Abstract
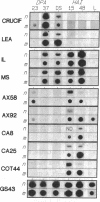
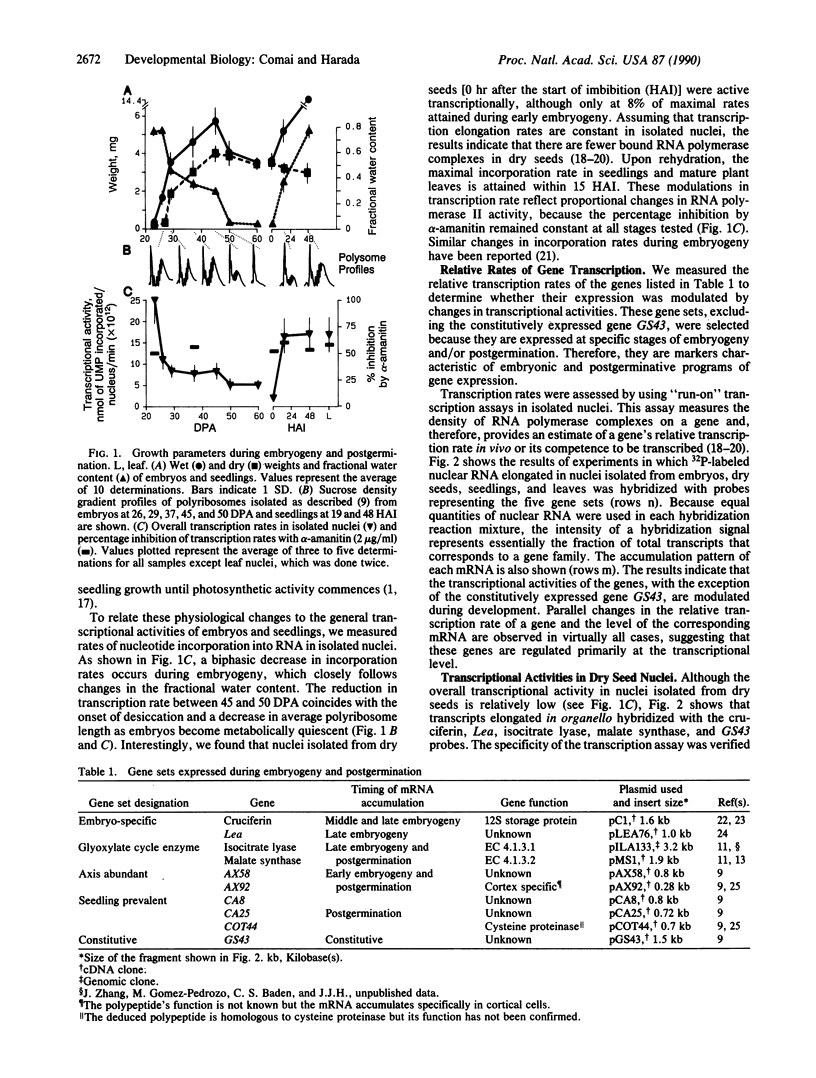
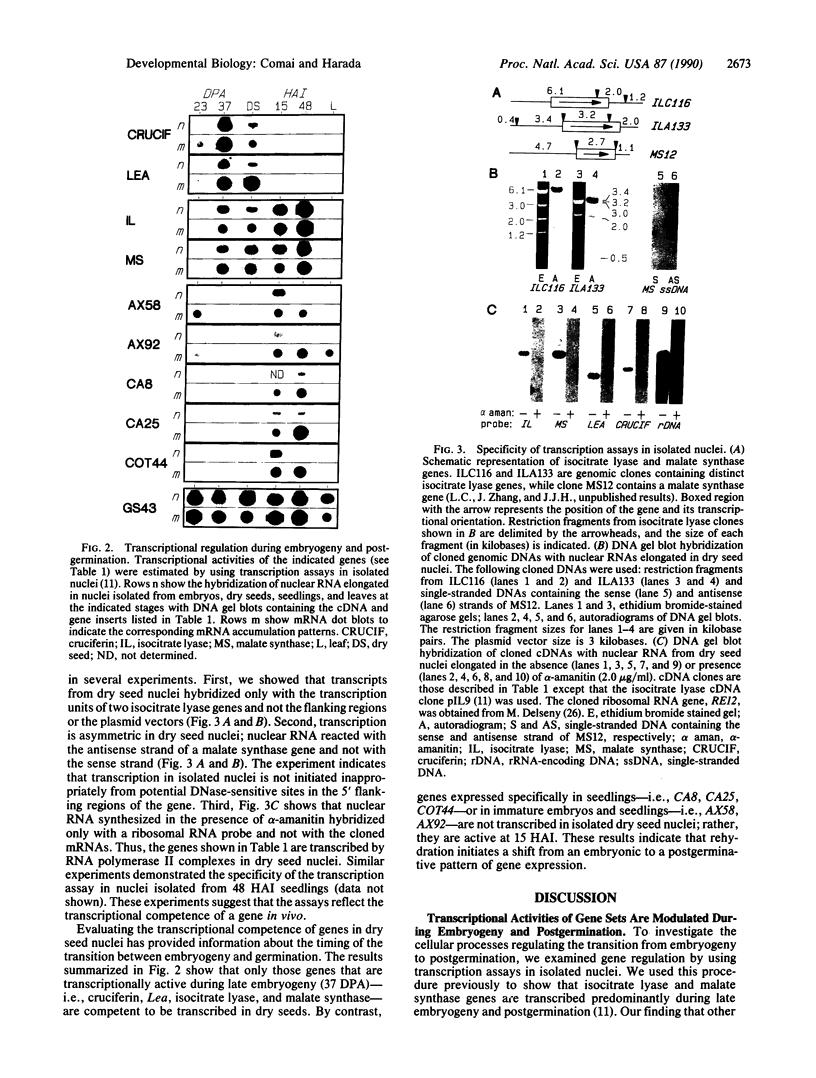
To investigate processes regulating the reinitiation of growth and differentiation during seed germination, we have studied the transcriptional activities of sets of genes that are expressed at specific stages of embryogeny and post-germination in the higher plant Brassica napus L. We show that transcripts from a subset of the genes are elongated in nuclei isolated from dry seeds, indicating that these genes are competent to be transcribed in desiccated and quiescent mature embryos. Analysis of the specific transcripts produced in dry seed nuclei indicates that the changes in gene expression patterns associated with germination are not initiated during late embryogeny. The results suggest that the transition from an embryonic to a postgerminative program of development occurs after seeds are rehydrated.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beach L. R., Spencer D., Randall P. J., Higgins T. J. Transcriptional and post-transcriptional regulation of storage protein gene expression in sulfur-deficient pea seeds. Nucleic Acids Res. 1985 Feb 11;13(3):999–1013. doi: 10.1093/nar/13.3.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk C. F., Jones K. C., James T. W. Assay for nanogram quantities of DNA in cellular homogenates. Anal Biochem. 1979 Jan 15;92(2):497–500. doi: 10.1016/0003-2697(79)90690-0. [DOI] [PubMed] [Google Scholar]
- Chappell J., Chrispeels M. J. Transcriptional and Posttranscriptional Control of Phaseolin and Phytohemagglutinin Gene Expression in Developing Cotyledons of Phaseolus vulgaris. Plant Physiol. 1986 May;81(1):50–54. doi: 10.1104/pp.81.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai L., Baden C. S., Harada J. J. Deduced sequence of a malate synthase polypeptide encoded by a subclass of the gene family. J Biol Chem. 1989 Feb 15;264(5):2778–2782. [PubMed] [Google Scholar]
