Abstract
Background:
Nonadherence to diabetes medication is prevalent and costly. MEssaging for Diabetes (MED), a mobile health (mHealth) intervention, identified and addressed user-specific barriers to medication adherence. We assessed whether MED reduced users’ targeted barriers and if barrier reductions were associated with within-participant improvements in adherence or glycemic control (HbA1c).
Methods:
Adults (N = 80) with type 2 diabetes completed self-report measures identifying barriers to adherence at baseline and monthly for 3 months. At each assessment, 17 barriers were assessed and ranked for each user. Each subsequent month, users received daily text messages addressing their 3 highest ranked barriers. Targeted barriers were different for each participant and could change monthly. Paired t-tests assessed within-participant improvement in targeted barriers each month, and nested regression models assessed if changes in a participant’s barrier scores were associated with improvements in adherence and HbA1c.
Results:
Participants were 69% non-white and 82% had incomes <$25K. Average HbA1c was 8.2 ± 2.0%. Assessment completion rates were 100% at baseline, 59% at 1 month, 30% at 2 months, and 65% at 3 months. The most commonly reported barriers were the cost of medications (76%), believing medications are harmful (58%), and lacking information about medications (53%). Participants’ barrier scores improved each month and barrier improvement predicted adherence assessed via nightly adherence assessment text messages (P < .001). Among participants who completed assessments each month, barrier improvement in months 2 and 3 (P < .05) predicted HbA1c improvement.
Conclusions:
Iterative, individual tailoring may overcome users’ barriers to adherence. Attrition is a challenge for mHealth interventions among low-income patients.
Keywords: type 2 diabetes, medication adherence, mHealth, mobile phone, text message, intervention
Nonadherence to diabetes medications is a problem,1 especially among low-socioeconomic, racially diverse populations.2,3 Medication nonadherence has costly consequences, including the human and economic burden of more diabetes-related complications and a higher risk of premature mortality.4 Mobile health (mHealth) interventions have the potential to reach the hardest-to-reach and most vulnerable populations to support adherence and improve health outcomes5,6 at low cost.7 Currently, 9 in 10 US adults use a mobile phone8 and more than 80% of mobile phone users text message.9 There are no disparities in using mobile phones for text messaging and phone calls by race/ethnicity or socioeconomic status (SES).9,10 Furthermore, mHealth interventions using text messaging and phone calls have been acceptable among low-income and racially diverse samples in the United States, and some have recently improved glycemic control among adults with diabetes.7,11 However, few mHealth interventions have targeted diabetes medication adherence, specifically.11-13
Mobile technology provides opportunities to tailor an intervention’s functionality (ie, timing and dose/frequency of messages and calls) and content to meet users’ needs and preferences.14 Tailored text messages have been repeatedly shown to be more engaging and more effective at changing health behavior than nontailored messages.13,15,16 Tailoring content to users’ barriers to adherence should be effective,17 but doing so only once may not be adequate for long-term improvements because (1) patient-reported barriers may change over time (eg, financial factors,18 social and environmental stressors,19 and regimen-specific challenges), and (2) interventions may be successful in reducing existing barriers to adherence and new barriers may arise. Tailoring throughout interventions (ie, dynamic tailoring) is increasingly being used to sustain patient engagement and improve intervention effectiveness,7,15,20 but its value is rarely empirically examined.
Despite increased interest in and success of mHealth interventions to support diabetes self-management, little is known about how mobile communications contribute to behavior change and improvements in clinical outcomes.21 Identifying whether interventions effectively improve the mechanisms they target can inform future interventions22 and ensure the most effective elements are retained.23 Few mHealth interventions have evaluated effects on the psychological mechanisms targeted by the content.24 Therefore, we conducted a 3-month evaluation of the MEssaging for Diabetes (MED) intervention, which identifies participants’ individual barriers to medication adherence and dynamically tailors text messages each month to improve patients’ adherence and glycemic control.25 Our objectives were to evaluate whether the MED intervention reduced user-specific barriers to adherence each month, and whether barrier reductions were associated with improvements in adherence and glycemic control.
