Abstract
Nanodiscs provide an excellent system for the structure-function investigation of membrane proteins. Its direct advantage lies in presenting a water soluble form of an otherwise hydrophobic molecule, making it amenable to a plethora of solution techniques. Nuclear Magnetic Resonance is one such high resolution approach that looks at the structure and dynamics of a protein with atomic level precision. Recently, there has been a breakthrough in making nanodiscs more susceptible for structure determination by solution NMR, yet it still remains to become the preferred choice for a membrane mimetic. In this practical review, we provide a general discourse on nanodisc and its application to solution NMR. We also offer potential solutions to remediate the technical challenges associated with nanodisc preparation and the choice of proper experimental set-ups. Along with discussing several structural applications, we demonstrate an alternative use of nanodiscs for functional studies, where we investigated the phosphorylation of a cell surface receptor, Integrin. This is the first successful manifestation of observing activated receptor phosphorylation in nanodiscs through NMR. We additionally present an on-column method for nanodisc preparation with multiple strategies and discuss the potential use of alternative nanoscale phospholipid bilayer systems like SMA lipid discs and Saposin-A lipoprotein discs.
Keywords: Nanodisc, Integrin, Saposin-A, SMALP, solution NMR, nanoscale phospholipid bilayers, Styrene Maleic Acid, beta barrel, transmembrane, membrane proteins
Graphical Abstract
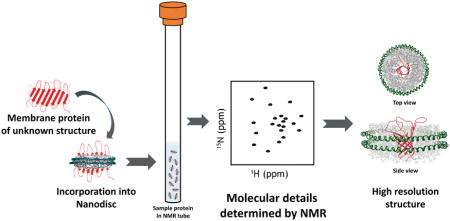
Introduction
Membrane proteins act like a conduit, communicating the internal of a cell with its outer environment. They make up for roughly 30% of the eukaryotic genome [1] and 50% of all drug targets [2]. Membrane proteins have long been the focal point of both academic and pharmaceutical research. Not surprisingly, their dysregulation, misfolding and/or mutations have been associated with numerous diseases [3, 4]. Despite their importance and relative abundance, very few have been structurally and functionally characterized, which reflects through the poor representation (less than 3%) of their available structures in the protein database. Membrane proteins are notorious for the difficulties associated with their overexpression, purification, low yield and stability. The inability to obtain protein samples which are stable, pure and in large quantities for X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy, and cryo electron microscopy (cryo-EM) greatly hampers the structural elucidation of these elusive proteins. Initial success in isolating membrane proteins was offered through the use of detergents, which helped in the biochemical investigation of several proteins. Detergents, however, do not provide an ideal solvent condition mimicking a “native like” environment for membrane proteins [5] and may not be optimal for studying signaling across the membrane as they may lead to the unfolding of the soluble interacting partners. Thus, there stands a need for a proper membrane mimetic that stably houses membrane proteins and makes them amenable to in-vitro investigations.
Solution NMR has been successfully used to study membrane proteins solubilized in different membrane mimicking systems, including organic solvent mixtures, amphipols, micelles, and bicelles [6, 7]. These media, though useful, present themselves with a number of caveats, including surface curvature artifacts, limited diversity of detergent or lipid molecules, heterogeneity of the sample preparation and a debilitating inability to study any interaction with their soluble binding partners. These problems were remedied by the introduction of nanodiscs, a class of soluble membrane mimetic which provided a stable lipid bilayer system quite close to its native environment. The success of nanodiscs has been vividly exemplified through the biophysical characterization of several receptors, enzymes, channels, and transporters [8, 9]. In this review, we discuss the application of nanodiscs to solution NMR. We also provide interesting strategies for incorporating membrane proteins into nanodiscs along with discussing other potential alternative nanoscale phospholipid bilayer systems. Finally, we present a previously unpublished original study of looking at the activation of a cell surface receptor, Integrin, encapsulated within nanodiscs. All together, we hope to make a compelling argument towards the use of nanodiscs in NMR and provide a lingering excitement that encourages researchers to appreciate and indulge in the use of this attractive system.
What are Nanodiscs?
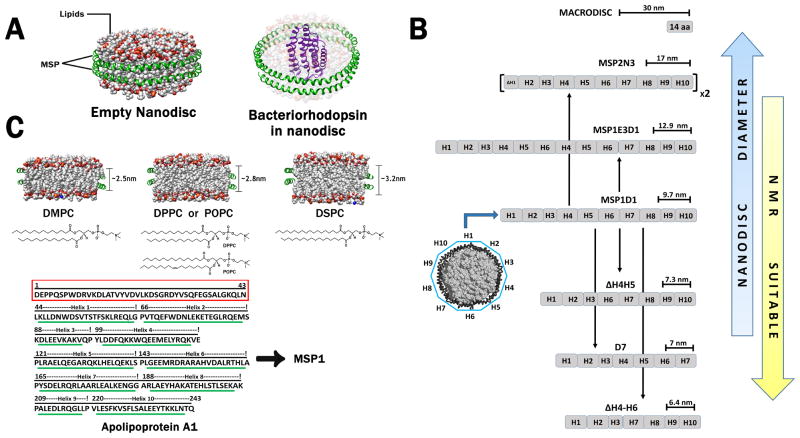
Nanodiscs are discoidal nanoscale lipoprotein complexes that are composed of a phospholipid bilayer held together by two anti-parallel strips of amphipathic helical Membrane Scaffold Protein (MSP) (Figure 1A). Nanodiscs were originally developed by Sligar and colleagues in the late nineties [10–13]. MSP is derived from Apolipoprotein A-1 (apoA-1), which normally functions to transport cholesterol from the tissue cells to the liver for excretion. The concept of a disc shaped structure came from the very nature of Apolipoprotein A-1 which forms nascent discoidal particles by utilizing phospholipids from the serum. These phospholipid particles later on ingests cholesterol from the tissue cells with the help of transporter ABCA1, growing from a discoidal to a spherical form and eventually ending up in the liver [14]. Full length apoA-1 is a helical protein with an N-terminal four helix bundle and two C-terminal helices (PDB: 2A01). An N-terminal deletion construct apoA-1 (Δ1-43) yielded a structure that was arranged in a circular belt of amphipathic helices (PDB: 1AV1) [15], which led to the advent of the present nanodisc technology. This deletion construct consisting of ten alpha helical repeats was referred to as MSP1, which (Figure 1C: Bottom panel) formed a belt encompassing lipid molecules. Another larger construct MSP2 was designed by joining two MSP1 molecules through a stable linker. MSP1, MSP2 [11] and other variants are broadly referred to as MSP. The variants are denoted by a letter and a number that follows the parent MSP1 or MSP2 from which they are derived through either deletions or insertions of helices. The presence of a protein belt constrains the dimensions of the bilayer, trapping lipid molecules within its central core. This way the system ensures a monodisperse size distribution. MSP variants differ in the size of their total length resulting in discs of different diameters. This in permutation with different types of lipid molecules, including DMPC (dimyristoyl-phosphocholine), DMPG (dimyristoyl-phosphorylglycerol), POPC (palmitoyl-oleoyl-phosphocholine), DPPC (dipalmitoyl-phosphocholine) and E. coli lipid extracts, etc. [16], offer a wide variety of customizable discs catering to the stability requirements of different membrane proteins. Lipid molecules differing in their acyl chain lengths provide a bilayer with altered thickness (Figure 1C: Top panel) which may be required for membrane proteins that either differ in their expanse of the bilayer region or undergo relative movements in the transmembrane region as a result of their activation. Nanodiscs provide a competitive edge over other systems due to their soluble nature, ease of concentration, monodispersity, temperature stability, and compatibility with cell free expression system offering themselves as a more viable alternative for investigation of membrane proteins.
Figure 1.
Nanodiscs models were constructed using the nanodisc builder from CHARMM GUI [62]. (A) Visual representation of a nanodisc that is either empty or contains bacteriorhodopsin (PDB: 1R2N). The outer belt protein MSP is shown in green while the lipid molecules are colored by atom type, where carbon is grey, oxygen is red, phosphorus is orange, and nitrogen is blue. The lipids are rendered partially transparent in order to distinguish bacteriorhodopsin from the rest of the nanodisc. (B) Schematic representation of the overall length and nanodisc diameters for several MSP1D1 variants. The arrow indicates the scheme for the addition or deletion of helices from the core MSP1D1 leading to MSP with different lengths. Nanodiscs with smaller diameters are preferable for NMR studies. The largest “MACRODISC” was obtained from a singular 14 amino acid peptide producing a disc with a diameter of 30nm while the smallest disc, with a diameter of 6.4 nm, was obtained from the deletion of helices 4 through 6 from the MSP1D1 construct. (C) Depiction of the approximate hydrocarbon thickness of nanodisc bilayers composed of commonly used phosphatidylcholines. As shown, DMPC is the smallest, DPPC and POPC have similar thickness, and DSPC is the largest [63]. The chemical structure of each lipid is presented underneath (Top Panel). The complete sequence of Apolipoprotein A1 is shown demarking each individual helices. The MSP1 construct, represented by the green lines, was created by deleting the N-terminal region highlighted in the red box (Bottom Panel).
