Abstract
Background: Visceral leishmaniasis (VL) is an emerging zoonosis, and Brazil harbors about 90% of those infected in Latin America. Since 1998, the disease has been spreading quickly in São Paulo state, and the western region is considered an emerging focus of VL in Brazil. Our aim was to evaluate the clinical characteristics and spatial distribution of VL in children referred to a public tertiary hospital located in the western region of São Paulo state, Brazil.
Methods: Medical records of children up to 18 years of age who were diagnosed with VL between January 2006 and December 2010 were reviewed. Geospatial analysis was performed using the ArcGIS 10.2 platform.
Results: Sixty-three patients were enrolled in the study; the median age was 3.3 ± 3.3 years. The median time interval between the onset of clinical symptoms and diagnosis was 16.1 ± 11.1 days, and the median time in the pediatric ward was 18.0 ± 9.4 days. Liposomal amphotericin B was the first-line treatment in 90.5% of the patients and 9.6% relapsed. One patient died (1.6%), and 19% were submitted to the pediatric intensive care unit.
Conclusion: The short interval between the onset of symptoms, diagnosis, and treatment and the reduced number of days of hospitalization certainly influenced the small number of deaths, relapses, and severity among the children infected with VL. However, the disease is spreading fast in the western region of São Paulo state. Thus, integrated actions and effective monitoring of the disease are needed to complement curative practices.
Keywords: Visceral leishmaniasis, clinical symptoms, diagnosis, treatment
Introduction
Visceral leishmaniasis (VL) is an emergent zoonosis of great significance for public health. It occurs especially in sub-tropical and tropical countries. Brazil harbors about 90% of those infected with VL in Latin America, and the disease is found in all regions of the country [1,2]. First, VL was restricted to rural areas of the northeastern region of Brazil, responsible for 70 to 90% of cases, but since the 1980s, important outbreaks in large Brazilian cities have been documents. This new scenario represents a challenge for VL control [3–5]. The state of São Paulo, located in the southeastern region, is the most populous and economically developed of the 26 states in Brazil. Two decades ago, VL was known only by imported cases. In 1998, the disease was found in dogs and in 1999 in humans in Araçatuba, northern São Paulo state. Thereafter, the infection spread throughout the neighboring administrative areas. In 2005, leishmaniasis was detected in humans and dogs in the municipality of Dracena, located in the western region of São Paulo state, considered by the Brazil Health Ministry to be an area with a high transmission rate [6–8]. Countrywide factors are related [7,8], however, paramount regional risk factors may be involved.
In developing countries, more than 50% of cases of VL occur in children, however, clinical and epidemiological data are scarce in the region [6,7]. Tertiary public hospitals play a key role in the Unified Public Health System (SUS) throughout Brazil. They support smaller hospitals and perform more complex treatment procedures [7,9]. Our aim was to evaluate the clinical characteristics and spatial distribution of VL in children referred to a public tertiary hospital located in the western region of São Paulo state, an emerging focus of VL in Brazil.
Patients and methods
Study design
This was a retrospective descriptive study of a cohort of children up to 18 years of age followed up at the pediatric ward of the regional hospital of Presidente Prudente (RH) located in Presidente Prudente municipality, western São Paulo state, Brazil. RH is a 450-bed not-for-profit tertiary care public university hospital providing care for SUS patients. Since 1999, it has been a reference center for the diagnosis and treatment of VL. Presidente Prudente covers the Regional Network of Health Care11 (RRAS11), which includes 45 municipalities and has a population of about 850,000 inhabitants. The study included patients diagnosed from January 2006 to December 2010. The onset of symptoms was analyzed at the time of admission, and the mean time between the onset of symptoms and hospital admission, treatment, medical reports, and clinical data were obtained from the medical records.
Cases of human VL were confirmed by clinical/epidemiological criteria plus laboratory diagnostics. The laboratory diagnostics recommended by the Manual of Surveillance and Control of Visceral Leishmaniasis of São Paulo state [10] included direct parasitology consisting of the presence of amastigotes of Leishmania in bone marrow aspirate (BMA) stained by Giemsa stain; a serological titer ≥1:80 in an indirect fluorescent antibody test (IFAT; Bio-Manguinhos/FIOCRUZ, Rio de Janeiro, Brazil); and in 2010, the rK39 rapid diagnostic test (Kalazar Detect, InBios, Seattle, Washington, U.S.A.) was implemented.
The kappa index was used to assess agreement between the tests. The significance was assessed by the chi-squared MacNemar test. The sensitivity and specificity of the IFAT and rK39 tests compared with the reference test, BMA, were estimated by point prevalence and 95% confidence interval. Statistical analysis was performed with R software [11].
