Abstract
It is widely appreciated that neuronal activity contributes to the development of brain representations of the external world. In the visual system, in particular, it is well known that activity cooperates with molecular cues to establish the topographic organization of visual maps on a macroscopic scale1,2, mapping axons in a retinotopic and eye-specific manner. In recent years, significant progress has been made in elucidating the role of activity in driving the finer-scale circuit refinement that shapes the receptive fields of individual cells. In this review, we focus on these recent breakthroughs – primarily in mice, but also in other mammals where noted.
Introduction
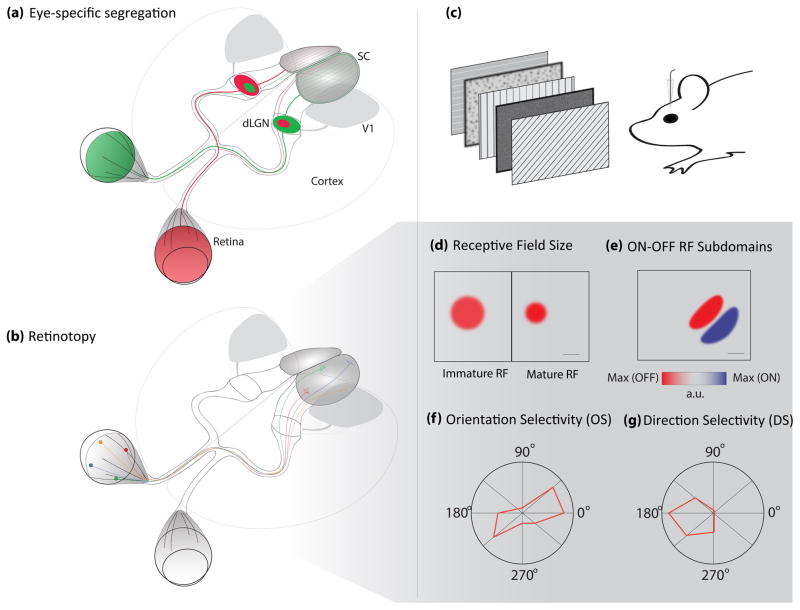
Classic experiments demonstrate that activity in the developing visual system can drive circuit refinement. In this review, we discuss the contribution of both pre-vision, spontaneous activity as well as early experience-driven activity to the refinement of receptive fields (RFs). The receptive field (RF) of a neuron refers to the attributes of a visual stimulus that generates a response in that cell, and typically includes a description of visual field location and preference for other specific features, such as preferred orientation or direction of visual stimuli. The RF of a neuron is determined by the connectivity of underlying neural circuits, starting in the retina, which can then be further modified or elaborated at additional stages of the visual system. A RF’s location in space is tied to the topographic organization of projections, which relay information from photoreceptors that tile the retina to sample the visual scene. A preference for increments or decrements of light (ON- or OFF-responsiveness) results from the organization of pathways carrying information from ON- or OFF-bipolar cells in the retina. Further downstream, RFs can be defined by an ocular dominance preference, resulting from the segregation or combination of inputs derived from each eye. Additionally, cells may prefer stimuli of a specific orientation or moving in a specific direction (orientation or direction selectivity, Figures 2f and 2g), either inheriting this property from presynaptic partners, or generating it de novo through the combination of untuned inputs. Here, we discuss recent reports exploring the contributions of activity-dependent interactions that regulate the development of visual receptive fields.
Figure 2.
Examples of the major receptive field properties referred to in this review. (a–b) Large scale circuit refinement properties. (a) Eye-specific lamination is here depicted as the projections from each retina in a different color to their targets in the LGN and SC. In higher mammals, thalamocortical projections also tessellate V1 with ocular dominance columns. (b) Projections from four retinal positions are shown with their corresponding targets in the SC where their retinotopic positions are preserved. Retinotopy is also present in dLGN, V1 and extrastriatal visual areas, but is not shown for clarity. (c) Schematic depicting common methodology for recording receptive fields in mice while presenting various visual stimuli. (d) Example of an RF measured from a single ON responsive cell before activity-dependent refinement (left) and that same cell after refinement (right). Scale bar indicates 2 visual degrees (see reference 34). (e) Schematic of the ON and OFF responses of a single neuron and their corresponding RF positions. In this example the neuron responds to an elongated ON (red) region in space (where an increment in light best produces a response), that is close but not overlapping with an OFF (blue) field (where a decrement in light best produces a response) Scale bar indicates 20 visual degrees (see reference 81). (f) Example of an orientation selective (OS) neuron that responds preferentially to gratings in two opposite directions, thus non-selective for direction but rather for the orientation of the moving bars. Each spoke on the rose plot represents a direction of motion of drifting gratings presented to the mouse (see 2c). The amplitude along each direction represents the relative strength of firing for a neuron to a given direction. (g) Lastly, a direction-selective (DS) response example where this neuron only responds to leftward movement. Both OS and DS are most highly tuned in V1 in all species studied, but also occur in subcortical regions, at a seemingly higher frequency in rodents and rabbits than other mammals with higher visual acuity. Likewise, OS in subcortical regions of mice is less sharp than OS in the cortex.
