Abstract
Toxoplasmic encephalitis (TE) is caused by Toxoplasma gondii infection and can be a life-threatening disease in immunocompromised patients. This study evaluated the rate of relapse associated with pyrimethamine-based maintenance therapy (i.e. secondary prophylaxis) in patients with human immunodeficiency virus (HIV) or AIDs treated prior to and after the common use (i.e. 1996) of highly active antiretroviral therapy (HAART) (pre-HAART and post-HAART, respectively). PubMed, Google Scholar, and Cochrane databases were searched to 6 June 2016 using search terms: pyrimethamine, Daraprim, Fansidar, Metakelfin, Fansimef, 5-(4-chlorophenyl)-6-ethyl-2,4-pyrimidinediamine, encephalitis, cerebral, toxoplasmosis, toxoplasmic, and gondii. Single-arm cohort, retrospective, and randomized studies were included. Twenty-six studies with 1,596 patients were included in the analysis; twenty pre-HAART (n = 1,228) studies and six post-HAART (n = 368) were performed. Pooled proportions test for pyrimethamine-based therapy from pre-HAART studies indicated a relapse rate of 19.2% and 18.9% from the fixed-effects and random-effects models, respectively. The relapse rate in the post-HAART studies was 11.1% (fixed and random effects). Continuous therapy was suggestive of lower incidence of relapse compared with intermittent therapy in the pre-HAART era (range, 18.7 to 17.3% vs. 20.9 to 25.6%, respectively). These findings indicate that the likelihood of relapse associated with pyrimethamine-based therepy in patients with HIV and TE decreased after the introduction of HAART to approximately 11%. The findings have important implications as relapse may affect a patient’s disease severity and prognosis, increase utilization of health care resources, and result in additional health care expenditure.
Keywords: Toxoplasmosis, Encephalitis, Toxoplasmis, Pyrimethamine, Human immunodeficiency virus, Meta-analysis, Relapse, Proportions
Introduction
Toxoplasmic encephalitis (TE) results from infection by Toxoplasma gondii (T. gondii), a ubiquitous obligate intracellular parasite with a worldwide prevalence that infects humans and other warm-blooded animals [1]. Approximately, one third of the population worldwide is chronically infected with T. gondii [2], T. gondii seroprevalence varies world wide. In the United States, it is estimated that 22.5% of the population 12 years and older are infected with the parasite [3]. It is estimated that 6.7% of people in Korea, 12.3% in China, 23.9% in Nigeria, 46% in Tanzania, and 47% in the rural areas of France are seropostive for T. gondii [4−8]. In Brazil, up to 50% of elementary school children and 50 to 80% of women of child-bearing age have antibodies to T. gondii [9]. T. gondii infection has two phases: active (acute) which is characterized by severe symptoms; and latent (dormant) which is typified by life-long persistence of cysts in tissues [10]. TE typically results from reactivation of the dormant organism [11].
TE is one of the most common opportunistic infections of the central nervous system (CNS) in patients with acquired immune deficiency syndrome (AIDS) worldwide, and in the United States between 10 and 40% of HIV-infected individuals have antibodies against T. gondii [12−14]. TE is a life-threatening disease, especially for immunocompromised patients, such as those with AIDS [13,15]. Encephalitis causes significant morbidity and mortality in patients with HIV, and toxoplasmosis was found to be the most common specified encephalitis-associated hospitalizations in patients with HIV [16].
The National Institutes of Health (NIH) Guidelines recommend the use of pyrimethamine, sulfadiazine, and leucovorin for the initial treatment of TE in HIV-infected pateints [17]. Pyrimethamine and sulfadiazine are thought to act synergistically to inhibit T. gondii proliferation and survival by blocking the folate metabolic pathway and consequently DNA synthesis [11]. Leucovorin is added to reduce potential adverse reactions associated with folic acid metabolism [11]. In case of allergy to sulfa drugs, the recommended alternative treatment is a combination of pyrimethamine, clindamycin, and leucovorin [17]. Sulfa drug allergies are observed in about 5% of the population and up to 30% of HIV-infected patients [18].
The incidence of TE in patients infected with HIV is closely related to the progression of immune deficiency and a reduction in CD4 T lymphocyte cell counts. TE is rare in patients with CD4 cell counts >200 cells/μL, while the risk of TE is greatest in patients with CD4 counts <50 cells/μL [19]. It is recommended by the NIH/AIDS Guidelines to continue chronic maintenance treatment in patients who have responded to initial TE therapy until the person remains asymptomatic and their CD4 counts are >200 cells/μL after highly active anti-retroviral therapy (HAART) for for six months [17].
Despite treatment efforts, a percentage of patients receiving maintenance therapy for TE will experience a relapse [13]. Relapse of TE can have significant implications for patients who often develop new lesions in areas of the brain previously free of infection [20], and for providers and the health care system as a relapse can result in rehospitalization and/or change in therapy.
Previous meta-analyses have assessed the efficacy and safety of pyrimethamine for acute TE [21−23]; however, the incidence of relapse during maintenance therapy following resolution of acute TE has not been assessed [21−24]. Therefore, to gain greater insight into the risk of TE relapse, we performed a systematic review and meta-analysis to evaluate the incidence of relapse associated with pyrimethamine-based maintenance therapy (i.e. secondary prophylaxis) in persons with HIV/AIDS. We also investigated whether the likelihood of relapse changed following the introduction of HAART therapy in the mid-1990s. This was of interest because HAART has greatly reduced AIDS-related morbidity and mortality and decreased the incidence of opportunistic infections in HIV-infected patients [25]. The impact of HAART on the incidence of relapse of TE in patients with HIV is not clear. In order to incorporate the broadest scope of available evidence, the analysis protocol allowed for inclusion of studies of varying design, such as single-arm observational cohort, randomized, and retrospective studies.
Methods
Search strategy
The study was performed in accordance with PRISMA [26], and the protocol can be found in Supplemental Material. PubMed, Google Scholar and Cochrane databases were searched up to 6 June 2016 using the following search terms: pyrimethamine, Daraprim, Fansidar, Metakelfin, Fansimef, 5-(4-chlorophenyl)-6-ethyl-2,4-pyrimidinediamine, encephalitis, cerebral, toxoplasmosis, toxoplasmic, gondii. Eligible studies included randomized controlled studies, observational prospective studies, and retrospective longitudinal studies. Studies had to report relapse of TE during maintenance therapy in patients with AIDS or HIV. All studies had to be published in peer-reviewed journals in English, Spanish, Portugueuse, or French. Studies that only provided relapse rates during acute treatment were rejected. Review articles, letters, comments, editorials, case reports, proceedings, personal communications, and preclinical studies were also excluded. Study eligibility for inclusion was determined by two independent reviewers, and in cases of uncertainty, a third reviewer was consulted.
Data extraction
Double data extraction was performed on all included studies to ensure data consistency. Discrepencies in extracted data between the two independent reviewers were resolved by a third reviewer. Non-English studies were reviewed by native speakers, and validated by a second reviewer for extracted data using Google Translator. The following data were extracted from the studies when reported: first author, publication year, study design, acute and maintence treatment regimens, number of patients, incidence of relapse, and duration of follow-up. For studies with insufficient reported data, we attempted to contact authors to obtain complete data. Studies were classified as being pre-HAART or post-HAART based on a cut-off date of 1996 for the conduct of the study (not publication date). The year 1996 is when the use of HAART therapy became widely available and dramatically decreased mortality of patients with AIDS (from 29.4/100 person years in 1995 to 8.8 per 100 person-years in the second quarter of 1997) [25]. HAART status was verified by patient baseline characteristics and current treatments reported, although not all patients treated in the HAART period were receiving antiretroviral therapy. The abstracts were screened and data were extracted by the authors MPC and EG.
Quality assessment
The quality of the included studies was evaluated using Study Quality Assessment Tools of the National Heart, Lung, and Blood Institute of the NIH for quality assessment of observational cohort, cross-sectional, before–after (pre-post) studies with no control group, and case series studies [27].