The clinical/epidemiological features of VL included fever, splenomegaly, and pancytopenia in individuals who reportedly came from an endemic VL region [10,12]. Patients diagnosed with VL were treated with liposomal amphotericin B (4 mg/kg/day for 5 days), and N-methylglucamineantimoniate (Glucantime) at a dosage of 20 mg/kg/day (maximum three ampoules) for 20 days, according to the instructions of São Paulo’s Health Department [10]. If warning signs of infection were present or the patient was referred from another RRAS11 hospital, cefepime was the chosen antibiotic (50 mg/kg/day, three times a day). Ceftriaxone (50–80 mg/kg/day) was chosen in community-associated infections. Alanine aminotransferase (ALT), aspartate aminotransferase (AST), and albumin were assessed using automated systems according to the manufacturer’s instructions; the normal ranges were 7–55 U/L, 8–43 U/L, and 3.5–5 g/dL, respectively. Spleen size below the costal margin was measured in centimeters and categorized based on distribution by age group. Age was stratified according to the Brazilian Health Ministry Manual [12]. All children up to 18 years old with confirmed VL infection from RRAS11 who completed treatment were included in the study. Children with missing data or incomplete treatment were excluded.
Spatial dispersion of canine and human VL in the western region of São Paulo state
The state of São Paulo comprises 645 municipalities, and the Health State Department is divided into 17 mesoregions termed regional health care networks (RRAS). Presidente Prudente covers RRAS11, which includes 45 counties. The geographic expansion of canine VL and human VL is based on the year in which the first case was detected. Data were obtained from the following Brazilian public health agencies: Supervision and Control of Epidemic (SUCEN); Center of Regional Laboratory of the Adolfo Lutz Institute of Presidente Prudente V (CRL-ALI-PPV); National System of Diseases Notification (SINAN), São Paulo Epidemiological Bulletin (BEPA), and Municipal Surveillance Epidemiology Center (MSEC). The databases for the maps were collected from the IBGE website. The maps showing the spatial distribution were made using the ArcGIS 10.2 platform (ESRI 2014).
Statistical analysis
The point prevalence of the clinical, laboratory, and epidemiological findings was estimated and 95% confidence intervals (95% CI) were calculated using the Wald method. Estimates were performed with R software (R Development Core Team, 2013) and the ArcGIS 10.2 platform (ESRI, 2014). Data are expressed as means ± SEM. The agreement between methods was assessed using a kappa analysis. The kappa values were interpreted as follows: <0, no agreement; 0–0.19, poor agreement; 0.20–0.39, fair agreement; 0.40–0.59, moderate agreement; 0.60–0.79, substantial agreement; 0.80–1.00, almost perfect agreement [11]. The minimum level of significance considered was 5%.
Study approval
This study was approved by the Ethics Committee of the University of Oeste Paulista, Presidente Prudente, São Paulo, Brazil (number 1070/2012).
Results
Epidemiological and treatment characteristics
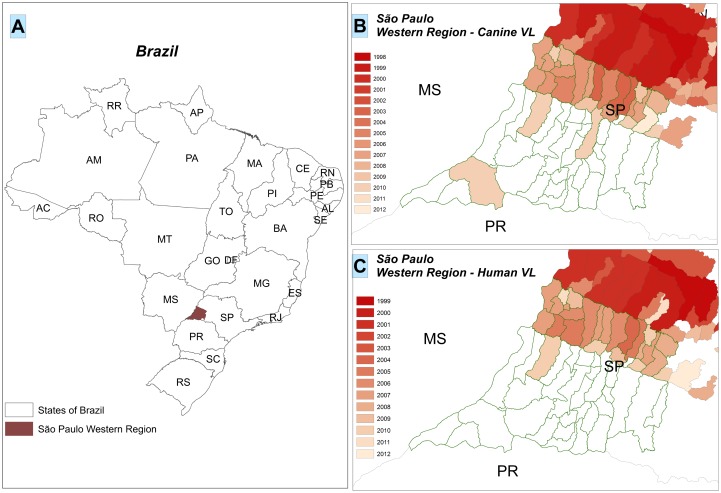
Between January 2006 and December 2010, 63 children aged from 29 days to 18 years (median age, 3.3 ± 3.3 years; 95% CI, 2.5–4.1) were referred to the pediatric ward of RH in Presidente Prudente, São Paulo state, Brazil. The median time interval between the onset of clinical symptoms and diagnosis was 16.1 ± 11.1 days (95% CI, 13.3–18.9). The average time that children remained in the pediatric ward was 18.0 ± 9.4 days (95% CI, 15.7–20.4). Children came from 12 (26.6%) of the 45 municipalities covered by RRAS11 and from 3 neighboring municipalities, Lucelia, Adamantina, and Osvaldo Cruz (Figure 1(C)). Most patients, 13 (20.6%), lived in the municipality of Panorama, followed by 10 (15.8%) in Tupi Paulista, 7 (11.2%) in Dracena, 4 (6.4%) in Paulicéia, and 4 (6.4%) in Irapuru, accounting for 60.3% of the infants (Figure 1(C)). In the western region, the disease is spreading from the north to the south going in the direction of Paraná state in the southern region of Brazil (Figure 1(B)).
