1. Introduction
Furan (Figure 1) is a volatile organic chemical used as a synthetic intermediate and in the production of pesticides, stabilizers, and pharmaceuticals (International Agency for Research on Cancer, 1995; Hazardous Substances Data Bank, 2011). The major sources of exposure for the general public to furan are tobacco products and food. Mainstream cigarette smoke is estimated to contain up to 65 μg furan/cigarette (Smith et al., 2000; International Agency for Research on Cancer, 2004). Furan is produced during the cooking of many common foods, including coffee, baked or fried cereal products, canned and jarred foods, baby food, and infant formula (Hasnip et al., 2006; Nyman et al., 2006; Zoller et al., 2007; Morehouse et al., 2008). The mean daily dietary consumption of furan in the U.S. has been estimated to be 0.25 μg/kg body weight (BW) in adults (Morehouse et al., 2008), 0.41 μg/kg BW in children during their first year of life, and 0.9 μg/kg BW in infants consuming only formula (DiNovi and Mihalov, 2007). Adults in Europe have been estimated to have a median dietary exposure to furan of 0.78 μg/kg BW (European Food Safety Authority, 2009). Coffee contributes approximately 50% of the total dietary exposure of adults to furan in the U.S. (Morehouse et al., 2008).
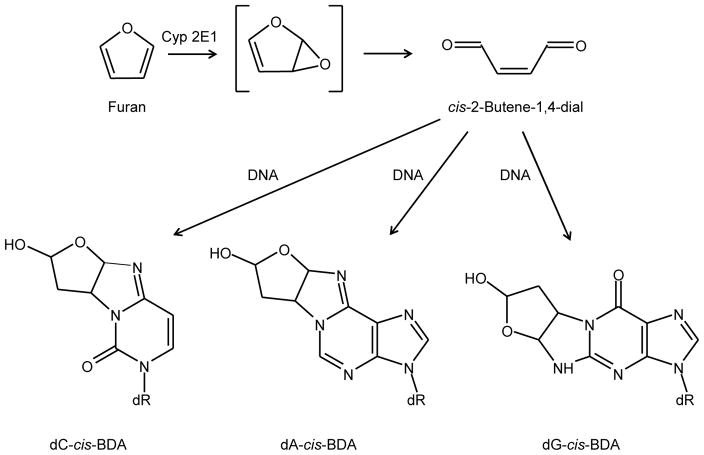
Figure 1.
Structures of furan, cis-2-butene-1,4-dial, and the DNA adducts resulting from reaction of cis-2-butene-1,4-dial with DNA. The abbreviations used are: Cyp2E1, cytochrome P450 2E1; dC, deoxycytidine; dA, deoxyadenosine; dG, deoxyguanosine; dR, deoxyribose; cis-BDA, cis-2-butene-1,4-dial.
Furan is rapidly absorbed from the gastro-intestinal tract and extensively metabolized, primarily by hepatic cytochrome P450 2E1, to cis-2-butene-1,4-dial (Figure 1; Kedderis et al., 1993; Chen et al., 1995), a highly reactive metabolite that can react with thiol and amino groups in glutathione and other peptides (Chen et al., 1997; Peterson et al., 2006; Kellert et al., 2008a; Lu and Peterson, 2010). In addition to forming amino acid adducts, cis-2-butene-1,4-dial can react with the exocyclic nitrogens of deoxycytidine, deoxyadenosine, and deoxyguanosine to form bicyclic hemiketal derivatives (Figure 1; Gingipalli and Dedon, 2001; Byrns et al., 2002; Bohnert et al., 2004). The deoxyadenosine and deoxyguanosine adducts are relatively unstable and undergo ring-opening and dehydration to form secondary etheno-type DNA adducts (Byrns et al., 2004). By using O-benzylhydroxylamine as a trapping agent, the deoxyadenosine and deoxycytidine adducts were detected in DNA reacted with cis-2-butene-1,4-dial (Byrns et al., 2006). Accelerator mass spectrometry was used to assess the formation of cis-2-butene-1,4-dial deoxynucleoside adducts in liver DNA from rats administered 0, 0.1, or 2 mg [3,4-14C]furan/kg BW. There was a dose-related increase in 14C associated with liver DNA; however, upon hydrolysis and chromatography of the DNA, only very limited amounts of the radioactivity corresponded to the previously characterized cis-2-butene-1,4-dial deoxynucleoside adducts (Neuwirth et al., 2012). More recently, the presence of deoxycytidine cis-2-butene-1,4-dial, the major and most stable of the DNA adducts obtained from furan, was examined in the livers of F344 rats treated 5 days/week by gavage for up to 360 days with 4.4 mg furan/kg BW (Churchwell et al., 2015). Formation of DNA adducts was not detected above the background level of 1 – 2 adducts/108 nucleotides.
The carcinogenicity of furan has been assessed in mice and rats. In male and female B6C3F1 mice treated by gavage 5 days/week with 0, 8, or 15 mg furan/kg BW for 2 years, there was a dose-dependent increase in hepatocellular adenoma, carcinoma, and combined adenoma or carcinoma. With the exception of hepatocellular carcinoma in female mice, the incidence of each of these neoplasms was significantly increased at both doses of furan (National Toxicology Program, 1993). In a subsequent study, female B6C3F1 mice were administered 0, 0.5, 1, 2, 4, or 8 mg furan/kg BW 5 days/week for 2 years. There was a dose-dependent increase in hepatocellular adenoma, carcinoma, and combined adenoma or carcinoma, with the incidence of adenoma and combined adenoma or carcinoma being significant at 4 and 8 mg furan/kg BW and the incidence of carcinoma being significant at 8 mg furan/kg BW (Moser et al., 2009). The carcinogenicity of furan has also been assessed in mice treated as newborns. Male B6C3F1 mice given a single intraperitoneal injection of 400 mg furan/kg BW on postnatal day 15 had a significant increase in liver adenoma. Likewise, male B6C3F1 mice given intraperitoneal injections of 200 mg furan/kg BW on postnatal days 3, 6, 9, 12, 15, and 18 had a significant increase in liver adenoma and combined liver adenoma or carcinoma (Johansson et al., 1997).
In male and female F344/N rats treated by gavage with 0, 2, 4, or 8 mg furan/kg BW 5 days/week for 2 years there was a dose-dependent increase in hepatocellular adenoma, carcinoma, and combined adenoma or carcinoma, liver cholangiocarcinoma, and mononuclear cell leukemia in both sexes. The incidence of each of the neoplasms, with the exception of hepatocellular carcinoma in female rats, was significantly increased at 4 and 8 mg furan/kg BW; liver cholangiocarcinoma was also significantly increased at 2 mg furan/kg BW. Likewise, the incidence of liver cholangiocarcinoma was significantly elevated in male and female rats treated by gavage with 2, 4, or 8 mg furan/kg BW 5 days/week for 9 or 15 months. In a subsequent experiment, male F344/N rats were administered by gavage 30 mg furan/kg BW 5 days/week for 13 weeks. When subgroups were assessed at the end of dosing and at 9 and 15 months after the initiation of dosing, the incidence of liver cholangiocarcinoma was 0, 100, and 100%, respectively (National Toxicology Program, 1993).
Although furan is carcinogenic in mice and rats (with rats being more sensitive than mice), the risk to humans from dietary exposure to furan cannot be estimated accurately because the lowest tested dose of furan (2 mg/kg BW) in the 2-year bioassay conducted by the National Toxicology Program (NTP) in rats gave nearly a 100% incidence of cholangiocarcinoma (86% in female F344/N rats and 98% in male F344/N rats; National Toxicology Program, 1993) leading to highly uncertain estimates of the cancer risk at lower doses. To provide bioassay data that can be used in preparing meaningful risk assessments, we determined the carcinogenicity of furan in F344/N Nctr rats at doses projected to give a wide range of carcinogenic responses. The bioassay was restricted to male rats because they appeared to be slightly more sensitive than female rats to the induction of cholangiocarcinoma (National Toxicology Program, 1993).
2. Materials and methods
2.1 Chemicals
Furan (CAS 110-00-9) was purchased as a single lot from Sigma-Aldrich (Milwaukee, WI). The acquired furan was stabilized with 0.025% butylated hydroxytoluene, a substance not present in the furan used in the previous NTP bioassay (National Toxicology Program, 1993). To prepare material that corresponded more closely to that used in the previous bioassay, the furan was distilled at atmospheric pressure to remove the butylated hydroxytoluene and stored at −20°C under nitrogen in sealed vials.
The identity and purity of the furan were assessed by gas chromatography coupled with electron impact mass spectrometry, NMR spectroscopy, and gas chromatography using flame ionization detection. These analyses indicated a purity of 99.5%, a value greater than the purity of the furan used in the previous NTP bioassay (National Toxicology Program, 1993). No butylated hydroxytoluene was detected, with a limit of detection of 0.003%.
Corn oil (CAS 8001-30-7), the same vehicle used in the previous NTP bioassay with furan, was obtained in 8 lots from Sigma Life Science (St. Louis, MO). The presence of peroxides was assessed by iodometric titration analysis in each lot of corn oil and values of < 2.9 mEq hydrogen peroxide/kg were obtained. These values were lower than the stated permissible value of < 10 mEq hydrogen peroxide/kg used in the previous NTP bioassay (National Toxicology Program, 1993).
2.2 Dose selection
Male F344/N rats administered 2, 4, or 8 mg furan/kg BW 5 days/week for 2 years (24 months) had an 86–98% incidence of liver cholangiocarcinoma (National Toxicology Program, 1993). Male F344/N rats treated with 2 or 4 mg furan/kg BW for 9 months had a liver cholangiocarcinoma incidence of 50 and 70%, respectively, while those given 2 mg furan/kg BW for 15 months had a liver cholangiocarcinoma incidence of 78% (National Toxicology Program, 1993). Based upon these data, a high dose of 2 mg furan/kg BW was selected for the current study.
In order to design a study that would allow the best characterization of the dose response, calculations were conducted to predict the cholangiocarcinoma incidence as a function of dose. Peto et al. (1984) derived an empirical relationship between tumor latency and dose that allows an estimation of the dose that would give a specific tumor incidence after 2 years of dosing based upon the tumor incidence obtained from shorter dosing periods. When this relationship was applied to the 50% tumor incidence observed after 9 months of dosing with 2 mg furan/kg BW (National Toxicology Program, 1993), it was estimated that a dose of 0.11 – 0.28 mg furan/kg BW would result in a 50% incidence of liver cholangiocarcinoma after 2 years of dosing. Similarly, it was estimated that a 70% tumor incidence would be associated with a dose of 0.21 – 0.56 mg furan/kg BW and a 78% tumor incidence would be associated with a dose of 0.49 – 0.78 mg furan/kg BW. Linear extrapolation of these values allowed an estimation of the 24-month liver cholangiocarcinoma incidences from specific doses of furan. The results of this extrapolation indicated that a dose of 0.02 mg furan/kg BW would be associated with a cholangiocarcinoma incidence of 0 – 23% (Table 1), whereas a dose of 1 mg furan/kg BW would be associated with a cholangiocarcinoma incidence of nearly 100%. Based upon these considerations, doses of 0, 0.02, 0.044, 0.092, 0.2, 0.44, 0.92 and 2.0 mg furan/kg BW were selected for the 2-year bioassay (Table 1).
