Abstract
Advances in insulin treatment options over recent decades have markedly improved the management of diabetes. Despite this, glycemic control remains suboptimal in many people with diabetes. Although postprandial glucose control has been improved with the development of subcutaneously injected rapid-acting insulin analogs, currently available insulins are not able to fully mimic the physiological time–action profile of endogenously secreted insulin after a meal. The delayed onset of metabolic action and prolonged period of effect induce the risk of postprandial hyperglycemia and late postprandial hypoglycemia. A number of alternative routes of insulin administration have been investigated over time in an attempt to overcome the limitations associated with subcutaneous administration and to provide an improved time–action insulin profile more closely simulating physiological prandial insulin release. Among these, pulmonary insulin delivery has shown the most promise. Technosphere® Inhaled Insulin (TI) is a rapid-acting inhaled human insulin recently approved by the FDA for prandial insulin therapy. In this article we discuss the pharmacokinetic and pharmacodynamic properties of TI, and, based on key studies performed during its clinical development, the implications for improved postprandial glucose control.
Keywords: inhaled insulins, insulin therapy, prandial insulin, pharmacokinetics, pharmacodynamics
The goal of prandial insulin therapy is to mimic the physiological metabolic effect induced by endogenously secreted insulin to limit postprandial glycemic excursions after a meal. Basal insulin requirements are covered by injection of long-acting insulin. The time–action profiles of modern long-acting insulin analogs are flatter and more reproducible compared with neutral protamine hagedorn (NPH) insulin, resulting in a lower risk of hypoglycemia.1-3 The development of rapid-acting insulin analogs (RAAs) with pharmacokinetic (PK)/pharmacodynamic (PD) properties that are improved compared with regular human insulin formulations has helped patients to achieve better prandial glucose control after subcutaneous injection.4 However, the slow absorption of insulin from the subcutaneous tissue remains a limiting factor, since the absorption rates of RAAs are not rapid enough to match physiological needs. These pharmacological disadvantages put people with diabetes at risk of postprandial hyperglycemia and late postprandial hypoglycemia.5-7 There is a need for an ultra-rapid-acting insulin (URAI) with a more rapid onset of action and a shorter duration of effect. A number of different URAIs are in clinical development.8 Most efforts focus on subcutaneous insulin administration, but alternative routes of administration have been investigated, including dosing insulin via the oral/buccal,9-11 nasal,12 and pulmonary routes. Of these, pulmonary delivery of insulin has shown the greatest promise, with a product delivered via this route already having reached the market.
The lungs have a number of characteristics which are advantageous to drug delivery. The approximately 480 million alveoli in the lungs13 provide a large, highly perfused surface area, with a thin alveolar epithelium which allows for rapid absorption of substances into the systemic circulation.14 An additional advantage of delivery through the lung is that “first pass” metabolism (hepatic degradation) is avoided.14,15
A number of attempts have been made to develop an inhaled insulin delivery system, including systems in which insulin was delivered as a dry powder (Exubera, AIR, and Technosphere®) or a liquid formulation (AERx® iDMS).16 Of these, Exubera (an inhaled insulin) was the first to be approved by the FDA (year of approval: 2006) for insulin therapy in patients with type 1 and type 2 diabetes, but its PK/PD profile was not markedly different from subcutaneously injected RAAs: onset of action was similar to insulin lispro.17 Pfizer withdrew Exubera from the market in 2007 due to poor sales. The reasons for the failure of Exubera were likely related to a number of factors, including the large size of the inhaler and the cumbersome handling procedure required for administration.6,18 Subsequently, development programs for inhaled insulin with similar PK/PD profiles were discontinued, while the development of Technosphere Inhaled Insulin (TI) continued. TI has a unique PK/PD profile, with a more rapid onset and shorter duration of action compared with subcutaneously injected RAAs and earlier inhaled insulins.19
Recently, a smaller, thumb-sized TI inhaler system (the Gen2 device) was approved by the FDA as a drug–device combination system (marketed as Afrezza® [insulin human] Inhalation Powder) for prandial insulin therapy in patients with diabetes.
Technosphere Inhaled Insulin
TI is an inhalation powder composed of recombinant human insulin adsorbed onto Technosphere microparticles formed by the inert excipient fumaryl diketopiperazine (FDKP). FDKP is highly soluble in water at neutral or basic pH. In mildly acidic conditions, FDKP undergoes intramolecular self-assembly and crystallizes into microparticles with a median diameter of around 2.0-2.5 μm.20,21 These particles are within the optimal size range for delivery to the deep lung; larger particles tend to be deposited in the mouth, throat, or upper airways, and smaller particles may be exhaled.14,15 The low-bulk density and regular particle size contribute to aerodynamic properties that facilitate the delivery of TI to the deep lung. Once in the deep lung, the particles rapidly dissolve in the neutral or basic physiological pH of the alveoli, allowing rapid absorption of insulin and FDKP into the systemic circulation; FDKP is biologically inactive and excreted unchanged in the urine.22
The inhaler is a key part of the TI drug–device combination system, and is essential to achieve consistent, reproducible insulin delivery. The TI inhalation system has been improved over the course of its clinical development to optimize performance and ease of use. Originally, TI was administered using the MedTone inhaler. The current Gen2 inhaler is a result of further development of this system, and consists of purpose-built, plastic, injection-molded components assembled with an ultrasonic weld. It is smaller and more discreet than the MedTone inhaler, and easier to use, requiring fewer steps prior to inhalation (4 compared with 8 for the MedTone inhaler). The Gen2 inhaler is more efficient (less powder per dose) and requires only 1 inhalation per cartridge (compared with 2 for the MedTone inhaler). The device is low maintenance (discarded and replaced every 15 days) and requires no cleaning.