Retina
Circuit development throughout the visual system is in part regulated by neuronal activity that originates in the retina before visual experience, where retinal ganglion cells (RGCs) periodically discharge correlated activity that propagates across the retina, commonly referred to as retinal waves3–5. Three separate developmental wave epochs have been described in the mammalian visual system, the first of which occurs before birth and is gap-junction dependent (stage I). Stage II waves, which are dependent on cholinergic receptors, propagate over large areas with low RGC recruitment, becoming smaller and denser as GABAA signaling matures around postnatal day (P)76,7. Glutamatergic influences dominate after P10 (stage III), causing profound changes in activity dynamics, with faster, smaller and more repetitive wave trajectories6. During glutamatergic waves, neighboring RGCs with opposite light responses (ON- vs. OFF-responsive) are recruited sequentially8, with AII amacrine cells coordinating a crossover circuit that allows ON CBCs to control glutamate release from OFF CBCs9,10. This suggests a role for stage III waves in building ON- and OFF-receptive subfields (Figure 2e), but it is still unclear how separate recruitment of ON and OFF RGCs in the retina affects RF properties in downstream circuits, or when ON and OFF subfields begin to form in primary visual cortex (V1)11. However, recent studies have shown that orientation selectivity in V1, which depends on separate ON and OFF subfields, matures rapidly around the time of eye opening but independent of vision12–15, suggesting a possible relationship with stage III waves. After eye opening, dark rearing suppresses the developmental decrease of ON-OFF-responsive RGCs and bistratified RGCs16, however, ON BC-specific silencing affects synapse number, but not stratification17. These observations may highlight a difference in the influence of spontaneously driven versus early visually evoked activity on circuit development.
Although the temporal properties of retinal wave bursts are important for activity-dependent refinement of retinal projections to central targets, they are not necessary for establishing direction selectivity observed in the retina, where a subpopulation of RGCs respond to movement in a preferred direction but not to stationary increments or decrements of light. CaV3.2 knockout mice exhibit disrupted waves during the period that direction selective circuits are established, from P11–P14, however, after eye-opening their direction selective ganglion cells (DSGCs) are indistinguishable from wild type mice18. Development of the retina’s direction selective circuits depends on an asymmetry in synapse number between inhibitory starburst amacrine cells (SACs) and DSGCs19, a circuit that emerges in an activity-independent manner20. However, a recent report found that the clustering of DSGCs preferred directions into cardinal axes does require vision during early development21. While studies have described orientation selectivity in rabbit and mouse RGCs22–26, the developmental mechanisms that give rise to this RF feature are not known. Thus, while spontaneous activity cannot account for all receptive field properties, activity-dependent processes play an important role in shaping some circuits in the retina.
After eye opening, recent studies have shown that early sensory stimulation regulates local connectivity as early as the first few synapses in the retina. Specifically, dark rearing mice reduces synaptic strength between cones and certain cone bipolar cell (CBC) types by means of light-dependent localization of metabotropic glutamate receptors27*. Interestingly, rod bipolar cells and type 6 ON- CBCs remain unaffected despite using the same glutamate receptor, underscoring the specificity of activity-dependent developmental mechanisms27,28. However, dendrites of type 6 CBCs recruit fewer inputs when their transmitter release is silenced throughout development, and more inputs when their neighbors are silenced29, suggesting an activity-dependent, population-based retrograde signal from CBC outputs can modify afferent inputs. In mice with selective silencing of CBCs, RFs of ON retinal ganglion cells (RGCs) are smaller and spatially less homogeneous compared to wildtype retinas but show similar kinetics, suggesting that the remaining ON BCs are capable of relaying normal photoreceptor signals. At the axonal output of BCs, cells with silenced transmitter release form fewer synapses onto RGC dendrites, but active BCs that target the same RGC dendrites do not compensate for this loss, while genetic ablation of some BC neighbors increases synaptogenesis of the remaining axons in an activity-independent manner30. These findings reveal that BC dendrites (but not axons) engage in activity-dependent competition, which ultimately can affect RF structure at even the first synapses in the retina.
Superior Colliculus
Further downstream in the visual pathway, recent work has more clearly defined the roles of molecular cues and spontaneous activity in circuit refinement. The expression of the axon guidance cue Ephrin-A controls RF size, whereas retinal waves guide the overlap of ON and OFF-responsive RF subfields31. Recent studies also demonstrate a direct, causal link between early synchronous retinal activity and the refinement of RGC axon projections32**. In the mouse, disrupting retinal waves causes a decoupling of activity in retinorecipient regions from their presynaptic RGC partners32**. On the other hand, stage III retinal waves are not necessary for normal eye-specific segregation (Figure 2a), as persistent stage II waves can compensate for the absence of stage III retinal waves in this process33. Eye-specific segregation, however, is disrupted in a retinal knockout of the β2-containing nicotinic acetylcholine receptor (which exhibits disrupted retinal waves), whereas retinotopy (Figure 2b) is surprisingly spared in a competition-dependent manner34. The relationship between activity and axon guidance cues can be quite complex, as another study showed that altering the relative levels of the Ephrin receptor, EphA3, and activity patterns can influence the variability in map formation35**. Taken together, these studies indicate that molecular guidance cues and spontaneous activity can interact, but appear to serve largely distinct roles in early retinotopic map formation in the SC.
After the onset of vision, long-term visual deprivation during development alters response polarity and spatial frequency preference of RF properties in mouse SC, but does not alter orientation, direction selectivity, or subfield size36. Sensory experience is, however, necessary for the maintenance of RF size in the SC of hamsters37, and short-term plasticity of inhibitory circuits in hamster SC is also altered by visual deprivation38, suggesting that inhibition contributes to the maintenance of refined RFs. Furthermore, in dark-reared mice, spontaneous or SC-evoked saccadic eye movements are larger than in controls, indicating that vision is required to fine-tune the gain of saccades and to establish normal eye movement maps in the SC39. The effects of dark rearing on SC neurons could result from changes in cortical inputs or intracollicular connections, but further experiments are necessary to isolate the role of cortical feedback on collicular RF development.
