Abstract
Preeclampsia (PE) is a multisystem disorder of pregnancy classically characterized with the onset of hypertension after 20 weeks gestation in the presence of proteinuria. PE typically affects 2–8% of pregnancies and is a leading cause of maternal and perinatal morbidity and mortality. This article reviews the most effective biomarkers used in first trimester screening for PE. It explores their use both in isolation and as part of an algorithm to yield the best detection rates. Screening by a combination of maternal risk factors, uterine artery Doppler, mean arterial pressure, maternal serum PAPP-A and PlGF can identify about 75% of cases of preterm PE for a false-positive rate of 10%. By identifying these patients at high risk for PE, appropriately tailored antenatal surveillance can be instigated and prophylactic pharmacological interventions can be prescribed to improve placentation and ultimately, the outcome for both the mother and fetus.
Keywords: first-trimester screening, mean arterial pressure, preeclampsia, pyramid of antenatal care, serum biochemistry, uterine artery Doppler
Preeclampsia (PE) is a medical condition in pregnancy characterized by high blood pressure and proteinuria after 20 weeks' gestation. It occurs in 2–8% of all pregnancies. The consequences of PE can be serious both for the mother and the fetus, especially when the disease is severe. Severe manifestations include delivery before 37 weeks gestation (preterm PE) and fetal growth restriction [1–7]. The condition is thought to be predominantly due to defective implantation of the placenta within the uterine endometrium. The quest to identify pregnant women that are at high risk of developing preterm PE is a major goal in modern obstetrics.
There is a need for early identification of these women as there is some evidence demonstrating that the prevalence of PE can be halved by commencing pregnant women on low-dose aspirin before 16 weeks gestation [8]. In the last 10 years, extensive research has shown, mainly as a consequence of the shift in screening for Down's syndrome from the second- to the first-trimester of pregnancy, that there are four potentially useful tests to screen for PE: measurements of blood pressure (BP) and the blood flow in the maternal blood vessels that supply the uterus (uterine artery pulsatility index [PI]), and the quantification of the levels of two placental proteins (PAPP-A and PlGF) in the mother's blood [9]. Using a novel mathematical model (Bayes theorem), that combines prior information from maternal characteristics, obstetric and medical history, uterine artery PI, mean arterial pressure (MAP) and maternal serum PAPP-A and PlGF, at 11–13 weeks gestation, can actually reveal a significant proportion of women who are at high risk for preterm PE during pregnancy [9,10]. The performance of these screening tests for PE are summarized in Table 1.
Table 1.
Estimated detection rates of all preeclampsia and preeclampsia requiring delivery before 37 and 34 weeks gestation, at false-positive rates of 5 and 10%.
| Screening test | FPR (%) | Detection rate, % (95% CI) |
||
|---|---|---|---|---|
| PE <34 weeks | PE <37 weeks | All PE | ||
| Maternal characteristics plus | 5 | 42 (33–51) | 36 (30–42) | 30 (27–33) |
| 10 | 58 (49–67) | 50 (44–56) | 41 (38–44) | |
|
| ||||
| Ut-PI | 5 | 57 (47–66) | 46 (40–53) | 33 (30–36) |
| 10 | 70 (61–78) | 59 (53–65) | 44 (41–47) | |
|
| ||||
| MAP | 5 | 49 (40–58) | 45 (39–52) | 35 (31–37) |
| 10 | 65 (56–73) | 60 (54–66) | 48 (45–51) | |
|
| ||||
| PAPP-A | 5 | 48 (38–57) | 42 (36–48) | 31 (28–34) |
| 10 | 60 (51–69) | 55 (49–61) | 44 (40–47) | |
|
| ||||
| PlGF | 5 | 57 (48–66) | 50 (44–56) | 35 (32–38) |
| 10 | 73 (64–81) | 66 (60–72) | 47 (43–50) | |
|
| ||||
| MAP and Ut-PI | 5 | 63 (54–72) | 53 (47–59) | 38 (35–41) |
| 10 | 80 (71–86) | 70 (65–76) | 52 (49–55) | |
|
| ||||
| PAPP-A and PlGF | 5 | 57 (48–66) | 49 (43–56) | 33 (30–36) |
| 10 | 77 (69–84) | 67 (61–73) | 48 (45–51) | |
|
| ||||
| Ut-PI, MAP and PAPP-A | 5 | 67 (58–75) | 56 (50–62) | 38 (34–40) |
| 10 | 80 (71–86) | 68 (62–74) | 52 (48–55) | |
|
| ||||
| Ut-PI, MAP and PlGF | 5 | 80 (72–87) | 66 (60–72) | 42 (38–45) |
| 10 | 89 (81–94) | 77 (71–82) | 54 (51–57) | |
|
| ||||
| Ut-PI, MAP, PAPP-A and PlGF | 5 | 76 (68–83) | 63 (57–69) | 40 (36–43) |
| 10 | 88 (81–93) | 75 (69–80) | 54 (50–56) | |
FPR: False-positive rate; MAP: Mean arterial pressure; PE: Preeclampsia; Ut-PI: Uterine artery pulsatility index.
