Abstract
In zones of violent conflict in the tropics, social disruption leads to elevated child mortality, of which malaria is the leading cause. Understanding the social determinants of malaria transmission may be helpful to optimize malaria control efforts. We conducted a cross-sectional study of healthy children aged 2 months to 5 years attending well-child and/or immunization visits in the Democratic Republic of Congo (DRC). Six hundred and forty-seven children were tested for malaria antigenemia by rapid diagnostic test and the accompanying parent or legal guardian simultaneously completed a survey questionnaire related to demographics, socioeconomic status, maternal education, as well as bednet use and recent febrile illness. We examined the associations between variables using multivariable logistic regression analysis, chi-squared statistic, Fisher’s exact test, and Spearman’s rank correlation, as appropriate. One hundred and twenty-three out of the 647 (19%) children in the study tested positive for malaria. Higher levels of maternal education were associated with a lower risk of malaria in their children. The prevalence of malaria in children of mothers with no education, primary school, and beyond primary was 41/138 (30%), 41/241 (17%), and 39/262 (15%), respectively (p = 0.001). In a multivariable logistic regression model adjusting for the effect of a child’s age and study site, the following remained significant predictors of malaria antigenemia: maternal education, number of children under five per household, and HIV serostatus. Higher maternal education, through several putative causal pathways, was associated with lower malaria prevalence among children in the DRC. Our findings suggest that maternal education might be an effective ‘social vaccine’ against malaria in the DRC and globally.
Keywords: Democratic Republic of Congo, childhood malaria, social determinants, maternal education, prevention, social vaccine
Introduction
Malaria is a vector-borne parasitic infection caused by the genus Plasmodium, which is transmitted through the bite of female Anopheles mosquitoes. In 2015, an estimated 249–303 million cases of malaria occurred globally, with an estimated 236–635 thousand deaths [1]. Eighty-eight percent of malaria cases and 90% of malaria-associated deaths occurred in Africa [1].
Social determinants of health are factors, such as gender, age, living conditions, socioeconomic status, and education, that influence health and risk of disease [2,3]. Unequal access to healthcare, education, sanitation, and adequate shelter all have an impact on the health disparities that impact disadvantaged populations [4]. Household wealth and maternal education are well-known examples of social factors that exert a profound ‘upstream’ influence on childhood mortality [5−8].
Malaria transmission is influenced by various social factors. Malaria and poverty are mutually amplifying, with a strong ecologic association [9]. The effect of malaria-related morbidity and mortality on the poor is disproportionately greater than that of the general population due to limited health care access [10]. Higher household wealth may be protective against malaria [11]. Sub-optimal living conditions may affect the risk of malaria infection due to increased vector exposure, reduced ability to use vector barrier methods (e.g. difficulty hanging bednet) and/or exposure to vector breeding sites [12,13]. Average household size is associated with malaria prevalence as the risk of transmission may increase when the number of people sleeping together in the same room increases [14]. Recent evidence suggests that a mother’s educational attainment and social network may be protective against malaria [7]. However, whether the protective effect of maternal education on childhood malaria persists in settings of violent conflict and social disruption has not been previously investigated, to our knowledge.
Civil and international conflict in the Democratic Republic of Congo (DRC) over the past two decades has decimated health care and civil infrastructure, with direct consequences on child mortality, malaria incidence, and education [15,16]. The DRC is ranked among the least operationally and technically feasible countries for malaria elimination due to the intensity of malaria transmission, armed conflict, and political instability [17]. Social upheaval has led to an epidemic of gender-based violence, large-scale population displacement, and disruption of education for girls and women [18,19]. In this context, we investigated determinants of childhood malaria infection, with particular attention to maternal education and putative causal pathways by which maternal education may modulate malaria risk. We hypothesized that higher maternal education would be associated with a lower childhood malaria prevalence in a resource-limited setting with social disruption due to violent conflict.
Methods
We conducted a cross-sectional observational study of afebrile, healthy children aged 2 months to 5 years attending well-child and/or immunization visits in the North Kivu province in eastern DRC from August to October 2010 to investigate the relationship between social determinants of health and malaria prevalence. All children who presented to the well-child and/or immunization clinics were eligible for the study, including those who tested positive for HIV. There were no exclusion criteria.
Study area and population
This study was conducted in three semi-urban cities of North Kivu, DRC: Butembo, Beni, and Goma. The estimated population of Butembo, Beni, and Goma are ~600 thousand, ~200 thousand, and 1 million, respectively. The population is primarily of Nande ethnicity, and the main languages spoken are Nande, Swahili and French. The DRC suffers from decimated civil and health care infrastructure, as well as social instability, after many years of civil and international conflict [15,16,20]. High mortality rates in the DRC can be attributed to limited infrastructure, poverty, violence, and especially, infectious diseases [15,16,19]. Malaria is the leading cause of death in this area and in children under 5 [15,16]. Although malaria incidence and prevalence in the DRC are not precisely known due to a lack of quality data, 97% of the population resides in high transmission regions [1]. The estimated number of cases and deaths caused by malaria in 2013 are estimated to be 26 million and 72 thousand, respectively [1]. Access to bednets is generally available free of charge through antenatal clinics, which are a part of the country’s national health care program. Bednets are also available for purchase at the local markets. There were no mass bednet distribution programs in the areas around the time of our study.
