Abstract
Introduction
The perineum stretches naturally during obstetrical labor, but it is unknown if this stretch has a negative impact on pelvic floor outcomes after a vaginal birth (VB). We aimed to evaluate whether perineal stretch was associated with postpartum pelvic floor dysfunction.
Materials and Methods
This was a prospective cohort study of primiparous women who had a VB. Perineal body (PB) length was measured antepartum, during labor, and 6 months postpartum. We determined the maximum PB (PB Max) measurements during the second stage of labor and PB change (ΔPB) between time points. Women completed functional questionnaires and had a POP-Q exam 6 months postpartum. We analyzed the relationship of PB measurements to perineal lacerations and postpartum outcomes including urinary, anal, or fecal incontinence, sexual activity and function, and POP-Q measurements.
Results
448 women with VB had a mean age of 24 ± 5.0 years and rare (5%) third or fourth degree lacerations. During the second stage of labor, 270/448 (60%) had perineal measurements. Mean antepartum PB length was 3.7 ± 0.8 cm with a maximum mean PB length (PB Max) during the second stage of 6.1 ± 1.5 cm, an increase of 65%. The change in PB length (ΔPB) from antepartum to 6 months postpartum was a net decrease (−0.39 ± 1.02 cm). PB at any time point and PB Max were not associated with perineal lacerations or outcomes postpartum (all p>0.05).
Discussion
PB stretch during labor is unrelated to perineal laceration or postpartum incontinence, sexual activity, or sexual function.
Keywords: incontinence, labor, perineal body, postpartum, sexual function, prolapse
Introduction
Vaginal birth has been associated with pelvic floor dysfunction, with past studies indicating that maternal expulsive efforts and perineal trauma are risk factors for postpartum pelvic floor disorders.1–4 Perineal and introital stretch in the second stage is thought to contribute to pelvic floor muscle damage. However, with the exception of overt levator damage and third and fourth degree perineal lacerations, the relationship of perineal injury to postpartum pelvic floor dysfunction has not been consistently demonstrated.5–8 Data are lacking to predict how stretching of the perineal structures in the second change of labor correlates to perineal laceration at delivery as well as postpartum functional outcomes such as urinary (UI), anal (AI) or fecal incontinence (FI), sexual dysfunction, and pelvic organ prolapse.
The primary aim of this study was to describe changes in the perineal body during the second stage of labor and evaluate whether perineal body stretch during labor is predictive of obstetrical lacerations or whether changes in perineal body length were predictive of 6 month postpartum pelvic floor outcomes. We also sought to determine if the maximum stretch of the introital opening and the anus during the second stage of labor were predictive of perineal lacerations and postpartum pelvic floor functional outcomes. We hypothesized that women with greater perineal body stretch during the second stage of labor, or greater change from antepartum to intrapartum perineal body length, would be at increased risk for UI, AI, FI, sexual dysfunction, and pelvic organ prolapse.
Materials and Methods
This is a planned secondary analysis of a large, prospective cohort evaluating postpartum pelvic floor changes following delivery of a first child, the full methods of which are described in prior publications.3,9 Nulliparous, healthy women who presented for prenatal care were recruited prenatally by the University of New Mexico midwives from December 2006 to January 2011 and were eligible for this secondary analysis if they had a vaginal birth (VB) at the study institution. This research was approved by the University of New Mexico Health Sciences Center Internal Review Board (IRB) prior to study initiation, and informed written consent was given by all participants.
The perineal body (PB) was measured (in centimeters) during Valsalva maneuver from the posterior fourchette to the center of the anal opening and recorded at several time points: during antepartum care, at the onset of labor, at the onset of pushing at the beginning of the second stage of labor, every 10 minutes after this during the second phase of labor until the delivery of the fetal head, and at 6 weeks and 6 months postpartum. Measurements were accomplished with a cotton-tipped swab marked to the centimeter and calibrated against a ruler, and all measurements were done in keeping with the standard methodology of the Pelvic Organ Prolapse Quantification (POP-Q) system outlined by Bump et al.10 The maximum perineal body measurement during the second phase of labor (PB Max) was calculated for each woman. The changes in PB between two time points (ΔPB) were calculated for each individual woman. During the antepartum visit and at 6 months postpartum, a full set of POP-Q measurements were also performed. All measurements were performed by specialists in the field of Female Pelvic Medicine and Reconstructive Surgery or by certified nurse-midwives who underwent POP-Q training with live models prior to study data collection. Standard deviation in this study of perineal body measurements has previously been reported to be 0.8 cm.3
While the fetal head was visible at the vaginal introitus, anterior-posterior and transverse measurements were taken (in centimeters) of the portion of the fetal head visible, and these measurements were repeated every 10 minutes until the fetal head was birthed. APIO Max was the maximum anterior-posterior measurement of the fetal head visualized during the second stage, and TIO Max was the maximum transverse measurement of the fetal head visualized. Using the formulaic calculation of the area of an ellipse, the surface area of fetal head exposed, or the area of the introital opening (AIO) was calculated for each patient. AIO Max was the maximum area of introital opening, as calculated using APIO Max and TIO Max. The anal opening (AnO) was also measured in centimeters every 10 minutes during the second stage of labor, and AnO Max was the maximum measurement during the second stage for each patient.