Table 1.
Doses of furan and group sizes for each dose administered to male F344/N Nctr rats for 2 years and the predicted incidence of cholangiocarcinoma at 2 yearsa
| Doses of furan (mg/kg BW) | Total number of rats/group | Number of rats examined at the 36-week interim sacrifice | Number of rats examined at the 60-week interim sacrifice | Number of rats remaining for the entire 2-year bioassay | Predicted cholangiocarcinoma incidence at 2 yearsb |
|---|---|---|---|---|---|
| 0 | 190 | 20 | 20 | 150 | 0% |
| 0.02 | 180 | 20 | 10 | 150 | 0 – 23% |
| 0.044 | 130 | 20 | 10 | 100 | 0 – 37% |
| 0.092 | 130 | 20 | 10 | 100 | 19 – 50% |
| 0.2 | 80 | 20 | 10 | 50 | 41 – 64% |
| 0.44 | 80 | 20 | 10 | 50 | 63 – 78% |
| 0.92 | 80 | 20 | 10 | 50 | 83 – 92% |
| 2 | 80 | 20 | 10 | 50 | 100% |
Furan was administered by gavage in corn oil vehicle 5 days/week for 2 years (104 weeks), with interim sacrifices conducted at 36 and 60 weeks.
The predicted cholangiocarcinoma incidences were derived with the empirical relationship of Peto et al. (1984), using exponents of 2 and 3.
2.3 Study design
This study was conducted under the auspices of the NTP in accordance with NTP specifications (http://ntp.niehs.nih.gov/ntp/test_info/finalntp_toxcarspecsjan2011.pdf) and in compliance with Food and Drug Administration (FDA) Good Laboratory Practice (GLP) Regulations (CFR 21, Part 58). The approved GLP report (NCTR Study E2168.02) is available from the National Center for Toxicological Research (NCTR) on request. The Institutional Animal Care and Use Committee at the NCTR reviewed and approved the protocol for this bioassay. The study was conducted at NCTR in accordance with all relevant FDA, National Institutes of Health (NIH), and NTP animal care and use policies and applicable federal, state, and local regulations and guidelines. The animal facility at NCTR is accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care International.
Male F344/N Nctr rats were obtained from the NCTR breeding colony. While the NCTR sub-strain differs from the Charles River sub-strain used in the previous furan bioassay (National Toxicology Program, 1993), the NCTR F344/N sub-strain has given tumorigenic responses comparable to those of other F344/N sub-strains in previously reported carcinogenicity bioassays (e.g., acrylamide; Beland et al., 2013). The treatment was initiated when the rats were 7 weeks of age. The rats were housed 2/cage in polycarbonate cages with hardwood chip bedding. Irradiated Purina 5K0L Diet (also designated NIH-41 Irradiated Rodent Diet) and Millipore-filtered tap water were provided ad libitum. The level of furan in feed was < 15 parts/billion as assessed by headspace gas chromatography mass spectral analysis. The animal rooms were maintained on a 12-hour light-dark cycle, with 10 – 15 air changes/hour. Environmental controls were set to maintain the temperature at 20 – 26°C, with a relative humidity of 30 – 70%.
The stability of furan in the corn oil vehicle was determined by capillary gas chromatography with flame ionization detection over a 14-day period and the concentrations were found to be within ± 10% of the target values. Furan dosing solutions were prepared weekly. Dose certification analyses were conducted on all doses, typically on each weekly preparation, and all were within ± 10% of the specified concentrations. Furan was not detected in the control corn oil, with the limit of quantitation being approximately 0.1 μg furan/mL of corn oil.
The furan was administered by gavage in corn oil 5 days/week for 2 years (104 weeks), with interim sacrifices conducted at 9 (36 weeks) and 15 (60 weeks) months. The dosing volume was 5.0 mL/kg BW. The number of rats in each group is given in Table 1. The group sizes were unbalanced, with more rats being assigned to the lower dose groups. The specific number of rats in each group was based upon power calculations to detect a significant difference between the projected tumor incidences in each treated group and the control group.
2.4 Necropsy and histopathology
Complete necropsies were performed on all animals, including those that died naturally or that were submitted moribund prior to the scheduled terminal sacrifice. Tissues were examined grossly, removed, and preserved in 10% neutral buffered formalin, except the eyes and testes, which were placed in modified Davidson’s fixative. The tissues were trimmed, processed, and embedded in Formula R® infiltrating medium (Leica Micro Biosystems Division, Richmond, IL), sectioned at approximately 5 microns, and stained with hematoxylin and eosin for microscopic evaluation. In a few cases, special staining procedures were applied to selected lesions to aid in characterizing the tissue changes.
A quality assessment pathologist evaluated slides of all proliferative lesions. Histopathology slides containing the diagnoses made by the study pathologist and quality assessment pathologist were reviewed by a Pathology Working Group. Final diagnoses of the reviewed lesions represented a consensus of the study pathologist, the reviewing pathologist, and the Pathology Working Group.
2.5 Statistical analyses
The effect of furan dose on body weight was investigated using a repeated measures, mixed models analysis of variance, with main effects of dose and week, and a dose x week interaction effect. Least squares estimates of mean body weight were obtained for each dose group in 4-week intervals starting at week 4 and continuing until the end of scheduled sacrifices. Dunnett’s adjusted pair-wise comparisons (Dunnett, 1955) of dose group to control group body weight means were performed to determine if there was a difference between the control and the respective dose group means.
Mean survival times and plots of rodent survival functions were obtained using the Kaplan-Meier estimation (Kaplan and Meier, 1958). Cox proportional hazards regression analyses (Cox, 1972) were conducted to compare the hazard function of each dose group to that of the control group and to test for a linear trend between the hazard and furan dose.
Continuity-corrected Poly-3 tests (Bailer and Portier, 1988), as modified by Bieler and Williams (1993), were used to assess prevalence of neoplasms and non-neoplastic lesions. For the interim sacrifices at 36 and 60 weeks, an exact Cochran-Armitage linear trend test was run, as well as Fisher’s Exact test, to compare the treatment groups to the control group. These results are referred to as CAFE, rather than Poly-3. The CAFE tests did not adjust for competing risks but there were generally very few early deaths before the interim sacrifices.
Benchmark doses (BMD) and the lower 95% confidence limits of the benchmark dose (BMDL) were calculated using Environmental Protection Agency Benchmark Dose software (version 2.1.1; http://www.epa.gov/ncea/bmds). The calculations were conducted using gamma, logistic, log-logistic, log-probit, multistage, probit, and Weibull models to fit the neoplastic incidences and administered dose of furan. The BMD10 was defined as the dose that caused a 10% excess risk of the specified adverse effect over that observed in the appropriate control group.
3. Results
Male F344/N Nctr rats were administered 0, 0.02, 0.044, 0.092, 0.2, 0.44, 0.92, and 2.0 mg furan/kg BW by gavage in corn oil 5 days/week for 2 years (104 weeks), with interim sacrifices conducted at 9 (36 weeks) and 15 (60 weeks) months (Table 1).
3.1 Body weights
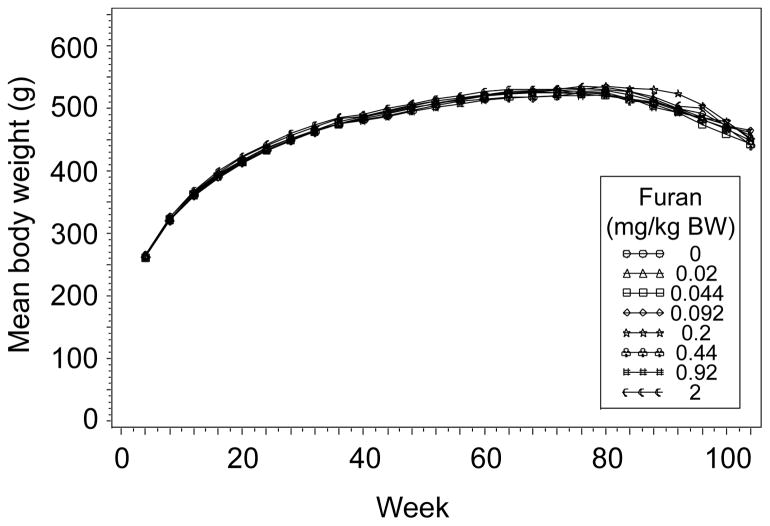
Administering furan to male F344/N Nctr rats by gavage caused only sporadic changes in body weight in the interim sacrifice groups (36 or 60 weeks) or in the groups treated for 2 years (104 weeks, Figure 2).
Figure 2.
Body weights of male F344/N Nctr rats administered 0, 0.02, 0.044, 0.092, 0.2, 0.44, 0.92, or 2.0 mg furan/kg BW for 2 years (104 weeks), as a function of the number of weeks on treatment.
3.2 Survival
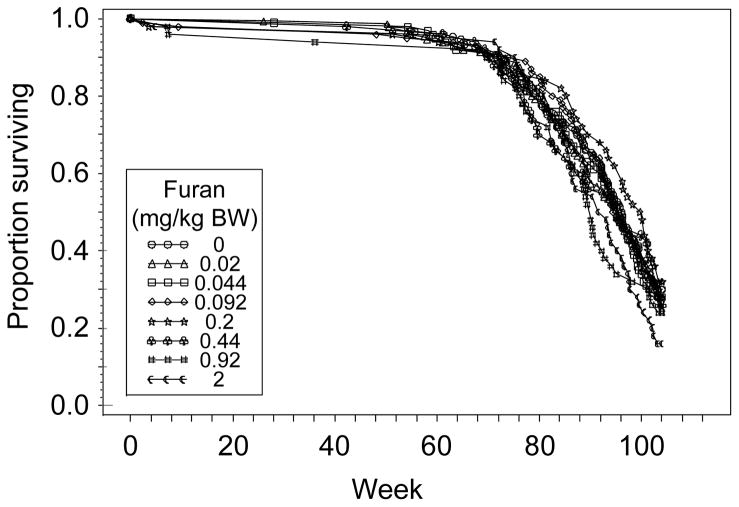
All rats in the 36-week interim sacrifice groups survived until the end of the treatment period. Survival in the 60-week interim sacrifice groups was ≥ 85%, with moribund and dead animals being distributed across the entire dose range. In the groups treated for 2 years, there was a significant (P = 0.021) dose-related decrease in survival (Figure 3); however, compared to the control group (0 mg furan/kg BW), none of the treatment groups had a significant decrease in survival.
