Abstract
Background:
Hypoglycemia remains a risk for closed loop insulin delivery particularly following exercise or if the glucose sensor is inaccurate. The aim of this study was to test whether an algorithm that includes a limit to insulin delivery is effective at protecting against hypoglycemia under those circumstances.
Methods:
An observational study on 8 participants with type 1 diabetes was conducted, where a hybrid closed loop system (HCL) (Medtronic™ 670G) was challenged with hypoglycemic stimuli: exercise and an overreading glucose sensor.
Results:
There was no overnight or exercise-induced hypoglycemia during HCL insulin delivery. All daytime hypoglycemia was attributable to postmeal bolused insulin in those participants with a more aggressive carbohydrate factor.
Conclusion:
HCL systems rely on accurate carbohydrate ratios and carbohydrate counting to avoid hypoglycemia. The algorithm that was tested against moderate exercise and an overreading glucose sensor performed well in terms of hypoglycemia avoidance. Algorithm refinement continues in preparation for long-term outpatient trials.
Keywords: type 1 diabetes, closed loop, hypoglycemia, continuous glucose monitoring
The ideal aim of closed loop insulin delivery is to normalize plasma glucose without increased exposure to hypoglycemia. To date, in-clinic and outpatient studies have demonstrated that closed loop insulin delivery reduces overall time spent in a hypoglycemic range.1-3 Closed loop therapy has also been shown to reduce hypoglycemia exposure in specific scenarios that are likely to precipitate hypoglycemia, for example exercise,4 and alcohol consumption5 when compared to standard insulin pump therapy. These were short term in-clinic studies, and severe hypoglycemia remains a risk during automated insulin delivery in free living patients. Exercise is particularly problematic because of the rapid falls in glucose that may develop and the inherent delay in response in current unihormonal artificial pancreas (AP) systems due to sensor function and subcutaneous insulin delivery. The combination of an overreading sensor and exercise may be particularly problematic and result in severe hypoglycemia. To counter these risks, limits to insulin delivery were generated and embedded in a hybrid closed loop insulin delivery algorithm. This study aimed to test the effectiveness of this strategy by challenging the performance of a hybrid closed loop system under conditions of overreading glucose sensor, exercise and both combined. This study was purely exploratory with no comparator and no specific endpoint. We report the results of a 4-day (and 3-night) in-clinic study using a hybrid closed loop system in patients with type 1 diabetes, when the continuous glucose monitoring data were intentionally calibrated to overread true blood glucose values, alone and then in combination with exercise.
Methods
Study Design/Subjects
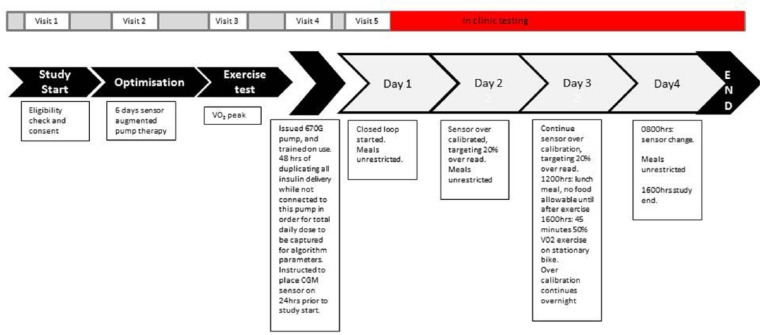
An in-clinic 4 day (and 3 night) observational study in 8 participants with type 1 diabetes (see Figure 1). Inclusion criteria were (1) type 1 diabetes greater than 2 years, (2) insulin pump therapy for at least 6 months, (3) HbA1c <9%, and (4) age 12-50 years. Ethics was granted by the Princess Margaret Hospital for Children Ethics Committee, and the trial is registered (ACTRN12614001005640).
Figure 1.
Study protocol flow diagram.