Labor and maternal characteristics were gathered at delivery as previously described; women with a second degree perineal laceration or greater were evaluated by a second examiner to ensure that lacerations were graded appropriately, with >90% agreement between observers.3,9,11 In our institution episiotomies are rare and operative delivery is uncommon and providers do not routinely perform perineal massage with delivery. Previous studies have reported our episiotomy rate at 2% and operative delivery rate at 6%.3,11 All third and fourth degree lacerations were repaired at the time of delivery, and second degree lacerations were repaired if they distorted anatomy, were actively bleeding, or there was a provider or patient preference for laceration repair.
Both antepartum (prior to the onset of labor) and six months postpartum, patients were given validated questionnaires to assess symptoms of urinary and fecal/anal incontinence: the Incontinence Severity Index (ISI)12 and the Wexner Fecal Incontinence Scale,13 respectively. The presence of urinary incontinence was considered to be any ISI score >0, and the presence of anal incontinence (AI) was considered to be a Wexner score >0, or any loss of flatal or fecal matter. Fecal incontinence (FI) was defined as both a Wexner score >0 and positive responses regarding involuntary loss of fecal matter. Sexual function was measured using Female Sexual Function Index (FSFI), a multi-domain questionnaire where higher scores confer better sexual function.14 A pelvic organ prolapse quantification (POP-Q) examination was performed both antepartum and six months postpartum.
PB, ΔPB, AnO Max, APIO Max, TIO Max values are reported as means ± standard deviations in centimeters, with AIO Max in square centimeters. PB for all measured time points, ΔPB values between all measured time points, AIO Max, APIO Max, TIO Max, and AnO Max are referred to henceforth as study measurements. Logistic regression was used to calculate the relationship between study measurements and the occurrence of the following binomial variables: second, third or fourth degree laceration, UI, AI, or FI at 6 months postpartum, and sexual activity at 6 months postpartum. Analysis of the relation of study measurements to perineal trauma was also repeated correcting for birth weight of the fetus (in grams).
Linear regression was utilized to correlate the relationship of the study measurements to continuous variables. The analysis was repeated correcting for the antepartum values of the outcome. For example, analysis of the relationship of study measurements to point C on the POP-Q exam 6 months postpartum was repeated correcting for antepartum point C in that patient. Where significant relationships were found with this correction, analysis was again repeated removing the patients with operative delivery and/or episiotomy and correcting for maternal age, BMI, length of the second stage of labor (in minutes), and infant birth weight (in grams).
The parent study was powered for differences in pelvic floor dysfunction amongst different birth modalities (Cesarean versus vaginal delivery), rather than for this analysis.3 However, with a study population of 270, we determined that we could detect an odds ratio of ≥2.0 of dichotomous outcomes with 10% or greater baseline prevalence (presence or absence of UI, AI, FI, or perineal laceration) for every 1 cm increase in the continuous independent variables (e.g. PB Max) with 80% power and α = 0.05. For continuous outcomes such as postpartum POP-Q measurements, our population of 199 women could detect a ≥0.2 cm descent in the POP-Q point with a 1 cm increase in PB Max, assuming the standard deviation of PB Max and point Aa in our population was ≤1.5 cm (80% power, α = 0.05).
Results
As reported previously,3,9,11 448 women in the parent study cohort had a vaginal birth (VB), with a mean age of 24 ± 5.0 years. Of the VB cohort, 270/448 (60%) of women had both antepartum perineal body measurements (PB-AP) and at least one perineal body measurement performed during the second stage of labor, and therefore a maximum PB (PB Max) and change in PB during the second stage could be recorded. These 270 women are the population of interest in this study (Figure 1). Of these 270 women, 83/270 (31%) of the population only had one repeat measurement of the perineum taken after the measurement at the onset of the second stage, meaning that the fetus delivered over 10 minutes after the second stage onset but not >=20 min after the onset of the second stage. There were 228/270 (84%) women who had PB measurements at 6 weeks postpartum and 199/270 (74% of the study population) had measurements 6 months postpartum. Patient characteristics of these 270 women are shown in Table 1, and the 199 women who presented at 6 months postpartum for repeat PB and POP-Q measurements were older (24.4 vs 23.0 years, p=0.02) and had less use of oxytocin during labor (40% vs 58%, p=0.01) than the 71 women who did not. Otherwise, women who had postpartum follow up had similar characteristics. Of note, none of these 270 women had operative vaginal delivery and 5/270 (2%) had episiotomy performed.
Figure 1.