Screening by maternal history
Several guidelines from different countries have emerged recommending that if a woman is at high risk of developing PE, she should be commenced on low-dose aspirin daily from early pregnancy until delivery of the baby [11–14]. However, the screening criteria in these guidelines are based on some maternal characteristics and medical history. Such an approach has been shown to identify only 35% of all cases of PE and about 40% of preterm PE, at false-positive rate of 10% [15].
It has been shown that maternal characteristics and medical history are useful in screening for PE. Box 1 shows the maternal risk factors for PE. However, it is only useful when these risk factors are extrapolated and incorporated into a specific mathematical formula designed to calculate the risk for PE [16]. In general, the maternal risk factor profiles vary between preterm and term PE. This resulted in a theory that preterm and term PE may in fact be different disorders. An alternative hypothesis is that PE is a spectrum disorder, and the more severe the disease is, the earlier the gestation at which the delivery will be. A novel approach for screening for PE involving multiple variables including maternal risk factors has since evolved into a new method in which, the gestation at the time of delivery for PE is treated as a continuous rather than a categorical variable. This approach, which allows estimation of individual patient-specific risks of PE requiring delivery before a specified gestation, is based on a survival time model. This model considers a situation that if a pregnancy continued indefinitely, all women would develop PE. Whether they develop PE or not depends on the competition between delivery before or after development of PE [17]. By applying this method, the impact of the various risk factors, alters the mean of the distribution of gestational age at delivery with PE. In those at low-risk for PE, the gestational age distribution is shifted to the right, with the implication that most women will actually deliver before developing of PE. In high-risk pregnancies, the distribution is shifted to the left and the earlier the mean gestational age, the higher the risk is for PE (Figure 1).
Box 1.
Recognized maternal risk factors for preeclampsia.
Previous PE
Previous early onset PE and preterm delivery at <34 weeks gestation
PE in more than one prior pregnancy
Chronic kidney disease
Autoimmune disease such as systemic lupus erythematosis or antiphospholipid syndrome
Heritable thrombophilias
Type 1 or Type 2 diabetes
Chronic hypertension
First pregnancy
Pregnancy interval of more than 10 years
New partner
Reproductive technologies
Family history of PE (mother or sister)
Excessive weight gain in pregnancy
Infection during pregnancy
Gestational trophoblastic disease
Multiple pregnancy
Age 40 years or older
Ethnicity – Nordic, black, south Asian or Pacific Island
BMI of 35 kg/m2 or more at first visit
Booking systolic blood pressure >130 mmHg or diastolic blood pressure >80 mmHg
Increased pre-pregnancy triglycerides
Family history of early onset cardiovascular disease
Lower socioeconomic status
Cocaine and methamphetamine use
Nonsmoking
Figure 1.
Gaussian distributions of gestational age at delivery for preeclampsia. In pregnancies at low-risk for preeclampsia (PE), the gestational age distribution is shifted to the right with the implication that most women will actually deliver before developing of PE. In pregnancies at high risk for PE the distribution is shifted to the left and the smaller the mean gestational age the higher is the risk for PE. The probability of PE occurring at or before a specified gestational age is given by the area under the curve (black). In the low-risk group the risk of PE at ≪34 weeks gestation is 1% and in the high-risk group the risk is 60%.
Adapted with permission from [17].
Using this approach, the mean gestational age for delivery with PE is 55 weeks with estimated standard deviation of 6.88 weeks. Those at high risk of PE have risk factors such as advanced maternal age, increased weight, Afro-Caribbean and South Asian racial origin, conception by IVF. A medical history of chronic hypertension, pre-existing diabetes mellitus and systemic lupus erythematosus or antiphospholipid syndrome, and personal and family history of PE will also contribute to them falling into the high-risk category. The risk for PE decreases with increasing maternal height and in parous women with no previous PE; in the latter, the protective effect is inversely related to the interpregnancy interval and persists beyond 15 years. Screening by maternal factors yields estimated detection rates of all PE and PE requiring delivery before 37 and 34 weeks gestation of about 41, 50 and 58%, respectively, at a false-positive rate of 10% (Table 1) [9].
Screening by maternal biophysical markers
Uterine artery Doppler
The most promising screening test for PE is uterine artery PI. The underlying mechanism for the development of PE is thought to be impaired trophoblastic invasion [18,19]. Doppler ultrasound is a noninvasive method for assessing the blood flow to the placenta. The finding that poor placental perfusion, demonstrated by increased uterine artery PI, is associated with the development of PE, supports the theory that PE is a consequence of impaired placentation. The results of previous first- and second-trimester Doppler studies as well as histological studies of the maternal spiral arteries within the wall of the uterus, also corroborate this hypothesis [20–22].
In order to obtain reliable and accurate measurements of uterine artery PI using Doppler ultrasound, appropriate training of sonographers and adherence to standard operating protocols for obtaining these measurements are essential. A process of training and quality assurance has been established by the Fetal Medicine Foundation [23]. Uterine artery PI is influenced by gestational age at screening, maternal age, weight, racial origin and history of PE in the previous pregnancy, and is therefore expressed as multiple of the median (MoM) after these factors are taken into consideration [24]. The uterine artery PI MoM is higher at 11–13 weeks gestation in those who subsequently develop PE and there is a significant negative linear correlation between the uterine artery PI MoM and gestational age at delivery [9]. Estimated detection rates of PE using maternal factors with uterine artery PI are shown in Table 1. This biophysical marker with maternal factors improves the detection rate from 41 to 44%, 50 to 59% and 58 to 70%, at a false-positive rate of 10%, for all PE and PE requiring delivery before 37 and 34 weeks gestation, respectively.