Preparation of nanodiscs
For NMR experiments, optimal sample preparation is paramount to getting good data. The following simple, but key considerations should be kept in mind.
1. Expression and Purification of MSP
Expression and purification of MSP is similar to any typical His-tag protein. We find “The QIAexpressionist™ (Qiagen, USA) as a good general handbook for His-tag protein purification. MSP in pET28a vector is transformed into E. coli BL-21(DE3) (Agilent, USA), grown to an optical density of 0.6, and induced with 1mm IPTG. The induction is continued for 4–6 hours. All expression steps are carried out at 37°C. We have found that overnight expression at room temperature leads to an increase in aggregation and impurities, reducing the overall yield of monomeric MSP. Post harvesting, the cells are lysed and passed over Ni-NTA resin (Qiagen, USA) following the protocol referenced in the methods paper [17]. Briefly, the purification process is performed in Tris-NaCl buffer at pH 8 and has four steps (three wash and one elution). The first buffer contains 1% Triton X-100 and the second contains 50mM Sodium Cholate as detergents. The second and third wash buffers each contain 20 and 50 mM Imidazole, respectively. Elution is achieved with 400mM Imidazole. MSP purification can be carried out both at room temperature and 4°C. A key consideration is to check the pH of the buffers at 4°C, since Tris is temperature sensitive and a change in pH might affect the final yield. We find that the wash steps along with removing impurities also washes out reasonable amount of MSP. This can be mitigated by reducing the salt concentration by half and having a single 20 mM Imidazole wash instead of 50 mM in buffer three. Imidazole is removed from the final elution and buffer exchanged through overnight dialysis in Tris-NaCl buffer at pH 7.5. For NMR applications, we perform additional Size Exclusion Chromatography (SEC) on a Superdex S-75 (GE, USA) column and discard the tailing end of the peak that contains degraded MSP. This helps us get rid of MSP molecules smaller than the optimal D7 construct which may form larger discs, increasing the heterogeneity of the sample. A further elaboration of this rationale can be found in the section (Size variation in nanodiscs). After completion of the purification process, MSP is concentrated to roughly 4 mg/ml and stored at 4°C. For long term storage, lyophilization has been recommended [17].
2. Incorporation into nanodiscs
2.1. Preparation of reagents
The primary reagents consists of two Tris-NaCl buffers with 20 mM Tris-HCl and 100 mM NaCl at pH 7.5. The second buffer, in addition, contains 60 mM Sodium Cholate detergent. NaCl concentration in the above buffers can be significantly reduced for membrane proteins that are sensitive to salt. The lipid stocks used for nanodisc preparations comes either in a soluble form, dissolved in chloroform or as solid powders (Avanti polar lipids, USA). Chloroform is removed from the solubilized lipid stock using a stream of nitrogen gas and later left in a vacuum desiccator to eliminate any residual amounts. Alternatively, one could use the powdered stock that can be directly weighted in appropriate quantities for disc preparation. Though we haven’t directly compared the soluble and the powder form, we routinely use the powdered form which has worked well without any drawbacks. Protein incorporation into discs can be carried out at different temperatures which may improve their stability or reduce protein degradation. Lipid molecules that differ in their phase transition temperatures viz., POPC at ~ 4°C, DMPC at ~ 25°C, and DPPC at ~ 37°C [18] are chosen based on the desirable operating temperature required for disc preparation. The most popular utilization has been DMPC at room temperature. Alternatively, one could also prepare or use lipid extracts from multiple sources to ensure proper stability of specific membrane proteins.
2.2. Preparation of nanodiscs
Lipid molecules are reconstituted in the cholate containing buffer. The amount of lipids required depends on the type of MSP molecule that is utilized. Generally, the reconstituted lipid molecules are added in molar excess as follows: 100–120 molar excess for MSP1E3D1, 80 molar excess for MSP1D1, 50 molar excess for ΔH5 and 20 molar excess for the smaller constructs, D7 or ΔH4H5. A typical reaction mixture consists of, MSP and lipid molecules for preparing empty discs; MSP, lipids, and target membrane protein for protein incorporated discs. After the addition of MSP and lipids in the desired molar ratios, the volume is made up by the aforementioned two buffers such that the final concentration of cholate in the reaction volume is between 12–40 mM. This reaction mixture is kept on a rocker for an hour. Meanwhile, pretreatment of Bio-beads SM-2 (Bio-Rad, USA), which functions to pull out the detergent and initiate nanodisc formation, are carried out. The beads are washed with Methanol (1x), Water (3x) and buffer one (1x) from the above two buffers. Bio-beads are added to the reaction mixture in a ratio of 0.5 to 1.5 grams per ml of the volume and left rocking at room temperature, such that the beads remain gently suspended in the solution, for 3–4 hours or overnight for samples with high amount of total detergents. Later, the sample is separated from beads, concentrated, and run on an SEC column to obtain purified nanodiscs. For incorporating protein into nanodiscs, the reaction mixture typically contains MSP at a concentration that is twice in molar excess of the target protein. A protein embeds itself into disc by occupying the volume of the lipid molecules that it displaces. Hence, the addition of lipids can be done at a reduced molar ratio than what is required for making empty discs. Empirical optimization to ensure maximum incorporation of the target protein into nanodiscs is achieved by monitoring peaks in the void volume arising from the aggregated or unincorporated membrane protein, and at the position of free MSP, implicating inefficient disc formation. Both can be overcome by adjusting the concentration of lipids, MSP, or target protein.
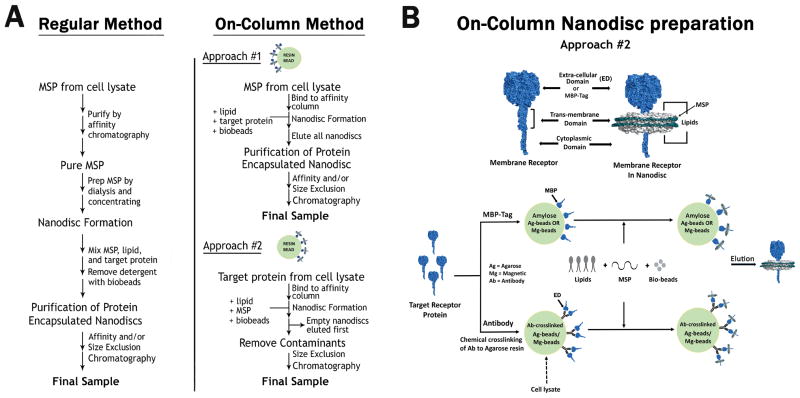
2.3 On-column nanodisc formation
As with any project, a typical NMR sample is prepared in several iterations to obtain the best NMR spectrum. Preparing nanodiscs samples for NMR applications are time intensive and requires multiple purifications to separate empty discs from the incorporated ones. We developed a quicker, alternative, on-column method where the protein incorporated discs were formed on the resin either through the bound MSP (approach-1) or the bound target protein (approach-2) [19]. The advantages to this method were several-fold, including reduced preparation time, concomitant separation of empty and incorporated discs and better yields, which collectively improves the quality of our NMR spectrum. For on-column preparation, the required reagents remain the same as in the original protocol while we explore the differential permutation of affinity tags to achieve our goals. Briefly, for approach-1, the harvested cells containing over expressed His-tagged MSP is bound to Ni-NTA resin in a gravity column and washed as per the regular protocol. Prior to elution, the resin is mixed with lipids and target protein in the same column for an hour before the addition of Bio-beads. After passage of the stipulated time, discs are formed on the resin attached through MSP (shown as inset in Figure 2A for approach-1). Once the reaction is over, elution is carried out in the same elution buffer previously used for the purification of MSP. The eluate contains a mixture of empty and protein incorporated discs, which are further separated through chromatography. For approach-2, we took the advantage of the MBP (Maltose binding protein) tag by engineering the transmembrane and cytoplasmic tail regions of Integrin-αIIb fused to MBP (in pMAL-C2 vector) with a TEV cut site present between the tag and the protein. MBP helps solubilize the otherwise insoluble transmembrane region of Integrin and the TEV cut site helps us remove the MBP tag post incorporation into discs using TEV protease (Figure 4A: Left Panel). Alternatively, other tags could be used in combination with a TEV cut site. The MBP-fused construct was expressed in E. coli BL-21 (DE3) similarly to MSP. Harvested cells were lysed and mixed with Amylose resin (NEB, USA), which is later washed with Tris-NaCl buffer containing 20 mM Tris-HCl and 100 mM NaCl at pH 7.5. Amylose resin retains the MBP fused protein through its affinity for MBP. Previously purified MSP and lipids are added to the resin, followed by the addition of Bio-beads. Discs are formed around the MBP fused protein attached to the Amylose resin (shown as inset in Figure 2A for approach-2). After the formation of discs, the resin is rinsed with Tris-NaCl buffer, which washes out the empty discs leaving behind the protein incorporated ones. Elution is carried out in Tris-NaCl buffer with 20mM maltose and the eluate is further subjected to SEC for buffer exchange and removal of unincorporated Integrin. For our NMR sample, elution was carried out in the presence of TEV to help remove the MBP tag. Approach-2 is faster and more efficient of the two approaches. A pictorial representation summarizing the different approaches is shown in Figure 2A. There are two caveats to the on-column method. (i) Estimation of the protein concentrations: we usually express MSP/target protein in large volumes of LB and aliquot 1L harvests in falcon tubes. From one such tube, we estimate the protein concentration and use that to decide the MSP to target protein or vice versa ratios. We admit that this may not be accurate, but the convenience of sampling through several proteins grossly outweighs the effects of inaccuracy arising from minor discrepancies in protein estimations. This problem is usually alleviated by the addition of MSP in excess to its required concentration. (ii) Retrieval of chromatography resin after mixing with Bio-beads: a simple solution to this problem is to use magnetic beads in lieu of agarose beads, which can be easily recovered from the solution mixture using any of the commercial magnetic apparatus. Alternatively, a net filter can be utilized with a pore size large enough for the resin but small enough for the beads, since both differ in size by several orders of magnitude.