Methods
We recruited patients receiving care from a federally qualified health center (FQHC) in Nashville, Tennessee from February to September 2013. Trained research assistants (RAs) worked with clinic staff and advertised the study on flyers in the clinic waiting area to recruit eligible and interested participants. Eligible patients were English-speaking adults (≥18 years old) diagnosed with and prescribed medications for type 2 diabetes mellitus (T2DM) who owned a cell phone with text messaging capability. Exclusion criteria included a diagnosis of dementia and visual or auditory limitations because participants needed to see text messages and hear automated calls. RAs took interested and eligible participants to a private clinic room to complete informed consent and to verbally administer self-report measures.26 RAs also completed medical chart reviews to collect the number and type of prescribed medications. The Vanderbilt University Institutional Review Board approved all study procedures prior to enrollment.
Intervention
The MED intervention includes daily text messages and weekly automated calls using interactive voice response (IVR) technology. The MED intervention sends: (1) a unique, daily, tailored text message addressing the user’s barriers to adherence, (2) a daily text message at the user’s bedtime asking if he or she has taken all diabetes medicines that day (requesting a yes/no response), and (3) a weekly IVR call. The IVR call provides feedback on the user’s adherence the previous week based on his or her responses to the daily 2-way text message, with encouragement and interactive problem-solving prompts.25 We used the SuperEgo© mobile communications platform27 to integrate self-reported barriers and preferences for timing of messages and IVR calls and to aggregate responses to 2-way text messages and communicate with Twilio©, which delivers corresponding text message and IVR communications.
Participants were exposed to MED for 3 months. Participants complete assessments with self-report measures at baseline, and at 1, 2, and 3 months postbaseline. Seventeen barriers to adherence are assessed and scores are standardized and ranked. Each barrier is “tagged” to sets of text messages with tips and information to reduce the barrier, and users get 1 text message per day addressing 1 of their 3 highest-ranked barriers over the course of 1 month.25 Text messages are sent randomly without replacement so users do not receive the same text message twice within the same month.25 At each assessment, all 17 barriers are reassessed and reranked, so a user might have 3 new barriers targeted by MED during the next month or 1 or more of the same barriers targeted previously, depending on whether the same barriers continued to rank highest for that user over time. If a user does not complete a monthly assessment, text messages continue to target barriers identified the previous month. For users who report less than 3 barriers, barriers are randomly selected, so MED addresses 3 barriers to adherence at any given time for all users.
Detailed descriptions of MED’s functionality, barriers, and associated text messages have been published.25 Example barriers and associated text messages included:
Barriers to accessing medication: “If you have a hard time getting to the pharmacy, you may be able to have your medications mailed to your home. Check your prescription plan.”
Fear of side effects: “If you’re afraid of having a side effect from your diabetes medications, talk with your doctor about how to handle it, so you’re prepared.”
Cost of medications: “If you are having trouble paying for your diabetes medications, ask your doctor if there are samples you can have in case of an emergency.”
Measures
Demographic and diabetes characteristics were assessed at baseline, including self-reported age, gender, race, ethnicity, income, education, and insurance status. Diabetes characteristics included self-reported diabetes duration and the number and type of medications collected via medical chart review.
Barriers to medication adherence were assessed at baseline and monthly for 3 months with validated scales: the Diabetes Medication Knowledge Questionnaire (DMKQ),28 the Medicines for Diabetes Questionnaire (MDQ),29 the Barriers to Diabetes Adherence measure,30 and the Medication Adherence Self-Efficacy Scale (MASES).31 Barriers were tagged to 1 or more items on the respective scales. For instance, the barrier Lack of information about medication was tagged to the DMKQ (all items), and the barrier Believing medications are harmful was tagged to 2 items on the MDQ assessing the degree to which respondents agreed with the statements, “If I were to take diabetes medication(s) regularly … it would cause me unpleasant side effects, such as feeling sick or boated” and “… it would lead to my gaining weight.” Responses were standardized (range 0-20), so each participant’s barriers could be ranked and the 3 highest scored barriers could be identified.25
Medication adherence was assessed with (1) the Summary of Diabetes Self-Care Activities (SDSCA) medications subscale32 at baseline and each follow-up assessment and (2) responses to daily 2-way text messages assessing adherence. The SDSCA uses 2 items to assess participants’ adherence to diabetes medications in the past 7 days. We asked both items for each prescribed diabetes medication, separately (eg, “On how many of the last 7 days did you take your Glumetza®?”) and then averaged items across all medications for an average adherence score ranging from 0-7, with higher scores indicating better adherence. This method of SDSCA administration has been previously validated.33 We also examined adherence using participants’ responses to the daily 2-way text message. We calculated percentage adherence as a function of the number of “yes” responses over the total number of daily 2-way adherence texts sent during the intervention for each user. This assessment method is relatively new and its validity and reliability is being explored in diabetes and in other contexts.34,35
Glycemic control (HbA1c) was assessed at baseline and 3 months. A clinic phlebotomist performed a blood draw HbA1c test approved by the National Glycohemoglobin Standardization Program.