A standard sample size calculation indicated that 627 children would be needed to show an absolute difference of 14% in malaria prevalence in children of mothers with education beyond primary level vs. those with primary level or no education, with 80% power at a level of significance α = 0.05. Previous studies in other countries noted a difference in malaria prevalence of 14% in children of mothers with primary or no education (30%) versus education beyond primary (16%) [7].
Ethical approval and consent
Ethics approval for the study was obtained from the Comité d’Éthique du Nord Kivu (Université Catholique du Graben), the University of Alberta Human Research Ethics Board, and regionally from the Médecin Chef de Zone, within the DRC Ministry of Health. A parent or legal guardian of each participating child provided written informed consent.
Study procedures
For each child, the accompanying parent or legal guardian completed a survey questionnaire including questions on demographics, household assets (motor vehicle, electricity, radio, television, refrigerator), household construction (brick and mortar versus thatch), household composition (number of people living in the house and number of children under 5), maternal education (no formal education, primary, beyond primary), household bednet ownership, and use of a bednet by the child the night prior to the survey, mobile phone ownership, recent (within past month) febrile illness, and quality of medical care accessed (diagnosis by a health care provider, blood test performed, treatment administered). A malaria rapid diagnostic test (RDT, ParaCheck-Pf ® kit, Orchid Biomedical Systems, Goa, India) and HIV test (Alere Determine ™, Orgenics, Ltd. Yavne, Israel) were performed on the child from a fingerpick. The RDT detects P. falciparum histidine-rich protein 2 (HRP2) antigens in the peripheral circulation and has a sensitivity of 98.9% and a specificity of 84.9% in field studies [21]. Study personnel were trained to safely and accurately use the RDTs with proficiency testing, as previously described [22]. Children with a positive RDT result were treated with artemisinin combination therapy (ACT) according to World Health Organization guidelines; all were asymptomatic or minimally symptomatic. HIV positive children were referred for care to the local mother–child HIV treatment program.
Statistical analysis
Our primary analysis focused on factors associated with malaria infection, using bivariate statistics and multivariable logistic regression analyses. To examine associations between dichotomous variables, we used chi-squared statistic or Fisher’s exact test, as appropriate. To examine associations between variables which were continuous or ordinal, we used Spearman’s rank correlation (ρ). Based on work by Njau et al. [7], we developed a conceptual framework (Supplemental Figure 1) linking maternal education to malaria infection in the child. Concepts in this framework and their proxy variables are defined in Table 1. We used a linear asset index, modified from Filmer and Pritchett [23], from a set of six household asset indicators. We used principal component analysis (PCA) to derive the weights for each asset, which allowed us to divide our participants into wealth quintiles. Previously, a similar asset index was validated, demonstrating close correspondence with state-level poverty in India, as well as correlation with household consumption and expenditures [23]. Missing data were excluded from statistical analysis. Statistics were computed using SPSS® version 23 (IBM®).
Table 1.
Variables of interest.
| Category | Variable (parallel concept in Njau et al. [7]) | Variable description |
|---|---|---|
| Outcome variable | Childhood malaria infection | Detection of HRP2 antigen in the blood of the child by RDT |
| Independent variable | Primary variable in putative causal pathway: | |
| Level of maternal education | Highest level of formal education completed by the mother of the child. The level of maternal education was divided into three categories: (1) no formal education; (2) only primary school; (3) any education beyond primary school | |
| Intermediate variables in putative causal pathways: | ||
| Uptake of malaria prevention and treatment strategies (child health knowledge) Assessed by two proxy variables: |
(1) Reported bednet (a) ownership and (b) use, defined as the child sleeping under a bednet the night before the survey (2) Access to quality medical care if the child had a febrile illness in the past month, which was determined by asking the mother if (a) there was a diagnosis by a health care provider, (b) a blood test was performed and (c) treatment was administered |
|
| Household wealth (economic empowerment) | A linear ‘asset index’ modified from [23], based on 6 household characteristics or assets. Principal component analysis was used to derive the weights for the index, which was used to divide households into wealth quintiles [23] | |
| Family formation pattern | Assessed by two proxy variables: (1) Household size, defined as the total number of people living in the house (2) Total number of children less than 5 years old per household |
|
| Social networking | The proxy variable used to assess a mother’s social network was mobile phone ownership | |
| HIV serostatus | Detection of the presence or absence of HIV antibodies in the blood of the child by RDT | |
| Covariates | Age | Due to the known age dependence of malaria prevalence, it was important to adjust for age in our analyses |
| Study site | Study was done at one of three sites (Butembo, Beni and Goma) |
Results
We enrolled 647 healthy children attending clinics for well-child visits and immunizations at three health facilities across the North Kivu province of the DRC (Butembo, Beni and Goma). A flow diagram is included in Supplemental Figure 2 to outline the study population at each stage of the study. The point prevalence of P. falciparum infection, in this asymptomatic study population was 123/647 (19%).