Figure 2.
General workflow for nanodisc preparation. (A) Comparison of the regular method to our on-column method. The on-column method is split into two approaches, depending on the immobilization of either MSP or the target protein on the resin. (B) Schematic representation of a target protein with its corresponding soluble Extra cellular/MBP tag domain, a transmembrane hydrophobic domain, and soluble cytoplasmic domain. Also shown is a nanodisc containing the encapsulated target protein with MSP and lipid molecules (Top Panel). A more detailed workflow for approach #2 from the on-column method is illustrated. The target protein is bound to the resin either through its affinity for the MBP tag or through the crosslinked antibody specific to the extracellular domain (ED). The addition of MSP, lipids, and bio-beads leads to the encapsulation of the target protein into nanodiscs while still bound to the resin. This nanodisc encapsulated protein is further eluted from the resin (Bottom Panel).
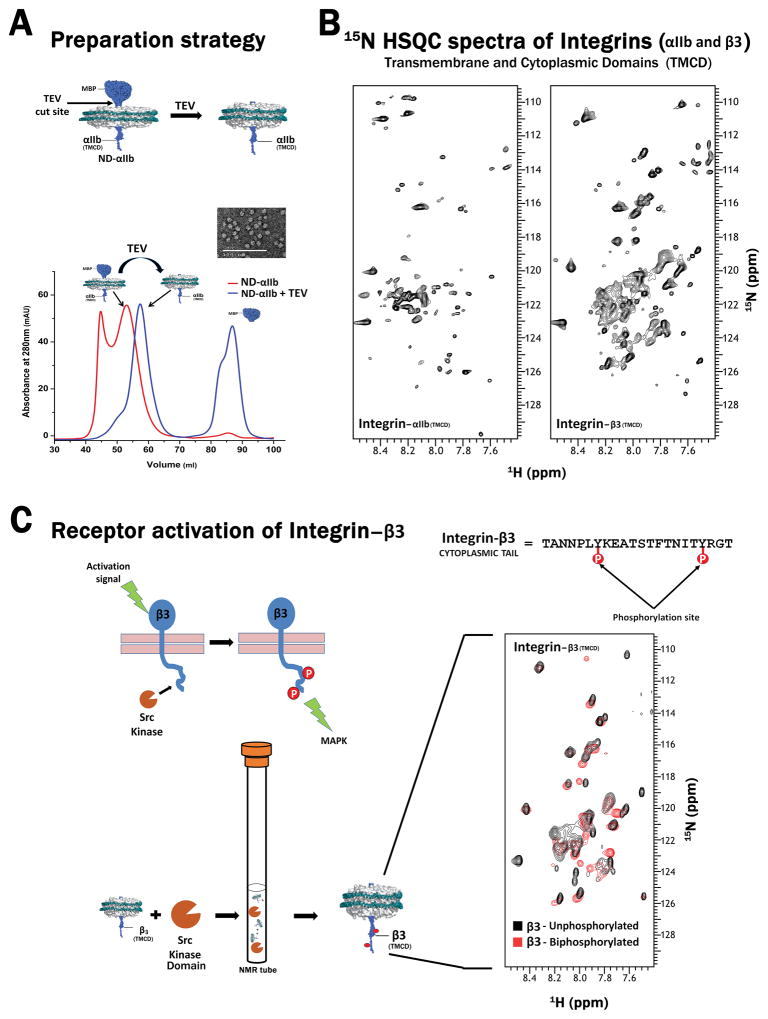
Figure 4.
(A) Overview of the procedure to obtain Integrin αIIb [trans-membrane and cytoplasmic domains (TMCD)] incorporated nanodiscs. TEV protease treatment of MBP- Integrin αIIb (TMCD) incorporated nanodiscs, cleaves the MBP–tag at the TEV cleavage site engineered between the tag and Integrin. The αIIb incorporated discs can then be purified from the rest of the cut products using SEC. Inset: Negatively stained TEM image of αIIb containing nanodiscs displaying a homogeneous population of discs. The exact same procedure can also be extended to obtain β3 (TMCD) incorporated nanodiscs. (B) 1H-15N- TROSY HSQC spectra of Integrin αIIb and β3 (TMCD) acquired on 800 MHz magnet (Agilent, USA). The sharp peaks correspond to the cytoplasmic tail region while the broader peaks correspond to the transmembrane region. (C) Applicability of nanodisc systems for studying signaling pathways. A schematic showing the activation events of Integrin β3 which is relayed intracellularly through the phosphorylation of its cytoplasmic tail by Src kinase (Top Panel). This event is mimicked (in vitro) in an NMR tube by mixing 15N labeled β3 incorporated discs with the kinase domain of Src kinase in the presence of ATP molecules. Phosphorylation is manifested through chemical shift perturbations (CSP) observed through the overlay of the 1H-15N- TROSY HSQC spectra obtained from the un-phosphorylated (black) and bi-phosphorylated (red) β3 (TMCD) collected on 600 MHz magnet (Agilent, USA). Also shown is the sequence of the β3 tail where the phosphorylation occurs at the two tyrosine residues.
An extension of approach-2, though not useful for NMR applications lies in the use of antibodies to pull larger protein receptors/complexes from any cell lysate. This strategy may find wide applicability among general and pharmaceutical researchers. Antibody based pull down is a routinely used well-established technique. We propose to utilize antibodies in conjunction with our on-column method by immobilizing antibodies through covalent crosslinking to the resin bead using Crosslink IP/Crosslink Magnetic IP Kit (Pierce, USA). The crosslinked antibodies (specific to the protein or a small tag) can then be used to fish out native receptors expressed in a mammalian cell line. This can be further washed and mixed with MSP and lipid molecules. Nanodiscs with the incorporated pulled receptor can be obtained either with the appropriate elution buffer or through TEV protease cutting at a TEV site engineered between the tag and the protein. A schematic representation, comparing the antibody versus the MBP tag using approach-2, is depicted in Figure 2B.
3. Considerations for NMR sample preparation
Deuteration is the sole key towards successful implementation of nanodiscs in solution NMR. The target receptor protein needs to be expressed in M9 Minimal media supplemented with [U-99%15N] NH4Cl and [U-99% 2H, 13C] Glucose in [99%] D2O (Cambridge Isotope Labs, USA). Nanodiscs should be prepared with deuterated lipids [eg. 1,2-dimyristoyl-d54-sn-glycero-3-phosphocholine; d54-DMPC (Avanti Polar, USA)] using any of the methods explained above with one exception. Typically, MSP is added twice in molar excess of the target protein. In case of NMR sample preparation, MSP should be added three–four times in molar excess of the target protein since the aim is to get monomeric incorporation of the target protein into nanodisc. The empty discs are successively removed thorough the employment of different chromatographic techniques.