Statistical Analyses
All analyses were conducted with Stata v13. We used descriptive statistics to characterize the sample, and identify the sample’s most common barriers to adherence. We used Mann-Whitney U tests and Fisher’s exact tests to compare assessment completers with noncompleters on demographics, clinical characteristics, and baseline medication adherence and glycemic control.
Paired (within-participant) t-tests assessed whether the barriers addressed by MED content each month were reduced, and included all participants who completed an assessment at the starting time and a follow-up assessment with the next 2 months. We used paired t-tests for each month separately instead of longitudinal analyses (which examine change in a single score over multiple time points) because the barriers targeted by the intervention changed each month for each user in response to assessments.
Next, a series of regression models assessed whether within-participant changes in barriers scores predicted within-participant change in adherence and HbA1c. Models were unadjusted because each participant was his or her own control. All models used clustered standard errors to account for shared within-participant variance (ie, barriers grouped within participants). The coefficients are interpreted the same as ordinary least squares regression nonstandardized coefficients, but the standard errors take into account that the observations are not independent. Standardized coefficients are nonsensical because standard errors vary across participants.
For medication adherence, we regressed SDSCA-reported adherence and, separately, responses to 2-way text messages assessing adherence onto changes in barrier scores. For the SDSCA analyses, we adjusted for baseline SDSCA scores and ran separate models in which barrier changes each month predicted change in the SDSCA score for that month. For the text message reporting, we examined whether change in barrier scores each month predicted adherence reported via text messages during MED exposure (ie, number of “yes” responses/number of 2-way texts sent).
For HbA1c, regression models assessed whether within-person change in barriers scores led to within-person change in HbA1c. These models were first unadjusted and then adjusted for baseline HbA1c. The first included all available data, regressing change in HbA1c from baseline to follow-up onto change in barrier scores (ie, number of barriers varied depending on the number of follow-up assessments each participant completed). This model was unable to isolate the effects of barrier reductions by month of MED exposure due to missed follow-up assessments. The second could isolate these effects, but only included data from 18 participants who completed all assessments. In this analysis, pre to post change in HbA1c was regressed onto change in each barrier score for all 3 months of MED exposure (ie, 9 values per participant—3 per month for 3 months). This model was able to isolate the effects of barrier reductions by month of MED exposure.
Results
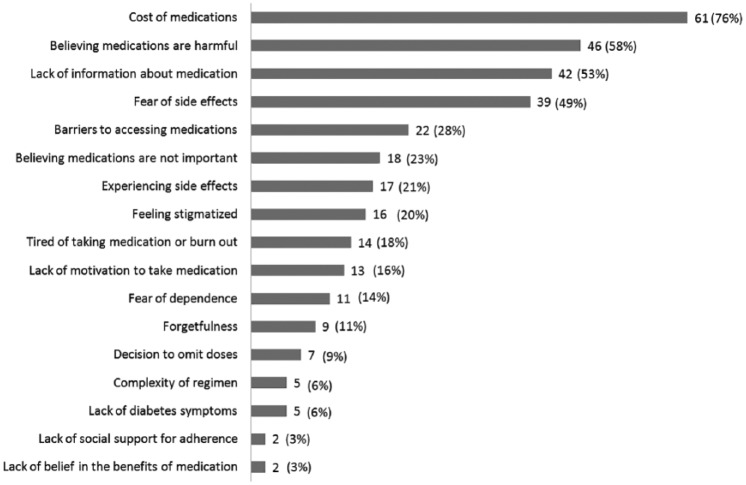
Participant characteristics are shown in Table 1. Participants were 80 adults (50.1 ± 10.5 years old), 68% were female and 69% were non-white. Most (82%) had an annual income <$25,000 (USD) and 35% were uninsured. Forty-three percent of participants were prescribed 2 diabetes medications (range 1-3) and 64% were prescribed insulin. Figure 1 depicts the number of participants who received intervention content addressing each barrier for at least 1 month during the 3-month MED trial. The most frequently reported (and therefore most commonly addressed) barriers were cost of medications (76% of participants), believing medications are harmful (58%), and lack of information about medication (53%). According to the SDSCA, out of the past 7 days, the sample was adherent for an average of 6.13 ± 1.22 days at baseline, 6.48 ± 1.36 days at 1 month, 6.82 ± 0.43 days at 2 months, and 6.18 ± 1.35 days at 3 months. Adherence according to the daily 2-way text message responses was 85.8% ± 20.5% (IQR 84.7-97.3%) over the 3-month MED trial. The average baseline HbA1c was 8.3 ± 2.0%; and the average change in HbA1c was −0.05 ± 1.04% (range −3.1 to 2.6%).