Three cartridges containing different amounts of insulin are approved for the Gen2 inhaler, labeled as approximating 4, 8, and 12 units (U) of subcutaneously administered insulin.23 The TI cartridge label is based on the transition (in clinical trials) of a patient on subcutaneous insulin to TI administered via the Gen2 inhaler; for every 4 U of subcutaneous insulin or part thereof, a patient was transferred to a dose containing 10 U of TI. Thus a cartridge with a fill content of 10 U of TI is now labeled 4 U. The subcutaneous insulin equivalents were instituted as a guide for switching patients from subcutaneous insulin to TI, or from TI to subcutaneous insulin. The first generation device, the MedTone inhaler, was less efficient, and required a cartridge fill content of 15 U to deliver the same amount of insulin to the bloodstream. With the MedTone inhaler the cartridge fill was 15/ 30 units RHI, with a nominal dose of 4 / 8 units, with the Gen2 this was 10 / 20 / 30 units RHI and 4 / 8 / 12 units. To deliver the labeled amount of TI in the cartridges to the patient, the inhaler is opened and the cartridge inserted. The inhalation effort lifts, deagglomerates, and disperses TI to the pulmonary tract. For doses above 12 U, inhalations from multiple cartridges are necessary.
Pharmacokinetic Properties of TI
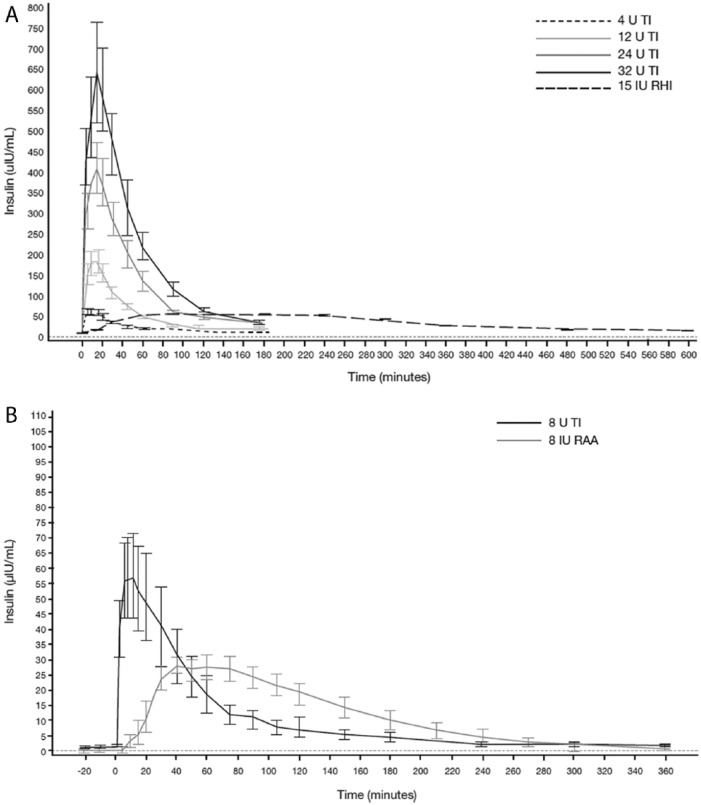
Two phase 1 studies have assessed the PK of TI administered using the Gen2 inhaler. The first was an open-label, randomized, 4-way crossover study in 32 healthy subjects to evaluate dose proportionality and linearity, relative bioavailability, and PD response of TI administered using the Gen2 inhaler compared with 15 U of subcutaneously administered regular human insulin (RHI; Study 176).24 The C-peptide corrected serum insulin concentration–time profiles after administration of 4, 12, 24, and 32 U of TI are shown in Figure 1A25,26 Across the 4 dose levels, the insulin kinetic profiles were similar, demonstrating rapid absorption, similar times to maximal insulin concentration (at approximately 15 minutes postdosing), and nearly complete return to predose (baseline) concentrations by 180 minutes (Figure 1A; Table 1).25,26 The median terminal half-life (t½) ranged from 28.2 to 38.8 minutes, compared to 144.6 minutes for RHI (Study 176; Data on file, MannKind Corporation, Danbury, CT, USA). C-peptide corrected insulin exposure over 3 hours postdosing (area under the serum concentration–time curve from time 0-180 minutes postdosing [AUC0-180]) showed a dose-proportional increase with TI dose (proportionality slope 1.00 [90% confidence interval 0.939-1.061]) from 4 to 32 U (Table 1).24 The second phase 1 study (Study 177) compared inhalation of 8 U of TI with subcutaneous injection of 8 U of insulin lispro in 12 patients with type 1 diabetes. Absorption of TI was again rapid following inhalation, with a median time of maximum plasma concentration (tmax) of 8 minutes compared with approximately 50 minutes for insulin lispro. Return to predose levels was observed at approximately 180-240 minutes for TI compared with approximately 280 minutes for insulin lispro (Figure 1B; Table 1). Peak serum concentration (Cmax) for TI was 51 μU/mL, compared with 34 μU/mL for insulin lispro.23,26
Figure 1.
Pharmacokinetics of TI administered using the Gen2 inhaler: mean (SE) C-peptide-corrected serum insulin concentration-time profiles in healthy subjects versus regular human insulin (Study 176) (A) and mean (SE) baseline-corrected serum insulin concentration over time profiles in subjects with type 1 diabetes, versus insulin lispro (Study 177) (B). IU, international units; SE, standard error.
Table 1.