Size variation in nanodiscs
NMR is a powerful high resolution technique that provides molecular details delineating both the structural and dynamic properties of a protein. The largest advantage lies in its ability to investigate the protein in solution conditions closely resembling a native environment, which is in sharp contrast to X-ray crystallography. However, the single most important limitation resides with the overall size of the protein. Previously, the size limitation for NMR susceptibility was capped at 50 kDa [20], but with multiple technological advances, such as TROSY [21]-based triple-resonance experiments at high magnetic fields strengths combined with extensive deuteration and innovative isotopic labeling schemes in sample preparations [22, 23], this size has been pushed to 100 kDa. Even with these advancements, it still remains challenging to study larger complexes at higher resolution.
1. Small discs
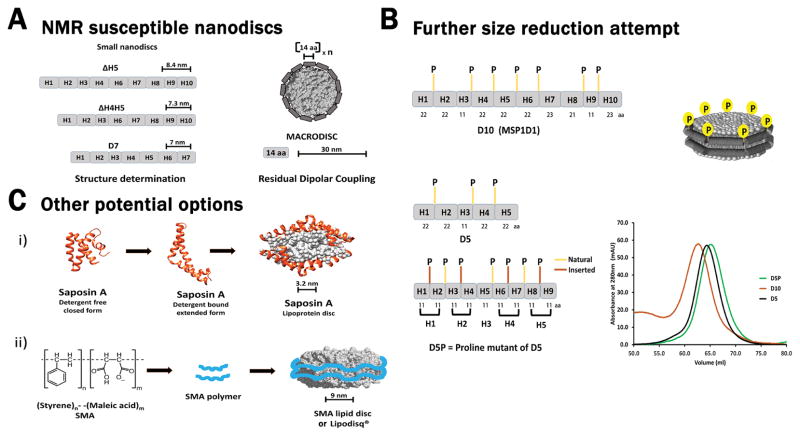
The original MSP construct, MSP1, that contained ten amphipathic helical repeats was modified at its first helix through partial deletion and further addition of a Histidine-tag to obtain the MSP1D1 construct [24], which formed nanodiscs with a diameter of ~10 nm and MW of ~150 kDa. This became the base construct from which most of the variant discs were generated (Figure 1B). For NMR applications, a larger disc size is unfavorable as its transverse relaxation rate is faster, leading to peak broadening and lower resolution. This problem was ameliorated by creating truncated constructs of MSP, which yielded smaller discs. Sligar’s group had previously developed MSP1E3D1 [24] which are larger discs of ~13 nm diameter, obtained through additional insertion of helices 4–6 (Figure 1B). This proved that repetition of helices in the middle region of the protein does not affect its property to form discs, which also indirectly meant that the middle region is amenable to alterations without impacting discs formation. Wagner’s group adopted this idea and successively removed the same three helices 4 through 6 obtaining smaller nanodiscs. The smallest obtained disc ΔH4-H6 was of ~6.4 nm, but was unstable and formed larger assemblies of ~11 nm over time. The next smaller disc, which was stable enough to retain its small size, was from the MSP construct ΔH4H5 with a diameter of ~7.3 nm and MW of ~70 kDa (Figure 3A) [25].
Figure 3.
(A) Small nanodiscs that have been employed in solution NMR studies. ΔH5, ΔH4H5, and D7 provide discs with smaller diameters that can be used for structural investigation of target proteins (Left Panel). Pictorial representation of larger “MACRODISCS” are shown where the outer annulus is formed by a 14 amino acid amphipathic peptide. These large 30 nm discs have been used to orient molecules for residual dipolar coupling (RDC) experiments (Right Panel). (B) Schematic representation of MSP1D1/D10 with the positioning of prolines and the number of amino acids present in each helix. Pictorial representation of a nanodisc showing the presence of prolines at helix turns (Top Panel). The scheme for incorporation of proline residues to further push nanodiscs to a smaller size is shown. An altered construct D5P, is created from D5 through the strategic placement of additional prolines, increasing the number of individual helices and potentially offering additional turns at proline residues. Each individual helix in the D5P construct is of identical length and is maintained at a size of 11 amino acids, representing the smallest building block in the MSP1D1 construct (Bottom Left Panel). The elution profile from size exclusion chromatography showing that the D5P nanodiscs (green) are approximately the same size as D5 discs (black). Furthermore, D5 and D5P nanodiscs are similar in size to the MSP1D1/D10 discs (brown) (Bottom Right Panel). (C) Alternative nanoscale phospholipid bilayer systems: Saposin-A lipoprotein disc formed by the addition of detergent molecules is shown. Saposin-A in its detergent free form adopts a closed conformation which becomes extended when bound to detergent molecules (PDB ID: 4DDJ). Saposin-A lipoprotein discs, with a diameter of 3.2 nm, are formed where two Saposin-A proteins are brought together by its lipid core (Top Panel). Styrene-Maleic Acid Lipid Particle (SMALP) is shown where the synthetic Styrene-Maleic Acid copolymer forms discs by encapsulating lipid within its central cavity. SMA lipid discs or Lipodisq® have a diameter of 9nm (Bottom Panel).
At the same time, our group independently arrived at a similar conclusion through a different scheme of deletion constructs. We successively removed individual helix from the C-terminal end and obtained the smallest disc from the deletion of the last three helices ΔH8-H10, which we refer to as D7 discs, since this deletion construct contained the first seven helices. D7 disc have a diameter of ~ 7nm with a molecular weight of ~ 62 kDa (Figure 3A). In the MSP1D1 construct, all helices are roughly of similar lengths except for Helix 3 and 9 which are half the standard length [Figure 3B: top panel (MSP1D1 or D10)]. Both D7 and ΔH4-H6 constructs were devoid of three helices, with our deletion ΔH8-H10 containing one short helix along with two standard helices while Wagner’s deletion removed three standard length helices. This translates into our D7 construct being slightly larger than the ΔH4-H6 construct though both have deletions by three helices. Interestingly, this was quite accurately corroborated in the distribution of disc sizes where our D7 (ΔH8-H10) disc size of ~7 nm falls right in between Wagner’s discs ΔH4H5 of ~7.3 nm and ΔH4-H6 of ~6.4 nm. Furthermore, we found that any successive reduction beyond seven helices lead to the formation of discs with larger diameters. For example, our D5 construct, which contains the first five helices, gave a size similar to the full length MSP1D1 (D10) construct with ten helices. This can be conceptualized as four D5 molecules coming together as two dimers of D5 forming either half of the circular belt. These rings present an edge to edge arrangement mimicking a single ring of 10 helices. The most intriguing finding from our D7 construct was that the terminal helices do not regulate the formation of nanodiscs, which we believe might have been the initial concern that led to the addition or deletion of helices exclusive to the middle region. A point to be noted here is that though Wagner’s ΔH4H5 gave the smallest disc, the ΔH5 construct with a slightly larger diameter of ~8.4 nm (Figure 3A: Left Panel) provided the best NMR spectrum and was adopted for structure determination.
Once we reached the smallest possible size through the deletion constructs, we still argued if we could possibly break the size barrier and force the system to even smaller diameters. A closer look at the protein sequence of MSP1D1 showed the strategic presence of proline residues present in between helices (Figure 3B: Top Panel). We hypothesize whether the inter-helical turns are an outcome of proline residues. Additionally, we observed that most helices had a standard length of ~ 22 amino acids (aa) with the smallest helix being 11 aa long. The natural presence of smaller helices led us to wonder whether reducing the helix length would facilitate further size reduction. Using all of this information, we attempted to redesign our D5 MSP construct, which previously gave us larger discs. D5 contains five helices with one 11 aa and four 22 aa blocks. Our idea was to break down D5 into blocks of 11 aa each, such that it gives us a total of 9 helices roughly resembling the natural 10 helix arrangement in MSP1D1. We achieved this by adding proline residues breaking the 22 aa bocks down to 11 aa and left the natural occurring 11 aa block untouched (Figure 3B: Bottom Left Panel). Unfortunately, this approach was unsuccessful as can be seen from the SEC data (Figure 3B: Bottom Right Panel), where the elution volumes of D5 and D5P (proline mutant) are nearly identical, indicating a similar stokes radius and hence similar diameters. In effect, we were unable to decrease the size of nanodisc any further through selective placement of additional prolines. While we tried several permutations of prolines insertion, we only present the case with maximum number of inserted prolines for the sake of simplicity.