Table 1.
Participant Characteristics.
| N = 80 | M ± SD or n (%) |
|---|---|
| Demographics | |
| Age, years | 50.0 ± 10.5 |
| Gender (female) | 54 (68) |
| Race (white/Caucasian) | 25 (31) |
| Black/African American | 50 (63) |
| Other | 5 (6) |
| Hispanic/Latino(a) | 6 (7) |
| Education, years | 12.9 ± 2.3 |
| Income (< $10K) | 29 (36) |
| $10K-$25K | 37 (46) |
| >$25K | 25 (18) |
| Private insurance | 14 (18) |
| Public | 38 (47) |
| Uninsured | 28 (35) |
| Clinical characteristics | |
| Diabetes duration, years | 9.6 ± 6.4 |
| Diabetes medications, # | 1.9 ± 0.8 |
| 1 | 26 (32) |
| 2 | 34 (43) |
| 3 | 20 (25) |
| Prescribed insulin | 51 (64) |
| Medication adherence | |
| SDSCA: Days adherent in past weeka at baseline | 6.1 ± 1.2 |
| Responses to daily 2-way text messageb | 85.8 ± 20.5% |
| Glycemic control, HbA1c at baselinec | 8.3 ± 2.0% |
Assessed with the Summary of Diabetes Self-Care Activities medications subscale administered separately for each prescribed diabetes medication and then averaged across medications.
Number of “yes” responses / total number of 2-way text messages sent during MED exposure. Two-way text messages were sent each night at each participant’s bedtime and asked if he or she took all his or her diabetes medications for that day.
One outlier was excluded from summary statistics and analyses for HbA1c. This participant had a baseline HbA1c value of 6.3% and a follow-up value of 11.4%, representing a change in HbA1c of 5.1%.
Figure 1.
Barriers to diabetes medication adherence assessed and the number of participants (out of 80) who received text messages targeting the barrier at any point during MEssaging for Diabetes.
Participants were invited to complete each assessment, regardless of whether they missed a prior one. Assessment completion rates were 100% at baseline, 59% at 1 month, 30% at 2 months, and 65% at 3 months. For each follow-up assessment, we compared completers with noncompleters on all variables in Table 1. We found no systematic differences: relative to noncompleters, more female, white, and adherent (all P = .03) participants completed the 1-month assessment; there were no differences between completers and noncompleters of the 2-month assessment; and more educated participants (P = .04) completed the 3-month assessment.
Figure 2 shows the average within-participant change (Δ, delta) in the standardized barrier scores MED addressed for each participant, depicted separately each month of the intervention. The within-participant reduction was significant for at least 2 of the 3 barriers addressed each month, with largest reductions in the first month (Figure 2). Within-participant barrier reductions were not associated with within-participant SDSCA changes overtime. However, within-participant barrier reductions by month predicted higher adherence assessed via text message responses (ie, regression coefficients at month 1 = −0.06; month 2 = −0.05; month 3 = −0.08, all P < .001). Among all participants, barrier reductions were not associated with HbA1c improvement. However, among the 18 participants who completed all assessments, barrier reductions in months 2 and 3 were associated with 3-month improvement in HbA1c (Table 2).
Figure 2.