Figure 1.
Spatial dispersion of canine and human visceral leishmaniasis in the western region of São Paulo state. (A) The 26 states of Brazil and São Paulo state located in the southeastern region, highlighting the western region. (B) The dispersion of canine visceral leishmaniasis in the western region of São Paulo state and bordering states: MS, Mato Grosso do Sul; PR, Paraná. (C) The dispersion of human visceral leishmaniasis in the western region of São Paulo state and bordering states. The colors represent the evolution of the events linked to the year. Data were obtained from the following Brazilian public health agencies: Brazilian Institute of Geography and Statistics; Supervision and Control of Epidemic; Center Regional Laboratory Adolfo Lutz Institute of Presidente Prudente V; National System of Diseases Notification; and São Paulo Epidemiological Bulletin.
Liposomal amphotericin B was chosen as first-line treatment in 90.5% of patients, with 9.6% relapses. One patient died. Antibiotic was administered to 90.5% of patients and 12 children (19%) were admitted to the pediatric intensive care unit, remaining there for 6 ± 2.4 days (95% CI, 0.5–11.4). Seven (11.1%) had infection in different sites, including urinary tract infection, septic shock, and tonsillitis. Skin conditions (rash, pruritus in the scalp, face and perineal hyperemia, macules and papules in the hands, enlarged lymph nodes, and petechiae in the back, face, and lips), apparently caused by adverse effects of liposomal amphotericin B and glucantime treatment, occurred in 13 (20.6%) of the infants. The epidemiological characteristics and details of treatment are presented in Table 1.
Table 1.
Main epidemiological characteristics of children admitted to RH with a diagnosis of VL from 2006 to 2010, showing the point prevalence estimates and 95% confidence intervals.
| Variables | No. of patients | 95% CI |
|---|---|---|
| Gender | ||
| Male | 36 (57.1) | 44.9–69.4 |
| Female | 27 (42.9) | 30.6–55.1 |
| Age | ||
| 29 days to <2 years | 28 (44.4) | 32.2–56.7 |
| >2 and <6 years | 24 (38.0) | 26.1–50.1 |
| >7 and <9 years | 3 (4.8) | NC |
| >10 and <18 years | 8 (12.8) | 4.5–20.9 |
| Time interval from onset of symptoms to diagnosis | ||
| <7 days | 16 (25.4) | 14.6–36.1 |
| >7 and <15 days | 30 (47.6) | 35.3–60.0 |
| >15 and <30 days | 14 (22.2) | 12.0–32.5 |
| >30 days | 3 (4.8) | NC |
| Hospitalization | ||
| <10 days | 3 (4.8) | NC |
| >10 and <20 days | 40 (63.5) | 51.6–75.4 |
| >20 and <30 days | 13 (20.6) | 10.6–30.6 |
| >30 days | 7 (11.1) | 3.4–18.9 |
| Treatment | ||
| Glucantime | 6 (9.5) | 2.3–16.8 |
| Liposomal amphotericin B | 57 (90.5) | 83.2–97.7 |
| Antibiotics | ||
| None | 6 (9.7) | 2.3–16.8 |
| Ceftriaxone | 35 (61.4) | 43.3–67.8 |
| Cefepime | 22 (38.6) | 23.1–46.7 |
| Ceftriaxone+cefepime | 9 (15.8) | 5.6–22.9 |
| Outcome | ||
| Alive | 62 (98.4) | NC |
| Lethality | 1 (1.6) | NC |
| Relapse | ||
| No | 57 (90.4) | 83.2–97.7 |
| Yes | 6 (9.6) | 2.3–16.8 |
Note: NC, not calculated (normal approximation by Wald method was not possible).
Table 2 presents the findings from the physical examinations on admission. The most prevalent symptoms were fever (95%) and pallor (93.6%). The most frequent signs on admission were splenomegaly (98%) and hepatomegaly (92%).
Table 2.
