Abstract
The FDA recently conducted an Advisory Panel meeting to evaluate the safety, efficacy, and benefits of granting a nonadjunctive label claim for the DEXCOM G5 Mobile continuous glucose monitoring (CGM) system. If approved, this claim will allow users to make day-to-day treatment decisions, including insulin dosing directly from the glucose values and rate of changes arrows generated by the CGM device, without the requirement of a confirmatory measurement with a self-monitoring blood glucose (SMBG) meter. Sporadic SMBG testing gives limited data, while CGM gives a value every 5 minutes and has alerts, alarms, trending information and allows caregivers to follow the user in real time 24/7. This indication will lead to more wide spread use of CGM and improve overall care with protection of hypoglycemia.
Keywords: nonadjunctive claim, CGM, FDA panel meeting, SMBG
The FDA recently conducted an Advisory Panel meeting to evaluate the safety, efficacy and benefits of granting a nonadjunctive label claim for the DEXCOM G5 Mobile continuous glucose monitoring (CGM) system.1
If approved, this claim will allow users to make day-to-day treatment decisions, including insulin dosing directly from the glucose values and rate of changes arrows generated by the CGM device, without the requirement of a confirmatory measurement with a self-monitoring blood glucose (SMBG) meter.
I was fortunate to have been invited to be part of the presentation team, along with a score of other hard working and dedicated individuals who spent months preparing for the July 21, 2016, Clinical Chemistry and Clinical Toxicology Devices Panel. We had 1 hour, and not 1 minute more, to present years of ‘blood, sweat and tears’ in terms of research and development, sensor accuracy studies, computer simulations, extensive data analysis, and human factors studies.
The reality of the current “real-world” situation is that among patients who are using the Dexcom G5 CGM, the vast majority including myself, have already transitioned to nonadjunctive use for most of our day-to-day treatment decisions. As the Dexcom CGM accuracy has improved, patient’s trust in this device has increased and the information provided has become an important component of their every day and night diabetes treatment decisions.
Published clinical trials and validated surveys have reported that patients are making CGM-based treatment decisions without confirmatory finger sticks and without reports of adverse events.2 In fact, they reported a lower rate of hypoglycemia after initiating CGM use, as well as making much larger than usual adjustments, upward and downward, to their insulin doses and timing, based on the trend or rate of change (ROC) arrows that accompany the glucose values.3
There are certainly times when patients should not rely on the CGM data to make treatment decisions. Adding this indication would allow DEXCOM to educate patients and health care providers (HCPs) about when and when not to use the CGM data nonadjunctively. Calibrating with an inaccurate SMBG value and less than 12 hours, as well as when a ROC arrow is not appearing on the screen, are some examples. In addition, if the user has symptoms that do not match the CGM value, there is always the option to test with a SMBG meter at any time.
Despite the advancements in technology and therapeutics only one-third of people with type 1 diabetes achieve the level of glycemic control needed in order to avoid long-term complications.2 And, for those who do get their A1c to goal, they commonly experience excessive episodes of mild to severe hypoglycemia leading to morbidity and, sadly at times, mortality.4 Every year a significant number of people die or suffer serious brain damage caused by hypoglycemia. The degree of frustration, poor quality of life, economic cost, and human suffering for the person with diabetes and the entire family is enormous.
One of the biggest issues we face in the management of diabetes is that we do not have enough glucose data throughout the day and especially at night. To be perfectly frank, SMBG is a pain. “Pricking” our fingers the required 6-10 times a day still leaves wide gaps of time with no information, and most people test far fewer times each day.
A major challenge of this meeting was to convince several of the FDA appointed panelists who knew very little about what it is like to live with type 1 diabetes. We needed to help them understand that the enhanced on-demand information provided by CGM, including a glucose value every 5 minutes along with trending information, ROC arrows, alerts, alarms and the ability to share this information in real time with caregivers 24/7, as opposed to an isolated sporadic SMBG measurement, allows for safer and better informed treatment decisions. This may sound like a no brainer, but it wasn’t.