Blood pressure
The importance of measuring maternal BP antenatally cannot be underestimated. Accurate assessment of BP can be challenging as each individual exhibits a considerable variability. The first BP recording at rest is often the highest one and it subsequently decreases as the patients become more familiar with the procedure [25]. Consequently, many professional bodies advocate that a series of BP measurements should be performed until a pre-specified level of stability is achieved [26,27]. The use of mercury sphygmomanometers remains the gold standard for noninvasive BP monitoring. However, concerns for both their clinical performance and safety have been raised [28]. Common reasons for inaccurate BP readings using this method include inter observer error and terminal digit preference. The rate at which the cuff deflates, the use the appropriate size cuff, the interarm difference in BP and the arm position and posture are all known to influence BP measurement.
Automated BP monitoring will mitigate many of these obstacles and allow simple, standardized and repeated measurements to be taken. However, their use still requires both the correct cuff size and patient positioning to achieve accurate measurements. It has, therefore, been proposed that MAP should be measured by validated automated devices, with women in sitting position with back supported and legs uncrossed, that two measurements should be taken from each arm simultaneously with each arm supported at the level of the heart, and the average of the four measurements should be used [25].
It has already been shown that women destined to develop PE will have elevated BP in the first- and second-trimesters of pregnancy [29]. A mixture of prospective and retrospective, cohort and case–control studies and randomized controlled trials, have reported varied results of screening performance (detection rate, median 43%; range: 5–100%; false-positive rate, median 16%; range: 0–66%), mainly due to major differences in their methodology. A systematic review of these studies, which included more than 60,000 women with 3300 cases of PE, concluded that the MAP is significantly better than systolic BP or diastolic BP in predicting PE [29].
MAP has been shown to be affected by gestational age at screening, maternal age, weight, height, Afro-Caribbean racial origin, cigarette smoking, family history of PE, history of PE in the previous pregnancy, interpregnancy interval, chronic hypertension and diabetes mellitus [30]. Similarly, as with uterine artery Doppler, it should be expressed as a MoM after adjustment for these factors. There is a significant negative linear correlation between the MAP MoM and gestational age at delivery [17] as seen with uterine artery PI. This is a result of the increased value of the MAP MoM at 11–13 weeks gestation in women who will develop PE. As seen in Table 1, screening by maternal factors with MAP improves the detection rate from 41 to 48%, 50 to 60% and 58 to 65%, at a false-positive rate of 10%, for all PE and PE requiring delivery before 37 and 34 weeks gestation, respectively.
As there is a significant association between these two biophysical markers in PE and unaffected pregnancies, the correlation factors for both uterine artery PI and MAP must be considered in the algorithm to avoid overestimating the contributions from each marker. This will ensure a more accurate risk assessment for PE. If both of these markers are used synergistically with maternal factors, estimated detection rates of all PE and PE requiring delivery before 37 and 34 weeks gestation are 38, 53 and 63%, respectively, at a false-positive rate of 5 and 52%, 70 and 80%, respectively, at a false-positive rate of 10% (Table 1).
Screening by maternal biochemical markers
There have been numerous biochemical markers studied and evaluated for clinical use in the prediction of PE. Due to the complexity of this disorder, it is not surprising that there is no single marker available to accurately diagnose or predict it. Many of these measurements are the sequelae of impaired placentation secondary to inadequate trophoblastic invasion of the maternal spiral arteries and impaired placental perfusion. These manifestations cause ischemic related damage with the release of inflammatory factors, platelet activation, endothelial dysfunction, maternal renal dysfunction or abnormal oxidative stress [18–19,31–34]. Maternal serum PAPP-A and PlGF are two biochemical markers that have been investigated extensively and have shown promising results in the early prediction of PE. They have both been shown to be useful in screening for Down's syndrome at 11–13 weeks gestation and they are now part of a platform of automated machines that provide reproducible results within 30–40 min of sampling.
PAPP-A is an IGF-binding protein secreted by the syncytiotrophoblast that plays an important role in placental growth and development. It enhances the mitogenic function of the IGFs. PE has been shown to be associated with a low level of circulating PAPP-A, which presumably is a consequence of a reduced availability of unbound IGFs to fulfil their functional role on a cellular level. In chromosomally normal pregnancies, a PAPP-A value below the 5th centile (0.4 MoM) is only present in 8–23% of women with PE. Therefore alone, this is not an accurate predictive test for PE [35–38].
PlGF is secreted by trophoblastic cells and is part of the angiogenic VEGF family. It binds to VEGFR1, which has been shown to increase in pregnancy. PlGF has both vasculogenetic and angiogenetic functions. Its angiogenetic abilities have been speculated to play a role in normal pregnancy and changes in the levels of PlGF or its inhibitory receptor have been implicated in the development of PE. In both the first and second trimesters of pregnancy a reduced concentration of serum PlGF have been shown to precede the clinical onset of PE [39–43].