Statistical analysis
Pooled proportions meta-analysis
The primary outcome was rate of relapse during pyrimethamine-based maintenance therapy. The secondary outcome was rate of relapse with continuous (daily) or intermittent (two to three times per week) pyrimethamine-based therapy. For each outcome, rates in each study were transformed using the Freeman-Tukey double arcsine method. This transformation stabilizes the variance estimates, which is important for proportions close to 0 or 1 [28]. Rates were then pooled using both fixed-effect and random-effects meta-analysis models. The fixed-effect model was performed using the inverse variance method and the random-effects model using the DerSimonian and Laird method, with the estimate of heterogeneity taken from the inverse variance model [29]. Heterogeneity across studies was assessed by quantifying I2 inconsistency scores [30]. I2 describes the percentage variation across studies attributed to heterogeneity rather than chance. Low I2 values (≤50%) suggest limited heterogeneity between studies, and I2 values >50 to 75% indicate moderate heterogeneity. Values >75% suggest high heterogeneity and the need to consider estimates from random-effects models [30]. The analysis was performed using StatsDirect (Altrincham, UK) statistical program version 2.8.0 (27 October 2013). For ease of interpretating, the reported proportions were converted to percentages. Because the studies are non-comparative, we assessed active treatment arms independently; therefore, no comparative statistics were performed and only the combined relapse rate is reported.
Bias of the results was evaluated by the funnel plot and the Egger’s test [31,32]. In the absence of bias, the graph is a symmetrical inverted funnel, and if there is bias, the funnel plot will appear asymmetrical [31,32]. Reporting bias was assessed for the pre-HAART analysis, but not the post-HAART analysis as the minumum studies for this analysis should be >10; below this value, the test power is usually too low to distinguish chance from real asymmetry [33].
Results
Search results
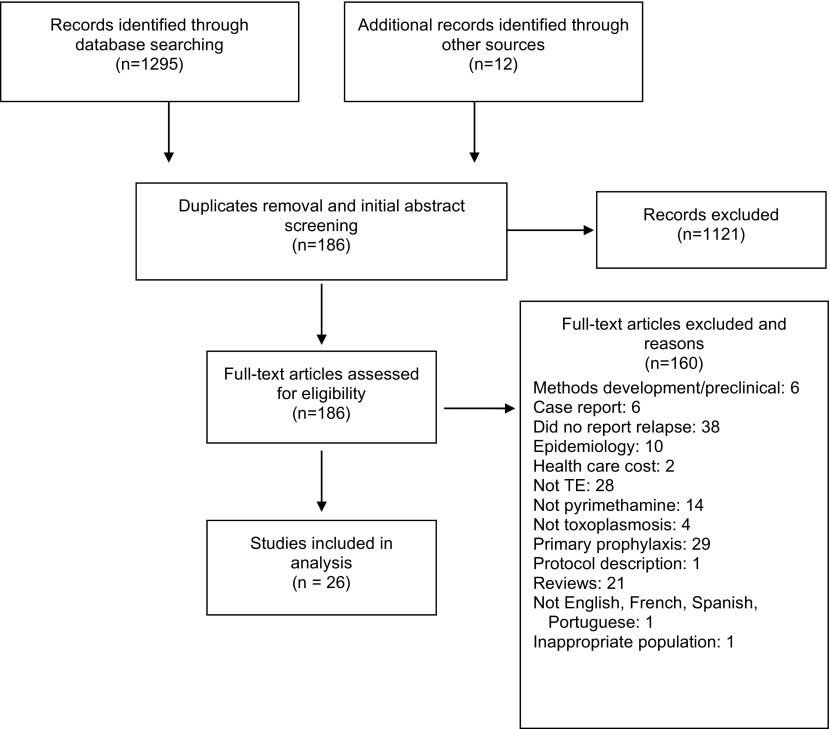
Of 1,307 studies initially identified, 1,121 were excluded following the ‘removal of duplicates and an initial abstract screen (Figure 1). After full-text review, an additional 160 were removed for only describing methodology or a study protocol, being a pre-clinical study, being a case report, not reporting relapse rates, being an epidemiology or health care cost study, not evaluating TE, not administering pyrimethamine-based regimens, not investigating toxoplasmosis, describing finding for primary prophylaxis of TE, being a review article, not being published in English, French, Spanish, or Portugeuse, or evaluting an inappropriate patient population.
Figure 1.
Search flow diagram.
Study characteristics
Twenty-six studies were included in the analysis with 1,596 patients (Tables 1 and 2). Twenty reported findings from studies that were performed up to 1996 (n = 1,228) (Table 1) [34−51], and six described results of studies performed after 1996 (n = 368) (Table 2) [52−57].
Table 1.
Summary of study characteristics performed until 1996.
| Author | Study Design | Demographics | Pyrimethamine-based therapy | No. patients evaluated for relapse, n | Relapse, n(%) | Follow-up | |
|---|---|---|---|---|---|---|---|
| Acute | Maintenance | ||||||
| Randomized studies | |||||||
| Katlama (1996) | Randomized prospective open-label | N = 299 | PS | PS | PS: 83 | PS: 17 (20.5%) | 89 to >130 weeks |
| PC (n = 152) | PYR (50 mg daily) plus SDZ (4 g daily in four divided doses) plus folinic acid (min 50 mg/wk) | PYR (25 mg/day) plus SDZ (500 mg four times/day) plus folinic acid 50 mg weekly | PC: 92 | ||||
| PC: 35 (38.0%) | |||||||
| Male:133 (88%) | |||||||
| Mean age: 34 yrs | |||||||
| PS (n = 147) | |||||||
| Male: 129 (88%) | PC | ||||||
| PYR (25 mg/day) plus CLI (300 mg four times/day) plus folinic acid 50 mg weekly | |||||||
| Mean age: 33 yrs | |||||||
| PC | |||||||
| PYR (50 mg daily) plus clindamycin (2.4 g daily in four divided doses) plus folinic acid (min 50 mg/wk) | |||||||
| Maintenance therapy was life-long. | |||||||
| Acute therapy was given for six weeks. | |||||||
| Podzamczer (1995) | Randomized open-label | N = 105 | n.a. | Daily PS | 105 | Total: 11 (10.4%) | median 11 months |
| Daily: (n = 60) | PYR (25 mg/day) plus SDZ (500 mg four times per day) and folinic acid (15 mg/day). | ||||||
| Male: 40 (66.7%) | Intermittent: 19.5 per 100 patient-yrs | ||||||
| Age: 34 yrs | |||||||
| Twice weekly: (n = 45) | |||||||
| Intermittent PS | |||||||
| Male: 34 (75.6%) | Daily: 4.4 per 100 patient-yrs | ||||||
| PYR (25 mg/day) twice weekly plus SDZ (500 mg four times per day) and folinic acid (15 mg/day). | |||||||
| Age: 32 yrs | |||||||
| Torre (1998) | Randomized, prospective, pilot | N = 77 | PS | Same regimens as above but at half of the original dosage given for three months | PS: n = 35 | PS: 0 | 3 months |
| PS (n = 37) | PYR (50 mg/day) plus SDZ (60 mg/kg/day) plus folinic acid (10 mg/day) | TMP-SMX: 1 (2.7%) | |||||
| TMP–SMX: n = 37 | |||||||
| Male: 29 (78.4%) | |||||||
| Mean age: 32.4 yrs | |||||||
| TMP-SMX (n = 40) | |||||||
| TMP-SMX | |||||||
| Male: 28 (70%) | TMP (10 mg/kg/day BID) plus SMX (50 mg/kg/d BID) | ||||||
| Mean age: 34.0 yrs | |||||||
| TX was for 30 days | |||||||
| Prospective studies | |||||||
| Bouree (1997) | Prospective observational | N = 60 | PS | PS | 60 | 11 (18.3%) | 7 years |
| Male: 46 (76.7) | PYR (100 mg/day) plus SDZ (6 g/day) plus folinic acid (50 mg/day) | PYR (100 mg/day) plus SDZ (6 g/day) plus folinic acid (50 mg/day) | |||||
| Mean age: 40 yrs. | |||||||
| Canessa (1992) | Prospective | N = 24 | TMP-SMX | PYR±SMP | 24 | 4 (17%) | n.r. |
| Male: 19 (79.2%) | TMP-SMX (40 mg/kg/day) or TMP-SMX (120 mg/kg/day) | Sulfamethoxpyrazine (500 mg) plus PYR (25 mg) twice weekly | |||||
| Age, range: 23–53 yrs | |||||||
| Folinic acid (15 mg/day) was also given | |||||||
| TX duration 25 day | |||||||
| Leport (1987) | Prospective observational | N = 12 | PS | Same as acute | 12 | 0 | n.r. |
| Male: 11 (91.7%) | PYR (average 50 mg [range 15–100 mg) plus SDZ (average 4 g (range 2–6 g), for 30 days. Folinic acid (range, 5 to 50 mg/day) was also administered. The 12th patient died during TX | ||||||
| Age, range: 20–47 yrs | |||||||
| Orefice (1992) | Prospective, observational | N = 15 | PSC | PYR (25 mg/day) plus SDZ (2 g/day) | 15 | 3 (20%) | n.r. |
