Abstract
Background
Endophthalmitis is a severe inflammation of the anterior or posterior (or both) chambers of the eye that may be sterile or associated with infection. It is a potentially vision‐threatening complication of cataract surgery. Prophylactic measures for endophthalmitis are targeted against various sources of infection.
Objectives
To evaluate the effects of perioperative antibiotic prophylaxis for endophthalmitis following cataract surgery compared with no prophylaxis or other form of prophylaxis.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register) (2016, Issue 12), Ovid MEDLINE, Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily (January 1946 to December 2016), Embase (January 1980 to December 2016), Latin American and Caribbean Health Sciences Literature Database (LILACS) (1982 to December 2016),the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We used no date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 6 December 2016. We also searched for additional studies that cited any included trials using the Science Citation Index.
Selection criteria
We included randomized controlled trials that enrolled adults undergoing cataract surgery (any method and incision type) for lens opacities due to any origin. We included trials that evaluated preoperative antibiotics, intraoperative (intracameral, subconjunctival or systemic), or postoperative antibiotic prophylaxis for acute endophthalmitis. We excluded studies that evaluated antiseptic preoperative preparations using agents such as povidone iodine or antibiotics for treating acute endophthalmitis after cataract surgery.
Data collection and analysis
Two review authors independently reviewed abstracts and full‐text articles for eligibility, assessed the risk of bias for each included study, and abstracted data.
Main results
Five studies met the inclusion criteria for this review, including 101,005 adults and 132 endophthalmitis cases. While the sample size was very large, the heterogeneity of the study designs and modes of antibiotic delivery made it impossible to conduct a formal meta‐analysis. Interventions investigated included the utility of adding vancomycin and gentamycin to the irrigating solution compared with standard balanced saline solution irrigation alone, use of intracameral cefuroxime with or without topical levofloxacin perioperatively, periocular penicillin injections and topical chloramphenicol‐sulfadimidine drops compared with topical antibiotics alone, and mode of antibiotic delivery (subconjunctival versus retrobulbar injections; fixed versus separate instillation of gatifloxacin and prednisolone). The risk of bias among studies was low to unclear due to information not being reported. We identified one ongoing study.
Two studies compared any antibiotic with no antibiotic. One study, which compared irrigation with antibiotics in balanced salt solution (BSS) versus BSS alone, was not sufficiently powered to detect differences in endophthalmitis between groups (very low‐certainty evidence). One study found reduced risk of endophthalmitis when combining intracameral cefuroxime and topical levofloxacin (risk ratio (RR) 0.14, 95% confidence interval (CI) 0.03 to 0.63; 8106 participants; high‐certainty evidence) or using intracameral cefuroxime alone (RR 0.21, CI 0.06 to 0.74; 8110 participants; high‐certainty evidence) compared with placebo, and an uncertain effect when using topical levofloxacin alone compared with placebo (RR 0.72, CI 0.32 to 1.61; 8103 participants; moderate‐certainty evidence).
Two studies found reduced risk of endophthalmitis when combining antibiotic injections during surgery and topical antibiotics compared with topical antibiotics alone (risk ratio (RR) 0.33, 95% confidence interval (CI) 0.12 to 0.92 (periocular penicillin and topical chloramphenicol‐sulfadimidine; 6618 participants; moderate‐certainty evidence); and RR 0.20, 95% CI 0.04 to 0.91 (intracameral cefuroxime and topical levofloxacin; 8101 participants; high‐certainty evidence)).
One study, which compared fixed versus separate instillation of gatifloxacin and prednisolone, was not sufficiently powered to detect differences in endophthalmitis between groups (very low‐certainty evidence). Another study found no evidence of a difference in endophthalmitis when comparing subconjunctival versus retrobulbar antibiotic injections (RR 0.85, 95% CI 0.55 to 1.32; 77,015 participants; moderate‐certainty evidence).
Two studies reported any visual acuity outcome; one study, which compared fixed versus separate instillation of gatifloxacin and prednisolone, reported only that mean visual acuity was the same for both groups at 20 days postoperation. In the other study, the difference in the proportion of eyes with final visual acuity greater than 20/40 following endophthalmitis between groups receiving intracameral cefuroxime with or without topical levofloxacin compared with no intracameral cefuroxime was uncertain (RR 0.69, 95% CI 0.22 to 2.11; 29 participants; moderate‐certainty evidence).
Only one study reported adverse events (1 of 129 eyes had pupillary membrane in front of the intraocular lens and 8 eyes showed posterior capsule opacity). No study reported outcomes related to quality of life or economic outcomes.
Authors' conclusions
Multiple measures for preventing endophthalmitis following cataract surgery have been studied. High‐certainty evidence shows that injection with cefuroxime with or without topical levofloxacin lowers the chance of endophthalmitis after surgery, and there is moderate‐certainty evidence to suggest that using antibiotic eye drops in addition to antibiotic injection probably lowers the chance of endophthalmitis compared with using injections or eye drops alone. Clinical trials with rare outcomes require very large sample sizes and are quite costly to conduct; thus, it is unlikely that many additional clinical trials will be conducted to evaluate currently available prophylaxis. Practitioners should rely on current evidence to make informed decisions regarding prophylaxis choices.
Plain language summary
Antibiotics at the time of cataract surgery to prevent bacterial infection of the eye
What is the aim of this review? The aim of this Cochrane Review was to find out if using antibiotics at the time of cataract surgery can prevent bacterial infection of the eye (endophthalmitis) after cataract surgery. Cochrane researchers collected and analyzed all relevant studies to answer this question and found five studies.
Key messages There is a very small chance of endophthalmitis after cataract surgery. Antibiotics injected into the eye during surgery lower this small chance of infection (high‐certainty evidence). Antibiotic injection and antibiotic eye drops given together probably lower the chance of infection compared with using either injection alone or eye drops alone. Information on adverse effects was not provided in most studies.
What was studied in this review? Endophthalmitis is a rare, but potentially serious, complication of cataract surgery that may lead to blindness. It is caused by bacteria that enter the eye during surgery or in the first few days after surgery. There are many ways to stop infection during and after surgery, such as using antibiotics at the time of surgery. There are several different types of antibiotic that can be used, and these may be used in different ways (either by injection into the eye, or infusion into the blood, or eye drops) or at different times (before, during, or after surgery).
What are the main results of the review? Cochrane researchers found five relevant studies. Two studies were conducted in Pakistan, one study in several European countries, one study in Brazil, and one study in Turkey. These studies all looked at different treatments: one study compared four different treatments ‐ antibiotic injection combined with antibiotic eye drops versus antibiotic injection alone versus antibiotic eye drops alone versus placebo eye drops; one study compared combined antibiotic injection and antibiotic eye drops versus antibiotic eye drops alone; one study compared combined antibiotics and steroids versus antibiotics and steroid given individually; one study compared two different locations for the antibiotic eye injection; one study compared adding antibiotics to the sterile fluid used during surgery versus not adding antibiotics to this fluid.
The review shows that:
• Antibiotic injection in the eye (cefuroxime) at the end of surgery lowers the chance of endophthalmitis after surgery (high‐certainty evidence). • Using antibiotic eye drops (either levofloxacin or chloramphenicol) in addition to antibiotic injection (either cefuroxime or penicillin) probably lowers the chance of endophthalmitis compared with using injections or eye drops alone (moderate certainty evidence). • It is very uncertain whether adding antibiotic to the sterile irrigating fluid used during cataract surgery lowers the chance of endophthalmitis (very low‐certainty evidence). • It is very uncertain if using antibiotics and steroids individually or in combination makes a difference to the chance of developing endophthalmitis (very low‐certainty evidence).
How up to date is this review? Cochrane researchers searched for studies that had been published up to December 2016.
Summary of findings
Summary of findings for the main comparison. Perioperative antibiotics for prevention of endophthalmitis after cataract surgery.
| Perioperative antibiotics for prevention of endophthalmitis after cataract surgery | ||||||||
|
Population: participants undergoing cataract surgery Settings: eye hospital or clinic Outcome: risk of endophthalmitis after surgery | ||||||||
| Perioperative prophylaxis versus no prophylaxis | ||||||||
| Study ID | No. eyes and participants | Follow‐up |
Comparison (intervention vs comparator) |
Risk of endophthalmitis by study group | RR (95% CI) Treatment vs control | Certainty of the evidence (GRADE) | ||
| Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | |||||
| Sobaci 2003 | 644 eyes of 640 participants | 6 weeks | Treatment: BSS with antibiotics (vancomycin 20 mg/mL and gentamicin 8 mg/mL) | Not reported | 0/322 (0%) eyes | Not reported | 0.20 (0.01 to 4.15) | ⊕⊝⊝⊝ Very low1,2 |
| Control: BSS‐only irrigating infusion fluid | Not reported | 2/322 (0.62%) eyes | ||||||
| ESCRS 2007 | 16,603 eyes of 16,603 participants | 6 weeks | Treatment 1: combined intracameral cefuroxime and topical levofloxacin | 2/4052 (0.05%) eyes | 1/4052 (0.02%) eyes | 0.14 (0.03 to 0.63) | 0.10 (0.01 to 0.78) | ⊕⊕⊕⊕ High |
| Treatment 2: intracameral cefuroxime 0.9% | 3/4056 (0.07%) eyes | 2/4056 (0.05%) eyes | 0.21 (0.06 to 0.74) | 0.20 (0.04 to 0.91) | ⊕⊕⊕⊕ High | |||
| Treatment 3: topical levofloxacin 0.5% | 10/4049 (0.25%) eyes | 7/4049 (0.17%) eyes | 0.72 (0.32 to 1.61) | 0.70 (0.27 to 1.84) | ⊕⊕⊕⊝ Moderate3 | |||
| Control: placebo drops | 14/4054 (0.35%) eyes | 10/4054 (0.25%) eyes | ||||||
| Comparisons of combinations of antibiotics with specific antibiotics | ||||||||
| Study ID | No. eyes and participants | Follow‐up | Interventions | Risk of endophthalmitis by study group | RR (95% CI) Treatment 1 vs treatment 2 | Certainty of the evidence (GRADE) | ||
| Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | |||||
| Christy 1979 | 6618 eyes of 6618 participants | 1 week | Treatment 1: combined prophylaxis (topical regimen + periocular penicillin at the time of surgery) | 5/3309 (0.15%) eyes | Not reported | 0.33 (0.12 to 0.92) | Not reported | ⊕⊕⊕⊝ Moderate4 |
| Treatment 2: topical regimen alone (chloramphenicol‐sulfadimidine) | 15/3309 (0.45%) eyes | Not reported | ||||||
| ESCRS 2007 | 16,603 eyes of 16,603 participants | 6 weeks | Treatment 1: combined intracameral cefuroxime and topical levofloxacin | 2/4052 (0.05%) eyes | 1/4052 (0.02%) eyes | Treatment 1 vs treatment 2: 0.67 (0.11 to 3.99) | Treatment 1 vs treatment 2: 0.50 (0.05 to 5.52) | ⊕⊕⊕⊝ Moderate3 |
| Treatment 2: intracameral cefuroxime 0.9% | 3/4056 (0.07%) eyes | 2/4056 (0.05%) eyes | Treatment 2 vs treatment 3: 0.30 (0.08 to 1.09) | Treatment 2 vs treatment 3: 0.29 (0.06 to 1.37) | ⊕⊕⊕⊝ Moderate3 | |||
| Treatment 3: topical levofloxacin 0.5% | 10/4049 (0.25%) eyes | 7/4049 (0.17%) eyes | Treatment 1 vs treatment 3: 0.20 (0.04 to 0.91) | Treatment 1 vs treatment 3: 0.14 (0.02 to 1.16) | ⊕⊕⊕⊕ High | |||
| Mode of antibiotic delivery | ||||||||
| Study ID | No. eyes and patients | Follow‐up | Interventions | Risk of endophthalmitis by study group | RR (95% CI) Mode 1 vs mode 2 | Certainty of the evidence (GRADE) | ||
| Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | |||||
| Christy 1986 | 77,015 eyes of 77,015 participants | 1 week | Mode 1: Anterior sub‐Tenon injections (subconjunctival) | 38/39,752 (0.10%) eyes | Not reported | 0.85 (0.55 to 1.32) | Not reported | ⊕⊕⊕⊝ Moderate4 |
| Mode 2: Posterior sub‐Tenon injections (retrobulbar) | 42/37,263 (0.11%) eyes | Not reported | ||||||
| Cunha 2013 | 108 eyes of 108 participants | 3 weeks | Treatment 1: fixed combination of topical gatifloxacin 0.3% and prednisolone acetate 1% | 0/47 (0%) eyes | Not reported | 0.43 (0.02 to 10.34) | Not reported | ⊕⊝⊝⊝ Very low1,5 |
| Treatment 2: individual instillation of topical gatifloxacin 0.3% and prednisolone acetate 1% | 1/61 (2%) eyes | Not reported | ||||||
| GRADE Working Group grades of evidence High‐certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: We are very uncertain about the estimate. | ||||||||
BSS: balanced salt solution; CI: confidence interval; RR: risk ratio.
*Presumed cases: includes both culture‐proven and clinically diagnosed cases of postoperative endophthalmitis.
**Proven cases: cases confirmed by at least one of Gram stain, culture, or polymerase chain reaction (PCR)
1 Downgraded for imprecision (‐2) as the study did not enroll a sufficient number of participants to detect differences between groups.
2 Downgraded for high risk of attrition bias (‐1) as the study authors excluded participants at the time of surgery based on the surgeon's discretion (number excluded not reported).
3 Downgraded for imprecision (‐1) as the confidence interval of the effect estimate between groups was wide.
4 Downgraded for indirectness (‐1) as the study was conducted more than 30 years ago and the techniques for cataract surgery have since changed substantially.