- Comai L., Dietrich R. A., Maslyar D. J., Baden C. S., Harada J. J. Coordinate expression of transcriptionally regulated isocitrate lyase and malate synthase genes in Brassica napus L. Plant Cell. 1989 Mar;1(3):293–300. doi: 10.1105/tpc.1.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Delisle A. J., Crouch M. L. Seed Storage Protein Transcription and mRNA Levels in Brassica napus during Development and in Response to Exogenous Abscisic Acid. Plant Physiol. 1989 Oct;91(2):617–623. doi: 10.1104/pp.91.2.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich R. A., Maslyar D. J., Heupel R. C., Harada J. J. Spatial patterns of gene expression in Brassica napus seedlings: identification of a cortex-specific gene and localization of mRNAs encoding isocitrate lyase and a polypeptide homologous to proteinases. Plant Cell. 1989 Jan;1(1):73–80. doi: 10.1105/tpc.1.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dure L., 3rd, Greenway S. C., Galau G. A. Developmental biochemistry of cottonseed embryogenesis and germination: changing messenger ribonucleic acid populations as shown by in vitro and in vivo protein synthesis. Biochemistry. 1981 Jul 7;20(14):4162–4168. doi: 10.1021/bi00517a033. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. R., Tenbarge K. M., Shumway J. E., Crouch M. L. Role of ABA in Maturation of Rapeseed Embryos. Plant Physiol. 1985 Jul;78(3):630–636. doi: 10.1104/pp.78.3.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galau G. A., Dure L., 3rd Developmental biochemistry of cottonseed embryogenesis and germination: changing messenger ribonucleic acid populations as shown by reciprocal heterologous complementary deoxyribonucleic acid--messenger ribonucleic acid hybridization. Biochemistry. 1981 Jul 7;20(14):4169–4178. doi: 10.1021/bi00517a034. [DOI] [PubMed] [Google Scholar]