Household assets were characteristic of a setting with severe resource limitations: only 49/646 (7.6%), 93/645 (14%), 104/646 (16%) 154/644 (24%), 169/646 (26%), and 433/647 (67%) owned a refrigerator, car, house built from high-quality material, television, electricity, and radio, respectively. Regarding bednets, 245/645 (38%) participants reported owning a bednet, and 169/643 (26%) children slept under the bednet the previous night. According to maternal report, 276/578 (48%) of children had experienced a febrile illness in the past month, including 87 cases of malaria. Of the 87 self-reported cases of malaria, 73/87 (84%), 54/87 (62%), and 70/87 (81%) had received a diagnosis of malaria by a health care professional, a blood test performed, and treatment administered, respectively.
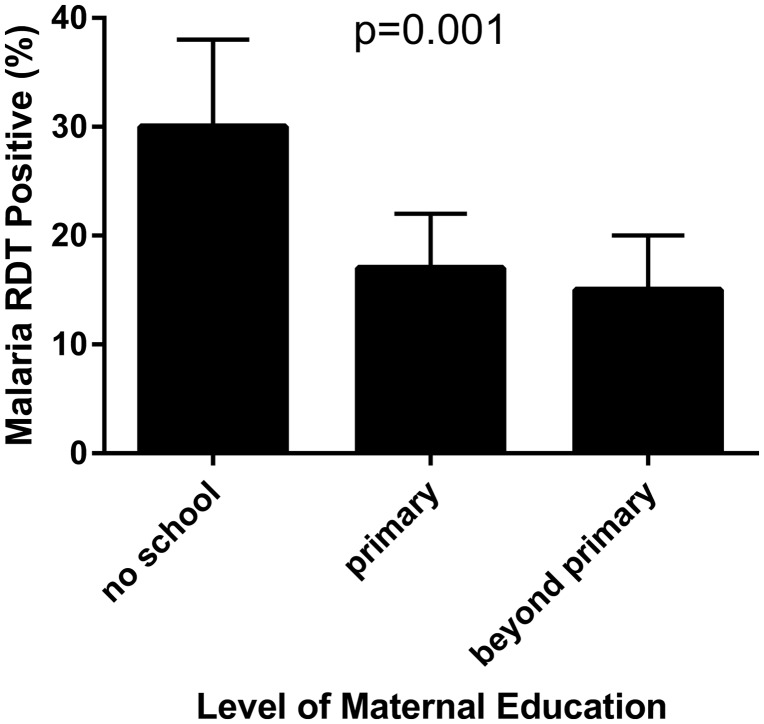
Higher levels of maternal education were associated with lower point prevalence of malaria in their children (Figure 1). Associations between malaria infection and maternal education, the number of children less than 5 years old per household, and HIV serostatus were all significant after multivariable logistic regression analysis accounting for possible confounding among co-variates and adjusting for the effect of child’s age and study site (Table 2). In a subgroup analysis of children in the poorest wealth quintile, the relationship between maternal education and malaria infection in children remained: 25/84 (30%), 7/54 (13%), 3/23 (13%) malaria prevalence among children of mothers with no, primary, and secondary or greater education, respectively (p = 0.014). To exclude possible persistence of HRP2 antigen following recently treated malaria episode as a cause of RDT positivity [24,25], we examined the subgroup of children who did not have a febrile illness in the past month and found that the association between maternal education and malaria remained significant (ρ = 0.13, p = 0.028).
Figure 1.
Impact of maternal education on childhood malaria. Data are based on a cross-sectional study population of 647 children attending vaccine clinics in the DRC tested for malaria using a RDT. Higher maternal education was associated with lower malaria prevalence.
Table 2.
Characteristics of research participants, according to malaria antigenemia.
| Entire study population N (%) | Children with malaria n (%) | Children without malaria n (%) | Odds ratio (OR) (95% CI) | OR adjusted (95% CI) | p-value* | |
|---|---|---|---|---|---|---|
| Study site | ||||||
| Butembo | 397 (61) | 86 (21) | 311 (79) | 1.0 | 1.0 | |
| Beni | 82 (13) | 19 (23) | 63 (77) | 1.1 (0.62–1.9) | 1.3 (0.66–2.4) | 0.47 |
| Goma | 168 (26) | 18 (11) | 150 (89) | 0.43 (0.25–0.74) | 0.45 (0.24–0.84) | 0.012 |
| Age (year) | ||||||
| <1 | 9 (1.4) | 1 (11) | 8 (89) | 1.1 (0.93–1.3) | 1.2 (0.96–1.4) | 0.13 |
| 1 | 142(22) | 14 (10) | 128 (90) | |||
| 2 | 183(28) | 42 (23) | 141 (77) | |||