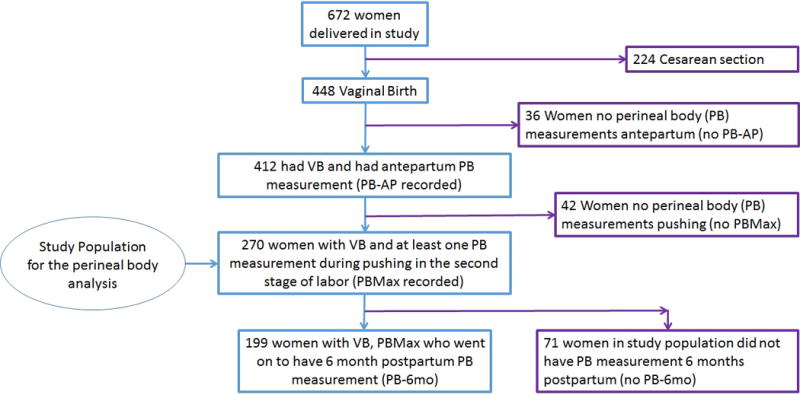
Populations of interest in the study cohort
Table 1.
Patient characteristics for the 270 women who had antepartum and intrapartum perineal body measurements before their vaginal birth, perineal body measurements over time, and 6 month postpartum pelvic floor outcomes and POP-Q measurements.
| Patient Characteristic | Mean Length ± SD, Medial (IQ range), or Frequency (%) |
|---|---|
|
| |
| Age (years) | 24.5 ± 5.0 |
|
| |
| Years of Education | 13.8 ± 2.6 |
|
| |
| BMI (kg/m2) | 24.3 ± 5.0 |
|
| |
| Race | |
| Non-Hispanic White | 127 (47) |
| Hispanic | 113 (42) |
| African American | 4 (1) |
| Asian | 10 (4) |
| Native American | 16 (6) |
|
| |
| Tobacco Use | 17 (6) |
|
| |
| Fetal birth weight (grams) | 3246 ± 415 |
|
| |
| Weight gain during pregnancy (pounds) | 35.9 ± 13.2 |
|
| |
| Oxytocin use in labor | 119 (45) |
|
| |
| Epidural anesthesia | 165 (61) |
|
| |
| Length of second stage of labor (min) | 72.3 ± 56.6 |
|
| |
| Episiotomy | 5 (2) |
|
| |
| Perineal laceration at birth | |
| Second degree or greater | 83 (31) |
| Third or fourth degree | 16 (6) |
|
| |
| Incontinence 6 months postpartum | |
| Urinary incontinence (UI) | 111 (54) |
| Fecal incontinence (FI) | 19 (9) |
| Anal incontinence (AI) | 103 (51) |
|
| |
| Sexually active 6 months postpartum | 173 (87) |
|
| |
| Mean PB measurements | |
| Antepartum (n=270) | 3.69 ± 0.84 |
| Onset of labor (n=262) | 4.20 ± 0.92 |
| PB Max (n=270) | 6.05 ± 1.48 |
| 6 weeks postpartum (n=228) | 3.87 ± 0.79 |
| 6 months postpartum (n=199) | 3.26 ± 0.76 |
|
| |
| Perineal body change (Δ PB) over time (cm) | |
| PB Onset Second Stage to PB Max (n=264) | 1.88 ± 1.50 |
| Antepartum to PB Max (n=270) | 2.41 ± 1.62 |
| Antepartum to 6 weeks postpartum (n=228) | 0.24 ± 0.89 |
| Antepartum to 6 months postpartum (n=199) | − 0.39 ± 1.02 |
|
| |
| Maximum genital opening in labor (n=269) | |
| TIO Max | 4.70 ± 1.90 |
| APIO Max | 7.50 ± 1.92 |
| AIO Max | 119 ± 68.9 |
|
| |
| AnO Max (n=248) | 1.99 ± 0.93 |
|
| |
| Mean POP-Q Measurements 6 mo postpartum (n=199) | |
| GH | 3.2 ± 0.7 |
| Aa | −1.9 ± 0.8 |
| Ba | −1.9 ± 0.8 |
| Ap | −2.6 ± 0.5 |
| Bp | −2.6 ± 0.5 |
| C | −5.2 ± 1.4 |
| D | −6.6 ± 1.7 |
| TVL | 7.3 ± 1.4 |
PB = perineal body length from the posterior vaginal fourchette to the anal opening (cm)
PB Max = Greatest PB length during the second stage of labor (cm)
ΔPB = Change in PB between two time points indicated (cm)
AnO Max = Maximum measurement of opening of the anus obtained during the second stage of labor (cm)
TIO Max = Maximum transverse measurement of the fetal head visualized during the seconds stage of labor (cm)
APIO Max = Maximum anterior-posterior measurement of the fetal head visualized during the seconds stage of labor (cm)
AIO Max = Maximum area of introital opening during the second stage of labor, calculated using APIO Max and TIO Max in the formula for area of an ellipse (cm2)
AnO Max = Maximum measurement of opening of the anus obtained during the second stage of labor (cm)
The means and standard deviations of PB lengths, ΔPB between various time points, and introital opening during the second stage of labor are shown in Table 1. The mean PB length antepartum was 3.7 ± 0.8 cm, which increased to 4.2 ± 1.0 cm at the onset of labor. The mean maximum perineal body length during the second stage of labor (PB Max) was 6.1 ± 1.5 cm. The greatest change in PB (ΔPB) was 2.4 ± 1.6 cm between PB Antepartum and PB Max, nearly a 65% increase from PB Antepartum. The mean ΔPB from PB Antepartum to PB 6 months Postpartum was small and demonstrated overall shortening in PB (−0.39 ± 1.02 cm). The mean maximum transverse diameter of the introitus during the second stage (TIO Max) was 4.72 ± 1.9 cm, and the mean maximum anterior-posterior diameter (APIO Max) was 7.5 ± 1.9 cm, yielding a mean AIO Max of 119 ± 69 cm2. The maximum anal opening during the second stage was 2.0 ± 0.9 cm in diameter.
