Abstract
Background: The emergence of novel strains of influenza A viruses with hemagglutinins (HAs) that are antigenically distinct from those circulating in humans, and thus have pandemic potential, pose concerns and call for the development of more broadly protective influenza vaccines. In the present study, modified vaccinia virus Ankara (MVA) encoding internal influenza antigens were evaluated for their immunogenicity and ability to protect HLA-A2.1 transgenic (AAD) mice from infection with influenza viruses.
Methods: MVAs expressing NP (MVA-NP), M1 (MVA-M1) or polymerase PB1 (MVA-PB1) of A/California/4/09 (CA/09) virus were generated and used to immunize AAD mice. Antibodies and CD8+T cell responses were assessed by ELISA and ELISPOT, respectively, and challenge experiments were performed by infecting vaccinated mice with CA/09 virus.
Results: CD8+T cells specific to immunodominant and subdominant epitopes on the internal influenza proteins were elicited by MVA-based vectors in AAD mice, whereas influenza-specific antibodies were detected only in MVA-NP-immunized mice. Both M1- and NP-based MVA vaccines, regardless of whether they were applied individually or in combination, conferred protection against lethal influenza virus challenge.
Conclusion: Our data further emphasize the promising potential of MVA vector expressing internal antigens toward the development of a universal influenza vaccine.
Keywords: Influenza virus, vaccine, internal proteins, MVA vector, transgenic mice
Introduction
Influenza A viruses are widely distributed in nature and can infect both animals and humans [1]. Occasionally, zoonotic spillover events may lead to the emergence of novel strains of influenza A viruses with hemagglutinins (HAs) that are antigenically distinct from those circulating in humans, and thus have pandemic potential [1,2]. Monovalent candidate vaccines that elicit HA-specific neutralizing antibodies have been produced against potentially pandemic strains [3]. Nevertheless, the unpredictable variability of influenza A viruses poses concerns and calls for the development of more broadly protective influenza vaccines [4].
Although neutralizing antibodies to the globular HA are most effective for the prevention of illness, new and innovative strategies aimed at inducing cross-reactive antibodies that bind to conserved portions of the HA stalk show promise for development of a universal influenza vaccine [5–8]. Furthermore, the induction or recall of CD8+T cells against conserved epitopes of the internal viral proteins, which are very poorly elicited by currently inactivated influenza vaccines, would effectively contribute to reducing viral load and limit disease severity after infection with a heterosubtypic influenza virus [9−13]. In particular, the rapid boosting of these cross-reactive influenza-specific memory T cells, which accumulate in the lungs and lymphoid tissues in humans after recovery from influenza virus infections, could be extremely beneficial to provide protection against severe influenza virus infections [14−16].
Several studies have been performed to determine the efficacy of viral vector vaccines that were engineered to express the conserved antigens of the influenza virus [17−19]. In particular, replication-deficient modified vaccinia virus Ankara (MVA) constructs encoding internal influenza proteins have been demonstrated to be safe and highly immunogenic in both animal models and human volunteers [20−23]. In this context, the co-administration of MVA, expressing a fusion protein of influenza A nucleoprotein (NP) and matrix protein (M1) (MVA-NP+M1), with seasonal vaccine simultaneously has been shown to boost T cell responses and increase some influenza-specific antibodies compared with a group receiving seasonal influenza vaccine alone [24]. However, little is known about the frequencies of the dominant and subdominant epitope-specific CD8+T cells that are elicited following vaccination with these recombinant MVA vectors.
Recently, we explored the CD8+T cell responses to selected epitopes of the internal proteins of both live and non-replicating influenza virus in HLA-A2.1 transgenic (AAD) mice [25]. In particular, we found that a non-replicating whole virus-based vaccine elicited CD8+T cell responses that were restricted predominantly to immunodominant epitopes from the most abundant internal proteins M1 and NP. In this study, we investigated the HLA-A*0201-restricted epitope specificities of CD8+T cells and antibody responses after vaccination of AAD mice with recombinant MVAs expressing NP (MVA-NP), M1 (MVA-M1) or polymerase PB1 (MVA-PB1) of the A/CA/04/09 virus (CA/09). Moreover, we determined the protective efficacy of these vaccine-induced influenza-specific CD8+T cell responses against lethal respiratory virus challenge in mice.
Methods
Generation and characterization of recombinant MVA viruses
The recombinant MVA viruses (MVA-NP, MVA-M1, MVA-PB1) expressing the internal proteins of CA/09 virus were generated as previously described [26]. Serum-free cultures of chicken embryo fibroblasts (CEF) were utilized for recombinant virus construction, terminal dilution cloning and virus stock production. The MVA wild type (MVA-wt) was also included in the study.
Western blot was performed by using polyclonal specific antisera to determine the transgene expression by MVA recombinant viruses [26].