Main symptoms and clinical findings of children admitted to RH with a diagnosis of VL from 2006 to 2010 showing the point prevalence estimates and 95% confidence intervals.
| Variables | No. of patients (%) | 95% CI |
|---|---|---|
| Fever* | ||
| 37.9–38.9 °C | 20 (31.7) | 21.7–45.0 |
| 39–39.9 °C | 39 (62.0) | 53.2–76.8 |
| ≥40 °C | 1 (6.3) | NC |
| Vomiting | ||
| Yes | 31 (49.2) | 36.9–61.6 |
| No | 32 (50.8) | 38.4–63.1 |
| Abdominal pain | ||
| Yes | 25 (39.7) | 27.6–51.8 |
| No | 38 (60.3) | 48.2–72.4 |
| Diarrhea | ||
| Yes | 12 (19) | 9.4–28.7 |
| No | 51 (81) | 71.3–90.6 |
| Loss of appetite | ||
| Yes | 24 (38.0) | 26.1–50.1 |
| No | 39 (62.0) | 49.9–73.9 |
| Weight loss | ||
| Yes | 14 (22.2) | 12.0–32.5 |
| No | 49 (77.8) | 67.5–88.0 |
| Pallor | ||
| Yes | 59 (93.6) | 87.6–99.7 |
| No | 4 (6.3) | 0.3–12.4 |
| Cough | ||
| Yes | 26 (41.3) | 29.1–53.4 |
| No | 37 (58.7) | 46.6–70.9 |
| Jaundice | ||
| Yes | 3 (4.7) | NC |
| No | 60 (95.3) | NC |
| Hepatomegaly* | ||
| >1–<3 cm | 30 (51.7) | 39.4–64.1 |
| ≥3–<6 cm | 22 (38.0) | 25.9–49.9 |
| ≥6 cm | 6 (10.3) | 2.8–17.9 |
| Splenomegaly* | ||
| >1–<6 cm | 26 (41.9) | 29.8–54.1 |
| ≥6–<10 cm | 29 (46.8) | 34.5–59.1 |
| ≥10 cm | 7 (11.3) | 3.5–19.1 |
Note: NC, not calculated (normal approximation by Wald method was not possible).
Only children who presented the symptoms.
Table 3 presents the prevalence of severe hematologic and biochemical features recorded in patients diagnosed with VL. Leukopenia (<2500 cells/mm3) and neutropenia (<20%/cells/mm3) were the most prevalent findings. Few patients had severe anemia.
Table 3.
Hematological and biochemical features recorded in 63 children diagnosed with VL in the western region of São Paulo State, 2006–2010
| Variables | No. of patients (%) | 95% CI | Normal range |
|---|---|---|---|
| Hemoglobin <5.0 g/Dl | 3 (2.17) | NC | 10.5–14.5 |
| Leukocytes <2500 cells/mm3 | 14 (22.2) | 12.0–32.5 | 6–17.5 |
| Neutrophils <20 cells/mm3 | 16 (25.4) | 14.6–36.1 | 54–62 |
| Platelets <50,000/mm3 | 7 (11.1) | 3.4–18.9 | 150–400 |
| AST (>185 UI) | 9 (14.3) | 5.6–22.9 | 0–35 |
| ALT (>105 UI) | 5 (7.9) | 1.3–14.6 | 0–40 |
| Prothrombin activity <60 | 2 (3.1) | NC | 100 |
| Albumin <2.5 g/Dl | 8 (12.7) | 4.5–20.9 | 3.3–4.7 |
Notes: NC, not calculated (normal approximation by Wald method was not possible). AST, Aspartate Aminotransferase; ALT, Alanine Aminotransferase.
Laboratory findings
BMA tests were performed in 63 children, and amastigote forms were identified in 40 (64.5%) of the infants. A laboratory diagnosis of VL was obtained in 54 (85.7%) of patients. The diagnosis relied on clinical and epidemiological criteria for 9 (14.3%) patients. Twenty-two patients underwent IFAT and BMA and 32 underwent rK39 and BMA, at the same time. The kappa analysis showing the agreement between methods is shown in Table 4. The sensitivity and specificity of the IFAT and rK39 methods used to confirm the diagnosis of VL, taking into account the BMA as the reference test, are described in Table 5.
Table 4.
Agreement (Kappa index), estimated by point and confidence interval (CI 95%) between IFAT and BMAs (n = 22) and rK39 and BMAs (n = 32) for diagnosis of VL.
| Diagnostic test | % agreement | Kappa | 95% CI | P* |
|---|---|---|---|---|
| IFAT × BMA | 72.7 | 0.23 | −0.17 to 0.63 | 0.40 |
| rK39 × BMA | 69.7 | 0.10 | −0.21 to 0.42 | 0.20 |
Significance for McNemar chi-squared test.
Table 5.
