We observed substantially improved glycemic control following addition of real-time continuous glucose monitoring (CGM) combined with structured education during run-in period in a study investigating prolonged use of day and night closed-loop.1 This was an open-label, 3-center, multinational randomized 2-period crossover study comparing automated closed-loop glucose control with sensor-augmented insulin pump therapy (referred to as control period hereinafter) for 12 weeks. Participants were adults with type 1 diabetes treated with continuous subcutaneous insulin infusion (CSII) for at least 6 months with glycated hemoglobin (HbA1c) between 58 mmol/mol (7.5%) and 86 mmol/mol (10%). The detailed study protocol has been published.2
All participants underwent a 4 to 6 weeks optimization period prior to randomization. Weekly study visits conducted by professional pump educators followed an agreed written curriculum, and data from the study pump (Dana R Diabecare, Sooil, Seoul, South Korea) and CGM device (FreeStyle Navigator II, Abbott Diabetes Care, Alameda, CA, USA) were downloaded and analyzed. Formal testing was undertaken to assess the adequacy of basal insulin delivery and bolus calculator settings (insulin-to-carbohydrate ratio and sensitivity factor).
Thirty-three adults (15 females, age 40.0 ± 9.4 years, BMI 25.5 ± 4.4 kg/m2, duration of diabetes 20.9 ± 9.3 years, and duration of pump therapy 7.8 ± 5.9 years) completed the optimization period. Baseline mean HbA1c at enrolment was 69 ± 7 mmol/mol (8.5 ± 0.7%). At the end of the 4 to 6 week optimization period mean HbA1c improved to 60 ± 9 mmol/mol (7.6 ± 0.8%) (see Figure 1) (paired difference, –9 mmol/mol [95% CI 6.8 to 11.2] or −0.8% [95% CI 0.6 to 1.0], P < .001). Mean sensor glucose (161 ± 27.6 mg/dl) and time below 50 mg/dl (0.4 [0.2 to 0.9]) during optimization were comparable to the control period. No severe hypoglycemia occurred during optimization. Total daily insulin dose was increased by a mean of 3 units (95% CI 0.3 to 5.8, P = .029). These improvements were maintained during the 3-month control period (HbA1c at end of control period 59 ± 12 mmol/mol [7.5 ± 1.1%], paired difference before and after the control period, 0 mmol/mol [95% CI −2 to 2] or 0.0% [95% CI −0.2 to 0.2], P = .79). Both groups of participants, that is, those who did the control period first (n = 15) and second after the closed-loop period (n = 18), had significantly lower HbA1c at the end of control period compared to baseline (P < .001 and P = .011 respectively).
Figure 1.
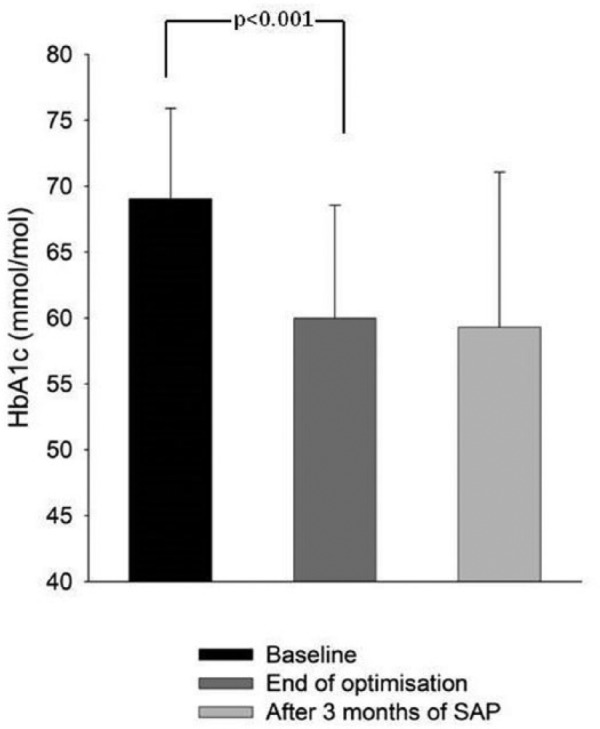
Mean HbA1c(SD) at baseline, after optimization and post 3 months of sensor augmented pump therapy.