Lateral Geniculate
In the dorsal lateral geniculate nucleus (dLGN), postnatal development encompasses a period of prolonged refinement of RGC inputs onto thalamic relay neurons, a process that can be divided into 3 phases40. The first two phases are driven by molecular cues and spontaneous activity and function to segregate retinal projections from each eye into separate domains (by around P10 in mice), and then prune excess inputs while strengthening those that remain (from P10 to P20). Retinal waves have long been thought to play a role in driving eye-specific segregation1, and experiments with the aforementioned retinal β2-nicotinic acetylcholine receptor knockout mice show that selectively disrupting retinal waves impairs eye-specific segregation. Pharmacological rescue of wave frequency improved this phenotype, but without the proper spatiotemporal character of the waves, retinotopic refinement remained abnormal, indicating the importance of different features of spontaneous activity on circuit development32**. Furthermore, in ferrets, increasing the frequency of waves accelerates the development of relay neuron receptive fields41. This effect is driven by a sharpening of the RF center (Figure 2d) rather than changes in the inhibitory surround, but recent work in mice also demonstrated a role for retinal activity in the initial recruitment of inhibitory interneurons into visual thalamic circuits42.
Much progress has also been made in elucidating the postsynaptic mechanisms underlying synapse refinement in dLGN relay neurons during these first two phases. The long-lasting plateau potentials mediated by L-type Ca2+ channels early in development43 appear to be necessary for proper retinogeniculate refinement, possibly due to CREB signaling44. Additionally, appreciation for the role of the immune system in synaptic refinement in the dLGN45,46 continues to grow, with the major histocompatibility complex I genes H2-Db and H2-Kb now implicated through their regulation of Hebbian plasticity mechanisms at the retinogeniculate synapse47.
In the third phase of refinement in the dLGN, retinal projections undergo experience-dependent rewiring. Visual deprivation in this phase, but not earlier in development, disrupts refinement48,49. This vision-dependent stage of remodeling is distinguished from earlier stages by distinct cellular mechanisms. Mice deficient for the transcriptional regulator MeCP250 or the AMPA receptor auxiliary subunit stargazin51 exhibit normal development up until P20, but demonstrate a common defect in experience-dependent refinement, such that between P20 and P30 additional RGC inputs are recruited and connectivity reverts to a high convergence state. In addition, feedback from primary visual cortex influences retinogeniculate refinement during this phase, as manipulations of activity in the corticothalamic pathway from layer 6 of V1 to the dLGN can also induce the recruitment of additional RGC inputs by relay neurons52**.
The purpose of this vision- and feedback-dependent remodeling of RGC to relay neuron connectivity is not entirely clear. Developmental pruning and strengthening at this synapse is typically thought to drive the sharpening of relay neuron RFs53. However, by P20 the number of functionally relevant RGC inputs to a relay neuron is substantially fewer than the ~30 RGCs with overlapping RFs24,54, making it unlikely this final stage of refinement functions merely to prune RGCs with non-aligned RFs. Recent changes in our understanding of the visual processing performed in thalamus raise an alternative possibility. Neurons that prefer stimuli with a specific orientation or direction of motion (Figure 2f and 2g), while sparse in the dLGN of cat and monkey55–57, are common in the visual thalamus of mice26,58–60 and persist even without cortical feedback26,59. As such, the feature selectivity of mature relay neurons may require precise combinations of specific subtypes of RGCs24,54,58,60, the relative weights of which could be optimized through vision-dependent refinement (and with feedback from developing cortical circuits). This hypothesis is particularly attractive given recent findings that RGC axon arbors remain broad throughout development, and functional pruning seems to occur via the rearrangement of presynaptic boutons within a large and relatively stable axon arbor61**. Furthermore, serial EM reconstruction of a mouse dLGN at postnatal day 32 revealed an unexpectedly high structural convergence of RGCs onto relay neurons62. Many of these axon arbors contact a given relay neuron with just a few boutons that form single release sites, which are likely vestiges of the vision-dependent fine-tuning of synaptic connections from an initially large pool of possible presynaptic partners. The role of activity in the development of relay neuron feature selectivity and structural connectivity will be important questions to address in the future.
Primary Visual Cortex
In primary visual cortex (V1), receptive fields depend on combinations of RFs inherited from the retina by way of the dLGN. In other words, activity anywhere in this pathway during development could affect cortical RFs. As such, it is remarkable that the orientation maps found in higher mammals, consisting of columns of cells tuned to stimuli of similar orientations, can develop without vision63–65 (though to varying degrees of maturity and reliability64–68). Similarly, orientation selectivity (OS) in rodent V1, which occurs without a clear columnar organization, matures substantially in the weeks after eye-opening12*,69, but does not initially require vision13–15,69–72. However, in all species studied, OS is plastic to changes induced by artificial visual stimulation73–75, and proper maintenance of OS requires visual experience63,76,77, perhaps implying a role for vision in finer-scale refinement of the OS initially constructed via experience-independent mechanisms. Two lines of evidence support this idea: vision is required in mice during the critical period for ocular dominance plasticity to maintain and enhance the matching of orientation preference from each eye that is otherwise present at eye opening15,72,78, and the development of visual acuity in binocular V1 is slowed by dark-rearing76. Direction selectivity (DS), on other hand, develops after OS and requires vision in higher mammals67,79–81, but appears mature at eye-opening in mice12,69. This may indicate a different strategy for constructing DS in the visual cortex of mice due to the presence of DSGCs and/or more highly tuned thalamic RFs.
Both of these features of cortical RFs (OS and DS) are directly related to the arrangement of a cell’s ON- and OFF- RF subregions (Figure 2e). Interestingly, two recent papers found that in cat and tree shrew, orientation hypercolumns are constructed in an “OFF-centric” fashion, with RFs in a given hypercolumn having clustered OFF subfields surrounded by ON subfields for a given localized region of visual space82*,83*. This suggests that clustering of OFF-driven thalamocortical afferents in a retinotopic manner seeds initial OS maps in V1 of higher mammals. Indeed, OFF-driven responses predominate in early postnatal V1 in cat84, lending further support to this model. It’s not yet clear whether an analogous process occurs in mice, where the organization of RFs into orientation columns is absent.