Sensitivity and specificity estimated by point prevalence and confidence interval (95% Ci) for IFAT and rK39 tests for the diagnosis of VL considering BMAs as a reference test.
| Diagnostic test | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|
| IFAT | 88 (62–98) | 33 (4–78) |
| rK39 | 87 (66–97) | 42 (15–72) |
The mean hemoglobin level was 8.0 ± 0.2 g/dL (95% CI, 7.7–8.5). The mean hematocrit level was 26.3 ± 4.5% (95% CI, 25.2–27.4). The mean platelet count was 113.1 ± 123.7 cells/mm3 (95% CI, 81.9–144.3). The mean leukocyte count was 3.8 ± 1.8 cells/mm3 (95% CI, 3.3–4.2). The neutrophil count was 25.7 ± 14.1% of cells/mm3 (95% CI, 22.2–29.3), and 19 (30.2%) of the infants had more than 5% juvenile, non-segmented neutrophils. The mean lymphocyte count was 59.7 ± 14.3% of cells/mm3 (95% CI, 56.1–63.3). The mean albumin level was 3.8 ± 1.8 g/dL (95% CI, 3.3–4.2).
Spatial dispersion of canine and human VL in the western region of São Paulo state
São Paulo (SP), located in the southeastern region, is one of the 26 states in Brazil, and the western region harbors de RRAS11 (Figure 1(A), in brown) as well as the bordering states (MS, Mato Grosso do Sul and PR, Paraná) (Figure 1(A)). The spatial distribution of canine VL is showed in Figure 1(B) and human VL is showed in Figure 1(C) throughout the study period in the western region of São Paulo state.
Discussion
Despite the measures adopted by the Visceral Leishmaniasis Control and Surveillance Program (VLCSP) to control VL throughout the country, which include early diagnosis and treatment of human VL, testing and euthanasia of seropositive dogs, control of sand-fly vectors, and health education initiatives [13], it is suspected that the disease is spreading fast throughout the western region of São Paulo state [6,7,14].
With increased time between onset of symptoms and the beginning of treatment and age (≥0.5 years) are considered to be important risk factor for the severity of the disease [15], the reduced time to diagnosis (16.1 days) is one of the hallmarks of VL infection in children in RRAS11 compared with studies from other regions and countries: 30 days in Recife [16]; 38.5 days in Fortaleza, northeastern Brazil [17], 5 months in children referred from the northeastern states to Clinics Hospital in São Paulo city, the biggest tertiary public hospital in South America [18]; 31 days in children from Portugal [19]; 60 days in Ethiopia [20]; and 59.6 days in Iran [21]. One hypothesis is that the number of patients with VL is increasing quickly in the municipalities of RRAS11, and thus physicians are more aware of the warning signs of the disease. Clinical suspicion occurs early after the onset of the symptoms, allowing for a prompt diagnosis and treatment. In addition, the average time that children remained in the pediatric ward was 18 days, a shorter period than in the studies conducted in other regions of Brazil [16,17,22,23]. A plausible reason is that liposomal amphotericin B was given to 90.5% of the infants. Following the guidelines of the Brazilian Ministry of Health, which recommend pentavalent antimony (SbV) as first-line therapy, the use of the liposomal formulation of amphotericin B was restricted to a few special conditions (e.g. children < 1 year and elderly persons) and patients with contraindications or who fail to respond to first-and second-line treatments. In São Paulo state, since the first patients were diagnosed and treated, the rate of case fatalities was 11.5%, about twice the Brazilian value. In 2006, in an effort to lower death rates, the state recommended therapy with liposomal amphotericin B for all children up to 10 years of age and adults over 50 years, those with VL/AIDS co-infection, in pregnancy, and after relapse; these recommendations were wider than those proposed by the Brazilian Health Ministry at that time [24]. In studies conducted in other states of Brazil, glucantime was the treatment available in 98% [16], 95.7% [22], and 90% [23] of the infants. Although limited by a small number of patients, we can suggest that for infants in RRAS11 referred to RH, VL is no longer a chronic disease but an acute or sub-acute disease.
Relapse after treatment with liposomal amphotericin B is associated with increased lethality rates and a factor of poor prognosis and disease severity among children [25,26]. In this study, few children relapsed and the lethality rate was one of the lowest (1.6%) found among children infected with VL in Brazil [16–18]. In the last decade, the reported lethality rates in the metropolitan areas of the north and northeast regions were 10.2% in children from Recife [16], 3.3% in Fortaleza [17], and 3.9% in São Luis [27]. In the central-western region, the lethality rate was 3.6% in children from Belo Horizonte [25] and 2.6% in children from Campo Grande [22]. The average lethality rate for the entire population infected with VL in São Paulo state was 14.1% in the 1999–2005. The average lethality rate for the entire country was 6.5% in 2000–2010 [28–29]. The importance of tertiary hospitals in Brazil in the context of SUS is overwhelming with regard to both short hospital stay and low lethality rates [7,9]. The classic signs and symptoms of the disease worldwide (discrete splenomegaly, hepatomegaly, fever, and pallor) were present in almost all the infants [1,2]. However, the presence of jaundice, increased size of the liver (≥6 cm) and spleen (≥10 cm), the hallmarks of VL infection and associated with increased mortality rates [30], were present in only 4.7, 10.3, and 11.3% of the infants, respectively.