Vaccination of AAD mice and virus challenge
AAD mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA), and animal experiments were performed at the Istituto Superiore di Sanità in compliance with institutional guidelines and approved protocols [25]. Groups of mice (6–8 weeks) were vaccinated intramuscularly (i.m.) twice, three weeks apart, with 107 pfu of the MVA-M1, MVA-NP or MVA-PB1 viruses, given as single or a three-MVA virus combination in separate injection sites. Four weeks after the last immunization, mice were anesthetized with Avertin and challenged intranasally (i.n.) with 2 50% lethal doses (LD50) of CA/09 virus in 45 μl volume. Control mice received MVA-wt virus or an equal volume of PBS. Mice were then monitored for survival and weight loss for 14 days after infection.
Peptides
Three peptide epitopes for mouse MHC class I molecules, H2-Db-NP366, H2-Kb-PB1703, and H2-Kb-M1128, and ten peptides that bind the HLA-A2.1 molecule and are conserved among diverse influenza subtypes were synthesized by Primm (Italy). Peptide purity was > 90% in all cases, and the identity of the peptides was verified by using spectrometry. The peptides were dissolved in DMSO at 10 mg/ml and stored at −20 °C.
IFN-γ ELISPOT assays
Spleens of mice (6–7 mice per group) were collected seven days after immunization or virus infection and immediately assayed for antigen-specific IFN-γ-producing cells using an IFN-γ ELISPOT assay. Single-cell suspensions from the lymphocytic populations were cultured with the indicated synthetic peptides or DMSO on anti-IFN-γ-coated plates at 37 °C for 36–40 h. Colored spots representing IFN-γ-releasing cells were reported as the number of spot-forming cells (SFC) per 106 spleen cells.
Antibody analysis
Serum samples were collected from mice vaccinated with MVA viruses immediately before virus challenge and tested for the presence of influenza-specific antibodies in ELISA using detergent–disrupted CA/09 virus [27].
Results
Generation and analysis of recombinant MVA viruses
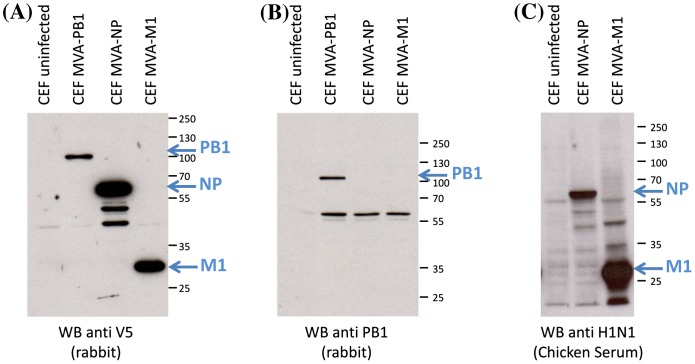
The MVA–NP, MVA–M1 and MVA–PB1 viruses were generated and the transgene expression was monitored by Western blot in infected CEF lysates with the anti-V5 antibody, which recognizes a tag at the C-terminus of the proteins. Chicken anti-influenza A H1N1 polyclonal antiserum and rabbit polyclonal PB1-specific antibodies confirmed the identity and expected molecular size of the influenza proteins (Figure 1).
Figure 1.
Western Blot analysis of influenza PB1, M1 and NP from recombinant MVA vectors. Cell lysates from infected CEF were analyzed 48 h p.i. Cell lysates from uninfected-CEF were used as controls. Protein expression and molecular weights were determined with a rabbit anti-V5 antibody (A), rabbit polyclonal PB1-specific antibodies (B) and chicken polyclonal H1N1-specific serum and (C). Position and size (kDa) of molecular weight markers are indicated on the right side of each panel. Specific bands are clearly distinguishable from a number of non-specific bands stained by the polyclonal antibodies.
Induction of influenza-specific humoral and cellular immune responses in AAD mice following vaccination with recombinant MVA viruses
AAD mice with the C57BL/6J genetic background express an interspecies hybrid class I molecule, composed of the alpha 1 and alpha 2 domains of the human HLA-A*0201 allele and the alpha 3 transmembrane and cytoplasmic domains of the mouse H-2Dd class I molecule [28]. Therefore, we first determined the breadth and specificity of primary CD8+T cell responses that were elicited against the internal proteins of influenza virus following immunization of the AAD mice with single recombinant MVA viruses. To this end, a panel of HLA-A2-restricted influenza derived CD8+T cell epitope peptides (see Table 1) consisting of 3 NP epitopes, 4 PB1 epitopes and 3 M1 epitopes was used in ex vivo IFN-γ ELISPOT assays because these epitopes are highly conserved among different influenza subtypes [29−31]. Moreover, the murine peptide epitopes, H2-Db-NP366, H2-Kb-PB1703, and H2-Kb-M1128, were also used in the assay to assess the specific H2-restricted-CD8+T cell responses [32−34].
Table 1.