Overview of Pharmacokinetic and Pharmacodynamics Parameters for TI Compared with Subcutaneous Insulin.
| Study | TI | Subcutaneous Insulin | Parameter |
|---|---|---|---|
| Study 176 (healthy subjects) | 4, 12, 24, and 32 U TI Gen2 inhaler |
15 U RHI | • tmax ~15 minutes postdosing and complete return to baseline by 180 minutes for TI • Dose-proportional increase in C-peptide corrected insulin exposure with TI dose from 4 to 32 U • Median terminal t½ ranged from 28.2-38.8 minutes compared to 144.6 minutes for RHI • Relative TI bioavailability of ~24% (range 20-27%) • FDKP tmax ~8-9 minutes across all dose levels of TI • Increases in GIR AUC0-240 and GIRmax were observed with each increasing dose of TI but were nonlinear, despite dose-proportionality seen in PK analyses |
| Study 177 (type 1 diabetes) | 8 U TI Gen2 inhaler |
8 U insulin lispro | • tmax ~8 minutes for TI compared with ~50 minutes for insulin lispro • Return to predose level by 180-240 minutes for TI compared with ~280 minutes for insulin lispro • Cmax for TI was 51 μU/mL, compared with 34 μU/mL for insulin lispro • Relative TI bioavailability of 33% • Maximum effect on GIR at 53 minutes with TI compared with 108 minutes for insulin lispro |
| Study 142 (healthy subjects) | 8 U TI MedTone inhaler versus Gen2 inhaler |
— | • AUC0-120 was 4,294 min·µU/mL for Gen2 inhaler versus 4,060 min·µU/mL for MedTone inhaler (ratio 1.060, 90% confidence interval 0.981-1.145) • Cmax was 105 µU/mL for Gen2 inhaler versus 97 µU/mL for MedTone inhaler (ratio 1.082, 90% CI 0.992 to 1.180) |
| Cassidy 2009 (type 1 diabetes) | Two 4 U cartridges of TI and one 8 U cartridge of TI MedTone inhaler |
10 U insulin lispro | • tmax 10 minutes for TI compared with 60 minutes for insulin lispro |
| Study 116 (type 1 diabetes) | 8 U TI MedTone inhaler |
10 U insulin lispro | • GIR reached a maximum by ~30 minutes after administration for TI compared with ~150 minutes for insulin lispro • GIR for TI returned to baseline by approximately 180 minutes versus 300 minutes for insulin lispro • Total dose-normalized glucose-lowering effect (GIR AUC0-360) of TI was approximately 20% that of insulin lispro |
| Rave 2009 (healthy subjects) | 25-100 U TI MedTone inhaler |
— | • Exposure linearly related to dose, effect expressed as GIR AUC0-360 not linear |
Initial PK/PD studies for TI were performed using the MedTone inhaler. Cmax was approximately 78 μU/mL and 49 μU/mL for one 12 U cartridge of TI compared to 10 U of subcutaneous RAA, and occurred earlier with a tmax of 10 and 60 minutes, respectively (Study 116; Data on file, MannKind Corporation). Following the development of the Gen2 inhaler, a phase 1 open-label, randomized, crossover “bridging” study in 46 healthy subjects was conducted to demonstrate equivalence for 8 U administered using the Gen2 inhaler and 8 U administered using the MedTone inhaler (Study 142; Data on file, MannKind Corporation). Geometric mean values for AUC0-120 were 4,294 min·µU/mL for the Gen2 inhaler versus 4,060 min·µU/mL for the MedTone inhaler (ratio 1.060, 90% confidence interval 0.981-1.145). Geometric mean values for Cmax were 105 µU/mL for the Gen2 inhaler versus 97 µU/mL for the MedTone inhaler (ratio 1.082, 90% CI 0.992-1.180) (Table 1). All serum insulin concentration values were C-peptide-corrected.
As seen in the equivalence study, the PK profile of TI administered with the Gen2 is similar to that seen in earlier studies using the MedTone inhaler.27-29
Once inhaled, TI disappears rapidly from the lung. In a serial assessment using bronchoscopy with bronchoalveolar lavage, following inhalation of TI, the concentration of insulin in the lung fell rapidly and was below limits of quantification (2 μU/mL) by 12 hours, with similarly rapid clearance of FDKP. The estimated clearance half-life from the lung of both insulin and FDKP is around 1 hour, suggesting the potential for accumulation on chronic administration of TI is minimal.30
Bioavailability
In the 2 phase 1 trials using the Gen2 inhaler, bioavailability compared to subcutaneous insulin was calculated. Note that in bioavailability calculations, the actual insulin content of the cartridges is used. In 1 study, conducted in healthy subjects, the median bioavailability of TI, based on AUC0-inf of C-peptide corrected serum insulin concentration data was approximately 24% relative to 15 U of subcutaneous RHI, with a range of 20-27% (Study 176; Data on file MannKind Corporation).24 In the other study (in patients with type 1 diabetes), the relative bioavailability against 8 U of subcutaneous insulin lispro was 33% (90% CI 0.23-0.49) based on AUC0-360 of baseline-corrected serum insulin concentration profiles (Study 177; Data on file, MannKind Corporation; Table 1).
