Abstract
Background:
Historically, intensive insulin therapy for type 1 diabetes (T1D) has improved glycemic control at the risk of adverse weight gain. The impact of continuous subcutaneous insulin infusion therapy (CSII) on weight in the current era remains unknown. We assessed changes in hemoglobin A1c (HbA1c) and weight in adults with T1D transitioning to CSII at 2 diabetes centers in Denmark and the United States.
Methods:
Patients with T1D, aged ≥18 years, managed with multiple daily injections (MDI) who transitioned to CSII between 2002 and 2013 were identified using electronic health record data from the Steno Diabetes Center (n = 600) and Joslin Diabetes Center (n = 658). Changes in HbA1c and weight after 1 year was assessed overall and by baseline HbA1c cut points. Multivariate regression assessed correlates of HbA1c reduction.
Results:
In adults with T1D transitioning to CSII, clinically significant HbA1c reductions were found in patients with baseline HbA1c 8.0-8.9% (Steno, –0.7%; Joslin, –0.4%) and baseline HbA1c ≥9.0% (Steno, –1.1%; Joslin, –0.9%) (P < .005 for all). Overall, there was no significant change in weight after 1 year at either center. Modest (<2%) weight gain was noted in patients with baseline HbA1c ≥9% at Steno (1.1 ± 0.3 kg, P < .0001) and Joslin (1.7 ± 1.1, P < .005). In multivariate models, HbA1c reduction was associated with higher HbA1c, older age, female sex at Steno (R2 = .28, P < .005), but only higher baseline HbA1c at Joslin (R2 = .19, P < .005).
Conclusion:
Adults with T1D with suboptimal glycemic control significantly improved HbA1c without a negative impact on weight 1 year after transitioning from MDI to CSII.
Keywords: continuous subcutaneous insulin infusion, HbA1c, insulin pumps, type 1 diabetes
Poor metabolic control in individuals with type 1 diabetes (T1D) is associated with increased risk for diabetes complications.1,2 Intensive insulin therapy has been associated with lower HbA1c, but also with greater risks of hypoglycemia and adverse weight gain.1 Continuous subcutaneous insulin infusion (CSII), or insulin pump, therapy has been associated with a reduced number of severe hypoglycemic events as well as reduced glycemic variability.3,4 Meta-analyses of randomized controlled trials show that CSII lowers HbA1c by 0.3% compared to multiple daily injections (MDI) in adults.3 As for any glucose-lowering treatment, the relative benefit of CSII over MDI increases with higher baseline HbA1c.5,6 Meanwhile, the effect of CSII treatment on body weight is controversial. To the best of our knowledge no multinational comparisons of health outcomes following pump initiation in adults with T1D has been presented. Steno Diabetes Center and Joslin Diabetes Center are 2 large specialized diabetes clinics situated in the Denmark and the United States, respectively. Steno and Joslin take care of about 5800 and 25 000 adult patients with diabetes on an ongoing basis, respectively. Electronic health records, available at both centers, provide the opportunity to assess outcomes associated with insulin pump use across large cohorts.
Our objective was to describe changes in HbA1c and weight after 1 year in adults with T1D transferring from MDI to CSII. Furthermore, we evaluated changes in HbA1c and weight according to baseline glycemic control and to assess patient-related parameters associated with change in HbA1c after 1 year.
Methods
Study Population
Eligible persons were aged 18 years or older and had type 1 diabetes managed using MDI therapy receiving care at the Steno Diabetes Center (Gentofte, Denmark) or the Adult Diabetes Section at the Joslin Diabetes Center (Boston, MA, USA). The Steno cohort initiated CSII between January 2002 and December 2013. The Joslin cohort transitioned to CSII between November 2002 and November 2012; patients who transitioned in Joslin’s Pediatric, Adolescent, and Young Adult Section were excluded.