Traditional academic types always want large randomized clinical trials but instead got a simulation study using a validated system designed by Claudio Cobelli and colleagues5,6 that, by the way, was previously accepted and requested by the FDA. This simulation system was also used in several other important projects, including the development of the artificial pancreas and testing of new insulin molecules.7,8 To be honest, it took me some time to understand the importance and necessity for these simulations, however several of the panelists just could not let go of their traditional way of evaluating studies. There are actually many advantages to using this method. It is possible to simulate the impact of multiple variables, including behavioral and physiological in isolation, testing results of different insulin treatment decisions, alert limits, using inaccurate glucose values to calibrate the CGM device, and the presence or absence of hypoglycemia awareness in ways that would not be possible or would be dangerous in a traditional clinical trial setting. An institutional review board would never have granted permission to do a clinical study looking at the variables that needed to be evaluated regarding nonadjunctive use. Furthermore, type 1 diabetes is an incredibly heterogeneous condition and trying to perform a randomized clinical trial looking at all of the different variables comparing CGM-based decisions with SMBG, is just not possible. These issues led 2 of the panelists (a statistician and academic pediatric endocrinologist) to cast dissenting votes on several of the ballot questions asked at the end of the day.
Dr Bruce Buckingham presented, giving the panel a realistic view from a very experienced, clinically oriented pediatric endocrinologist, and showed how any type of schmutz (Yiddish term for garbage) on one’s fingers can lead to very inaccurate, usually high, SMBG measurements. This one fact, a common occurrence, has serious ramifications in terms of over insulinization and unpredictable mild to severe hypoglycemic events. None of the panelists had major issues with the accuracy and human factors associated with testing. In fact, the mean absolute relative difference (MARD) of the DEXCOM G5 was in the range of conventional meters and is not influenced by hand washing.
The most significant and influential part of the day, in my opinion, was the open public testimony when more than 30 individuals gave heartfelt testimonials describing how the DEXCOM CGM has made transformative changes in their lives, as well as their loved ones. They had been uniformly using their CGM devices nonadjunctively for years, and they repeatedly mentioned the word “trust” in relying on the CGM-generated numbers for diabetes decision-making. The hypoglycemia alerts and alarms were “lifesavers” for so many of the folks who spoke. Young children as well as 70 and 80 year old individuals living with type 1 diabetes for 50 or more years spoke on the values of their DEXCOM. The passion and urgency in the room was palpable.
Dr Robert Ratner, the chief medical officer of the American Diabetes Association, said the issue was “just plain silly,” and Bill Tamberlane reminded the panel that “the FDA has already approved a CGM device that turns off a persons insulin pump without even letting them know,” referring to the low glucose suspend feature of the Medtronic Enlite 530g. Adam Brown, representing diaTribe, made the analogy of CGM versus SMBG using a story of 2 pilots flying different planes. One plane gave basic flight information to the pilot every 3-4 hours and he/she had to walk to the back of the aircraft to get the information. The other plane gave the pilot more sophisticated information in the cockpit in real time. He ended with, “Which plane would you rather be on?” Bill Polonsky, founder of the Behavioral Diabetes Institute, spoke about the burden of finger sticks and the heavy toll they have on diabetes distress as well as overall control.
Finally, at the end of the day, it was time for an open discussion among the panelists. It was held in front of everyone in the room, and the only thing we could do was listen. There were several times I wanted to get out of my chair, stand up and scream, “You just don’t get it!” People with diabetes are not testing enough now, or not at all, and they are making insulin treatment decisions with little or no information, however you are worried about them using data from CGM devices that is just as accurate as SMBG meters, which do not have alerts or trending information or alarms to protect against prolonged hyperglycemia and dangerous hypoglycemia?
The voting came next, and I felt like a person on trial waiting for the judge to announce the verdict after jury deliberations. The panel voted 8-2 in favor of the DEXCOM G5 being safe for nonadjunctive use, 9-1 in favor of the G5 being effective for non-adjunctive use and, finally, 8-2 in favor of the benefits of the G5 outweighing the risks for non-adjunctive use. The FDA officials were impressively informed and fair in their presentations and questions regarding the information submitted and presented. They also helped the panel members perform their responsibilities with appropriate guidance, which was obviously needed.
Where do we go from here? It is time to educate people who are using CGM devices about how to make better treatment decisions in order to spend more time in range, minimizing the duration and severity of hyper- and hypoglycemic excursions. Some of the important educational issues include setting the upper and lower alert values, responding to the ROC arrows with emphasis on insulin timing and dosing, meal timing and nutrient composition, dealing with the type, duration and intensity of exercise, and so on. Additional important topics requiring user education include the prevention of alarm fatigue, utilizing the Share feature with loved ones, teaching them to evaluate and use their own glucose trend information with the Clarity application, and using the estimated A1c value as a point of care motivator.