Influenza A virus-derived MHC class I restricted T cell epitopes included in the study.
| Peptides | Sequence | Position | MHC restriction |
|---|---|---|---|
| M1-58 | GILGFVFTL | 58–66 | HLA-A2.1 |
| M1-59 | ILGFVFTLTV | 59–68 | HLA-A2.1 |
| M1-128 | MGLIYNRM | 128–135 | H2-Kb |
| M1-130 | GLIYNRMGA | 130–138 | HLA-A2.1 |
| PB1-407 | MMMGMFNML | 407–415 | HLA-A2.1 |
| PB1-413 | NMLSTVLGV | 413–421 | HLA-A2.1 |
| PB1-501 | FVANFSMEL | 501–509 | HLA-A2.1 |
| PB1-505 | FSMELPSFGV | 505–514 | HLA-A2.1 |
| PB1-703 | SSYRRPVGI | 703–711 | H2-Kb |
| NP-275 | CLPACVYGL | 275–283 | HLA-A2.1 |
| NP-329 | QLVWMACHSAA | 329–339 | HLA-A2.1 |
| NP-458 | FQGRGVFEL | 458–466 | HLA-A2.1 |
| NP-366 | ASNENVETM | 366–374 | H2-Db |
Murine MHC haplotypes are indicated in bold.
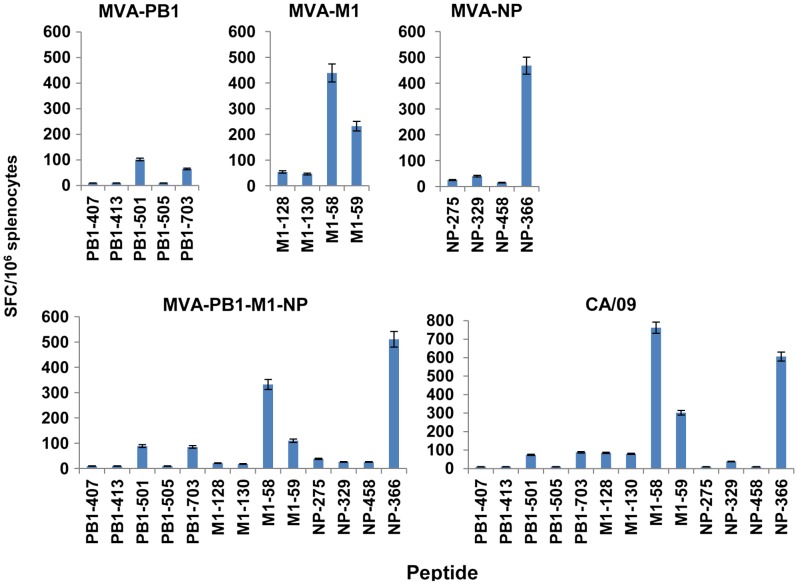
Mice immunization with either MVA-M1 or MVA-NP virus elicited CD8+T cell responses that were mainly detectable for the immunodominant epitopes A2-M158 and H2-Db-NP366, respectively. Reproducible T cell responses were also observed for the overlapping peptide A2-M59 in the MVA-M1-immunized mice, whereas lower numbers of responder T cells were consistently measured for the other subdominant epitopes (Figure 2). Interestingly, vaccination of mice with MVA-PB1 virus elicited detectable responses specific to peptide epitopes A2-PB1501 and H2-Kb-PB1703, whereas no significant T cell frequencies were revealed against the other subdominant epitopes of PB1 protein. When mice were vaccinated with the three-virus combination, CD8+T cell responses to the specific peptides were similar to those measured in mice vaccinated with the single MVA viruses (Figure 2). Infection of AAD mice with sublethal doses (0.1 LD50) of CA/09 virus generated A2-M158-specific- and H2-Db-NP366-specific CD8+T cells with similar frequency. In addition, significant numbers of responding T cells to at least six subdominant epitopes of those listed in Table 1 were measured in these mice. Among them, CD8+T cells specific to the peptide A2-M159 overlapping the immunodominant A2-M158 were prevalent, followed by those specific to H2-Kb-PB1703, A2-PB1501, H2-Kb-M1128, A2-M1130 and A2-NP329. Thus, a similar pattern in reactivity was detected in mice following MVA-based immunization or influenza virus respiratory infection.
Figure 2.
Vaccine-induced CD8+T cell responses in AAD mice. Groups of AAD mice (6/group) were immunized twice, three weeks apart, with MVA-PB1, MVA-M1, MVA-NP, or a three-virus combination. A group of mice received, i.n. under anesthesia, a sublethal dose (0.1 LD50) of CA/09 virus. Seven days later, influenza-specific CD8+T cell responses were measured from bulk splenocytes by use of an ex vivo IFN-γ-ELISPOT assay with the indicated peptides. Bars represent the mean values ± standard deviation (SD) of triplicate cultures. The data are representative of three independent experiments that gave similar results.
To investigate whether the MVA-based vaccines generated influenza A virus-specific antibodies, sera from vaccinated mice were tested in ELISA against detergent-disrupted CA/09 virus. The results showed that MVA-NP vaccination, as a single virus and in combination with the other MVAs, induced robust serum IgG titers, whereas no detectable IgG responses were induced by the PB1- and M1-based vaccines (Figure 3).
Figure 3.