Biopotency
The cartridge label for TI provides a basis for transitioning a patient from subcutaneous prandial insulin to TI: the low-, middle-, and high-dose Gen2 cartridges containing 10 U, 20 U, and 30 U of insulin are used to replace subcutaneous insulin doses of 4 U, 8 U, and 12 U, respectively. As discussed above, the cartridges are therefore labeled as 4 U, 8 U, and 12 U. The metabolic effect of a cartridge tends to be less than its nominal dose, so the labeling reflects a conservative approach that should reduce the risk of hypoglycemia during the transition to TI. The starting dose for a patient beginning therapy with TI would, therefore, tend to be somewhat less effective than the subcutaneous insulin dose, so an up-titration is expected. Indeed, patients with type 1 diabetes in a phase 3 study increased their TI doses to achieve glycemic control similar to that observed in patients treated with subcutaneous insulin aspart.31 The mean daily prandial insulin aspart dose increased by 9.1% from randomization to Week 12, and TI dose increased by 43%.31 In the same study, a small increase in basal insulin requirement was also seen, as would be expected when transitioning to an insulin with a shorter duration of action.32,33
Pharmacokinetics and Toxicology of FDKP
FDKP absorbed into the systemic circulation is not metabolized, and is eliminated via the renal route.34 Around 20% of FDKP is deposited in the throat and subsequently swallowed after TI inhalation.30 FDKP has no biological activity. In vitro, FDKP does not facilitate drug absorption, but functions solely as the particle matrix to carry the insulin to the lung. In vitro studies show no evidence that FDKP is cytotoxic to human lung cells, with no indication of any effect on airway epithelial tight junction integrity, cell viability, or cell permeability.20
Concentration changes of FDKP following inhalation of TI have been shown to follow a similar pattern to that of insulin (Table 1). In a dose-response study the mean tmax for FDKP was approximately 8-9 minutes across all dose levels (4, 8, 24, and 32 U of TI) (Study 176; Data on file, MannKind Corporation). The mean t1/2 ranged between 120 and 190 minutes, was dose independent, and was characterized by a 1-compartment model. The total exposure (AUC0-inf) demonstrated direct proportionality to the administered dose of TI.
In a randomized, 4-period crossover, double-blind, double-dummy, placebo- and active-controlled cardiac safety study, 48 healthy subjects (26 men, 22 women) were dosed with 20 mg and 40 mg doses of FDKP, placebo, and oral 400 mg moxifloxacin (active control) in a randomized sequence (with a 3-day interdose washout period). Electrocardiograms were obtained for each treatment at 45, 30, and 15 minutes before predose, and at 5, 10, 15, 20, and 30 minutes and 1, 2, 3, 4, 8, 12, and 23 hours postdose. Therapeutic and supratherapeutic doses of TI had no effect on QTc interval, heart rate, atrioventricular conduction, cardiac depolarization (measured in terms of PR and QRS), or interval.35
Pharmacodynamic Properties of TI
TI exhibits a more rapid onset of action and shorter duration of metabolic effect than subcutaneously injected RAA. In a euglycemic glucose clamp study, the baseline-corrected glucose infusion rate (GIR) of 25 patients with type 1 diabetes receiving 8 U of TI (administered using the MedTone inhaler) rose earlier and declined sooner than the GIR following 10 U of subcutaneous insulin lispro (Figure 2).36 The GIR after 8 U of TI reached a maximum approximately 30 minutes after administration, whereas GIR peaked approximately 150 minutes after subcutaneous administration of insulin lispro. The time to 50% of maximal effect (T50% Cmax) in this study was 19 minutes for TI and 50 minutes for insulin lispro. At 120 minutes, TI had delivered 60% of the total glucose lowering effect (area under the GIR versus time curve [AUC GIR]), compared to 33% for insulin lispro (Figure 3). The GIR for TI returned to baseline at approximately 180 minutes versus 300 minutes for insulin lispro. (Study 116; Data on file, MannKind Corporation; Table 1). In a study using the Gen2 inhaler in 12 patients with type 1 diabetes, maximum effect on GIR occurred at a median 53 minutes with TI compared with 108 minutes for insulin lispro (Study 177; Data on File, MannKind Corporation; Table 1). There was considerable variability in GIR in this study, and the PK data presented in the label also originate from this study, the results of which diverge from the majority of other studies performed with TI, indicating that better data are required. An earlier study (described above) in 25 patients with type 1 diabetes also compared the PD of TI and insulin lispro, but was conducted with the original MedTone inhaler. Given that the PK characteristics of both TI and insulin lispro were similar between studies, it is likely that the larger study (Study 116; Figure 2) more accurately describes the action profile expected from TI in the type 1 diabetes population.
Figure 2.
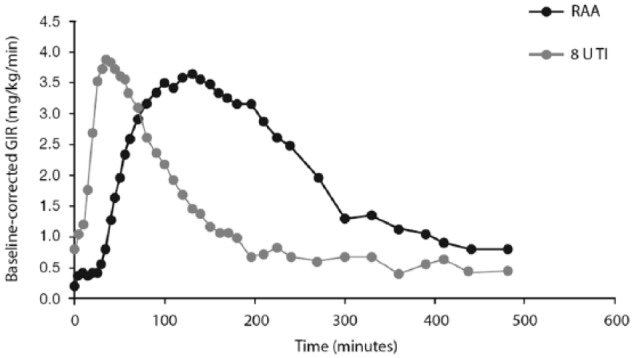
Pharmacodynamics of TI: mean baseline-corrected glucose infusion rate of TI versus RAA in type 1 diabetes (Study 116).
Figure 3.
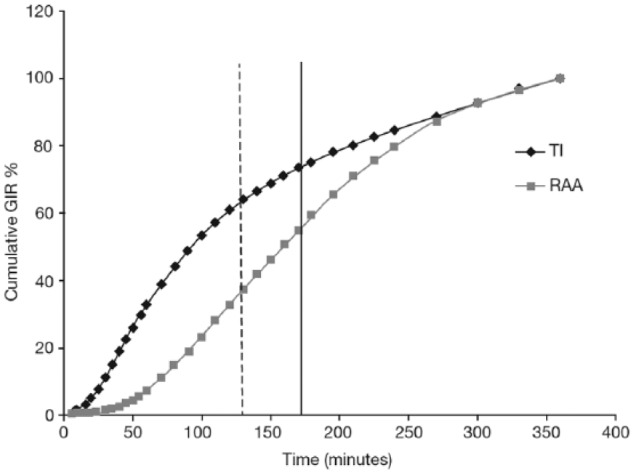
Cumulative effect on glucose infusion rate of 8 U of TI and 10 IU of insulin lispro.