2. Large discs
The other end of the spectrum, with respect to size, has also been worked out through the development of discs with larger diameters. Sligar’s lab had successfully developed large discs by fusing two MSP1 molecules together to obtain discs of 17 nm diameter, which were named MSP2N3 [26]. This is most likely useful for incorporating large macromolecular complexes. A separate use of larger nanodiscs in NMR has been demonstrated by Opella’s group. They developed a 30 nm MACRODISC using multiple copies of an amphipathic 14-residue peptide, which formed a discoidal bilayer particle when mixed with phospholipids at a certain ratio (Figure 3A: Right Panel). The authors found that increasing the discs diameter slows their reorientation rate and generates sufficient magnetic susceptibility anisotropy, thus allowing them to align in an external magnetic field [27]. These discs can be used for residual dipolar coupling measurements [28] by providing partial alignment of macromolecules. A summary of all discs relevant to the above discussion are presented in Figure 1B as a quick comparison of their diameters and suitability to solution NMR applications.
3. Disc thickness
Another key characteristic for consideration is the thickness of the lipid bilayer, which is defined by the length of the aliphatic chain from the participating lipid molecules. It is important to have a bilayer thickness appropriate enough to accommodate the entire hydrophobic length of a membrane protein. Mismatches may occur when the hydrophobic thickness is less or greater than the lipid bilayer. This anomaly may cause curvature and/or disorder of the bilayer in close proximity to the protein’s core surface, leading to distortions in the lipid molecules around the hydrophobic area [29]. In few certain cases, this could be favorable for studying the folding of β-barrel protein [30]. In most other cases, the functional states of channels or receptors may be affected through the distortion of their structure [31].
The appropriate thickness required for the protein of interest would have to be experimentally explored by testing various lipid compositions. Nanodiscs are resilient towards accommodating a wide range of lipids, which could differ in their alkyl chain length, degree of saturation, and polarity of head groups. A complex lipid composition might be essential to mimic natural bilayers and maintain the functional activity of a particular membrane protein [32].
4. Alternative bilayer systems
Besides MSP derived nanodiscs, other existing nanoscale phospholipid bilayer systems can serve as potential alternatives. Here we discuss two such systems: SMALPs (Styrene Maleic Acid Lipid Particles) or Lipodisq ® and Saposin-A lipoprotein discs (Salipro). Of the two, Saposin-A lipoprotein discs might offer a greater potential for NMR applications.
SMALPs or Lipodisq® are discoidal lipid-polymer complexes, where the outer annulus is formed by Styrene-Maleic acid (SMA) copolymer composed of Styrene and Maleic acid in ratios of either 2:1 or 3:1 [33]. These self-assembling water-soluble particles contain about 140 lipids and have a median diameter of ~10 nm for an empty disc (Figure 3C: Bottom Panel). SMA is required in very low concentrations for the formation of lipid particles and is reflected through the extremely low molar ratios of lipid to polymer. SMALPs have been successfully used with bilayer forming lipids like POPC [34] and DMPC [35]. An intriguing feature of SMA copolymer lies in its ability to directly solubilize membrane proteins from its native membrane, forming nanoscale discs with endogenous lipids surrounding the protein. This ability has been exemplified through the incorporation of mitochondrial respiratory Complex IV, which was shown to retain its enzymatic activity [36]. SMA precipitates at pH values lower than its pKa of 6.5. Thus, NMR experiments involving SMALPs need to be executed within a narrow pH range of 6.8–7.5. Additionally, the use of divalent cations should be omitted as they destabilize the SMA copolymer causing them to dissociate from the lipid molecules. The major drawback of using SMALPs in NMR studies resides in its ability to form heterogeneous disc population as can be visualized from the TEM data referenced in [35]. The presence of a relatively lighter copolymer belt, in comparison to protein nanodiscs, might offer some advantages with respect to its relaxation properties. It still remains to be seen whether SMALPs incorporating a deuterated protein can lead to an encouraging NMR spectrum.
Saposin-A lipoprotein discs (Salipro) are nanoscale discs, where two Saposin-A molecules assemble together to produce a disc like system with lipid molecules in its core. The difference between the Saposin-A discs and nanodiscs lies in the arrangement of the outer annulus protein surrounding the lipid molecules. For nanodiscs, the outer protein forms a continuous belt surrounding the lipid molecules while Saposin-A proteins are present in a head to tail arrangement forming a discontinuous envelope around lipids. The structure of Saposin-A bound to Lauryldimethylamine-N-Oxide (LDAO) solved at 1.9 Å shows that it forms a closed structure in its apo form (detergent free) and becomes extended on binding to LDAO molecules [37]. Two such detergent bound Saposin-A proteins are brought together through the core hydrophobic interactions with acyl chains, forming a discontinuous structure with a distorted disc shape (Figure 3C: Top Panel). Saposin-A discs have a diameter of ~3.2 nm with a molecular weight of 43 kDa. In total, there are 40 detergent molecules arranged asymmetrically with 24 in the upper and 16 in the lower leaflet of the bilayer as seen in case of LDAO. This system is also compatible with a wide range of lipid molecules. Recently, Saposin-A discs were utilized for cryo-EM study of large membrane protein complexes like archaeal mechanosensitive channel T2 (32.9 kDa), with four predicted transmembrane helices existing as a putative homo pentamer, and bacterial peptide transporter PepTSo2 (56 kDa), with 14 transmembrane helices existing as a homo tetramer [38].
This paper brings to light certain key features of Saposin-A discs that could be useful for NMR applications. First, Saposin-A system provides a wide range of pH applicability: reactions can be carried out at physiological pH or, as shown in the original paper [37], at acidic pH. Second, it has exceptional temperature stability: Saposin-A discs can withstand freeze-thaw cycles, extending the storage capability of a sample. They are highly thermostable (0–95°C), allowing for NMR experiments to be conducted at higher temperatures as is required for larger systems to improve spectral quality. Third, sample homogeneity: The negatively stained TEM image shows the presence of homogeneous population of Saposin-A disc containing the incorporated protein. However, this system also presents two major concerns: (a) the propensity to allow spontaneous oligomerization of inserted proteins. The cryo-EM paper demonstrates more than two Saposin-A molecules coming together to accommodate a large membrane complex. NMR studies are preferentially carried out on monomeric proteins as oligomeric forms of proteins increase the ambiguity of the results; (b) potential interaction between the incorporated protein and Saposin-A. The presence of low number of lipid molecules might lead to protein-protein interaction between the incorporated protein and Saposin-A. Moreover, there seems to be a gap between the two discontinuous Saposin-A proteins, which may allow for the loss of lipid molecules at higher temperatures, reducing their effective concentration. If these concerns contribute to the final dataset collected from NMR experiments, the resulting structure may not represent its true form in a native environment. Nevertheless, these are conjectures and need to be confirmed (or rejected) through rigorous experimentation. In summary, Saposin-A discs have a significantly smaller size with higher pH variability, thermostability and homogeneous distribution. Taken together, these might offer an advantage for Saposin-A discs over nanodiscs for solution NMR applications. During the preparation of this review, we did not come across any NMR data using Saposin-A discs, but it won’t be surprising to see one in the very near future.
Application of nanodiscs for structural studies by solution NMR
Though presently there are few structure papers, there still are several examples of successful application of nanodiscs with solution NMR. The initial use of nanodiscs were demonstrated for two membrane proteins: (i) CD4 mutant, containing a single transmembrane and cytoplasmic tail, where the aliphatic resonances in 1H-13C HSQC spectra were compared to that in DPC micelles [39]; and (ii) VDAC-1, human anion channel protein, where 1H-15N TROSY HSQC spectra were compared between LDAO isotropic bicelles and MSP1D1 nanodiscs [40]. Additionally, the occurrence of Chemical shift perturbations (CSP) due to NADH binding was demonstrated. A similar study was done later with VDAC-2 [41]. Following this, several other proteins were later investigated in nanodiscs. The Voltage sensing domain (VSD) of KvAP channel, containing four consecutive transmembrane helices, was incorporated into (MSP1D1) nanodiscs with different types of lipid molecules and was found to maintain a proper conformation only in zwitterionic environments [42]. A 30 nm MACRODISC, discussed earlier, was developed as a medium for residual dipolar coupling measurements [27]. Bacteriorhodopsin, a hepta-helical transmembrane protein, was studied comparatively across different mimetics comprising of micelles, amphipols, and (MSP1D1) nanodiscs [43]. 1H-15N TROSY HSQC spectra of the trans-membrane and cytoplasmic domains of Integrin αIIb spectra was obtained in smaller D7 nanodiscs[19]. YgaP, an E. coli integral membrane protein composed of two transmembrane helices and cytoplasmic Rhodanese domain, was studied in mixed micelles, FC12 micelles, and MSP1D1 nanodiscs. This study demonstrated the improvement in quality of 1H-15N TROSY HSQC spectra in the presence of deuterated d54-DMPC lipids in nanodiscs. Interestingly, the spectral quality in nanodiscs was relatively better in comparison to FC12, which reflects the perturbed structural integrity of Rhodanese domain in micelles [44]. Sequential assignments of OmpA, an E. coli beta barrel protein, were achieved in (MSP1D1) nanodiscs albeit with the help of assignments from Fos-10 micelles. Additionally, application of micro-coil NMR was demonstrated to find optimized solution conditions for OmpA in nanodiscs [45]. 1H-15N TROSY HSQC spectra of two beta-barrel outer membrane proteins OprG and OprH from P. aeruginosa were compared between DHPC micelles and different truncation mutants of MSP ΔH4, ΔH5, ΔH4H5 and ΔH4-H6 (developed by Wagner’s group[46]). In agreement with Wagner’s findings, it was found that ΔH5 offered the best spectra [47]. Protein–protein interactions have also been studied through the incorporation of P450-cytb in a 22 amino acid long peptide based nanodisc [46]. Another approach, using HADDOCK simulations [48] with distance restraints derived from paramagnetic relaxation enhancement (PRE), allowed to distinguish the reorientation of the effector binding site on K-RAS4B GTPase anchored to (MSP1D1) nanodiscs that was previously occluded through the anionic membrane [49]. The most rigorous and compelling study came from Wagner’s group, where they solved the structure of a beta barrel protein, OmpX, in nanodiscs using small discs formed by the ΔH5 deletion construct of MSP [25]. A later study performing RDC measurements of OmpX nanodiscs carried out using Pf1 phage alignment medium, has helped better define the orientation of N-H bonds thereby improving the overall structure of OmpX in ΔH5 discs [50].
