatry Neurosci. 2012;37(4):241–9. doi: 10.1503/jpn.110069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Management of Substance Abuse Use Disorders Working Group; The Office of Quality and Safety VA Washington DC and Quality Management Division United States Army MEDCOM, editor. VA/DoD Clinical Practice Guidelines for Management of Substance Use Disorders (SUD) Washington DC: 2009. pp. 1–158. [Google Scholar]
- Torrens M, Fonseca F, Mateu G, Farre M. Efficacy of antidepressants in substance use disorders with and without comorbid depression. A systematic review and meta-analysis. Drug Alcohol Depend. 2005;78(1):1–22. doi: 10.1016/j.drugalcdep.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Watts BV, Schnurr PP, Mayo L, Young-Xu Y, Weeks WB, Friedman MJ. Meta-analysis of the efficacy of treatments for posttraumatic stress disorder. J Clin Psychiatry. 2013;74(6):e541–50. doi: 10.4088/JCP.12r08225. [DOI] [PubMed] [Google Scholar]
- Woodworth AM, McLellan AT. Converging advances in science, policy and public awareness: A time of great opportunity and change in addiction treatment. Brain Res Bull. 2016;123:110–3. doi: 10.1016/j.brainresbull.2016.05.005. [DOI] [PubMed] [Google Scholar]