Toward a complete understanding of the development of V1 RFs, exciting recent work has progressed beyond the traditional characterization of single-cell responses to a standard battery of stimuli. Combined in vivo imaging and in vitro slice recordings from the same neurons in mouse V1 demonstrated that cells with similar visual responses preferentially form recurrent connections after eye opening, concomitant with a decrease in the variability of responses to drifting gratings13,85. Notably, this process still occurs in dark-reared animals, implying spontaneous activity may contribute, but the full extent of circuit reorganization requires visual experience14*. These findings are reminiscent of those in cat and ferret where horizontal axons linking matching orientation columns initially cluster with spontaneous activity, but further refine with visual experience65,86,87. Potentially related work in ferret reveals that population coding of visual responses matures rapidly after eye-opening, with variability and noise correlations decreasing in an experience-dependent manner as the population response becomes increasingly sparse67**. Finally, offering insight into how such distributed yet fine-scale circuit refinement may relate to RF changes at the single-cell level, it was shown in mice that visual experience drives the maturation of precise surround suppression onto V1 neurons, imparting a sensitivity specifically for the higher-order structure of natural stimuli88**. Taken together, these results indicate that understanding the maturation of visual function and its dependence on activity will require carefully characterizing responses of neural ensembles to naturalistic stimuli, rather than averaging responses from single units to repeated presentations of drifting gratings.
Collectively these findings suggest a general model for the development of visual RFs, wherein nuanced interactions between molecular cues and spontaneous activity guide the establishment of initial RFs, while vision subsequently refines these immature circuits to improve the selectivity and reliability of both single cell and population responses. This model appears to hold true across species, despite the fact that the region of the visual system where certain RF properties first emerge differs. Future studies will be required to further test this model, to characterize the emergence and activity-dependence of finer scale RF properties, the effects of retinal RF refinement on downstream visual areas, and to clarify the similarities and differences in these processes across species.
Figure 1.
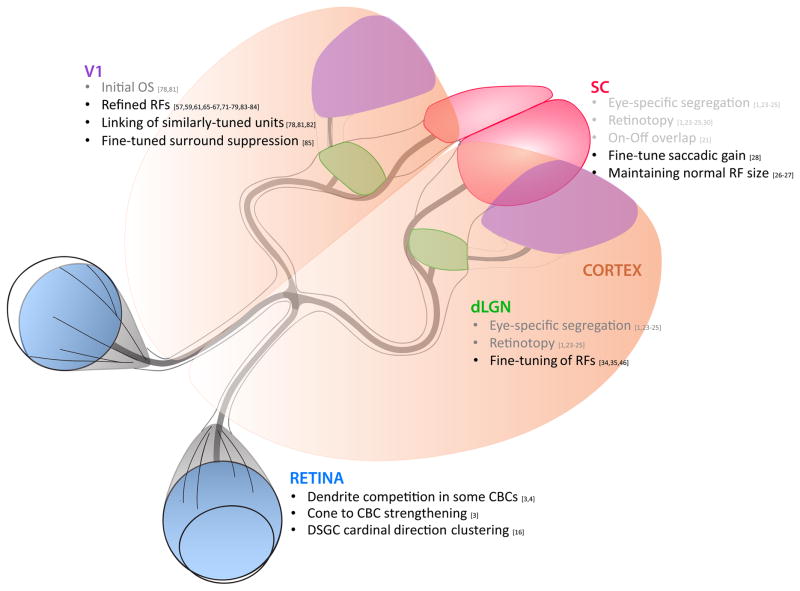
Summary of major activity-dependent receptive field features at each anatomical station in the mouse visual pathway, from the retina to the dorsal lateral geniculate nucleus (dLGN) and superior colliculus (SC), and dLGN to visual cortex (V1). Items listed in grey appear to be reliant on activity prior to the onset of vision (such as spontaneous retinal waves), whereas items listed in black appear to depend on vision for proper development.
Highlights.
Molecular cues and activity together shape visual circuit development.
Activity influences development at all levels of the visual system.
Prior to vision, activity is generated spontaneously in the retina.
Sensory experience also fine-tunes development throughout the visual system.
Acknowledgments
NIH F31NS083437 to Andrew Thompson, NIH F31EY025968 to Alexandra Gribizis, NIH R01EY013613 and P30HD018655 to Chinfei Chen and NIH R01EY015788, R01EY023105, P30EY026878 to Michael Crair.