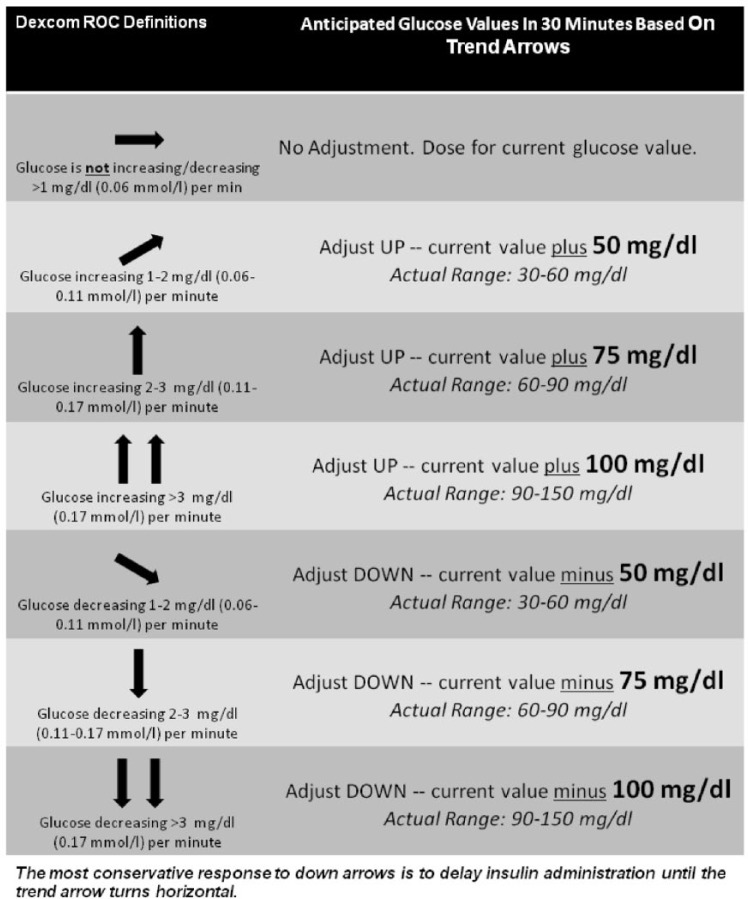
This indication of nonadjunctive use opens the door for so many important issues and advancements. With only 16% of people with type 1 diabetes utilizing CGM, it should lead to wider adoption.9 It is hard for me to fathom how many patients with type 1 diabetes have not even been exposed to this life-changing technology. Diabetes specialists will now be driven to develop more specific instructions about how to use the ROC curves to make insulin dosing decisions and other nonpharmacologic manipulations (Table 1, Figure 1). The utilization of medical services as will as glucose strips will drop and so will those costs. Nonadjunctive use should open the door for Medicare approval of CGM, however I suspect they will find some other excuse to hide behind in order to deny coverage. I tell my friends that I can’t wait until I turn 65 years old since, according to Medicare, my type 1 diabetes goes away. I feel strongly that this indication will help advance the field of artificial pancreas projects around the world.
Table 1.
Advice and Recommendations for Safe, Effective CGM Use.
|
1. Users should be encouraged to wear the CGM as much as possible and look at the receiver frequently. The clinical benefits of rtCGM are only realized with frequent, persistent use. 2. Users should set reasonable expectations for their CGM. CGM is an excellent tool, but it is not perfect. It is important to calibrate as instructed by the manufacturer to minimize false readings and alarms. 3. Confirmation with SMBG is sometimes needed. If the CGM device does not display a sensor glucose reading or is displaying inconsistent readings, users should perform a fingerstick blood glucose value for diabetes treatment decisions. Fingerstick testing should also be performed if glucose alerts and readings do not match symptoms or expectations. 4. Alerts and alarms should be viewed as critical components of CGM use. Users should be encouraged to use the high and low alerts and modify them over time. However, setting the alarms too aggressively at initiation can result in alarm fatigue. Therefore, it is recommended that as control improves, the alarms can be narrowed to encourage tighter control. 5. Users should have a plan for preventing or responding to low glucose. Users should be instructed to respond immediately to low glucose but not overreact. Users may not see the effects of treatment with carbohydrates for more than 15 minutes. Additionally, as CGM devices alert caregivers, a discussion with friends and family on how to respond to alarms is encouraged. 6. Respond to high glucose between meals but avoid “stacking” insulin. Constantly seeing high glucose values can lead to frustration and inclination to bolus repeatedly. Therefore, users should be reminded that rapid-acting insulin can take up to 90-120 minutes to peak and may still be working 3-5 hours after their last injection. Stacking insulin poses a high risk for hypoglycemia; whereas, administration of conservative insulin doses, guided by CGM data, mitigates this risk. 7. Users should be encouraged to utilize the ROC information to make treatment decisions. As mentioned in this article, this trend affects meal timing, insulin dosing, and many other aspects of T1D management. Doing “correctly” takes time, but will come with experience and guidance from their clinician. |
Seven important points that HCPs should review with their patients using CGM. The success of the patient will be much greater with education regarding the best practices for CGM.