Figure 3.
Survival of male F344/N Nctr rats administered 0, 0.02, 0.044, 0.092, 0.2, 0.44, 0.92, or 2 mg furan/kg BW for 2 years (104 weeks), as a function of the number of weeks on treatment. There was a significant (P = 0.021) dose-related decrease in survival; however, compared to the control group (0 mg furan/kg BW), none of the treatment groups had a significant decrease in survival. The Kaplan-Meier estimates for the percent probability of survival at the end of the study were 30, 28, 28, 28, 32, 26, 24, and 16% for 0, 0.02, 0.044, 0.092, 0.2, 0.44, 0.92, and 2 mg furan/kg BW, respectively.
3.3 Neoplasms
There were no treatment-related neoplastic findings in male F344/N Nctr rats treated with furan by gavage for 36 or 60 weeks. In the 36-week interim sacrifice, 8 spontaneous tumors (adrenal cortex adenoma; benign adrenal medulla pheochromocytoma; preputial gland adenoma, carcinoma, and papilloma; subcutaneous skin lipoma; benign neural crest ear tumor; and testicular interstitial cell adenoma) were evident across various treatment groups, including the control group. In the 60-week interim sacrifice, spontaneous neoplasms were observed in all treatment groups, including the control group.
The gavage administration of furan to male F344/N Nctr rats for 2 years was associated with the development of malignant mesothelioma on membranes surrounding the epididymis and on the testicular tunics (Table 2). The survival adjusted incidence of malignant mesothelioma was significantly increased in the combined testes or epididymis of rats administered 2 mg furan/kg BW. Microscopically, malignant mesotheliomas in the epididymis and testicular tunics were characterized by complex papillary surface growths of one to several layers of polyhedral-to-cuboidal mesothelial cells on pedunculated fibrovascular stalks. The neoplastic cells had either abundant weakly eosinophilic cytoplasm and ovoid nuclei with one or more nucleoli or scanty cytoplasm and numerous small basophilic nuclei. In some rats, malignant mesothelioma was associated with other organs or tissues including the large and small intestine, liver, mesentery, pancreas, stomach, adrenal gland, peritoneum, prostate, seminal vesicle, mesenteric lymph node, spleen, scrotum, skeletal muscle, and urinary bladder.
Table 2.
Incidences of malignant mesothelioma, mononuclear cell leukemia, and hepatocellular neoplasms in male F344/N Nctr rats administered 0, 0.02, 0.044, 0.092, 0.2, 0.44, 0.92, or 2 mg furan/kg BW for 2 years.
| Neoplasm | Furan (mg/kg BW) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.02 | 0.044 | 0.092 | 0.2 | 0.44 | 0.92 | 2 | ||
| Malignant mesothelioma, epididymis | Overall ratea | 6/149 (4%) | 8/149 (5%) | 1/98 (1%) | 2/100 (2%) | 0/50 (0%) | 2/50 (4%) | 2/50 (4%) | 5/50 (10%) |
| Poly-3 testb | P = 0.018 | P = 0.371 | P = 0.163N | P = 0.301N | P = 0.155N | P = 0.647 | P =0.617 | P = 0.087 | |
| Malignant mesothelioma, testes | Overall rate | 5/150 (3%) | 7/149 (5%) | 1/98 (1%) | 1/100 (1%) | 0/50 (0%) | 2/49 (4%) | 2/50 (4%) | 4/50 (8%) |
| Poly-3 test | P = 0.032 | P = 0.362 | P = 0.235N | P = 0.222N | P = 0.202N | P = 0.569 | P = 0.544 | P = 0.142 | |
| Malignant mesothelioma, epididymis or testes | Overall rate | 6/150 (4%) | 8/150 (5%) | 1/98 (1%) | 2/100 (2%) | 0/50 (0%) | 2/50 (4%) | 2/50 (4%) | 6/50 (12%) |
| Poly-3 test | P = 0.003 | P = 0.373 | P = 0.164N | P = 0.303N | P = 0.156N | P = 0.645 | P = 0.616 | P = 0.033 | |
| Malignant mesothelioma, all organs | Overall rate | 6/150 (4%) | 9/150 (6%) | 1/100 (1%) | 2/100 (2%) | 0/50 (0%) | 3/50c (6%) | 2/50 (4%) | 6/50 (12%) |
| Poly-3 test | P = 0.005 | P = 0.283 | P = 0.161N | P = 0.303N | P = 0.156N | P = 0.402 | P = 0.616 | P = 0.033 | |
| Mononuclear cell leukemia | Overall rate | 47/150 (31%) | 56/150 (37%) | 36/100 (36%) | 44/100 (44%) | 29/50 (58%) | 18/50 (36%) | 27/50 (54%) | 28/50 (56%) |
| Poly-3 test | P < 0.001 | P = 0.182 | P = 0.275 | P = 0.037 | P = 0.001 | P = 0.341 | P < 0.001 | P = 0.001 | |
| Hepatocellular adenoma | Overall rate | 2/149 (1%) | 2/150 (1%) | 1/99 (1%) | 2/100 (2%) | 0/50 (0%) | 1/49 (2%) | 1/50 (2%) | 3/49 (6%) |
| Poly-3 test | P = 0.017 | P = 0.690 | P = 0.639N | P = 0.549 | P = 0.485N | P = 0.624 | P = 0.603 | P = 0.086 | |
| Hepatocellular carcinoma | Overall rate | 3/149 (2%) | 0/150 (0%) | 0/99 (0%) | 0/100 (0%) | 0/50 (0%) | 0/49 (0%) | 0/50 (0%) | 0/49 (0%) |
| Poly-3 test | P = 0.392N | P = 0.125N | P = 0.206N | P = 0.200N | P = 0.352N | P = 0.377N | P = 0.398N | P = 0.386N | |
| Hepatocellar adenoma or carcinoma | Overall rate | 5/149 (3%) | 2/150 (1%) | 1/99 (1%) | 2/100 (2%) | 0/50 (0%) | 1/49 (2%) | 1/50 (2%) | 3/49 (6%) |
| Poly-3 test | P = 0.066 | P = 0.226N | P = 0.224N | P = 0.399N | P = 0.199N | P = 0.514N | P = 0.544N | P = 0.318 | |
Number of animals with neoplasm/number of animals examined microscopically.
Beneath the 0 mg furan/kg BW incidences are the P-values associated with the trend test. Beneath the treated group incidences are the P-values corresponding to pair-wise comparisons between the 0 mg furan/kg BW group and each treated group. The Poly-3 test accounts for differential mortality in animals that do not reach the terminal sacrifice. P-values < 0.05 were considered significant and are bolded. An N indicates a lower incidence compared to the 0 mg furan/kg BW group.
Includes a mesothelioma (not otherwise specified) of the heart atrium.
Male F344/N Nctr rats had dose-related increases in the incidence of mononuclear cell leukemia, with the increase in incidence being significant at 0.092, 0.2, 0.92, and 2 mg furan/kg BW (Table 2). There was a dose-related increasing trend in the incidence of hepatocellular adenoma; however, none of the treatment groups had a significant increase compared to the control group (0 mg furan/kg BW; Table 2). Likewise, the incidence of hepatocellular carcinoma and combined hepatocellular adenoma or carcinoma was not affected by treatment with furan (Table 2). Based upon the International Harmonization of Nomenclature and Diagnostic Criteria, as described by Thoolen et al. (2010), cholangiocarcinoma was not detected in any dose group.
3.4 Non-neoplastic lesions
The gavage administration of furan to male F344/N Nctr rats for 36 weeks was associated with dose-related increasing trends in liver lesions including cholangiofibrosis, mixed cell foci, biliary tract hyperplasia, bile duct subcapsular hyperplasia, oval cell hyperplasia, hepatocyte hypertrophy, periportal cytoplasmic alteration, and subcapsular fibrosis, chronic inflammation, and pigmentation (Table 3). Significant increases in cholangiofibrosis, bile duct subcapsular hyperplasia, and subcapsular fibrosis, chronic inflammation, and pigmentation were observed at 0.44 mg furan/kg BW. The average severity of each of these lesions increased from minimal to mild as the dose increased.
Table 3.
Incidences of non-neoplastic lesions in the liver of male F344/N Nctr rats administered 0, 0.02, 0.044, 0.092, 0.2, 0.44, 0.92, or 2 mg furan/kg BW for 36 or 60 weeks or 2 years.