| 3 | 161(25) | 35 (22) | 126 (78) | |||
| 4 | 121(19) | 28 (23) | 93 (77) | |||
| 5 | 30(4.6) | 3 (10) | 27 (90) | |||
| Household size | ||||||
| 1 to 5 | 253(39) | 40 (16) | 213 (84) | 1.1 (0.98–1.1) | 1.0 (0.96–1.1) | 0.28 |
| 6 to 10 | 348(54) | 73 (21) | 275 (79) | |||
| >10 | 41(6.4) | 8 (20) | 33 (80) | |||
| Children <5 years old per household | ||||||
| 1 | 236(37) | 31 (13) | 205 | 1.3 (1.0–1.7) | 1.4 (1.0–1.9) | 0.026 |
| 2 | 326(51) | 75 (23) | 251 (77) | |||
| 3 | 62(9.8) | 13 (21) | 49 (79) | |||
| 4 | 10(1.6) | 1 (10) | 9 (90) | |||
| 7 | 1 (0.16) | 0 (0) | 1 (100) | |||
| Wealth quintile | ||||||
| 1st (poorest) | 161(25) | 35 (22) | 126 (78) | 1.0 | 1.2 (0.94–1.5) | 0.14 |
| 2nd | 211(33) | 40 (19) | 171 (81) | 0.82 (0.51–1.4) | ||
| 3rd | 18(2.8) | 4 (22) | 14 (78) | 1.0 (0.32–3.3) | ||
| 4th | 126(20) | 18 (14) | 108 (86) | 0.60 (0.32–1.1) | ||
| 5th (wealthiest) | 128(20) | 25 (20) | 103 (80) | 0.87 (0.49–1.6) | ||
| Maternal education | ||||||
| No formal education | 138(22) | 41 (30) | 97 (70) | 1.0 | 1.0 | |
| Primary | 241(38) | 41 (17) | 200 (83) | 0.49 (0.30–0.80) | 0.46 (0.27–0.80) | 0.005 |
| Beyond primary | 262(41) | 39 (15) | 223(85) | 0.41 (0.25–0.68) | 0.41(0.22–0.77) | 0.005 |
| Bednet | ||||||
| Do not use | 473(74) | 95 (20) | 378 (80) | 1.0 | 0.87 (0.52–1.4) | 0.58 |
| Use | 169(26) | 27 (16) | 142 (84) | 0.76 (0.47–1.2) | ||
| Mobile phone ownership | ||||||
| Does not own | 319 (49) | 71 (58) | 248 (47) | 1.0 | 0.68 (0.34–1.3) | 0.26 |
| Own | 328 (51) | 52 (42) | 276 (53) | 0.66 (0.44–0.98) | ||
| HIV serostatus | ||||||
| Positive | 8(1.2) | 4 (50) | 4 (50) | 4.3 (1.0–17) | 5.1 (1.1–24) | 0.039 |
| Negative | 636(99) | 121 (19) | 515 (81) | 1.0 | ||
p-value refers to statistical significance in the multivariable model
The number of children with missing data for each variable can be found in Supplemental Table 1.
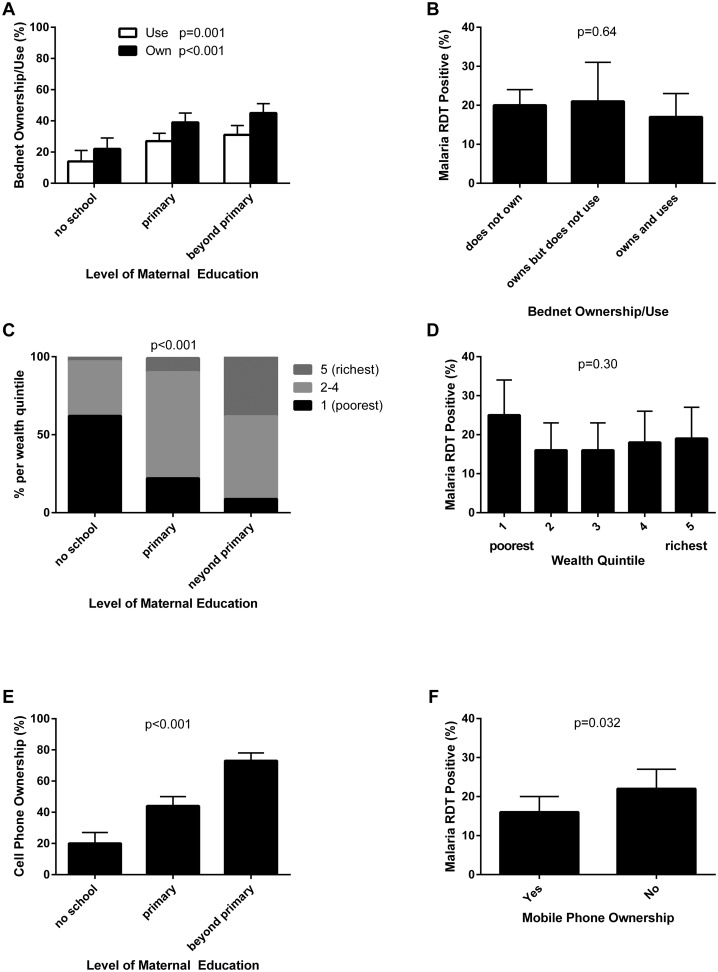
Variables in putative causal pathways linking maternal education to malaria infection in children were examined [7]. Statistically significant associations between maternal education and bednet ownership and use, household wealth, and social networking were found (Figure 2 and Table 3). Additionally, a statistically significant association between malaria infection and family formation patterns, social networking, and HIV serostatus (Table 3).
Figure 2.
Correlations between variables in putative causal pathways linking maternal education to childhood malaria infection. (A) Bednet ownership/use was positively associated with maternal education. (B) Malaria antigenemia and bednet ownership/use. (C) Household wealth and maternal education were positively correlated. (D) Malaria antigenemia and household wealth. (E) Social networking (proxy: mobile phone ownership) was positively associated with maternal education. (F) Malaria antigenemia and mobile phone ownership were inversely correlated.