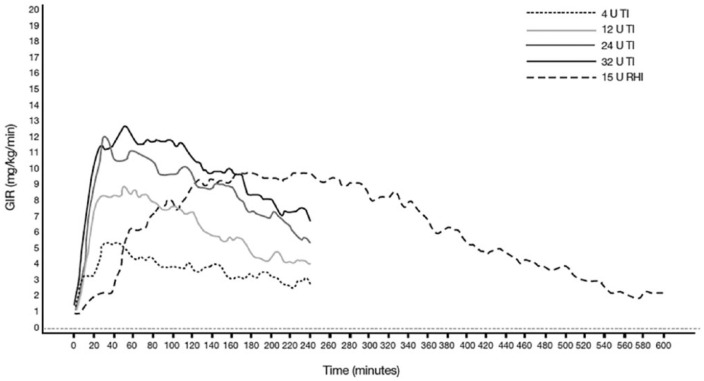
The dose–effect relationship has been studied for doses ranging from 4-32 U of TI in healthy subjects (Figure 4).24 In this study, the time to maximal effect (GIRmax) was 58-73 minutes for TI and 223 minutes for RHI. The time to 50% of maximal effect (T50% GIRmax) was 8-12 minutes for TI and 50 minutes for RHI. Increases in maximal effect (GIRmax) were observed with each increasing dose of TI; however, these increases were nonlinear, despite the dose-proportionality in terms of systemic insulin exposure seen in the PK analyses (Table 1). GIR was not back to baseline at the end of the clamp therefore, especially for larger doses, parts of the effect are not recorded. Increases in the total effect during the initial 4 hours (GIR AUC0-240) were also observed with each increasing dose of TI.
Figure 4.
Pharmacodynamics of TI: mean baseline-corrected glucose infusion rate of TI versus regular human insulin in healthy subjects (Study 176).
The effect of 12 U of TI on endogenous glucose production (EGP) compared with 12 U of subcutaneous insulin lispro and 4 mg of inhaled Exubera was investigated in an open label, single-dose, 3-way crossover study incorporating a standardized meal challenge in 18 insulin-treated patients with type 2 diabetes and normal pulmonary function.37 EGP suppression occurred markedly earlier with TI relative to subcutaneous insulin lispro and Exubera (a median of 40, 75, and 130 minutes postdose, respectively), with significant differences in median EGP up to 40 minutes postdose (P < .002) between insulin lispro compared with TI and up to 2 hours postdose for Exubera compared with TI (P < .05; Figure 5). Maximum EGP suppression was comparable across treatments in the postmeal challenge period (Figure 5).
Figure 5.
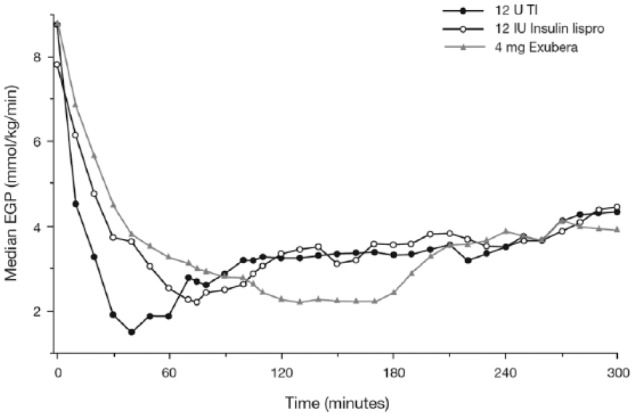
Median EGP versus time after a meal challenge in subjects with type 2 diabetes.37
Source: Republished with permission of the American Diabetes Association from Potocka et al;37 permission conveyed through Copyright Clearance Center, Inc. Figure legend represents dosing per current TI cartridge labeling.
Discussion
TI has a more rapid absorption and clearance profile compared with subcutaneously injected RAAs. As a consequence of this, TI has a faster onset of action and a shorter duration of action, partly due to a more rapid effect on the liver, resulting in a more rapid EGP suppression and coverage of prandial insulin needs (Table 1).24,27-29,37,38 These effects are independent of inhaler used for delivery or subject population. Though TI is labeled as rapid-acting insulin, its unique PK/PD profile supports previous characterization as an URAI.36 While the dose exposure relationship is linear, the dose–effect relationship on the maximal glucose infusion rate at very high doses is not. This is in keeping with the known dose–response characteristics of insulin in general, which are nonlinear. The maximal effect is achieved at insulin concentrations around 200-300 µU/mL and declines at higher insulin levels.39 Studies with subcutaneously administered insulin have also shown a nonproportional increase in effect with increasing insulin dose, particularly at higher doses.40 This phenomenon is thought to relate to a saturable-receptor-mediated biological response and clearance following insulin administration40 and may explain why TI has a somewhat lower biopotency (AUC GIR) than sc injected insulin relative to the total absorbed insulin (AUCinsulin).