Hybrid Closed Loop System
The components of the hybrid closed loop system (HCL) were the Minimed Medtronic’s next generation insulin pump (670G) and fourth-generation glucose sensor (Enlite III). The insulin pump housed a hybrid closed-loop algorithm comprised of a modified PID controller with an insulin feedback component, and additional safety settings—primarily a maximum delivery rate. This maximum delivery rate is a function of the total daily dose, requires a sensor glucose value and insulin delivery profile. It is recalculated every 24 hours, and therefore has the ability to adapt. Micro boluses of insulin were delivered every 5 minutes according to the algorithm output representing “basal” insulin delivery. Meals were announced by entering the carbohydrate content of the food and a capillary blood glucose level for which an insulin bolus was delivered according to the participant’s individualized carbohydrate ratio and insulin sensitivity factor (correction factor). The controller gains and the safety thresholds of the system were determined based on a minimum of 2 days of historical insulin and sensor data from the pump. This is automatically updated every 24 hours, and hence is adaptive to changing insulin requirements. The glucometer used was the Contour® next link 2.4 from Bayer.
Study Protocol (Figure 1)
All participants had insulin pump settings optimized with 6 days of sensor augmented pump therapy and clinical review prior to the study. Optimization was from a single clinician (MDB) interpreting the data and changing insulin pump settings in a patient-centered approach. Participants underwent an incremental exercise test to determine their peak rate of oxygen consumption (VO2 peak). This test involves cycling on an ergometer (Lode, Corival; InMed Pty Ltd, Seven Hills, NSW, Australia) at a fixed cadence against a resistance that increases every 3 minutes until the participant can no longer sustain the required workload. The initial workload was set at 50 W and increased in 25 W increments. During the test, the expired air of the participants was collected and analyzed using an indirect calorimetry system (Vmax Spectra; SensorMedics Corp, Yorba Linda, CA, USA).
At least 48 hours of insulin requirements were collected on to the HCL insulin pump by duplicating the participants’ regular insulin pump settings (basal rates, carbohydrate ratio, and insulin sensitivity factor), and then instructing the study participants to duplicate all insulin pump actions they entered into their own pump, on to the HCL pump. The “carbohydrate factor” was calculated for each participant (carbohydrate factor = individualized carbohydrate ratio × total daily dose) so that the aggressiveness of the carbohydrate ratios could be compared between participants—which is particularly useful if patients are on a low carbohydrate diet. Typically the carbohydrate factor is 500 when patients begin carbohydrate counting but changes with time with individual requirements. A low carbohydrate factor would deliver more insulin for ingested carbohydrate (a more aggressive carbohydrate ratio with respect to total daily dose) and a high value would deliver less insulin for ingested carbohydrate (a less aggressive carbohydrate ratio with respect to total daily dose). Participants were not physically attached to the HCL insulin pump during this period as it was an investigation device at the time. Participants started the in-clinic phase only if the total daily doses between their own pump and the HCL insulin pump were >95% concordant to ensure the controller gains and the safety thresholds of the HCL were appropriate for the individual. Participants were instructed to avoid extreme physical exercise in the 48 hours prior to the in-clinic phase.
Participants presented fasted to the Clinical Children’s Research Facility at 7:30 am on the day of the in-clinic phase.
On day 1, participants had an IV cannula inserted, and the HCL pump was then attached to the participant, and HCL (Automode) initiated. Food intake was unrestricted throughout the study, and was bolused for using the participant’s individual carbohydrate ratio. Capillary blood glucose values were taken prior to each meal, and prior to sleep, and once overnight, and the sensor calibrated with each blood glucose level (at least 6 times per day). Auto-calibration was disabled in anticipation of the requirement to artificially calibrate the sensor on day 2. Plasma glucose was taken every 30 minutes for 2 hours after each meal, hourly during waking hours, and 2 hourly overnight.
On day 2, the glucose sensor was artificially overcalibrated by entering glucose values 20% greater than the true capillary glucose value. Bolus delivery was calculated using the normal blood capillary blood glucose value. The association between the plasma glucose and sensor glucose was then monitored, and the sensor calibrated with each capillary glucose time point in an attempt to keep it 20% greater than the plasma value.
On day 3, the sensor overcalibration was maintained. Food was restricted on day 3 to ensure that lunch was given at noon, with no additional food until exercise 4 hours later. At 4:00 pm, participants exercised at 55% of their previously ascertained peak V02 on a stationary bicycle for 45 minutes. Plasma glucose and lactate were measured every 15 minutes during exercise. Overcalibration continued all night.
On day 4, the sensor was changed (during closed loop the HCL insulin pump delivers a predetermined safe basal rate for up to 1.5 hours before reverting to open loop settings while it waits for a new sensor value to become available). The study ended at 4:00 pm on day 4.