Table 3.
Correlations between variables and putative causal pathways linking maternal education to childhood malaria infection.
| Maternal Education | Malaria Infection | |
|---|---|---|
| Putative Causal Pathways | ρ | ρ |
| Uptake of malaria prevention and treatment strategies | ||
| (a) Bednet ownership/use | ||
| (i) Ownership | 0.17*** | –0.18 |
| (ii) Use | 0.14** | –0.046 |
| (b) Access to quality medical care | ||
| (i) Diagnosis by healthcare professional | 0.026 | –0.13 |
| (ii) Blood test performed | –0.042 | –0.19 |
| (iii) Treatment given | 0.080 | 0.071 |
| Household wealth | 0.48*** | –0.041 |
| Family formation patterns | ||
| (a) Household size | 0.027 | 0.064 |
| (b) Number of children < 5 years old per household | 0.038 | 0.11* |
| Social networking | 0.41*** | –0.084* |
| HIV serostatus | –0.045 | 0.089* |
p-value < 0.05
p-value = 0.001
p-value < 0.001.
Discussion
In this cross-sectional study of 647 children in a sub-Saharan African context characterized by ongoing conflict and insecurity, higher maternal education appeared to be a protective social determinant of childhood malaria. Noteworthy aspects of this study include the highly statistically significant association between maternal education and malaria, which was robust after the adjustments of co-variates in a multivariable statistical model, and which had plausibility, based on multiple significant associations between variables in previously described putative causal pathways. Our findings are noteworthy, demonstrating that maternal education remains a strong determinant of child health even under conditions of social disruption due to violent conflict.
Maternal education is a well-known social determinant of numerous childhood health outcomes. Both general educational attainment and a mother’s specific health-related knowledge affect the risk of malaria in her child [7,26]. In high- and low-income countries, higher general maternal education has been linked to lower infant and child mortality [27−30], improved nutritional status [31], and reduced rates of injuries, respiratory conditions, hospital admission, and infection [32−35]. Specific infections that have been linked to maternal education include: diarrhea and pneumonia [32], chronic suppurative otitis media [33], Helicobacter pylori [33,35], and malaria [7]. A cross-sectional study of over 10,000 households in Tanzania, Uganda, and Angola investigating the relationship between maternal education and childhood malaria infection demonstrated that there is a negative relationship between maternal education and the risk of childhood malaria infection [7]. Our study confirms and extends these findings to another sub-Saharan African context, characterized by social disruption due to violent conflict. Additionally, a considerable proportion of the relationship between maternal education and malaria was independent of the mother’s income and assets in a previous report [27]. Consistent with this finding, maternal education was an independent predictor of malaria in a multi-variable model adjusting for household wealth in our study. Maternal education also influenced childhood malaria within each wealth stratum, including the poorest subgroup.
In addition to maternal education, we identified multiple other independent predictors of childhood malaria (Table 3). In order to integrate these co-variates into a cohesive structure, we adapted a conceptual framework first proposed by Njau et al. [7], linking maternal education to childhood malaria though several putative causal pathways (Supplemental Figure 1). Mothers with higher education: (1) may be more likely to access malaria prevention measures (e.g. bednets) and quality treatment strategies; (2) may be wealthier and better able to provide for health needs of their children; (3) may have different family formation patterns (e.g. longer birth interval and fewer children); (4) may have improved social networks to access information and health services; and (5) may have lower HIV prevalence and lower HIV vertical transmission rates to their children. One or more of these factors may influence the risk of childhood malaria. We quantified the association between maternal education and proxy variables for each putative causal pathway, as well as the association between these intermediate variables and malaria positivity in the child (Table 3).
Educated mothers are more likely to participate in behaviors that promote health such as vaccination [28,36−38]. Likewise, malaria prevention using bednets was more common among educated mothers in previous studies in Tanzania and Kenya [7,39,40]. In our study, higher maternal education was associated with bednet ownership and use (Figure 2(A)). We observed an inverse relationship between bednet use and malaria infection (Table 3), although this did not reach statistical significance. However, a previous meta-analysis of randomized trials has clearly demonstrated that long-lasting insecticidal bednets significantly reduce malaria-related morbidity and mortality [41]. Our findings support a putative causal pathway whereby maternal education is associated with bednet ownership and use, which in turn is protective against malaria in their children.
We also explored whether mothers with different levels of education attainment accessed medical care for their child for recent febrile illness, and the quality of the care they received. We did not find evidence that mothers with higher education sought better quality care, as indicated by: (1) diagnosis by a healthcare professional; (2) blood test performed; and (3) treatment administered.
On a global macroeconomic scale, there is a strong association between poverty and malaria burden [9,42]. However, at the household level, studies examining the association between malaria incidence and socioeconomic status have not demonstrated a consistent relationship [42]. Several studies found no relationship between socioeconomic status and malaria incidence [42]. On the other hand, another study found that ‘economic empowerment,’ which described household wealth based on asset ownership, was the single most important factor in reducing childhood malaria prevalence [7]. We found a strong association between maternal education and household wealth (Table 3), although household wealth was not statistically significantly associated with childhood malaria, consistent with some previous reports [42].