The time point at which the maximal metabolic effect of the applied prandial insulin is achieved is crucial to achieve good control of postprandial glycemic excursions. Use of TI has been shown to reduce the early postprandial increase in glycemia when used in combination with the long-acting insulin analog insulin glargine (compared with twice-daily biaspart insulin in patients with type 2 diabetes41 and RAAs in patients with type 1 diabetes).36 Furthermore, addition of prandial TI to an automated closed-loop artificial pancreas system has been also shown to result in superior postprandial glycemic control without increased risk of hypoglycemic events.42 The relatively short duration of action of TI is assumed to be the reason for the reduced risk of hypoglycemia that has been observed in clinical studies, particularly in the late postprandial period.31,41,43 This is somewhat in contrast to what one would expect that can happen within the first hour of taking this very rapidly acting insulin, that is, an increased risk of early hypoglycemic events, due to the rapid absorption (this has been observed with other faster acting insulins like nasally administered insulin). In a phase 3 study conducted in patients with type 1 diabetes, 0-2-hour postprandial hypoglycemia event rates were comparable for TI and insulin aspart, but were 2- to 3- fold higher in the insulin aspart group in the period from >2-5 hours postmeal.31 Although subcutaneously administered RAA might still be too long-acting for optimal prandial coverage, the metabolic effect of TI may, at least in some cases, not be long-acting enough.36 In patients with type 2 diabetes treated with prandial TI added to insulin glargine, glucose excursions were significantly lower compared with twice-daily biaspart insulin for the first postprandial hour, but higher with TI after 2 hours.41
To handle this prandial insulin requirement adequately it is possible to apply a second dose of TI without inducing late hypoglycemia. This has been investigated in a 45-day single-arm treat-to-target pilot study in subjects with type 1 diabetes, where participants were instructed to take a second dose if blood glucose was ≥180 mg/dL 2 hours after meals.44 Over the course of the study the second dose was taken 38% of the time. Overall, treat-to-target with TI improved glucose control without increasing the time spent with blood glucose levels <60 mg/dL. In addition, patients with type 1 diabetes treated with TI in the phase 3 study using the Gen2 inhaler were instructed to take a supplemental dose (4 U of TI) if a 90-minute postmeal self-monitored blood glucose value was ≥180 mg/dL.31 A post hoc analysis of hypoglycemia event rates in patients taking ≥1 supplemental dose of TI or insulin aspart during this study showed that there was no significant difference in the mean number of supplemental doses taken by TI and insulin aspart-treated patients, and that hypoglycemia event rates were significantly higher in patients taking insulin aspart compared with TI (P <0.05).45
The distinct dose increments for TI (labeled as 4, 8, and 12 U) have not lead to increased hypoglycemic event rates in patients with either type 1 diabetes or type 2 diabetes;31,41 this is likely due to the shorter duration of action. The fact that the labeling was based on a relative transition dose when initiating TI and not the actual bioavailability (25-30%) has also led to a requirement to considerably up-titrate doses when patients transfer to TI, unit to unit, from subcutaneous insulin.
Clinical trials have also indicated that TI may have other potential benefits (which may be related to its unique PK/PD profile) in terms of weight change and fasting plasma glucose levels;28,43 however, further studies are required to determine the precise mechanisms underlying these clinical findings. Further studies with larger patient numbers should also evaluate whether demographic and baseline characteristics features (ethnicity, gender, BMI, etc) affect the PK and PD properties of TI. Discussion of the data obtained in a series of studies with TI in special populations (for example, those with asthma or chronic obstructive pulmonary disease) is also warranted, but falls outside of the scope of this article.
In summary, TI has a PK profile that is distinct from both subcutaneously injected RAAs and other inhaled insulins that were previously in development. The PD profile confers a faster onset and shorter duration of action that permits better synchronization of prandial insulin action with glucose absorption in the gut. In clinical practice the intrapatient and interpatient variability of PK and PD of a given prandial insulin (expressed as CV) is less than the variability in glucose absorption from the gut after meals with quite diverse carbohydrate content and preparation: for example, pizza versus a Japanese meal with carbohydrates all at end versus an extended Spanish lunch. The error made with carbohydrate estimation for dose finding comes on top of this. A rapid and short acting prandial insulin (a “precise tool”) is of help to cover the insulin requirement with all different types of meals to enable better (= reproducible) postprandial glycemic control, especially in the early phase. This leads also to a reduced risk of late postprandial hypoglycemia. An increase in TI dose relative to subcutaneous insulin is required, and it is feasible to introduce a supplementary postmeal dose if required—for example, in case of a heavy meal to manage extended postprandial hyperglycemia.
Acknowledgments
The contents of the article and the opinions expressed within are those of the authors, and it was the decision of the authors to submit the manuscript for publication. The authors took responsibility for the writing of this manuscript, including critical review and editing of each draft, and approval of the submitted version. The authors received writing/editorial support in the preparation of this manuscript provided by Katherine Roberts, PhD, of Excerpta Medica, funded by Sanofi US, Inc. The authors have not received any funding from Sanofi US, Inc for writing this manuscript. Afrezza and Technosphere are registered trademarks of MannKind Corporation.
Footnotes
Abbreviations: AUC, area under the serum concentration–time curve; Cmax, peak serum concentration; EGP, endogenous glucose production; FDKP, fumaryl diketopiperazine; GIR, glucose infusion rate; NPH, neutral protamine hagedorn; PD, pharmacodynamic; PK, pharmacokinetic; RAA, rapid-acting insulin analog; RHI, regular human insulin; t½, terminal half-life; T50%, time to 50% of maximal effect; TI, Technosphere Inhaled Insulin; tmax, maximum plasma concentration; U, units; URAI, ultra-rapid-acting insulin.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: LH receives, or has received, funding from companies manufacturing insulin or developing novel insulins including Biodel, Eli Lilly, Halozyme, Novo Nordisk, and Sanofi. RB is an employee of MannKind Corporation. AB is an employee of Sanofi US, Inc. MH is an employee and shareholder at Profil Institute for Clinical Research.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors received writing/editorial support in the preparation of this manuscript provided by Katherine Roberts, PhD, of Excerpta Medica, funded by Sanofi US, Inc. The authors have not received any funding from Sanofi US, Inc for writing this manuscript.
