ABSTRACT
Anti-human immunodeficiency virus type 1 (HIV-1) nonneutralizing antibodies (nnAbs) capable of antibody-dependent cellular cytotoxicity (ADCC) have been identified as a protective immune correlate in the RV144 vaccine efficacy trial. Broadly neutralizing antibodies (bNAbs) also mediate ADCC in cell culture and rely on their Fc region for optimal efficacy in animal models. Here, we selected 9 monoclonal nnAbs and 5 potent bNAbs targeting various epitopes and conformations of the gp120/41 complex and analyzed the potency of the two types of antibodies to bind and eliminate HIV-1-infected cells in culture. Regardless of their neutralizing activity, most of the selected antibodies recognized and killed cells infected with two laboratory-adapted HIV-1 strains. Some nnAbs also bound bystander cells that may have captured viral proteins. However, in contrast to the bNAbs, the nnAbs bound poorly to reactivated infected cells from 8 HIV-positive individuals and did not mediate effective ADCC against these cells. The nnAbs also inefficiently recognize cells infected with 8 different transmitted-founder (T/F) isolates. The addition of a synthetic CD4 mimetic enhanced the binding and killing efficacy of some of the nnAbs in an epitope-dependent manner without reaching the levels achieved by the most potent bNAbs. Overall, our data reveal important qualitative and quantitative differences between nnAbs and bNAbs in their ADCC capacity and strongly suggest that the breadth of recognition of HIV-1 by nnAbs is narrow.
IMPORTANCE Most of the anti-HIV antibodies generated by infected individuals do not display potent neutralizing activities. These nonneutralizing antibodies (nnAbs) with antibody-dependent cellular cytotoxicity (ADCC) have been identified as a protective immune correlate in the RV144 vaccine efficacy trial. However, in primate models, the nnAbs do not protect against simian-human immunodeficiency virus (SHIV) acquisition. Thus, the role of nnAbs with ADCC activity in protection from infection remains debatable. In contrast, broadly neutralizing antibodies (bNAbs) neutralize a large array of viral strains and mediate ADCC in cell culture. We analyzed the capacities of 9 nnAbs and 5 bNAbs to eliminate infected cells. We selected 18 HIV-1 strains, including virus reactivated from the reservoir of HIV-positive individuals and transmitted-founder isolates. We report that the nnAbs bind poorly to cells infected with primary HIV-1 strains and do not mediate potent ADCC. Overall, our data show that the breadth of recognition of HIV-1 by nnAbs is narrow.
KEYWORDS: ADCC, HIV-1, monoclonal antibodies, neutralizing antibodies, nonneutralizing antibodies, reservoir
INTRODUCTION
Broadly neutralizing antibodies (bNAbs) targeting the envelope of human immunodeficiency virus type 1 (HIV-1) are highly efficacious when passively administered in vivo. The most active bNAbs protect against virus acquisition or dampen viral replication in humanized mice and in macaques (1–6). Clinical trials in viremic patients revealed that 3BNC117 or VRC01, two bNAbs that target the CD4 binding site (CD4bs) of the envelope, reduce viremia by up to 2.5 logs (7, 8). 3BNC117 and, to a lesser extent, VRC01 can also delay viral rebound after antiretroviral treatment interruption (9, 10). A vaccine that could generate such bNAbs is likely to be protective (11), yet this is a challenging goal to achieve due to the rare elicitation of bNAbs during natural infection (12) and the unprecedented level of affinity maturation observed in the most active ones (13, 14).
The RV144 vaccine trial performed in Thailand achieved a modest, but significant, 31% protection (15). This is the only evidence of vaccine-induced protection against HIV-1 acquisition to date. Protection was not associated with the presence of broadly neutralizing antibodies in the serum of vaccinated persons but rather with anti-HIV-1 antibody-dependent cellular cytotoxicity (ADCC) activity in the absence of potentially competing anti-HIV-1 IgA antibodies (16–19). This raised the interesting possibility that non-broadly neutralizing but potent ADCC-mediating antibodies may have protective potential.
Nonneutralizing monoclonal antibodies (nnAbs) bind to numerous regions of the gp120/gp41 complex (20–22). Targeted epitopes include a gp41 immunodominant domain (gp41ID) that corresponds to a buried loop under the gp120 trimer (23) and different conformational CD4-induced (CD4i) epitopes revealed after Env binding to CD4 (21, 22, 24). Prototypic examples of CD4i antibodies are A32, belonging to the so-called cluster A antibodies and targeting the C1/C2 region, and 17b, targeting the coreceptor binding site (CoRBS) (25, 26). Other nnAbs target the CD4bs or the V3 loop of gp120, without preventing virus binding or entry of most HIV-1 strains (20, 27–29). Of note, nnAbs and bNAbs display differential binding to native-like soluble HIV-1 Env trimers, uncleaved Env proteins, and monomeric subunits (30). Moreover, the nnAbs tested so far do not protect against simian-human immunodeficiency virus (SHIV) acquisition in primate models, although they may marginally reduce viral loads or limit the number of founder viruses in a fraction of treated animals (23, 31–39).
Several lines of evidence suggest that ADCC plays a protective role in the host response to HIV-1 infection. For instance, viral escape has been reported for ADCC-targeted epitopes, suggesting the existence of ADCC-related immune pressure on the virus (40). Some but not all studies have correlated ADCC responses with lower viremia (41–43), and HIV controllers display elevated ADCC activity (44, 45). Regarding the virus itself, the accessory proteins Vpu and Nef decrease ADCC mediated by some monoclonal or polyclonal antibodies, likely by limiting the amount of Env at the surface of infected cells (24, 46–51). Mutation of the internalization motif in the gp41 cytoplasmic tail also increases Env surface exposure and susceptibility to ADCC (46). The kinetics of HIV-1 suppression in infected individuals by passively administered 3BNC117 suggest that the effects of the antibody are not limited to the neutralization of viral particles but also include an acceleration of the clearance of infected cells (52). Consistently, optimal therapeutic efficacy requires the Fc region of bNAbs (53–55).