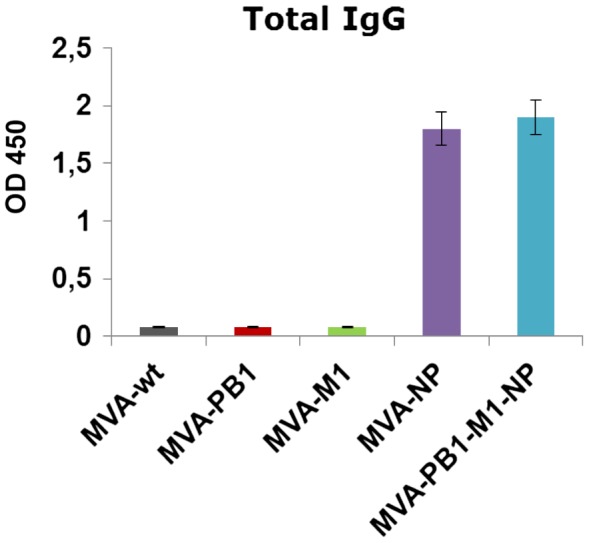
Serum IgG titers in mice following immunization with MVA vectors. Groups of mice were immunized twice with MVA constructs and the titers of anti-influenza specific antibodies were determined with use of 100-fold-diluted samples obtained three weeks after immunization by titration on ELISA plates coated with CA/09 virus. Values represent mean of 6 mice per group ± SD. OD 450 = Optical density at 450 nm.
Together, these data indicate that vaccination with MVA-based vectors expressing the internally conserved influenza proteins elicits CD8+T cell responses against immunodominant and subdominant epitopes and antibodies against NP protein, regardless of whether they were applied alone or in combination.
Protective efficacy against a lethal influenza virus challenge
To determine whether MVA-based vaccines could elicit protective immunity, AAD mice were immunized and then challenged with 2 LD50 of the CA/09 virus. All mice that received PBS or MVA-wt experienced rapid weight loss and succumbed to influenza virus challenge before day 8 (Figure 4). Mice vaccinated with MVA-M1 or MVA-NP virus had 72% and 100% survival rates, respectively, whereas no protection was achieved in mice vaccinated with MVA-PB1. Mild weight loss occurred in the MVA-M1 (20%) or MVA-NP (14%) groups, whereas all mice immunized with MVA-PB1 vaccine exhibited weight loss greater than 25% of their initial weight and were euthanized. Mice vaccinated with the three-MVA virus combination showed 16% weight loss and 100% survival rate (Figure 4).
Figure 4.
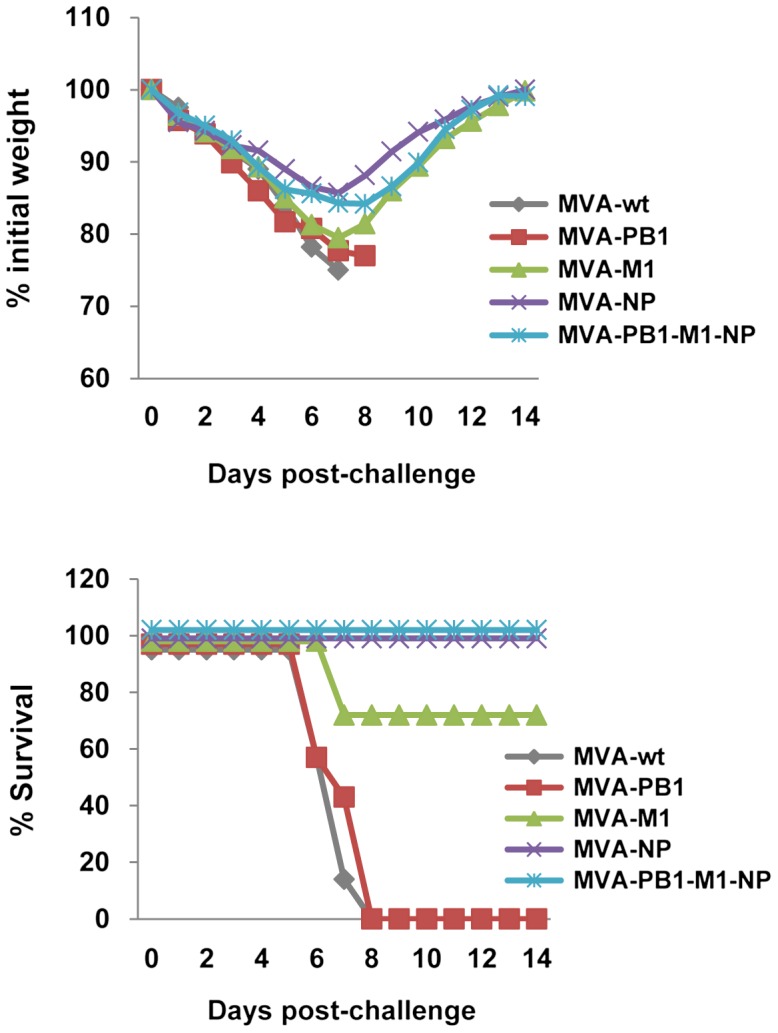
Protective effect against influenza virus challenge. Mice (7/group) were immunized twice with MVA vectors and challenged i.n. four weeks later with 2 LD50 of CA/09 virus. Body weight and survival were monitored for fourteen days after virus infection, and mice were sacrificed when body weight reached 75% of starting weight. One of three similar experiments is shown.