With the aim to further nanodisc application beyond structure determination using NMR based approaches, we hereby demonstrate how nanodiscs can be used to study the functional activity of a membrane protein. Membrane protein phosphorylation has been previously reported in nanodiscs [51]. In our present study we show, for the first time, the molecular details of phosphorylation through NMR. To demonstrate this, we used the major platelet Integrin αIIbβ3 as an example. Integrins represent a class of human cell surface receptor heterodimers, formed by a combination of alpha and beta subunits. Upon stimulation with the extracellular domain, Integrin undergoes the phosphorylation of the β3 cytoplasmic tail at two tyrosine residues by Src kinase, schematically shown in Figure 4C (Top Panel). Integrin constructs, used in our study, consists of an MBP tag (maltose binding protein) fused to the transmembrane and cytoplasmic domain (TMCD) of either αIIb or β3 [52]. MBP tag enhances the solubility of the TMCD region, which aids in the purification and incorporation of αIIb or β3 into nanodiscs. Once Integrin subunits are incorporated into the disc, the tag is excised with TEV protease which acts on the TEV cut site engineered between MBP and the rest of the protein, providing nanodiscs containing just the TMCD region (Figure 4A). We employed our small D7 MSP construct (roughly similar to Wagner’s ΔH4H5 construct) to form nanodiscs. By themselves, αIIb forms homo-dimers and β3 forms homo-trimers [19, 53]. Thus, at least two or three transmembrane helixes fit comfortably within the internal lipid area of the discs, which have an estimated inner diameter of ~60 Å. The 1H-15N TROSY HSQC spectra of the individual αIIb or β3 incorporated discs were collected on 800 MHz magnet (Agilent, USA) and are shown in Figure 4B. The overall size of the assembly, even within the smallest nanodiscs, is still quite large, resulting in broader peaks corresponding to the transmembrane portion of the protein. Additional local motion of cytoplasmic tails, which protrudes out from the discs, are manifested by sharper peaks. We exploited this advantage to study the downstream effect of Integrin activation through the phosphorylation of Integrin β3 on 600 MHz magnet (Agilent, USA). Since the sample was not deuterated, it becomes difficult to observe peaks from the transmembrane region, making it easier to selectively focus only on the cytoplasmic tail region. We simply mixed the 15N-labeled β3 discs with the kinase domain of Src kinase along with ATP molecules in an NMR tube and collected a 1H-15N TROSY HSQC spectrum. By overlaying the un-phosphorylated and bi-phosphorylated spectra, one can clearly see shifts arising due to the phosphorylation of β3 tails in nanodiscs (Figure 4C: Right Panel). The shifts correspond to the region around the tyrosine residues at the C-terminus of the cytoplasmic tail where phosphorylation occurs as shown from our previous studies using just the short cytoplasmic tail regions [54]. Further assignments will be required to exactly pinpoint the specific residues perturbed in the process. Thus, here we show a simple application, free from the need for deuteration, which can be routinely applied with a medium field NMR magnet to study receptors signaling. It is important to note that our D7 disc is similar in size to Wagner’s ΔH4H5 disc. Since Wagner’s ΔH5 construct, which is of a larger diameter than the ΔH4H5 construct, gave a better NMR spectra for OmpX, further experimentation would be required to ascertain the ability of our D7 discs to study beta barrel proteins, though our larger D5 discs definitely offers an equal advantage as the ΔH5 discs. Considering all the above different examples, we truly believe that nanodiscs are a promising membrane mimetic system that can been utilized successfully for a multitude of NMR applications.
Technical challenges and possible solutions
Despite the benefits of using nanodiscs, there are certain challenges that need to be overcome. We categorize them into: a) preparation of protein incorporated nanodiscs, and b) uncertainties with experimental setups. We have previously discussed potential solution to the former in the section “Preparation of nanodiscs”. We now address some strategies to overcome the latter.
Smaller discs are definitely more advantageous than larger ones. One can easily employ the smallest nanodiscs developed specifically for this purpose either through our (D7) or Wagner’s (ΔH4H5 or ΔH5) constructs. If the plasmid for MSP1D1 is readily available, D7 construct can be easily generated by placing a stop codon at the end of the seventh helix using site directed mutagenesis. The ~7 nm diameter discs reflects sufficient lateral space to incorporate smaller porins, such as 8-stranded OmpX [25], or helical membrane proteins with at least two or three transmembrane helices, as has been demonstrated above through αIIb homo-dimers and β3 homo-trimers (Figure 4B). To make assignments feasible, complete deuteration of the system is required along with the use of deuterated lipids to minimize dipolar-dipolar relaxations. NMR experiments need to be carried out on high field magnets like 800 MHz or above to take full advantage of the TROSY-based experiments for complete backbone assignments [55]. Furthermore, novel pulse sequences specifically designed for Ile/Leu/Val side-chain assignments in perdeuterated samples with selectively protonated methyl groups can be used [23, 56]. These assigned methyls, in addition to the backbone amides utilized for the structural characterization of β-barrel porins [25], are absolutely necessary for the high resolution structure determination of α–helical proteins as the number of long distance restraints obtained exclusively from HN-HN would be insufficient. Interestingly, chemicals shifts of membrane protein acquired in other membrane mimetic systems, such as micelles, bicelles or amphipols, often overlap (or are similar) with corresponding chemical shifts in nanodiscs, especially for the residues located within the hydrophobic core, and could potentially be utilized to aid the assignment process [25, 57].
Nanodiscs are especially advantageous in their ability to achieve high concentrations in the high μM to low mM ranges. A sample with higher concentration drastically increases the signal to noise ratio and provides better spectrum quality. Nevertheless, proper caution should be taken to maintain the homogeneity of these concentrated samples. We have shown that smaller constructs of MSP form larger discs [19]. This suggests that the purity of your MSP preparation is very crucial. Impure preparations with degraded MSP might lead to a heterogeneous population consisting of a mixture of small and large discs. This will effectively reduce the concentration of small discs, compromising sensitivity. An easy way to check this is through negatively stained TEM (Transmission Electron Microscopy) images, which clearly display sample heterogeneity (Inset in Figure 4A). Moreover, if your sample contains a beta barrel porin, you can check the number of pores present in the population of your discs which will manifest itself as black dots within your disc’s image. As an example, demonstrating the visualization of pores in nanodiscs, we have previously carried out image analysis of a trimeric beta barrel protein TprC (C-Terminal domain) in large MSP1E3D1 discs. The image averaged result shows the presence of three pores in the backdrop of a nanodisc [58].