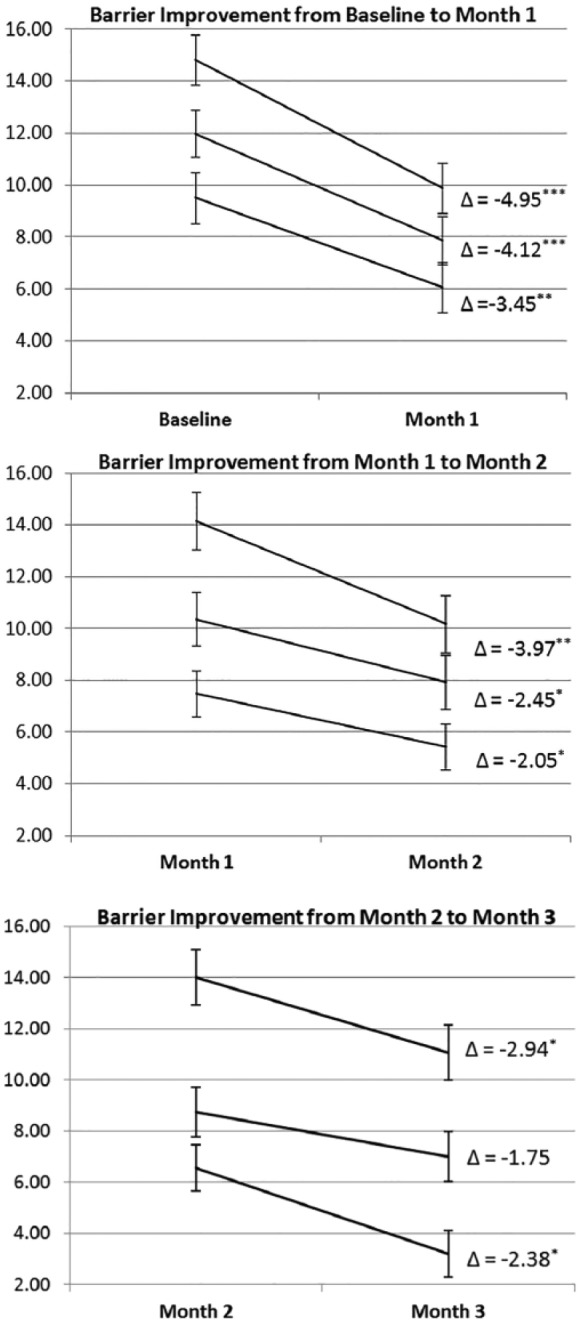
Changes in standardized scores for the top 3 barriers to diabetes medication adherence addressed by the intervention each month. Paired (within-participant) t-tests assessed changes in barrier scores for the 3 barriers addressed during each month of the intervention, and included all participants who completed an assessment at the starting time and a follow-up assessment with the next 2 months (eg, change from baseline to month 1 included participants who completed a follow-up assessment at month 1 or month 2, and so on). Higher scores indicated a barrier was more of a problem. Δ indicates the average within-participant change in standardized barrier scores (possible standardized scores range 0-20); standard error bars indicate standard errors of within-participant differences. *P < .05. **P < .01. ***P < .001.
Table 2.
Do Changes (Δ) in Barrier Scores During Each Month of the Intervention Predict Change in HbA1c From Baseline to 3-Month Follow-Up Assessment Among Participants Who Completed Each Assessment (n = 18)?
| Model 1 Δ in barrier scores each month |
Model 2 Model 1 adjusted for baseline HbA1c |
|||
|---|---|---|---|---|
| Outcome: Δ in HbA1c | Coeff | Robust SEa | Coeff | Robust SEa |
| Month 1 Δ in barrier scores | .018 | .019 | .037 | .020 |
| Month 2 Δ in barrier scores | .068** | .022 | .081** | .024 |
| Month 3 Δ in barrier scores | .048* | .022 | .048† | .025 |
| Baseline HbA1c | — | — | .049 | .038 |
Robust standard errors allow for intragroup correlation (ie, 3 barriers for each participant, each month) using Stata option cluster. Among the 18 participants who completed all assessments, the average change in HbA1c was −0.22 ± 1.28%.
Boldface indicates statistical significance. †P < .07. *P < .05. **P < .01.