From 2002 to 2005, patients at Steno were admitted to the ward and insulin pump therapy was started on an individual basis. After 2005, carbohydrate counting is optimized before CSII initiation. Pump treatment is initiated in a group setting during a 4-day course. Following the pump course, the patient is seen in consultations with a diabetes nurse, an endocrinologist and a dietitian after 1 and 2 months before resuming regular consultations with the diabetes team. At Joslin, the patient’s clinical and educational needs are evaluated during a pump assessment appointment. Foundations of pump therapy are taught in a 4-hour course before pump treatment is started in individual or group sessions. Two follow-up visits are performed, along with nutrition education and exercise fine-tuning prior to returning to routine follow-up with the diabetes team.
Data Collection
Demographic, clinical, and laboratory data were extracted from the diabetes centers’ electronic health records. Steno uses a customized electronic patient record system based on DocuLive (version 4.9.3.0 DLEPR, Siemens Nixdorf, Munich, Germany) implemented in 2001. Joslin uses a customized electronic health record based on the NextGen EHR platform (version 5.6, Horsham, PA, USA) implemented in 1999.
Primary outcome measures were change in HbA1c and change in weight (kg) 1 year following transition from MDI to CSII overall and according to HbA1c cut points: <7.0%, 7.0-7.9%, 8.0-8.9%, ≥9.0%. At Steno and Joslin, baseline HbA1c and weight were the most recent values within 12 months prior to pump initiation. At Steno, follow-up HbA1c and weight after 1 year was defined using the first value 9 months following pump start date. At Joslin, follow-up HbA1c after 1 year was defined using the value closest to 12 months after start date, ranging from 9 to 15 months (Table 1) and follow-up weight was defined using the value closest to 12 months after start date, ranging from 6 to 18 months. Suboptimal glycemic control was defined as HbA1c ≥8%.
Table 1.
Characteristics of Adults With Type 1 Diabetes Transitioning to CSII.
| Steno (n = 600) | Joslin (n = 658) | |
|---|---|---|
| Age (years) | 40 ± 14 (18-76) | 42 ± 13 (19-75) |
| Sex (% male) | 38 | 47 |
| Diabetes duration (years) | 22 ± 13 (1-69) | 19 ± 13 (0-64) |
| Insulin dose (units/kg/day) | 0.7 ± 0.3 (0.2-2.1) | N/A |
| HbA1c (%) | ||
| Baseline | 8.4 ± 1.2 (4.2-13.1) | 7.8 ± 1.2 (4.8-14.1) |
| Follow-up | 7.8 ± 1.1 (4.7-12.4) | 7.7 ± 1.1 (4.0-12.8) |
| Weight (kg) | ||
| Baseline | 76 ± 14 (36-121) | 78 ± 17 (46-155) |
| Follow-up | 76 ± 14 (37-126) | 78 ± 18 (41-169) |
| Interval from CSII start (days) (median [IQR]) | ||
| Baseline | −3 (−11 to −1) | −66 (−105 to −36) |
| Follow-up | 302 (271 to 341) | 357 (322 to 399) |
Data are mean ± SD (range) unless otherwise noted. N/A, not available.
Statistical Analysis
Steno and Joslin data were analyzed as separate cohorts. Descriptive statistics were used for patient characteristics. Spearman correlations and t-tests were used to determine univariate associations. Multivariable linear regression was used to assess factors associated with change in HbA1c. A P value < .05 determined statistical significance. Statistical software included SAS v.9.2 (SAS Institute, Cary, NC, USA) and NCSS (NCSS Software, Kaysville, UT, USA). The analysis was conducted as a quality improvement project at Steno and did not require review by the local Ethics Committee. This study was approved by the Joslin Diabetes Center’s Committee on Human Studies and was determined to meet the definition of research not requiring informed consent.
Results
Patient characteristics are shown in Table 1. Overall, patients were 42 ± 13 and 40 ± 14 years of age at Joslin and Steno, respectively, with a long duration of diabetes. The percentage of males was higher in the Joslin cohort compared to the Steno cohort (47% vs 38%). Baseline HbA1c was higher at Steno compared to Joslin (8.4 ± 1.2% vs 7.8 ± 1.2%). HbA1c levels at follow-up were comparable (Steno, 7.8 ± 1.2% vs Joslin, 7.7 ± 1.1%). Baseline weight was similar at both centers.