| Non-neoplastic liver lesion |
Treatment duration |
Furan (mg/kg BW) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.02 | 0.044 | 0.092 | 0.2 | 0.44 | 0.92 | 2 | |||
| Cholangio-fibrosis | 36 weeks | Overall ratea | 0/20 (0%) | 0/20 (0%) | 0/20 (0%) | 0/20 (0%) | 0/20 (0%) | 6/20 (30%) | 17/20 (85%) | 19/20 (95%) |
| CAFEb | P < 0.001 | P = 0.010 | P < 0.001 | P < 0.001 | ||||||
| Average severityc | 1.0 | 1.1 | 1.2 | |||||||
| 60 weeks | Overall ratea | 0/20 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 10/10 (100%) | 10/10 (100%) | 8/10 (80%) | |
| CAFEb | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | ||||||
| Average severityc | 1.1 | 1.6 | 2.1 | |||||||
| 2 years | Overall ratea | 0/149 (0%) | 0/150 (0%) | 0/99 (0%) | 1/100 (1%) | 38/50 (76%) | 49/49 (100%) | 47/50 (94%) | 49/49 (100%) | |
| Poly-3 testd | P < 0.001 | P = 0.422 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | ||||
| Average severityc | 1.0 | 1.7 | 2.6 | 3.4 | 3.9 | |||||
| Mixed cell foci | 36 weeks | Overall rate | 0/20 (0%) | 0/20 (0%) | 0/20 (0%) | 0/20 (0%) | 1/20 (5%) | 0/20 (0%) | 1/20 (5%) | 5/20 (25%) |
| CAFE | P < 0.001 | P = 0.500 | P = 0.500 | P = 0.024 | ||||||
| Average severity | -e | - | - | - | - | - | - | - | ||
| 60 weeks | Overall rate | 1/20 (5%) | 0/10 (0%) | 1/10 (10%) | 0/10 (0%) | 1/10 (10%) | 1/10 (10%) | 3/10 (30%) | 6/10 (60%) | |
| CAFE | P < 0.001 | P = 0.667N | P = 0.563 | P = 0.667N | P = 0.563 | P = 0.563 | P = 0.095 | P = 0.002 | ||
| Average severity | -e | - | - | - | - | - | ||||
| 2 years | Overall rate | 7/149 (5%) | 7/150 (5%) | 6/99 (6%) | 3/100 (3%) | 5/50 (10%) | 3/49 (6%) | 6/50 (12%) | 13/49 (27%) | |
| Poly-3 test | P < 0.001 | P = 0.588 | P =0.418 | P = 0.364N | P = 0.170 | P = 0.485 | P = 0.056 | P < 0.001 | ||
| Average severity | -e | - | - | - | - | - | - | - | ||
| Biliary tract hyperplasia | 36 weeks | Overall rate | 1/20 (5%) | 1/20 (5%) | 0/20 (0%) | 1/20 (5%) | 0/20 (0%) | 0/20 (0%) | 6/20 (30%) | 19/20 (95%) |
| CAFE | P < 0.001 | P = 0.756 | P = 0.500N | P = 0.756 | P = 0.500N | P = 0.500N | P = 0.046 | P < 0.001 | ||
| Average severity | 1.0 | 1.0 | 1.0 | 1.3 | 1.8 | |||||
| 60 weeks | Overall rate | 7/20 (35%) | 3/10 (30%) | 1/10 (10%) | 2/10 (20%) | 1/10 (10%) | 2/10 (20%) | 3/10 (30%) | 8/10 (80%) | |
| CAFE | P < 0.001 | P = 0.560N | P = 0.154N | P = 0.344N | P = 0.154N | P = 0.344N | P = 0.560N | P = 0.025 | ||
| Average severity | 1.0 | 1.0 | 2.0 | 1.0 | 1.0 | 1.0 | 1.3 | 2.5 | ||
| 2 years | Overall rate | 89/149 (60%) | 86/150 (57%) | 59/99 (60%) | 56/100 (56%) | 29/50 (58%) | 25/49 (51%) | 32/50 (64%) | 43/49 (88%) | |
| Poly-3 test | P < 0.001 | P = 0.308N | P =0.440N | P = 0.303N | P = 0.419N | P = 0.195N | P = 0.341 | P = 0.002 | ||
| Average severity | 1.4 | 1.6 | 1.5 | 1.6 | 2.0 | 2.9 | 3.2 | 3.6 | ||
| Oval cell hyperplasia | 36 weeks | Overall rate | 0/20 (0%) | 0/20 (0%) | 0/20 (0%) | 0/20 (0%) | 0/20 (0%) | 0/20 (0%) | 1/20 (5%) | 16/20 (80%) |
| CAFE | P < 0.001 | P = 0.500 | P < 0.001 | |||||||
| Average severity | 1.0 | 2.2 | ||||||||
| 60 weeks | Overall rate | 0/20 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 3/10 (30%) | 7/10 (70%) | |
| CAFE | P < 0.001 | P = 0.030 | P < 0.001 | |||||||
| Average severity | 1.3 | 2.3 | ||||||||
| 2 years | Overall rate | 14/149 (9%) | 15/150 (10%) | 10/99 (10%) | 8/100 (8%) | 7/50 (14%) | 6/49 (12%) | 14/50 (28%) | 33/49 (67%) | |
| Poly-3 test | P < 0.001 | P = 0.484 | P =0.500 | P = 0.434N | P = 0.284 | P = 0.360 | P < 0.001 | P < 0.001 | ||
| Average severity | 2.2 | 2.5 | 2.0 | 2.1 | 2.1 | 2.7 | 3.1 | 3.1 | ||
| Bile duct subcapsular hyperplasia | 36 weeks | Overall rate | 0/20 (0%) | 0/20 (0%) | 0/20 (0%) | 0/20 (0%) | 1/20 (5%) | 12/20 (60%) | 19/20 (95%) | 20/20 (100%) |
| CAFE | P < 0.001 | P = 0.500 | P < 0.001 | P < 0.001 | P < 0.001 | |||||
| Average severity | 1.0 | 1.1 | 1.2 | 1.8 | ||||||
| 60 weeks | Overall rate | 0/20 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 1/10 (10%) | 9/10 (90%) | 10/10 (100%) | 7/10 (70%) | |
| CAFE | P < 0.001 | P = 0.333 | P < 0.001 | P < 0.001 | P < 0.001 | |||||
| Average severity | 1.0 | 1.1 | 1.5 | 1.9 | ||||||
| Hepatocyte hypertrophy | 36 weeks | Overall rate | 0/20 (0%) | 0/20 (0%) | 0/20 (0%) | 0/20 (0%) | 1/20 (5%) | 0/20 (0%) | 2/20 (10%) | 12/20 (60%) |
| CAFE | P < 0.001 | P = 0.500 | P = 0.244 | P < 0.001 | ||||||
| Average severity | 2.0 | 1.5 | 1.8 | |||||||
| 60 weeks | Overall rate | 0/20 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 5/10 (50%) | 4/10 (40%) | 8/10 (80%) | |
| CAFE | P < 0.001 | P = 0.002 | P = 0.008 | P < 0.001 | ||||||
| Average severity | 2.2 | 1.5 | 2.1 | |||||||
| Periportal cytoplasmic alteration | 36 weeks | Overall rate | 0/20 (0%) | 0/20 (0%) | 0/20 (0%) | 0/20 (0%) | 0/20 (0%) | 0/20 (0%) | 7/20 (35%) | 19/20 (95%) |
| CAFE | P < 0.001 | P = 0.004 | P < 0.001 | |||||||
| Average severity | 1.1 | 2.0 | ||||||||
| 60 weeks | Overall rate | 0/20 (0%) | 0/10 (0%) | 0/10 (0%) | 1/10 (10%) | 0/10 (0%) | 1/10 (10%) | 7/10 (70%) | 8/10 (80%) | |
| CAFE | P < 0.001 | P = 0.333 | P = 0.333 | P < 0.001 | P < 0.001 | |||||
| Average severity | 2.0 | 1.0 | 1.6 | 2.0 | ||||||
| Subcapsular fibrosis | 36 weeks | Overall rate | 0/20 (0%) | 0/20 (0%) | 0/20 (0%) | 0/20 (0%) | 3/20 (15%) | 14/20 (70%) | 20/20 (100%) | 20/20 (100%) |
| CAFE | P < 0.001 | P = 0.115 | P < 0.001 | P < 0.001 | P < 0.001 | |||||
| Average severity | 1.3 | 1.1 | 1.3 | 1.8 | ||||||
| 60 weeks | Overall rate | 0/20 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 4/10 (40%) | 9/10 (90%) | 10/10 (100%) | 7/10 (70%) | |
| CAFE | P < 0.001 | P = 0.008 | P < 0.001 | P < 0.001 | P < 0.001 | |||||
| Average severity | 1.0 | 1.1 | 1.5 | 1.9 | ||||||
| Subcapsular chronic inflammation | 36 weeks | Overall rate | 0/20 (0%) | 0/20 (0%) | 0/20 (0%) | 0/20 (0%) | 2/20 (10%) | 14/20 (70%) | 20/20 (100%) | 20/20 (100%) |
| CAFE | P < 0.001 | P = 0.244 | P < 0.001 | P < 0.001 | P < 0.001 | |||||
| Average severity | 1.0 | 1.1 | 1.3 | 1.8 | ||||||
| 60 weeks | Overall rate | 0/20 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 8/10 (80%) | 9/10 (90%) | 10/10 (100%) | 7/10 (70%) | |
| CAFE | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | |||||
| Average severity | 1.0 | 1.1 | 1.5 | 1.9 | ||||||
| Subcapsular pigmentation | 36 weeks | Overall rate | 0/20 (0%) | 0/20 (0%) | 0/20 (0%) | 0/20 (0%) | 2/20 (10%) | 14/20 (70%) | 20/20 (100%) | 20/20 (100%) |
| CAFE | P < 0.001 | P = 0.244 | P < 0.001 | P < 0.001 | P < 0.001 | |||||
| Average severity | 1.0 | 1.1 | 1.3 | 1.8 | ||||||
| 60 weeks | Overall rate | 0/20 (0%) | 0/10 (0%) | 0/10 (0%) | 0/10 (0%) | 8/10 (80%) | 9/10 (90%) | 10/10 (100%) | 7/10 (70%) | |
| CAFE | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | P < 0.001 | |||||
| Average severity | 1.0 | 1.1 | 1.5 | 1.9 | ||||||
| Basophilic foci | 2 years | Overall rate | 28/149 (19%) | 16/150 (11%) | 19/99 (19%) | 18/100 (18%) | 11/50 (22%) | 13/49 (27%) | 10/50 (20%) | 21/49 (43%) |
| Poly-3 test | P < 0.001 | P = 0.036N | P = 0.536 | P = 0.445N | P = 0.485 | P = 0.164 | P = 0.390 | P < 0.001 | ||
| Average severity | -e | - | - | - | - | - | - | - | ||
| Regenerative hyperplasia | 2 years | Overall rate | 0/149 (0%) | 1/150 (1%) | 1/99 (1%) | 2/100 (2%) | 1/50 (2%) | 1/49 (2%) | 7/50 (14%) | 12/49 (24%) |
| Poly-3 test | P < 0.001 | P = 0.498 | P = 0.419 | P = 0.158 | P = 0.290 | P = 0.276 | P < 0.001 | P < 0.001 | ||
| Average severity | 4.0 | 4.0 | 2.5 | 3.0 | 2.0 | 3.6 | 3.9 | |||
| Cytoplasmic vacuolization | 2 years | Overall rate | 23/149 (15%) | 23/150 (15%) | 14/99 (14%) | 19/100 (19%) | 12/50 (24%) | 18/49 (37%) | 23/50 (46%) | 37/49 (76%) |
| Poly-3 test | P < 0.001 | P = 0.548 | P = 0.493N | P = 0.280 | P = 0.153 | P = 0.001 | P < 0.001 | P < 0.001 | ||
| Average severity | 2.3 | 2.2 | 2.1 | 2.3 | 3.0 | 3.0 | 2.8 | 3.3 | ||
Number of animals with lesion/number of animals examined microscopically.
Beneath the 0 mg furan/kg BW incidences are the P-values associated with a Cochran-Armitage linear trend test. Beneath the treated group incidences are the P-values corresponding to pair-wise comparisons, as assessed by a Fisher’s Exact test, between the 0 mg furan/kg BW group and each treated group. CAFE refers to Cochran-Armitage linear trend test/Fisher’s Exact test. P-values < 0.05 were considered significant and are bolded. An N indicates a lower incidence compared to the 0 mg furan/kg BW group.
Average severity of the observed lesions. The severity of the lesions was graded as 1, minimal; 2, mild; 3, moderate; and 4, marked.