Laboratory parameters provide warning signs of progression to severe illness and worse prognosis and are associated with increased mortality rates for VL infection in children, including bacterial infections (pneumonia and/or urinary tract infection), sepsis, neutrophil count < 500/mm3, and platelet count < 50,000/mm3 [25,26,30,31]. In the current study, few children fulfilled the criteria for high severity, 11.1% of the infants presented a neutrophil count < 500/mm3; 11% presented a platelet count < 50,000/mm3, 19% were admitted to the pediatric intensive care unit, 6.3% evolved to septic shock, and 11.1% had infection in different sites, including the urinary tract. One hypothesis for our results relies on the short period from the onset of symptoms to diagnosis and treatment of infants. Furthermore, bacterial infections are typical secondary infections during VL, and are often the immediate cause of death [32]. Antibiotic was applied in 90.5% of our patients, and in 15.8% combined ceftriaxone plus cefepime was needed. Certainly, this procedure helped to reduce the rates of lethality and length of hospital stay. Different aspects must be highlighted when comparing data from children and adults infected with VL worldwide [15,31]. In a reference center in the northeast of Brazil, adults showed a longer time between the onset of symptoms and the beginning of treatment, treatment failure, increased kidney injury, worse prognosis, and mortality rates followed by increased laboratory abnormalities compared with children. One possible explanation was the delay in prognosis [15].
BMA was performed in the whole study population and amastigotes were identified in 64.5%. However, the rapid diagnostic test has the cornerstone of serological diagnosis of VL in Brazil since 2011. Distributed to states by the Brazil Ministry of Health, it has been used routinely in hospitals, including the RH. In individuals suspected of having VL, if a rapid test yields a positive result, BMA, an invasive procedure, is not necessary [10,12]. However, on the basis of BMA positivity, we found poor agreement between BMA and rK39 tests (69.7%; kappa 0.10), with a sensitivity and specificity of 87% and 42%, respectively. These results are similar to the results of a retrospective analysis of 94 patients with clinically suspected VL in India (sensitivity, 87%) in which rK39 falsely detected 6 cases (6.25%) [33], similar to the data for 310 patients with clinically suspected VL in Nepal yielding a sensitivity of rK39 of 87.4% and specificity of 77% [34]. A shortcoming of our results is the small number of samples, and they should be confirmed by testing a large number of confirmed VL and negative samples. Diagnosis relied on clinical and epidemiological criteria in only 14.3% of the infants, similar to the results reported by others in Brazil [16,17,23].
The reasons why VL is spreading in a fast and alarming way in the western region of São Paulo and in the direction of Paraná state are not well understood, however, a complex array of factors may be fueling the epidemic and sustained endemic transmission [35]. Since 2005, when the first case of VL was reported, up to 2012, of the 45 municipalities that comprise RRAS11, located in Presidente Prudente’s mesoregion, Lutzimia longipalpis was found in 27 (60.0%), canine VL was found in 15 (33.3 %), and human VL was found in 13 (29%) of the 45 counties; there have been 330 human cases reported and 19 (5.8%) deaths. This is considered the poorest region of the state with sandy/dry soil in the winter and rainy/wet soil in the summer, and increasing temperatures in the last decades. Nevertheless, VL dissemination could be aggravated by the extensive amount of watersheds flowing into three biggest rivers of southwestern and southern Brazil, with nine big lakes and a flooded area of 2384 square miles, supporting hydroelectric plants and bridges. These bridges link dozens of small towns and villages connected by a large number of highways (1480 miles). The bridges and highways support a daily flow of people, cars, animals, and goods linking endemic regions to VL-free areas. Since 2008, canine VL has been found in Teodoro Sampaio county, located in the border of Paraná state (Figure 1(B)). A bridge over the Paranapanema river links the states. We suggest that these environmental factors are new concepts not present in other regions of São Paulo state.
Conclusion
The short interval between the onset of symptoms, diagnosis, and treatment with liposomal amphotericin B and the reduced number of days of hospitalization certainly influenced the small number of deaths, relapses, and severity among children infected with VL referred to the regional hospital. However, like the shadows of a ghost, the disease is spreading fast in the western region of São Paulo state. Thus, integrated actions and effective monitoring of the disease are needed to complement curative practices.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This study was supported by Fundação de Amparo a Pesquisa no Estado de São Paulo [grant number 13/20781-7].
References
- [1].Alvar J, Vélez ID, Bern C, et al. WHO Leishmaniasis control team. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7:e35671 10.1371/journal.pone.0035671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Leishmaniasis, World Health Organization [cited 2016 Jan]. Available from: http://www.who.int/mediacentre/factsheets/fs375/en/.