Historically, malaria eradication in many countries was strongly associated with a reduction in average household size below four persons [14]. A previous study showed a significant relationship between family formation patterns (including household size and number of children under five), maternal education, and malaria infection [7]. In our study, a high number of children less than five per household was associated with higher malaria prevalence. We speculate, as have previous authors [7], that families with a greater number of children may be less able than smaller families to provide bednets and other prevention and treatment measures for all the children. Alternatively, households with a higher density of susceptible hosts may be more conducive to malaria transmission.
As a woman expands her social network, she is more likely to use utilize healthcare services [27]. Social networking has been previously described as an important mechanism through which maternal education decreases the risk of malaria infection in children [7]. We also observed significant relationships between maternal education and social networking using mobile phone ownership as a proxy variable, as in another study [7]. In addition, social networking was associated with lower malaria prevalence in our study. These data support a putative causal pathway whereby maternal education is associated with better social networks, and lower malaria prevalence in their children.
HIV seropositivity was a risk factor for malaria in our study, as previously shown [43]. A woman’s education level may influence her risk of HIV infection [44], as a precursor to vertical transmission. We did not observe a statistically significant association between maternal education and the child’s HIV status in our study, although the number of HIV-infected children was small.
Our study is subject to several limitations. We used data from a cross-sectional observational study, which allowed us to explore associations, but does not definitively establish a causal relationship between variables. We studied children attending well-child or immunization clinics in three cities in the province of North Kivu, DRC, which may not be fully representative of the country or region. Nonetheless, the immunization rates in the DRC are between 70 and 86% [45] and the prevalence of malaria in our study population was similar to that in the most recent Demographic and Health Survey in the DRC [46], suggesting that the children visiting well-child or immunization clinics may provide a fair sample of Congolese children. Our findings should be extrapolated with caution to large urban or remote rural settings, which may differ from the cities in this study. We used mobile phone ownership as a proxy for social networking as in previous studies [7], although this is an imperfect proxy and may also reflect household wealth or maternal access to household wealth. Our household asset index differs in some details from that of Filmer and Pritchett, incorporating fewer variables into our principal component analysis [23]. Finally, limited sample size may have reduced the statistical power to identify some associations (e.g. maternal education and childhood HIV seroprevalence).
Conclusion
Our study demonstrated that maternal education was strongly associated with childhood malaria prevalence in a resource-limited tropical setting characterized by social disruption due to violent conflict. Evidence from this study and others supports the plausibility of a causal relationship between maternal education and malaria through bednet use, higher household wealth, greater social networking, family structure, and possibly lower HIV rates. If indeed maternal education is a determinant of malaria infection in her child, this suggests that education of girls and women may represent a ‘social vaccine’ against one of the leading infectious causes of global childhood mortality [47]. In our study, maternal education beyond primary school, relative to no education, was associated with a reduction in malaria prevalence from 30 to 14%, corresponding to a relative reduction of 53%. This effect size is of similar magnitude to that of the leading biomedical malaria vaccine, RTS,S/AS01E, which had a protective efficacy of 26–30% in recent phase three clinical trials [48]. The role of maternal education is indeed strong enough that even major social disruption as seen in the DRC did not abrogate its protective effect. Given the broad impact of maternal education on a host of health outcomes in their children, including malaria, investment in the education of girls and women is likely to yield substantial returns in terms of child survival in challenging environments like the DRC and globally [49,50].
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
The work was supported by the Association for Health Innovation in Africa (AFHIA). The funder had no role in the design of the study, in the collection, analysis, and interpretation of data, or in writing the manuscript.
Supplemental data
The supplemental material for this paper is available online at http://dx.doi.org/10.1080/20477724.2017.1288971
Supplementary Material
Acknowledgments
We would like to thank Dr. Stan Houston and Rhianna Charchuk from the University of Alberta for critically revising this paper.