Discussion
MEssaging for Diabetes (MED) delivers daily text messages addressing user’s individually identified barriers to diabetes medication adherence. In this diverse, low-income sample the most common barriers were the cost of medications, believing medications are harmful, and lack of information about medication. MED retailored text message content to each participant’s barriers each month they completed an assessment—continuing to address barriers that remained a problem and/or identifying new barriers. Participants experienced reductions in barriers addressed each month of the intervention. Furthermore, barrier reductions each month of MED exposure predicted greater adherence assessed via participants’ responses to daily text messages. Barrier reductions were not associated with glycemic control improvement in the full sample, but were associated with glycemic control improvement among participants who completed all assessments.
We found barrier reductions tapered throughout the intervention, with the largest effects in the first month and the weakest effects in the third month. There are several potential explanations for this. First, the novelty of MED may have worn off and participants quit reading and/or thinking about the text messages due to alert fatigue or repetitiveness of the messages (message content was repeated if targeted barriers did not change). Second, MED may have addressed the most salient barriers for participants at the beginning of the study. Figure 2 shows empirical support for this hypothesis; barriers with higher initial scores (ie, more potential for reduction) were addressed in the first 2 months. Third, we had less power to detect change in participants’ barrier scores in later months due to fewer participants completing follow-up assessments.
Our measures of adherence have limitations. Barrier reductions did not predict improvement in participant-reported adherence on the SDSCA, but did predict rate of yes responses to daily 2-way text messages. The SDSCA assesses adherence during the past 7 days only, and the average baseline adherence in our sample was more than 6 days per week, leaving little room for improvement. Others have noted the SDSCA’s lack of sensitivity, limited variability, and ceiling effects.32 Adherence via daily 2-way texts messages also has limitations. There is no baseline level using this measure, and this text message was an intervention component in itself—many participants said they used the message as a reminder to take their evening doses—so responses may have little to do with those text messages addressing barriers.
Engagement is an important concern in behavioral interventions, especially for patients with low SES.26 Participants who completed monthly assessments did not differ systematically from those who did not on demographic characteristics, adherence or HbA1c. Thus, MED was successful in retaining the most at-risk participants (ie, those with the lowest incomes, no insurance, less adherence, and high HbA1c). However, getting participants to complete follow-up assessments was still a problem. Nonparticipation in follow-up assessments led to a more static user experience, which may have, in turn, led to further nonparticipation. When MED content was redundant due to nonparticipation, it may have addressed already-overcome barriers, potentially making users less interested in MED and/or less interested in completing additional assessments. This may have reduced MED’s efficacy as well. Barrier reductions were associated with improvements in HbA1c only among participants who completed all follow-up assessments and thus had the content dynamically tailored each month.
Conclusions
Understanding the pathways by which mHealth interventions affect behavior change and clinical outcomes is critical to improving their reach and impact.21 To advance the field of mHealth and inform behavior change efforts, studies should assess the degree to which interventions effectively change the targeted mechanisms, and whether changes in those targeted mechanism drive changes in behavioral and clinical outcomes.22 Our as-treated analyses allowed for examining the intervention as intended, and provides a more mechanistic understanding of how this type of intervention may impact behaviors and clinic outcomes (or not). Although it is not possible to disentangle which elements of MED produced changes, by evaluating whether the barriers targeted by MED changed over time, we can begin to understand whether and how text messages can effectively address patients’ barriers to adherence. We do not yet know what types of barriers text messaging can address, and this study contributes to that pursuit as well. The most common barrier for MED participants, cost of medications, is often considered unchangeable by psychoeducational interventions. However, we found participants receiving text messages reported reductions in this barrier, indicating such barriers may be addressable with a low-touch mHealth intervention. Finally, our findings add to a literature suggesting mHealth interventions that dynamically tailor intervention content to users’ changing needs are most effective for supporting diabetes self-management and improving clinical outcomes.36 However, dynamically tailored mHealth interventions must find ways to keep participants engaged to maximize long-term efficacy.
Acknowledgments
The authors thank Cecilia C. Quintero for her role in data collection, and the Vine Hill Community Clinic personnel and the participants for their contributions to this research. This work was presented as a poster at the 75th Annual Meeting of the American Diabetes Association in June, 2015 with the title “Texting Intervention Overcomes Barriers to Medication Adherence Among Low-Income, Diverse Adults With Type 2 Diabetes.”