Overall, changes in HbA1c were inversely correlated with baseline HbA1c for both cohorts (Steno, r = –.46, P < .0001 and Joslin, r = –.43, P < .0001). Change in HbA1c was modestly correlated with change in weight at Steno (r = –.12, P < .05), but not at JDC (r = –.03, P = .4). In both cohorts, clinically significant reductions in HbA1c were noted in patients with baseline HbA1c ≥8% (Figure 1). For Joslin patients with baseline HbA1c <7%, the mean HbA1c increased, but remained <7% at follow-up (6.4 ± 0.5%). Overall, weight was stable in both cohorts 1 year after pump initiation. In both groups, modest weight gain was only noted in patients with baseline HbA1c ≥9% representing an average increase of <2% in total body weight.
Figure 1.
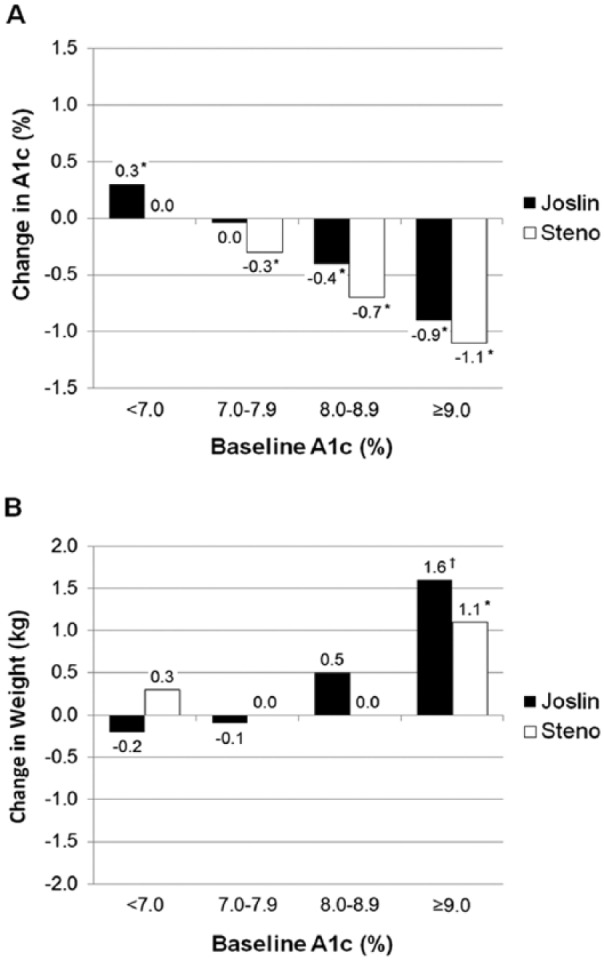
Clinical outcomes 1 year after transition to CSII: (A) change in HbA1c by baseline HbA1c cut points (*P < .0001 for difference between baseline and 1-year outcome) and (B) change in weight (kg) by baseline HbA1c cut points. *P < .0001, †P < .005 for difference between baseline and 1-year outcome.
In a significant multivariate model (R2 = .28, P < .0001) controlling for age, sex, diabetes duration, and baseline weight, higher baseline HbA1c (β = −0.4, P < .0001), older age (β = −0.07, P = .03) and female sex (β = −2.6, P = .02) were significantly associated with change in HbA1c 1 year following CSII initiation at Steno. In a significant multivariate model (R2 = .19, P < .0001) controlling for age, sex, diabetes duration, and baseline weight, only higher baseline HbA1c (β = −0.3, P < .0001) was significantly associated with change in HbA1c 1 year following CSII initiation at Joslin.
Discussion
CSII treatment is a well-established tool to improve the metabolic control of individuals with T1D. We conducted a retrospective, observational investigation of the effect of transitioning from MDI to CSII on 1-year changes in HbA1c and weight in 2 large, real-world multidisciplinary diabetes centers in Europe and the United States. We found clinically significant reductions in HbA1c in patients with suboptimal glycemic control without adverse effects on weight that are commonly associated with insulin-induced improvement of glycemic control.