To assess the epitope specificity of CD8+T cells after viral challenge, mice of each group were sacrificed on day 7 p.i., and IFN-γ production was measured by ELISPOT assay from splenocytes in the presence of the selected peptides. High levels of CD8+T cells specific to A2-M158 and H2-Db-NP366 were found in mice previously vaccinated with the three-MVA virus combination that correlated with the survival rate of these mice (Figure 5). Notably, the frequencies of immunodominant epitopes A2-M158 and H2-Db-NP366 increased at least five-fold in the spleens after viral challenge, whereas the frequencies of the subdominant epitopes were similar to those observed in unvaccinated mice. Similar results were obtained from mice immunized with MVA-M1 or MVA-NP virus (data not shown).
Figure 5.
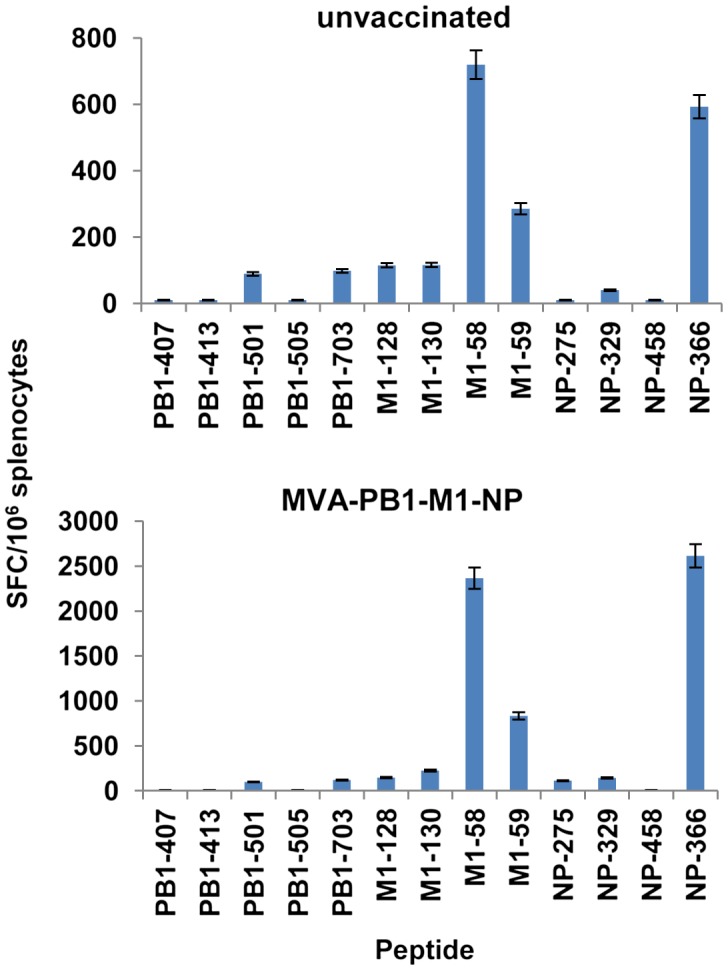
MHC class I-restricted recall responses following influenza virus challenge. Naïve mice or mice vaccinated with two doses of the three-MVA virus combination four weeks earlier (6/group) were challenged i.n. under anesthesia with 2 LD50 of CA/09 virus. Seven days after challenge, mice were sacrificed and influenza-specific CD8+T cell responses were measured in cells from bulk splenocytes by means of an ex vivo IFN-γ-ELISPOT assay with the indicated peptides. Bars represent the means ± SD of triplicate cultures. The data are representative of two independent experiments that gave similar results.
Overall, our results show that protective cellular immune responses can be induced in AAD mice following systemic immunization with MVA-based vectors expressing the conserved internal antigens M1 and NP.
Discussion
In the present study, we evaluated the CD8+T cell responses to internal viral proteins elicited by MVA-based vectors in AAD mice. We found that CD8+T cells specific to immunodominant and subdominant epitopes could be detected in these mice. Importantly, such CD8+T cell-mediated immunity appears to be associated with the protection of mice against a lethal influenza virus challenge.