Footnotes
Abbreviations: DMKQ, Diabetes Medication Knowledge Questionnaire; FQHC, federally qualified health center; HbA1c, hemoglobin A1C; IVR, interactive voice response; MASES, Medication Adherence Self-Efficacy Scale; MDQ, Medicines for Diabetes Questionnaire; MED, MEssaging for Diabetes; mHealth, mobile health; RA, research assistant; SDSCA, Summary of Diabetes Self-Care Activities; SES, socioeconomic status; T2DM, type 2 diabetes mellitus.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by a McKesson Foundation mHealth Award to CYO and SAM. LSM was supported by career development award K01DK106306 from the National Institute of Diabetes and Digestive and Kidney Diseases, and by the Vanderbilt Center for Diabetes Translational Research P30DK092986. CYO was also supported by a career development award K01DK087894 and R01DK100694-01A1 from the National Institute of Diabetes and Digestive and Kidney Diseases. The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent official views of the National Institutes of Health or the McKesson Foundation. The funders played no role in study design; collection, analysis, and interpretation of data; writing the report; or the decision to submit the report for publication.
References
- 1. Aikens J, Piette J. Longitudinal association between medication adherence and glycaemic control in type 2 diabetes. Diabetic Med. 2013;30(3):338-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ngo-Metzger Q, Sorkin DH, Billimek J, Greenfield S, Kaplan SH. The effects of financial pressures on adherence and glucose control among racial/ethnically diverse patients with diabetes. J Gen Intern Med. 2012;27(4):432-437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kirkman MS, Rowan-Martin MT, Levin R, et al. Determinants of adherence to diabetes medications: findings from a large pharmacy claims database. Diabetes Care. 2015;38(4):604-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hong JS, Kang HC. Relationship between oral antihyperglycemic medication adherence and hospitalization, mortality, and healthcare costs in adult ambulatory care patients with type 2 diabetes in South Korea. Med Care. 2011;49(4):378-384. [DOI] [PubMed] [Google Scholar]
- 5. Krishna S, Boren SA, Balas EA. Healthcare via cell phones: a systematic review. Telemed J E Health. 2009;15(3):231-240. [DOI] [PubMed] [Google Scholar]
- 6. Krishna S, Boren SA. Diabetes self-management care via cell phone: a systematic review. J Diabetes Sci Technol. 2008;2(3):509-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nundy S, Dick JJ, Chou CH, Nocon RS, Chin MH, Peek ME. Mobile phone diabetes project led to improved glycemic control and net savings for Chicago plan participants. Health Affairs. 2014;33(2):265-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pew Research Center. Technology devise ownership: 2015. 2015. Available at: http://www.pewinternet.org/2015/10/29/technology-device-ownership-2015/.
- 9. Duggan M. Cell phone activities 2013. Washington, DC: Pew Research Center; 2013. [Google Scholar]
- 10. Chakkalakal RJ, Kripalani S, Schlundt DG, Elasy TA, Osborn CY. Disparities in using technology to access health information: race versus health literacy. Diabetes Care. 2014;37(3):e53-e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arora S, Peters AL, Burner E, Lam CN, Menchine M. Trial to examine text message-based mHealth in emergency department patients with diabetes (TExT-MED): a randomized controlled trial. Ann Emerg Med. 2014;63(6):745-754e6. [DOI] [PubMed] [Google Scholar]
- 12. Arora S, Peters AL, Agy C, Menchine M. A mobile health intervention for inner city patients with poorly controlled diabetes: proof-of-concept of the TExT-MED program. Diabetes Technol Ther. 2012;14(6):492-496. [DOI] [PubMed] [Google Scholar]
- 13. Park LG, Howie-Esquivel J, Dracup K. A quantitative systematic review of the efficacy of mobile phone interventions to improve medication adherence. J Adv Nurs. 2014;70(9):1932-1953. [DOI] [PubMed] [Google Scholar]
- 14. Patrick K, Griswold WG, Raab F, Intille SS. Health and the mobile phone. Am J Prev Med. 2008;35(2):177-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fjeldsoe BS, Marshall AL, Miller YD. Behavior change interventions delivered by mobile telephone short-message service. Am J Prev Med. 2009;36(2):165-173. [DOI] [PubMed] [Google Scholar]
- 16. Head KJ, Noar SM, Iannarino NT, Harrington NG. Efficacy of text messaging-based interventions for health promotion: a meta-analysis. Soc Sci Med. 2013;97:41-48. [DOI] [PubMed] [Google Scholar]