References
- [1].World Health Organization World malaria report. Geneva, Switzerland: World Health Organization; 2015. 2015. Report No. [Google Scholar]
- [2].Roberts B, Odong VN, Browne J, et al. . An exploration of social determinants of health amongst internally displaced persons in northern Uganda. Confl Health. 2009;3:10 10.1186/1752-1505-3-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Braveman P. Accumulating knowledge on the social determinants of health and infectious disease. Public Health Rep. 2011;126(Suppl 3):28–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Marmot M, Friel S, Bell R, et al. . Closing the gap in a generation: health equity through action on the social determinants of health. Lancet. 2008;372(9650):1661–1669. 10.1016/S0140-6736(08)61690-6 [DOI] [PubMed] [Google Scholar]
- [5].Caldwell J, McDonald P. Influence of maternal education on infant and child mortality: levels and causes. Health Policy Educ. 1982;2(3–4):251–267. 10.1016/0165-2281(82)90012-1 [DOI] [PubMed] [Google Scholar]
- [6].Quansah E, Ohene LA, Norman L, et al. . Social factors influencing child health in Ghana. PLos One. 2016;11(1):e0145401 10.1371/journal.pone.0145401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Njau JD, Stephenson R, Menon MP, et al. . Investigating the important correlates of maternal education and childhood malaria infections. Am J Trop Med Hyg. 2014;91(3):509–519. 10.4269/ajtmh.13-0713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Williams DR, Costa MV, Odunlami AO, et al. . Moving upstream: how interventions that address the social determinants of health can improve health and reduce disparities. J Public Health Manag Pract. 2008;14(Suppl):S8–S17. 10.1097/01.PHH.0000338382.36695.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sachs J, Malaney P. The economic and social burden of malaria. Nature. 2002;415(6872):680–685. 10.1038/415680a [DOI] [PubMed] [Google Scholar]
- [10].Suh KN, Kain KC, Keystone JS. Malaria. CMAJ. 2004;170(11):1693–1702. 10.1503/cmaj.1030418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Graves PM, Richards FO, Ngondi J, et al. . Individual, household and environmental risk factors for malaria infection in Amhara, Oromia and SNNP regions of Ethiopia. Trans R Soc Trop Med Hyg. 2009;103(12):1211–1220. 10.1016/j.trstmh.2008.11.016 [DOI] [PubMed] [Google Scholar]
- [12].Bates I, Fenton C, Gruber J, et al. . Vulnerability to malaria, tuberculosis, and HIV/AIDS infection and disease. Part 1: determinants operating at individual and household level. Lancet Infect Dis. 2004;4(5):267–277. 10.1016/S1473-3099(04)01002-3 [DOI] [PubMed] [Google Scholar]
- [13].Bizimana JP, Twarabamenye E, Kienberger S. Assessing the social vulnerability to malaria in Rwanda. Malar J. 2015;14:2 10.1186/1475-2875-14-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Huldén L, McKitrick R, Huldén L. Average household size and the eradication of malaria. J R Stat Soc Stat. 2014;177(3):725–742. 10.1111/rssa.12036 [DOI] [Google Scholar]
- [15].Coghlan B, Brennan RJ, Ngoy P. Mortality in the Democratic Republic of Congo: a nationwide survey (vol 367, pg 44, 2006). Lancet. 2006;367(9510):568. [DOI] [PubMed] [Google Scholar]
- [16].Coghlan B, Ngoy P, Mulumba F, et al. . Update on mortality in the Democratic Republic of Congo: results from a third nationwide survey. Disaster Med Public. 2009;3(02):88–96. 10.1097/DMP.0b013e3181a6e952 [DOI] [PubMed] [Google Scholar]
- [17].Tatem AJ, Smith DL, Gething PW, et al. . Ranking of elimination feasibility between malaria-endemic countries. Lancet. 2010;376(9752):1579–1591. 10.1016/S0140-6736(10)61301-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Malemo Kalisya L, Lussy Justin P, Kimona C, et al. . Sexual violence toward children and youth in war-torn Eastern Democratic Republic of Congo. PLos One. 2011;6(1):e15911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Carrion Martın A, Bil K, Salumu P, et al. . Mortality rates above emergency threshold in population affected by conflict in North Kivu, Democratic Republic of Congo, July 2012–April 2013. PLoS Negl Trop Dis. 2014;8(9): e3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Central Intelligence agency. The world Factbook: Democratic Republic of the Congo; 2015; [cited 2016 Aug]. Available from: https://www.cia.gov/library/publications/the-world-factbook/geos/cg.html.
- [21].Brooker S, Kolaczinski JH, Gitonga CW, et al. . The use of schools for malaria surveillance and programme evaluation in Africa. Malar J. 2009;8:231. 10.1186/1475-2875-8-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hawkes M, Katsuva JP, Masumbuko CK. Use and limitations of malaria rapid diagnostic testing by community health workers in war-torn Democratic Republic of Congo. Malaria J. 2009;8:308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Filmer D, Pritchett LH. Estimating wealth effects without expenditure data–or tears: an application to educational enrollments in states of India. Demography. 2001;38(1):115–132. [DOI] [PubMed] [Google Scholar]
- [24].Humar A, Ohrt C, Harrington MA, et al. . Parasight F test compared with the polymerase chain reaction and microscopy for the diagnosis of Plasmodium falciparum malaria in travelers. Am J Trop Med Hyg. 1997;56(1):44–48. [DOI] [PubMed] [Google Scholar]