Beneath the 0 mg furan/kg BW incidences are the P-values associated with the trend test. Beneath the treated group incidences are the P-values corresponding to pair-wise comparisons between the 0 mg furan/kg BW group and each treated group. The Poly-3 test accounts for differential mortality in animals that do not reach the terminal sacrifice. P-values < 0.05 were considered significant and are bolded. An N indicates a lower incidence compared to the 0 mg furan/kg BW group.
Severity score not recorded; the lesion was indicated as present or absent.
The subcapsular changes, usually noted along the margins of the left and caudate lobes, were focal to segmentally continuous and featured subcapsular bile duct hyperplasia, inflammatory cell infiltrates, pigmentation, and fibrosis, along with periportal proliferation of oval cells and bile ducts. In many rats, hypertrophy was noted along the bordering layers of hepatocytes and these usually displayed a basophilic cytoplasm. A diagnosis of cholangiofibrosis was made only when the normal hepatic parenchyma was replaced by a combination of the features noted above, without markedly disturbing the lobe outline.
Rats treated for 60 weeks had a spectrum of liver lesions similar to that found after dosing for 36 weeks (Table 3), but the incidences tended to become significant at lower doses. For example, in the 60-week rats, the incidence of subcapsular fibrosis, chronic inflammation, and pigmentation became significant at 0.2 mg furan/kg BW (as compared to 0.44 mg furan/kg BW at 36 weeks).
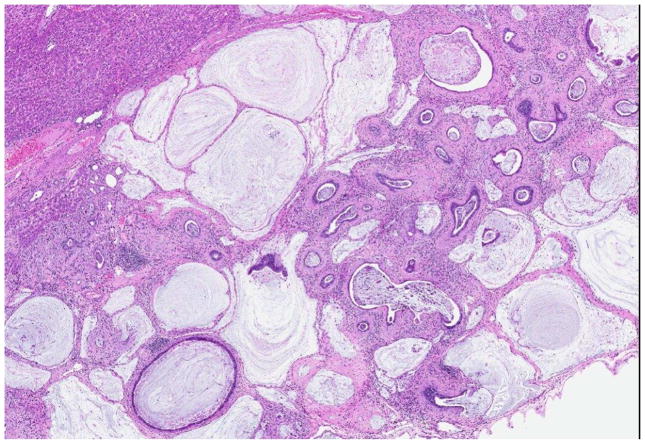
Rats administered furan for 2 years had dose-related increasing trends in liver cholangiofibrosis, mixed cell foci, basophilic foci, biliary tract hyperplasia, oval cell hyperplasia, regenerative hyperplasia, and cytoplasmic vacuolization (Table 3). The incidence of cholangiofibrosis was significantly increased at 0.2 mg furan/kg BW, and a single occurrence was observed at 0.092 mg furan/kg BW. At 2 years, the cholangiofibrosis was characterized by dilated to cystic bile ducts, often irregular, filled with mucinous and cellular debris, surrounded by dense collagenous connective tissue, with a prominent inflammatory cell infiltrate (Figure 4). The epithelium varied from low cuboidal to tall columnar, with hyperbasophilic and pleomorphic cells, along with goblet and Paneth cells. Large sclerotic lesions, displaying only epithelial remnants or crescent-shaped structures, were observed in the higher dose groups. Regenerative hepatocellular hyperplasia was sometimes associated with these extensive lesions.
Figure 4.
Cholangiofibrosis observed in a male rat treated with 2 mg furan/kg BW for 2 years. The lesion is characterized by dilated and cystic bile ducts filled with mucinous and cellular debris surrounded by dense collagenous connective tissue with a prominent inflammatory cell infiltrate. The epithelium varies from low cuboidal to tall columnar, with hyperbasophilic and pleomorphic cells along with goblet cells and Paneth cells.
The severity of the cholangiofibrosis increased with dose, with the severity being minimal-to-mild at 0.2 mg furan/kg BW and moderate-to-marked at 2 mg furan/kg BW. Significant oval cell hyperplasia, regenerative hyperplasia, and cytoplasmic vacuolization were noted in the higher dose groups. At the highest dose of 2 mg furan/kg BW, there was an increase in biliary tract hyperplasia, basophilic foci, and mixed cell foci. Lesions with diagnostic features of cholangiocarcinoma (e.g. invasion into adjacent liver tissue; Thoolen et al., 2010) were not evident in any of the treatment groups.
In addition to non-neoplastic lesions of the liver, rats treated with furan for 2 years had dose-related increasing trends in bone marrow hyperplasia, cataracts of the eye, and forestomach edema, epithelium hyperplasia, inflammation, and ulceration (Supplementary Table 1). The incidence of these lesions was significantly increased at 2 mg furan/kg BW and the severity tended to be mild-to-moderate. Slight, but statistically significant, increases in non-neoplastic lesions were also observed in other tissues and organs (Supplementary Table 1).
4. Discussion
Furan is produced during the cooking of many common foods, including coffee, baked or fried cereal products, canned and jarred foods, baby food, and infant formula. Previous bioassays have demonstrated furan to be hepatocarcinogenic in mice and rats (Maronpot et al., 1991; Elmore and Sirica, 1993; National Toxicology Program, 1993; Johansson et al., 1997; Moser et al., 2009), with rats appearing to be more sensitive than mice due to the formation of cholangiocarcinoma. For example, when B6C3F1 mice (Moser et al., 2009) were administered furan for 2 years, the incidence of combined hepatocellular adenoma or carcinoma became significantly increased at a dose of 4 mg furan/kg BW. F344/N rats treated in a similar manner also had a significant increase in hepatocellular adenoma or carcinoma at 4 mg furan/kg BW; however, they also had nearly a 100% incidence of cholangiocarcinoma at the lowest dose tested, which was 2 mg furan/kg BW (National Toxicology Program, 1993). In order to provide bioassay data for use in risk assessments of dietary exposures to furan, we have investigated the carcinogenicity of furan in male F344/N Nctr rats at doses anticipated to give a wide range of carcinogenic responses (Table 1).
Furan at doses of up to 2 mg/kg BW for 2 years had no effect upon body weight in male F344/N Nctr rats (Figure 2), and while there was a dose-related decrease in survival (Figure 3), none of the furan treatment groups differed significantly from the control group. This is similar to what was observed in the previous NTP bioassay with furan: 2 mg furan/kg BW did not affect the body weight or survival of the F344/N rats; these parameters were only affected at 8 mg furan/kg BW (National Toxicology Program, 1993).
The administration of furan caused a dose-related increase in malignant mesothelioma of the epididymis or testes. This neoplasm was not reported in the previous NTP bioassay with furan, even at doses up to 8 mg furan/kg BW (National Toxicology Program, 1993). In the current bioassay, a statistically significant increase in malignant mesothelioma was observed at 2 mg furan/kg BW, with the incidence being 12% compared to 4% in the control group (Table 2). The incidence in the 2 mg furan/kg BW group exceeded the historical control range (mean, 4.2% (31/742); range, 0 – 6.4% for 10 studies) observed for malignant mesothelioma in male F344/N Nctr rats in bioassays conducted at the NCTR.
The mechanism for the induction of malignant mesothelioma of the epididymis or testes is currently not known. This neoplasm has been reported in F344 rats administered the dietary contaminant acrylamide (Johnson et al., 1986; Friedman et al., 1995; National Toxicology Program, 2012; Beland et al., 2013), and benchmark dose modeling indicates that furan (Table 4 and Supplementary Figure 1) and acrylamide (BMD10, 29.90 – 31.04 μmol/kg BW; BMDL10 18.08 – 23.71 μmol/kg BW; Beland et al., 2013) have similar potencies for the induction of this neoplasm. DNA adducts derived from the electrophilic metabolite glycidamide have been detected in DNA from the testes and other tumor target tissues (i.e., thyroid and mammary gland) of F344 rats administered acrylamide (Doerge et al., 2005), which suggests a genotoxic mechanism for tumor induction by acrylamide; in contrast, evidence for the formation of DNA adducts derived from cis-2-butene-1,4-dial, the presumed electrophilic metabolite of furan, is minimal in tissues from rats treated with furan, even at high doses and for extended exposure times (Neuwirth et al., 2012; Churchwell et al., 2015). Although glycidamide-DNA adducts have been detected in testicular DNA of acrylamide-treated rats (Doerge et al., 2005), Big Blue rats administered acrylamide did not show an increased mutant frequency in the testes (Mei et al., 2010); likewise, increased mutant frequencies were not observed in various tissues of Big Blue rats administered furan (McDaniel et al., 2012).
Table 4.
Benchmark dose modeling of incidences of epididymis or testes malignant mesothelioma and cholangiofibrosis in male F344/N Nctr rats administered 0, 0.02, 0.044, 0.092, 0.2, 0.44, 0.92, or 2 mg furan/kg BW for 2 years.
| Neoplasm/non-neoplastic lesion | Model | AICa | Fitted modelb | GOFc | BMD10d | BMDL10e |
|---|---|---|---|---|---|---|
| Malignant mesothelioma | Gamma | 227.9 | 0.154 | 0.279 | 30.47 | 21.86 |
| Logistic | 226.8 | 0.182 | 0.306 | 33.10 | 22.48 | |
| Log-Logistic | 227.9 | 0.154 | 0.279 | 30.38 | 21.93 | |
| Log-Probit | 227.9 | 0.154 | 0.279 | 30.62 | 21.13 | |
| Multistage | 226.1 | 0.225 | 0.368 | 31.70 | 22.05 | |
| Probit | 226.9 | 0.175 | 0.298 | 34.20 | 22.08 | |
| Weibull | 227.9 | 0.154 | 0.279 | 30.34 | 22.12 | |
| Cholangiofibrosisf | Gamma | 70.3 | 1.000 | 1.000 | 1.81 | 1.60 |
| Logistic | 70.5 | 0.995 | 0.998 | 2.04 | 1.79 | |
| Log-Logistic | 70.4 | 0.999 | 1.000 | 1.88 | 1.65 | |
| Log-Probit | 70.3 | 1.000 | 1.000 | 1.76 | 1.59 | |
| Multistage | -g | |||||
| Probit | 70.3 | 1.000 | 1.000 | 1.90 | 1.69 | |
| Weibull | 70.3 | 1.000 | 1.000 | 1.95 | 1.70 |
AIC, Akaike information criterion.
P-value of fitted model compared to the full model.
GOF, Goodness-of-fit P-value.
BMD10, benchmark dose (μmol furan/kg BW) that caused a 10% excess risk of the specified adverse effect over that observed in the appropriate control group.
BMDL10, lower 95% confidence limit of benchmark dose (μmol furan/kg BW).