- [3].Albuquerque PL, Silva Júnior GB, Freire CC, et al. Urbanization of Visceral leishmaniasis (kala-azar) in Fortaleza, Ceará. Brazil Rev Panam Salud Publica. 2009;26:330–333. 10.1590/S1020-49892009001000007 [DOI] [PubMed] [Google Scholar]
- [4].Araújo VE, Pinheiro LC, Almeida MC, et al. Relative risk of Visceral leishmaniasis in Brazil: a spatial analysis in urban area. PLoS Negl Trop Dis. 2013;7(11):e2540 10.1371/journal.pntd.0002540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Cavalcante IJ, Vale MR. Epidemiological aspects of Visceral leishmaniasis (kala-azar) in Ceará in the period 2007–2011. Rev Bras Epidemiol. 2014;17:911–924. 10.1590/1809-4503201400040010 [DOI] [PubMed] [Google Scholar]
- [6].D’Andrea LAZ, Camargo-Neves VLF, Sampaio SMP, et al. American Visceral leishmaniasis: disease control strategies in Dracena micro region in Alta Paulista, SP, Brazil. J Venom Anim Toxins Trop Dis. 2009;15:305–324. 10.1590/S1678-91992009000200012 [DOI] [Google Scholar]
- [7].Naufal Spir PR, Zampieri D’Andrea LA, Fonseca ES, et al. Epidemiology of human immunodeficiency virus-Visceral leishmaniasis-co-infection. J Microbiol Immunol Infect. 2013;49:295–299. [DOI] [PubMed] [Google Scholar]
- [8].Cardim MF, Guirado MM, Dibo MR, et al. Visceral leishmaniasis in the state of Sao Paulo, Brazil: spatial and space-time analysis. Rev Saude Publica. 2016;50:48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Prestes-Carneiro LE, Vieira JTM, Isaac LB, et al. Clinical, demographic, and epidemiologic characteristics of hepatitis B virus-infected patients at a tertiary public hospital in Presidente Prudente, State of São Paulo. Brazil Rev Soc Bras Med Trop. 2016;49:24–28. 10.1590/0037-8682-0315-2015 [DOI] [PubMed] [Google Scholar]
- [10].Manual de Vigilância e Controle da Leishmaniose Visceral Americana do Estado de São Paulo 2006. [cited 2016 Oct]. Available from: ftp://ftp.cve.saude.sp.gov.br/doc_tec/zoo/lva06_manual.pdf.
- [11].R Development Core Team R Software: a language and environment for statistical computing; 2013. [cited 2016 Jul]. Available from: http://www.r-project.org.
- [12].Manual de Vigilância e controle de Leishmaniose Visceral, Ministério da Saúde; 2014. [cited 2016 Oct]. Available from: https://www.icict.fiocruz.br/sites/www.icict.fiocruz.br/files/Manual_Vigilancia_Controle_Leishmaniose_Visceral.pdf.
- [13].Romero GA, Boelaert M. Control of Visceral leishmaniasis in Latin America – a systematic review. PLoS Negl Trop Dis. 2010;4:e584 10.1371/journal.pntd.0000584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].D’Andrea LA, da Silva Fonseca ES, Prestes-Carneiro LE, et al. The shadows of a ghost: a survey of canine leishmaniasis in Presidente Prudente and its spatial dispersion in the western region of São Paulo state, an emerging focus of Visceral leishmaniasis in Brazil. BMC Vet Res. 2015;11(1):e584 10.1186/s12917-015-0583-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rocha NA, Oliveira MJ, Franco LF, et al. Comparative analysis of pediatric and adult Visceral leishmaniasis in Brazil. Pediatr Infect Dis J. 2013;32:e182–e185. 10.1097/INF.0b013e3182814eae [DOI] [PubMed] [Google Scholar]
- [16].Queiroz MJ, Alves JG, Correia JB. Visceral leishmaniasis: clinical and epidemiological features of children in an endemic area. J Pediatr (Rio J). 2004;80:141–6. Portuguese 10.2223/JPED.1154 [DOI] [PubMed] [Google Scholar]
- [17].Rey LC, Martins CV, Ribeiro HB, et al. American Visceral leishmaniasis (kala-azar) in hospitalized children from an endemic area. J Pediatr (Rio J). 2005;81:73–78. Portuguese 10.2223/JPED.1286 [DOI] [PubMed] [Google Scholar]
- [18].Pastorino AC, Jacob CM, Oselka GW, et al. Visceral leishmaniasis: clinical and laboratorial aspects. J Pediatr (Rio J). 2002;78:120–127. Portuguese. [PubMed] [Google Scholar]
- [19].Dionísio MT, Dias A, Rodrigues F, et al. Paediatric Visceral leishmaniasis: experience of a paediatric referral center 1990–2009. Acta Med Port. 2011;24:399–399. Portuguese. [PubMed] [Google Scholar]