Recombinant MVAs have been widely recognized as suitable vaccine vectors for their safety and immunogenic properties, even in the presence of pre-existing MVA immunity [35,36]. Although MVA vectors expressing various combinations of the internal viral proteins have already been evaluated in other animal models, there is a lack of information on the specificity for both dominant and subdominant epitopes that could be targeted by CD8+T cells following vaccination. Immune responses to subdominant epitopes may contribute to fight a viral infection, especially in the case of the emergence of cytotoxic T lymphocyte escape variants with mutations in a dominant epitope [37,38]. We recently described that immunization of AAD mice with inactivated virus induced CD8+T cell responses mainly to the immunodominant epitopes [25]. Here, we investigated whether MVA-delivered antigens could improve the breadth and specificity of primary CD8+T cell responses that are elicited in AAD mice following vaccination. Our data show that immunization with MVA-M1 virus elicited CD8+T cells specific to the HLA-A2-restricted immunodominant M158 epitope and, to a lesser extent, the overlapping peptide epitope M159, whereas lower reactivity was measured against the subdominant epitopes A2-M1130 and H2-Kb-M1128. Mice immunized with MVA-NP virus showed significant serum levels of antibodies against NP protein and high numbers of spleen cells reactive to the immunodominant epitope H2-Db-NP366. Additionally, we could detect the expression of PB1 in cells infected by MVA-PB1 and consistently measure a reactivity to the peptide epitopes A2-PB1501 and H2-Kb-PB1703 in mice vaccinated with this viral vector. Interestingly, a similar pattern in reactivity to all the peptides under study was also measured in mice vaccinated in combination with MVA-M1 and MVA-NP viruses. In contrast to a non-replicating whole virus-based vaccine, these data show that CD8+T cells to subdominant epitopes could be elicited when expressed by MVA vectors [25]. Considering the impact of multiple factors on establishing immunodominance hierarchies generated during the primary T cell responses, these results are also of interest because they show that a similar profile of CD8+T cells against dominant and subdominant HLA-A2-restricted epitopes of internal influenza proteins was elicited by either MVA-based vaccines or influenza virus infection.
Although we could not assess the real contribution of HLA-A2-restricted CD8+T cell responses for the presence of endogenous murine MHC class I molecules in AAD transgenic mice, it is conceivable that the A2-restricted subdominant epitopes on NP may only marginally contribute to survival against virus challenge. In addition to the contribution of CD8+T cells specific to the H2-Db-NP366 epitope, anti-NP antibodies most likely conferred effective anti-viral activities by promoting FcR-mediated phagocytosis and complement-mediated lysis of virus-infected cells [39]. Here, we have been able to show that MVA-Μ1 vaccine alone can induce a protective immunity against influenza in AAD mice. This finding is consistent with previous studies showing that M1-based vaccines are capable of inducing protection against influenza in HLA-A2 transgenic mice that generate A2-M158-specific CD8+T cells [40–42]. We could not detect M1-specific antibodies, as also reported previously in BALB/c mice immunized with the MVA-M1 vector [22]. Recent studies show that M1-specific antibodies elicited by DNA prime-vaccinia virus boost regimens based on M1 protein could, in the absence of specific T cells, confer protection (70% survival rate) after infection of BALB/c mice with 1.7 LD50 of the A/PR/8 virus [19]. Thus, strategies to ameliorate the M1-specific antibody response induced by MVA-based vaccine alone or in combination with other antigens would be extremely beneficial for these promising viral vectors. Previous studies have shown that NP protein accumulates in lipid rafts at the apical cell membrane, even when expressed alone, whereas the M1 protein seems to bind cell membranes through electrostatic interactions [43,44]. Thus, different localization and interaction of the M1 protein with the cell membrane may affect antigen presentation to B cells, and explain the lack of induction of M1-specific antibodies, compared to the NP protein [45]. In this context, it would be interesting to determine if the fusion of M1 with portions of NP or other conserved antigens, such as the transmembrane and extracellular domains of M2 protein, would increase the partition of M1 into the cell membrane and thus improve the immunogenicity of MVA-M1 vectors expressing these chimeric constructs.
Finally, our data reveal that both M1- and NP-based MVA vaccines, regardless of whether they were applied individually or in combination, conferred protection against lethal virus challenge in AAD mice and clearly correlated with the high level of recall responses to the immunodominant epitopes in these animals. Although mouse models do not closely reflect human influenza virus infection, we can provide further evidence of the efficacy of NP- and M1-based influenza vaccines. We could not demonstrate a protective efficacy of an MVA vector expressing PB1, as also reported by others [19,22]. Nevertheless, the inclusion of PB1 in the vaccine may still help boost pre-existing immune T cells in the context of different haplotypes and thus contributes to virus clearance.
In summary, we show that MVA vectors expressing influenza proteins could induce high numbers of influenza-specific CD8+T cells to epitope peptides, which are conserved among different viral strains, and provide a significant level of protection against lethal challenge in AAD mice. Although we are still far away from the ‘one-shot for life,’ boosting of T cell responses to conserved viral antigens is, together with cross-reactive antibodies, an essential step toward a successful universal vaccine against influenza.
Competing interests
The authors declare that they have no competing interests.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was funded by the Ministry of Health, Italy [grant number RF-2010-2318269] and Fondazione Cariplo [grant number 2009-3594].
Acknowledgment
We thank Andrea Giovannelli for assistance with the animal experiments.