The models were constructed using the cholangiofibrosis incidence in male rats administered 0, 0.02, 0.044, 0.092, 0.2, or 0.44 mg furan/kg BW for 2 years. Satisfactory fits could not be obtained when the 2 highest doses (0.92 and 2 mg furan/kg BW) were included in the calculations.
Model rejected.
As an alternative to a genotoxic mechanism, malignant mesothelioma of the epidydimis or testes has been proposed to be a consequence of Leydig cell tumor progression resulting from carcinogen-induced decreases in testosterone and increases in luteinizing hormone (Shipp et al., 2006; Maronpot et al., 2009; Maronpot et al., 2016). Maronpot et al. (2015) have recently evoked such an explanation when assessing the induction of malignant mesothelioma in male Wistar Han rats treated with acrylamide. F344 rats administered relatively high doses of acrylamide (≥ 10 mg acrylamide/kg BW) do have decreased serum levels of testosterone and increased serum levels of luteinizing hormone; nonetheless, Leydig cell proliferation was not evident (Camacho et al., 2012). In contrast, F344 rats treated with furan had increased serum levels of testosterone and decreased serum levels of luteinizing hormone, but again, Leydig cell proliferation was not evident (Gill et al., 2010). Thus, while the induction of malignant mesothelioma is clearly related to treatment with furan, the mechanism for the induction of these tumors is still uncertain.
Mononuclear cell leukemia was observed in all dose groups of male F344/N Nctr rats, including the control group. Although statistically significant increases in prevalence were observed in the higher dose groups (0.092, 0.2, 0.92, and 2 mg furan/kg BW; Table 2), the incidences were within the historical control range (mean, 46.9% (348/742); range, 31.3 – 64.6% for 10 studies) observed for mononuclear cell leukemia in male F344/N Nctr rats in bioassays conducted at the NCTR. An increased incidence of mononuclear cell leukemia was observed in the previous NTP furan bioassay, with the increase in male F344/N rats being significant at 4 and 8 mg furan/kg BW (National Toxicology Program, 1993). Carthew et al. (2010) performed benchmark dose modeling on the mononuclear cell leukemia in male F344/N rats from the NTP bioassay and obtained a BMD10 of 34.21 μmol furan/kg BW and a BMDL10 of 16.88 μmol furan/kg BW. These values are quite close to those calculated for malignant mesothelioma of the epididymis or testes in the current furan bioassay (Table 4 and Supplementary Figure 1). Attempts to model the mononuclear cell leukemia data from the current furan bioassay were not successful (data not shown).
A major goal of this project was to define the dose response for the formation of cholangiocarcinoma upon the administration of furan. The criteria used to characterize cholangiocarcinoma were those recently specified by Thoolen et al. (2010) as part of the International Harmonization of Toxicologic Pathology Nomenclature initiative (Mann et al., 2012). As a consequence, the diagnostic criteria used to identify cholangial lesions in the present study were not the same as those used in the previous study of furan by the NTP (National Toxicology Program, 1993). Based upon these updated criteria, there was no evidence for cholangiocarcinoma in any of the male F344/N Nctr rats treated with furan; instead, the major hepatic lesion detected was cholangiofibrosis, a precursor lesion to cholangiocarcinoma.
In the previous NTP furan bioassay, 43 of 50 male rats treated with 2 mg furan/kg BW were diagnosed with cholangiocarcinoma (National Toxicology Program, 1993). A re-assessment of 23 livers diagnosed with cholangiocarcinoma from the 2 mg furan/kg BW dose group in the previous NTP furan bioassay, based upon the updated criteria specified by Thoolen et al. (2010), resulted in 20 of the lesions being reclassified as cholangiofibrosis, while only 3 retained the designation of cholangiocarcinoma. By contrast, when livers from the 8 mg furan/kg BW group were re-examined, all retained the classification of cholangiocarcinoma (Malarkey et al., 2014). From these data, it appears that, in both bioassays, male F344/N rats administered 2 mg furan/kg BW developed a very high incidence of cholangiofibrosis, with cholangiocarcinoma becoming prevalent at higher doses.
The induction of cholangiofibrosis in the male F344/N Nctr rats in the current 2-year bioassay was markedly non-linear. The lesion was not detected in the control rats or in rats administered 0.02 or 0.044 mg furan/kg BW for 2 years (Table 3). A very low incidence (1%) occurred in rats given 0.092 mg furan/kg BW, whereas a very high prevalence (≥ 76%) was detected at higher doses (0.2, 0.44, 0.92, and 2 mg furan/kg BW). Furthermore, as the dose increased, so did the severity of the lesion, from minimal at 0.092 mg furan/kg BW to marked at 2 mg furan/kg BW.
Cholangiofibrosis was also present in the livers of the F344/N Nctr rats examined after 36 and 60 weeks of dosing. As with the rats treated for 2 years, the dose-response was markedly non-linear: cholangiofibrosis was not detected at doses ≤ 0.2 mg furan/kg BW, while at higher doses, the incidence increased dramatically (Table 3). The severity of the lesion also increased with the length of dosing, being minimal after 36 weeks of dosing and minimal-to-mild after 60 weeks of treatment.
The induction of hepatobiliary lesions in F344/N rats treated with furan has been the subject of intense investigations (reviewed by Bakhiya and Appel, 2010; Moro et al., 2012a). A substantial number of these investigations have been conducted at doses that far exceed those used in the current study, which makes extrapolation to lower doses problematic. Two mechanisms have been considered to account for the hepatobiliary lesions: a genotoxic pathway, as a consequence of DNA adduct formation, and a non-genotoxic pathway involving furan-induced necrosis, inflammation, and proliferative regeneration. These mechanisms are not necessarily mutually exclusive.
Furan is known to undergo hepatic cytochrome P450 2E1-catalyzed oxidation to cis-butene-1,4-dial, a reactive dialdehyde (Kedderis et al., 1993; Chen et al., 1995). Inhibition of this metabolic oxidation through the use of cytochrome P450 inhibitors suppresses the hepatotoxicities associated with furan, thus reinforcing the importance of cis-butene-1,4-dial in the toxic manifestations of the compound (Fransson-Steen et al., 1997). cis-Butene-1,4-dial will react with DNA and a number of DNA adducts resulting from the reaction have been characterized in vitro (Figure 1; Gingipalli and Dedon, 2001; Byrns et al., 2002; Bohnert et al., 2004; Byrns et al., 2004; Byrns et al., 2006). cis-Butene-1,4-dial was mutagenic in S. typhimurium TA104 (Peterson et al., 2000) and increased the mutant frequency in L5158Y tk+/− mouse lymphoma cells (Kellert et al., 2008b), which supports the concept of a genotoxic pathway. Nonetheless, as noted above, there is minimal evidence for the formation of cis-butene-1,4-dial adducts in hepatic DNA from rats treated with furan (Neuwirth et al., 2012; Churchwell et al., 2015). Likewise, there was no increase in the mutant frequency in the cII transgene in DNA isolated from the livers of Big Blue rats treated with up to 8 mg furan/kg BW (McDaniel et al., 2012).
cis-Butene-1,4-dial bears a C=C double bond conjugated with two aldehyde groups; as such, it will readily react with nucleophilic sites (thiols and amino groups) in proteins, which may play a role in furan toxicities. Extensive binding of furan to proteins has been demonstrated in rats treated with furan, with the highest levels being detected in the liver (Burka et al., 1991). Substantial hepatic protein binding has been detected at doses as low as 0.1 mg furan/kg BW, with the binding increasing 10-fold at 2 mg/kg BW (Moro et al., 2012b). Mass spectral analyses indicated that the targeted proteins were localized primarily in the cytosol and mitochondria, with fewer furan-modified nuclear proteins being detected. The targeting of mitochondrial proteins by cis-butene-1,4-dial could contribute to the impairment of oxidative phosphorylation that has been observed in F344 rats treated with furan (Mugford et al., 1997). This interpretation is supported by the observation that the depletion of ATP could be prevented by prior treatment with the cytochrome P450 inhibitor 1-phenylimidazole. Other low-dose effects that have been observed upon administration of furan to F344/N rats include an increase in cell proliferation, as indicated by an increase in bromodeoxyuridine incorporation in the subcapsular area of the caudate liver lobe (Mally et al., 2010), and an increased expression of hepatic genes associated with cell cycle and apoptosis (Chen et al., 2010).
More recently, dose-dependent and time-dependent epigenetic changes have been examined in the livers of male F344 rats exposed to 0, 0.92, 2, and 4 mg furan/kg BW for up to 360 days (de Conti et al., 2014). Promoter region hypermethylation was observed in the tumor suppressor genes p16IKN4a and Rassf1a, and this was accompanied by a decreased expression of Rassf1a. Subsequently, the persistence of the epigenetic changes was investigated in the livers of male F344 rats treated for 90 days with 8 mg furan/kg BW and then monitored for up to an additional 360 days (de Conti et al., 2015). Upon the discontinuation of the treatment, most of the furan-associated changes were reversible; nonetheless, there was a sustained decrease in the acetylation of histone H3 lysines 9 and 56, and this was associated with the formation of heterochromatin and decreased gene expression. In further work, cholangiofibrotic lesions were examined in the livers of male F344 rats exposed to 0.92 and 2 mg furan/kg BW for 2 years (Tryndyak et al., 2016). A number of genes involved in key pathways associated with different aspects of liver pathology showed alterations in promoter-region DNA methylation and gene expression. For example, decreased promoter methylation and increased gene expression were observed with Areg and Jag1, key components of the Hippo and Notch signaling pathways. Over-expression of these genes has been involved in the pathogenesis of liver fibrosis, activation of hepatic progenitor cells, and the development of liver cancer. In contrast to Areg and Jag 1, hypermethylation of the promoter region and decreased gene expression was found with Foxe1, which encodes a thyroid-specific forkhead transcription factor that represses transcription of several rat genes, including fibrogenesis-associated Duox2 and Adamts1 genes. Therefore, hypermethylation-associated inhibition of Foxe1 expression may cause activation of these genes and stimulation of liver fibrogenesis. These results indicate that gene-specific changes in DNA methylation have functional consequences that may be important for the induction of cholangiofibrosis and subsequently cholangiocarcinoma.
Non-neoplastic lesions were also detected in non-hepatic tissues, including the kidney, eye, forestomach, and bone marrow (Supplementary Table 1). The incidences of these lesions tended to become significant at only the highest dose of 2 mg furan/kg BW, which is probably a reflection of the fact that much higher levels of furan-protein binding are detected in the liver as compared to other organs (Burka et al., 1991; Moro et al., 2012b).