5 Downgraded for high risk of attrition bias (‐1) as the study authors excluded participants who did not return for follow‐up (16% of study population).
Background
Description of the condition
Age‐related cataract is a leading cause of reduced vision in both high‐income and low‐income countries (Friedman 2004; Resnikoff 2004). Surgery for cataract involves removal of the opaque lens and replacement with an intraocular lens (IOL). In the few cases where IOL implantation is not possible, contact lenses and glasses are valid options for the correction of the refractive error that results from being aphakic (without a lens). Endophthalmitis is a potentially vision‐threatening complication of cataract surgery. Endophthalmitis is a severe inflammation of the anterior or posterior (or both) chambers of the eye and may be sterile or associated with infection. It most commonly occurs as a complication of cataract surgery, but also may occur following other ocular procedures, trauma to the eye, metastatic systemic infections, and systemic inflammatory disorders.
Epidemiology
Reported endophthalmitis rates vary substantially, with some individual centers reporting no endophthalmitis in a several‐year period (Galvis 2014; Monica 2005), while others report rates as high as 1 in 200 or 300 surgeries (ESCRS 2007; Garcia‐Arumi 2007). One systematic review that included studies from high‐income and low‐income countries indicated a decreasing incidence of endophthalmitis following cataract surgery until the early 1990s, followed by an increase in incidence (Taban 2005a). The pooled estimate for incidence of endophthalmitis was 1.09 per 1000 surgeries from 1963 to 1999 and 2.65 per 1000 surgeries from 2000 to 2003 (Taban 2005a). In addition, an analysis of US Medicare data reported a 40% increase in the adjusted risk of endophthalmitis comparing data from 1998 to 2001 against 1994 to 1997, with annual rates ranging from 1.79 to 2.47 cases per 1000 surgeries (West 2005). An analysis of Medicare fee‐for‐service cataract surgeries reported that rates declined to 1.32 per 1000 surgeries in 2003 and 1.11 per 1000 surgeries in 2004 (Keay 2012). Rates appear to have remained relatively consistent, with two more recent Medicare analyses showing rates of 1.2 per 1000 surgeries (Coleman 2015; Du 2014). Other national‐level data have shown a decline, with Sweden's reported rate dropping from 0.48 per 1000 surgeries in 2002 through 2004 to 0.29 per 1000 surgeries for 2005 through 2010 (Friling 2013), and Iran reports an overall rate of 0.02% (Jabbarvand 2016). Furthermore, India has recently reported a rate of 0.08% among patients not receiving intracameral antibiotics and 0.02% among those receiving intracameral antibiotics (Haripriya 2016).
Presentation and diagnosis
Endophthalmitis usually presents within a few days following cataract surgery, and 80% of cases present within six weeks. Presenting features include decreased visual acuity (VA), pain, swelling and redness of the eyelids, redness of the conjunctiva, haziness of the cornea due to edema, and increased cellularity of fluid in the anterior chamber of the eye with or without hypopyon (pus). Signs of infection and inflammation of the retina and vitreous usually are observed during exam. Although endophthalmitis is a rare infection, it often results in significant long‐term morbidity, even when treated appropriately. Approximately 50% of people do not regain vision of 20/40 or better despite treatment (Gower 2015; Lalwani 2008), and often nearly one‐third have acuity worse than 20/200 following treatment (Gower 2015; Ng 2005; Sheng 2011).
Description of the intervention
Several factors are thought to contribute to the incidence of endophthalmitis following cataract surgery. One primary factor is the type of incision used for surgery (Lundstrom 2007; Taban 2005a; Taban 2005b). In addition, many research studies have focused on the role of antibiotics used prophylactically to target ocular surface flora. Examples of prophylactic measures include preoperative lash‐trimming and irrigation of the lacrimal drainage system with antibiotics, antiseptic preparation of the operative site using agents such as povidone iodine, and preoperative, intraoperative, and postoperative administration of antibiotics. Perioperative antibiotics may be administered through parenteral, topical, or intravitreal routes, using a variety of antibiotics. This review focuses only on perioperative antibiotic use as a prophylactic measure.
How the intervention might work
The vast majority of culture‐proven postoperative endophthalmitis cases are caused by gram‐positive bacteria, with most cases caused by Staphylococcus epidermidis and other coagulase‐negative staphylococci, flora commonly found on the ocular surface (EVSG 1995; Mollan 2007; Ng 2005; Schimel 2013). Other gram‐positive organisms and gram‐negative agents are less frequently associated with acute endophthalmitis; however, some of the less common gram‐negative organisms are associated with the worst visual outcomes. Streptococci are considered the most virulent and have the worst outcomes (Barry 2009; Gower 2015; Miller 2004; Simunovic 2012; Soriano 2006). The conjunctiva and eyelids are the most common sources of infection. The bacteria from these sites are presumably introduced into the anterior chamber through the surgical incision. Endophthalmitis occurs when the intrinsic immune defenses fail to eliminate the virulent bacterial inoculum. To assist the inborn bactericidal processes of the eye, perioperative antibiotics are used to decrease intraocular microbial contamination. Decreasing contamination can be accomplished in several ways, depending on the route of drug administration. Topical antibiotics directly penetrate the ocular surface to enter the aqueous humor of the eye (i.e. the fluid in the anterior chamber). Some antibiotics administered orally achieve intraocular concentrations via systemic delivery, while other antibiotics do not effectively penetrate the eye. Intracameral antibiotic administration is the most direct route of delivery to the site of potential infection.
Perioperative antibiotics eliminate etiologic organisms by either bacteriostatic or bactericidal mechanisms. Bacteriostatic agents arrest the growth and replication of bacteria found on the ocular surface, eyelids, or those already iatrogenically introduced into the aqueous humor. Thus, these drugs limit the spread of infection while the body's immune system eliminates the nonproliferating pathogens. Bactericidal antibiotics kill the bacteria directly, decreasing the total concentration of viable microorganisms. Bactericidal agents are more commonly used in ocular surgery as they can achieve more rapid destruction of invading bacteria. Frequently used perioperative antibiotics with bactericidal properties include fluoroquinolones, vancomycin, aminoglycosides, and cephalosporins.
Why it is important to do this review
Cataract surgery is the most common operative procedure in the aged population. While endophthalmitis is relatively rare, the frequency of the procedure makes the absolute number of cases significant enough to be a public health problem. In 2003 to 2004, nearly 1.6 million cases of cataract surgery were performed annually in the US Medicare fee‐for‐service population alone (Schein 2012), and an estimated 10 million procedures were performed worldwide annually in the 1990s (Foster 2001). Experts estimate that the annual target for cataract surgery should be above 30 million surgeries (Foster 2001). At that rate, and assuming an incidence of one case per 1000 surgeries, 30,000 cases of postcataract surgery endophthalmitis would occur annually, with about 10,000 leading to blindness in the operated eye. Visual recovery following acute postoperative endophthalmitis remains poor across different clinical settings, despite advances in treatment (Lalitha 2005; Miller 2005; Ng 2005; Sheng 2011). The extensive use of surgery to provide better vision for people with cataracts across the world calls for adoption of evidence‐based methods to prevent acute endophthalmitis. This systematic review update aims to identify the current evidence to facilitate the adoption of evidence‐based practices for prophylaxis of acute endophthalmitis after cataract surgery.
Objectives
To evaluate the effects of perioperative antibiotic prophylaxis for endophthalmitis following cataract surgery compared with no prophylaxis or other form of prophylaxis.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs). We employed no date or language restrictions.
Types of participants
We included trials enrolling adults undergoing cataract surgery with any procedure for lens opacities due to any origin.
Types of interventions
We included trials evaluating preoperative antibiotics, intraoperative (intracameral, subconjunctival, or systemic), or postoperative antibiotic prophylaxis for acute endophthalmitis. Comparisons of interest included:
any prophylaxis versus no prophylaxis;
preoperative versus postoperative or intraoperative prophylaxis or combinations;
specific antibiotics used in included trials;
mode of perioperative antibiotic delivery.
We excluded studies that evaluated antiseptic preoperative preparation using agents such as povidone iodine. In addition, excluded studies that evaluated antibiotics for treating acute endophthalmitis after cataract surgery.
We excluded studies with less than one week of follow‐up after surgery.
Types of outcome measures
Primary outcomes
Endophthalmitis: both presumed and culture‐proven endophthalmitis within six weeks after cataract surgery. Our primary analysis was based on six‐week outcomes; however, we also evaluated data from weeks one to four.
Visual acuity (VA) measured either as a mean logMAR score or as the number of participants with best‐corrected VA better than 20/40 and those worse than 20/200 at the different follow‐up times. Whenever multiple VA measures were available, we used acuity at six weeks after diagnosis as the primary outcome measure.
Secondary outcomes
Adverse effects: specific adverse effects of interest were postoperative bacterial keratitis, antibiotic resistance if documented, allergy and anaphylaxis. We also summarized other adverse effects as reported in included trials.
Quality of life measures.
Economic data
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Trials Register) (2016, Issue 12), Ovid MEDLINE, Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE Daily (January 1946 to December 2016), Embase (January 1980 to December 2016), Latin American and Caribbean Health Sciences Literature Database (LILACS) (1982 to December 2016),the ISRCTN registry (www.isrctn.com/editAdvancedSearch), ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We used no date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 6 December 2016.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), LILACS (Appendix 4), the ISRCTN (Appendix 5), ClinicalTrials.gov (Appendix 6) and the ICTRP (Appendix 7).
Searching other resources
We searched for additional studies that cited any included references using the Science Citation Index Expanded database (Web of Science).
Data collection and analysis
Selection of studies
Two review authors independently reviewed the titles and abstracts resulting from the literature searches according to the inclusion criteria. We classified abstracts as 'definitely exclude', 'unsure' or 'definitely include'. We obtained the full‐text for articles in the 'unsure' category and reassessed them for inclusion. A third review author resolved any disagreement between the two review authors. Studies excluded after full‐text review are listed in the Characteristics of excluded studies table along with the reasons for exclusion.
Data extraction and management
We developed data extraction forms to collect data from the included studies. We tested the forms using a few studies prior to extracting data for all included studies. Two review authors independently extracted study characteristics, methods, and outcomes data, and assessed risk of bias for all included studies. The two review authors compared data extraction forms and resolved discrepancies between them by discussion. One review author entered the data into Review Manager 5 (RevMan 2014), and a second review author checked the entered data for accuracy.
Assessment of risk of bias in included studies
Two review authors independently assessed the included studies for risk of bias according to guidelines set out in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), and a third review author resolved any discrepancies. For each domain related to systematic biases, we made judgments of 'low risk of bias', 'unclear risk of bias', or 'high risk of bias' for each included study.
Selection bias: adequate sequence generation and allocation concealment. Examples of adequate sequence generation included using computerized randomization or random number lists. Methods such as centralized randomization and sequentially numbered, sealed, opaque envelopes provided adequate allocation concealment.
Performance bias: masking of study participants and personnel. For studies in which masking was not done or not possible (e.g. surgeons administering subconjunctival versus retrobulbar injections), we considered whether the person knowing the treatment assignment could have influenced the treatment effects.
Detection bias: masking of outcome assessors.
Attrition bias: incomplete outcome data. We assessed whether follow‐up rates and reasons for losses to follow‐up were similar in the comparison groups and whether all participants were analyzed in the group to which they were randomized.
Reporting bias: selective outcome reporting. Studies that reported results for all study outcomes described in the methods section of the included papers were considered to have low risks of reporting bias.
Other sources of bias: other potential sources of bias that were considered included, but were not limited to, funding source, study design, and imbalance in baseline characteristics.
A third review author resolved any disagreement in assessments by the two review authors. In the event of missing or unclear data, we contacted the primary investigators for additional information. We allowed six weeks for a response; failing that, we used the information as available in identified reports.
Measures of treatment effect
For individual studies, we presented dichotomous outcomes as risk ratios (RR) with 95% confidence intervals (CI). We did not conduct meta‐analyses as part of this review.
Unit of analysis issues
The unit of analysis was the individual (one eye per participant) in four studies (Christy 1979; Christy 1986; Cunha 2013; ESCRS 2007). In the Sobaci 2003 study, both eyes of 4/640 (less than 1%) participants were included in the analysis; for the remaining 636 participants, only one eye was included.
Dealing with missing data
In the event of missing or unclear data, we contacted the primary investigators for additional information. We allowed six weeks for a response; failing that, we used the information as available in identified reports. We analyzed outcome data using the available data, assuming data were missing at random. We did not perform missing data statistics as the proportion of missing data was low (less than 1% of included participants) in most studies.
Assessment of heterogeneity
We assessed clinical heterogeneity using qualitative information on trial methodology, participant characteristics, interventions compared, routes of administration of prophylactic measures, duration of follow‐up, and losses to follow‐up. We performed no statistical tests for heterogeneity.
Assessment of reporting biases
Typically, funnel plots are used to examine reporting biases when 10 or more studies contribute to a given outcome. In this review with only five included studies and no meta‐analysis, funnel plots were not appropriate.
Data synthesis
Because of the small number and heterogeneity of the included studies, we described data for each study narratively.
Subgroup analysis and investigation of heterogeneity
We performed no subgroup analyses.
Sensitivity analysis
We conducted no sensitivity analyses, given the small number of included studies.
'Summary of findings' table
We prepared a 'Summary of findings' table including relative and absolute effects for the outcome of endophthalmitis for all comparisons. We assessed the certainty of evidence for all outcomes in this review using the GRADE classification system (GRADEpro 2014).
Results
Description of studies
Results of the search
Electronic literature searches as of 25 October 2012 identified 491 potentially relevant titles and abstracts for this review (Gower 2013). After duplicate independent abstract review, 12 records were assessed at the full‐text level, of which four were excluded and eight were included in the review. The eight records reported four studies. A review of references that cited the included studies and the reference lists of included studies identified one additional record that was excluded after full‐text assessment.