It has been proposed that potent ADCC-mediating antibodies mainly target regions of Env that are recognized by nonneutralizing antibodies (gp120 CD4i or gp41ID epitopes) (21, 26, 50, 56–58). The ADCC activities mediated by nnAbs differ based on the epitope that they recognize (56, 57, 59). Moreover, the addition of CD4 mimetics to infected cells modifies the conformation of Env at the surface, allowing the exposure of CD4i epitopes and sensitizing the cells to nonneutralizing monoclonal or polyclonal antibodies (60–62). We and other demonstrated that a subset of bNAbs targeting the CD4bs, the V3 and V1V2 loops, and the membrane-proximal external region of gp41 (MPER) also exert a high level of ADCC in culture (51, 59, 63, 64). This indicates that Env epitopes can be targeted by antibodies with both neutralizing and ADCC functions. We also showed that the landscape of Env epitope exposure at the surface of infected cells and the subsequent sensitivity to ADCC vary considerably between viral strains (63). It is noteworthy that most of the studies regarding the ADCC activity of nnAbs in animal models or in cell culture systems have been performed by using a relatively limited number of different HIV-1 strains (24, 39, 49–51, 59, 60, 65–70). Moreover, the frequent use of gp120-coated cells (26, 57, 68, 71, 72) as targets in ADCC assays is a convenient tool, but it does not fully recapitulate the levels or conformation of Env at the surface of infected cells. Indeed, levels of Env at the surface of cells infected with a wild-type virus are relatively low. Moreover, the presence of CD4 may promote the transition of recombinant Env toward an open state, which is not usually observed with HIV-1-infected cells.
Here, we analyzed the ability of anti-HIV-1 nnAbs to perform ADCC in cell culture. We selected a panel of nnAbs isolated from elite neutralizers and displaying high-affinity binding to YU-2 gp140 trimers (20, 27–29). We performed a side-by-side comparison of the abilities of these nnAbs and of several of the most potent bNAbs to bind and kill infected cells through NK cell engagement. Almost all antibodies were able to trigger ADCC of cells infected by laboratory-adapted strains. However, the nnAbs were generally poorly active against primary HIV-1 reactivated from the reservoir or against transmitted-founder (T/F) viruses.
RESULTS
Presentation of nonneutralizing and broadly neutralizing antibodies used in this study.
We and other previously isolated a large panel of anti-HIV-1 Env monoclonal antibodies from elite neutralizers by the capture of a single memory B cell using a YU-2 gp140 bait (20, 27–29). Some of the antibodies displayed a broad neutralization profile (such as 3BNC117 or 10-1074), whereas others were ineffective or poorly active in neutralization assays (20, 27–29). Within this second category, we selected a panel of 9 nnAbs that bind with high affinity to canonical epitopes of the gp120/gp41 complex. These epitopes include the linear immunodominant domain on gp41 (gp41ID) (4-20 and 5-25), the CD4bs (1-863 and 2-1262), the CD4i epitopes in the CoRBS (4-8 and 4-42), and the variable loop 3 crown (V3c) (2-59, 10-188, and 11-340) (Table 1). In some of the experiments, we used the well-characterized A32 antibody, which binds another CD4i epitope localized in C1/C2 regions (22), as a control. All antibodies were cloned into the same IgG1 backbone. As expected, the gp41ID-specific antibodies did not neutralize the NL4.3 and NLAD8 strains or the CH058 T/F strain (Table 1). The other antibodies showed neutralization of some tier 1 and, more rarely, tier 2 viruses (Table 1) (20, 27–29). For comparison purposes, we also selected 5 potent bNAbs with previously described ADCC activity (59, 63), targeting the CD4bs (3BNC117 and NIH45-46), the N332/V3 loop (10-1074 and PGT128), and glycan-V1/V2 loops (PG16) (Table 1) (27, 29, 73, 74).
TABLE 1.
Epitope specificity and neutralization activity of the anti-HIV-1 monoclonal antibodies used in this study
| Antibody | Epitope | Reference | Neutralization IC50 (μg/ml) |
||
|---|---|---|---|---|---|
| NL4.3 | NLAD8 | CH058 | |||
| nnAbs | |||||
| 5-25 | gp41 immunodominant | 20 | >15 | >15 | >15 |
| 4-20 | gp41 immunodominant | 20 | >15 | >15 | >15 |
| 4-42 | CD4-induced CoRBS | 20 | 0.1 | >15 | >15 |
| 4-8 | CD4-induced CoRBS | 20 | 1.1 | >15 | >15 |
| 2-1262 | CD4 binding site | 20 | 0.24 | >15 | >15 |
| 1-863 | CD4 binding site | 20 | 0.84 | >15 | >15 |
| 2-59 | V3 crown | 20 | >15 | >15 | >15 |
| 10-188 | V3 crown | 28 | >15 | >15 | >15 |
| 11-340 | V3 crown | 28 | >15 | >15 | >15 |
| bNAbs | |||||
| 3BNC117 | CD4 binding site | 29 | 0.05 | 0.1 | 0.1 |
| NIH45-46 | CD4 binding site | 29 | 0.06 | 0.2 | 0.2 |
| 10-1074 | N322-glycan supersite | 27 | >15 | 0.1 | 0.2 |
| PGT128 | N322-glycan supersite | 73 | >15 | 0.2 | 0.02 |
| PG16 | V1/V2 glycans | 74 | 0.7 | 0.05 | >15 |
Binding and ADCC activity of nnAbs on cells infected with laboratory-adapted HIV-1 strains NLAD8 and NL4.3.