A previous study in pediatric and adolescent cohorts found a slightly lower HbA1c following CSII initiation in Italy compared to Spain and Canada, but to the best of our knowledge no previous multinational comparisons of health outcomes following pump initiation in adults with T1D has been published.7-9 We found that HbA1c prior to CSII initiation was lower in the American population than in the Danish. This may be related to differences in indication for CSII use at both centers. In Denmark, patients with T1D often start CSII treatment if the HbA1c is above 7% despite attempts optimize treatment on MDI, whereas HbA1c <7% is not viewed as contraindication to CSII consideration at Joslin.
Patients with an HbA1c ≥8% achieved clinically significant HbA1c reductions 1 year after pump initiation at both centers. This is in accordance with meta-analyses5,6 and a recent retrospective study,10 showing that the relative benefit of CSII over MDI is increased with higher baseline HbA1c.
We found body weight to be stable in both cohorts 1 year after CSII initiation. In a meta-analysis including 2 studies of 1 to 4 years of duration, the mean difference of weight was estimated to be −1.6 kg in favor of CSII compared to MDI.3 These studies were small, involving only 23-30 participants each. Additional studies have shown an increased8,11 (n = 138 and 295) or unchanged12,13 (n = 126 and 23) bodyweight after 2 to 10 years of treatment. In comparison, our study included 1,258 patients and represent what might be expected in a real-life clinical setting. In addition, insulin dose may impact changes in weight during intensification of diabetes management. Meta-analyses have shown a reduction in insulin doses of 0.14 U/kg when CSII is initiated.3 At Steno, where information on insulin doses was available, insulin doses were routinely reduced by 20% when CSII was initiated.
A strength of the present study was the comparison of 2 large, real-world adult T1D cohorts over a 10-year period. Patients in both cohorts were receiving care by multidisciplinary care teams prior to CSII transition which should limit the impact of the specialty care setting on observed outcomes. To the best of our knowledge, the present analysis is one of the largest, multinational evaluations of clinical outcomes following pump initiation. Limitations include lack of information on adverse events, such as hypoglycemia and diabetic ketoacidosis, or quality of life, which help inform the overall clinical effectiveness of CSII use. Greater motivation for self-management may be present among those choosing to transition to CSII therapy. The frequency of blood glucose monitoring and use of continuous glucose monitoring was not available and have been shown to impact glycemic control.12,13 Harmonizing definitions of baseline and follow-up measures will facilitate statistical comparisons of pump programs between diabetes centers in future evaluations.
Conclusions
We conducted a contemporary analysis of health outcomes in adults with T1D 1 year after transitioning from MDI to CSII in 2 large T1D cohorts from multidisciplinary diabetes centers over a 10-year period. Patients with an HbA1c ≥8% achieved clinically significant HbA1c reductions 1 year after pump initiation. Similar to published clinical trials, higher baseline HbA1c was the main predictor of HbA1c reduction after 1 year. Overall, patients at Steno achieved a larger reduction in HbA1c 1 year after pump initiation but this is likely to be due to a baseline HbA1c average which is slightly higher due to differences in CSII indication criteria. There was no adverse weight gain for either cohort after 1 year; modest (<2 kg) weight gain was noted among patients with baseline HbA1c ≥9%. CSII represents an important tool for improving HbA1c in adults with T1D who fail to achieve optimal glycemic control using MDI.
Acknowledgments
This was an investigator-initiated study. An abstract from the preliminary analysis was presented at the 74th Scientific Sessions of the American Diabetes Association, June 13-17, 2013, San Francisco, CA.