Accurate determination of the measured maternal serum metabolite concentration requires adjusting for certain maternal and pregnancy characteristics, as well as, the machine and reagents used for the assays. Their concentrations can then be expressed in the MoM of the normal. Maternal serum concentrations of PAPP-A are affected by gestational age at screening, maternal weight, height, racial origin, cigarette smoking, diabetes mellitus, method of conception, previous pregnancy with PE and birth weight Z-score of the neonate in the previous pregnancy [44]. Similarly, maternal serum concentrations of PlGF are influenced by gestational age at screening, maternal age, weight and racial origin, cigarette smoking, diabetes mellitus and gestational age at delivery and birth-weight Z-score of the neonate in the previous pregnancy [45]. Contrary to the findings with biophysical markers, the MoM values of PAPP-A and PlGF are lower at 11–13 weeks gestation in women who subsequently develop PE. There is a significant positive linear correlation between the MoM values of these markers and the gestational age at which delivery occurs [10]. This observation further confirms that PE is a single pathophysiological entity with a wide spectrum of severity.
Screening by maternal factors with biochemical markers improves the detection rates of PE from 41 to 48%, 50 to 67% and 58 to 77%, at a false-positive rate of 10%, for all PE and PE requiring delivery before 37 and 34 weeks gestation, respectively.
Screening by maternal biochemical & biophysical markers
Effective screening for PE, in a similar manner to first-trimester screening for Down's syndrome, can also be achieved using a combination of maternal factors, biochemical and biophysical markers. Using the competing risk model, the gestational age at the time of delivery for PE is treated as a continuous variable. Bayes' theorem is then used to combine prior information from maternal characteristics, obstetric and medical history with the MoM values of uterine artery PI, MAP, serum PAPP-A and PlGF. In contrast to the other published models [46,47], this model uniquely, offers the option to clinicians and researchers to select their own gestational age cut-off to define the high-risk group that could potentially benefit from therapeutic interventions starting from the first-trimester of pregnancy [8].
As there are significant associations between these biochemical and biophysical markers in PE and unaffected pregnancies, therefore when combining the four markers in calculating the patient-specific risk for PE, it is important that the correlation factors are taken into account when calculating the risk for PE. Screening by maternal factors with biochemical and biophysical markers yields detection rates of all PE and PE requiring delivery before 37 and 34 weeks gestation of 54, 75 and 88%, respectively, at a false-positive rate of 10% (Table 1).
Screening by cell-free DNA
Several studies have reported that in women with established PE, the plasma or serum concentrations of both total and fetal cell-free (cf)DNA are higher than in normotensive controls and the increase is particularly marked in those with severe PE [48–54]. These findings have been attributed to accelerated apoptosis of trophoblastic cells resulting from placental ischemia [48] and reduced clearance of the cfDNA from the maternal circulation in women with PE [55]. However, there are conflicting data as to whether these altered levels precede the onset of the disease.
A recent systematic review examined fetal cfDNA quantification in the prediction of PE [56]. The review included three prospective cohort studies and ten case–control studies with a total of 440 cases of PE and 2576 controls. It was reported that 11 of the 13 studies found significantly higher concentrations of fetal cfDNA in women who developed PE. The authors suggested that most of the included studies did not adequately control for possible confounding factors such as BMI, smoking status and racial origin, and that the definitions of PE and its severity varied. Due to the significant heterogeneity between the published studies, a clinically meaningful meta-analysis could not be performed, and therefore no precise conclusions could be drawn. Our group has also demonstrated that, at 11–13 weeks gestation, in pregnancies that subsequently develop early PE, the median maternal plasma concentration of total cfDNA is increased and fetal fraction is reduced. In pregnancies that develop late PE the median fetal fraction at 20–24 weeks is reduced. However, both total cfDNA and fetal fraction are affected by maternal characteristics and when these associations are taken into account, the MoMs in PE are not significantly different from normotensive controls. A beneficial consequence of using fetal cfDNA has yet to be proved in clinical practice [57].
Conclusion & future perspective
In a proposed new approach to antenatal care, the potential value of an integrated clinic at 11–13 weeks gestation in which maternal characteristics and history are combined with the results of a series of biophysical and biochemical markers to assess the risk for a wide range of pregnancy complications, has been extensively documented [58]. Effective screening for preterm PE can be achieved in this clinic with a detection rate of about 75% at a false-positive rate of 10%. Another potential approach to this would be a contingent screening model, in which women who are identified at high risk for PE in the first trimester, are reassessed in both the second and third trimester using both the biophysical and biochemical markers.
There is now emerging evidence that metabolomic markers offer the potential for accurate screening markers for PE. A recent study has shown that a metabolite-only model consisting of glycerol, 3-hydroxyisovalerate, 2-hydroxybutyrate, acetone and citrate can achieve a detection rate of 75% at a false-positive rate of 25% in the prediction of early PE requiring delivery before 34 weeks [59]. Interestingly, combining metabolomic analysis with clinical and ultrasound characteristics could potentially improve screening for PE. A combined logistic regression model with glycerol, 3-hydroxyisovalerate, arginine, and uterine artery PI achieved a detection rate of 90% at a false-positive rate of 12% for the prediction of early PE [59]. These results have been achieved with low numbers so further prospective validation studies will be required. The Bayes' theorem-based model of screening for PE would be the basis for evaluation and inclusion of any new potentially useful biomarkers that could improve the performance of screening. In summary, the primary aim of first-trimester screening for PE is to identify high-risk women who would potentially benefit from appropriately tailored antenatal surveillance and prophylactic pharmacological interventions to improve placentation if necessary.