The prevalence of perineal lacerations (≥3rd degree or ≥2nd degree) during labor and 6 month postpartum UI, AI, FI, and sexual activity in the study population are shown in Table 1. Third and fourth degree lacerations were rare and were not significantly associated with any study measurements (all p>0.05). Second, third, and fourth degree lacerations were analyzed in combination as well, and also were not associated with any study measurements, with or without correction for infant birth weight (all p>0.05). With or without correction for antepartum UI, no study measurements were correlated with the occurrence of UI at 6 months postpartum. Furthermore, no study measurements were related to postpartum FI or AI, with or without correction for antepartum symptoms and/or perineal lacerations (all 95% CIs crossing 1.0).
Sexual activity in this population increased from antepartum to postpartum as reported previously, with 76% of women sexually active antepartum (in the third trimester) and 88% sexually active postpartum.3 Adjusting for antepartum sexual activity and perineal trauma, no study measurements were related to sexual activity at 6 months postpartum (all 95% CIs crossing 1.0). After correction for antepartum FSFI scores, measurements of introital opening (APIO Max and AIO Max) were correlated with slightly improved satisfaction scores on the 6 month postpartum FSFI.Table 2 demonstrates the correlation of study measurements to FSFI sexual function scores within individual domains of sexual function and overall, corrected in all cases for antepartum FSFI scores..
Table 2.
Study measurements as correlated to sexual function postpartum by the FSFI score (domains and total score), corrected for antepartum FSFI scores.
| Measurement and Time Point | Arousal Domain p-value |
Desire Domain p-value |
Lubrication Domain p-value |
Orgasm Domain p-value |
Pain Domain p-value |
Satisfaction Domain p-value (slope ± SE where p<0.05) |
FSFI Total Score p-value |
|---|---|---|---|---|---|---|---|
| PB Max (n=127) |
0.44 | 0.33 | 0.69 | 0.42 | 0.21 | 0.46 | 0.21 |
| ΔPB, PB Antepartum to PB Max (n=127) |
0.33 | 0.41 | 0.40 | 0.46 | 0.27 | 0.44 | 0.14 |
| TIO Max (n=127) |
0.27 | 0.85 | 0.65 | 0.49 | 0.06 | <0.01 (+0.15 ± 0.05) |
0.09 |
| APIO Max (n=127) |
0.43 | 0.46 | 0.91 | 0.23 | 0.49 | 0.11 | 0.12 |
| AIO Max (n=125) |
0.26 | 0.95 | 0.56 | 0.37 | 0.22 | 0.01 (+<0.01 ± <0.01) |
0.19 |
| AnO Max (n=118) |
0.71 | 0.27 | 0.87 | 0.90 | 0.74 | 0.37 | 0.43 |
PB = perineal body length from the posterior vaginal fourchette to the anal opening
PB Max = Greatest PB length during the second stage of labor
ΔPB = Change in PB between two time points indicated
TIO Max = Maximum transverse measurement of the fetal head visualized during the seconds stage of labor
APIO Max = Maximum anterior-posterior measurement of the fetal head visualized during the seconds stage of labor
AIO Max = Maximum area of introital opening during the second stage of labor, calculated using APIO Max and TIO Max in the formula for area of an ellipse
AnO Max = Maximum measurement of opening of the anus obtained during the second stage of labor
With statistical correction for confounding variables, postpartum POP-Q values were not related to stretching in the introitus during labor (APIO Max, TIO Max, AIO Max, or AnO Max). Once corrected for confounders, study measurements were also not correlated to POP-Q points GH, Aa, Ba, Ap, D, or TVL at 6 months postpartum.Table 3 demonstrates the correlation of all study measurements to postpartum POP-Q measurements, corrected for antepartum POP-Q measurements and relevant confounders in all cases. Point Bp was positively correlated to PB Max and point C was positively correlated to ΔPB from PB Antepartum to PB Max, but the degree of the correlation was extremely small for both of these relationships.
Table 3.