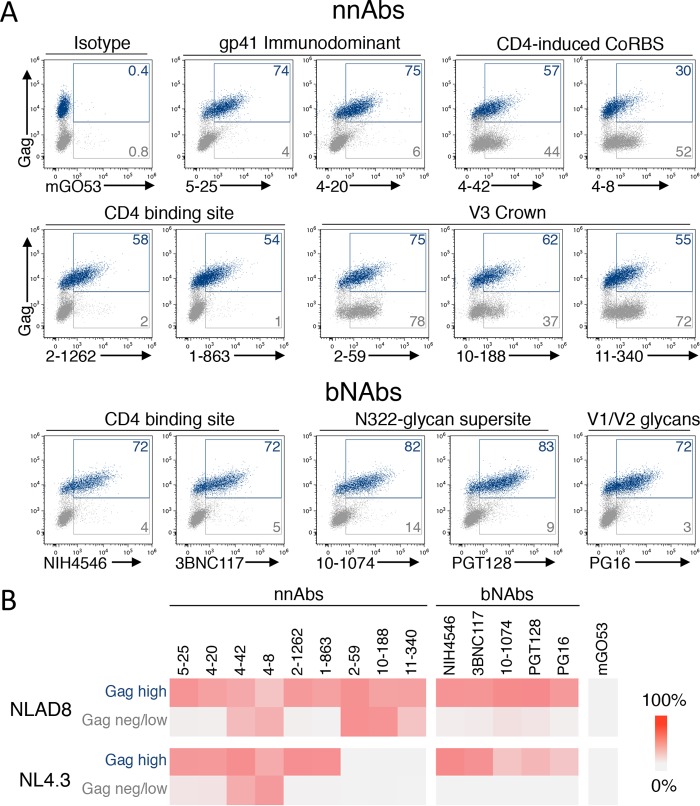
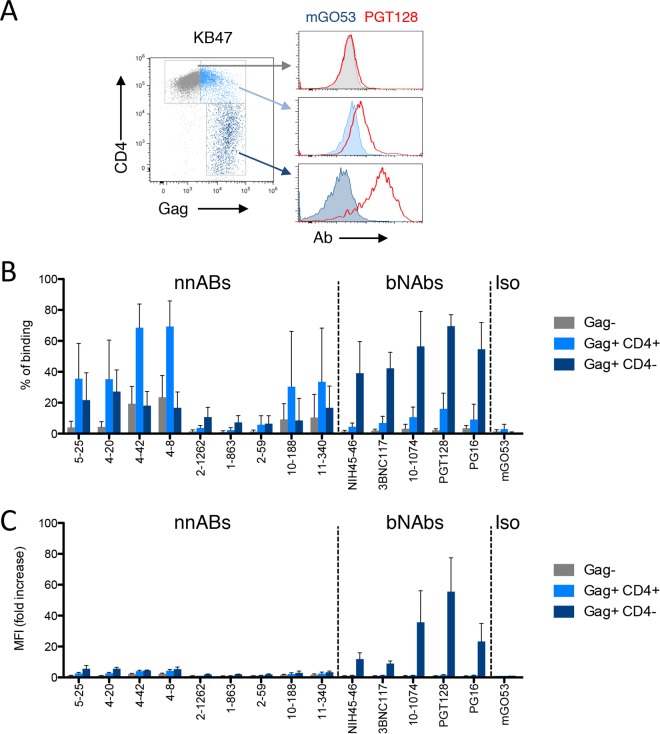
Strong antibody binding to HIV-1-infected cells is a prerequisite for ADCC activity (63, 75). We first asked whether the selected antibodies bound to T cells (CEM-NKR cell line) infected with the NLAD8 or NL4.3 reference strain. All tested antibodies bound to the fraction of cells productively infected (Gag high) with NLAD8 (Fig. 1A and B). Some of the nnAbs also bound to the fraction of Gag-low or Gag-negative (Gag−) cells present in the culture. For instance, the anti-gp41ID antibodies showed a “diagonal” population on Gag-low cells, while anti-CD4i antibodies equally bound to Gag-positive (Gag+) and Gag− cells. In contrast, the bNAbs efficiently bound to Gag+ cells without staining the bystander cell population (Fig. 1A and B). A similar profile of binding was observed with NL4.3-infected cells, except for the three V3 loop-targeting nnAbs, which did not recognize this X4-tropic envelope (Fig. 1B).
FIG 1.
Antibody binding at the surface of cells infected with two HIV-1 reference strains. (A) CEM-NKR cells infected with HIV-1 (NLAD8) were incubated with 15 μg/ml of anti-Env monoclonal antibodies. The levels of antibody bound on infected (Gag-high) and bystander (Gag-low and Gag-negative) cells were then evaluated by flow cytometry. Dead cells were excluded based on morphological criteria using side and forward scatters. A representative dot plot of each indicated antibody is presented. (B) CEM-NKR cells infected with HIV-1 (NLAD8 or NL4.3) were stained with the indicated antibodies, and the percentage of antibody-positive cells was measured by flow cytometry. The heat map represents the mean percentage of Ab+ cells in infected (Gag-high) or bystander (Gag-negative/low) cells obtained from 3 independent experiments.
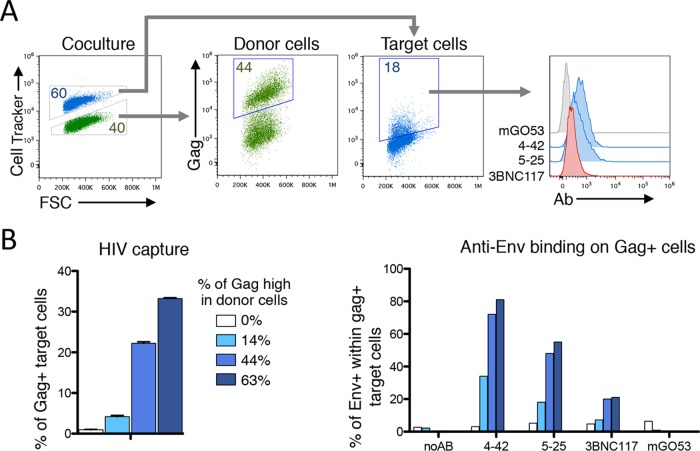
To further describe the binding of some antibodies to cells that do not express high levels of Gag, we cocultivated infected cells with target cells for 2 h and stained the cells with anti-Gag and anti-Env antibodies (Fig. 2). Low Gag staining was detected in a fraction of target cells, representing viral particles being transferred from infected cells to uninfected cells (76). The CD4i and gp41ID nnAbs bound to these Gag-low or Gag-negative target cells (Fig. 2). These results confirmed that some nnAbs bind to bystander uninfected cells that have captured HIV virions or that may be covered with shed gp120, likely because binding to CD4 induced conformational changes exposing the cryptic epitopes (61).
FIG 2.