References
- [1].Wright PF, Neumann G, Kawaoka Y. Orthomyxoviruses In: Knipe DM, Howley PM, editors. Fields Virology. 5th ed. Philadelphia, USA: Lippincot Williams & Wilkins; 2007. p. 1691-740. [Google Scholar]
- [2].Webster RG, Govorkova EA. Continuing challenges in influenza. Ann NY Acad Sci. 2014;1323:115–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Abt M, de Jonge J, Laue M, et al. Improvement of H5N1 influenza vaccine viruses: influence of internal gene segments of avian and human origin on production and hemagglutinin content. Vaccine. 2011;29:5153–62. [DOI] [PubMed] [Google Scholar]
- [4].Cox NJ, Hickling J, Jones R, et al. Report on the second WHO integrated meeting on development and clinical trials of influenza vaccines that induce broadly protective and long-lasting immune responses: Geneva, Switzerland, 5–7 May 2014. Vaccine. 2015; 33(48):6503-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Krammer F, Palese P. Influenza virus hemagglutinin stalk-based antibodies and vaccines. Curr Op Virol. 2013;3:521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Impagliazzo A, Milder F, Kuipers H, et al.. A stable trimeric influenza hemagglutinin stem as a broadly protective immunogen. Science. 2015; 349(6254):1301-6. 10.1126/science.aac7263 [DOI] [PubMed] [Google Scholar]
- [7].Yassine HM, Boyington JC, McTamney PM, et al.. Hemagglutinin-stem nanoparticles generate heterosubtypic influenza protection. Nat Med. 2015; 21(9):1065-70. 10.1038/nm.3927 [DOI] [PubMed] [Google Scholar]
- [8].Krammer F, Palese P. Advances in the development of influenza virus vaccines. Nat Rev. 2015;14:167–82. [DOI] [PubMed] [Google Scholar]
- [9].McMichael AJ, Gotch FM, Noble GR, et al. Cytotoxic T-cell immunity to influenza. N Engl J Med. 1983;309:13–17. [DOI] [PubMed] [Google Scholar]
- [10].Sridhar S, Begom S, Bermingham A, et al. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat Med. 2013;19:1305–12. [DOI] [PubMed] [Google Scholar]
- [11].Lee LY, Ha do LA, Simmons C, et al. Memory T cells established by seasonal human influenza A infection cross-react with avian influenza A (H5N1) in healthy individuals. J Clin Invest. 2008;118:3478–90. 10.1111/j.1365-2362.1990.tb01797.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].van de Sandt CE, Kreijtz JH, de Mutsert G, et al. Human cytotoxic T lymphocytes directed to seasonal influenza A viruses cross-react with the newly emerging H7N9 virus. J Virol. 2014;88:1684–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Altenburg AF, Rimmelzwaan GF, de Vries RD. Virus-specific T cells as correlate of cross-protective immunity against influenza. Vaccine. 2015;33:500–6. [DOI] [PubMed] [Google Scholar]
- [14].Kohlmeier JE, Woodland DL. Immunity to respiratory viruses. Annu Rev Immunol. 2009;27:61–82. [DOI] [PubMed] [Google Scholar]
- [15].de Bree GJ, van Leeuwen EM, Out TA, et al. Selective accumulation of differentiated CD8+ T cells specific for respiratory viruses in the human lung. J Exp Med. 2005;202:1433–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Purwar R, Campbell J, Murphy G, et al. Resident memory T cells (T(RM)) are abundant in human lung: diversity, function, and antigen specificity. PLoS ONE. 2011;6:e16245. 10.1371/journal.pone.0016245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Vitelli A, Quirion MR, Lo CY, et al. Vaccination to conserved influenza antigens in mice using a novel Simian adenovirus vector, PanAd3, derived from the bonobo pan paniscus. PLoS ONE. 2013;8(3):e55435. 10.1371/journal.pone.0055435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Antrobus RD, Coughlan L, Berthoud TK, et al. Clinical assessment of a novel recombinant Simian adenovirus ChAdOx1 as a vectored vaccine expressing conserved influenza A antigens. Mol Ther. 2014;22(3):668–74. 10.1038/mt.2013.284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wang W, Li R, Deng Y, et al. Protective efficacy of the conserved NP, PB1, and M1 proteins as immunogens in DNA- and vaccinia virus-based universal influenza A virus vaccines in mice. Clin Vacc Immunol. 2015;22(6):618–30. 10.1128/CVI.00091-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lillie PJ, Berthoud TK, Powell TJ, et al. Preliminary assessment of the efficacy of a T-cell-based influenza vaccine, MVA-NP+M1, in humans. Clin Infect Dis. 2012;55:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Boyd AC, Ruiz-Hernandez R, Peroval MY, et al. Towards a universal vaccine for avian influenza: protective efficacy of modified Vaccinia virus Ankara and Adenovirus vaccines expressing conserved influenza antigens in chickens challenged with low pathogenic avian influenza virus. Vaccine 2013;31:670–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hessel A, Savidis-Dacho H, Coulibaly S, et al. MVA vectors expressing conserved influenza proteins protect mice against lethal challenge with H5N1, H9N2 and H7N1 viruses. PLoS ONE. 2014;9(2):e88340. 10.1371/journal.pone.0088340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Altenburg AF, Kreijtz JH, de Vries RD, et al. Modified vaccinia virus Ankara (MVA) as production platform for vaccines against influenza and other viral respiratory diseases. Viruses. 2014;6:2735–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mullarkey CE, Boyd A, van Laarhoven A, et al. Improved adjuvanting of seasonal influenza vaccines: preclinical studies of MVA-NP+M1 coadministration with inactivated influenza vaccine. Eur J Immunol. 2013;43:1940–52. [DOI] [PubMed] [Google Scholar]