Correlation of perineal body (PB) measurements or PB changes over time (ΔPB) with POP-Q measurements 6 months postpartum, after correction for the confounding variables of the corresponding antepartum POP-Q measurement, maternal age and BMI at delivery, birth weight of infant, and length of the second stage of labor.
| Measurement and Time Point | GH p-value |
Aa p-value |
Ap p-value |
Ba p-value |
Bp p-value (slope ± SE where p<0.05) |
C p-value (slope ± SE where p<0.05) |
D p-value |
|---|---|---|---|---|---|---|---|
| PB Max (n=194) |
0.12 | 0.06 | 0.06 | 0.06 | 0.02 (+0.06 ± 0.03) |
0.11 | 0.29 |
| ΔPB, PB Antepartum to PB Max (n=193) |
0.94 | 0.08 | 0.21 | 0.09 | 0.09 | 0.02 (+0.17 ± 0.07) |
0.08 |
| TIO Max (n=193) |
0.38 | 0.93 | 0.24 | 0.93 | 0.36 | 0.96 | 0.44 |
| APIO Max (n=193) |
0.88 | 0.46 | 0.86 | 0.50 | 0.49 | 0.97 | 0.62 |
| AIO Max (n=192) |
0.80 | 0.49 | 0.43 | 0.50 | 0.49 | 0.90 | 0.35 |
| AnO Max (n=177) |
0.34 | 0.60 | 0.65 | 0.56 | 0.63 | 0.80 | 0.84 |
PB = perineal body length from the posterior vaginal fourchette to the anal opening
PB Max = Greatest PB length during the second stage of labor
ΔPB = Change in PB between two time points indicated
TIO Max = Maximum transverse measurement of the fetal head visualized during the seconds stage of labor
APIO Max = Maximum anterior-posterior measurement of the fetal head visualized during the seconds stage of labor
AIO Max = Maximum area of introital opening during the second stage of labor, calculated using APIO Max and TIO Max in the formula for area of an ellipse
AnO Max = Maximum measurement of opening of the anus obtained during the second stage of labor
Discussion
In this secondary analysis of a large cohort of healthy, low risk women having their first delivery, we found that the perineal body stretches significantly, increasing in size by 65% during delivery. Nonetheless, individual perineal body measurements and maximum perineal stretch did not correlate with functional outcomes such as UI, FI, AI, sexual activity, or sexual function at 6 months postpartum, nor did they correlate to perineal laceration intrapartum. Maximum introital opening measurements during the second stage were also not correlated to perineal lacerations or postpartum functional changes, with the exception of a weak relationship to improved sexual function scores. Providers can offer women reassurance that stretching during the second stage of labor is normal and does not seem to negatively affect pelvic floor function or anatomy at 6 months postpartum. Stretching during labor also does not increase the risk of perineal trauma at delivery, even in this setting of sparing use of episiotomy.
Risk factors for perineal lacerations have been well studied and include nulliparity, older maternal age, Asian race, increasing gestational age and birth weight, instrumented delivery, prolonged second stage of labor, occiput posterior fetal position, and midline episiotomy.15–17 While some of these indicate that a greater degree of perineal stretch may correspond to injury, it is unclear if stretch during labor always is pathological. Short perineal body length (PBL) has been implicated as a predictor of severe perineal injury, but these data are conflicting. A recent retrospective study reported that women with a PBL ≤ 2.5cm had a 40% higher chance of sustaining 3rd or 4th degree lacerations.18 Additionally, a prospective cohort found an increased risk of anal sphincter injury if the PBL was < 3 cm.19 However, another study found that PBL and pelvic floor muscle strength were not predictive of sphincter trauma,20 and prospective data from a nulliparous cohort of women at our institution demonstrated no correlation between antepartum PBL and perineal tearing.9
We found that, despite significant stretching of the perineal body, introitus, and anal opening in labor, maximum measurements during the second stage were not associated with anal incontinence postpartum. It has been indicated that a short PBL is related to sonographic anal sphincter defects,19 which are correlated to postpartum FI in some studies1–2,20 but ultrasound findings are inconsistent predictors of postpartum FI pathology.21,22 This indicates that anatomic changes, or even damage, do not always result in poor function. Our large, prospective study confirms that even a large degree of stretch during labor has no association with AI or FI symptoms.
Our data indicate that sexual activity and sexual function were not associated with stretching of the perineal body, including the maximum stretch of the perineum (PB Max), but greater introital stretch was positively correlated to improved satisfaction domain scores on the FSFI. This may be due to the fact that women with better tissue compliance at the introitus in labor may have tissue properties that improve sexual satisfaction, such as better tissue stretch and comfort during coitus. However, if this were true, one would expect the pain domain on the FSFI to be related to introital stretch, which this data did not detect. While some data indicate a relationship between anatomic changes during VB and decreased sexual function postpartum,3,23,24 recent studies have found that similar postpartum sexual function between vaginal birth and Cesarean birth25 or between women that did and did not have levator avulsion.26 These mixed findings indicate that tissue changes in labor may be related to sexual function, but any negative effects of tissue stretch may be mitigated by the multitude of other factors that influence sexual function. Overall, the findings of this study can provide women reassurance that maximum stretch in labor is unlikely to negatively impact their sexual function.