Binding of anti-Env antibodies to bystander cells. CEM-NKR donor cells infected with HIV-1 (NL4.3 strain) were cocultivated for 2 h with uninfected target CEM-NKR cells stained with a fluorescent dye. Cells were then stained for Gag and Env and analyzed by flow cytometry. FSC, forward scatter. (A) Gating strategy for donor and target cells and levels of Gag and Env from one representative experiment. (B) The frequency of target cells that become Gag+ cells varies with the extent of infection of donor cells. (C) Levels surface binding of the indicated anti-Env antibodies among Gag+ target cells. mGO53 was used as a control.
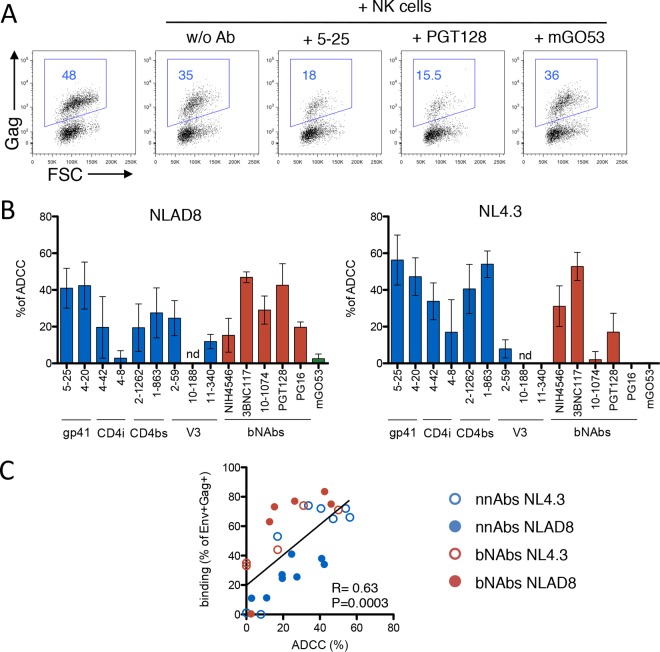
We then examined the ability of nnAbs to trigger ADCC against HIV-1-infected cells. CEM-NKR cells infected with NLAD8 or NL4.3 were preincubated with the different antibodies before coculture with primary NK cells for 4 h. We evaluated the disappearance of Gag+ target cells as a readout of ADCC activity (63). To facilitate comparisons, all antibodies were tested at high concentrations (15 μg/ml) (59, 63). A typical experiment showed that isotype control antibody mGO53 was inactive, whereas 5-25 and PGT128 induced the disappearance of about half of the NLAD8-infected cells (Fig. 3A). All nnAbs (except 4-8 and 10-188) and the 5 bNAbs induced ADCC against NLAD8-infected cells (Fig. 3B). NL4.3-infected cells were also sensitive to ADCC (Fig. 3B). The levels of ADCC were variable among the antibodies tested, ranging from 0% to 60%, and were positively correlated with the extent of binding to infected cells (Fig. 3C). Altogether, these data show that the majority of nnAbs tested bound to cells infected with the two laboratory-adapted HIV-1 strains and induced their killing through ADCC, displaying efficacies similar to those of bNAbs.
FIG 3.
Analysis of ADCC activities of nnAbs and bNAbs. (A) CEM-NKR cells infected with HIV-1 (NLAD8 strain) were incubated with 5-25, PGT128, or the mGO53 isotype antibody (all at 15 μg/ml) and with heterologous NK cells. After 4 h, the percentage of Gag+ CEM-NKR target cells (indicated in blue) was measured by flow cytometry. Data from one representative experiment (out of six) are shown. (B) CEM-NKR cells were infected by two laboratory-adapted HIV-1 strains (NLAD8 or NL4.3), and each antibody was tested with heterologous NK cells from at least three healthy donors. ADCC was calculated as the disappearance of infected cells with or without antibody. Negative values were set to zero (means ± standard errors of the means are shown). nd, not determined. (C) Correlates of ADCC activity. ADCC means were calculated and plotted with mean binding values (from Fig. 1B) for each antibody and the two viruses. Correlation was calculated by the Spearman correlation coefficient (r). nnAbs are color-coded in blue, bNAbs are in red, and the isotypic control is in green.
Activity of nnAbs against reactivated HIV-1-infected cells.
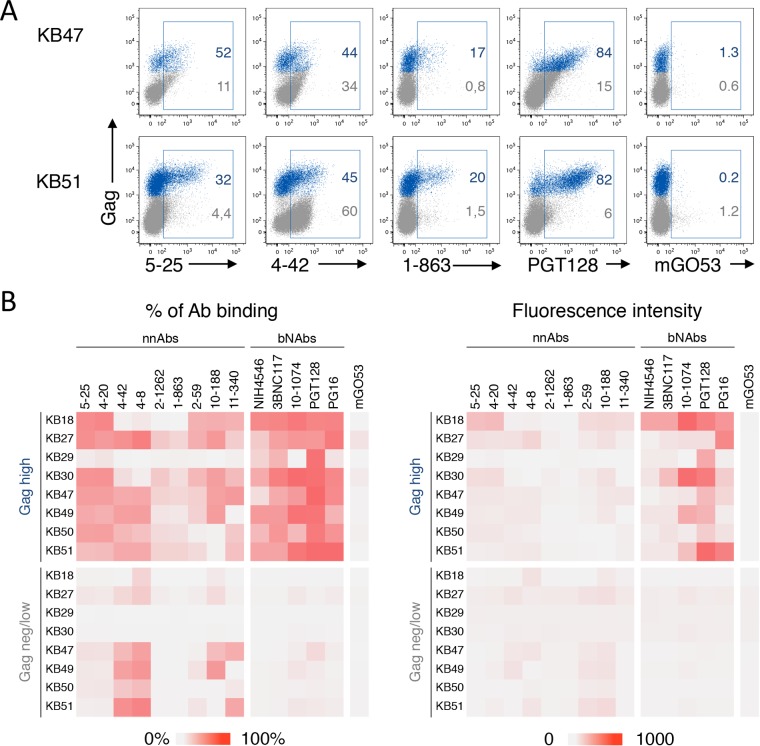
Primary HIV-1 isolates may be less sensitive to ADCC than laboratory-adapted strains (59, 63). To explore the sensitivity of primary HIV-1 to nnAbs, we first measured the exposure of Env epitopes at the surface of activated CD4 T cells isolated from patients' blood. We selected infected individuals under suppressive antiretroviral treatment (ART) (viral loads of <40 copies/ml) (Table 2). We used a viral outgrowth assay in which phytohemagglutinin (PHA) treatment activates resting CD4+ T cells and induces HIV-1 spread from latently infected cells (63). HIV-1 Gag+ cells began to be detected at days 7 to 12 postreactivation in 8 individuals (not shown), and their numbers increased over time, indicating that viruses were infectious. Cell surface Env expression was assessed with the panel of nnAbs and bNAbs (Fig. 4). Representative stainings of cells from two donors (donors KB47 and KB51) with 5 antibodies are presented in Fig. 4A, whereas a summary of all stainings, including both the percentage of infected cells positive for Env staining and the median fluorescence intensity (MFI) of Env staining, is displayed in Fig. 4B. In the representative examples, the nnAbs (5-25, 4-42, and 1-863) bound to Gag+ cells, but the MFI of binding was low. As observed with the laboratory-adapted strains, the CD4i nnAbs also bound to a subset of cells expressing undetectable or low levels of Gag, likely corresponding to bystander cells that may have captured viral material and/or to cells at an early stage of the viral life cycle. The PGT128 bNAb bound with high intensity to Gag+ cells and not to Gag− cells (Fig. 4A). A comprehensive analysis of the binding of the 8 nnAbs and 5 bNAbs on cells from the 8 patients confirmed these results (Fig. 4B). Some of the nnAbs bound to cells infected by the reactivated virus, but the intensity of binding remained low. The nnAbs also bound with low intensity to bystander cells. In contrast, the bNAbs displayed broader coverage and a higher intensity of binding to reactivated Gag+ cells, with minimal attachment to Gag− cells (Fig. 4).