Footnotes
Conflict of Interest Statement: Nothing declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huberman AD, Feller MB, Chapman B. Mechanisms underlying development of visual maps and receptive fields. Annu Rev Neurosci. 2008;31:479–509. doi: 10.1146/annurev.neuro.31.060407.125533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cang J, Feldheim DA. Developmental Mechanisms of Topographic Map Formation and Alignment. Annu Rev Neurosci. 2013;36:51–77. doi: 10.1146/annurev-neuro-062012-170341. [DOI] [PubMed] [Google Scholar]
- 3.Meister M, Wong RLOL, Baylor DA, Shatz CJ. Synchronous Bursts of Action Potentials in Ganglion Cells of the Developing Mammalian Retina. Science (80-) 1991;252:939–943. doi: 10.1126/science.2035024. [DOI] [PubMed] [Google Scholar]
- 4.Feller MB, Wellis DP, Stellwagen D, Werblin FS, Shatz CJ. Requirement for cholinergic synaptic transmission in the propagation of spontaneous retinal waves. Science (80-) 1996;272:1182–1187. doi: 10.1126/science.272.5265.1182. [DOI] [PubMed] [Google Scholar]
- 5.Ackman JB, Burbridge TJ, Crair MC. Retinal waves coordinate patterned activity throughout the developing visual system. Nature. 2012;490:219–25. doi: 10.1038/nature11529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maccione A, et al. Following the ontogeny of retinal waves: pan-retinal recordings of population dynamics in the neonatal mouse. J Physiol. 2014;592:1545–63. doi: 10.1113/jphysiol.2013.262840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arroyo Da, Feller MB. Spatiotemporal Features of Retinal Waves Instruct the Wiring of the Visual Circuitry. Front Neural Circuits. 2016;10:1–7. doi: 10.3389/fncir.2016.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kerschensteiner D, Wong ROL. A Precisely Timed Asynchronous Pattern of ON and OFF Retinal Ganglion Cell Activity during Propagation of Retinal Waves. Neuron. 2008;58:851–858. doi: 10.1016/j.neuron.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Firl A, et al. Elucidating the role of AII amacrine cells in glutamatergic retinal waves. J Neurosci. 2015;35:1675–1686. doi: 10.1523/JNEUROSCI.3291-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akrouh A, Kerschensteiner D. Intersecting circuits generate precisely patterned retinal waves. Neuron. 2013;79:322–334. doi: 10.1016/j.neuron.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kerschensteiner D. Glutamatergic Retinal Waves. Front Neural Circuits. 2016;10:38. doi: 10.3389/fncir.2016.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *12.Hoy JL, Niell CM. Layer-specific refinement of visual cortex function after eye opening in the awake mouse. J Neurosci. 2015;35:3370–83. doi: 10.1523/JNEUROSCI.3174-14.2015. The authors measure receptive field properties from awake mouse visual cortex starting at eye opening and find responses for orientation, direction, and spatial frequency at eye opening. Over the following week after eye opening, layer-specific maturation of orientation selectivity, direction selectivity, and linearity was observed, as well as behavioral-state modulation of responsiveness. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ko H, et al. The emergence of functional microcircuits in visual cortex. Nature. 2013;496:96–100. doi: 10.1038/nature12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *14.Ko H, Mrsic-Flogel TD, Hofer SB. Emergence of feature-specific connectivity in cortical microcircuits in the absence of visual experience. J Neurosci. 2014;34:9812–9816. doi: 10.1523/JNEUROSCI.0875-14.2014. This study shows that neurons with similar responses to oriented gratings or natural movies become preferentially connected in V1 (see also reference 81). This connectivity pattern still emerges in dark-reared mice, though the degree of matching is degraded. Dark-rearing also prevents the pruning of connections between nonresponsive visual neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarnaik R, Wang BS, Cang J. Experience-dependent and independent binocular correspondence of receptive field subregions in mouse visual cortex. Cereb Cortex. 2014;24:1658–1670. doi: 10.1093/cercor/bht027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian N, Copenhagen DR. Visual stimulation is required for refinement on ON and OFF pathways in postnatal retina. Neuron. 2003;39:85–96. doi: 10.1016/s0896-6273(03)00389-1. [DOI] [PubMed] [Google Scholar]
- 17.Kerschensteiner D, Morgan JL, Parker ED, Lewis RM, Wong ROL. Neurotransmission selectively regulates synapse formation in parallel circuits in vivo. Nature. 2009;460:1016–1020. doi: 10.1038/nature08236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamby AM, Rosa JM, Hsu CH, Feller MB. CaV3.2 KO mice have altered retinal waves but normal direction selectivity. Vis Neurosci. 2015;32:E003. doi: 10.1017/S0952523814000364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morrie RD, Feller MB. An Asymmetric Increase in Inhibitory Synapse Number Underlies the Development of a Direction Selective Circuit in the Retina. J Neurosci. 2015;35:9281–6. doi: 10.1523/JNEUROSCI.0670-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei W, Hamby AM, Zhou K, Feller MB. Development of asymmetric inhibition underlying direction selectivity in the retina. Nature. 2011;469:402–406. doi: 10.1038/nature09600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bos R, Gainer C, Feller MB. Role for visual experience in the development of direction-selective circuits. Curr Biol. 2016:1–9. doi: 10.1016/j.cub.2016.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levick WR. Receptive fields and trigger features of ganglion cells in the visual streak of the rabbits retina. J Physiol. 1967;188:285–307. doi: 10.1113/jphysiol.1967.sp008140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen H, Liu X, Tian N. Subtype-Dependent Postnatal Development of Direction- and Orientation-Selective Retinal Ganglion Cells in Mice. J Neurophysiol. 2014:2092–2101. doi: 10.1152/jn.00320.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baden T, et al. The functional diversity of mouse retinal ganglion cells. Nature. 