- Goldberg R. B., Barker S. J., Perez-Grau L. Regulation of gene expression during plant embryogenesis. Cell. 1989 Jan 27;56(2):149–160. doi: 10.1016/0092-8674(89)90888-x. [DOI] [PubMed] [Google Scholar]
- Goldberg R. B., Hoschek G., Tam S. H., Ditta G. S., Breidenbach R. W. Abundance, diversity, and regulation of mRNA sequence sets in soybean embryogenesis. Dev Biol. 1981 Apr 30;83(2):201–217. doi: 10.1016/0012-1606(81)90467-x. [DOI] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada J. J., Barker S. J., Goldberg R. B. Soybean beta-conglycinin genes are clustered in several DNA regions and are regulated by transcriptional and posttranscriptional processes. Plant Cell. 1989 Apr;1(4):415–425. doi: 10.1105/tpc.1.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes D. W., Galau G. A. Temporally modular gene expression during cotyledon development. Genes Dev. 1989 Mar;3(3):358–369. doi: 10.1101/gad.3.3.358. [DOI] [PubMed] [Google Scholar]
- Irifune M., Ogino S., Harada T., Matsunaga T., Sakai K. [The desensitization therapy in children with nasal allergy to house dust]. Nihon Jibiinkoka Gakkai Kaiho. 1989 Mar;92(3):395–401. doi: 10.3950/jibiinkoka.92.395. [DOI] [PubMed] [Google Scholar]
- Kodrzycki R., Boston R. S., Larkins B. A. The opaque-2 mutation of maize differentially reduces zein gene transcription. Plant Cell. 1989 Jan;1(1):105–114. doi: 10.1105/tpc.1.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight G. S., Palmiter R. D. Transcriptional regulation of the ovalbumin and conalbumin genes by steroid hormones in chick oviduct. J Biol Chem. 1979 Sep 25;254(18):9050–9058. [PubMed] [Google Scholar]
- Walling L., Drews G. N., Goldberg R. B. Transcriptional and post-transcriptional regulation of soybean seed protein mRNA levels. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2123–2127. doi: 10.1073/pnas.83.7.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]