- [20].Diro E, Lynen L, Gebregziabiher B, et al. Clinical aspects of paediatric Visceral leishmaniasis in North-west Ethiopia. Trop Med Int Health. 2015;20:8–16. 10.1111/tmi.2014.20.issue-1 [DOI] [PubMed] [Google Scholar]
- [21].TofighiNaeem A, Mahmoudi S, Saboui F, et al. Clinical features and laboratory findings of Visceral leishmaniasis in children referred to Children Medical Center Hospital, Tehran, Iran during 2004–2011. Iran J Parasitol. 2014;9:1–5. [PMC free article] [PubMed] [Google Scholar]
- [22].Brustoloni YM, Cunha RV, Cônsolo LZ, et al. Treatment of Visceral leishmaniasis in children in the Central-West Region of Brazil. Infection. 2010;38:261–267. 10.1007/s15010-010-0022-3 [DOI] [PubMed] [Google Scholar]
- [23].Rocha NA, Silva GB, Oliveira MJ, et al. Visceral leishmaniasis in children: a cohort of 120 patients in a metropolitan city of Brazil. Turk J Pediatr. 2011;53:154–160. [PubMed] [Google Scholar]
- [24].Melo EC, Fortaleza CM. Challenges in the therapy of Visceral leishmaniasis in Brazil: a public health perspective. J Trop Med. 2013;2013:319234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Braga AS, Toledo Junior AC, Rabello A. Factors of poor prognosis of Visceral leishmaniasis among children under 12 years of age. A retrospective monocentric study in Belo Horizonte, State of Minas Gerais, Brazil, 2001-2005. Rev Soc Bras Med Trop. 2013;46(1):55–59. 10.1590/0037-868216432013 [DOI] [PubMed] [Google Scholar]
- [26].Burza S, Sinha PK, Mahajan R, et al. Risk factors for Visceral leishmaniasis relapse in immunocompetent patients following treatment with 20 mg/kg liposomal Amphotericin B (Ambisome) in Bihar, India. PLoS Negl Trop Dis. 2014;8(1):e2536 10.1371/journal.pntd.0002536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Silva AR, Tauil PL, Cavalcante MN, et al. Epidemiological situation of Visceral leishmaniasis on the Island of São Luis, State of Maranhão. Rev Soc Bras Med Trop. 2008;41:358–364. Portuguese. [DOI] [PubMed] [Google Scholar]
- [28].SUCEN - Superintendência de Controle de Endemias 2014 doi: 10.1590/0037-868216432013. [cited 2016 Dec]. Available from: http://www.saude.sp.gov.br/sucen-superintendencia-de-controle-de-endemias/programas/leishmaniose-visceral/situacao-atual. [DOI] [Google Scholar]
- [29].Madalosso G, Fortaleza CM, Ribeiro AF, et al. American Visceral leishmaniasis: factors associated with lethality in the state of São Paulo, Brazil. J Trop Med. 2012;2012:281572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Sampaio MJ, Cavalcanti NV, Alves JG, et al. Risk factors for death in children with Visceral leishmaniasis. PLoS Negl Trop Dis. 2010;4(11):e877 10.1371/journal.pntd.0000877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Mueller Y, Mbulamberi DB, Odermatt P, et al. Risk factors for in-hospital mortality of Visceral leishmaniasis patients in eastern Uganda. Trop Med Int Health. 2009;14:910–917. 10.1111/tmi.2009.14.issue-8 [DOI] [PubMed] [Google Scholar]
- [32].Barati M, Sharifi I, Daie Parizi M, et al. Bacterial infections in children with Visceral leishmaniasis: observations made in Kerman province, southern Iran, between 1997 and 2007. Ann Trop Med Parasitol. 2008;102:635–641. 10.1179/136485908X311858 [DOI] [PubMed] [Google Scholar]
- [33].Mandal J, Khurana S, Dubey ML, et al. Evaluation of direct agglutination test, rk39 Test, and ELISA for the diagnosis of Visceral leishmaniasis. Am J Trop Med Hyg. 2008;79:76–78. [PubMed] [Google Scholar]
- [34].Boelaert M, Rijal S, Regmi S, et al. A comparative study of the effectiveness of diagnostic tests for Visceral leishmaniasis. Am J Trop Med Hyg. 2004;70:72–77. [PubMed] [Google Scholar]
- [35].Prestes-Carneiro LE. The role of environment in the spreading of Visceral leishmaniasis in western São Paulo, Brazil. J Bacteriol Parasitol. 2015;6:41. [Google Scholar]