References
- 1. Heise T, Pieber TR. Towards peakless, reproducible and long-acting insulins. An assessment of the basal analogues based on isoglycaemic clamp studies. Diabetes Obes Metab. 2007;9:648-659. [DOI] [PubMed] [Google Scholar]
- 2. Owens DR, Matfin G, Monnier L. Basal insulin analogues in the management of diabetes mellitus: What progress have we made? Diabetes Metab Res Rev. 2014;30:104-119. [DOI] [PubMed] [Google Scholar]
- 3. Pettus J, Santos Cavaiola T, Tamborlane WV, Edelman S. The past, present, and future of basal insulins. Diabetes Metab Res Rev. 2016;32:478-96. [DOI] [PubMed] [Google Scholar]
- 4. Heinemann L, Heise T, Wahl CH, et al. Prandial glycaemia after a carbohydrate-rich meal in type I diabetic patients using the rapid acting insulin analogue [Lys(B28), Pro(B29)] human insulin. Diabet Med. 1996;3:625-629. [DOI] [PubMed] [Google Scholar]
- 5. Chen JW, Christiansen JS, Lauritzen T. Limitations to subcutaneous insulin administration in type 1 diabetes. Diabetes Obes Metab. 2003;5:223-233. [DOI] [PubMed] [Google Scholar]
- 6. Cavaiola T, Edelman S. Inhaled insulin: a breath of fresh air? A review of inhaled insulin. Clin Ther. 2014;36:1275-1289. [DOI] [PubMed] [Google Scholar]
- 7. Home PD. Plasma insulin profiles after subcutaneous injection: how close can we get to physiology in people with diabetes? Diabetes Obes Metab. 2015;17(11):1011-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heinemann L, Muchmore DB. Ultrafast-acting insulins: state of the art. J Diabetes Sci Technol. 2012;6:728-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bernstein G. Delivery of insulin to the buccal mucosa utilizing the RapidMist system. Expert Opin Drug Deliv. 2008;5:1047-1055. [DOI] [PubMed] [Google Scholar]
- 10. Pozzilli P, Raskin P, Parkin CG. Review of clinical trials: update on oral insulin spray formulation. Diabetes Obes Metab. 2010;12:91-96. [DOI] [PubMed] [Google Scholar]
- 11. Kapitza C, Zijlstra E, Heinemann L, Castelli MC, Riley G, Heise T. Oral insulin: a comparison with subcutaneous regular human insulin in patients with type 2 diabetes. Diabetes Care. 2010;33:1288-1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duan X, Mao S. New strategies to improve the intranasal absorption of insulin. Drug Discov Today. 2010;15:416-427. [DOI] [PubMed] [Google Scholar]
- 13. Ochs M, Nyengaard JR, Jung A, et al. The number of alveoli in the human lung. Am J Respir Crit Care Med. 2004;169:120-124. [DOI] [PubMed] [Google Scholar]
- 14. Patton JS, Byron PR. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov. 2007;6:67-74. [DOI] [PubMed] [Google Scholar]
- 15. Cefalu WT. Concept, strategies, and feasibility of noninvasive insulin delivery. Diabetes Care. 2004;27:239-246. [DOI] [PubMed] [Google Scholar]
- 16. Mastrandrea LD. Inhaled insulin: overview of a novel route of insulin administration. Vasc Health Risk Manag. 2010;6:47-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rave K, Bott S, Heinemann L, et al. Time-action profile of inhaled insulin in comparison with subcutaneously injected insulin lispro and regular human insulin. Diabetes Care. 2005;28:1077-1082. [DOI] [PubMed] [Google Scholar]
- 18. Bailey CJ, Barnett AH. Why is Exubera being withdrawn? BMJ. 2007;335:1156. [Google Scholar]
- 19. Heinemann L. Current status of the development of inhaled insulin. Br J Diabetes Vasc Dis. 2004; 4:295-301. [Google Scholar]
- 20. Angelo R, Rousseau K, Grant M, Leone-Bay A, Richardson P. Technosphere insulin: defining the role of Technosphere particles at the cellular level. J Diabetes Sci Technol. 2009;3:545-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Raskin P, Heller S, Honka M, et al. Pulmonary function over 2 years in diabetic patients treated with prandial inhaled Technosphere Insulin or usual antidiabetes treatment: a randomized trial. Diabetes Obes Metab. 2012;14:163-173. [DOI] [PubMed] [Google Scholar]
- 22. Richardson PC, Boss AH. Technosphere insulin technology. Diabetes Technol Ther. 2007;9(suppl 1):S65-S72. [DOI] [PubMed] [Google Scholar]
- 23. MannKind Corporation. Afrezza highlights of prescribing information. June 2014. Updated June 2016. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2014/022472lbl.pdf. Accessed January 29, 2016.
- 24. Baughman RA, Amin N, Watkins E, Chung P-C, Nezamis JP, Boss AH. A phase 1, open-label, randomized dose proportionality study of Technosphere Insulin Inhalation Powder (TI) doses up to 80 U administered with the Gen2 inhaler in healthy subjects. Diabetes. 2013;62(suppl 1):A251 abstract 982-P. [Google Scholar]
- 25. Baughman RA, Amin N, Watkins E, Chung PC, Nezamis JP, Boss AH. A phase 1, open-label, randomized dose proportionality study of Technosphere Insulin Inhalation Powder (TI) doses up to 80 U administered with the Gen2 inhaler in healthy subjects. Poster presented at: American Diabetes Association; Chicago, IL; June 21, 2013. [Google Scholar]
- 26. FDA Briefing Document. Endocrinologic and Metabolic Drug Advisory Committee. April 1, 2014. NDA 022472. AFREZZA (insulin human [rDNA origin]) inhalation powder: an ultra-rapid acting insulin treatment to improve glycemic control in adult patients with diabetes mellitus. Available at: http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/EndocrinologicandMetabolicDrugsAdvisoryCommittee/UCM390865.pdf. Accessed January 29, 2016.