Footnotes
Abbreviations: CSII, continuous subcutaneous insulin infusion; HbA1c, hemoglobin A1c; MDI, multiple daily injections; T1D, type 1 diabetes.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: HUA owns stock in Novo Nordisk and has served on an advisory board for Abbott. MR and EEH have served on an advisory board for Medtronic. MJA has served as a consultant for Novo Nordisk and WebMD Health Services. HAW has served as a consultant for Abbott Diabetes Care, Dexcom, Insulet, and Novo Nordisk and has received grant support from Abbott Diabetes Care and Dexcom. No other authors reported conflicts of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Joslin Diabetes Center’s Diabetes and Endocrinology Research Center (NIH P30DK036836).
References
- 1. Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977-986. [DOI] [PubMed] [Google Scholar]
- 2. Nathan DM, Cleary PA, Backlund JY, et al. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353(25):2643-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Misso ML, Egberts KJ, Page M, O’Connor D, Shaw J. Continuous subcutaneous insulin infusion (CSII) versus multiple insulin injections for type 1 diabetes mellitus. Cochrane Database Syst Rev. 2010(1):CD005103. [DOI] [PubMed] [Google Scholar]
- 4. Maiorino MI, Bellastella G, Petrizzo M, et al. Treatment satisfaction and glycemic control in young type 1 diabetic patients in transition from pediatric health care: CSII versus MDI. Endocrine. 2014;46(2):256-262. [DOI] [PubMed] [Google Scholar]
- 5. Pickup JC, Sutton AJ. Severe hypoglycaemia and glycaemic control in type 1 diabetes: meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med. 2008;25(7):765-774. [DOI] [PubMed] [Google Scholar]
- 6. Retnakaran R, Hochman J, DeVries JH, et al. Continuous subcutaneous insulin infusion versus multiple daily injections: the impact of baseline A1c. Diabetes Care. 2004;27(11):2590-2596. [DOI] [PubMed] [Google Scholar]
- 7. Lepore G, Dodesini AR, Nosari I, Trevisan R. Both continuous subcutaneous insulin infusion and a multiple daily insulin injection regimen with glargine as basal insulin are equally better than traditional multiple daily insulin injection treatment. Diabetes Care. 2003;26(4):1321-1322. [DOI] [PubMed] [Google Scholar]
- 8. Bruttomesso D, Pianta A, Crazzolara D, et al. Continuous subcutaneous insulin infusion (CSII) in the Veneto region: efficacy, acceptability and quality of life. Diabet Med. 2002;19(8):628-634. [DOI] [PubMed] [Google Scholar]
- 9. Mameli C, Scaramuzza AE, Ho J, Cardona-Hernandez R, Suarez-Ortega L, Zuccotti GV. A 7-year follow-up retrospective, international, multicenter study of insulin pump therapy in children and adolescents with type 1 diabetes. Acta Diabetologica. 2014;51(2):205-210. [DOI] [PubMed] [Google Scholar]
- 10. Clements M, Matuleviciene V, Attvall S, et al. Predicting the effectiveness of insulin pump therapy on glycemic control in clinical practice: a retrospective study of patients with type 1 diabetes from 10 outpatient diabetes clinics in Sweden over 5 years. Diabetes Technol Ther. 2015;17(1):21-28. [DOI] [PubMed] [Google Scholar]
- 11. Joubert M, Morera J, Vicente A, Rod A, Parienti JJ, Reznik Y. Cross-sectional survey and retrospective analysis of a large cohort of adults with type 1 diabetes with long-term continuous subcutaneous insulin infusion treatment. J Diabetes Sci Technol. 2014;8(5):1005-1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cohen ND, Hong ES, Van Drie C, Balkau B, Shaw J. Long-term metabolic effects of continuous subcutaneous insulin infusion therapy in type 1 diabetes. Diabetes Technol Ther. 2013;15(7):544-549. [DOI] [PubMed] [Google Scholar]
- 13. Pozzilli P, Crino A, Schiaffini R, et al. A 2-year pilot trial of continuous subcutaneous insulin infusion versus intensive insulin therapy in patients with newly diagnosed type 1 diabetes (IMDIAB 8). Diabetes Technol Ther. 2003;5(6):965-974. [DOI] [PubMed] [Google Scholar]