Throughout the study, plasma glucose values were taken every 20 minutes if the glucose value was <72 mg/dL. Participants were treated for hypoglycemia if the plasma glucose was <62 mg/dL, or if they felt hypoglycemic above this value. The primary outcome was hypoglycemic events, defined as plasma glucose <62 mg/dL. Hypoglycemia events were checked for their temporal association with an insulin bolus for carbohydrate ingestion, and time since the algorithm delivery insulin.
Results
Eight participants (3 male, 5 female) completed the study. Mean age was 18 years (range 14-36), mean HbA1c was 7.9% (range 6.3-8.8). One participant did not complete the study due to failure of the glucose sensor and came out of closed loop function on day 3 immediately prior to the exercise phase (and is excluded from data analysis). Demographic and clinical data are presented in Table 1. Overall, the mean plasma glucose was 148 ± 14 mg/dL. Percentage time in plasma glucose ranges <72 mg/dL, 72-144 mg/dL, 72-180 mg/dL, 180-270 mg/dL, and >270 mg/dL were 1.5 ± 1.4%, 50.9 ± 11.6%, 75.2 ± 10.3%, 22.0 ± 10.5%, and 1.3 ± 1.6%, respectively. There were 9 hypoglycemic events (plasma glucose <63 mg/dL) during the study. The distribution of these events is shown in Table 2. Individual patient CGM, YSI glucose, and insulin delivery data over the entire study are presented in the supplementary material.
Table 1.
Demographics.
| Participant | Sex | HbA1c | Age (years) | Length of diabetes (years) | BMI (kg/m2) | Total daily dose (U/kg/d) (mean last 5 days) | Carbohydrate ratio (mean) | Carbohydrate factor | Insulin sensitivity factor (correction factor) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 6.3 | 14 | 3 | 17.8 | 0.83 | 8 | 424 | 2.2 |
| 2 | M | 8.0 | 18 | 8.4 | 28.9 | 0.96 | 4 | 328 | 1 |
| 3 | F | 7.9 | 14 | 2.6 | 22.3 | 0.91 | 7 | 413 | 1.8 |
| 4 | F | 7.1 | 17 | 2.5 | 26.3 | 0.70 | 7 | 350 | 2 |
| 5 | F | 8.3 | 18 | 9 | 28.4 | 0.44 | 9 | 333 | 2.3 |
| 6 | M | 8.5 | 36 | 23 | 29.1 | 0.85 | 6.8 | 551 | 1.5 |
| 7 | F | 8.8 | 16 | 6.2 | 26.9 | 1.02 | 6 | 462 | 1.5 |
| 8 | F | 7.1 | 14 | 9.5 | 24.2 | 0.79 | 8.5 | 400 | 3 |
Table 2.
Summary of Results.
| Participant | Hypoglycemic eventsa |
Mean time (minutes) hypoglycemic event from last bolus | Mean time (minutes) algorithm stopped delivering insulin prior to hypoglycemic event | Mean plasma glucose Mg/dL | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Nocturnal hypoglycemia | Exercise hypoglycemia | |||||
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | N/A | N/A | 119 | |
| 2 | 0 | 1 | 1 | 0 | 0 | 0 | 153 | 90 | 146 | |
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | N/A | N/A | 164 | |
| 4 | 0 | 2 | 0 | 0 | 0 | 0 | 130 | 60 | 144 | |
| 5 | 0 | 0 | 1 | 0 | 0 | 0 | 136 | 184 | 146 | |
| 6 | 0 | 0 | 0 | 0 | 3 | 0 | N/A | N/A | 153 | |
| 7 | 0 | 0 | 0 | 0 | 0 | 0 | N/A | N/A | 160 | |
| 8 | 2 | 0 | 1 | 0 | 1b | 0 | 40 | 105 | 153 | |
| Total | 2 | 3 | 3 | 0 | 1b | 0 | Mean ± SD | 115 ± 50 | 110 ± 53 | 148 ± 14 |
Data are given as mean ± SD where appropriate.
Hypoglycemic event defined as plasma glucose <62 mg/dL.
Hypoglycemic event occurred after the patient exited closed loop due to a pump site failure and hyperglycemia, and a manual correction of insulin was given.
On day 1 (no hypoglycemic stimuli), there were 2 hypoglycemic events. Both of these events occurred in a single participant, and were closely related to an insulin bolus for ingested carbohydrate (15 and 65 minutes after bolus), no algorithm calculated insulin had been delivered for 105 and 70 minutes prior to the hypoglycemia respectively. There was no overnight hypoglycemia on the first night.