An updated search as of December 2016 identified 157 new records (Figure 1). The Cochrane Information Specialist removed 50 duplicate records and we screened the remaining 107 reports. We rejected 101 records after reading the abstracts and obtained the full‐text reports of six references for further assessment. We identified one new study which met the inclusion criteria (Cunha 2013), and one ongoing trial (NCT02770729). We excluded four studies (Carron 2013; Cetinkaya 2015; Li 2015; Pérez‐Canales 2015; see Characteristics of excluded studies table for details).
1.
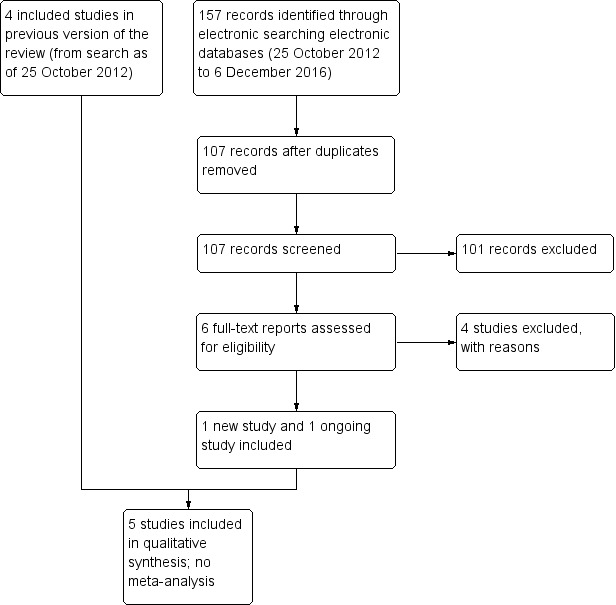
Study flow diagram.
Included studies
We include five RCTs in this review (see Characteristics of included studies table). The studies enrolled 101,005 adults undergoing cataract surgery. The five studies varied widely in the approaches and prophylactic measures examined.
The first two studies were conducted at cataract surgery camps in northern Pakistan where intracapsular cataract extraction was performed, and participants were followed postoperatively for one week (Christy 1979; Christy 1986). Endophthalmitis diagnosis was made based on clinical signs. Although intracapsular cataract extraction is rarely performed in the 21st century, and hence the relevance of these studies for contemporary consideration is reduced, the role of prophylactic antibiotics remains relevant to contemporary practice, and surgical camps remain a mainstay in many low‐income countries. Thus, these studies are described briefly.
Christy 1979 compared combined chloramphenicol‐sulfadimidine drops and periocular injection of 500,000 units of benzyl penicillin with chloramphenicol‐sulfadimidine drops alone in 6618 people/eyes. All participants were provided with a single dose of antibiotic ointment on the day prior to surgery and immediately after surgery; sulfadimidine 5% drops were instilled on subsequent days.
Christy 1986 compared subconjunctival versus retrobulbar injection of antibiotics in 77,015 people/eyes. All participants received five applications of a sulfadimidine‐chloramphenicol solution in the 20 hours before surgery. In both studies, participants were followed for one week after surgery and evaluated for endophthalmitis based on clinical signs.
The three more recent studies employed phacoemulsification.
Sobaci 2003 was conducted in Turkey and compared antibiotics (vancomycin and gentamycin) in balanced salt solution (BSS) irrigating infusion fluid with BSS‐only irrigating infusion fluid in 644 eyes of 640 participants. All were treated with ofloxacin and diclofenac sodium four times on the day prior to surgery. Povidone iodine was utilized for antisepsis at the time of surgery and a solution of ofloxacin, dexamethasone, and indomethacin was given postoperatively. Follow‐up was for six weeks postoperation. Since the incidence of endophthalmitis following cataract surgery is low (the study authors of Sobaci 2003 reported the rate of postoperative endophthalmitis at their institution was 0.109%) and because only 644 eyes were included in the study (with less than one eye expected to be affected), the study lacked sufficient power to detect valid differences between treatments.
ESCRS 2007 conducted at multiple sites throughout Europe and Turkey, implemented a two‐by‐two factorial design to evaluate intracameral cefuroxime injected at the end of surgery and topical levofloxacin given immediately preoperatively (within one hour of surgery) and up to 15 minutes following surgery in 16,603 participants. In a factorial design studying two drugs or procedures that are expected to act independently, treatment arms were allocated such that both drugs could be evaluated alone and in combination. In ESCRS 2007, the two interventions studied were intracameral cefuroxime and topical levofloxacin. One group received only intracameral cefuroxime, one group received only topical levofloxacin, one group received both intracameral cefuroxime and topical levofloxacin, and one group received neither intervention. Povidone iodine was used for antisepsis at the time of surgery and topical levofloxacin was given to all participants starting the morning after surgery. Follow‐up was for six weeks postoperation.
In Cunha 2013, all participants underwent phacoemulsification with IOL implantation. The use of a fixed combination of gatifloxacin 0.3% and prednisolone acetate 1% (i.e. both drugs in a single bottle) was compared with the administration of gatifloxacin 0.3% alone and prednisolone acetate 1% alone. Participants instilled the drops beginning one day before the cataract surgery until 15 days postoperation. Although the study authors reported endophthalmitis as an adverse outcome, the study was not designed to assess differences in endophthalmitis rates between intervention groups. Further, with only 129 enrolled participants, the study did not have sufficient power to detect valid differences between treatment groups.
Excluded studies
We excluded nine studies overall: six were not RCTs and three did not evaluate the risk of endophthalmitis (see Characteristics of excluded studies table).
Risk of bias in included studies
Allocation
Although all the included studies were RCTs, only ESCRS 2007 reported sufficient detail to be judged as having adequate sequence generation and allocation concealment (Figure 2). ESCRS 2007 used computerized randomization for sequence generation and coded droppers to conceal treatment assignments. Cunha 2013 reported using an adequate sequence generation method, but did not report whether the allocation sequence was concealed. We judged the remaining three studies as having unclear sequence generation and allocation concealment. Christy 1986 reported that a deck of cards marked with the treatment assignments was used to randomize participants to treatment groups. The treatment administered to the participant was determined by the card that was on the top of the deck at the time of surgery; however, it was not clear whether the markings on the cards were concealed prior to surgery. Sobaci 2003 reported that participants were randomly allocated to treatment group according to the scheduled day of surgery. However, it is unclear whether the treatment assignment was stratified by the day of surgery, or whether the day's treatment assignment was revealed at the start of the operative day and alternated from day to day. Christy 1979 did not report any information regarding allocation other than that the study was randomized.
2.
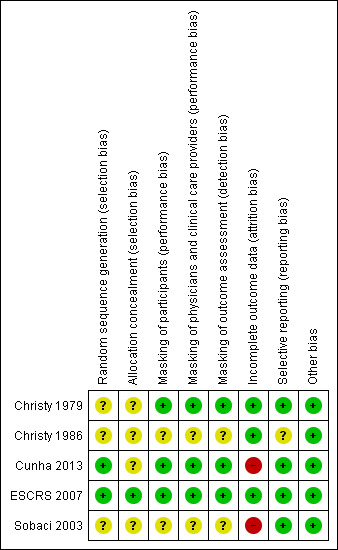
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Masking (performance bias and detection bias)
One study reported masking all study participants and personnel by distributing identical bottles with masked labels (Cunha 2013). ESCRS 2007 masked participants and clinicians by using coded droppers with either active or placebo drops. Participants and physicians were not masked for the injections since no sham injections were performed for those not receiving the intracameral cefuroxime injection. Participants and physicians who were present during the surgery were masked to the drops, and other clinical partners were masked to both drops and injections throughout the study. It was unclear whether the physicians who were present during the surgery or their clinical partners were assessing the outcomes for the study. One other study was reported to be masked, but details of who was masked and how masking was accomplished were not reported (Christy 1979). We assessed these three studies as having low risks of performance and detection bias, since masking was reported and we would not expect the diagnosis of endophthalmitis to be affected if masking was broken. The two remaining included studies did not report masking (Christy 1986; Sobaci 2003).
Incomplete outcome data
Risk of bias due to incomplete outcome data was low in three studies and high in two studies (Figure 2). In Sobaci 2003, judged as having a high risk of bias, eyes of participants for which the surgical procedure was modified according to physician discretion during surgery were excluded from the study. Reasons for modifying the protocol included administering subconjunctival antibiotics and adding a suture. The number of excluded participants was not reported. In Cunha 2013, more than 15% of participants were not included in the analyses, most due to missing the follow‐up visit. Christy 1979 and Christy 1986 reported no exclusions or losses to follow‐up; however, the study authors noted that data were limited to early postoperative infections occurring one week after surgery, since most participants lived too far away for follow‐up visits once discharged. The studies undertook no bacteriologic confirmation of infection. Although ESCRS 2007 reported following intent‐to‐treat analyses, 324 (2%) participants who were lost to follow‐up and 68 (0.4%) participants who did not undergo the planned surgery or withdrew consent (timing of withdrawal not specified) were excluded from the analyses.
Selective reporting
Risk of selective reporting bias in these studies was low. All studies employed commonly used methods for reporting endophthalmitis cases. Two studies reported results for suspected cases without bacteriological confirmation (Christy 1979; Christy 1986). Sobaci 2003 reported results for bacteriologically confirmed cases, and ESCRS 2007 reported results for all suspected cases as well as for bacteriologically confirmed cases. Cunha 2013 did not report how endophthalmitis was diagnosed.
Christy 1986 used two types of antibiotics for injections, determined by the surgeon doing the operation. The study authors reported that infection rates were similar between the two types of antibiotics and the two surgeons, but did not report infection rates for treatment groups (anterior versus posterior injections) separately by type of antibiotic.
Other potential sources of bias
We did not identify other potential sources of bias for the included studies.
Effects of interventions
See: Table 1
The results of the five studies are described individually below. Interventions differed between them. Given the heterogeneity of study designs and modes of antibiotic delivery, we decided against conducting meta‐analyses. We describe outcome data and present a summary of postoperative endophthalmitis for all comparisons in Table 1.
The primary outcome for four studies was postoperative endophthalmitis following cataract surgery; the fifth study investigated prophylaxis and control of inflammation following cataract surgery with endophthalmitis reported as an adverse outcome. The two earliest studies relied on clinical diagnosis of endophthalmitis (Christy 1979; Christy 1986). Sobaci 2003 reported results for bacteriologically confirmed cases only, ESCRS 2007 reported results for all suspected cases as well as the subset of bacteriologically confirmed cases, and Cunha 2013 did not report how endophthalmitis was defined.
Perioperative prophylaxis versus no prophylaxis
Irrigation with antibiotics in balanced salt solution versus balanced salt solution alone
In Sobaci 2003, at six weeks 0/322 (0%) eyes that received vancomycin and gentamycin in BSS irrigating infusion fluid had postoperative endophthalmitis compared with 2/322 (0.62%) eyes that received BSS‐only irrigating infusion fluid. The between‐group difference reflected the small number of cases that the study was not powered to detect a difference (RR 0.20, 95% CI 0.01 to 4.15). We assessed the certainty of evidence for this outcome as very low, downgrading for imprecision of the effect estimate and high risk of attrition bias in the study.
Intracameral with or without topical antibiotics versus no antibiotics
In ESCRS 2007, the risk of clinically diagnosed (presumed) postoperative endophthalmitis at six weeks was significantly reduced for eyes that received intracameral cefuroxime injections, with or without topical levofloxacin, compared with no prophylaxis (neither injection nor topical levofloxacin) (RR 0.14, 95% CI 0.03 to 0.63 with topical levofloxacin; RR 0.21, 95% CI 0.06 to 0.74 without topical drops). There were similar results when analyzing culture‐proven cases of postoperative endophthalmitis. We assessed the certainty of evidence for these outcomes as high, finding no reason to downgrade the assessment.
The effect of topical levofloxacin alone compared with no prophylaxis to reduce the risk of postoperative endophthalmitis was less certain (RR 0.72, 95% CI 0.32 to 1.61 for presumed cases; RR 0.70, 95% CI 0.27 to 1.84 for culture‐proven cases). We assessed the certainty of evidence for this outcome as moderate, downgrading for imprecision.
Comparisons of combinations of antibiotics with specific antibiotics
Chloramphenicol‐sulfadimidine drops with versus without periocular penicillin
In the Christy 1979 study of chloramphenicol‐sulfadimidine drops with or without periocular penicillin injection, 5/3309 (0.15%) eyes that received combined prophylaxis (drops and injection at the time of surgery) had postoperative endophthalmitis at one week, compared with 15/3309 (0.45%) eyes that received the topical regimen alone (RR 0.33, 95% CI 0.12 to 0.92). We assessed the certainty of evidence for this outcome as moderate, downgrading for indirectness as the study was conducted in the mid‐to‐late 1970s and the techniques for cataract surgery have since changed substantially.
Intracameral and topical antibiotics versus either antibiotic alone
In ESCRS 2007, a risk reduction was observed for eyes treated with combined intracameral cefuroxime and topical levofloxacin compared with eyes treated with topical levofloxacin alone for presumed cases of postoperative endophthalmitis (RR 0.20, 95% CI 0.04 to 0.91), but this difference was less precise for culture‐proven cases (RR 0.14, 95% CI 0.02 to 1.16). We assessed the certainty of evidence for this outcome as high, finding no reason to downgrade the assessment.
When comparing combined intracameral cefuroxime and topical levofloxacin with eyes treated with intracameral cefuroxime alone, the difference of postoperative endophthalmitis was unclear (RR 0.67, 95% CI 0.11 to 3.99 for presumed cases; RR 0.50, 95% CI 0.05 to 5.52 for proven cases). We assessed the certainty of evidence for this outcome as moderate, downgrading for imprecision.