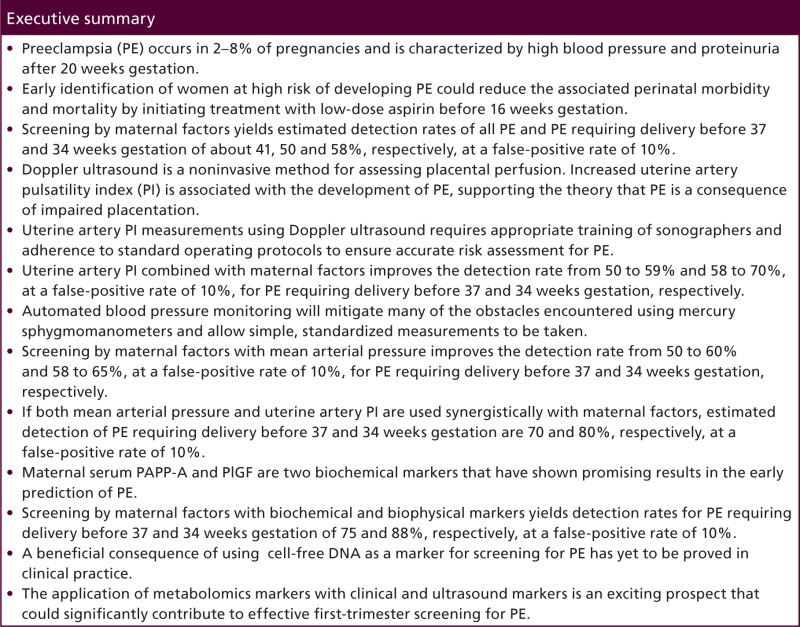
Executive summary
Preeclampsia (PE) occurs in 2–8% of pregnancies and is characterized by high blood pressure and proteinuria after 20 weeks' gestation.
Early identification of women at high risk of developing PE could reduce the associated perinatal morbidity and mortality by initiating treatment with low-dose aspirin before 16 weeks gestation.
Screening by maternal factors yields estimated detection rates of all PE and PE requiring delivery before 37 and 34 weeks gestation of about 41, 50 and 58%, respectively, at a false-positive rate of 10%.
Doppler ultrasound is a noninvasive method for assessing placental perfusion. Increased uterine artery pulsatility index (PI) is associated with the development of PE, supporting the theory that PE is a consequence of impaired placentation.
Uterine artery PI measurements using Doppler ultrasound requires appropriate training of sonographers and adherence to standard operating protocols to ensure accurate risk assessment for PE.
Uterine artery PI combined with maternal factors improves the detection rate from 50 to 59% and 58 to 70%, at a false-positive rate of 10%, for PE requiring delivery before 37 and 34 weeks gestation, respectively.
Automated blood pressure monitoring will mitigate many of the obstacles encountered using mercury sphygmomanometers and allow simple, standardized measurements to be taken.
Screening by maternal factors with mean arterial pressure improves the detection rate from 50 to 60% and 58 to 65%, at a false-positive rate of 10%, for PE requiring delivery before 37 and 34 weeks gestation, respectively.
If both mean arterial pressure and uterine artery PI are used synergistically with maternal factors, estimated detection of PE requiring delivery before 37 and 34 weeks gestation are 70 and 80%, respectively, at a false-positive rate of 10%.
Maternal serum PAPP-A and PlGF are two biochemical markers that have shown promising results in the early prediction of PE.
Screening by maternal factors with biochemical and biophysical markers yields detection rates for PE requiring delivery before 37 and 34 weeks gestation of 75 and 88%, respectively, at a false-positive rate of 10%.
A beneficial consequence of using cell-free DNA as a marker for screening for PE has yet to be proved in clinical practice.
The application of metabolomics markers with clinical and ultrasound markers is an exciting prospect that could significantly contribute to effective first-trimester screening for PE.
Financial & competing interests disclosure
The Fetal Medicine Foundation (UK Charity No: 1037116) provided funding for training in fetal medicine to the research fellows involved in the collection of the data. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.WHO. Make Every Mother and Child Count. World Health Report, 2005. WHO, Geneva, Switzerland: (2005). www.who.int [Google Scholar]
- 2.Confidential Enquiry into Maternal and Child Health (CEMACH) Perinatal Mortality 2006. England, Wales and Northern Ireland. CEMACH, London, UK: (2008). www.publichealth.hscni.net [Google Scholar]
- 3.Duley L. The global impact of pre-eclampsia and eclampsia. Semin. Perinatol. 33, 130–137 (2009). [DOI] [PubMed] [Google Scholar]
- ••.This article provides an excellent background of preeclampsia (PE). It describes the seriousness of the disorder and its impact on a global scale.