Using data from the previous NTP bioassay on furan (National Toxicology Program, 1993), Carthew et al. (2010) applied a margin of exposure (MOE) approach to assess the risk associated with dietary exposures to furan. Their assessment was based upon furan-induced combined hepatocellular adenoma or carcinoma in male F344/N rats, for which benchmark dose modeling gave a BMDL10 of 1.23 mg furan/kg BW (18.13 μmole furan/kg BW). Applying this BMDL10 led to MOEs of 750 – 4,300, depending upon age and geographic location. Benchmark dose modeling of the furan-induced malignant mesothelioma of the epididymis or testes in the currently bioassay gave BMDL10 values of 1.44 – 1.53 mg furan/kg BW (21.13 – 22.48 μmole furan/kg BW; Table 4; Supplementary Figure 1), which would afford MOEs similar to those calculated by Carthew et al. (2010). The application of benchmark dose modeling to the furan-induced cholangiofibrosis led to BMDL10 values of 0.11 – 0.12 mg furan/kg BW (1.59 – 1.79 μmole furan/kg BW; Table 4; Supplementary Figure 2), which would result in an approximately 10-fold reduction in the MOEs compared to the values obtained based upon hepatocellular neoplasms or malignant mesothelioma.
Supplementary Material
Highlights.
Furan is a contaminant in many common foods
The carcinogenicity of furan was assessed in male F344/N rats
Exposure to furan induced malignant mesothelioma and mononuclear cell leukemia
The most sensitive non-neoplastic lesion was cholangiofibrosis
Acknowledgments
We thank F. Michelle McLellen and Matthew S. Bryant for conducting the chemical analyses and Andy Matson and James Carson for preparing dosing solutions and providing animal care. This study was supported by the Intramural Research Program of the NIH/National Institute of Environmental Health Sciences (NIEHS) via an Interagency Agreement between the NTP/NIEHS and the NCTR/FDA (NCTR/FDA IAG #224-12-0003; NIH/NTP IAG #AES12013). Financial support was also provided by Fundação para a Ciência e a Tecnologia, Portugal (Grants RECI/QEQ-MED/0330/2012 and UID/QUI/00100/2013). The opinions expressed in this paper do not necessarily represent those of the U.S. Food and Drug Administration.
Abbreviations
- BMD
benchmark dose
- BMDL
lower limit of benchmark dose
- BW
body weight
- FDA
Food and Drug Administration
- GLP
Good Laboratory Practice
- MOE
margin of exposure
- NCTR
National Center for Toxicological Research
- NIEHS
National Institute of Environmental Health Sciences
- NIH
National Institutes of Health
- NTP
National Toxicology Program
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bailer AJ, Portier CJ. Effects of treatment-induced mortality and tumor-induced mortality on tests for carcinogenicity in small samples. Biometrics. 1988;44:417–431. [PubMed] [Google Scholar]
- Bakhiya N, Appel KE. Toxicity and carcinogenicity of furan in human diet. Arch Toxicol. 2010;84:563–578. doi: 10.1007/s00204-010-0531-y. [DOI] [PubMed] [Google Scholar]
- Beland FA, Mellick PW, Olson GR, Mendoza MCB, Marques MM, Doerge DR. Carcinogenicity of acrylamide in B6C3F1 mice and F344/N rats from a 2-year drinking water exposure. Food Chem Toxicol. 2013;51:149–159. doi: 10.1016/j.fct.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Bieler GS, Williams RL. Ratio estimates, the delta method, and quantal response tests for increased carcinogenicity. Biometrics. 1993;49:793–801. [PubMed] [Google Scholar]
- Bohnert T, Gingipalli L, Dedon PC. Reaction of 2′-deoxyribonucleosides with cis- and trans-1,4-dioxo-2-butene. Biochem Biophys Res Commun. 2004;323:838–844. doi: 10.1016/j.bbrc.2004.08.164. [DOI] [PubMed] [Google Scholar]
- Burka LT, Washburn KD, Irwin RD. Disposition of [14C]furan in the male F344 rat. J Toxicol Environ Health. 1991;34:245–257. doi: 10.1080/15287399109531564. [DOI] [PubMed] [Google Scholar]
- Byrns MC, Predecki DP, Peterson LA. Characterization of nucleoside adducts of cis-2-butene-1,4-dial, a reactive metabolite of furan. Chem Res Toxicol. 2002;15:373–379. doi: 10.1021/tx0101402. [DOI] [PubMed] [Google Scholar]
- Byrns MC, Vu CC, Peterson LA. The formation of substituted 1,N6-etheno-2′-deoxyadenosine and 1,N2-etheno-2′-deoxyguanosine adducts by cis-2-butene-1,4-dial, a reactive metabolite of furan. Chem Res Toxicol. 2004;17:1607–1613. doi: 10.1021/tx049866z. [DOI] [PubMed] [Google Scholar]
- Byrns MC, Vu CC, Neidigh JW, Abad JL, Jones RA, Peterson LA. Detection of DNA adducts derived from the reactive metabolite of furan, cis-2-butene-1,4-dial. Chem Res Toxicol. 2006;19:414–420. doi: 10.1021/tx050302k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho L, Latendresse JR, Muskhelishvili L, Patton R, Bowyer JF, Thomas M, Doerge DR. Effects of acrylamide exposure on serum hormones, gene expression, cell proliferation, and histopathology in male reproductive tissues of Fischer 344 rats. Toxicol Lett. 2012;211:135–143. doi: 10.1016/j.toxlet.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carthew P, DiNovi M, Setzer RW. Application of the margin of exposure (MoE) approach to substances in food that are genotoxic and carcinogenic. Example: furan (CAS No 110-00-9) Food Chem Toxicol. 2010;48:S69–S74. doi: 10.1016/j.fct.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Chen LJ, Hecht SS, Peterson LA. Identification of cis-2-butene-1,4-dial as a microsomal metabolite of furan. Chem Res Toxicol. 1995;8:903–906. doi: 10.1021/tx00049a001. [DOI] [PubMed] [Google Scholar]
- Chen LJ, Hecht SS, Peterson LA. Characterization of amino acid and glutathione adducts of cis-2-butene-1,4-dial, a reactive metabolite of furan. Chem Res Toxicol. 1997;10:866–874. doi: 10.1021/tx9700174. [DOI] [PubMed] [Google Scholar]
- Chen T, Mally A, Ozden S, Chipman JK. Low doses of the carcinogen furan alter cell cycle and apoptosis gene expression in rat liver independent of DNA methylation. Environ Health Perspect. 2010;118:1597–1602. doi: 10.1289/ehp.1002153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchwell MI, Scheri RC, Von Tungeln LS, Gamboa da Costa G, Beland FA, Doerge DR. Evaluation of serum and liver toxicokinetics for furan and liver DNA adduct formation in male Fischer 344 rats. Food Chem Toxicol. 2015;86:1–8. doi: 10.1016/j.fct.2015.08.029. [DOI] [PubMed] [Google Scholar]
- Cox DR. Regression models and life-tables. J Royal Stat Soc B. 1972;34:187–220. [Google Scholar]
- de Conti A, Kobets T, Escudero-Lourdes C, Montgomery B, Tryndyak V, Beland FA, Doerge DR, Pogribny IP. Dose- and time-dependent epigenetic changes in the livers of Fisher 344 rats exposed to furan. Toxicol Sci. 2014;139:371–380. doi: 10.1093/toxsci/kfu044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Conti A, Kobets T, Tryndyak V, Burnett SD, Han T, Fuscoe JC, Beland FA, Doerge DR, Pogribny IP. Persistence of furan-induced epigenetic aberrations in the livers of F344 rats. Toxicol Sci. 2015;144:217–226. doi: 10.1093/toxsci/kfu313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNovi M, Mihalov J. An updated exposure assessment for furan from the consumption of adult and baby foods. Center for Food Safety and Applied Nutrition, U.S. Food and Drug Administration; 2007. [Accessed 24 August 2015]. http://www.fda.gov/Food/FoodborneIllnessContaminants/ChemicalContaminants/ucm110770.htm. [Google Scholar]
- Doerge DR, Gamboa da Costa G, McDaniel LP, Churchwell MI, Twaddle NC, Beland FA. DNA adducts derived from administration of acrylamide and glycidamide to mice and rats. Mutat Res. 2005;580:131–141. doi: 10.1016/j.mrgentox.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Dunnett CW. A multiple comparison procedure for comparing several treatments with a control. J Am Stat Assoc. 1955;50:1096–1121. [Google Scholar]
- Elmore LW, Sirica AE. “Intestinal-type” of adenocarcinoma preferentially induced in right/caudate liver lobes of rats treated with furan. Cancer Res. 1993;53:254–259. [PubMed] [Google Scholar]
- European Food Safety Authority. Technical report of EFSA prepared by Data Collection and Exposure Unit (DATEX) on “Monitoring of furan levels in food”. The EFSA Scientific Report. 2009;304:1–23. [Google Scholar]
- Fransson-Steen R, Goldsworthy TL, Kedderis GL, Maronpot RR. Furan-induced liver cell proliferation and apoptosis in female B6C3F1 mice. Toxicology. 1997;118:195–204. doi: 10.1016/s0300-483x(97)03618-4. [DOI] [PubMed] [Google Scholar]
- Friedman MA, Dulak LH, Stedham MA. A lifetime oncogenicity study in rats with acrylamide. Fundam Appl Toxicol. 1995;27:95–105. doi: 10.1093/toxsci/27.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill S, Bondy G, Lefebvre DE, Becalski A, Kavanagh M, Hou Y, Turcotte AM, Barker M, Weld M, Vavasour E, Cooke GM. Subchronic oral toxicity study of furan in Fischer-344 rats. Toxicol Pathol. 2010;38:619–630. doi: 10.1177/0192623310368978. [DOI] [PubMed] [Google Scholar]
- Gingipalli L, Dedon PC. Reaction of cis- and trans-2-butene-1,4-dial with 2′-deoxycytidine to form stable oxadiazabicyclooctaimine adducts. J Am Chem Soc. 2001;123:2664–2665. doi: 10.1021/ja0056421. [DOI] [PubMed] [Google Scholar]
- Hasnip S, Crews C, Castle L. Some factors affecting the formation of furan in heated foods. Food Add Contam. 2006;23:219–227. doi: 10.1080/02652030500539766. [DOI] [PubMed] [Google Scholar]
- Hazardous Substances Data Bank. [Accessed 17 November 2014];Furan. 2011 http://toxnet.nlm.nih.gov.