Additionally, the head‐to‐head comparison of intracameral cefuroxime alone compared with topical levofloxacin alone suggested that intracameral cefuroxime may perform better or as good as topical levofloxacin for preventing postoperative endophthalmitis (RR 0.30, 95% CI 0.08 to 1.09 for presumed cases; RR 0.29, 95% CI 0.06 to 1.37 for proven cases). We assessed the certainty of evidence for this outcome as moderate, downgrading for imprecision.
Mode of antibiotic delivery
Subconjunctival versus retrobulbar antibiotic injection
In Christy 1986, at one week after surgery, 38/39,752 (0.10%) eyes receiving subconjunctival injection had presumed postoperative endophthalmitis, compared with 42/37,263 (0.11%) eyes receiving retrobulbar antibiotic injection. The risk of postoperative endophthalmitis was similar between groups (RR 0.85, 95% CI 0.55 to 1.32). We assessed the certainty of evidence for this outcome as moderate, downgrading for indirectness as the techniques of the cataract surgery used in the study were different compared with current cataract surgery methods.
Fixed combination versus individual instillation of topical antibiotic and corticosteroid
Cunha 2013 compared fixed combination versus individual instillation of gatifloxacin 0.3% and prednisolone acetate 1%. None of 47 eyes that received the fixed combination had postoperative endophthalmitis compared with 1/61 (2%) eyes that received the individual drops up to 20 days postoperation (RR 0.43, 95% CI 0.02 to 10.34). Due to the small number of participants and events in the study, the analysis was not powered to detect a difference between groups. We assessed the certainty of evidence for this outcome as very low, downgrading for imprecision of the effect estimate and high risk of attrition bias in the study.
Visual acuity
Only one study reported VA outcomes for postoperative endophthalmitis (ESCRS 2007). Cunha 2013 reported that mean VA was the same for both groups at baseline (0.3 logMAR) and 20 days postoperation (0.1 logMAR). No other study reported VA outcomes.
ESCRS 2007 presented outcomes in a combined manner for both intracameral cefuroxime injection groups compared to topical levofloxacin or no prophylaxis groups combined (Table 2).
1. Visual acuity following endophthalmitis.
| Comparisons of specific antibiotics or combinations of antibiotics | |||||||||
| Study ID | Groups | Proportion of eyes with final VA > 20/40 following endophthalmitis | RR (95% CI) Group 1 vs group 2 | Proportion of eyes with final VA < 20/200 following endophthalmitis | RR (95% CI) Group 1 vs group 2 | ||||
| Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | Presumed cases* | Proven cases** | ||
| ESCRS 2007 | Group 1: intracameral cefuroxime injection, with or without topical levofloxacin drops | 2/5 (40%) eyes | 1/3 (33.3%) eyes | 0.69 (0.22 to 2.11) | 0.57 (0.11 to 2.95) | 0/5 (0%) eyes | 0/3 (0%) eyes | 0.46 (0.03 to 7.48) | 0.50 (0.03 to 7.54) |
| Group 2: no injection, with or without topical levofloxacin drops | 14/24 (58.3%) eyes | 10/17 (58.1%) eyes | 4/24 (16.7%) eyes | 4/17 (23.5%) eyes | |||||
CI: confidence interval; final VA: visual acuity at time of last follow‐up visit (range 3 weeks to 8 months); VA: visual acuity.
*Presumed cases: includes both culture‐proven and clinically diagnosed cases of postoperative endophthalmitis.
**Proven cases: cases confirmed by at least one of Gram stain, culture, or polymerase chain reaction (PCR).
Proportion of eyes with final visual acuity greater than 20/40 following endophthalmitis
Among the five presumed cases of postoperative endophthalmitis who received intracameral cefuroxime injections, two (40%) had final VA better than 20/40. Among the 24 presumed cases of postoperative endophthalmitis who did not receive intracameral cefuroxime injections, 14 (58.3%) had final VA better than 20/40. The difference between antibiotic injection and no injection groups was uncertain (RR 0.69, 95% CI 0.22 to 2.11). There were similar results for culture‐proven cases between antibiotic injection (1/3 (33.3%) eyes) and no injection (10/17 (58.1%) eyes) groups (RR 0.57, 95% CI 0.11 to 2.95). We assessed the certainty of evidence for this outcome as moderate, downgrading for imprecision.
Proportion of eyes with final visual acuity less than 20/200 following endophthalmitis
Among the five presumed cases of postoperative endophthalmitis who received intracameral cefuroxime injections, none had final VA worse than 20/200. Among the 24 presumed cases of postoperative endophthalmitis who did not receive intracameral cefuroxime injections, four (16.7%) had final VA worse than 20/200. This difference between injection and no‐injection groups was very imprecise (RR 0.46, 95% CI 0.03 to 7.48). There were similar results for proven cases between the injection (0/3 (0%) eyes) and no injection (4/17 (23.5%) eyes) groups (RR 0.50, 95% CI 0.03 to 7.54). We assessed the certainty of evidence for this outcome as moderate, downgrading for imprecision.
Adverse effects
Cunha 2013 was the only study to report adverse events.
Cunha 2013 did not report information specific to postoperative bacterial keratitis, antibiotic resistance, allergy, or anaphylaxis. At 20 days postoperation, the one eye in the individual drops group with endophthalmitis had pupillary membrane in front of the IOL. Three eyes (6%) in the fixed combination group compared with five eyes (8%) in the individual drops group showed posterior capsule opacity. The study authors reported no cases of hypopyon or IOL pigmentation and no statistically significant difference between group with respect to central or incisional corneal edema.
Quality of life
No study reported outcomes related to quality of life measures.
Economic outcomes
No study reported outcomes related to economic data.
Discussion
Summary of main results
The studies included in this review were too heterogeneous for us to perform a meta‐analysis. The five included studies tested three modes of delivery for antibiotic prophylaxis measures: intraocular injection, topical drops, and antibiotics in the irrigating solution. The two studies that reported statistically significant differences among treatment arms both included antibiotic injection during surgery (one intraocular and the other periocular), and the treatment arms that included ocular injection had the lowest rates of endophthalmitis, ranging from 0.14 to 1.5 endophthalmitis cases per 1000 surgeries (Christy 1979; ESCRS 2007). Within the ESCRS 2007 study, the primary results paper combined the two intracameral injection groups for comparison against placebo and reported a 4.9‐fold increased risk of endophthalmitis when not using intracameral injection, which can be translated to an 80% decrease in endophthalmitis risk when using intracameral injection.
In this review, we calculated RRs and CIs for multiple comparisons within ESCRS 2007 using the data provided in the study reports; we compared both intracameral injection of cefuroxime and topical drops individually to placebo and to the combined regimen. Both the combined prophylaxis and the intracameral injection alone showed a reduced risk of both presumed and culture‐proven endophthalmitis compared with no prophylaxis. Comparison of the combined regimen against topical drops alone showed a reduction in risk for presumed cases, but not for culture‐proven cases only.
Sobaci 2003 compared antibiotics in the irrigating solution against BSS alone, and reported a lower endophthalmitis rate (0/322 eyes versus 2/322 eyes up to six weeks after surgery) in the treatment arm that included antibiotics (RR 0.20, 95% CI 0.01 to 4.15). If the study were adequately powered, this difference would translate to an 80% reduction in endophthalmitis. However, the overall sample size for this study was quite small (644 eyes) for a rare outcome like endophthalmitis, limiting the ability to evaluate statistical significance, as noted by the wide CIs (95% CI 0.01 to 4.15). Similarly, Cunha 2013 enrolled 129 participants, with only 108 participants analyzed at 20 days postoperation, resulting in a high degree of imprecision among outcomes.
Christy 1986 investigated the mode of delivery of antibiotics injected during surgery, and found no significant difference between subconjunctival and retrobulbar injection. The rates of endophthalmitis (1.0 to 1.1 per 1000 surgeries) in that study were comparable to endophthalmitis rates reported in the 21st century, even though surgery was performed in surgical camp settings in a developing country and that intracapsular surgery was performed. However, it is notable that this study only followed participants for one week, so some cases likely were missed.
Overall completeness and applicability of evidence
The five studies included 101,005 adults and 132 total endophthalmitis cases. While the overall sample size was quite large, the heterogeneity of the study settings, designs, and modes of antibiotic delivery made it impossible to combine the studies and make direct comparisons. Two studies were conducted in the late 1970s and early 1980s. Cataract surgery practice has changed substantially since that time, making the results of these studies less applicable today. Povidone iodine is now used routinely in most countries and is a proven measure for reducing intraocular infection (Speaker 1991). In addition, wound construction is quite different. In the 1970s and early 1980s large (180°) incisions were used routinely. Today, even in the most remote centers, much smaller incisions are employed. Small‐incision manual surgery is now the procedure of choice in the majority of surgical camp settings in low‐income countries. Despite these changes in surgical technique, Christy 1979 suggested that adding periocular penicillin injection substantially reduced the risk of endophthalmitis.
Among the five studies, the ESCRS 2007 results are most applicable to 21st century surgical practice, as it used contemporary surgical techniques and study drugs that are readily available in Europe. Its design allowed for examination of both topical and intracameral antibiotics, and included a sample size sufficient to yield statistically significant results. Thus, among the studies reviewed, it provided the firmest evidence upon which to recommend a prophylactic regimen in these settings, and suggested that intracameral antibiotic injection is useful in reducing the risk of postcataract surgery endophthalmitis. However, the choice of antibiotic remains a question for many physicians. Since the publication of ESCRS 2007, uptake of intracameral cefuroxime has varied widely. In the UK, approximately 50% of providers reported intracameral antibiotic use (Gore 2009). A retrospective analysis of billing codes in France suggested that the use of intracameral antibiotics increased from 0.60% to 80% between 2005 and 2014, likely due to the ESCRS recommendations (Creuzot‐Garcher 2016). Uptake has been more limited in the US. Results of the 2011 American Society of Cataract and Refractive Surgery (ASCRS) member survey showed less than 20% of physicians utilizing intracameral antibiotics (Leaming 2012; Vazirani 2013). By the ASCRS 2014 survey, 50% of US physicians reported use of intracameral antibiotics (Chang 2015); however, this percentage remains well below the rates in Europe, Australia and New Zealand (Behndig 2015; Meyer 2016;Schwartz 2016), and US physicians continue to express concerns about the lack of a commercially available preparation. In the US, incorporating intracameral antibiotics into standard prophylaxis practice appears to be related to surgeon volume (Chang 2008), and increased surgeon volume has been reported to be associated with reduced risk of postoperative endophthalmitis (Keay 2012). In both the US and UK, physicians not using intracameral antibiotics cite concerns regarding dilution errors and risk of contamination when compounding the drugs for doses needed for ocular injection (Gore 2009; Leaming 2012). These factors are important to consider when evaluating the applicability of the current evidence. Further discussion on this issue is provided below in the Agreements and disagreements with other studies or reviews section.
Quality of the evidence
The five studies included in this review varied substantially in the prophylaxis measures that they compared and the way data were reported. Two of these studies, Christy 1979 and Christy 1986, were conducted over 30 years ago, when standards for randomization and reporting in clinical trials were less stringent and less well‐defined. To the credit of the Christy team, they provided detailed information on both the operative procedure and the follow‐up procedures. All five studies randomized participants, but random sequence generation and allocation concealment were described fully only in ESCRS 2007. Hence, we cannot judge whether some selection bias may have occurred in the other studies.
Masking of the intervention was not complete in most of these studies. In some, the person in charge of outcome assessment was unaware of the randomization and did not actively participate in the surgery visits. These studies may be less prone to bias than those in which the examiner was present at all times and was aware of the treatment assignment.
Because of differences in the interventions used and outcomes assessed among studies, we performed no meta‐analysis. Overall, one study provided moderate‐ to high‐certainty evidence (ESCRS 2007); two studies, which were downgraded for indirectness, provided moderate‐certainty evidence (Christy 1979; Christy 1986); and two studies, which were downgraded for high risk of attrition bias and imprecision, provided very low‐certainty evidence (Cunha 2013; Sobaci 2003).
Potential biases in the review process
To minimize bias with regard to selecting studies for this review, we devised a highly sensitive search strategy to identify relevant studies from the published literature. We also searched other sources such as the reference lists of included studies, the Science Citation Index, and clinical trial registries. We imposed no date or language restrictions.
Two review authors independently performed major steps in the review process to minimize bias and errors when screening studies for inclusion, recording study characteristics, extracting quantitative data, and assessing risks of bias. The review team included clinicians, researchers, and methodologists.
Agreements and disagreements with other studies or reviews
The rare nature of endophthalmitis makes RCTs difficult to conduct, because of the very large sample sizes needed to make statistically valid comparisons. Thus, few trials have been conducted, and all that we are aware of are included here. Worldwide, numerous single‐center retrospective analyses have been conducted to examine whether changes in practice patterns resulted in reduced endophthalmitis rates. Several studies have reported reduced rates of endophthalmitis following adoption of intracameral or subconjunctival antibiotics (Beselga 2014; Garat 2009; Garcia‐Saenz 2010; Montan 2002; Myneni 2013; Packer 2011; Shorstein 2013). The antibiotic of choice has varied across studies of intracameral injections, with moxifloxacin, vancomycin, and cefuroxime all showing a reduction compared with no antibiotic injection (Packer 2011). Most notably, numerous studies have investigated the benefit of intracameral cefuroxime in light of the ESCRS study and increased availability of the antibiotic in Europe. Data from Sweden have shown extremely low rates of postoperative endophthalmitis (0.029%) in the presence of intracameral cefuroxime (Friling 2013). In Portugal, a center found that endophthalmitis decreased from 0.26% to 0.0% after introduction of the ESCRS protocol and use of intracameral cefuroxime (Beselga 2014). Several other studies also have reported declines in endophthalmitis. Across these studies, the general consensus has been that intracameral antibiotic use reduces the risk of endophthalmitis, which provides further support to the ESCRS 2007 study findings.