| Male: 12 (80%) | PYR (50 mg/day) plus SDZ (4 g/day) plus CLI (1200 mg/day) plus folinic acid | ||||||
| PSC (n = 5) | |||||||
| PS (n = 10) | |||||||
| PS | |||||||
| PYR (50 mg/day) plus SDZ (4 g/day) plus folinic acid | |||||||
| TX was for 4–6 weeks | |||||||
| Ruf (1991) | Prospective, observational | N = 51 | Three-drug regimen (PC ± SPY) | PYR ± SDX (Fansidar) | 39 | 4 (10%) | Median 13 months |
| Three-drug regimen (PC ± SPY) (n = 25) | |||||||
| Two-drug regimen (PC) (n = 26) | |||||||
| PYR (15 mg) plus SDX (500 mg) plus folinic acid (15 mg) twice a week. | |||||||
| PYR (1.5 mg/kg/day) plus CLI (2400 mg/day) plus SPY (9 × 106 IU/day) | |||||||
| Two-drug regimen (PC) | |||||||
| PYR (50 mg/day for body weight <65 kg or 75 mg/day for body weight >65 kg) plus CLI | |||||||
| (2400 mg/day) | |||||||
| Both TX arms received folinic acid 45 mg/day (although some patients only received drug after myelosuppression became apparent) | |||||||
| TX was for three weeks | |||||||
| Ruf (1993) | Prospective, open-label | N = 56 | PC | PYR ± SDX (Fansidar) | 56 | 4 (7.1%) | n.r. |
| Male: 48 (85.7%) | PYR (50 mg/day body weight <65 kg or 75 mg/day body weight >65 kg) plus CLI (2400 mg/day) plus LEU 30 mg/day. | ||||||
| 25 mg PYR/SDX 500 mg plus LEU (15–30 mg) twice a week | |||||||
| Median age: 39 yrs | |||||||
| Retrospective studies | |||||||
| Altes (1989) | Retrospective | N = 70; 13 with TE | PS | PYR 25 mg/day | 13 | 7 (54%) | n.r. |
| PYR (50–100 mg) plus SDZ (4–6 g) and folinic acid (15–45 mg) daily. | |||||||
| Dannemann (1988) | Retrospective, case review | N = 15 | CLI | PC | PC: 4 | PC: 1 (25%) | Mean 7.5 months |
| Mean age:47 yrs | Various IV dosages of CLI (–) from 1200–4.8 g/day given from every 6-8 h) | PYR (25–75 mg/day plus CLI (300 to 900 every 6-8 h) or PYR (75 mg every other day) plus CLI 450 mg every 6 h) | PYR: 4 | ||||
| PYR: 1 (25%) | |||||||
| CLI (n = 9) | CLI: 3 | ||||||
| SPY: 1 | CLI: 3 (100%) | ||||||
| PC (n = 6) | |||||||
| SPY: 0 | |||||||
| PC | |||||||
| Various IV dosages of CLI (–from 1200–4.8 g/day given every 6-8 h) with PYR (25–100 mg/day) | |||||||
| PYR | |||||||
| PYR (25–100 mg/day) | |||||||
| CLI | |||||||
| CLI (300–450 mg every 6 h or 900 every 8 h) | |||||||
| Folinic acid (5–10 mg/day) was administered to 11 patients | SPY | ||||||
| SPY (500 mg every 8 h) | |||||||
| TX length variable across patients (range 3 to 300 days) | |||||||
| de Gans (1992) | Retrospective | N = 38 | PS | PYR (25 or 50 mg/day) plus folinic acid (15 mg/day) | 38 | 12 (31.6%) | n.r. |
| Male: 37 (97.7%) | PYR (25–75 mg/day) plus SDZ (2–6 mg/day) plus folinic acid (15 mg/day) | ||||||
| Median age: 38.5 | |||||||
| Acute treatment was for six weeks. | |||||||
| Ferrer (1996) | Retrospective | N = 63 | PS | PS | 63 | Total: 19 (30.2%) | n.r. |
| Male: 46 (73%) | PYR (50–75 mg/day) plus | PYR (25 mg/day) plus SDZ (2 g/day) | |||||
| Mean age for both: 21–65 years | PS: 12 | ||||||
| SDZ (4 g/day) plus folinic acid (15 mg/day) QID PO for 4–6 weeks | PC | PC: 7 | |||||
| PYR (25 mg/day) plus CLI (1200 mg/day) | |||||||
| PS (n = 49) | |||||||
| PC (n = 14) | |||||||
| PC (n = 14) | |||||||
| PYR (50–75 mg/day) plus folinic acid (15 mg/day) QID PO for 4–6 weeks plus CLI (600 mg/q6 h) PO or IV | |||||||
| Pts w/ intracranial hypertension and perilesional edema: | |||||||
| Dexamethasone (12- 16 mg/day) | |||||||
| Foppa (1991) | Retrospective | N = 14 | PC | PC | 14 | 0 | Mean 7.7 months |
| Male: 13 (92.9%) | PYR (100 mg loading dose, followed by 50 mg/d) plus CLI (600–900 mg orally TID) plus folinic acid (15 mg/day) for 6–8 weeks | PYR (25 mg/d) plus CLI (300 mg QID or 450 mg TID) plus folinic acid (15 mg/day) | |||||
| Age, range: 29–39 yrs | |||||||
| Duration of acute therapy was from 6 to 8 weeks | |||||||
| Gonzalez-Clemente (1990) | Retrospective | N = 57 | PS | PS | PS: n = 15 | PS: 0 | PS: Median 12.3 months |
| PYR 5–100 mg for one day followed by 25–50 mg/day, SDZ 75 mg/kg/day divided into four doses, plus folinic acid (10–20 mg/day) | PYR 5–100 mg for one day followed by 25–50 mg/day, SDZ 75 mg/kg/day divided into four doses, plus folinic acid (10–20 mg/day) two days per week (Tuesday and Friday) | PC: 4 (40%) | |||||
| PC: n = 10 | |||||||
| PC: Median 13.3 months | |||||||
| PC | |||||||
| If patient had sulfa allergy, clindamycin (600 mg/every 6 h) was substituted for SDZ. | |||||||
| PC | |||||||
| If patient had sulfa allergy, clindamycin (600 mg/every 6 h) was substituted for SDZ | |||||||
| Acute treatment was for 3 to 6 weeks | |||||||
| Leport (1988) | Retrospective chart review | N = 35 | PS | PS | 24 | 6 (25%) | Mean 6 months |
| Male: 33 (94.3%) | PYR (loading doses 100–200 mg for first 1–2 days, followed by 50–100 mg/day) plus SDZ (2–6 g/day) plus folinic acid (5–50 mg/day) | PYR (25–50 mg/day) plus SDZ (1–4 g/day) plus folinic acid (5–50 mg/day) | |||||
| Mean age: 37.0 yrs | |||||||
| Leport (1991) | Retrospective | N = 35 | n.a. | PS (n = 13)a | 35 | Total: 6 (17%) | Mean 13 months |
| PC: 4 | |||||||
| PYR: 1 | |||||||
| PYR (25–100 mg/day) plus SDZ (2–4 g/day) | |||||||
| PS: 1 | |||||||
| PC (n = 20)a PYR (25–100 mg/day) plus CLI (450–1200 mg/day) | |||||||
| PYR (n = 11)a | |||||||
| PYR 50–100 mg/day | |||||||
| Pedrol (1990) | Retrospective | N = 43 | PS | PS | 12 | 6 (50.0%) | 10.3–13.7 months |
| Male: 35 (81.4%) | PYR 50–75 mg loading dose, followed by 25 mg/day plus SDZ 75 mg/day plus folinic acid (10–20 mg/day) | Same as acute dosage but given only 2 days/wk (Tues and Fri) | |||||
| Acute treatment was for 3 weeks | |||||||
| Ragnaud (1993) | Retrospective | N = 73 | PS | PS | 60 | 12 (20%) | 8 months |
| Sex ratio: 2.8:1 | PYR (100–200 mg/day for 1–3 days followed by 75 mg/day) plus SDZ (4–8 g/day) plus folinic acid (10–50 mg/day) | PYR 50 mg / day plus SDZ 2 to 3 g / day | |||||
| Mean age: 36.2 yrs | |||||||
| PC | |||||||
| PYR 50 mg/day plus CLI 1.2 g/day | |||||||
| Renold (1992) | Retrospective chart review | N = 86 | PS | PS | 82 | 16 (19.5%) | n.r. |
| Male: 69 (80%) | PC | PC | |||||
| Acute treatment was defined as antitoxoplasma drugs received within 42 days of diagnosis | Maintenance therapy was calculated from the 42nd day following diagnosis to death, loss to follow-up, relapse, or December 31, 1990, or whichever came first. | ||||||
| Mean age: 36.4 yrs |
n indicates number of courses. Forty-four treatment courses were evaluated in 35 patients.