- 4.Yu CK, Khouri O, Onwudiwe N, Spiliopoulos Y, Nicolaides KH, Fetal Medicine Foundation Second-Trimester Screening Group. Prediction of pre-eclampsia by uterine artery Doppler imaging: relationship to gestational age at delivery and small-for-gestational age. Ultrasound Obstet. Gynecol. 31, 310–313 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Witlin GA, Saade GR, Mattar FM, Sibai BM. Predictors of neonatal outcome in women with severe pre-eclampsia or eclampsia between 24 and 33 weeks' gestation. Am. J. Obstet. Gynecol. 182, 607–611 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Irgens HU, Reisaeter L, Irgens LM, Lie RT. Long term mortality of mothers and fathers after pre-eclampsia: population based cohort study. BMJ 323, 1213–1217 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.von Dadelszen P, Magee LA, Roberts JM. Subclassification of pre-eclampsia. Hypertens. Pregnancy 22, 143–148 (2003). [DOI] [PubMed] [Google Scholar]
- 8.Bujold E, Roberge S, Lacasse Y, et al. Prevention of preeclampsia and intrauterine growth restriction with aspirin started in early pregnancy: a meta-analysis. Obstet. Gynecol. 116, 402–414 (2010). [DOI] [PubMed] [Google Scholar]
- ••.This meta-analysis shows that low-dose aspirin started before 16 weeks significantly reduces the incidence of preterm PE, gestational hypertension, preterm birth and IUGR.
- 9.O'Gorman N, Wright D, Syngelaki A, Akolekar R, Wright A, Poon L, Nicolaides KH. Competing risks model in screening for preeclampsia by maternal factors and biomarkers at 11–13 weeks' gestation. Am. J. Obstet. Gynecol. 214(1), 103.e1–103.e12 (2015). [DOI] [PubMed] [Google Scholar]
- •.A prospective analysis of a 1st trimester screening model for PE. This study has large numbers with excellent detection rates, especially for PE <34 weeks.
- 10.Akolekar R, Syngelaki A, Poon L, Wright D, Nicolaides KH. Competing risks model in early screening for preeclampsia by biophysical and biochemical markers. Fetal Diagn. Ther. 33, 8–15 (2013). [DOI] [PubMed] [Google Scholar]
- 11.National Collaborating Centre for Women's and Children's Health (UK). Hypertensionin Pregnancy: The Management of Hypertensive Disorders During Pregnancy. RCOG Press, London, UK: (2010) [PubMed] [Google Scholar]
- 12.WHO, Department. of Reproductive Health and Research, Department. of Maternal, Newborn, Child and Adolescent Health, Department. of Nutrition for Health and Development. WHO recommendations for prevention and treatment of pre-eclampsia and eclampsia (2011). www.preeclampsia.org
- 13.Magee LA, Helewa M, Moutquin JM, von Dadelszen P, Hypertension Guideline Committee, Strategic Training Initiative in Research in the Reproductive Health Sciences (STIRRHS) Scholars. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. J. Obstet. Gynaecol. Can. 30 (Suppl. 3), S1–S48 (2008).18817592 [Google Scholar]
- 14.American College of Obstetricians and Gynecologists, Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 122, 1122–1131 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Wright D, Syngelaki A, Akolekar R, Poon LC, Nicolaides KH. Competing risks model in screening for preeclampsia by maternal characteristics and medical history. Am. J. Obstet. Gynecol. 213(1), 62.e1–62.e10 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Poon LC, Kametas NA, Chelemen T, Leal A, Nicolaides KH. Maternal risk factors for hypertensive disorders in pregnancy: a multivariate approach. J. Hum. Hypertens. 24, 104–110 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Wright D, Akolekar R, Syngelaki A, Poon LC, Nicolaides KH. A competing risks model in early screening for preeclampsia. Fetal Diagn. Ther. 32, 171–178 (2012). [DOI] [PubMed] [Google Scholar]
- 18.Khong TY, De Wolf F, Robertson WB, Brosens I. Inadequate maternal vascular response to placentation in pregnancies complicated by pre-eclampsia and by small-for-gestational age infants. BJOG 93, 1049–1059 (1986). [DOI] [PubMed] [Google Scholar]
- 19.Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA. Pathophysiology of hypertension during preeclampsia linking placental ischemia with endothelial dysfunction. Hypertension 38, 718–722 (2001). [DOI] [PubMed] [Google Scholar]
- 20.Papageorghiou AT, Yu CK, Cicero S, Bower S, Nicolaides KH. Second-trimester uterine artery Doppler screening in unselected populations: a review. J. Matern. Fetal. Neonatal. Med. 12, 78–88 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Plasencia W, Maiz N, Bonino S, Kaihura C, Nicolaides KH. Uterine artery Doppler at 11 + 0 to 13 + 6 weeks in the prediction of pre-eclampsia. Ultrasound Obstet. Gynecol. 30, 742–749 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Olofsson P, Laurini RN, Marsal K. A high uterine artery pulsatility index reflects a defective development of placental bed spiral arteries in pregnancies complicated by hypertension and fetal growth retardation. Eur. J. Obstet. Gynecol. Reprod. Biol. 49, 161–168 (1993). [DOI] [PubMed] [Google Scholar]
- 23.The Fetal Medicine Foundation. www.fetalmedicine.org
- 24.Tayyar A, Guerra L, Wright A, Wright D, Nicolaides KH. Uterine artery pulsatility index in the three trimesters of pregnancy: effects of maternal characteristics and medical history. Ultrasound. Obstet. Gynecol. 45(6), 689–697 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Poon LC, Zymeri NA, Zamprakou A, Syngelaki A, Nicolaides KH. Protocol for measurement of mean arterial pressure at 11–13 weeks' gestation. Fetal Diagn. Ther. 31, 42–48 (2012). [DOI] [PubMed] [Google Scholar]
- 26.National Heart Foundation of Australia. Hypertension Management Guide for Doctors (2004). www.heartfoundation.org.au
- 27.Pickering TG, Hall JE, Appel LJ, et al. Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension 45, 142–161 (2005). [DOI] [PubMed] [Google Scholar]
- 28.Markandu ND, Whitcher F, Arnold A, Carney C. The mercury sphygmomanometer should be abandoned before it is proscribed. J. Hum. Hypertens. 14, 31–36 (2000). [DOI] [PubMed] [Google Scholar]
- 29.Cnossen JS, Vollebregt KC, de Vrieze N, et al. Accuracy of mean arterial pressure and blood pressure measurements in predicting pre-eclampsia: meta-analysis and systematic review. BMJ 336, 1117–1120 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- •.When blood pressure is measured in the 1st trimester, the mean arterial pressure is a better predictor for preeclampsia than systolic blood pressure, diastolic blood pressure, or an increase of blood pressure.