Authors' conclusions
Implications for practice.
This systematic review underscores the broad scope of prophylaxis regimens considered to be of potential use in preventing endophthalmitis following cataract surgery. Among the included studies, the mode of antibiotic administration ranged widely from topical administration preoperatively to intraocular injections during surgery. Given our decision not to conduct a meta‐analysis of the accumulated data, we are unable to report a direct comparison of the effectiveness of these prophylactic measures. However, among the individual studies evaluated, ESCRS 2007 provides the best evidence for antibiotic prophylaxis against postcataract surgery endophthalmitis. Clinical trials of this magnitude are costly and take many years to conduct. Hence, decisions currently need to be made based on the available evidence, with the possibility of conducting future trials as new and improved prophylactic treatments become available (antibiotics or otherwise).
ESCRS 2007 demonstrated the efficacy of using intracameral antibiotics for reducing endophthalmitis. However, the antibiotic of choice may differ based on the clinical setting. Concerns of toxicity, contamination, and other problems associated with compounding and diluting remain with the use of cefuroxime (Chang 2015; Delyfer 2011; Olavi 2012). US physicians indicate they may increase their use of intracameral antibiotics if a single‐dose vial were commercially available (Chang 2015). Hence, the American Society of Cataract and Refractive Surgeons (ASCRS) has called for the pharmaceutical industry and US Food and Drug Administration to prioritize development and approval of single‐dose intracameral antibiotics (Braga‐Mele 2014; Chang 2015). One report of ocular toxicity in a cluster of people following a dilution error highlights the need for clinicians to remain vigilant in monitoring preparation of intracameral antibiotics for use during surgery. Resistance of endophthalmitis‐causing organisms to moxifloxacin appears to be increasing (Schimel 2013). In determining whether to use intracameral injections and if so which antibiotic to use, individual surgical centers should evaluate the risks and benefits associated with the various available intracameral antibiotics and the resources available for ensuring appropriate dilution and sterility based on their setting (Olavi 2012).
Implications for research.
This review highlights the limited amount of randomized controlled trial data available for evaluating measures used to prevent endophthalmitis following cataract surgery. Intraocular antibiotics have been used for decades, with multiple studies conducted in surgical camp settings in low‐income countries. However, speculation remains over which antibiotics to use and what mode of intraocular delivery is best.
Since the mid‐2000s, a great deal of attention has been placed on the value of adding an intracameral injection to the perioperative regimen, and the findings of ESCRS 2007 support the effectiveness of this practice. Several case reports and other retrospective analyses have examined a variety of antibiotics for use in intracameral injection at the close of cataract surgery (Packer 2011). To date, however, the ESCRS 2007 study provides the only prospective clinical trial evidence for the use of intracameral antibiotics. The reluctance of many physicians to utilize intracameral injections with currently available antibiotic preparations highlights the need for the development of commercially available single‐use vials of antibiotics for intracameral injection. A single‐use vial of cefuroxime, which requires only reconstitution and not dilution, was approved in Europe in 2012 (Thea Laboratories; Keating 2013). Numerous studies in Europe have reported increases in the use of intracameral antibiotics and corresponding decreases in rates of endophthalmitis following the increased use of cefuroxime (Barreau 2012; Beselga 2014; Daien 2016; Haripriya 2016; Jabbarvand 2016). Surveys should be conducted in one to two years to determine whether increased utilization of intracameral antibiotics leads to increased antibiotic resistance or other ocular complications, or both. Additionally, single‐use vials of antibiotics for intracameral use are needed in the US and other countries. Some ophthalmologists currently utilize moxifloxacin (Alcon) topical drops for intracameral injection without dilution. Given that the coverage spectrum of moxifloxacin is somewhat broader than that of cefuroxime and available for use without dilution, it would be of interest to research the comparative effectiveness of these two drugs. However, such a study would require a sample size of over 100,000 participants, making it unlikely that it will ever be conducted. On a longer view, new approaches to instilling antibiotics into the anterior chamber are needed. Sustained drug‐delivery devices provide one example of an area ripe for additional research.
What's new
| Date | Event | Description |
|---|---|---|
| 6 December 2016 | New search has been performed | Issue 2, 2017: searches updated |
| 6 December 2016 | New citation required but conclusions have not changed | Issue 2, 2017: one new study added (Cunha 2013); one ongoing trial identified (NCT02770729) |
History
Protocol first published: Issue 1, 2007 Review first published: Issue 7, 2013
| Date | Event | Description |
|---|---|---|
| 19 August 2008 | Amended | Converted to new review format. |
Acknowledgements
We acknowledge Iris Gordon, Information Specialist for Cochrane Eyes and Vision (CEV), who designed strategies for and conducted the electronic searches. We acknowledge Niall Crosby, Barbara Hawkins, and Tianjing Li for their comments on the protocol and Lisa Herrinton, Neal Shorstein, and Barbara Hawkins for peer reviewing the original review.
We are grateful to Roy Chuck (RC), Ashley Behrens (AB), and Satyanarayana Vedula (SV) for their contributions to developing and publishing the protocol for this review.
Appendices
Appendix 1. CENTRAL search strategy
IDSearch #1 MeSH descriptor: [Ophthalmologic Surgical Procedures] explode all trees #2 MeSH descriptor: [Cataract] explode all trees #3 MeSH descriptor: [Cataract Extraction] explode all trees #4 cataract* near/3 extract* or aspirat* or operat* or remov* or surg* or excis* or implant* #5 lens* near/3 extract* or aspirat* or operat* or remov* or surg* or excis* or implant* #6 pha?oemulsif* #7 lensectomy #8 #1 or #2 or #3 or #4 or #5 or #6 or #7 #9 MeSH descriptor: [Endophthalmitis] explode all trees #10 endophthalmitis #11 ophthalmia #12 #9 or #10 or #11 #13 MeSH descriptor: [Anti‐Bacterial Agents] explode all trees #14 antibiotic* #15 bacteri* #16 chloramphenicol* #17 MeSH descriptor: [Ciprofloxacin] explode all trees #18 ciprofloxacin* #19 fusidic acid* #20 gentamicin* #21 levofloxacin* #22 neomycin* #23 ofloxacin* #24 polymyxin* B #25 cefazolin* #26 MeSH descriptor: [Cefuroxime] explode all trees #27 cefuroxime* #28 moxifloxacin* #29 norfloxacin* #30 MeSH descriptor: [Vancomycin] explode all trees #31 vancomycin* #32#13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 #33 MeSH descriptor: [Antibiotic Prophylaxis] explode all trees #34 prophyla* #35 prevent* #36 #33 or #34 or #35 #37 #8 and #12 and #32 #38 #36 and #37
Appendix 2. MEDLINE Ovid search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp ophthalmologic surgical procedure/ 14. exp cataract/ 15. exp cataract extraction/ 16. ((cataract$ adj3 extract$) or aspirat$ or operat$ or remov$ or surg$ or excis$ or implant$).tw. 17. ((lens$ adj3 extract$) or aspirat$ or operat$ or remov$ or surg$ or excis$ or implant$).tw. 18. pha?oemulsif$.tw. 19. lensectomy.tw. 20. or/13‐19 21. exp endophthalmitis/ 22. endophthalmitis.tw. 23. ophthalmia.tw. 24. or/21‐23 25. exp anti bacterial agents/ 26. antibiotic$.tw. 27. bacteri$.tw. 28. chloramphenicol$.tw. 29. exp ciprofloxacin/ 30. ciprofloxacin.tw. 31. (fusidic adj2 acid$).tw. 32. exp gentamicin/ 33. gentamicin$.tw. 34. exp levofloxacin/ 35. levofloxacin$.tw. 36. neomycin$.tw. 37. ofloxacin$.tw. 38. (polymyxin$ adj1 B).tw. 39. cefazolin$.tw. 40. exp cefuroxime/ 41. cefuroxime$.tw. 42. moxifloxacin$.tw. 43. norfloxacin$.tw. 44. exp vancomycin/ 45. vancomycin$.tw. 46. or/21‐45 47. exp antibiotic prophylaxis/ 48. prophyla$.tw. 49. prevent$.tw. 50. or/47‐49 51. 20 and 24 and 46 52. 50 and 51 53. 12 and 52
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase Ovid search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. exp cataract/ 34. exp cataract extraction/ 35. ((cataract$ adj3 extract$) or aspirat$ or operat$ or remov$ or surg$ or excis$ or implant$).tw. 36. ((lens$ adj3 extract$) or aspirat$ or operat$ or remov$ or surg$ or excis$ or implant$).tw. 37. pha?oemulsif$.tw. 38. lensectomy.tw. 39. or/33‐38 40. exp endophthalmitis/ 41. endophthalmitis.tw. 42. ophthalmia.tw. 43. or/40‐42 44. exp antiinfective agent/ 45. antibiotic$.tw. 46. bacteri$.tw. 47. chloramphenicol$.tw. 48. ciprofloxacin.tw. 49. (fusidic adj2 acid$).tw. 50. gentamicin$.tw. 51. levofloxacin$.tw. 52. neomycin$.tw. 53. ofloxacin$.tw. 54. (polymyxin$ adj1 B).tw. 55. cefazolin$.tw. 56. cefuroxime$.tw. 57. moxifloxacin$.tw. 58. norfloxacin$.tw. 59. vancomycin$.tw. 60. or/44‐59 61. exp antibiotic prophylaxis/ 62. prophyla$.tw. 63. prevent$.tw. 64. or/61‐63 65. 39 and 43 and 60 66. 64 and 65 67. 32 and 66
Appendix 4. LILACS search strategy
cataract$ or phacoemulsification or IOL and endophthalmitis
Appendix 5. ISRCTN Trials search strategy
(cataract OR phacoemulsification OR IOL) AND endophthalmitis
Appendix 6. ClinicalTrials.gov search strategy
(cataract OR phacoemulsification OR IOL) AND endophthalmitis
Appendix 7. ICTRP search strategy
cataract AND endophthalmitis
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Christy 1979.
| Methods |
Study design: randomized controlled trial Exclusions and loss to follow‐up: none reported Study follow‐up: 1 week |
|
| Participants |
Setting: cataract surgery camp at Christian Hospital, Taxila, Pakistan Enrollment: 6618 people undergoing cataract surgery Age: not reported Gender: not reported Inclusion criteria: normal intraocular pressure; patent lacrimal drainage system Exclusion criteria: active signs of ocular infection or inflammation |
|
| Interventions |
Intervention 1: topical regimen alone (chloramphenicol‐sulfadimidine drops) Intervention 2: combined prophylaxis (topical regimen + periocular penicillin during surgery) General: all surgeries were performed by 1 surgeon; surgical technique, postoperative treatment, and follow‐up were identical for both groups. Preoperative treatment: on the day prior to surgery, all participants' faces were washed with soap and water, eyelashes were clipped, and antibiotic ointment was applied to the conjunctival sac. At the time of surgery, procaine 2% and retrobulbar blocks (lidocaine 2 mL of 2% with hyaluronidase 6 units/mL) were administered, participants' eyelids and surrounding face washed with sterile water, a lid speculum was inserted, and the conjunctival sac irrigated with sterile water. Surgical technique: the surgeon used intracapsular cataract extraction procedure and did not rescrub hands between cases or use gloves. All instruments were sterilized with a speed autoclave. Operative technique included a 180° von Graefe knife incision; 1 peripheral iridectomy; 1 to 3 virgin silk corneoscleral sutures placed after the iridectomy but before the lens extraction and forceps delivery of the lens. After the operation, 1 drop of medication (pilocarpine 4%, polymyxin B sulfate 5000 IU/mL, neomycin sulfate 2.5 mg/mL, and hydrocortisone acetate 5 mg/mL) was placed in the conjunctival sac and a sterile pad placed over the eye. Postoperative treatment: eyes were examined and dressed daily. Antibiotic ointment was instilled on the first day and on subsequent days a drop of sulfadimidine 5% and 1 drop of atropine 1% were instilled. Participants without complications were hospitalized for 1 week. |
|
| Outcomes |
Primary outcome: risk of clinical postoperative endophthalmitis within 1 week after surgery; diagnosis was determined by slit lamp evaluation showing significant inflammation in the anterior chamber; no bacterial cultures were taken. Unit of analysis: the participant (1 eye per person) |
|
| Notes |
Study dates: March to November 1977 Funding source: not reported Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomization was not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported. |
| Masking of participants (performance bias) | Low risk | Although the study was reported to be masked, details of masking or the use of placebo were not reported. |
| Masking of physicians and clinical care providers (performance bias) | Low risk | Although the study was reported to be masked, details of masking or the use of placebo were not reported. |
| Masking of outcome assessment (detection bias) | Low risk | Although the study was reported to be masked, details of masking were not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions or loss to follow‐up were reported; however, the study authors noted that data were limited to early postoperative infections occurring 1 week after surgery since most participants lived too far away for follow‐up visits once discharged. |
| Selective reporting (reporting bias) | Low risk | Results were reported for the primary outcome. |
| Other bias | Low risk | No other potential sources of bias identified. |
Christy 1986.