Past investigations with imaging technology have indicated that damage to the levator plate during labor predisposes women to pelvic organ prolapse, and tissue stretching in labor is a known risk factor for levator muscle damage.27,28 We found that stretching in the perineum is only weakly correlated to some of the postpartum POP-Q measurements (Bp and C), unlikely to be clinically meaningful at 6 months postpartum. Prior studies are conflicting regarding whether or not PB length antepartum can predict intrapartum perineal trauma,9,18,19 a known factor for prolapse, but a single measurement does not reflect the complexity of anatomic changes in pregnancy and labor.
Limitations of this study include the fact that no formal inter-rater or intra-rater analysis could be made to validate the reproducibility of these measurements, particularly in the second stage of labor, where the measurements change so rapidly that multiple measurements are not feasible. Furthermore, to make this study more clinically realistic, we did not utilize a pressure catheter in the vagina or rectum to standardize the level of Valsalva during the POP-Q measurements. Limitations of this study also include the shorter duration of the study, which only allows us to detect pelvic floor disease that arises in the first six months postpartum. However, there are data to support that a substantial portion of postpartum stress incontinence resolves by 6 months after delivery,29 and recent imaging data confirm that tissue recovery has mostly occurred by 8 months postpartum,30 leading us to believe that our time frame was relevant for the postpartum period. Second, given the low-risk population in this study (midwifery patients delivering by VB), these results may not be generalizable to populations at increased risk of instrumented deliveries or episiotomy. Third, while our analysis corrected for several suspected confounders, we cannot correct for all possible unmeasured or unknown confounders that could affect the results. Fourth, our population was only large enough to detect significant changes in risk with a 1 cm change in PB Max, so more subtle relationships between perineal changes and the outcomes may have been missed.
Lastly, it is worth noting that the maximum stretch of the perineum during the second stage may not have been captured by the last measurement taken before the birth of the neonate in this study. As can be seen by the TIO Max and APIO Max measurements, which are not the width and breadth of a typical neonatal head, measurements were taken as close to the birth of the neonate as feasibility and protocol would allow, but not at the exact moment of delivery. However, based on the cardinal movements of labor, the maximum stretch of the PB likely existed before the fetal head passed under the pubic bone and underwent extension and external rotation, which would allow the perineal to shorten again. Moreover, even if the greatest extent of stretch was not captured by this study, our data indicate that the perineal body would have had to stretch to over double the length in this data (to over 12 cm long, which is not anatomically feasible) in order to become mathematically related to any of the pelvic floor outcomes study. This upholds our primary conclusion that even extreme elongation of the perineal body in the second stage of labor does not negatively impact pelvic floor outcomes in the postpartum months.
The strengths of this study include the large cohort size and the inclusion of low-risk women typical of many obstetrics and midwifery practices. Moreover, this trial utilized a standardized method of measurement of the perineal body supported by the literature, and all measurements were performed by personnel given formal training on methods of measurements. We also performed anatomical measurements at many time points in order to provide a full description of anatomy through pregnancy, labor, and postpartum. This better illuminates what providers and patients can expect during this time period, and can reassure patients that certain anatomic changes in labor are normal and do not affect postpartum function or intrapartum lacerations. The variety of measurements taken allowed evaluate different aspects of posterior compartment anatomy (introital, perineal, anal), and describe in detail any relationships to functional outcomes. The extent of this database also allowed us to correct for maternal characteristics and labor factors suspected to be confounders.
Conclusion
Perineal anatomy undergoes a large degree of spontaneous stretching during the course of labor, and an overall resolution of this stretch can be expected postpartum. While spontaneous stretching is extensive in the second stage, this stretch did not correlate with perineal lacerations, UI, FI, AI, or sexual activity postpartum. These data allow providers to better counsel women about normal changes in pregnancy and reassure women that perineal stretch during labor does not appear to have negative impact on postpartum pelvic floor function.
Brief Summary.
In primiparous women having a vaginal birth, perineal body stretch during labor is not related to perineal lacerations or pelvic dysfunction 6 months postpartum.
Acknowledgments
Funding
The parent study, Pelvic floor changes before and after birth (PI Rebecca G. Rogers, M.D.), is funded by NICHHD 1R01HD049819-01A2 and by the National Center for Research Resources and the National Center for Advancing Translational Sciences of the NIH (Grant Number 8UL1TR000041).