Figure 1.
One suggestion for patients using CGM on how to adjust their meal and correction insulin does based on trend arrows. The concept is to use the anticipated glucose value in 30 minutes based on the ROC curves when calculating how much insulin to give at meal time and when correcting for incidental hyperglycemia.10
My most important conflict to report in writing this commentary is that I have been living with type 1 diabetes for 47 years, coming from a time of urine testing only: no pumps, no insulin pens, no A1c assays or designer insulins. I am also the founder of a national, nonprofit, patient-oriented organization called Taking Control Of Your Diabetes, where I interact with thousands of people struggling with this condition across our country every year. It is about time that our regulations catch up with reality.
Acknowledgments
The author would like to thank Annamarie Sucher for her editorial assistance.
Footnotes
Abbreviations: CGM, continuous glucose monitoring; FDA, Food and Drug Administration; HCP, health care provider; ROC, rate of change; SMBG, self-monitoring blood glucose.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Disclosures: SE consults for several CGM and SMBG companies, including Dexcom.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Food and Drug Administration. Advisory committees. Available at: http://www.fda.gov/AdvisoryCommittees/default.htm. Accessed August 12, 2016.
- 2. Beck RW, Tamborlane WV, Bergenstal RM, et al. The T1D Exchange Clinic Registry. J Clin Endocrinol Metab. 2012;97:4383-4389. [DOI] [PubMed] [Google Scholar]
- 3. Pettus J, Price DA, Hill KJ, Edelman S. How people use direction and rate of change information provided by real-time continuous glucose monitoring (RT-CGM) to adjust insulin dosing. Diabetes Technol Ther. 2014;16(S1):198.24401008 [Google Scholar]
- 4. Pettus JH, Hirsch IB, Edelman SV. Practical Management of Type 1 Diabetes. 2nd ed. Greenwich, CT: Professional Communications; 2013. [Google Scholar]
- 5. Kovatchev BP, Breton MD, Dalla Man C, Cobelli C. In silico preclinical trials: a proof of concept in closed-loop control of type 1 diabetes. J Diabetes Sci Technol. 2009;3:44-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dalla Man C, Micheletto F, Lv D, Breton M, Kovatchev B, Cobelli C. The UVA/PADOVA Type 1 Diabetes Simulator: new features. J Diabetes Sci Technol. 2014;8:26-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Viceconti M, Cobelli M, Haddad T, Himes A, Kovatchev B, Palmer M. In silico assessment of biomedical products: the conundrum of rare but not so rare events. J Eng Med (In press). [DOI] [PubMed] [Google Scholar]
- 8. Visentin R, Giegerich C, Jäger R, et al. Improving efficacy of inhaled technosphere insulin (Afrezza) by postmeal dosing: in-silico clinical trial with the University of Virginia/Padova Type 1 Diabetes Simulator. Diabetes Technol Ther. 2016;18:574-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Miller KM, Foster NC, Beck RW, et al. Current state of type 1 diabetes treatment in the US: updated data from the T1D Exchange Clinic Registry. Diabetes Care. 2015;38(6):971-978. [DOI] [PubMed] [Google Scholar]
- 10. Pettus J, Edelman SV. Recommendations for Using Real-Time Continuous Glucose Monitoring (rtCGM) Data for Insulin Adjustments in Type 1 Diabetes [published online August 16, 2016]. J Diabetes Sci Technol. [DOI] [PMC free article] [PubMed] [Google Scholar]