- 30.Wright A, Wright D, Ispas CA, Poon LC, Nicolaides KH. Mean arterial pressure in the three trimesters of pregnancy: effects of maternal characteristics and medical history. Ultrasound. Obstet. Gynecol. 45(6), 698–706 (2015). [DOI] [PubMed] [Google Scholar]
- 31.Pijnenborg R. The placental bed. Hypertens. Pregnancy 15, 7–23 (1996). [Google Scholar]
- 32.Meekins JW, Pijnenborg R, Hanssens M, McFayden IR, van Assche A. A study of placental bed spiral arteries and trophoblastic invasion in normal and severe pre-eclamptic pregnancies. BJOG 101, 669–674 (1994). [DOI] [PubMed] [Google Scholar]
- 33.Redman CWG. Pre-eclampsia and the placenta. Placenta 12, 301–308 (1991). [DOI] [PubMed] [Google Scholar]
- 34.Roberts JM, Redman CW. Pre-eclampsia: more than pregnancy-induced hypertension. Lancet 341, 1447–1451 (1993). [DOI] [PubMed] [Google Scholar]
- •.This article describes the unique systemic manifestations of PE and why it is distinct from pregnancy-induced hypertension.
- 35.Yaron Y, Heifetz S, Ochshorn Y, Lehavi O, Orr-Urtreger A. Decreased first trimester PAPP-A is a predictor of adverse pregnancy outcome. Prenat. Diagn. 22, 778–782 (2002). [DOI] [PubMed] [Google Scholar]
- 36.Smith GCS, Stenhouse EJ, Crossley JA, Aitken DA, Cameron AD, Connor JM. Early pregnancy levels of pregnancy associated plasma protein A and the risk of intrauterine growth restriction, premature birth, preeclampsia and stillbirth. J. Clin. Endocrinol. Metab. 87, 1762–1767 (2002). [DOI] [PubMed] [Google Scholar]
- 37.Dugoff L, Hobbins JC, Malone FD, et al. First trimester maternal serum PAPP-A and free beta subunit human chorionic gonadotropin concentrations and nuchal translucency are associated with obstetric complications: a population based screening study (The FASTER Trial). Am. J. Obstet. Gynecol. 191, 1446–1451 (2004). [DOI] [PubMed] [Google Scholar]
- 38.Spencer K, Yu CKH, Cowans NJ, Otigbah C, Nicolaides KH. Prediction of pregnancy complications by first trimester maternal serum PAPP-A and free β-hCG and with second trimester uterine artery Doppler. Prenat. Diagn. 25, 949–953 (2005). [DOI] [PubMed] [Google Scholar]
- 39.Tidwell SC, Ho HN, Chiu WH, Torry RJ, Torry DS. Low maternal serum levels of placenta growth factor as an antecedent of clinical preeclampsia. Am. J. Obstet. Gynecol. 184, 1267–1272 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Krauss T, Pauer HU, Augustin HG. Prospective analysis of placenta growth factor (PlGF) concentrations in the plasma of women with normal pregnancy and pregnancies complicated by preeclampsia. Hypertens. Pregnancy 23, 101–111 (2004). [DOI] [PubMed] [Google Scholar]
- 41.Crispi F, Llurba E, Domínguez C, Martín-Gallán P, Cabero L, Gratacós E. Predictive value of angiogenic factors and uterine artery Doppler for early- versus late-onset pre-eclampsia and intrauterine growth restriction. Ultrasound Obstet. Gynecol. 31, 303–309 (2008). [DOI] [PubMed] [Google Scholar]
- 42.Erez O, Romero R, Espinoza J, et al. The change in concentrations of angiogenic and anti-angiogenic factors in maternal plasma between the first and second trimesters in risk assessment for the subsequent development of preeclampsia and small-for-gestational age. J. Matern. Fetal. Neonatal. Med. 21, 279–287 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akolekar R, Zaragoza E, Poon LC, Pepes S, Nicolaides KH. Maternal serum placental growth factor at 11 + 0 to 13 + 6 weeks of gestation in the prediction of pre-eclampsia. Ultrasound Obstet. Gynecol. 32, 732–739 (2008). [DOI] [PubMed] [Google Scholar]
- 44.Wright D, Silva M, Papadopoulos S, Wright A, Nicolaides KH. Serum pregnancy associated plasma protein-A in the three trimesters of pregnancy: effects of maternal characteristics and medical history. Ultrasound Obstet. Gynecol. 46(1), 42–50 (2015). [DOI] [PubMed] [Google Scholar]