On day 2 (overreading sensor), there were 3 hypoglycemic events in 2 participants. As observed in day 1, the hypoglycemic events were closely related to an insulin bolus delivered for an ingested meal (120, 140 and 185 minutes after bolus), and no algorithm calculated insulin had been delivered for 55, 65, and 110 minutes prior to the hypoglycemia, respectively. There was no hypoglycemia on the second night.
On day 3 (overreading sensor, and afternoon exercise) there were 3 hypoglycemic events that occurred in 3 participants. One event proceeded exercise, and occurred 136 minutes after an insulin bolus for carbohydrate, and no algorithm calculated insulin had been delivered for 184 minutes. One event occurred 305 minutes after exercise, 121 minutes after an insulin bolus, and no algorithm calculated insulin had been delivered for 70 minutes. The third event occurred after exercise and at night, however this occurred after the participant exited closed loop due to a pump site failure and resultant hyperglycemia that was corrected with a manual bolus of insulin (50% of the suggested correction according to the patient insulin sensitivity factor). The mean plasma glucose at the beginning of exercise was 137 mg/dL (range 85 to 236). The mean fall in blood glucose during the 45 minutes of exercise was 38 mg/dL (range −2 to 68). On day 2 and day 3, during the sensor overcalibration period, the mean sensor glucose overread achieved was 14% above the plasma glucose value.
On day 4 (sensor change), there was no hypoglycemia.
Examining the temporal relationship between bolused insulin and hypoglycemia, it was noted that participants who experienced daytime hypoglycemia had a carbohydrate factor lower (mean 353, range 328-399) than those who did not experience daytime hypoglycemia (mean 462, range 413-550)
Discussion
The results demonstrate that the insulin limit strategy was effective in avoiding overnight and exercise-induced hypoglycemia even in the presence of an overreading glucose sensor. The hypoglycemia events observed were related to excessive meal boluses. Avoidance of overnight hypoglycemia and exercise-induced hypoglycemia even with an overcalibrated sensor confirmed the effectiveness of the strategy. Observation that time in target range was 75%, demonstrated that insulin limits did not compromise control.
Hypoglycemia was always observed during the daytime and related to the meal bolus. The individual carbohydrate ratios varied between patients for different times of the day, depending on the patient’s insulin requirement (range 1 unit for 6 grams to 1 unit for 15 grams). Using a carbohydrate factor (carbohydrate ratio × TDD) is a useful way to compare patients. The relationship between hypoglycemic events and bolused insulin for meals is highlighted by a clear separation with only participants having a carbohydrate factor < 400 experiencing hypoglycemia. Review of carbohydrate ratios and education on accurate carbohydrate counting are therefore still important when using HCL systems. Premeal blood glucose level did not predict subsequent hypoglycemia, and therefore any correction for hyperglycemia according to the patient insulin sensitivity factor did not contribute to the hypoglycemic events.
The average cessation of insulin prior to hypoglycemia was 110 minutes, but cessation of insulin delivery alone was unable to prevent the hypoglycemic event. HCL systems are reliant on a preset carbohydrate ratio, therefore if this setting is too aggressive (as seen in this study), hypoglycemia is more likely.
Exercise-induced hypoglycemia is a significant challenge for people with type 1 diabetes, and is a major contributor to low rates of physical activity in this population. Many strategies and position statements have been published describing strategies to reduce this risk—which include eating carbohydrate, reducing insulin, and using sprints.6,7 Application of this in real life, when exercise can be spontaneous, is challenging. The observation of no exercise-induced hypoglycemia using this HCL system (even with an overcalibrated sensor) suggests that it may help patients exercise with renewed confidence.
The performance of the fourth-generation sensor in terms of accuracy can not be defined in this study. Typically, sensors oscillate between under- and overreading from a reference glucose value (plasma sample or glucometer), hence the absolute value is used to express the mean absolute relative difference (MARD). In this study, we artificially calibrated the sensor to only overread. The 14% overread that we sustained would therefore correlate to a much higher MARD in practice (as any underreading values would add to this). Recent literature reported a 12.6 ± 11.0% MARD using the same sensor.8 The implication is that while we simulated a hypothetical situation of sustained glucose sensor overread, this (1) is unlikely to occur in real-world scenarios and (2) suggests that sensor accuracy is currently robust enough to not contribute to hypoglycemia. The caveat to this is short-term very poor accuracy; however, the Medtronic HCL system has built-in safety that monitors for extreme sensor discordance, and automatically exits the closed loop function.