Large macromolecular systems, including nanodiscs, are notorious for peak broadenings in most of the triple-resonance NMR experiments, resulting in a low signal–to-noise ratio. Even though nanodiscs with incorporated membrane proteins can be easily concentrated, the problem of sensitivity still remains. This can be partially alleviated by data acquisition at higher temperatures with longer time durations. The downside of higher temperatures lies in the detrimental effect it has on protein stability. Another approach to increase the sensitivity (and resolution if necessary) without significantly raising acquisition times is through the utilization of non-uniform sampling (NUS) [59, 60]. NUS has recently emerged as a powerful techniques to speed up the acquisition of multidimensional NMR experiments. It is based on the rationale where only a subset of the linearly sampled data in the Nyquist grid is measured. For this reason, NUS requires processing algorithms different from the normal Fourier transform. A number of sophisticated methods have been developed for reconstructing sparsely sampled NMR data with minimal artifacts. NMRPipe (http://www.nmrscience.com/nmrpipe.html), a UNIX-based collection of programs and scripts developed by Frank Delaglio [61], provides tools for reconstruction of NUS data, including Iterative Shrinkage Thresholding (IST) decomposition and options for Maximum Entropy Reconstruction (MEM). The NMRPipe compatible scripts for istHMS, developed by Wagner’s group, could be found at their website: http://gwagner.med.harvard.edu/intranet/istHMS/index.html. The IST software itself may be requested from the same group. MEM processing script generator, developed by Jeff Hoch and coworkers as a part of RNMRTK (http://rnmrtk.uchc.edu/rnmrtk/RNMRTK.html) can be found at website: http://sbtools.uchc.edu/nmr/nmr_toolkit/.
Conclusion
In this review, we discuss the advantages and challenges associated with nanodiscs for studying membrane proteins. We provide an extensive view on the preparation and application of nanodiscs. This could serve as a general guide that should aid any future user in successfully carrying out nanodisc based experiments; either through the selection of specific discs or through the strategies offered for the incorporation of typical membrane proteins. The feasibility of NMR based experiments have been clearly emphasized with the need for deuteration to achieve desirable results for assignments or structure solution. We also demonstrate a simple application that can be used for studying protein signaling events. Finally, we offer other promising alternatives that may provide better viability with NMR applications and be suitable for any tailor made requirements needed for specific membrane proteins. Nanodisc based systems clearly have the potential for both structural and functional investigations and is a system that is constantly progressing in terms of utilization and development. With the current pace of developments, we are hopeful that nanodiscs will become the membrane mimetic system of choice for investigating membrane proteins.
Acknowledgments
We would like to acknowledge and thank Drs. Nathan Alder and Jun Qin for providing us with the MSP1D1 and MBP-Integrin constructs, Dr. Vitaliy Gorbatyuk for discussion and help with NMR experiments, Dr. Xiaochen Lin for help with Src phosphorylation experiments, and NMRbox, the National Center for Biomolecular NMR Data Processing and Analysis, which is supported by NIH grant P41GM111135 (NIGMS). We would like to finally acknowledge partial support for this work which came from the NIH grant HL098777.
Abbreviations
- NMR
nuclear magnetic resonance
- TROSY
transverse relaxation optimized spectroscopy
- RDC
residual dipolar coupling
- PRE
paramagnetic relaxation enhancement
- EM
electron microscopy
- HSQC
heteronuclear single quantum coherence spectroscopy
- TMCD
transmembrane and cytoplasmic domains
- SMA
Styrene Maleic Acid
References
- 1.Krogh A, et al. Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. Journal of molecular biology. 2001;305(3):567–80. doi: 10.1006/jmbi.2000.4315. [DOI] [PubMed] [Google Scholar]
- 2.Drews J. Drug discovery: a historical perspective. Science. 2000;287(5460):1960–4. doi: 10.1126/science.287.5460.1960. [DOI] [PubMed] [Google Scholar]
- 3.Myers JK, Beihoffer LA, Sanders CR. Phenotology of disease-linked proteins. Hum Mutat. 2005;25(1):90–7. doi: 10.1002/humu.20118. [DOI] [PubMed] [Google Scholar]
- 4.Stenson PD, et al. The Human Gene Mutation Database: building a comprehensive mutation repository for clinical and molecular genetics, diagnostic testing and personalized genomic medicine. Human genetics. 2014;133(1):1–9. doi: 10.1007/s00439-013-1358-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang Z, et al. Membrane protein stability can be compromised by detergent interactions with the extramembranous soluble domains. Protein science: a publication of the Protein Society. 2014;23(6):769–89. doi: 10.1002/pro.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanders CR, Oxenoid K. Customizing model membranes and samples for NMR spectroscopic studies of complex membrane proteins. Biochimica et biophysica acta. 2000;1508(1–2):129–45. doi: 10.1016/s0005-2736(00)00308-4. [DOI] [PubMed] [Google Scholar]
- 7.Liang B, Tamm LK. NMR as a tool to investigate the structure, dynamics and function of membrane proteins. Nature structural & molecular biology. 2016;23(6):468–74. doi: 10.1038/nsmb.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denisov IG, Sligar SG. Nanodiscs for structural and functional studies of membrane proteins. Nature structural & molecular biology. 2016;23(6):481–6. doi: 10.1038/nsmb.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viegas A, Viennet T, Etzkorn M. The power, pitfalls and potential of the nanodisc system for NMR-based studies. Biological chemistry. 2016 doi: 10.1515/hsz-2016-0224. [DOI] [PubMed] [Google Scholar]
- 10.Carlson JW, Jonas A, Sligar SG. Imaging and manipulation of high-density lipoproteins. Biophysical journal. 1997;73(3):1184–9. doi: 10.1016/S0006-3495(97)78150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayburt TH, Grinkova YV, Sligar SG. Self-Assembly of Discoidal Phospholipid Bilayer Nanoparticles with MembraneScaffold Proteins. NANO Letters. 2002;2(8):853–856. [Google Scholar]
- 12.Sligar SG. Finding a single-molecule solution for membrane proteins. Biochemical and biophysical research communications. 2003;312(1):115–9. doi: 10.1016/j.bbrc.2003.09.188. [DOI] [PubMed] [Google Scholar]
- 13.Bayburt TH, Sligar SG. Self-assembly of single integral membrane proteins into soluble nanoscale phospholipid bilayers. Protein science: a publication of the Protein Society. 2003;12(11):2476–81. doi: 10.1110/ps.03267503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohashi R, et al. Reverse cholesterol transport and cholesterol efflux in atherosclerosis. QJM. 2005;98(12):845–56. doi: 10.1093/qjmed/hci136. [DOI] [PubMed] [Google Scholar]
- 15.Nath A, Atkins WM, Sligar SG. Applications of phospholipid bilayer nanodiscs in the study of membranes and membrane proteins. Biochemistry. 2007;46(8):2059–69. doi: 10.1021/bi602371n. [DOI] [PubMed] [Google Scholar]
- 16.Bayburt TH, Sligar SG. Membrane protein assembly into Nanodiscs. FEBS Lett. 2010;584(9):1721–7. doi: 10.1016/j.febslet.2009.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ritchie TK, et al. Chapter 11 - Reconstitution of membrane proteins in phospholipid bilayer nanodiscs. Methods Enzymol. 2009;464:211–31. doi: 10.1016/S0076-6879(09)64011-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw AW, McLean MA, Sligar SG. Phospholipid phase transitions in homogeneous nanometer scale bilayer discs. FEBS Lett. 2004;556(1–3):260–4. doi: 10.1016/s0014-5793(03)01400-5. [DOI] [PubMed] [Google Scholar]
- 19.Puthenveetil R, Vinogradova O. Optimization of the design and preparation of nanoscale phospholipid bilayers for its application to solution NMR. Proteins. 2013;81(7):1222–31. doi: 10.1002/prot.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clore GM, Gronenborn AM. Determining the structures of large proteins and protein complexes by NMR. Trends Biotechnol. 1998;16(1):22–34. doi: 10.1016/S0167-7799(97)01135-9. [DOI] [PubMed] [Google Scholar]
- 21.Pervushin K, et al. Attenuated T2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(23):12366–71. doi: 10.1073/pnas.94.23.12366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kay LE. NMR studies of protein structure and dynamics - a look backwards and forwards. Journal of magnetic resonance. 2011;213(2):492–4. doi: 10.1016/j.jmr.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Tugarinov V, Kay LE. Ile, Leu, and Val methyl assignments of the 723-residue malate synthase G using a new labeling strategy and novel NMR methods. Journal of the American Chemical Society. 2003;125(45):13868–78. doi: 10.1021/ja030345s. [DOI] [PubMed] [Google Scholar]
- 24.Denisov IG, et al. Directed self-assembly of monodisperse phospholipid bilayer Nanodiscs with controlled size. Journal of the American Chemical Society. 2004;126(11):3477–87. doi: 10.1021/ja0393574. [DOI] [PubMed] [Google Scholar]