2016:1–21. doi: 10.1038/nature16468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nath A, Schwartz GW. Cardinal Orientation Selectivity Is Represented by Two Distinct Ganglion Cell Types in Mouse Retina. J Neurosci. 2016;36:3208–21. doi: 10.1523/JNEUROSCI.4554-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao X, Chen H, Liu X, Cang J. Orientation-selective Responses in the Mouse Lateral Geniculate Nucleus. J Neurosci. 2013;33:12751–12763. doi: 10.1523/JNEUROSCI.0095-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *27.Dunn FA, DellaSantina L, Parker ED, Wong ROL. Sensory experience shapes the development of the visual system’s first synapse. Neuron. 2013;80:1159–1166. doi: 10.1016/j.neuron.2013.09.024. The authors show that dark rearing reduces synaptic strength between cones and cone bipolar cells, although this effect was most pronounced in type 8 CBCs, and not observed in type 6 CBCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tian N, Copenhagen DR. Visual deprivation alters development of synaptic function in inner retina after eye opening. Neuron. 2001;32:439–449. doi: 10.1016/s0896-6273(01)00470-6. [DOI] [PubMed] [Google Scholar]
- 29.Johnson RE, Kerschensteiner D. Retrograde plasticity and differential competition of bipolar cell dendrites and axons in the developing retina. Curr Biol. 2014;24:2301–2306. doi: 10.1016/j.cub.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okawa H, Della Santina L, Schwartz GW, Rieke F, Wong ROL. Interplay of cell-autonomous and nonautonomous mechanisms tailors synaptic connectivity of converging axons in vivo. Neuron. 2014;82:125–137. doi: 10.1016/j.neuron.2014.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu M, Wang L, Cang J. Different roles of axon guidance cues and patterned spontaneous activity in establishing receptive fields in the mouse superior colliculus. Front Neural Circuits. 2014;8:23. doi: 10.3389/fncir.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **32.Burbridge TJ, et al. Visual circuit development requires patterned activity mediated by retinal acetylcholine receptors. Neuron. 2014;84:1049–1064. doi: 10.1016/j.neuron.2014.10.051. This paper conclusively demonstrates a causal link between retinal waves and normal development of visual circuits, and distinguishes between different aspects of retinal wave activity in driving eye-specific segregation and retinotopic refinement in both SC and dLGN. The authors also report a decoupling of activity between retina and its downstream targets whereby neurons in the developing SC generate intrinsic spontaneous activity patterns when receiving reduced retinal drive. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu HP, et al. Retinal Wave Patterns Are Governed by Mutual Excitation among Starburst Amacrine Cells and Drive the Refinement and Maintenance of Visual Circuits. J Neurosci. 2016;36:3871–86. doi: 10.1523/JNEUROSCI.3549-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu HP, et al. Spatial pattern of spontaneous retinal waves instructs retinotopic map refinement more than activity frequency. Dev Neurobiol. 2015;75:621–640. doi: 10.1002/dneu.22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **35.Owens MT, Feldheim DA, Stryker MP, Triplett JW. Stochastic Interaction between Neural Activity and Molecular Cues in the Formation of Topographic Maps. Neuron. 2015;87:1261–1273. doi: 10.1016/j.neuron.2015.08.030. The authors show that in heterozygous transgenic mice expressing a single extra copy of EphA3 in Isl2+ RGCs, azimuth maps in SC are variable –– some are single, others duplicated (as in mice that express two extra copies), or a mixture of the two. When retinal waves are disrupted in these mice, this stochasticity disappears, demonstrating a delicate balance between the influence of molecular cues and activity in guiding map formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Sarnaik R, Rangarajan K, Liu X, Cang J. Visual receptive field properties of neurons in the superficial superior colliculus of the mouse. J Neurosci. 2010;30:16573–84. doi: 10.1523/JNEUROSCI.3305-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carrasco MM, Razak Ka, Pallas SL. Visual Experience Is Necessary for Maintenance But Not Development of Receptive Fields in Superior Colliculus. Visual Experience Is Necessary for Maintenance But Not Development of Receptive Fields in Superior Colliculus. 2013;94:1962–1970. doi: 10.1152/jn.00166.2005. [DOI] [PubMed] [Google Scholar]
- 38.Balmer TS, Pallas SL. Visual experience prevents dysregulation of GABAB receptor-dependent short-term depression in adult superior colliculus. J Neurophysiol. 2015;113:2049–2061. doi: 10.1152/jn.00882.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang L, Liu M, Segraves MA, Cang J. Visual Experience Is Required for the Development of Eye Movement Maps in the Mouse Superior Colliculus. J Neurosci. 2015;35:12281–6. doi: 10.1523/JNEUROSCI.0117-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hong YK, Chen C. Wiring and rewiring of the retinogeniculate synapse. Curr Opin Neurobiol. 2011;21:228–237. doi: 10.1016/j.conb.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis ZW, Chapman B, Cheng HJ. Increasing Spontaneous Retinal Activity before Eye Opening Accelerates the Development of Geniculate Receptive Fields. J Neurosci. 2015;35:14612–23. doi: 10.1523/JNEUROSCI.1365-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Golding B, et al. Retinal input directs the recruitment of inhibitory interneurons into thalamic visual circuits. Neuron. 2014;81:1057–1069. doi: 10.1016/j.neuron.2014.01.032. [DOI] [PubMed] [Google Scholar]
- 43.Lo FS, Ziburkus J, Guido W. Synaptic mechanisms regulating the activation of a Ca(2+)-mediated plateau potential in developing relay cells of the LGN. J Neurophysiol. 2002;87:1175–1185. doi: 10.1152/jn.00715.1999. [DOI] [PubMed] [Google Scholar]