HbA1c improvements during optimization was negatively related to weight (r =−0.35, P = .044) and positively related to duration of pump therapy (r = .5, P = .002). However improvements were not related to baseline HbA1c (P = .43), age (P = .48), BMI (P = .40), duration of diabetes (P = .50), or total daily dose of insulin (P = .95).
Our result highlights the incremental value of real-time CGM combined with structured education in improving glycemic control in CSII patients with suboptimally controlled type 1 diabetes (HbA1c ≥ 7.5%). We observed impressive reductions of HbA1c levels, with very low time spent in biochemical hypoglycemia with no severe hypoglycemia in a relatively short period of time, not associated with baseline HbA1c levels. Experienced pump users benefitted the most. Our data adds to existing evidence showing benefit of real-time CGM in improving glucose control in those already using CSII.3,4 We acknowledge that attention bias related to study participation may have contributed to these improvements. Further investigations are warranted.
Acknowledgments
We are grateful to study volunteers for their participation. We acknowledge support by the staff at the Addenbrooke’s Wellcome Trust Clinical Research Facility. Josephine Hayes (University of Cambridge) provided administrative support. Karen Whitehead (University of Cambridge) provided laboratory support. We acknowledge support by the staff at Profil Institut; Krisztina Schmitz-Grozs provided support as a research physician, Martina Haase supported the study as an insulin pump expert, and Maren Luebkert, Kirstin Kuschma, and Elke Przetak provided administrative, coordinating, and documentation support. Barbara Semlitsch and Markus Schauer (both from Medical University of Graz) supported the study as insulin pump experts. LL and HT contributed equally to this article.
Footnotes
Abbreviations: CGM, continuous glucose monitoring; CI, confidence interval; CSII, continuous subcutaneous insulin infusion.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: RH reports having received speaker honoraria from Minimed Medtronic, Eli Lilly, BBraun, and Novo Nordisk, serving on advisory panel for Eli Lilly, Novo Nordisk and Merck, receiving license fees from BBraun and Medtronic; and having served as a consultant to BBraun and Profil. MEW has received license fees from Becton Dickinson and has served as a consultant to Beckton Dickinson. MLE reports having received speaker honoraria from Abbott Diabetes Care, Novo Nordisk and Animas, serving on advisory panels for Novo Nordisk, Abbott Diabetes Care, Medtronic, Roche and Cellnovo and holding stock options in Cellnovo. SH serves as a consultant for Novo-Nordisk and for the ONSET group, and reports having received speaker/training honoraria from Medtronic. RH and MEW report patents and patent applications. JKM reports having received speaker honoraria from AstraZeneca, NintaMed, NovoNordisk, Roche Diabetes Care, Sanofi, Takeda, and serving on advisory panel for MSD, Sanofi, and Boehringer Ingelheim. TRP is an advisory board member of Novo Nordisk A/S, a consultant for Roche, Novo Nordisk A/S, Eli Lilly & Co, Infineon, Carnegie Bank, and on speaker’s bureau of Novo Nordisk A/S and Astra Zeneca. HT, LL, SD, CB, MH, HK, and SA declare no competing financial interests exist.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Seventh Framework Programme of the European Union (ICT FP7- 247138). Additional support for the Artificial Pancreas work by Juvenile Diabetes Research Foundation, National Institute for Health Research Cambridge Biomedical Research Centre and Wellcome Strategic Award (100574/Z/12/Z). Abbott Diabetes Care supplied discounted continuous glucose monitoring devices, sensors, and communication protocol to facilitate real-time connectivity.
References
- 1. Thabit H, Tauschmann M, Allen JM, et al. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med. 2015;373:2129-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leelarathna L, Dellweg S, Mader JK, et al. Assessing the effectiveness of 3 months day and night home closed-loop insulin delivery in adults with suboptimally controlled type 1 diabetes: a randomised crossover study protocol. BMJ Open. 2014;4:e006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tamborlane WV, Beck RW, Bode BW, et al. Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med. 2008;359:1464-1476. [DOI] [PubMed] [Google Scholar]
- 4. Battelino T, Conget I, Olsen B, et al. The use and efficacy of continuous glucose monitoring in type 1 diabetes treated with insulin pump therapy: a randomised controlled trial. Diabetologia. 2012;55:3155-3162. [DOI] [PMC free article] [PubMed] [Google Scholar]