- 45.Tsiakkas A, Duvdevani N, Wright A, Wright D, Nicolaides KH. Serum placental growth factor in the three trimesters of pregnancy: effects of maternal characteristics and medical history. Ultrasound Obstet. Gynecol. 45(5), 591–598 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Akolekar R, Syngelaki A, Sarquis R, Zvanca M, Nicolaides KH. Prediction of early, intermediate and late pre-eclampsia from maternal factors, biophysical and biochemical markers at 11–13 weeks. Prenat. Diagn. 31, 66–74 (2011). [DOI] [PubMed] [Google Scholar]
- 47.Scazzocchio E, Figueras F, Crispi F, et al. Performance of a first-trimester screening of preeclampsia in a routine care low-risk setting. Am. J. Obstet. Gynecol. 208, 203.e1–203.e10 (2013). [DOI] [PubMed] [Google Scholar]
- 48.Lo YM, Leung TN, Tein MS, Sargent IL, Zhang J, Lau TK, Haines CJ, Redman CW. Quantitative abnormalities of fetal DNA in maternal serum in preeclampsia. Clin. Chem. 45, 184–188 (1999). [PubMed] [Google Scholar]
- 49.Smid M, Vassallo A, Lagona F, et al. Quantitative analysis of fetal DNA in maternal plasma in pathological conditions associated with placental abnormalities. Ann. NY Acad. Sci. 945, 132–137 (2001). [DOI] [PubMed] [Google Scholar]
- 50.Zhong XY, Laivuori H, Livingston JC, et al. Elevation of both maternal and fetal extracellular circulating deoxyribonucleic acid concentrations in the plasma of pregnant women with preeclampsia. Am. J. Obstet. Gynecol. 184, 414–419 (2001). [DOI] [PubMed] [Google Scholar]
- 51.Farina A, Sekizawa A, Rizzo N, et al. Cell-free fetal DNA (SRY locus) concentration in maternal plasma is directly correlated to the time elapsed from the onset of preeclampsia to the collection of blood. Prenat. Diagn. 24, 293–297 (2004). [DOI] [PubMed] [Google Scholar]
- 52.Alberry MS, Maddocks DG, Hadi MA, et al. Quantification of cell free fetal DNA in maternal plasma in normal pregnancies and in pregnancies with placental dysfunction. Am. J. Obstet. Gynecol. 200, 98.e1–98.e6 (2009). [DOI] [PubMed] [Google Scholar]
- 53.Miranda ML, Macher HC, Munoz-Hernandez R, et al. Role of circulating cell-free DNA levels in patients with severe preeclampsia and HELLP syndrome. Am. J. Hypertens. 26, 1377–1380 (2013). [DOI] [PubMed] [Google Scholar]
- 54.Zeybek YG, Gunel T, Benian A, Aydinli K, Kaleli S. Clinical evaluations of cell-free fetal DNA quantities in pre-eclamptic pregnancies. J. Obstet. Gynaecol. Res. 39, 632–640 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Lau TW, Leung TN, Chan LY, et al. Fetal DNA clearance from maternal plasma is impaired in preeclampsia. Clin. Chem. 48, 2141–2146 (2002). [PubMed] [Google Scholar]
- 56.Martin A, Krishna I, Martina B, Samuel A. Can the quantity of cell-free fetal DNA predict preeclampsia: a systematic review. Prenat. Diagn. 34, 685–691 (2014). [DOI] [PubMed] [Google Scholar]
- 57.Rolnik DL, O'Gorman N, Fiolna M, van den Boom D, Nicolaides KH, Poon LC. Maternal plasma cell-free DNA in the prediction of pre-eclampsia. Ultrasound Obstet. Gynecol. 45(1), 106–11 (2015). [DOI] [PubMed] [Google Scholar]
- 58.Nicolaides KH. Turning the pyramid of prenatal care. Fetal Diagn. Ther. 29, 183–196 (2011). [DOI] [PubMed] [Google Scholar]
- •.This reviews illustrates how many of the common pathologies encountered in pregnancy can be screened for in the first trimester. Consequently, antenatal care can be tailored accordingly and interventions initiated as appropriate.
- 59.Bahado-Singh RO, Syngelaki A, Akolekar R, et al. Validation of metabolomic models for prediction of early-onset preeclampsia. Am. J. Obstet. Gynecol. 213(4), 530.e1–530. e1 (2015). [DOI] [PubMed] [Google Scholar]