| Methods |
Study design: randomized controlled trial Exclusions and loss to follow‐up: none reported Study follow‐up: 1 week |
|
| Participants |
Setting: cataract surgery camp at Christian Hospital, Taxila, Pakistan Enrolment: 77,015 people undergoing cataract surgery Age: not reported Gender: not reported Inclusion criteria: adults having nonimplant intracapsular cataract extractions Exclusion criteria: people receiving intraocular lenses and children with congenital or juvenile cataracts |
|
| Interventions |
Intervention 1: anterior sub‐Tenon injections (subconjunctival); given beside the limbus exactly subconjunctival or beneath the anterior part of Tenon's capsule Intervention 2: posterior sub‐Tenon injections (retrobulbar); given beside the eye behind the equator of the globe General: 2 types of antibiotics were used for the injections: benzyl penicillin 500,000 units/0.5 mL or ampicillin 200 mg/0.5 mL Preoperative treatment: all participants received 5 applications of 1 drop of sulfadimidine 10% and chloramphenicol 0.5% solution between the first preoperative examination and surgery (about a 13‐ to 22‐hour period). Surgical technique: the surgeons used intracapsular cataract extraction and did not rescrub hands between cases or use gloves. Surgeons were careful not to touch any needle, suture, or part of any instrument that would come into contact with the participants' eyes. Operations were performed quickly to keep the eye open for only 3 to 4 minutes. Most operations included a 180° von Graefe knife incision; 1 peripheral iridectomy; 3 to 5 virgin silk sutures placed after the incision but before the lens extraction; and intracapsular lens extraction performed with a simplified efficient cryoprobe. |
|
| Outcomes |
Primary outcome: risk of clinical postoperative endophthalmitis 1 week after surgery; diagnosis was determined by slit lamp evaluation showing significant inflammation in the anterior chamber, no bacterial cultures were taken Unit of analysis: the participant (1 eye per person) |
|
| Notes |
Study dates: January 1979 to June 1985 Funding source: not reported Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A deck of marked cards was used to randomize participants to treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | A deck of marked cards was shuffled daily and the top card at the time of surgery was used to allocate participants to treatment group. It is unclear whether the marks were concealed (face‐down) prior to allocation or whether the study personnel could preview the order of cards prior to allocation. |
| Masking of participants (performance bias) | Unclear risk | Masking of participants was not reported. |
| Masking of physicians and clinical care providers (performance bias) | Unclear risk | Surgeons could not be masked to the interventions. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions or loss to follow‐up were reported; however, follow‐up was only for 1 week after surgery. |
| Selective reporting (reporting bias) | Unclear risk | 2 types of antibiotics were used for the injections depending on the surgeon doing the operation. The study authors reported that infection rates were similar between the 2 types of antibiotics and the 2 surgeons, but did not report infection rates by treatment group (anterior vs posterior injections) separately by type of antibiotic. |
| Other bias | Low risk | No other potential sources of bias identified. |
Cunha 2013.
| Methods |
Study design: randomized controlled trial Exclusions and loss to follow‐up: 21 (16%) participants were excluded; 20 because they missed a scheduled follow‐up visit and 1 due to ocular trauma requiring another surgery Study follow‐up: 20 days |
|
| Participants |
Setting: Hospital of the Medical School of the University of São Paulo, São Paulo, Brazil Enrolment: 129 people undergoing cataract surgery Age: group 1: 71 ± 10 years (range 44 to 88); group 2: 71 ± 10 years (range 41 to 88) Gender: 35/108 (32%) men and 73/108 (68%) women Inclusion criteria: participants undergoing phacoemulsification and intraocular lens implantation. Exclusion criteria: "history of uveitis or chronic ocular inflammation, pseudoexfoliation syndrome, history of ocular trauma, uncontrolled diabetes, pregnant and nursing women, allergy or sensitivity to any component of the medications, serious systemic diseases and perioperative complications, such as anterior capsule rupture and vitreous loss." |
|
| Interventions |
Intervention 1: fixed combination of gatifloxacin 0.3% and prednisolone acetate 1% (Zypred, Allergan) Intervention 2: individual instillation of gatifloxacin 0.3% and prednisolone acetate 1% (Zypred and Predfort) General: each participant received 2 bottles; drops instilled every 6 hours 1 day prior to surgery to 15 days postoperation Preoperative treatment: not reported Surgical technique: the surgeons performed phacoemulsification and intraocular lens implantation using the "phaco chop technique" under topical anesthesia. |
|
| Outcomes |
Outcomes assessed: best‐corrected visual acuity, tolerability (pain, photophobia, burning sensation, itching, foreign body sensation), signs of ocular inflammation (redness, edema, tearing, discharge), conjunctival hyperemia, central and incisional corneal edema, anterior chamber cells, intraocular pressure, presence of hypopyon, posterior capsule opacity, pigments or membrane in front of the intraocular lens, compliance, and adverse events; no bacterial cultures were taken Participants were seen on days 1, 7, 15, and 20 Unit of analysis: the participant (1 eye per person) |
|
| Notes |
Study dates: not reported Funding source: not reported, but drugs were provided by Allergan Laboratories, Inc Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned using the Research Randomizer software (site: www.randomizer.org); the value 1 was assigned to patients enrolled in Group I, and the value 2 was assigned to patients enrolled in Group II." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Masking of participants (performance bias) | Low risk | "The group assignment was masked from all patients and investigators. Each patient was given two identical bottles labeled according to their group assignment. All bottles were opaque and patients were instructed to apply one drop from each bottle in the operated eye every 6 h with a 5‐min interval between drops, beginning one day prior to the surgery until the 15th day." |
| Masking of physicians and clinical care providers (performance bias) | Low risk | "The group assignment was masked from all patients and investigators." |
| Masking of outcome assessment (detection bias) | Low risk | "The group assignment was masked from all patients and investigators." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 21 (16%) participants were excluded from the analyses. |
| Selective reporting (reporting bias) | Low risk | Results were reported for the outcomes assessed. |
| Other bias | Low risk | No other potential sources of bias identified. |
ESCRS 2007.
| Methods |
Study design: randomized controlled trial Exclusions and loss to follow‐up: 324 (2%) participants were lost to follow‐up; 68 participants were excluded because they did not undergo the planned surgery or they withdrew consent. Study follow‐up: 6 weeks |
|
| Participants |
Setting: 24 ophthalmology units in Austria, Belgium, Germany, Italy, Poland, Portugal, Spain, Turkey, and the UK Enrolment: 16,603 people undergoing phacoemulsification cataract surgery Age: median for men was 73 years; for women was 75 years Gender: 42% men and 58% women Inclusion criteria: participants having routine cataract surgery at any study unit. Exclusion criteria: participants allergic to penicillins and cephalosporins, people in long‐term nursing homes, pregnant, or < 18 years; people severely at risk of infection (i.e. atopic keratoconjunctivitis or active blepharitis). |
|
| Interventions |
Intervention 1: intracameral cefuroxime 0.9% (injected into the anterior chamber at the end of surgery) Intervention 2: topical levofloxacin 0.5% (instilled 1 drop 1 hour before surgery, 1 drop 30 minutes before surgery, and 3 more drops at 5‐minute intervals immediately after surgery) Intervention 3: combined intracameral cefuroxime and topical levofloxacin Intervention 4: placebo drops (no sham injection was given) General: all study centers used povidone iodine 5% for antisepsis. Some centers additionally performed skin cleansing procedures; no detergents were used. Postoperative treatment: all participants were given topical levofloxacin 0.5% starting the morning after surgery (approximately 18 hours after surgery) and 4 times daily for 6 days. |
|
| Outcomes |
Primary outcomes (at 6 weeks' postsurgery):
overall number of participants with presumed infectious postoperative endophthalmitis;
number of participants with infectious endophthalmitis as proven by at least 1 of Gram stain, culture, or polymerase chain reaction Secondary outcomes: other risk factors for increased susceptibility, such as clear corneal incision or surgery during summer months, or decreased risk, such as foldable intraocular lenses inserted with sterile injector, etc. Unit of analysis: the participant (1 eye per person) |
|
| Notes |
Study dates: September 2003 to January 2006 Full study name: European Society of Cataract and Refractive Surgeons Study on the Antibiotic Prophylaxis of Post‐operative Endophthalmitis Funding source: European Society of Cataract and Refractive Surgeons and Santen GmbH, Germany Publication language: English |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 12‐block computerized randomization stratified by study center was used. |
| Allocation concealment (selection bias) | Low risk | An electronic database was used to conceal the treatment assignments for each participant. Droppers were labeled with sequential subject IDs, which were entered into the database at the time of surgery to determine whether or not an injection should be given. Treatment allocation codes were held in a central randomization file. |
| Masking of participants (performance bias) | Low risk | Partial masking of participants was done with use of placebo drops. No sham injection was performed. |
| Masking of physicians and clinical care providers (performance bias) | Low risk | Partial masking of physicians was done by using identically labeled droppers. No sham injection was performed. |
| Masking of outcome assessment (detection bias) | Low risk | Physicians were partially masked and it was reported that clinical partners were masked throughout the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 324 (2%) participants who were lost to follow‐up and 68 (0.4%) participants who did not undergo the planned surgery or withdrew consent were excluded from the intention‐to‐treat analyses. |
| Selective reporting (reporting bias) | Low risk | Study outcomes were published in study protocols, trial registrations and methods papers prior to the study beginning. Results were reported for these primary and secondary outcomes. |
| Other bias | Low risk | Performed power calculations to enroll a study size to detect a 4‐fold reduction in risk at 5% significance level. The study chairman, coordinator, clinical partners, and data monitoring committee were masked while the study was running. |
Sobaci 2003.
| Methods |
Study design: randomized controlled trial Exclusions and loss to follow‐up: eyes for which the surgical procedure was modified due to physician discretion at time of surgery were excluded from the study Study follow‐up: 6 weeks |
|
| Participants |
Setting: Gülhane Military Medical Academy and Medical School Hospital, Ankara, Turkey Enrolment: 644 eyes of 640 participants undergoing phacoemulsification cataract surgery Age: group 1: 64.2 ± 14.3 years (range 43 to 87); group 2: 61.2 ± 14.2 years (range 40 to 81) Gender: not reported Inclusion criteria: people scheduled to undergo phacoemulsification surgery Exclusion criteria: people with previous history of immunosuppressive treatment, diabetes mellitus, ocular surgery, recent infection, or inflammation |
|
| Interventions |
Intervention 1: balanced salt solution‐only irrigating infusion fluid (n = 322 eyes) Intervention 2: balanced salt solution with antibiotics (vancomycin 20 mg/mL and gentamicin 8 mg/mL; 322 eyes) General: interventions were given intraoperatively. Preoperative treatment, postoperative treatment, and follow‐up were identical for both groups. Preoperative treatment: 1‐day course of topical ofloxacin 0.3% and diclofenac sodium 1 mg/mL 4 times a day; conjunctival smears were obtained just before povidone iodine instillation at time of surgery. Surgical technique: phacoemulsification with a standard 3.2‐mm clear corneal incision, circular capsulotomy, and stop‐chop technique followed by foldable hydrophobic acrylic intraocular lens implantation; no sutures, subconjunctival antibiotics, or steroid injections were used. Postoperative treatment: eyes were treated with ofloxacin 0.3%, dexamethasone 1 mg/mL, and indomethacin 0.1% drops with a 4‐week tapering dose; participants were discharged the day after surgery. |
|
| Outcomes |
Primary outcomes:
risk of postoperative endophthalmitis;
aqueous humor contamination during phacoemulsification Participants were seen on days 2, 5, 10, 15, 30, and 45 Unit of analysis: the eye (both eyes of 4 participants were included separately in the analysis) |
|
| Notes |
Study dates: May 2000 to June 2002 Funding source: not reported Publication language: English The study authors reported the rate of postoperative endophthalmitis at their institution was 0.109%, but only 644 eyes were included in the study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Patients were randomly allocated to irrigating infusion fluid containing either balanced salt solution (BSS)‐only (group 1; 322 eyes of 320 patients) or BSS with antibiotics (20 mg/ml vancomycin and 8 mg/ml gentamicin) (group 2; 322 eyes of 320 patients), according to the scheduled day of surgery, which was performed one after another. (1:1)." |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported. |
| Masking of participants (performance bias) | Unclear risk | Masking of participants was not reported. |
| Masking of physicians and clinical care providers (performance bias) | Unclear risk | Masking of physicians was not reported. |
| Masking of outcome assessment (detection bias) | Unclear risk | Masking of outcome assessors was not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Eyes for which the surgical procedure was modified due to physician discretion at time of surgery were excluded from the study. The number of excluded participants was not reported. |
| Selective reporting (reporting bias) | Low risk | Results were reported for both primary outcomes. |
| Other bias | Low risk | No other potential sources of bias identified. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Camesasca 2007 | Endophthalmitis was not an outcome of the study: 2 different postcataract surgery antibiotic/steroid therapeutic combinations were compared in an intra‐individual randomized controlled trial; 142 participants (284 eyes) completed the 15‐day study; the study outcomes were efficacy of treatment, frequency of complications, and participant satisfaction. |
| Carron 2013 | Endophthalmitis was not an outcome of the study: topical ciprofloxacin 0.3% prior to cataract surgery was compared with no antibiotics in a randomized controlled trial; 46 participants completed the 1‐day study; the study outcomes were the presence of bacteria in cultures taken the day prior to surgery, the morning of surgery, immediately before surgery, and at the end of surgery. |
| Cetinkaya 2015 | Not a randomized controlled trial: intracameral moxifloxacin was administered following standard cataract surgery in some eyes, but not others; all participants received topical moxifloxacin for 1 week after surgery; data were reviewed retrospectively and participants with intraoperative complications were excluded from analyses; the study outcomes were postoperative best‐corrected visual acuity, anterior chamber cell and flare, intraocular pressure, and corneal edema. |
| Kolker 1967 | Not a randomized controlled trial: subconjunctival injections of antibiotics were administered following intraocular surgical procedures (including cataract, glaucoma, corneal transplant, pupillary membrane needling, etc.) to alternate participants during the first phase of the study and to all participants subsequently; rates for postoperative endophthalmitis were not reported separately for people with cataract who did not receive antibiotics in the first phase of the study. |
| Li 2015 | Not a randomized controlled trial: topical neomycin/polymyxin‐B was administered either 1 day or 1 hour prior to cataract surgery; the authors reported the study as a prospective comparative case series; the study outcomes were the presence of bacteria in cultures taken prior to the application of povidone‐iodine before surgery, after the application of povidone‐iodine before surgery, and at the end of surgery. |
| Maloof 2004 | Not a randomized controlled trial: letter reporting changes made by a hospital following an increased rate of endophthalmitis; changes included reorganizing the layout of the operating theater and administering postoperative intracameral vancomycin. |
| Paganelli 2009 | Endophthalmitis was not an outcome of the study: an intraoperative injection of triamcinolone and ciprofloxacin in a controlled‐release system (DuoCat) was compared with prednisolone and ciprofloxacin eye drops after cataract surgery in a randomized controlled trial; 135 participants completed the 4‐week study; the study outcomes were postoperative anterior chamber cell and flare, intraocular pressure, lack of anti‐inflammatory response, and presence of infection. |
| Peyman 1977 | Not a randomized controlled trial: South Indian eye camps were sequentially divided into 3 groups of treatment regimens: group 1: no prophylactic intracameral gentamicin was used, but oral and topical chloramphenicol was given to all participants; group 2: female participants received prophylactic intracameral gentamicin, but no chloramphenicol and male participants received oral and topical chloramphenicol, but no prophylactic intracameral gentamicin; group 3: all participants received prophylactic intracameral gentamicin, but no chloramphenicol or any other antibiotic was given. |
| Pérez‐Canales 2015 | Not a randomized controlled trial: intracameral injections of vancomycin versus cefuroxime were administered at the end of cataract surgery; the authors reported the study as a prospective comparative case series; the study outcomes were postoperative uncorrected and corrected distance visual acuity, refraction, anterior chamber cell and flare, intraocular pressure, endothelial specular microscopy, and corneal edema and thickness. |
Characteristics of ongoing studies [ordered by study ID]
NCT02770729.