Footnotes
Author Conflict of Interest Statement:
RG Rogers: DSMB chair for American Medical Systems, royalties from UptoDate and McGraw Hill publishing
GD Dunivan: Research support from Pelvalon, American Urogynecologic Society research committee
KV Meriwether, C Qualls, L Migliaccio, JK Alldredge, LM Leeman: Nothing to disclose
Author Participation:
KV Meriwether: Protocol/project development, data analysis, manuscript writing/editing
RG Rogers: Protocol/project development, data collection/management, manuscript editing
GD Dunivan: Protocol/project development, data analysis, manuscript writing/editing
JK Alldredge: Data analysis, manuscript writing/editing
C Qualls: Data analysis, manuscript editing
L Migliaccio: Protocol/project development, data collection/management, manuscript editing
LM Leeman: Protocol/project development, data collection/management, manuscript editing
References
- 1.Bols EM, Hendriks EJ, Berghmans BC. A systematic review of etiological factors for postpartum fecal incontinence. Acta Obstet Gynecol Scand. 2010;89:302–314. doi: 10.3109/00016340903576004. [DOI] [PubMed] [Google Scholar]
- 2.Fenner DE, Genberg B, Brahma P, Marek L, DeLancey JO. Fecal and urinary incontinence after vaginal delivery with anal sphincter disruption in an obstetrics unit in the United States. Am J Obstet Gynecol. 2003 Dec;189(6):1543–9. doi: 10.1016/j.ajog.2003.09.030. discussion 1549–50. [DOI] [PubMed] [Google Scholar]
- 3.Rogers RG, Leeman LM, Borders N, Qualls C, Fullilove AM, Teaf D, Hall RJ, Bedrick E, Albers LL. Contribution of the second stage of labour to pelvic floor dysfunction: a prospective cohort comparison of nulliparous women. BJOG. 2014 Aug;121(9):1145–53. doi: 10.1111/1471-0528.12571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hall W, McCracken K, Osterweil P, Guise JM. Frequency and predictors for postpartum fecal incontinence. Am J Obstet Gynecol. 2003 May;188(5):1205–7. doi: 10.1067/mob.2003.333. [DOI] [PubMed] [Google Scholar]
- 5.Leeman LM, Rogers RG, Greulich B, Albers LL. Do unsutured second-degree perineal lacerations affect postpartum functional outcomes? J Am Board Fam Med. 2007 Sep-Oct;20(5):451–7. doi: 10.3122/jabfm.2007.05.060222. [DOI] [PubMed] [Google Scholar]
- 6.Rogers RG, Borders N, Leeman LM, Albers LL. Does spontaneous genital tract trauma impact postpartum sexual function? J Midwifery Womens Health. 2009 Mar-Apr;54(2):98–103. doi: 10.1016/j.jmwh.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheer I, Andrews V, Thakar R, Sultan AH. Urinary incontinence after obstetric anal sphincter injuries (OASIS)–is there a relationship? Int Urogynecol J Pelvic Floor Dysfunct. 2008 Feb;19(2):179–83. doi: 10.1007/s00192-007-0431-8. [DOI] [PubMed] [Google Scholar]
- 8.Handa VL, Blomquist JL, McDermott KC. Pelvic Floor Disorders after Vaginal Birth Effect of Episiotomy, Perineal Laceration, and Operative Birth. Obstet Gynecol. 2012;119:233–239. doi: 10.1097/AOG.0b013e318240df4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Komorowski LK, Leeman LM, Fullilove AM, Bedrick EJ, Migliaccio LD, Rogers RG. Does a large infant head or a short perineal body increase the risk of obstetrical perineal trauma? See comment in PubMed Commons below Birth. 2014 Jun;41(2):147–52. doi: 10.1111/birt.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bump RC, Mattiasson A, Bø K, Brubaker LP, DeLancey JO, Klarskov P, Shull BL, Smith AR. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996 Jul;175(1):10–7. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 11.Meriwether KV, Hall RJ, Leeman LM, Migliaccio L, Qualls C, Rogers RG. Postpartum translabial 2D and 3D ultrasound measurements of the anal sphincter complex in primiparous women delivering by vaginal birth versus Cesarean delivery. Int Urogynecol J. 2014 Mar;25(3):329–36. doi: 10.1007/s00192-013-2215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanley J, Capewell A, Hagen S. Validity study of the severity index, a simple measure of urinary incontinence in women. Br Med J. 2001;322:1096–7. doi: 10.1136/bmj.322.7294.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jorge J, Wexner S. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36:77–97. doi: 10.1007/BF02050307. [DOI] [PubMed] [Google Scholar]