BID: twice daily; CLI, clindamycin; IV, intravenous; LEU, leucovorin; n.a., not applicable; n.r., not reported; PC, pyrimethamine plus clindamycin; PK, pharmacokinetic; PS, pyrimethamine plus sulfadiazine; PSC: pyrimethamine plus sulfadiazine, plus clindamycin; PYR, pyrimethamine; QID, four times/day; SDX, sulfadoxine; SDZ, sulfadiazine; SMP, sulphamethoxpyrazine; SMX, sulfamethoxazole; SPY, spiramycin; TMP, trimethoprim; TMP-SMX, trimethoprim-sulfamethoxazole (co-trimoxazole); TX, treatment.
Table 2.
Summary of study characteristics after 1996.
| Author | Study design | Demographics | Pyrimethamine-based therapy | No. patients evaluated for relapse, n | Relapse, n(%) | Follow-up | |
|---|---|---|---|---|---|---|---|
| Acute | Maintenance | ||||||
| Randomized studies | |||||||
| Chirgwin (2002) | Randomized, open-label, phase 2 | N = 39 | ATO ± PYR | 42 weeks after acute treatment with same treatment regimen as acute therapy | 15 | 1 (6.7%) | 48 weeks |
| ATO±PYR (n = 28) | |||||||
| ATO (1500 mg BID) plus PYR (200 mg loading dose, followed by 75 mg/day) plus LEU 10 mg daily | |||||||
| Male: 22 (79%) | |||||||
| Median age: 35 yrs | |||||||
| ATO±SDZ (n = 12) | |||||||
| Male: 10 (83%) | |||||||
| Median age: 37 yrs | |||||||
| ATO ± SDZ | |||||||
| ATO (1500 mg BID) plus SDZ (1500 mg QID) plus LEU 10 mg/day. | |||||||
| Acute treatment was administered for six weeks | |||||||
| Podzamczer (2000) | Randomized open-label | N = 124 | n.a. | PS Daily Regimen:PYR | 124 | Total: 16 (12.9%) | Median 11 months |
| Daily: 14.9 episodes per 100 patient yrs | |||||||
| (25 mg/day) plus SDZ (1 g BID) plus folinic acid (15 mg/day) | |||||||
| Daily (n = 58) | |||||||
| Male/female ratio: 3.5 | |||||||
| Intermittent: 14.1 episodes per 100 patient yrs | |||||||
| Age: 35.7 yrs | |||||||
| Thrice weekly (n = 66) | PS Three times/wk regimen: | ||||||
| Male/female ratio:5.0 | PYR (50 mg/day) plus (SDZ: 1 g BID) plus folinic acid (15 mg)/day | ||||||
| Age: 34.9 yrs. | |||||||
| Prospective studies | |||||||
| Langmann (2004) | Prospective | N = 6 (3 received secondary prophylaxis) | n.a. | PYR: 50 mg/day or 37.5 mg/day depending on body weight plus folinic acid (7.5 mg/day) | 3 | 0 | Mean 22 ± 13 months |
| Age, range: 35–59 yrs | |||||||
| Vidal (2005) | Prospective longitudinal | N = 55 | PS | PS | 46 | 2 (4%) | One year from diagnosis |
| Male: 33 (60%) Mean age: | PYR + SDZ + folinic acid | PYR + SDZ + folinic acid | |||||
| 36 yrs | |||||||
| Retrospective study | |||||||
| Arendt (1999) | Retrospective | N = 106 | P ± SND | PYR: 75 mg/day | 106 | 13 (12%) | n.r. |
| Male: 101 (95.3%) | PYR (75 mg/day) plus sulfonamides (2 g/day) or clindamycin (2400 mg/day). | ||||||
| Mean age: 40.4 yrs | |||||||
| P ± SND (n = 98) | |||||||
| PC (n = 6) | |||||||
| PC | |||||||
| PYR (75 mg/day) plus clindamycin (2400 mg/day). | |||||||
| Davarpanah (2007) | Retrospective | N = 38 | PS | PS | 4 | 0 | n.r. |
| Male: 30 (78.9%) | PYR + SDZ + leucovorin | PYR + SDZ | |||||
| Age: 38 yrs |
ATO, atovaquone; LEU, leucovorin; n.a., not applicable; n.r., not reported; PC, pyrimethamine plus clindamycin; PS, pyrimethamine plus sulfadiazine; P + SND, pyrimethamine plus a sulfonamide; PYR, pyrimethamine; SDZ, sulfadiazine.
Study designs differed across both pre- and post-HAART studies. The pre-HAART studies included three randomized, six prospective, and 11 retrospective studies (Table 1), and the post-HAART studies included two randomized, two prospective, and two retrospective studies (Table 2). All studies included patients with AIDS or infected with HIV and who had received maintenance therapy for TE.
Among the studies, the pyrimethamine-based treatment regimens were heterogeneous, both for acute and maintenance therapy (Tables 1 and 2). In the pre- and post-HAART groups, the most common therapy both for acute and maintenance therapy was the combination of pyrimethamine and sulfadiazine. Other investigated pyrimethamine-based regimens included clindamycin, sulphamethopyrazine, sulfadoxine, clarithromycin, and atovaquone. Four studies in the pre-HAART group [41,42,45,47] and two in the post-HAART group [52,55] administered pyrimethamine alone for maintenance therapy. Most studies co-administered folinic acid (leucovorin) with pyrimethamine for acute therapy; only four did not report co-administering folinic acid with pyrimethamine – three in the pre-HAART group [35,58,59] and one in the post-HAART group [52]. Of the included studies, 11 did not report co-administering folinic acid with pyrimethamine during maintenance therapy [34,35,38,41,45,47,49,51,52,54,60].
The follow-up for pre-HAART studies ranged from 3 months to 7 years and for the post-HAART studies from 4 to 22 months. For the pre-HAART studies, the rate of relapse ranged from 0 to 67% and for the post-HAART studies ranged from 0 to 12.9%.
Quality assessment
The majority of included studies obtained scores of medium–to-low quality due the varied trial designs. Despite the overall quality assessment, the relapse data reported were of sufficient quality for inclusion in the proportions analysis.