- 17. Krueger KP, Berger BA, Felkey B. Medication adherence and persistence: a comprehensive review. Adv Ther. 2005;22(4):313-356. [DOI] [PubMed] [Google Scholar]
- 18. Piette JD, Heisler M, Harand A, Juip M. Beliefs about prescription medications among patients with diabetes: variation across racial groups and influences on cost-related medication underuse. J Health Care Poor Underserved. 2010;21(1):349-361. [DOI] [PubMed] [Google Scholar]
- 19. Osborn CY, Mayberry LS, Wagner JA, Welch GW. Stressors may compromise medication adherence among adults with diabetes and low socioeconomic status. West J Nurs Res. 2014;36(9):1091-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Quinn CC, Shardell MD, Terrin ML, Barr EA, Ballew SH, Gruber-Baldini AL. Cluster-randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes Care. 2011;34(9):1934-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nundy S, Mishra A, Hogan P, Lee SM, Solomon MC, Peek ME. How do mobile phone diabetes programs drive behavior change? Evidence from a mixed methods observational cohort study. Diabetes Educ. 2014;40(6):806-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. Int J Nurs Stud. 2013;50(5):587-592. [DOI] [PubMed] [Google Scholar]
- 23. Glasgow RE, Fisher L, Strycker LA, et al. Minimal intervention needed for change: definition, use, and value for improving health and health research. Transl Behav Med. 2014;4(1):26-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Riley WT, Rivera DE, Atienza AA, Nilsen W, Allison SM, Mermelstein R. Health behavior models in the age of mobile interventions: are our theories up to the task? Transl Behav Med. 2011;1(1):53-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Osborn CY, Mulvaney SA. Development and feasibility of a text messaging and interactive voice response intervention for low-income, diverse adults with type 2 diabetes mellitus. J Diabetes Sci Technol. 2013;7(3):612-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Nelson LA, Mulvaney SA, Gebretsadik T, Ho Y-X, Johnson KB, Osborn CY. Disparities in the use of a mHealth medication adherence promotion intervention for low-income adults with type 2 diabetes. J Am Med Inform Assoc. 2016;23:12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mulvaney SA, Anders S, Smith AK, Pittel EJ, Johnson KB. A pilot test of a tailored mobile and web-based diabetes messaging system for adolescents. J Telemed Telecare. 2012;18(2):115-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McPherson ML, Smith SW, Powers A, Zuckerman IH. Associations between diabetes patients’ knowledge about medications and their blood glucose control. Res Social Adm Pharm. 2008;4:37-45. [DOI] [PubMed] [Google Scholar]
- 29. Farmer A, Kinmonth AL, Sutton S. Measuring beliefs about taking hypoglycaemic medication among people with type 2 diabetes. Diabet Med. 2006;23(3):265-270. [DOI] [PubMed] [Google Scholar]
- 30. Mulvaney SA, Hood KK, Schlundt DG, et al. Development and initial validation of the barriers to diabetes adherence measure for adolescents. Diabetes Res Clin Pract. 2011;94(1):77-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ogedegbe G, Mancuso CA, Allegrante JP, Charlson ME. Development and evaluation of a medication adherence self-efficacy scale in hypertensive African-American patients. J Clin Epidemiol. 2003;56(6):520-529. [DOI] [PubMed] [Google Scholar]
- 32. Toobert D, Hampson S, Glasgow R. The Summary of Diabetes Self-Care Activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23:943-950. [DOI] [PubMed] [Google Scholar]
- 33. Mayberry LS, Gonzalez JS, Wallston KA, Kripalani S, Osborn CY. The ARMS-D out performs the SDSCA, but both are reliable, valid, and predict glycemic control. Diabetes Res Clin Pract. 2013;102(2):96-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Runyan JD, Steenbergh TA, Bainbridge C, Daugherty DA, Oke L, Fry BN. A smartphone ecological momentary assessment/intervention “app” for collecting real-time data and promoting self-awareness. PLOS ONE. 2013;8(8):e71325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Heron KE, Smyth JM. Ecological momentary interventions: incorporating mobile technology into psychosocial and health behaviour treatments. Br J Health Psychol. 2010;15(1):1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hall AK, Cole-Lewis H, Bernhardt JM. Mobile text messaging for health: a systematic review of reviews. Annu Rev Public Health. 2015;36:393. [DOI] [PMC free article] [PubMed] [Google Scholar]