- 44.Dilger EK, et al. Absence of Plateau Potentials in dLGN Cells Leads to a Breakdown in Retinogeniculate Refinement. J Neurosci. 2015;35:3652–3662. doi: 10.1523/JNEUROSCI.2343-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bialas AR, Stevens B. TGF-β signaling regulates neuronal C1q expression and developmental synaptic refinement. Nat Neurosci. 2013;16:1773–82. doi: 10.1038/nn.3560. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Stevens B, et al. The Classical Complement Cascade Mediates CNS Synapse Elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 47.Lee H, et al. Synapse elimination and learning rules co-regulated by MHC class I H2-Db. Nature. 2014;509:195–200. doi: 10.1038/nature13154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hooks BM, Chen C. Vision triggers an experience-dependent sensitive period at the retinogeniculate synapse. J Neurosci. 2008;28:4807–4817. doi: 10.1523/JNEUROSCI.4667-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hooks BM, Chen C. Distinct roles for spontaneous and visual activity in remodeling of the retinogeniculate synapse. Neuron. 2006;52:281–291. doi: 10.1016/j.neuron.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 50.Noutel J, Hong YK, Leu B, Kang E, Chen C. Experience-Dependent Retinogeniculate Synapse Remodeling Is Abnormal in MeCP2-Deficient Mice. Neuron. 2011;70:35–42. doi: 10.1016/j.neuron.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Louros SR, Hooks BM, Litvina L, Carvalho A, Chen C. A role for stargazin in experience-dependent plasticity. Cell Rep. 2014;7:1614–1625. doi: 10.1016/j.celrep.2014.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **52.Thompson AD, Picard N, Min L, Fagiolini M, Chen C. Cortical Feedback Regulates Feedforward Retinogeniculate Refinement. Neuron. 2016;91:1021–1033. doi: 10.1016/j.neuron.2016.07.040. This study demonstrates that corticothalamic feedback, from L6 of V1 to the dLGN, is necessary for proper refinement of the retinogeniculate projection during the window for experience-dependent plasticity at this synapse. Manipulations of cortical activity patterns during this window lead to an increased RGC to relay neuron convergence, suggesting subcortical circuits can incorporate cortically-processed sensory input to optimize their feedforward wiring. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tavazoie SF, Reid RC. Diverse receptive fields in the lateral geniculate nucleus during thalamocortical development. Nat Neurosci. 2000;3:608–616. doi: 10.1038/75786. [DOI] [PubMed] [Google Scholar]
- 54.Sanes JR, Masland RH. The Types of Retinal Ganglion Cells: Current Status and Implications for Neuronal Classification. Annu Rev Neurosci. 2014;38:150421150146009. doi: 10.1146/annurev-neuro-071714-034120. [DOI] [PubMed] [Google Scholar]
- 55.Vidyasagar TR, Urbas JV. Orientation sensitivity of cat LGN neurones with and without inputs from visual cortical areas 17 and 18. Exp Brain Res. 1982;46:157–169. doi: 10.1007/BF00237172. [DOI] [PubMed] [Google Scholar]
- 56.Albus K, Wolf W, Beckman R. Orientation bias in the response of kitten LGNd neurons to moving light bars. Dev Brain Res. 1983;6:308–313. doi: 10.1016/0165-3806(83)90071-8. [DOI] [PubMed] [Google Scholar]
- 57.Cheong SK, Tailby C, Solomon SG, Martin PR. Cortical-like receptive fields in the lateral geniculate nucleus of marmoset monkeys. J Neurosci. 2013;33:6864–76. doi: 10.1523/JNEUROSCI.5208-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Piscopo DM, El-Danaf RN, Huberman AD, Niell CM. Diverse visual features encoded in mouse lateral geniculate nucleus. J Neurosci. 2013;33:4642–56. doi: 10.1523/JNEUROSCI.5187-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scholl B, Tan AYY, Corey J, Priebe NJ. Emergence of orientation selectivity in the Mammalian visual pathway. J Neurosci. 2013;33:10616–24. doi: 10.1523/JNEUROSCI.0404-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marshel JH, Kaye AP, Nauhaus I, Callaway EM. Anterior-Posterior Direction Opponency in the Superficial Mouse Lateral Geniculate Nucleus. Neuron. 2012;76:713–720. doi: 10.1016/j.neuron.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **61.Hong YK, et al. Refinement of the retinogeniculate synapse by bouton clustering. Neuron. 2014;84:332–9. doi: 10.1016/j.neuron.2014.08.059. This study characterized RGC axon arbor morphology during developmental refinement in the dLGN. Contrary to the expected pruning and retraction of arbors to correlate with functional refinement, axon arbors remained broad; refinement occurred by the spatial reorganization and clustering of presynaptic boutons. This process was disrupted by visual deprivation, suggesting a structural basis (redistribution of synapses within a large axon scaffold) for experience-dependent plasticity at this synapse. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morgan JL, Berger DR, Wetzel AW, Lichtman JW. The Fuzzy Logic of Network Connectivity in Mouse Visual Thalamus. Cell. 2016;165:192–206. doi: 10.1016/j.cell.2016.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Crair MC, Gillespie DC, Stryker MP. The role of visual experience in the development of columns in cat visual cortex. Science (80-) 1998;279:566–570. doi: 10.1126/science.279.5350.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chapman B, Stryker MP, Bonhoeffer T. Development of orientation preference maps in ferret primary visual cortex. J Neurosci. 1996;16:6443–6453. doi: 10.1523/JNEUROSCI.16-20-06443.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.White LE, Coppola DM, Fitzpatrick D. The contribution of sensory experience to the maturation of orientation selectivity in ferret visual cortex. Nature. 2001;411:1049–1052. doi: 10.1038/35082568. [DOI] [PubMed] [Google Scholar]
- 66.Sherk H, Stryker MP. Quantitative study of cortical orientation selectivity in visually inexperienced kitten. J Neurophysiol. 1976;39:63–70. doi: 10.1152/jn.1976.39.1.63. [DOI] [PubMed] [Google Scholar]