Meta-analysis
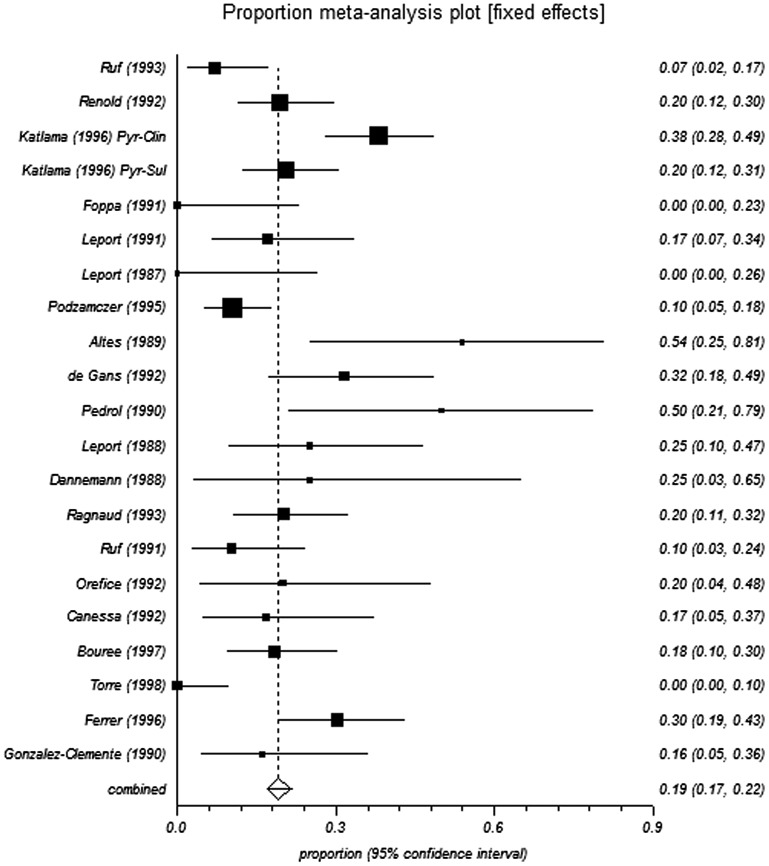
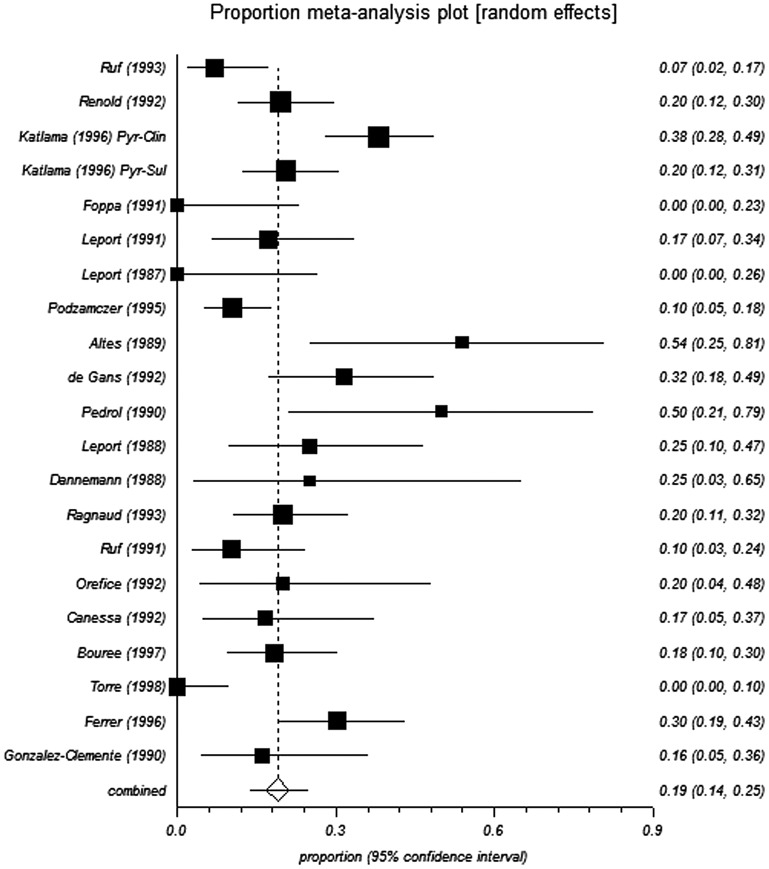
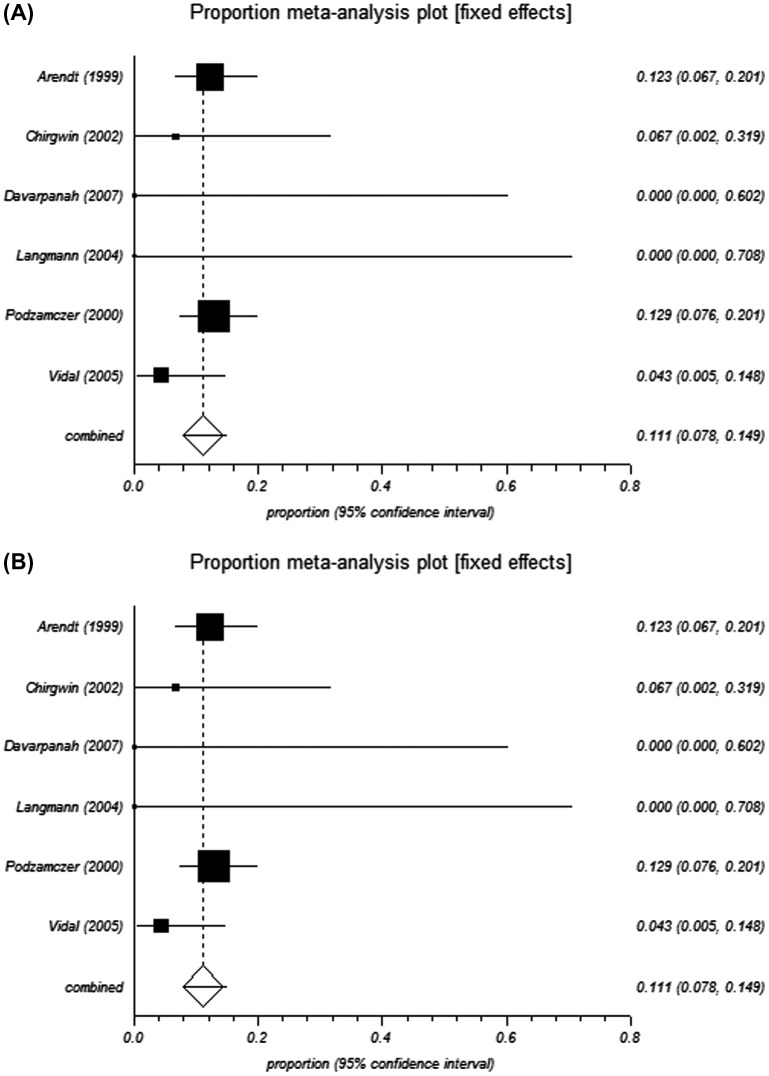
The pooled proportions test for pyrimethamine-based therapy in pre-HAART studies indicated a relapse rate of 19.2% and 18.9% from the fixed-effects and random-effects models, respectively (Table 3 and Figure 2 and Figure 3). In the post-HAART period, the pooled proportions relapse rate was 11.1%, and was the same from both the fixed- and random-effect models due to limited heterogeneity in the data (I2 = 0%) (Table 3 and Figure 4).
Table 3.
Pyrimethamine-based maintenance therapy: relapse rates in pre-HAART and post-HAART period.
| Proportions Fixed-effect | Proportions Random-effect | I2 | |
|---|---|---|---|
| Pre-HAART | 0.192 (95% CI = 0.167 to 0.218) | 0.189 (95% CI = 0.137 to 0.247) | 76.4% (95% CI = 62.8 to 83.5%) |
| Post-HAART | 0.111 (95% CI = 0.078 to 0.149) | 0.111 (95% CI = 0.078 to 0.149) | 0% (95% CI = 0 to 61%) |
| Intermittent Pre-HAART | 0.209 (95% CI = 0.139 to 0.287) | 0.256 (95% CI = 0.104 to 0.445) | 72.9% (95% CI = 17.9 to 86.7%) |
| Intermittent Post-HAARTa | – | – | |
| Continuous Pre-HAART | 0.187 (95% CI = 0.161 to 0.214) | 0.173 (95% CI = 0.119 to 0.234) | 77.5% (95% CI = 64.4 to 84.3%) |
| Continuous Post-HAART | 0.105 (95% CI = 0.069 to 0.146) | 0.105 (95% CI = 0.069 to 0.146) | 0% (95% CI = 0 to 61%) |
Analysis was not performed as only one study in the post-HAART timeframe evaluated intermittent pyrimethamine-based therapy.