TABLE 2.
Biological characteristics of the 8 patients with detectable HIV-1 reactivation
| Patient | Age (yr) | Duration of HAART (yr) | CD4 T cell count (cells/mm3) | RNA level (copies/ml) |
|---|---|---|---|---|
| KB18 | 55 | 9 | 2002 | <40 |
| KB27 | 54 | 4 | 495 | <40 |
| KB29 | 45 | 8 | 366 | <40 |
| KB30 | 39 | 5 | 730 | <40 |
| KB47 | 46 | 8 | 638 | <40 |
| KB49 | 50 | 7 | 1329 | <40 |
| KB50 | 43 | 5 | 684 | <40 |
| KB51 | 69 | 26 | 536 | <40 |
FIG 4.
Binding of anti-Env antibodies on reactivated HIV-1-infected cells from the viral reservoir in patients on HAART. (A) Purified CD4+ T cells from the 2 indicated patients on HAART (patients KB47 and KB51) were activated, and viral replication was monitored by flow cytometry. When the percentage of Gag+ cells was >5%, cells were stained with the indicated Abs. The data indicate the percentage of NAb+ cells among Gag+ cells. Infected cells (Gag high) are shown in blue, and bystander cells (Gag negative/low) are shown in gray. Data from one representative experiment (out of 2 to 3 for each patient) are shown. (B) Heat maps representing the percentage of Ab+ cells (left) and the median fluorescence intensity of Ab staining (right) in infected or bystander cells obtained from each patient.
We further documented the binding of anti-Env antibodies to reactivated lymphocytes from patients by performing costaining with an anti-CD4 antibody. We reasoned that cells that have downregulated CD4 are likely to be productively infected and in an advanced stage of the viral life cycle, since Vpu, Nef, and Env are each able to interfere with CD4 cell surface expression (77, 78). The Gag+ cells were clearly composed of two populations of cells, expressing CD4 or not at the cell surface (Fig. 5). As expected, the Gag MFI was higher in CD4− cells, which were preferentially and strongly bound by bNAbs. The situation was different with the nnAbs, which preferentially bound to CD4+ cells expressing low levels of Gag (Fig. 5). The MFI of nnAb binding to CD4+ cells was low, confirming our results obtained with the whole cell population (Fig. 4B).
FIG 5.
Binding of anti-Env antibodies to reactivated HIV-1-infected cells from the viral reservoir. Purified CD4+ T cells from a patient on HAART (patient KB47) were activated, and viral replication was monitored by flow cytometry. When the percentage of Gag+ cells was >5%, cells were stained with the indicated Abs. (A) Cells were stained with anti-CD4 and anti-Gag antibodies and with PGT128 or the isotype control mGO53. Three cell populations were defined, depending on the levels of Gag and CD4. (B and C) Frequencies of antibody-positive cells (B) and median fluorescence intensities of staining (C) for each of the three cell populations (n = 4; means ± standard errors of the means).
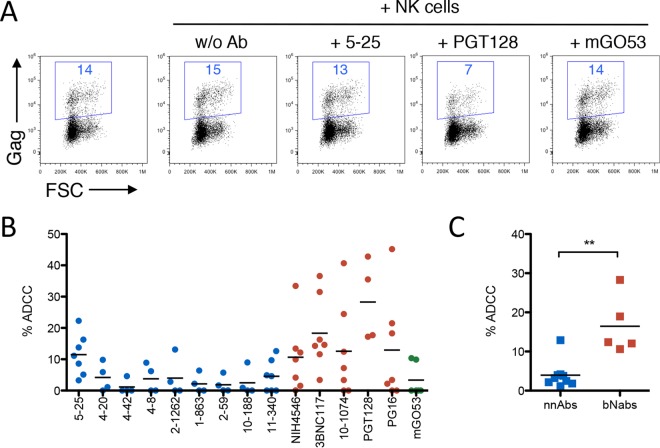
We then used our panel of antibodies to test the sensitivity of reactivated cells to ADCC. In a representative example with cells from donor KB51, the 5-25 nnAb was ineffective, whereas PGT128 depleted half of the infected cells (Fig. 6A). Each antibody from the panel was then tested against reactivated cells from 4 to 6 donors (Fig. 6B). None of the nnAbs tested displayed detectable ADCC activity against these primary isolates, except for 5-25, which was moderately active against cells from some donors. In contrast, bNAbs were generally ADCC potent on cells from the majority of donors tested. Collectively, the bNAbs display significantly higher levels of ADCC than do the nnAbs (Fig. 6C). Altogether, these results indicate that nnAbs may bind to some cells expressing reactivated viral strains, but the intensity of binding is generally not sufficient to allow ADCC.