- **67.Smith GB, et al. The development of cortical circuits for motion discrimination. Nat Neurosci. 2015;18:252–61. doi: 10.1038/nn.3921. In this study, the authors use in vivo calcium imaging to study the maturation of population responses in ferret V1 during the weeks after eye-opening. They find that responses to moving stimuli become more sparse, and variability and noise correlations in the response decrease over development, improving the ability of cortical activity to discriminate motion direction. Furthermore, experience with a moving stimulus drove a rapid reduction in noise correlations, indicating a role for vision in these developmental changes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Buisseret P, Imbert M. Visual cortical cells: their developmental properties in normal and dark reared kittens. J Physiol. 1976;255:511–525. doi: 10.1113/jphysiol.1976.sp011293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rochefort NL, et al. Development of direction selectivity in mouse cortical neurons. Neuron. 2011;71:425–432. doi: 10.1016/j.neuron.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 70.Hagihara KM, Murakami T, Yoshida T, Tagawa Y, Ohki K. Neuronal activity is not required for the initial formation and maturation of visual selectivity. Nat Neurosci. 2015;18:1780–8. doi: 10.1038/nn.4155. [DOI] [PubMed] [Google Scholar]
- 71.Li Y-t, Ma W-p, Pan C-j, Zhang LI, Tao HW. Broadening of Cortical Inhibition Mediates Developmental Sharpening of Orientation Selectivity. J Neurosci. 2012;32:3981–3991. doi: 10.1523/JNEUROSCI.5514-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang BS, Sarnaik R, Cang J. Critical Period Plasticity Matches Binocular Orientation Preference in the Visual Cortex. Neuron. 2010;65:246–256. doi: 10.1016/j.neuron.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Weliky M, Katz LC. Disruption of orientation tuning in visual cortex by artificially correlated neuronal activity. Nature. 1997;386:680–685. doi: 10.1038/386680a0. [DOI] [PubMed] [Google Scholar]
- 74.Sengpiel F, Stawinski P, Bonhoeffer T. Influence of experience on orientation maps in cat visual cortex. Nat Neurosci. 1999;2:727–732. doi: 10.1038/11192. [DOI] [PubMed] [Google Scholar]
- 75.Kreile A, Bonhoeffer T, Hubener M. Altered Visual Experience Induces Instructive Changes of Orientation Preference in Mouse Visual Cortex. J Neurosci. 2011;31:13911–13920. doi: 10.1523/JNEUROSCI.2143-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kang E, et al. Visual acuity development and plasticity in the absence of sensory experience. J Neurosci. 2013;33:17789–96. doi: 10.1523/JNEUROSCI.1500-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fagiolini M, Pizzorusso T, Berardi N, Domenici L, Maffei L. Functional postnatal development of the rat primary visual cortex and the role of visual experience: dark rearing and monocular deprivation. Vis Res. 1994;34:709–720. doi: 10.1016/0042-6989(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 78.Wang BS, Feng L, Liu M, Liu X, Cang J. Environmental Enrichment Rescues Binocular Matching of Orientation Preference in Mice that Have a Precocious Critical Period. Neuron. 2013;80:198–209. doi: 10.1016/j.neuron.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Y, Fitzpatrick D, White LE. The development of direction selectivity in ferret visual cortex requires early visual experience. Nat Neurosci. 2006;9:676–681. doi: 10.1038/nn1684. [DOI] [PubMed] [Google Scholar]
- 80.Li Y, Van Hooser SD, Mazurek M, White LE, Fitzpatrick D. Experience with moving visual stimuli drives the early development of cortical direction selectivity. Nature. 2008;456:952–956. doi: 10.1038/nature07417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Berman N, Daw NW. Comparison of the critical periods for monocular and directional deprivation in cats. J Physiol. 1977;265:249–259. doi: 10.1113/jphysiol.1977.sp011715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *82.Kremkow J, Jin J, Wang Y, Alonso JM. Principles underlying sensory map topography in primary visual cortex. Nature. 2016;533:52–57. doi: 10.1038/nature17936. This study in cat shows that the cortical maps for orientation, direction and retinal disparity are related to the organization of light (ON) and dark (OFF) stimuli. This organization is OFF-dominated, OFF-centric and runs orthogonal to ocular dominance columns. See also *Lee et al. 2016 below. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *83.Lee K-S, Huang X, Fitzpatrick D. Topology of ON and OFF inputs in visual cortex enables an invariant columnar architecture. Nature. 2016;533:90–94. doi: 10.1038/nature17941. This study in tree shrew shows the centers of OFF subfields for neurons in a given region of cortex are confined to a compact region of visual space and display a smooth visuotopic progression, whereas the centers of the ON subfields are distributed over a wider region of visual space, display substantial visuotopic scatter, and have an orientation-specific displacement consistent with orientation preference map structure. See also *Kremkow et al. 2016 above. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Albus K, Wolf W. Early post-natal development of neuronal function in the kitten’s visual cortex: a laminar analysis. J Physiol. 1984;348:153–85. doi: 10.1113/jphysiol.1984.sp015104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ko H, et al. Functional specificity of local synaptic connections in neocortical networks. Nature. 2011;473:87–91. doi: 10.1038/nature09880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Callaway EM, Katz LC. Effects of binocular deprivation on the development of clustered horizontal connections in cat striate cortex. Proc Natl Acad Sci U S A. 1991;88:745–749. doi: 10.1073/pnas.88.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ruthazer ES, Stryker MP. The role of activity in the development of long-range horizontal connections in area 17 of the ferret. J Neurosci. 1996;16:7253–7269. doi: 10.1523/JNEUROSCI.16-22-07253.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **88.Pecka M, Han Y, Sader E, Mrsic-Flogel TD. Experience-Dependent Specialization of Receptive Field Surround for Selective Coding of Natural Scenes. Neuron. 2014;84:457–469. doi: 10.1016/j.neuron.2014.09.010. Pecka et al. conducted in vivo whole cell recordings from layer 2/3 of V1 and found that neuronal firing in response to natural scenes presented to a cell’s RF was more selective when the RF surround was also stimulated with natural scenes, rather than phase-scrambled movies lacking complex structure. The authors report a circuit mechanism for this specificity of surround modulation, and show that it requires visual experience to develop. [DOI] [PMC free article] [PubMed] [Google Scholar]