| Trial name or title | Use of Intracameral Moxifloxacin for the Prevention of Acute Endophthalmitis Following Cataract Surgery: a Controlled and Randomized Clinical Trial |
| Methods |
Study design: parallel group, randomized controlled trial Study follow‐up: 8 weeks |
| Participants |
Setting: University of Campinas, Brazil Estimated enrolment: 6000 eyes of 6000 participants undergoing cataract surgery Inclusion criteria: people aged 50 to 100 years scheduled to undergo cataract surgery Exclusion criteria: vulnerable people; people with allergy to moxifloxacin; people with ocular or periocular infection, advanced glaucoma, or severe dry eye, or undergoing cataract surgery for traumatic cataract with ocular perforation or other reasons (e.g. glaucoma filtering surgery, vitreoretinal surgery, and cornea surgery |
| Interventions |
Intervention 1: intracameral injection of moxifloxacin 0.5% at conclusion of cataract surgery Intervention 2: no intracameral injection |
| Outcomes | Primary outcome: risk of postoperative endophthalmitis at 1 month Secondary outcome: endothelial cell count at 2 months |
| Starting date | Study dates: May 2016 to May 2018 |
| Contact information | Principal investigator: Mathias V Mélega, MD University of Campinas, Brazil |
| Notes | Study sponsor: University of Campinas, Brazil |
Differences between protocol and review
We added methods for the assessment of the certainty of evidence and presentation of outcomes in a 'Summary of findings' table according to revised Cochrane standards.
Contributions of authors
Conceiving the review: EG, AB, RC, IL, PM, SV.
Designing the review: EG, AB, RC, IL, PM, SV.
Coordinating the review: EG.
Data collection for the review:
Undertaking manual searches: EG, KL, IL, AN;
Screening search results: EG, KL, IL, RC, AN;
Organizing retrieval of papers: KL, AN;
Screening retrieved papers against inclusion criteria: EG, KL, IL, AN;
Appraising quality of papers: EG, KL, AB;
Extracting data from papers: EG, KL, IL, AN;
Writing to authors of papers for additional information: not applicable;
Providing additional data about papers: not applicable;
Obtaining and screening data on unpublished studies: not applicable.
Data management for the review:
Entering data into Review Manager 5: KL, EG.
Analysis of data: EG, KL.
Interpretation of data: EG, KL, AN, IL, PM.
Writing the review: EG, KL.
Securing funding for the review: PM.
Performing previous work that was the foundation of current study: AB, PM, EG.
Updating the review: EG, KL, ST, AN, IL, PM.
Sources of support
Internal sources
Discretionary funds, Wilmer Eye Institute, Johns Hopkins University, USA.
External sources
Cochrane Eyes and Vision US Project supported by grant 1 U01 EY020522, National Eye Institute, National Institutes of Health, USA.
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- The NIHR also funds the CEV Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, National Health Service, or the Department of Health.
Declarations of interest
EG: none known. KL: none known. ST: none known. AN: none known. IL: none known. PM: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Christy 1979 {published data only}
- Christy NE, Sommer A. Antibiotic prophylaxis of postoperative endophthalmitis. Annals of Ophthalmology 1979;11(8):1261‐5. [PubMed] [Google Scholar]
Christy 1986 {published data only}
- Christy NE, Lall P. A randomized, controlled comparison of anterior and posterior periocular injection of antibiotic in the prevention of postoperative endophthalmitis. Ophthalmic Surgery 1986;17(11):715‐8. [PubMed] [Google Scholar]
Cunha 2013 {published data only}
- Cunha PA, Shinzato FA, Tecchio GT, Weber SP, Brasil A, Avakian A. Efficacy and tolerability of a gatifloxacin/prednisolone acetate fixed combination for topical prophylaxis and control of inflammation in phacoemulsification: a 20‐day‐double‐blind comparison to its individual components. Clinics 2013;68(6):834‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
ESCRS 2007 {published data only}
- Barry P, Gardner S, Seal D, Gettinby G, Lees F, Peterson M, et al. Clinical observations associated with proven and unproven cases in the ESCRS study of prophylaxis of postoperative endophthalmitis after cataract surgery. Journal of Cataract and Refractive Surgery 2009;35(9):1523‐31. [DOI] [PubMed] [Google Scholar]
- Barry P, Seal DV, Gettinby G, Lees F, Peterson M, Revie CW, et al. ESCRS study of prophylaxis of postoperative endophthalmitis after cataract surgery: preliminary report of principal results from a European multicenter study. Journal of Cataract and Refractive Surgery 2006;32(3):407‐10. [DOI] [PubMed] [Google Scholar]
- Endophthalmitis Study Group, European Society of Cataract and Refractive Surgeons. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. Journal of Cataract and Refractive Surgery 2007;33(6):978‐88. [DOI] [PubMed] [Google Scholar]
- Pleyer U, Geldsetzer K. Will intracameral cefuroxime become the new standard in endophthalmitis prevention?. Klinische Monatsblätter für Augenheilkunde 2008;225(11):934‐40. [DOI] [PubMed] [Google Scholar]
- Seal DV, Barry P, Gettinby G, Lees F, Peterson M, Revie CW, et al. ESCRS study of prophylaxis of postoperative endophthalmitis after cataract surgery: case for a European multicenter study. Journal of Cataract and Refractive Surgery 2006;32(3):396‐406. [DOI] [PubMed] [Google Scholar]
Sobaci 2003 {published data only}
- Sobaci G, Tuncer K, Taş A, Ozyurt M, Bayer A, Kutlu U. The effect of intraoperative antibiotics in irrigating solutions on aqueous humor contamination and endophthalmitis after phacoemulsification surgery. European Journal of Ophthalmology 2003;13(9‐10):773‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Camesasca 2007 {published data only}
- Camesasca FI, Bianchi C, Beltrame G, Caporossi A, Piovella M, Rapisarda A, et al. Control of inflammation and prophylaxis of endophthalmitis after cataract surgery: a multicenter study. European Journal of Ophthalmology 2007;17(5):733‐42. [DOI] [PubMed] [Google Scholar]
Carron 2013 {published data only}
- Carron A, Samudio M, Laspina F, Fariña N, Sanabria RR, Cibils D, et al. Efficacy of topical 0.3% ciprofloxacin application in reducing the conjunctival biota of patients undergoing cataract extraction. Archivos de la Sociedad Espanola de Oftalmologia 2013;88(9):345‐51. [DOI] [PubMed] [Google Scholar]
Cetinkaya 2015 {published data only}
- Cetinkaya S, Cetinkaya YF, Acir NO, Dadaci Z. Application of intracameral moxifloxacin to prevent endophthalmitis in cataract surgery. International Eye Science 2015;15(10):1680‐3. [Google Scholar]
Kolker 1967 {published data only}
- Kolker AE, Freeman MI, Pettit TH. Prophylactic antibiotics and postoperative endophthalmitis. American Journal of Ophthalmology 1967;63(3):434‐9. [DOI] [PubMed] [Google Scholar]
Li 2015 {published data only}
- Li B, Miño de Kaspar H, Haritoglou C, Kook D, Kampik A, Sheng M, et al. Comparison of 1‐day versus 1‐hour application of topical neomycin/polymyxin‐B before cataract surgery. Journal of Cataract and Refractive Surgery 2015;41(4):724‐31. [DOI] [PubMed] [Google Scholar]
Maloof 2004 {published data only}
- Maloof A, Saw V. Prophylactic intracameral vancomycin. Journal of Cataract and Refractive Surgery 2004;30(8):1610‐1. [DOI] [PubMed] [Google Scholar]
Paganelli 2009 {published data only}
- Paganelli F, Cardillo JA, Melo LAS, Lucena DR, Silva AA, Oliveira AG, et al. A single intraoperative sub‐Tenon's capsule injection of triamcinolone and ciprofloxacin in a controlled‐release system for cataract surgery. Investigative Ophthalmology and Visual Science 2009;50(7):3041‐7. [DOI] [PubMed] [Google Scholar]
Pérez‐Canales 2015 {published data only}
- Pérez‐Canales JL, Pérez‐Santonja JJ, Campos‐Mollo E. Corneal endothelial changes after intracameral vancomycin injection in cataract surgery. Journal of Cataract and Refractive Surgery 2015;41(1):126‐34. [DOI] [PubMed] [Google Scholar]
Peyman 1977 {published data only}
- Peyman GA, Sathar ML, May DR. Intraocular gentamicin as intraoperative prophylaxis in South India eye camps. British Journal of Ophthalmology 1977;61(4):260‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT02770729 {published data only}
- NCT02770729. Evaluation of efficacy and safety of intracameral moxifloxacin for prevention of postcataract endophthalmitis. clinicaltrials.gov/show/NCT02770729 (accessed 11 December 2016).