FIG 6.
ADCC activity of nnAbs and bNAbs on reactivated HIV-1-infected cells from patients. (A) Reactivated cells from one representative patient (patient KB51) were incubated with 5-25, PGT128, or the mGO53 isotype antibody (all at 15 μg/ml) and with heterologous NK cells. After 6 h, the percentage of Gag+ target cells (indicated in blue) was measured by flow cytometry. (B) Summary of ADCC observed for each antibody against reactivated CD4 T cells isolated from patients. Broadly neutralizing antibodies are color-coded in red, nonneutralizing antibodies are in blue, and the isotype control is in green. Each dot represents data for one patient, tested with the indicated Abs and NK cells isolated from 2 to 3 heterologous healthy donor cells. (C) Comparison of ADCC activities observed with nnAbs and bNAbs. Each dot represents the mean ADCC activity of each Ab, tested on cells from 6 to 8 patients. Black bars indicate the means (**, P < 0.005 by a Mann-Whitney test).
Binding of nnAbs to cells infected with T/F HIV-1 strains.
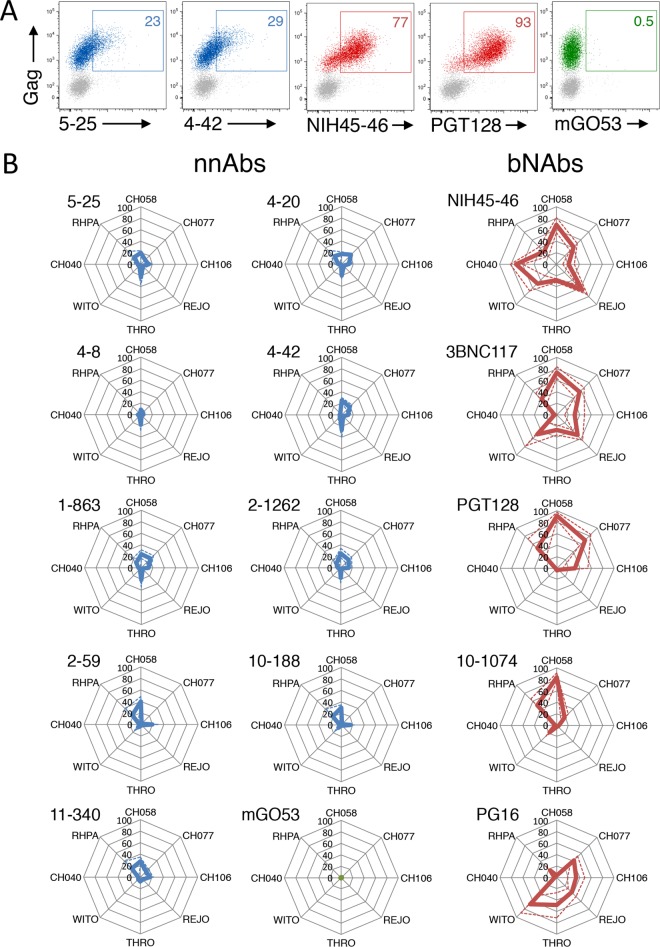
We extended our analysis of the recognition of infected cells by nnAbs to other primary HIV-1 strains that might be relevant during transmission between individuals. CEM-NKR cells were infected with 8 T/F HIV-1 strains (79) and costained with anti-Gag and anti-Env antibodies. A representative example with CH058 shows that two nnAbs (5-25 and 4-42) bound poorly to infected cells, whereas the NIH45-46 and PGT128 bNAbs bound strongly (Fig. 7A). When the whole panel of nnAbs and bNAbs was tested against the 8 T/F viruses, we observed a strong dichotomy between the two categories of antibodies. The percentage of infected cells stained with nnAbs remained consistently low (Fig. 7B), and among positive cells, the MFI of staining was also low for nnAbs (not shown). These results confirmed that the binding of bNAbs to T/F HIV-1 strains is variable (63). However, each of the 5 bNAbs bound to 3 to 5 of the 8 T/F strains, and a strong intensity of staining was often observed (Fig. 7A and data not shown). Conversely, each of the 8 T/F viruses was targeted by 1 to 5 of the 5 bNAbs (Fig. 7B and data not shown).
FIG 7.
Limited recognition of T/F HIV-1-infected cells by nnAbs. (A) CEM-NKR cells infected with T/F HIV-1 (strain CH058) were incubated with the indicated Abs (at 15 μg/ml). The numbers indicate the percentages of bNAb+ cells among infected (Gag+) cells. Data from one representative experiment (out of 4) are shown. (B) CEM-NKR cells infected with 8 T/F HIV-1 strains (CH040, CH058, CH077, CH106, THRO, REJO, RHPA, and WITO) were incubated with the indicated Abs (at 15 μg/ml). Radar plots represent the mean percentages (plain lines) and standard deviations (dashed lines) of Ab+ cells among infected (Gag+) cells evaluated by flow cytometry, from 3 independent experiments. nnAbs are in blue, and bNAbs are in red.
Therefore, the breadth of recognition of cells infected with primary HIV isolates, including reactivated virus from the reservoir and T/F strains, is particularly limited for nnAbs.
A small CD4-mimetic sulfopeptide conjugate modulates the activity of nnAbs.