CI, confidence interval; I2, inconsistency.
Figure 2.
Forest plot of fixed for analysis of pre-HAART studies.
Figure 3.
Forest plot of random effect model for analysis of pre-HAART studies.
Figure 4.
Forest plot of (A) fixed and (B) random effect model for analysis of post-HAART studies.
Six studies reported relapse rates of patients who received intermittent pyrimethamine-based therapy (two to three times per week); five pre-HAART [40,43,45,49,61] and one post-HAART [56]. We evaluated the rate of relapse in these studies compared with studies with continuous (daily) dosing. The relapse rate with intermittent pyrimethamine-based therapies in pre-HAART studies was 20.9% from the fixed-effect and 25.6% from the random-effect models, and for continous therapy was 18.7–17.3%, respectively (Table 3). The rates for continuous therapy for post-HAART were 10.5%. Intermittent therapy was not analyzed in the post-HAART studies as only one study [56] evaluated this type of treatment regimen.
Analysis of reporting bias
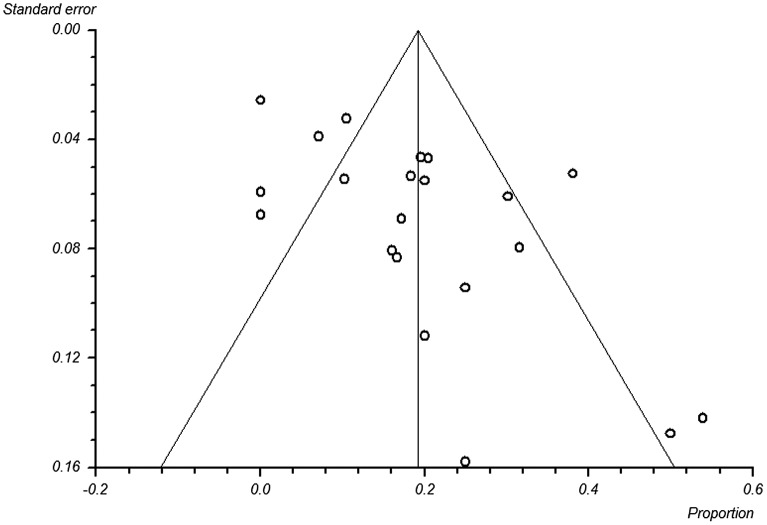
Funnel plot analysis was performed for the pre-HAART studies (Figure 5). The analysis indicated the presence of reporting bias as indicated by significant funnel plot asymmetry (Eggar bias: 3.12386 (95% CI = 1.196098 to 5.051621); P = 0.0031).
Figure 5.
Funnel plot analysis of reporting bias.
Discussion
This systematic review and meta-analysis assessed the incidence of TE relapse following pyrimethamine-based therapy in patients with HIV or AIDS. The anlysis found that the incidence of relapse was higher in pre-HAART (19.2% for fixed effect model and 18.9% for random effect model) compared with post-HAART (11.1% for both fixed- and random-effect models). The drop in the incidence of relapse with the use of HAART therapy is consistent with the impact of HAART on the immune status of patients with HIV or AIDs. To our knowledge, this is the first meta-analysis to evaluate the rate of relapse of TE in HIV-infected or AIDS patients, and specifically illustrates the benefits of HAART therapy for preventing relapse.
Relapse during maintenance therapy may result from a number of causes including treatment efficacy, dosing regimen, patient characteristics, and treatment adherence. The importance of the dosing regimen is indicated by our results that sugggest intermittent therapy administered two to three times per week was associated with a higher relapse rate compared with continuous therapy, and supports the NIH Guidelines of daily dosing of pyrimethamine plus sulfadiazine for maintenance treatment [17].
The analysis described here focused on pyrimethamine-based therapy for the prevention of relapse due to the extensive history of the use of pyrimethamine in the clinic and the significant number of observational and retrospective clinical studies that have evaluated the effect of pyrimethamine-based therapy in treating TE. Much less-published information is available regarding the use of other off-label treatments for TE, such as the trimethoprim–sulfamethoxazole (TMP-SMX) combination. A similar search identified only five studies that evaluated TMP-SMX for maintenance therapy and reported relapse of HIV-infected patients with TE [48,62−65], four of which were performed post-1996 [62−65]. Across the five studies, the rate of TE relapse ranged from 3 to 39%.
Evidence from randomized controlled trials serve as the gold-standard for studies to be included in meta-analyses to reduce bias in the results. However, challenges exist in applying randomized control trial results to the real-world clinical setting due to differences between subjects recruited and the types of patients encountered in clinical practice [66]. We included both prospective observational and retrospective studies due to the shortage of available randomized studies with pyrimethamine relative to the number of other study designs in TE. The inclusion of these studies in our analysis broadens the range of evidence and may improve the generalizability of the findings. This approach is consistent with other health service researchers who have included observational study designs in meta-analyses, and has been applied in other disease areas [67,68].
The analysis described here has several limitations that are worth considering. Significant heterogeneity across studies was present with respect to study design, dosing regimen, and defintion of relapse, and the majority of studies were of mediumto-low quality. Also, the size of the patient population for the post-HAART analysis was small. Furthermore, not all patients were receiving HAART therapy for various reasons, and the results were not presented by HAART precluding an assessment of how this would influence results. Although, based on the analysis described here it is likely to have diminished effectiveness and increased likelihood of relapse. Reporting bias was also present which may have confounded the results. In addition, duration of follow-up varied between studies which could influence the rate of relapse. It is also important to emphasize that the proportions reported here are independently evaluated; therefore, it was not feasible to infer whether differences in relapse rates reported here are significantly different.
This study comprehensively evaluated the relapse rate of TE in patients with HIV or AIDS who received pyrimethamine-based maintenance therapy. The findings indicate that the incidence of relapse decreased after the introduction of HAART. The reduction in relapse following the introduction of HAART is consistent with the ability of HAART to control HIV infection. In addition, this study suggests that daily pyrimethamine-based maintenance therapy is likely more efficacious in preventing TE relapse compared with intermittent therapy. These findings have important medical and cost implications as relapse may affect a patient’s prognosis and healthcare resource utilization, and result in additional healthcare expenditure.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by Turing Pharmaceuticals.
Supplementary data
Supplemental data for this article can be accessed here.
Supplementary Material
Acknowledgment
The authors would like to thank Melissa Giraldo for assisting with the literature search, and Julio Casoy, MD and Rubben Ben-Harari, PhD for critically reading and reviewing the manuscript.
References
- [1].Holland GN. Ocular toxoplasmosis: a global reassessment. Part I: epidemiology and course of disease. Am J Ophthalmol. 2003;136(6):973–88. 10.1016/j.ajo.2003.09.040 [DOI] [PubMed] [Google Scholar]
- [2].Dalimi A, Abdoli A. Latent toxoplasmosis and human. Iran J Parasitol. 2012;7(1):1–17. [PMC free article] [PubMed] [Google Scholar]
- [3].Parasites – Toxoplasmosis (Toxoplasma infection) : Resources for Health Professionals: Centers for Disease Control and Prevention; [Page last updated [cited 2015 December 14]].