Additional references
Barreau 2012
- Barreau G, Mounier M, Marin B, Adenis JP, Robert PY. Intracameral cefuroxime injection at the end of cataract surgery to reduce the incidence of endophthalmitis: French study. Journal of Cataract and Refractive Surgery 2012;38(8):1370‐5. [DOI] [PubMed] [Google Scholar]
Barry 2009
- Barry P, Gardner S, Seal D, Gettinby G, Lees F, Peterson M, et al. Clinical observations associated with proven and unproven cases in the ESCRS study of prophylaxis of postoperative endophthalmitis after cataract surgery. Journal of Cataract and Refractive Surgery 2009;35(9):1523‐31. [DOI] [PubMed] [Google Scholar]
Behndig 2015
- Behndig A, Cochener‐Lamard B, Güell J, Kodjikian L, Mencucci R, Nuijts R, et al. Surgical, antiseptic, and antibiotic practice in cataract surgery: results from the European Observatory in 2013. Journal of Cataract and Refractive Surgery 2015;41(12):2635‐43. [DOI] [PubMed] [Google Scholar]
Beselga 2014
- Beselga D, Campos A, Castro M, Fernandes C, Carvalheira F, Campos S, et al. Postcataract surgery endophthalmitis after introduction of the ESCRS protocol: a 5‐year study. European Journal of Ophthalmology 2014;24(4):516‐9. [DOI] [PubMed] [Google Scholar]
Braga‐Mele 2014
- Braga‐Mele R, Chang DF, Henderson BA, Mamalis N, Talley‐Rostov A, Vasavada A, et al. Intracameral antibiotics: safety, efficacy, and preparation. Journal of Cataract and Refractive Surgery 2014;40(12):2134‐42. [DOI] [PubMed] [Google Scholar]
Chang 2008
- Chang MA, Congdon NG, Baker SK, Bloem MW, Savage H, Sommer A. The surgical management of cataract: barriers, best practices and outcomes. International Ophthalmology 2008;28(4):247‐60. [DOI] [PubMed] [Google Scholar]
Chang 2015
- Chang DF, Braga‐Mele R, Henderson BA, Mamalis N, Vasavada A, ASCRS Cataract Clinical Committee. Antibiotic prophylaxis of postoperative endophthalmitis after cataract surgery: results of the 2014 ASCRS member survey. Journal of Cataract and Refractive Surgery 2015;41(6):1300‐5. [DOI] [PubMed] [Google Scholar]
Coleman 2015
- Coleman AL. How big data informs us about cataract surgery: The LXXII Edward Jackson Memorial Lecture. American Journal of Ophthalmology 2015;160(6):1091‐1103. [DOI] [PubMed] [Google Scholar]
Creuzot‐Garcher 2016
- Creuzot‐Garcher C, Benzenine E, Mariet AS, Lazzer A, Chiquet C, Bron AM, et al. Incidence of acute postoperative endophthalmitis after cataract surgery: a nationwide study in France from 2005 to 2014. Ophthalmology 2016;123(7):1414‐20. [DOI] [PubMed] [Google Scholar]
Daien 2016
- Daien V, Papinaud L, Gillies MC, Domerg C, Nagot N, Lacombe S, et al. Effectiveness and safety of an intracameral injection of cefuroxime for the prevention of endophthalmitis after cataract surgery with or without perioperative capsular rupture. JAMA Ophthalmology 2016;134(7):810‐6. [DOI] [PubMed] [Google Scholar]
Delyfer 2011
- Delyfer MN, Rougier MB, Leoni S, Zhang Q, Dalbon F, Colin J, et al. Ocular toxicity after intracameral injection of very high doses of cefuroxime during cataract surgery. Journal of Cataract and Refractive Surgery 2011;37(2):271‐8. [DOI] [PubMed] [Google Scholar]
Du 2014
- Du DT, Wagoner A, Barone SB, Zinderman CE, Kelman JA, Macurdy TE, et al. Incidence of endophthalmitis after corneal transplant or cataract surgery in a medicare population. Ophthalmology 2014;121(1):290‐8. [DOI] [PubMed] [Google Scholar]
EVSG 1995
- Anonymous. Results of the Endophthalmitis Vitrectomy Study. A randomized trial of immediate vitrectomy and of intravenous antibiotics for the treatment of postoperative bacterial endophthalmitis. Endophthalmitis Vitrectomy Study Group. Archives of Ophthalmology 1995;113(12):1479‐96. [PubMed] [Google Scholar]
Foster 2001
- Foster A. Cataract and "Vision 2020 ‐ the right to sight" initiative. British Journal of Ophthalmology 2001; Vol. 85, issue 6:635‐7. [DOI] [PMC free article] [PubMed]
Friedman 2004
- Friedman DS, West SK, Munoz B, Park W, Deremeik J, Massof R, et al. Racial variations in causes of vision loss in nursing homes: the Salisbury Eye Evaluation in Nursing Home Groups (SEEING) Study. Archives of Ophthalmology 2004;122(7):1019‐24. [DOI] [PubMed] [Google Scholar]
Friling 2013
- Friling E, Lundstrom M, Stenevi U, Montan P. Six‐year incidence of endophthalmitis after cataract surgery: Swedish national study. Journal of Cataract and Refractive Surgery 2013;39(1):15‐21. [DOI] [PubMed] [Google Scholar]
Galvis 2014
- Galvis V, Tello A, Sánchez MA, Camacho PA. Cohort study of intracameral moxifloxacin in postoperative endophthalmitis prophylaxis. Ophthalmology and Eye Diseases 2014;6:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garat 2009
- Garat M, Moser CL, Martin‐Baranera M, Alonso‐Tarres C, Alvarez‐Rubio L. Prophylactic intracameral cefazolin after cataract surgery: endophthalmitis risk reduction and safety results in a 6‐year study. Journal of Cataract and Refractive Surgery 2009;35(4):637‐42. [DOI] [PubMed] [Google Scholar]
Garcia‐Arumi 2007
- Garcia‐Arumi J, Fonollosa A, Sararols L, Fina F, Martinez‐Castillo V, Boixadera A, et al. Topical anesthesia: possible risk factor for endophthalmitis after cataract extraction. Journal of Cataract and Refractive Surgery 2007;33(6):989‐92. [DOI] [PubMed] [Google Scholar]
Garcia‐Saenz 2010
- Garcia‐Saenz MC, Arias‐Puente A, Rodriguez‐Caravaca G, Banuelos JB. Effectiveness of intracameral cefuroxime in preventing endophthalmitis after cataract surgery: ten‐year comparative study. Journal of Cataract and Refractive Surgery 2010;36(2):203‐7. [DOI] [PubMed] [Google Scholar]
Glanville 2006
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
Gore 2009
- Gore DM, Angunawela RI, Little BC. United Kingdom survey of antibiotic prophylaxis practice after publication of the ESCRS Endophthalmitis Study. Journal of Cataract and Refractive Surgery 2009;35(4):770‐3. [DOI] [PubMed] [Google Scholar]
Gower 2015
- Gower EW, Keay LJ, Stare DE, Arora P, Cassard SD, Behrens A, et al. Characteristics of endophthalmitis after cataract surgery in the United States Medicare population. Ophthalmology 2015;122(8):1625‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro. Version accessed 11 December 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Haripriya 2016
- Haripriya A, Chang DF, Namburar S, Smita A, Ravindran RD. Efficacy of intracameral moxifloxacin endophthalmitis prophylaxis at Aravind Eye Hospital. Ophthalmology 2016;123(2):302‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JAC editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Jabbarvand 2016
- Jabbarvand M, Hashemian H, Khodaparast M, Jouhari M, Tabatabaei A, Rezaei S. Endophthalmitis occurring after cataract surgery: outcomes of more than 480 000 cataract surgeries, epidemiologic features, and risk factors. Ophthalmology 2016;123(2):295‐301. [DOI] [PubMed] [Google Scholar]
Keating 2013
- Keating GM. Intracameral cefuroxime: prophylaxis of postoperative endophthalmitis after cataract surgery. Drugs 2013;73(2):179‐86. [DOI] [PubMed] [Google Scholar]
Keay 2012
- Keay L, Gower EW, Cassard SD, Tielsch JM, Schein OD. Postcataract surgery endophthalmitis in the United States: analysis of the complete 2003 to 2004 Medicare database of cataract surgeries. Ophthalmology 2012;119(5):914‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lalitha 2005
- Lalitha P, Rajagopalan J, Prakash K, Ramasamy K, Prajna NV, Srinivasan M. Postcataract endophthalmitis in South India: incidence and outcome. Ophthalmology 2005;112(11):1884‐9. [DOI] [PubMed] [Google Scholar]
Lalwani 2008
- Lalwani GA, Flynn HW Jr, Scott IU, Quinn CM, Berrocal AM, Davis JL, et al. Acute‐onset endophthalmitis after clear corneal cataract surgery (1996‐2005). Clinical features, causative organisms, and visual acuity outcomes. Ophthalmology 2008;115(3):473‐6. [DOI] [PubMed] [Google Scholar]
Leaming 2012
- Leaming D. Report on the 2011 ESCRS and ASCRS member practice style surveys with comparisons and trends. European Society of Cataract and Refractive Surgeons. www.escrs.org/milan2012/programme/free‐paper‐details.asp?id=14025&day=0 (accessed 30 January 2013).
Lundstrom 2007
- Lundstrom M, Wejde G, Stenevi U, Thorburn W, Montan P. Endophthalmitis after cataract surgery: a nationwide prospective study evaluating incidence in relation to incision type and location. Ophthalmology 2007;114(5):866‐70. [DOI] [PubMed] [Google Scholar]
Meyer 2016
- Meyer JJ, Polkinghorne PJ, McGhee CN. Cataract surgery practices and endophthalmitis prophylaxis by New Zealand ophthalmologists. Clinical & Experimental Ophthalmology 2016;44(7):643‐5. [DOI] [PubMed] [Google Scholar]
Miller 2004
- Miller JJ, Scott IU, Flynn HW Jr, Smiddy WE, Corey RP, Miller D. Endophthalmitis caused by Streptococcus pneumoniae. American Journal of Ophthalmology 2004;138(2):231‐6. [DOI] [PubMed] [Google Scholar]
Miller 2005
- Miller JJ, Scott IU, Flynn HW Jr, Smiddy WE, Newton J, Miller D. Acute‐onset endophthalmitis after cataract surgery (2000‐2004): incidence, clinical settings, and visual acuity outcomes after treatment. American Journal of Ophthalmology 2005;139(6):983‐7. [DOI] [PubMed] [Google Scholar]
Mollan 2007
- Mollan SP, Gao A, Lockwood A, Durrani OM, Butler L. Postcataract endophthalmitis: incidence and microbial isolates in a United Kingdom region from 1996 through 2004. Journal of Cataract and Refractive Surgery 2007;33(2):265‐8. [DOI] [PubMed] [Google Scholar]
Monica 2005
- Monica ML, Long DA. Nine‐year safety with self‐sealing corneal tunnel incision in clear cornea cataract surgery. Ophthalmology 2005;112(6):985‐6. [DOI] [PubMed] [Google Scholar]
Montan 2002
- Montan PG, Wejde G, Koranyi G, Rylander M. Prophylactic intracameral cefuroxime. Efficacy in preventing endophthalmitis after cataract surgery. Journal of Cataract and Refractive Surgery 2002;28(6):977‐81. [DOI] [PubMed] [Google Scholar]
Myneni 2013
- Myneni J, Desai SP, Jayamanne DG. Reduction in postoperative endophthalmitis with intracameral cefuroxime. Journal of Hospital Infection 2013;84(4):326‐8. [DOI] [PubMed] [Google Scholar]
Ng 2005
- Ng JQ, Morlet N, Pearman JW, Constable IJ, McAllister IL, Kennedy CJ, et al. Management and outcomes of postoperative endophthalmitis since the endophthalmitis vitrectomy study: the Endophthalmitis Population Study of Western Australia (EPSWA)'s fifth report. Ophthalmology 2005;112(7):1199‐206. [DOI] [PubMed] [Google Scholar]
Olavi 2012
- Olavi P. Ocular toxicity in cataract surgery because of inaccurate preparation and erroneous use of 50mg/ml intracameral cefuroxime. Acta Ophthalmologica 2012;90(2):e153‐4. [DOI] [PubMed] [Google Scholar]
Packer 2011
- Packer M, Chang DF, Dewey SH, Little BC, Mamalis N, Oetting TA, et al. Prevention, diagnosis, and management of acute postoperative bacterial endophthalmitis. Journal of Cataract and Refractive Surgery 2011;37(9):1699‐714. [DOI] [PubMed] [Google Scholar]
Resnikoff 2004
- Resnikoff S, Pascolini D, Etya'ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bulletin of the World Health Organization 2004;82(11):844‐51. [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schein 2012
- Schein OD, Cassard SD, Tielsch JM, Gower EW. Cataract surgery among Medicare beneficiaries. Ophthalmic Epidemiology 2012;19(5):257‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schimel 2013
- Schimel AM, Miller D, Flynn HW Jr. Endophthalmitis isolates and antibiotic susceptibilities: a 10‐year review of culture‐proven cases. American Journal of Ophthalmology 2013;156(1):50‐2. [DOI] [PubMed] [Google Scholar]
Schwartz 2016
- Schwartz SG, Grzybowski A, Flynn HW Jr. Antibiotic prophylaxis: different practice patterns within and outside the United States. Clinical Ophthalmology 2016;10:251‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheng 2011
- Sheng Y, Sun W, Gu Y, Lou J, Liu W. Endophthalmitis after cataract surgery in China, 1995‐2009. Journal of Cataract and Refractive Surgery 2011;37(9):1715‐22. [DOI] [PubMed] [Google Scholar]
Shorstein 2013
- Shorstein NH, Winthrop KL, Herrinton LJ. Decreased postoperative endophthalmitis rate after institution of intracameral antibiotics in a Northern California eye department. Journal of Cataract and Refractive Surgery 2013;39(1):8‐14. [DOI] [PubMed] [Google Scholar]
Simunovic 2012
- Simunovic MP, Rush RB, Hunyor AP, Chang AA. Endophthalmitis following intravitreal injection versus endophthalmitis following cataract surgery: clinical features, causative organisms and post‐treatment outcomes. British Journal of Ophthalmology 2012;96(6):862‐6. [DOI] [PubMed] [Google Scholar]
Soriano 2006
- Soriano F, Pérez‐Trallero E, Pallarés R, Meseguer MA, Fleites A, Gené A, et al. Streptococcus pneumoniae endophthalmitis: a study of 36 cases with special reference to antibiotic resistance and treatment options. Clinical Microbiology and Infection 2006;12(6):519‐26. [DOI] [PubMed] [Google Scholar]
Speaker 1991
- Speaker MG, Menikoff JA. Prophylaxis of endophthalmitis with topical povidone‐iodine. Ophthalmology 1991;98(12):1769‐75. [DOI] [PubMed] [Google Scholar]
Taban 2005a
- Taban M, Behrens A, Newcomb RL, Nobe MY, Saedi G, Sweet PM, et al. Acute endophthalmitis following cataract surgery: a systematic review of the literature. Archives of Ophthalmology 2005;123(5):613‐20. [DOI] [PubMed] [Google Scholar]
Taban 2005b
- Taban M, Sarayba MA, Ignacio TS, Behrens A, McDonnell PJ. Ingress of India ink into the anterior chamber through sutureless clear corneal cataract wounds. Archives of Ophthalmology 2005;123(5):643‐8. [DOI] [PubMed] [Google Scholar]
Vazirani 2013
- Vazirani J, Basu S. Role of topical, subconjunctival, intracameral, and irrigative antibiotics in cataract surgery. Current Opinion in Ophthalmology 2013;24(1):60‐5. [DOI] [PubMed] [Google Scholar]
West 2005
- West ES, Behrens A, McDonnell PJ, Tielsch JM, Schein OD. The incidence of endophthalmitis after cataract surgery among the U.S. Medicare population increased between 1994 and 2001. Ophthalmology 2005;112(8):1388‐94. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Gower 2013
- Gower EW, Lindsley K, Nanji AA, Leyngold I, McDonnell PJ. Perioperative antibiotics for prevention of acute endophthalmitis after cataract surgery. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD006364.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leyngold 2007
- Leyngold I, Nanji AA, Chuck RS, Behrens A, Vedula SS, McDonnell PJ, et al. Perioperative antibiotics for prevention of acute endophthalmitis after cataract surgery. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD006364] [DOI] [PMC free article] [PubMed] [Google Scholar]