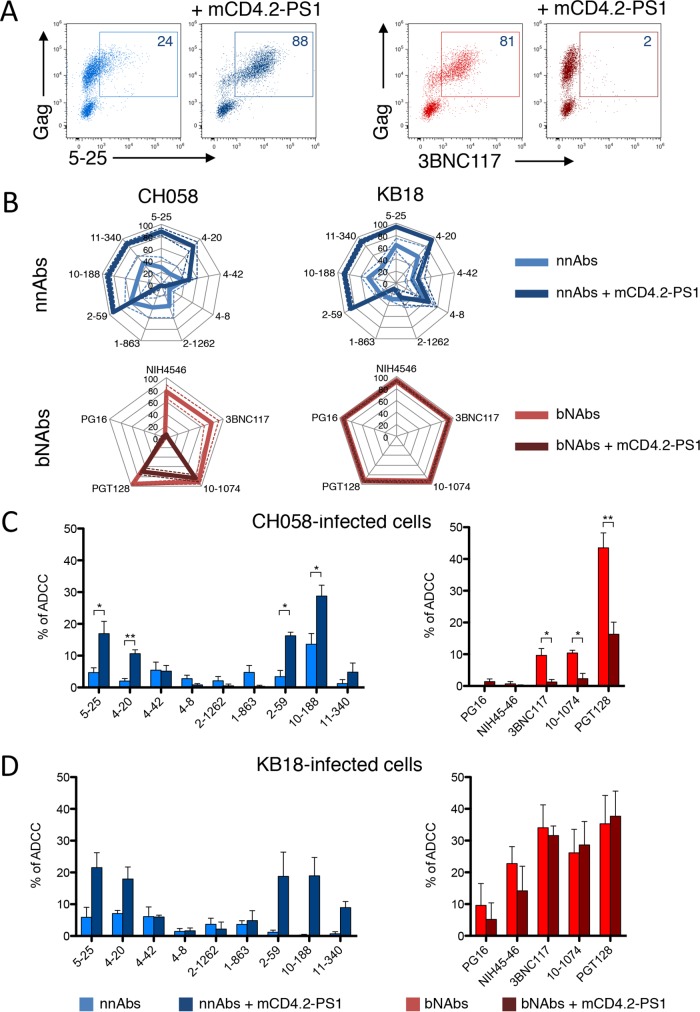
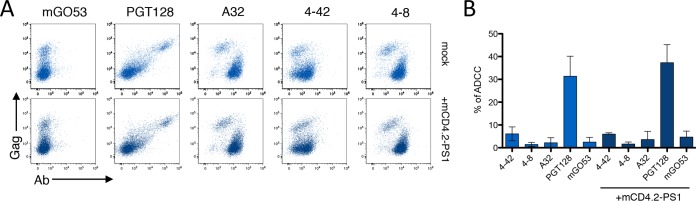
Small CD4 mimetics modify the conformation of Env at the cell surface and sensitize HIV-1-infected cells to ADDC mediated by nonneutralizing polyclonal antibodies present in sera and other fluids from HIV-1-infected individuals (60). We thus examined the effect of mCD4.2-PS1 on the binding and ADCC activity of our panel of nnAbs and bNAbs. mCD4.2-PS1 is a recently described CD4-mimetic sulfopeptide conjugate that binds to HIV-1 Env and inhibits cell-free and cell-associated HIV-1 with particularly low 50% inhibitory concentrations (IC50s), in the picomolar-to-nanomolar range (80). mCD4.2-PS1 binds gp120 through its mCD4 moiety and induces the structural modifications necessary to expose the coreceptor binding domain, which, as a result, becomes available to be blocked by the PS1 moiety (81). We first examined the effect of mCD4.2-PS1 on Env epitope exposure. CEM cells infected with NLAD8 or CH058 and primary CD4+ T cells infected with the reactivated virus from patient KB18 (KB18v) were incubated with mCD4.2-PS1 (10 nM) for 10 min before staining with the panel of nnAbs and bNAbs. A representative example with CH058-infected cells shows that the binding of the 5-25 nnAb was strongly enhanced by mCD4.2-PS1, whereas the staining of the 3BNC117 bNAb was decreased, likely because both molecules compete for the CD4bs on gp120 (Fig. 8A). An analysis of the panel of antibodies confirmed that for cells infected with the CH058 and KB18v viruses, the staining of some nnAbs (mostly those targeting the V3 loop and the gp41ID epitopes) was increased (Fig. 8B). As expected, the nnAbs targeting the CD4bs were inhibited in their binding, whereas the CD4i epitope was not significantly enhanced. The sensitivity of bNAbs to mCD4.2-PS1 varied depending on the viral strain, but none of the 5 bNAbs tested displayed enhanced binding after treatment with mCD4.2-PS1 (Fig. 8B).
FIG 8.
A synthetic CD4 mimetic modulates binding of nnAbs and bNAbs and ADCC. (A) CEM-NKR cells infected with T/F HIV-1 (strain CH058) were incubated with mCD4.2-PS1 (10 nM) for 10 min before measurement of Ab binding by flow cytometry. Data from one representative experiment (out of 3) are shown. (B) CEM-NKR cells infected with T/F HIV-1 (strain CH058) and primary CD4 T cells infected with HIV strain KB18v from patient KB18 were incubated or not with mCD4.2-PS1 before measurement of Ab binding by flow cytometry. nnAbs (top) (in blue) and bNAbs are depicted separately. Radar plots represent the mean percentages (plain lines) and standard deviations (dashed lines) of Ab+ cells among infected (Gag+) cells evaluated by flow cytometry, from 3 independent experiments. (C and D) ADCC was evaluated in the presence or absence of mCD4.2-PS1 by using CEM-NKR cells infected with T/F HIV-1 (strain CH058) (C) or CD4 T cells infected with HIV-1 strain KB18v (D). Results are means ± standard errors of the means with NK cells from three donors (*, P < 0.05; **, P < 0.005 [by a Mann-Whitney test]).
We then assessed the effect of mCD4.2-PS1 on the ADCC activity of the nnAbs and bNAbs. T cells infected with either CH058 or KB18v were incubated or not with mCD4.2-PS1 before being used as targets in ADCC assays (Fig. 8C and D). The small CD4 mimetic enhanced the ADCC potency of some nnAbs, reflecting their increased binding to infected cells. The ADCC efficacy of the bNAbs was decreased with CH058 and not affected with KB18v, mirroring the effect of mCD4.2-PS1 on bNAb binding.
We then examined how the prototypic CD4i anti-cluster A A32 antibody (25) behaves in our assays, since A32-like antibodies constitute the majority of the ADCC activity observed in HIV-1-infected or RV144-vaccinated individuals (21, 26, 50, 68, 71, 82). Using lymphocytes infected with KB18v, we observed that the profile of A32 binding was comparable to that of CD4i CoRBS antibodies 4-8 and 4-42, with preferential staining for Gag-low or -negative cells (Fig. 9). The intensity of staining was not enhanced by mCD4.2-PS1 (Fig. 9). Accordingly, none of the three CD4i nnAbs (targeting either cluster A or the CoRBS) induced efficient ADCC against KB18v-infected cells in the absence or presence of mCD4.2-PS1 (Fig. 9).