- [25].Kyabayinze DJ, Tibenderana JK, Odong GW, et al. . Operational accuracy and comparative persistent antigenicity of HRP2 rapid diagnostic tests for Plasmodium falciparum malaria in a hyperendemic region of Uganda. Malar J. 2008;7:221 10.1186/1475-2875-7-221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Chirdan OO, Zoakah AI, Ejembi CL. Impact of health education on home treatment and prevention of malaria in Jengre, North Central Nigeria. Ann Afr Med. 2008;7(3):112–119. [DOI] [PubMed] [Google Scholar]
- [27].Cleland JG, van Ginneken JK. Maternal education and child survival in developing countries: the search for pathways of influence. Soc Sci Med. 1988;27(12):1357–1368. 10.1016/0277-9536(88)90201-8 [DOI] [PubMed] [Google Scholar]
- [28].Desai S, Alva S. Maternal education and child health: is there a strong causal relationship? Demography. 1998;35(1):71–81. 10.2307/3004028 [DOI] [PubMed] [Google Scholar]
- [29].Gage TB, Fang F, O’Neill E, et al. . Maternal education, birth weight, and infant mortality in the United States. Demography. 2013;50(2):615–635. 10.1007/s13524-012-0148-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shoham-Yakubovich I, Barell V. Maternal education as a modifier of the association between low birthweight and infant mortality. Int J Epidemiol. 1988;17(2):370–377. 10.1093/ije/17.2.370 [DOI] [PubMed] [Google Scholar]
- [31].Abuya BA, Ciera J, Kimani-Murage E. Effect of mother’s education on child’s nutritional status in the slums of Nairobi. BMC Pediatr. 2012;12:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Victoria CG, Huttly SRA, Barros FC, et al. . Maternal education in relation to early and late child health outcomes: findings from a Brazilian cohort study. Soc Sci Med. 1992;34(8):899–905. 10.1016/0277-9536(92)90258-R [DOI] [PubMed] [Google Scholar]
- [33].Shaheen MM, Raquib A, Ahmad SM. Prevalence and associated socio-demographic factors of chronic suppurative otitis media among rural primary school children of Bangladesh. Int J Pediatr Otorhi. 2012;76(8):1201–1204. 10.1016/j.ijporl.2012.05.006 [DOI] [PubMed] [Google Scholar]
- [34].Davey TM, Cameron CM, Ng SK, et al. . The relationship between maternal education and child health outcomes in urban Australian children in the first 12 months of life. Matern Child Health J. 2015;19(11):2501–2511. 10.1007/s10995-015-1771-5 [DOI] [PubMed] [Google Scholar]
- [35].Bures J, Kopacova M, Koupil I, et al. . Epidemiology of helicobacter pylori infection in the Czech Republic. Helicobacter. 2006;11(1):56–65. 10.1111/hel.2006.11.issue-1 [DOI] [PubMed] [Google Scholar]
- [36].Abuya BA, Onsomu EO, Kimani JK, et al. . Influence of maternal education on child immunization and stunting in Kenya. Matern Child Health J. 2011;15(8):1389–1399. 10.1007/s10995-010-0670-z [DOI] [PubMed] [Google Scholar]
- [37].Onsomu EO, Abuya BA, Okech IN, et al. . Maternal education and immunization status among children in Kenya. Matern Child Hlth J. 2015;19(8):1724–1733. 10.1007/s10995-015-1686-1 [DOI] [PubMed] [Google Scholar]
- [38].Racine AD, Joyce TJ. Maternal education, child immunizations, and public policy: evidence from the US National Immunization Survey. Soc Sci Med. 2007;65(8):1765–1772. 10.1016/j.socscimed.2007.06.004 [DOI] [PubMed] [Google Scholar]
- [39].Mazigo HD, Obasy E, Mauka W, et al. . Knowledge, attitudes, and practices about malaria and its control in rural Northwest Tanzania. Malar Res Treat. 2010;2010:794261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Noor AM, Omumbo JA, Amin AA, et al. . Wealth, mother’s education and physical access as determinants of retail sector net use in rural Kenya. Malar J. 2006;5:5 10.1186/1475-2875-5-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst Rev. 2004(2):CD000363. [DOI] [PubMed] [Google Scholar]
- [42].Worrall E, Basu S, Hanson K. Is malaria a disease of poverty? a review of the literature. Trop Med Int Health. 2005;10(10):1047–1059. 10.1111/tmi.2005.10.issue-10 [DOI] [PubMed] [Google Scholar]
- [43].Abu-Raddad LJ, Patnaik P, Kublin JG. Dual infection with HIV and malaria fuels the spread of both diseases in sub-Saharan Africa. Science. 2006;314(5805):1603–1606. 10.1126/science.1132338 [DOI] [PubMed] [Google Scholar]
- [44].Hargreaves JR, Bonell CP, Boler T, et al. . Systematic review exploring time trends in the association between educational attainment and risk of HIV infection in sub-Saharan Africa. AIDS. 2008;22(3):403–414. [DOI] [PubMed] [Google Scholar]
- [45].UNICEF Democratic Republic of the Congo: the United Nations Children’s Fund; 2013. [updated 2013; cited 2016 Jun 9].
- [46].Ministère du Plan et Suivi de la Mise en oeuvre de la Révolution de la Modernité (MPSMRM), Ministère de la Santé Publique (MSP) and ICF International Democratic Republic of Congo Demographic and Health Survey 2013–14: key findings. Rockville (MA): MPSMRM, MSP et ICF International; 2014. [Google Scholar]
- [47].Baum F, Narayan R, Sanders D, et al. . Social vaccines to resist and change unhealthy social and economic structures: a useful metaphor for health promotion. Health Promot Int. 2009;24(4):428–433. 10.1093/heapro/dap026 [DOI] [PubMed] [Google Scholar]
- [48].Rts SCTP, Agnandji ST, Lell B, et al. . A phase 3 trial of RTS, S/AS01 malaria vaccine in African infants. N Engl J Med. 2012;367(24):2284–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].UNICEF Strategies for girls’ education. New York (NY): The United Nations Children’s Fund; 2004. [Google Scholar]
- [50].Odhiambo G. Higher education in Kenya: an assessment of current responses to the imperative of widening access. J High Educ Policy M. 2016;38(2):196–211. 10.1080/1360080X.2016.1150551 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.