- 14.Rosen R, Brown C, Heiman J, Leiblum S, MEston C, Shabsigh R, et al. The female sexual function index (FSFI): A multidimensional self-report instrument for the assessment of female sexual function. J Sex and Marital Ther. 2000;26:191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 15.Schmitz T, Alberti C, Andriss B, Moutafoff C, Oury JF, Sibony O. Identification of women at high risk for severe perineal lacerations. Eur J Obstet Gynecol Reprod Biol. 2014 Aug 29;182C:11–15. doi: 10.1016/j.ejogrb.2014.08.031. [DOI] [PubMed] [Google Scholar]
- 16.Angioli R, Gómez-Marín O, Cantuaria G, O’Sullivan MJ. Severe perineal lacerations during vaginal delivery: the University of Miami experience. Am J Obstet Gynecol. 2000 May;182(5):1083–5. doi: 10.1067/mob.2000.105403. [DOI] [PubMed] [Google Scholar]
- 17.Groutz A, Hasson J, Wengier A, Gold R, Skornick-Rapaport A, Lessing JB, Gordon D. Third- and fourth-degree perineal tears: prevalence and risk factors in the third millennium. Am J Obstet Gynecol. 2011 Apr;204(4):347.e1–4. doi: 10.1016/j.ajog.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Deering, et al. Perineal body length and lacerations at delivery. J Reprod Med. 2004 Apr;49(4):306–10. [PubMed] [Google Scholar]
- 19.Geller EJ, Robinson BL, Matthews CA, Celauro KP, Dunivan GC, Crane AK, Ivins AR, Woodham PC, Fielding JR. Perineal body length as a risk factor for ultrasound-diagnosed anal sphincter tear at first delivery. Int Urogynecol J. 2014 May;25(5):631–6. doi: 10.1007/s00192-013-2273-x. [DOI] [PubMed] [Google Scholar]
- 20.Roos AM, Abdool Z, Thakar R, Sultan AH. Predicting anal sphincter defects: the value of clinical examination and manometry. Int Urogynecol J. 2012 Jun;23(6):755–63. doi: 10.1007/s00192-011-1609-7. [DOI] [PubMed] [Google Scholar]
- 21.Oude Lohuis EJ, Everhardt E. Outcome of obstetric anal sphincter injuries in terms of persisting endoanal ultrasonographic defects and defecatory symptoms. Int J Gynaecol Obstet. 2014 Jul;126(1):70–3. doi: 10.1016/j.ijgo.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 22.Reid AJ, Beggs AD, Sultan AH, Roos AM, Thakar R. Outcome of repair of obstetric anal sphincter injuries after three years. Int J Gynaecol Obstet. 2014 Oct;127(1):47–50. doi: 10.1016/j.ijgo.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laganà AS, Burgio MA, Ciancimino L, Sicilia A, Pizzo A, Magno C, Butticè S, Triolo O. Evaluation of recovery and quality of sexual activity in women during postpartum in relation to the different mode of delivery: a retrospective analysis. Minerva Ginecol. 2015 Aug;67(4):315–20. [PubMed] [Google Scholar]
- 24.Safarinejad MR, Kolahi AA, Hosseini L. The effect of the mode of delivery on the quality of life, sexual function, and sexual satisfaction in primiparous women and their husbands. J Sex Med. 2009 Jun;6(6):1645–67. doi: 10.1111/j.1743-6109.2009.01232.x. [DOI] [PubMed] [Google Scholar]
- 25.Dabiri F, Yabandeh AP, Shahi A, Kamjoo A, Teshnizi SH. The effect of mode of delivery on postpartum sexual functioning in primiparous women. Oman Med J. 2014 Jul;29(4):276–9. doi: 10.5001/omj.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laterza RM, Schrutka L, Umek W, Albrich S, Koelbl H. Pelvic floor dysfunction after levator trauma 1-year postpartum: a prospective case-control study. Int Urogynecol J. 2015 Jan;26(1):41–7. doi: 10.1007/s00192-014-2456-0. [DOI] [PubMed] [Google Scholar]
- 27.Heilbrun ME, Nygaard IE, Lockhart ME, Richter HE, Brown MB, Kenton KS, Rahn DD, Thomas JV, Weidner AC, Nager CW, Delancey JO. Correlation between levator ani muscle injuries on magnetic resonance imaging and fecal incontinence, pelvic organ prolapse, and urinary incontinence in primiparous women. Am J Obstet Gynecol. 2010 May;202(5):488.e1–6. doi: 10.1016/j.ajog.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeLancey JO, Morgan DM, Fenner DE, Kearney R, Guire K, Miller JM, Hussain H, Umek W, Hsu Y, Ashton-Miller JA. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol. 2007 Feb;109(2 Pt 1):295–302. doi: 10.1097/01.AOG.0000250901.57095.ba. [DOI] [PubMed] [Google Scholar]
- 29.Martin-Martin S, Pascual-Fernandez A, Alvarez-Colomo C, Calvo-Gonzalez R, Muñoz-Moreno M, Cortiñas-Gonzalez JR. Urinary incontinence during pregnancy and postpartum. Associated risk factors and influence of pelvic floor exercises. Arch Esp Urol. 2014 May;67(4):323–30. [PubMed] [Google Scholar]
- 30.Miller JM, Low LK, Zielinski R, Smith AR, DeLancey JO, Brandon C. Evaluating maternal recovery from labor and delivery: bone and levator ani injuries. See comment in PubMed Commons below Am J Obstet Gynecol. 2015 Aug;213(2):188.e1–188.e11. doi: 10.1016/j.ajog.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