- [25].Di Mario G, Garulli B, Sciaraffia E, et al. A heat-inactivated H7N3 vaccine induces cross-reactive cellular immunity in HLA-A2.1 transgenic mice. Virol J. 2016;13:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Di Lullo G, Soprana E, Panigada M, et al. The combination of marker gene swapping and fluorescence-activated cell sorting improves the efficiency of recombinant modified Vaccinia virus Ankara vaccine production for human use. J Virol Meth. 2010;163:195–204. [DOI] [PubMed] [Google Scholar]
- [27].World Health Organization 2011. Global influenza surveillance network. Manual for the laboratory diagnosis and virological surveillance of influenza. http://whqlibdoc.who.int/publications/2011/9789241548090_eng.pdf
- [28].Newberg MH, Smith DH, Haertel SB, et al. Importance of MHC class I α2 and α3 domains in the recognition of self and non-self MHC molecules. J Immunol. 1996;156:2473–80. [PubMed] [Google Scholar]
- [29].Gianfrani C, Oseroff C, Sidney J, et al. Human memory CTL response specific for influenza A virus is broad and multispecific. Human Immunol. 2000;61:438–52. [DOI] [PubMed] [Google Scholar]
- [30].Assarsson E, Bui HH, Sidney J, et al. Immunomic analysis of the repertoire of T-cell specificities for influenza A virus in humans. J Virol. 2008;2:12241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Alexander J, Bilsel P, del Guercio MF, et al. Identification of broad binding class I HLA supertype epitopes to provide universal coverage of influenza A virus. Human Immunol. 2010;71:468–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Townsend AR, Rothbard J, Gotch FM, et al. The epitopes of influenza nucleoprotein recognized by cytotoxic T lymphocytes can be defined with short synthetic peptides. Cell. 1986;44:959–68. [DOI] [PubMed] [Google Scholar]
- [33].Vitiello A, Yuan L, Chesnut RW, et al. Immunodominance analysis of CTL responses to influenza PR8 virus reveals two new dominant and subdominant Kb-restricted epitopes. J Immunol. 1996;157:5555–5562. [PubMed] [Google Scholar]
- [34].Belz GT, Xie W, Doherty PC. Diversity of epitope and cytokine profiles for primary and secondary influenza A virus-specific CD8+ T cell responses. J Immunol. 2001;166:4627–33. [DOI] [PubMed] [Google Scholar]
- [35].Drexler I, Staib C, Sutter G. Modified vaccinia virus Ankara as antigen delivery system: how can we best use its potential? Curr Op Biotechnol. 2004;15:506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Gilbert SC. Clinical development of modified Vaccinia virus Ankara vaccines. Vaccine. 2013;31:4241–46. [DOI] [PubMed] [Google Scholar]
- [37].Oukka M, Manuguerra JC, Livaditis N, et al. Protection against lethal viral infection by vaccination with nonimmunodominant peptides. J Immunol. 1996;157:3039–45. [PubMed] [Google Scholar]
- [38].Holtappels R, Simon CO, Munks MW, et al. Subdominant CD8 T-cell epitopes account for protection against cytomegalovirus independent of immunodomination. J Virol. 2008;82:5781–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].LaMere MW, Lam H, Moquin A, et al. Contributions of antinucleoprotein IgG to heterosubtypic immunity against influenza virus. J Immunol. 2011;186:4331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Plotnicky H, Cyblat-Chanal D, Aubry JP, et al. The immunodominant influenza matrix T cell epitope recognized in human induces influenza protection in HLA-A2/Kb transgenic mice. Virology. 2003;309:320–9. [DOI] [PubMed] [Google Scholar]
- [41].Matsui M, Kohyama S, Suda T, et al. A CTL-based liposomal vaccine capable of inducing protection against heterosubtypic influenza viruses in HLA-A*0201 transgenic mice. Biochem Biophys Res Commun. 2010;391:1494–9. [DOI] [PubMed] [Google Scholar]
- [42].Suda T, Kawano M, Nogi Y, et al. The route of immunization with adenoviral vaccine influences the recruitment of cytotoxic T lymphocytes in the lung that provide potent protection from influenza A virus. Antiviral Research. 2011;91:252–8. [DOI] [PubMed] [Google Scholar]
- [43].Carrasco M, Amorim MA, Digard P. Lipid raft-dependent targeting of the influenza A virus nucleoprotein to the apical plasma membrane. Traffic. 2004;5:979–92. [DOI] [PubMed] [Google Scholar]
- [44].Ruigrok RW, Barge A, Durrer P, et al. Membrane interaction of influenza virus M1 protein. Virology. 2000;267:289–98. [DOI] [PubMed] [Google Scholar]
- [45].Heesters BA, van der Poel CE, Das A, et al. Antigen presentation to B cells. Trend Immunol. 2016; 37(12):844–54. [DOI] [PubMed] [Google Scholar]