- International Agency for Research on Cancer. Furan. International Agency for Research on Cancer; Lyon: 1995. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 63. Dry Cleaning, Some Chlorinated Solvents and Other Industrial Chemicals; pp. 393–407. [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer. Production, composition, use and regulations. International Agency for Research on Cancer; Lyon: 2004. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Volume 83. Tobacco Smoke and Involuntary Smoking. Tobacco Smoke. 1; pp. 53–119. [PMC free article] [PubMed] [Google Scholar]
- Johansson E, Reynolds S, Anderson M, Maronpot R. Frequency of Ha-ras-1 gene mutations inversely correlated with furan dose in mouse liver tumors. Mol Carcinog. 1997;18:199–205. doi: 10.1002/(sici)1098-2744(199704)18:4<199::aid-mc3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Gorzinski SJ, Bodner KM, Campbell RA, Wolf CH, Friedman MA, Mast RW. Chronic toxicity and oncogenicity study on acrylamide incorporated in the drinking water of Fischer 344 rats. Toxicol Appl Pharmacol. 1986;85:154–168. doi: 10.1016/0041-008x(86)90109-2. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Stat Assoc. 1958;53:457–481. [Google Scholar]
- Kedderis GL, Carfagna MA, Held SD, Batra R, Murphy JE, Gargas ML. Kinetic analysis of furan biotransformation by F-344 rats in vivo and in vitro. Toxicol Appl Pharmacol. 1993;123:274–282. doi: 10.1006/taap.1993.1246. [DOI] [PubMed] [Google Scholar]
- Kellert M, Wagner S, Lutz U, Lutz WK. Biomarkers of furan exposure by metabolic profiling of rat urine with liquid chromatography-tandem mass spectrometry and principal component analysis. Chem Res Toxicol. 2008a;21:761–768. doi: 10.1021/tx7004212. [DOI] [PubMed] [Google Scholar]
- Kellert M, Brink A, Richter I, Schlatter J, Lutz WK. Tests for genotoxicity and mutagenicity of furan and its metabolite cis-2-butene-1,4-dial in L5178Y tk+/− mouse lymphoma cells. Mutat Res. 2008b;657:127–132. doi: 10.1016/j.mrgentox.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Lu D, Peterson LA. Identification of furan metabolites derived from cysteine-cis-2-butene-1,4-dial-lysine cross-links. Chem Res Toxicol. 2010;23:142–151. doi: 10.1021/tx9003215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malarkey DE, Willson GA, Osborne TS. PWG review of cholangioproliferative lesions from selected 2 mg/kg and 8 mg/kg furan-dosed rats from the previously reported NTP study named: Toxicology and Carcinogenesis Studies of Furan (CAS No. 110-00-9) in F344 rats and B6C3F1 Mice (Gavage Studies) (TR #402) Experimental Pathology Laboratories, Inc; Research Triangle Park, NC: 2014. pp. 1–10. [Google Scholar]
- Mally A, Graff C, Schmal O, Moro S, Hamberger C, Schauer UM, Brück J, Özden S, Sieber M, Steger U, Schrenk D, Hard GC, Chipman JK, Dekant W. Functional and proliferative effects of repeated low-dose oral administration of furan in rat liver. Mol Nutr Food Res. 2010;54:1556–1567. doi: 10.1002/mnfr.201000064. [DOI] [PubMed] [Google Scholar]
- Mann PC, Vahle J, Keenan CM, Baker JF, Bradley AE, Goodman DG, Harada T, Herbert R, Kaufmann W, Kellner R, Nolte T, Rittinghausen S, Tanaka T. International harmonization of toxicologic pathology nomenclature: an overview and review of basic principles. Toxicol Pathol. 2012;40:7S–13S. doi: 10.1177/0192623312438738. [DOI] [PubMed] [Google Scholar]
- Maronpot RR, Giles HD, Dykes DJ, Irwin RD. Furan-induced hepatic cholangiocarcinomas in Fischer 344 rats. Toxicol Pathol. 1991;19:561–570. doi: 10.1177/019262339101900401. [DOI] [PubMed] [Google Scholar]
- Maronpot RR, Zeiger E, McConnell EE, Kolenda-Roberts H, Wall H, Friedman MA. Induction of tunica vaginalis mesotheliomas in rats by xenobiotics. Crit Rev Toxicol. 2009;39:512–537. doi: 10.1080/10408440902969430. [DOI] [PubMed] [Google Scholar]
- Maronpot RR, Thoolen RJMM, Hansen B. Two-year carcinogenicity study of acrylamide in Wistar Han rats with in utero exposure. Exp Toxicol Pathol. 2015;67:189–195. doi: 10.1016/j.etp.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Maronpot RR, Nyska A, Foreman JE, Ramot Y. The legacy of the F344 rat as a cancer bioassay model (a retrospective summary of three common F344 rat neoplasms) Crit Rev Toxicol. 2016;46:641–675. doi: 10.1080/10408444.2016.1174669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel LP, Ding W, Dobrovolsky VN, Shaddock JG, Jr, Mittelstaedt RA, Doerge DR, Heflich RH. Genotoxicity of furan in Big Blue rats. Mutat Res. 2012;742:72–78. doi: 10.1016/j.mrgentox.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Mei N, McDaniel LP, Dobrovolsky VN, Guo X, Shaddock JG, Mittelstaedt RA, Azuma M, Shelton SD, McGarrity LJ, Doerge DR, Heflich RH. The genotoxicity of acrylamide and glycidamide in Big Blue rats. Toxicol Sci. 2010;115:412–421. doi: 10.1093/toxsci/kfq069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morehouse KM, Nyman PJ, McNeal TP, DiNovi MJ, Perfetti GA. Survey of furan in heat processed foods by headspace gas chromatography/mass spectrometry and estimated adult exposure. Food Add Contam. 2008;25:259–264. doi: 10.1080/02652030701552949. [DOI] [PubMed] [Google Scholar]
- Moro S, Chipman JK, Wegener JW, Hamberger C, Dekant W, Mally A. Furan in heat-treated foods: formation, exposure, toxicity, and aspects of risk assessment. Mol Nutr Food Res. 2012a;56:1197–1211. doi: 10.1002/mnfr.201200093. [DOI] [PubMed] [Google Scholar]
- Moro S, Chipman JK, Antczak P, Turan N, Dekant W, Falciani F, Mally A. Identification and pathway mapping of furan target proteins reveal mitochondrial energy production and redox regulation as critical targets of furan toxicity. Toxicol Sci. 2012b;126:336–352. doi: 10.1093/toxsci/kfs005. [DOI] [PubMed] [Google Scholar]
- Moser GJ, Foley J, Burnett M, Goldsworthy TL, Maronpot R. Furan-induced dose-response relationships for liver cytotoxicity, cell proliferation, and tumorigenicity (furan-induced liver tumorigenicity) Exp Toxicol Pathol. 2009;61:101–111. doi: 10.1016/j.etp.2008.06.006. [DOI] [PubMed] [Google Scholar]
- Mugford CA, Carfagna MA, Kedderis GL. Furan-mediated uncoupling of hepatic oxidative phosphorylation in Fischer-344 rats: an early event in cell death. Toxicol Appl Pharmacol. 1997;144:1–11. doi: 10.1006/taap.1997.8121. [DOI] [PubMed] [Google Scholar]
- National Toxicology Program. Toxicology and Carcinogenesis Studies of Furan (CAS No. 110-00-9) in F344/N rats and B6C3F1 Mice (Gavage Studies) U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health; 1993. NTP TR 402 (NIH Publication No. 93-2857) [PubMed] [Google Scholar]
- National Toxicology Program. Toxicology and Carcinogenesis Studies of Acrylamide (CAS No. 79-06-1) in F344/N Rats and B6C3F1 Mice (Feed and Drinking Water Studies) U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health; 2012. NTP TR 575 (NIH Publication No. 12-5917) [PubMed] [Google Scholar]
- Neuwirth C, Mosesso P, Pepe G, Fiore M, Malfatti M, Turteltaub K, Dekant W, Mally A. Furan carcinogenicity: DNA binding and genotoxicity of furan in rats in vivo. Mol Nutr Food Res. 2012;56:1363–1374. doi: 10.1002/mnfr.201200226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyman PJ, Morehouse KM, McNeal TP, Perfetti GA, Diachenko GW. Single-laboratory validation of a method for the determination of furan in foods by using static headspace sampling and gas chromatography/mass spectrometry. J AOAC Internat. 2006;89:1417–1424. [PubMed] [Google Scholar]
- Peterson LA, Naruko KC, Predecki DP. A reactive metabolite of furan, cis-2-butene-1,4-dial, is mutagenic in the Ames assay. Chem Res Toxicol. 2000;13:531–534. doi: 10.1021/tx000065f. [DOI] [PubMed] [Google Scholar]
- Peterson LA, Cummings ME, Chan JY, Vu CC, Matter BA. Identification of a cis-2-butene-1,4-dial-derived glutathione conjugate in the urine of furan-treated rats. Chem Res Toxicol. 2006;19:1138–1141. doi: 10.1021/tx060111x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto R, Pike MC, Bernstein L, Gold LS, Ames BN. The TD50: a proposed general convention for the numerical description of the carcinogenic potency of chemicals in chronic-exposure animal experiments. Environ Health Perspect. 1984;58:1–8. doi: 10.1289/ehp.84581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipp A, Lawrence G, Gentry R, McDonald T, Bartow H, Bounds J, Macdonald N, Clewell H, Allen B, Van Landingham C. Acrylamide: review of toxicity data and dose-response analyses for cancer and noncancer effects. Crit Rev Toxicol. 2006;36:481–608. doi: 10.1080/10408440600851377. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Perfetti TA, Mullens MA, Rodgman A, Doolittle DJ. “IARC Group 2B carcinogens” reported in cigarette mainstream smoke. Food Chem Toxicol. 2000;38:825–848. doi: 10.1016/s0278-6915(00)00066-1. [Corrected and republished in Food Chem. Toxicol. 39, 183–205 (2001)] [DOI] [PubMed] [Google Scholar]
- Thoolen B, Maronpot RR, Harada T, Nyska A, Rousseaux C, Nolte T, Malarkey DE, Kaufmann W, Küttler K, Deschl U, Nakae D, Gregson R, Vinlove MP, Brix AE, Singh B, Belpoggi F, Ward JM. Proliferative and nonproliferative lesions of the rat and mouse hepatobiliary system. Toxicol Pathol. 2010;38:5S–81S. doi: 10.1177/0192623310386499. [DOI] [PubMed] [Google Scholar]
- Tryndyak V, de Conti A, Doerge DR, Olson GR, Beland FA, Pogribny IP. Furan-induced transcriptomic and gene-specific DNA methylation changes in the livers of Fischer 344 rats in a two-year carcinogenicity study. Arch Toxicol. 2016 doi: 10.1007/s00204-016-1786-8. in press. [DOI] [PubMed] [Google Scholar]
- Zoller O, Sager F, Reinhard H. Furan in food: headspace method and product survey. Food Add Contam. 2007;24(S1):91–107. doi: 10.1080/02652030701447389. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.