- [4].Shin DW, Cha DY, Hua QJ, et al. Seroprevalence of Toxoplasma gondii Infection and characteristics of seropositive patients in general hospitals in Daejeon, Korea. Korean J Parasitol. 2009;47(2):125–30. 10.3347/kjp.2009.47.2.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Xiao Y, Yin J, Jiang N, et al. Seroepidemiology of human Toxoplasma gondii infection in China. BMC Infect Dis. 2010;10:4. 10.1186/1471-2334-10-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kamani J, Mani AU, Egwu GO, et al. Seroprevalence of human infection with Toxoplasma gondii and the associated risk factors, in Maiduguri, Borno state, Nigeria. Ann Trop Med Parasitol. 2009;103(4):317–21. 10.1179/136485909X435094 [DOI] [PubMed] [Google Scholar]
- [7].Swai ES, Schoonman L. Seroprevalence of Toxoplasma gondii infection amongst residents of Tanga district in North-East Tanzania. Tanzan J Health Res. 2009;11(4):205–9. [DOI] [PubMed] [Google Scholar]
- [8].Fromont EG, Riche B, Rabilloud M. Toxoplasma seroprevalence in a rural population in France: detection of a household effect. BMC Infect Dis. 2009;9:1217. 10.1186/1471-2334-9-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dubey JP, Lago EG, Gennari SM, et al. Toxoplasmosis in humans and animals in Brazil: high prevalence, high burden of disease, and epidemiology. Parasitology. 2012;139(11):1375–424. 10.1017/S0031182012000765 [DOI] [PubMed] [Google Scholar]
- [10].Derouin F, Pelloux H. Parasitology ESGoC. Prevention of toxoplasmosis in transplant patients. Clin Microbiol Infect. 2008;14(12):1089–101. 10.1111/j.1469-0691.2008.02091.x [DOI] [PubMed] [Google Scholar]
- [11].Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363(9425):1965–76. 10.1016/S0140-6736(04)16412-X [DOI] [PubMed] [Google Scholar]
- [12].Pereira-Chioccola VL, Vidal JE, Su C. Toxoplasma gondii infection and cerebral toxoplasmosis in HIV-infected patients. Future Microbiol. 2009;4(10):1363–79. 10.2217/fmb.09.89 [DOI] [PubMed] [Google Scholar]
- [13].Luft BJ, Remington JS. Toxoplasmic encephalitis in AIDS. Clin Infect Dis. 1992;15(2):211–22. 10.1093/clinids/15.2.211 [DOI] [PubMed] [Google Scholar]
- [14].Grant IH, Gold JW, Rosenblum M, et al. Toxoplasma gondii serology in HIV-infected patients: the development of central nervous system toxoplasmosis in AIDS. AIDS. 1990;4(6):519–22. 10.1097/00002030-199006000-00004 [DOI] [PubMed] [Google Scholar]
- [15].Ruskin J, Remington JS. Toxoplasmosis in the compromised host. Ann Intern Med. 1976;84(2):193–9. 10.7326/0003-4819-84-2-193 [DOI] [PubMed] [Google Scholar]
- [16].Vora NM, Holman RC, Mehal JM, et al. Burden of encephalitis-associated hospitalizations in the United States, 1998–2010. Neurology. 2014;82(5):443–51. 10.1212/WNL.0000000000000086 [DOI] [PubMed] [Google Scholar]
- [17].Panel on antiretroviral guidelines for adults and adolescents; guidelines for the prevention and treatment of opportunistic infections in HIV-infected adults and adolescents. Washington, DC: Department of Health and Human Services; [updated [cited 2015 October 19]]. Available from: https://aidsinfo.nih.gov/guidelines/html/4/adult-and-adolescent-oi-prevention-and-treatment-guidelines/322/toxo. [Google Scholar]
- [18].Brackett CC, Singh H, Block JH. Likelihood and mechanisms of cross-allergenicity between sulfonamide antibiotics and other drugs containing a sulfonamide functional group. Pharmacotherapy. 2004;24(7):856–70. 10.1592/phco.24.9.856.36106 [DOI] [PubMed] [Google Scholar]
- [19].Waxman S, Metz J, Herbert V. Defective DNA synthesis in human megaloblastic bone marrow: effects of homocysteine and methionine. J Clin Invest. 1969;48(2):284–9. 10.1172/JCI105984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Martin-Blondel G, Alvarez M, Delobel P, et al. Toxoplasmic encephalitis IRIS in HIV-infected patients: a case series and review of the literature. J Neurol Neurosurg Psychiatry. 2011;82(6):691–3. 10.1136/jnnp.2009.199919 [DOI] [PubMed] [Google Scholar]
- [21].Wei HX, Wei SS, Lindsay DS, et al. A systematic review and meta-analysis of the efficacy of anti-Toxoplasma gondii medicines in humans. PLoS One. 2015;10(9):e0138204. 10.1371/journal.pone.0138204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Yan J, Huang B, Liu G, et al. Meta-analysis of prevention and treatment of toxoplasmic encephalitis in HIV-infected patients. Acta Trop. 2013;127(3):236–44. 10.1016/j.actatropica.2013.05.006 [DOI] [PubMed] [Google Scholar]
- [23].Hernandez AV, Thota P, Pellegrino D, et al. A systematic review and meta-analysis of the relative efficacy and safety of treatment regimens for HIV-associated cerebral toxoplasmosis: is trimethoprim-sulfamethoxazole a real option? HIV Med. doi: 10.1111/hiv.12402. [DOI] [PubMed] [Google Scholar]
- [24].Rajapakse S, Chrishan Shivanthan M, Samaranayake N, et al. Antibiotics for human toxoplasmosis: a systematic review of randomized trials. Pathog Glob Health. 2013;107(4):162–9. 10.1179/2047773213Y.0000000094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Palella FJ Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV outpatient study investigators. N Engl J Med. 1998;338(13):853–60. 10.1056/NEJM199803263381301 [DOI] [PubMed] [Google Scholar]
- [26].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- [27].National heart lung and blood institute study quality assessment tools. Available from: https://www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools
- [28].Freeman FM, Tukey JW. Transformations related to the angular and the square root. Ann Math Stat. 1950;21(4):607–11. 10.1214/aoms/1177729756 [DOI] [Google Scholar]
- [29].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- [30].Cochrane handbook for systematic reviews of interventions Available from: http://training.cochrane.org/handbook.
- [31].Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–55. 10.1016/S0895-4356(01)00377-8 [DOI] [PubMed] [Google Scholar]
- [32].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- [34].Ruf B, Schürmann D, Bergmann F, et al. Efficacy of pyrimethamine/sulfadoxine in the prevention of toxoplasmic encephalitis relapses and pneumocystis carinii pneumonia in HIV-infected patients. Eur J Clin Microbiol Infect Dis. 1993;12(5):325–9. 10.1007/BF01964427 [DOI] [PubMed] [Google Scholar]
- [35].Renold C, Sugar A, Chave JP, et al. Toxoplasma encephalitis in patients with the acquired immunodeficiency syndrome. Medicine (Baltimore). 1992;71(4):224–39. 10.1097/00005792-199207000-00005 [DOI] [PubMed] [Google Scholar]
- [36].Katlama C, Wit S, O’Doherty E, et al. Pyrimethamine-clindamycin vs. pyrimethamine-sulfadiazine as acute and long-term therapy for toxoplasmic encephalitis in patients with AIDS. Clinical Infect Dis. [Internet]. 1996; 22(2):268–75. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/960/CN-00130960/frame.html. [DOI] [PubMed] [Google Scholar]
- [37].Foppa CU, Bini T, Gregis G, et al. A retrospective study of primary and maintenance therapy of toxoplasmic encephalitis with oral clindamycin and pyrimethamine. Eur J Clin Microbiol Infect Dis. 1991;10(3):187–9. 10.1007/BF01964458 [DOI] [PubMed] [Google Scholar]
- [38].Leport C, Tournerie C, Raguin G, et al. Long-term follow-up of patients with AIDS on maintenance therapy for toxoplasmosis. Eur J Clin Microbiol Infect Dis. 1991;10(3):191–3. 10.1007/BF01964460 [DOI] [PubMed] [Google Scholar]