- 27. Rave K, Heise T, Pfützner A, Boss AH. Coverage of postprandial blood glucose excursions with inhaled technosphere insulin in comparison to subcutaneously injected regular human insulin in subjects with type 2 diabetes. Diabetes Care. 2007;30:2307-2308. [DOI] [PubMed] [Google Scholar]
- 28. Rave K, Heise T, Heinemann L, Boss AH. Inhaled Technosphere insulin in comparison to subcutaneous regular human insulin: time action profile and variability in subjects with type 2 diabetes. J Diabetes Sci Technol. 2008;2:205-212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rave K, Potocka E, Heinemann L, et al. Pharmacokinetics and linear exposure of AFRESA compared with the subcutaneous injection of regular human insulin. Diabetes Obes Metab. 2009;11:715-720. [DOI] [PubMed] [Google Scholar]
- 30. Cassidy JP, Amin N, Marino M, et al. Insulin lung deposition and clearance following Technosphere insulin inhalation powder administration. Pharm Res. 2011;28:2157-2164. [DOI] [PubMed] [Google Scholar]
- 31. Bode BW, McGill JB, Lorber DL, et al. Inhaled Technosphere insulin compared with injected prandial insulin in type 1 diabetes: a randomized 24-week trial. Diabetes Care. 2015;38:2266-2273. [DOI] [PubMed] [Google Scholar]
- 32. Del Sindaco P, Ciofetta M, Lalli C, et al. Use of the short-acting insulin analogue lispro in intensive treatment of type 1 diabetes mellitus: importance of appropriate replacement of basal insulin and time-interval injection-meal. Diabet Med. 1998;15:592-600. [DOI] [PubMed] [Google Scholar]
- 33. Tamás G, Marre M, Astorga R, et al. Glycaemic control in type 1 diabetic patients using optimised insulin aspart or human insulin in a randomised multinational study. Diabetes Res Clin Pract. 2001;54:105-114. [DOI] [PubMed] [Google Scholar]
- 34. Potocka E, Cassidy JP, Haworth P, Heuman D, van Marle S, Baughman RA., Jr. Pharmacokinetic characterization of the novel pulmonary delivery excipient fumaryl diketopiperazine. J Diabetes Sci Technol. 2010;4:1164-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baughman RA, Haworth PC, Litwin JS, McDowell JA, Cassidy JP, Boss AH. No cardiac effects found with therapeutic and supratherapeutic doses of Technosphere inhalation powder: results from a thorough QTc clinical study. Diabetes. 2011;60(suppl 1):A255 abstract 933-P. [Google Scholar]
- 36. Boss AH, Petrucci R, Lorber D. Coverage of prandial insulin requirements by means of an ultra-rapid-acting inhaled insulin. J Diabetes Sci Technol. 2012;6:773-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Potocka E, Hovorka R, Baughman RA, et al. AFRESA Suppresses endogenous glucose production earlier than a rapid-acting analog (lispro) and inhaled exubera. Diabetes. 2009;58(suppl 1):abstract 232-OR. [Google Scholar]
- 38. Cassidy JP, Baughman RA, Schwartz SL, Haworth PC, Boss AH, Richardson PC. AFRESA (Technosphere Insulin) dosage strengths are interchangeable. Diabetes. 2009;58(suppl 1):abstract 425-P. [Google Scholar]
- 39. Rizza RA, Mandarino LJ, Gerich JE. Dose-response characteristics for effects of insulin on production and utilization of glucose in man. Am J Physiol. 1981;240:E630-E639. [DOI] [PubMed] [Google Scholar]
- 40. Becker RH, Frick AD. Clinical pharmacokinetics and pharmacodynamics of insulin glulisine. Clin Pharmacokinet. 2008;47:7-20. [DOI] [PubMed] [Google Scholar]
- 41. Rosenstock J, Lorber DL, Gnudi L, et al. Prandial inhaled insulin plus basal insulin glargine versus twice daily biaspart insulin for type 2 diabetes: a multicentre randomised trial. Lancet. 2010;375:2244-2253. [DOI] [PubMed] [Google Scholar]
- 42. Zisser H, Dassau E, Lee JJ, Harvey RA, Bevier W, Doyle FJ., III Clinical results of an automated artificial pancreas using technosphere inhaled insulin to mimic first-phase insulin secretion. J Diabetes Sci Technol. 2015;9:564-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kapsner P, Bergenstal R, Rendell M, et al. Comparative efficacy and safety of technosphere insulin and a rapid-acting analogue both given with glargine in subjects with type 1 diabetes mellitus in a 52-week study. Diabetologia. 2009;52(suppl 1):S386. [Google Scholar]
- 44. Garg SK, Kelly WC, Freson BJ, Petrucci RE, Ritchie PJ. Treat-to-target Technosphere insulin in patients with type 1 diabetes. Diabetes. 2011;60(suppl 1):A257 (941-P). [Google Scholar]
- 45. Blonde L, Gill J, Nikonova E, Stewart J, Bode B. Reduced hypoglycemia is observed with inhaled insulin versus subcutaneous insulin aspart in type 1 diabetes mellitus. Late breaking abstract #1220 presented at the American Association of Clinical Endocrinologists 24th Annual Meeting & Clinical Congress, 2015. Available at: http://am2015.aace.com/sites/all/files/Late-Breaking.pdf. Accessed January 2016.