This study has a major limitation with respect to having no comparator. Nevertheless, this study was not designed to compare the HCL system to standard pump therapy with respect to hypoglycemia reduction, as this has been shown in numerous inpatient and outpatient studies. The purpose was to explore the performance of the algorithm with a view to modifying parameters within it, and also to uncover important lessons while we prepare for a long-term outpatient trial.
Conclusions
We conclude that (1) algorithm refinement for this HCL system continues to be required in preparation for long-term home trials, (2) HCL systems that rely on accurate carbohydrate counting and appropriate carbohydrate ratios are vulnerable to daytime hypoglycemia and therefore clinical review and patient education will remain important, and (3) avoidance of hypoglycemia during exercise suggests that closed loop systems should give patients renewed confidence to exercise and therefore benefit from a more active lifestyle. Future studies to compare the HCL systems against currently available technology such as the “predictive low glucose suspend”9 function are required to demonstrate incremental improvement and are occurring in long-term outpatient trials currently.
Supplementary Material
Acknowledgments
The authors acknowledge the study participants who volunteered their time to this study. They also acknowledge funding from the Diabetes Australia Research Trust, and the support in kind from Medtronic for the supply of the hybrid closed loop system.
Footnotes
Abbreviations: ACTRN, Australasian Clinical Trials Register Number; AP, artificial pancreas; HCL, hybrid closed loop; MARD, mean absolute relative difference; PID, proportional integrative derivative; TDD, total daily dose.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MDB reported he was supported by the Juvenile Diabetes Research Foundation mentored fellowship, and had received honoraria for scientific lectures (Medtronic) and travel reimbursement by Novo-Nordisk and the Juvenile Diabetes Research Foundation mentored fellowship. TJ reported receiving honoraria for scientific lectures and travel reimbursement from Medtronic, Sanofi-aventis, Eli Lilly, and Novo-Nordisk. AR, BG, and NK are employees of Medtronic.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding was provided by the Diabetes Australia Research Grant, with support in kind from Medtronic.
Supplemental Material: The supplemental material is available at http://journals.sagepub.com/doi/suppl/10.1177/1932296816668876
References
- 1. Leelarathna L, Dellweg S, Mader JK, et al. Day and night home closed-loop insulin delivery in adults with type 1 diabetes: three-center randomized crossover study. Diabetes Care. 2014;37:1931-1937. [DOI] [PubMed] [Google Scholar]
- 2. Russell SJ, El-Khatib FH, Sinha M, et al. Outpatient glycemic control with a bionic pancreas in type 1 diabetes. N Engl J Med. 2014;371:313-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thabit H, Tauschmann M, Allen JM, et al. Home use of an artificial beta cell in type 1 diabetes. N Engl J Med. 2015;373: 2129-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sherr JL, Cengiz E, Palerm CC, et al. Reduced hypoglycemia and increased time in target using closed-loop insulin delivery during nights with or without antecedent afternoon exercise in type 1 diabetes. Diabetes Care. 2013;36:2909-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hovorka R, Kumareswaran K, Harris J, et al. Overnight closed loop insulin delivery (artificial pancreas) in adults with type 1 diabetes: crossover randomised controlled studies. BMJ. 2011;342:d1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Basu R, Johnson ML, Kudva YC, Basu A. Exercise, hypoglycemia, and type 1 diabetes. Diabetes Technol Ther. 2014;16:331-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pivovarov JA, Taplin CE, Riddell MC. Current perspectives on physical activity and exercise for youth with diabetes. Pediatr Diabetes. 2015;16:242-255. [DOI] [PubMed] [Google Scholar]
- 8. Ly TT, Roy A, Grosman B, et al. Day and night closed-loop control using the integrated Medtronic hybrid closed-loop system in type 1 diabetes at diabetes camp. Diabetes Care. 2015;38:1205-1211. [DOI] [PubMed] [Google Scholar]
- 9. Buckingham BA, Raghinaru D, Cameron F, et al. Predictive low-glucose insulin suspension reduces duration of nocturnal hypoglycemia in children without increasing ketosis. Diabetes Care. 2015;38:1197-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.