- [39].Leport C, Vilde JL, Katlama C, et al. Cerebral toxoplasmosis in immunosuppressed patients: diagnosis and treatment. Ann Med Interne (Paris). 1987;138(1):30–3. [PubMed] [Google Scholar]
- [40].Podzamczer D, Miro JM, Bolao F, et al. Twice-weekly maintenance therapy with sulfadiazine-pyrimethamine to prevent recurrent toxoplasmic encephalitis in patients with AIDS. Spanish toxoplasmosis study group. Ann Intern Med. 1995;123(3):175–80. 10.7326/0003-4819-123-3-199508010-00003 [DOI] [PubMed] [Google Scholar]
- [41].Altes J, Salas A, Ricart C, et al. Cerebral toxoplasmosis in patients with AIDS. Arch Neurobiol (Madr). 1989;52(Suppl 1):121–6. [PubMed] [Google Scholar]
- [42].de Gans J, Portegies P, Reiss P, et al. Pyrimethamine alone as maintenance therapy for central nervous system toxoplasmosis in 38 patients with AIDS. J Acquir Immune Defic Syndr. 1992;5(2):137–42. [PubMed] [Google Scholar]
- [43].Pedrol E, Gonzalez-Clemente JM, Gatell JM, et al. Central nervous system toxoplasmosis in AIDS patients: efficacy of an intermittent maintenance therapy. AIDS. 1990;4(6):511–8. 10.1097/00002030-199006000-00003 [DOI] [PubMed] [Google Scholar]
- [44].Leport C, Raffi F, Matheron S, et al. Treatment of central nervous system toxoplasmosis with pyrimethamine/sulfadiazine combination in 35 patients with the acquired immunodeficiency syndrome. Efficacy of long-term continuous therapy. Am J Med. 1988;84(1):94–100. 10.1016/0002-9343(88)90014-9 [DOI] [PubMed] [Google Scholar]
- [45].Dannemann BR, Israelski DM, Remington JS. Treatment of toxoplasmic encephalitis with intravenous clindamycin. Arch Intern Med. 1988;148(11):2477–82. 10.1001/archinte.1988.00380110109023 [DOI] [PubMed] [Google Scholar]
- [46].Ruf B, Pohle H. Role of clindamycin in the treatment of acute toxoplasmosis of the central nervous system. Eur J Clin Microbiol Infect Dis. 1991;10(3):183–6. 10.1007/BF01964457 [DOI] [PubMed] [Google Scholar]
- [47].Orefice G, Carrieri PB, Chirianni A, et al. Cerebral toxoplasmosis and AIDS. Clinical, neuroradiological and immunological findings in 15 patients. Acta Neurol. 1992;14(4–6):493–502. [PubMed] [Google Scholar]
- [48].Torre D, Casari S, Speranza F, et al. Randomized trial of trimethoprim-sulfamethoxazole versus pyrimethamine-sulfadiazine for therapy of toxoplasmic encephalitis in patients with AIDS. Antimicrob Agents Chemother. 1998;42(6):1346–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Canessa A, Del Bono V, De Leo P, et al. Cotrimoxazole therapy of Toxoplasma gondii encephalitis in AIDS patients. Eur J Clin Microbiol Infect Dis. 1992;11(2):125–30. 10.1007/BF01967063 [DOI] [PubMed] [Google Scholar]
- [50].Bouree P, Dumazedier D, Magdeleine C, et al. Cerebral toxoplasmosis and AIDS in martinique. Med Trop (Mars). 1997;57(3):259–61. [PubMed] [Google Scholar]
- [51].Ragnaud JM, Morlat P, Dupon M, et al. Cerebral toxoplasmosis in AIDS. 73 cases. Clinical epidemiology group on AIDS in aquitania. Presse Med. 1993;22(19):903–8. [PubMed] [Google Scholar]
- [52].Arendt G, von Giesen HJ, Hefter H, et al. Long-term course and outcome in AIDS patients with cerebral toxoplasmosis. Acta Neurol Scand. 1999;100(3):178–84. [DOI] [PubMed] [Google Scholar]
- [53].Chirgwin K, Hafner R, Leport C, et al. Randomized phase II trial of atovaquone with pyrimethamine or sulfadiazine for treatment of toxoplasmic encephalitis in patients with acquired immunodeficiency syndrome: ACTG 237/ANRS 039 Study. AIDS Clinical Trials Group 237/Agence Nationale de Recherche sur le SIDA, Essai 039. Clinical Infect Dis. [Internet]. 2002; 34(9):1243–50. Available from: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/407/CN-00379407/frame.html. [DOI] [PubMed] [Google Scholar]
- [54].Davarpanah M, Mehrabani D, Neirami R, et al. Toxoplasmosis in HIV/AIDS patients in Shiraz, southern Iran. Iran Red Crescent Med J. 2007;2007(1):22–7. [Google Scholar]
- [55].Langmann P, Schirmer D, Zilly M, et al. Drug monitoring of pyrimethamine during maintenance therapy of toxoplasmic encephalitis in patients with advanced HIV infection during HAART. Med Sci Monit. 2004;10(5):PI65–9. [PubMed] [Google Scholar]
- [56].Podzamczer D, Miro JM, Ferrer E, et al. Thrice-weekly sulfadiazine-pyrimethamine for maintenance therapy of toxoplasmic encephalitis in HIV-infected patients. Spanish toxoplasmosis study group. Eur J Clin Microbiol Infect Dis. 2000;19(2):89–95. 10.1007/s100960050436 [DOI] [PubMed] [Google Scholar]
- [57].Vidal JE, Hernandez AV, De Oliveira ACP, et al. Cerebral toxoplasmosis in HIV-positive patients in Brazil: clinical features and predictors of treatment response in the HAART era. AIDS Patient Care St. 2005;19(10):626–34. 10.1089/apc.2005.19.626 [DOI] [PubMed] [Google Scholar]
- [58].Ragnaud J, Morlat P, Dupon M, et al. Relapse of brain toxoplasmosis in 25 AIDS patients. Med Mal Infect. 1993;23(11):791–5. 10.1016/S0399-077X(05)81283-1 [DOI] [Google Scholar]
- [59].Walckenaer G, Leport C, Longuet P, et al. Recurrence of cerebral toxoplasmosis in 15 AIDS patients. Ann Med Interne (Paris). 1994;145(3):181–4. [PubMed] [Google Scholar]
- [60].Ferrer S, Fuentes I, Domingo P, et al.. Cerebral toxoplasmosis in patients with human immunodeficiency virus (HIV) infection. Clinico-radiological and therapeutic aspects in 63 patients. Anales de medicina interna (Madrid, Spain: 1984). 1996;13(1):4–8. [PubMed] [Google Scholar]
- [61].Gonzalez-Clemente JM, Miro JM, Pedrol E, et al. Encephalic toxoplasmosis in patients with the acquired immunodeficiency syndrome. A clinico-radiological study and the therapeutic results in 78 cases. Med Clin (Barc). 1990;95(12):441–6. [PubMed] [Google Scholar]
- [62].Beraud G, Pierre-Francois S, Foltzer A, et al. Cotrimoxazole for treatment of cerebral toxoplasmosis: an observational cohort study during 1994–2006. Am J Trop Med Hyg. 2009;80(4):583–7. [PubMed] [Google Scholar]
- [63].Chaddha DS, Kalra SP, Singh AP, et al. Toxoplasmic encephalitis in acquired immunodeficiency syndrome. J Assoc Physicians India. 1999;47(7):680–4. [PubMed] [Google Scholar]
- [64].Duval X, Pajot O, Le Moing V, et al. Maintenance therapy with cotrimoxazole for toxoplasmic encephalitis in the era of highly active antiretroviral therapy. Aids. 2004;18(9):1342–4. 10.1097/00002030-200406180-00016 [DOI] [PubMed] [Google Scholar]
- [65].Smadja D, Fournerie P, Cabre P, et al. Efficacy and good tolerance of cotrimoxazole as treatment of cerebral toxoplasmosis in AIDS. Presse Med. 1998;27(26):1315–20. [PubMed] [Google Scholar]
- [66].Rothwell PM. External validity of randomised controlled trials: “to whom do the results of this trial apply?”. Lancet. 2005;365(9453):82–93. 10.1016/S0140-6736(04)17670-8 [DOI] [PubMed] [Google Scholar]
- [67].Shrier I, Boivin JF, Steele RJ, et al. Should meta-analyses of interventions include observational studies in addition to randomized controlled trials? a critical examination of underlying principles. Am J Epidemiol. 2007;166(10):1203–19. 10.1093/aje/kwm189 [DOI] [PubMed] [Google Scholar]
- [68].Cox H, Ford N. Linezolid for the treatment of complicated drug-resistant tuberculosis: a systematic review and meta-analysis. Int J Tuberc Lung Dis. 2012;16(4):447–54. 10.5588/ijtld.11.0451 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.