- 25.Hagn F, et al. Optimized Phospholipid Bilayer Nanodiscs Facilitate High-Resolution Structure Determination of Membrane Proteins. J Am Chem Soc. 2013 doi: 10.1021/ja310901f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grinkova YV, I, Denisov G, Sligar SG. Engineering extended membrane scaffold proteins for self-assembly of soluble nanoscale lipid bilayers. Protein Eng Des Sel. 2010;23(11):843–8. doi: 10.1093/protein/gzq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park SH, et al. Nanodiscs versus macrodiscs for NMR of membrane proteins. Biochemistry. 2011;50(42):8983–5. doi: 10.1021/bi201289c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bax A, Kontaxis G, Tjandra N. Dipolar couplings in macromolecular structure determination. Methods Enzymol. 2001;339:127–74. doi: 10.1016/s0076-6879(01)39313-8. [DOI] [PubMed] [Google Scholar]
- 29.Fernandez C, et al. Lipid-protein interactions in DHPC micelles containing the integral membrane protein OmpX investigated by NMR spectroscopy. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(21):13533–7. doi: 10.1073/pnas.212515099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamm LK, Hong H, Liang B. Folding and assembly of beta-barrel membrane proteins. Biochimica et biophysica acta. 2004;1666(1–2):250–63. doi: 10.1016/j.bbamem.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Lee AG. How lipids affect the activities of integral membrane proteins. Biochimica et biophysica acta. 2004;1666(1–2):62–87. doi: 10.1016/j.bbamem.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 32.Roos C, et al. Characterization of co-translationally formed nanodisc complexes with small multidrug transporters, proteorhodopsin and with the E. coli MraY translocase. Biochimica et biophysica acta. 2012;1818(12):3098–106. doi: 10.1016/j.bbamem.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Knowles TJ, et al. Membrane proteins solubilized intact in lipid containing nanoparticles bounded by styrene maleic acid copolymer. Journal of the American Chemical Society. 2009;131(22):7484–5. doi: 10.1021/ja810046q. [DOI] [PubMed] [Google Scholar]
- 34.Vargas C, et al. Nanoparticle self-assembly in mixtures of phospholipids with styrene/maleic acid copolymers or fluorinated surfactants. Nanoscale. 2015;7(48):20685–96. doi: 10.1039/c5nr06353a. [DOI] [PubMed] [Google Scholar]
- 35.Orwick MC, et al. Detergent-free formation and physicochemical characterization of nanosized lipid-polymer complexes: Lipodisq. Angewandte Chemie. 2012;51(19):4653–7. doi: 10.1002/anie.201201355. [DOI] [PubMed] [Google Scholar]
- 36.Long AR, et al. A detergent-free strategy for the reconstitution of active enzyme complexes from native biological membranes into nanoscale discs. BMC Biotechnol. 2013;13:41. doi: 10.1186/1472-6750-13-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Popovic K, et al. Structure of saposin A lipoprotein discs. Proc Natl Acad Sci U S A. 2012;109(8):2908–12. doi: 10.1073/pnas.1115743109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frauenfeld J, et al. A saposin-lipoprotein nanoparticle system for membrane proteins. Nat Methods. 2016;13(4):345–51. doi: 10.1038/nmeth.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gluck JM, et al. Integral membrane proteins in nanodiscs can be studied by solution NMR spectroscopy. Journal of the American Chemical Society. 2009;131(34):12060–1. doi: 10.1021/ja904897p. [DOI] [PubMed] [Google Scholar]
- 40.Raschle T, et al. Structural and functional characterization of the integral membrane protein VDAC-1 in lipid bilayer nanodiscs. Journal of the American Chemical Society. 2009;131(49):17777–9. doi: 10.1021/ja907918r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu TY, et al. Solution NMR spectroscopic characterization of human VDAC-2 in detergent micelles and lipid bilayer nanodiscs. Biochimica et biophysica acta. 2012;1818(6):1562–9. doi: 10.1016/j.bbamem.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shenkarev ZO, et al. NMR structural and dynamical investigation of the isolated voltage-sensing domain of the potassium channel KvAP: implications for voltage gating. Journal of the American Chemical Society. 2010;132(16):5630–7. doi: 10.1021/ja909752r. [DOI] [PubMed] [Google Scholar]
- 43.Etzkorn M, et al. Cell-free expressed bacteriorhodopsin in different soluble membrane mimetics: biophysical properties and NMR accessibility. Structure. 2013;21(3):394–401. doi: 10.1016/j.str.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tzitzilonis C, et al. Detergent/nanodisc screening for high-resolution NMR studies of an integral membrane protein containing a cytoplasmic domain. PloS one. 2013;8(1):e54378. doi: 10.1371/journal.pone.0054378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Susac L, Horst R, Wuthrich K. Solution-NMR characterization of outer-membrane protein A from E. coli in lipid bilayer nanodiscs and detergent micelles. Chembiochem: a European journal of chemical biology. 2014;15(7):995–1000. doi: 10.1002/cbic.201300729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang M, et al. Reconstitution of the Cytb5-CytP450 Complex in Nanodiscs for Structural Studies using NMR Spectroscopy. Angewandte Chemie. 2016;55(14):4497–9. doi: 10.1002/anie.201600073. [DOI] [PubMed] [Google Scholar]
- 47.Kucharska I, et al. Optimizing nanodiscs and bicelles for solution NMR studies of two beta-barrel membrane proteins. Journal of biomolecular NMR. 2015;61(3–4):261–74. doi: 10.1007/s10858-015-9905-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Vries SJ, van Dijk M, Bonvin AM. The HADDOCK web server for data-driven biomolecular docking. Nat Protoc. 2010;5(5):883–97. doi: 10.1038/nprot.2010.32. [DOI] [PubMed] [Google Scholar]
- 49.Mazhab-Jafari MT, et al. Oncogenic and RASopathy-associated K-RAS mutations relieve membrane-dependent occlusion of the effector-binding site. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(21):6625–30. doi: 10.1073/pnas.1419895112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bibow S, et al. Measuring membrane protein bond orientations in nanodiscs via residual dipolar couplings. Protein science: a publication of the Protein Society. 2014;23(7):851–6. doi: 10.1002/pro.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Inagaki S, et al. G Protein-Coupled Receptor Kinase 2 (GRK2) and 5 (GRK5) Exhibit Selective Phosphorylation of the Neurotensin Receptor in Vitro. Biochemistry. 2015;54(28):4320–9. doi: 10.1021/acs.biochem.5b00285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang J, et al. Structure of an integrin {alpha}IIb{beta}3 transmembrane-cytoplasmic heterocomplex provides insight into integrin activation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(42):17729–34. doi: 10.1073/pnas.0909589106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li R, et al. Oligomerization of the integrin alphaIIbbeta3: roles of the transmembrane and cytoplasmic domains. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(22):12462–7. doi: 10.1073/pnas.221463098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deshmukh L, et al. Tyrosine phosphorylation as a conformational switch: a case study of integrin beta3 cytoplasmic tail. The Journal of biological chemistry. 2011;286(47):40943–53. doi: 10.1074/jbc.M111.231951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang D, Kay L. Improved HN-detected triple resonance TROSY-based experiments. Journal of biomolecular NMR. 1999;13:3–10. doi: 10.1023/A:1008329230975. [DOI] [PubMed] [Google Scholar]
- 56.Yang D, et al. Sequence-specific assignments of methyl groups in high-molecular weight proteins. Journal of the American Chemical Society. 2004;126(12):3710–1. doi: 10.1021/ja039102q. [DOI] [PubMed] [Google Scholar]
- 57.Yu TY, et al. Solution NMR spectroscopic characterization of human VDAC-2 in detergent micelles and lipid bilayer nanodiscs. Biochim Biophys Acta. 2012;1818(6):1562–9. doi: 10.1016/j.bbamem.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anand A, et al. Bipartite Topology of Treponema pallidum Repeat Proteins C/D and I: OUTER MEMBRANE INSERTION, TRIMERIZATION, AND PORIN FUNCTION REQUIRE A C-TERMINAL beta-BARREL DOMAIN. The Journal of biological chemistry. 2015;290(19):12313–31. doi: 10.1074/jbc.M114.629188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hyberts SG, Arthanari H, Wagner G. Applications of non-uniform sampling and processing. Topics in current chemistry. 2012;316:125–48. doi: 10.1007/128_2011_187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoch JC, Maciejewski MW, Filipovic B. Randomization improves sparse sampling in multidimensional NMR. Journal of magnetic resonance. 2008;193(2):317–20. doi: 10.1016/j.jmr.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Delaglio F, et al. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. Journal of biomolecular NMR. 1995;6(3):277–93. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 62.Jo S, et al. CHARMM-GUI: a web-based graphical user interface for CHARMM. Journal of computational chemistry. 2008;29(11):1859–65. doi: 10.1002/jcc.20945. [DOI] [PubMed] [Google Scholar]
- 63.Kucerka N, Nieh MP, Katsaras J. Fluid phase lipid areas and bilayer thicknesses of commonly used phosphatidylcholines as a function of temperature. Biochimica et biophysica acta. 2011;1808(11):2761–71. doi: 10.1016/j.bbamem.2011.07.022. [DOI] [PubMed] [Google Scholar]