FIG 9.
A synthetic CD4 mimetic modulates binding of nnAbs and bNAbs and ADCC. (A) Primary CD4 T cells infected with HIV-1 (KB18v isolate) were incubated with mCD4.2-PS1 (10 nM) for 10 min before measurement of Ab binding by flow cytometry. Data from one representative experiment (out of 3) are shown. (C) ADCC was evaluated in the presence or absence of mCD4.2-PS1. Results are means ± standard errors of the means from three independent experiments. Results are means ± standard errors of the means with NK cells from three donors.
Altogether, these results indicate that a small CD4 mimetic enhances the binding and ADCC activity of some but not all nnAbs. However, the combination of mCD4.2-PS1 and nnAbs does not seem to be superior to bNAbs used without the mimetic.
DISCUSSION
A correlate analysis of the RV144 vaccine trial suggested a protective role of nnAbs displaying ADCC activity in the prevention of HIV-1 acquisition (17–19). However, subsequent studies in primates failed to demonstrate a protective role of passively administered nonneutralizing single or pooled monoclonal antibodies or polyclonal antibodies (23, 33, 34, 37, 38). The transfer of polyclonal, ADCC-inducing antibodies isolated from an elite controller that exhibited nonneutralizing control of infection also did not protect against SHIV challenge (36). In contrast, the passive transfer of the most active bNAbs mediates sterilizing protection in primate models (1–6). Thus, the role of nonneutralizing antibodies with ADCC activity in protection from infection or slowing disease progression remains debatable and partly understood. Here, we have addressed this question by characterizing the activity of a panel of nine nonneutralizing monoclonal antibodies in cell culture systems. We analyzed the ability of the nnAbs to bind to HIV-1-infected cells and to mediate ADCC through NK lysis. The nnAbs were selected to cover some of the known nonneutralizing epitopes present on the gp120/gp41 complex and to bind with high affinity to the HIV-1 YU-2 gp140 trimer (20, 27–29). Their activity was compared to those of five of the most active bNAbs, including those that we and others recently demonstrated to be potent in eliminating HIV-1-infected cells through ADCC (51, 59, 63).
We analyzed the binding activity of the nonneutralizing and neutralizing antibodies in lymphocytes infected with up to 18 different HIV-1 strains, including 2 HIV-1 reference strains (NL4.3 and NLAD8), 8 isolates reactivated from patients under suppressive treatment, and 8 T/F viruses. Strikingly, the nnAbs efficiently bound to cells infected with the reference strains but were generally modestly able to bind to T cells infected with the primary isolates. Our results reveal an unexpected lack of breadth of nnAbs, a phenomenon that cannot be observed in classical neutralization assays with cell-free viral particles, since the antibodies are, by definition, nonneutralizing. Moreover, we report that nnAbs often bind preferentially to bystander cells present in the culture. Bystanders are defined as cells displaying low levels of Gag and an absence of CD4 downregulation and likely correspond to cells that have recently captured viral particles or are covered by shed gp120 from neighboring productively infected cells. This preferential binding to bystander cells was previously reported for other monoclonal or polyclonal nonneutralizing antibodies (61, 83). This is likely the consequence of intrinsic properties of nnAbs, which are not able to bind to the fully closed state of the viral envelope (84). The binding of Env to CD4 molecules present on noninfected cells will induce conformational changes, revealing the hidden epitopes targeted by some of the nnAbs. For instance, the binding of gp41ID nnAbs to both Gag+ and Gag− cells is probably due to the recognition of a nonfunctional trimeric Env spike, more specifically gp41 stumps (83). In comparison with the nnAbs, the recognition profile of bNAbs is wide, and they preferentially target productively infected cells rather than neighboring cells.
We and other previously reported that a prerequisite for the ADCC activity of a given bNAb is its ability to stably bind to infected cells (46, 56, 59, 63). The affinity of binding to target cells, measured as the MFI of staining analyzed by flow cytometry, also correlates with the efficacy of ADCC (63). In agreement with the poor binding of the nnAbs to most of the viral isolates tested, we show that these antibodies are inefficient at eliminating cells producing primary virus from the viral reservoir or cells infected with T/F strains. This is again in contrast to the ability of bNAbs to eliminate infected cells, as demonstrated here and in recent studies in cell culture and in vivo (51, 52, 59, 63).
Small CD4 mimetics with the capacity to trigger the CD4-bound conformation of Env enhance the recognition of infected cells by serum or other antibody-containing fluids from HIV-infected individuals (60–62). Here, we have tested the impact of mCD4.2-PS1, a CD4-mimetic sulfopeptide conjugate, on the efficacy of our panel of nnAbs. In agreement with data from previous reports, we observed an enhancement of binding and ADCC activity by some of the nnAbs, indicating that the compound induced conformational changes in Env at the surface of infected cells. However, the increased efficacy was somewhat modest and did not reach the antiviral effect observed with the bNAbs. Interestingly, mCD4.2-PS1 facilitated the binding of nnAbs targeting the gp41ID or the V3 epitopes but did not induce the exposition of the CD4i epitope (using either anti-CoRBS or anti-cluster A antibodies), at least with the 2 viral strains tested. This can be due to the structure of mCD4.2-PS1, including a sulfopeptide conjugate targeting the CD4i epitope, thus competing with the binding of CD4i antibodies (81). However, we cannot exclude that this compound may stabilize Env in a conformation different from that observed for other CD4 mimetics. It has also been reported that small CD4 mimetics require the addition of antibodies targeting the coreceptor binding site to facilitate recognition by CD4i nnAbs (62).
It has been proposed that CD4i antibodies or other nnAbs that recognize Env epitopes exposed after virus binding to uninfected cells may mediate ADCC at an early step of the viral replication cycle (21, 57, 85, 86). Such antibodies might thus mediate the elimination of infected cells more efficiently than those targeting epitopes exposed at later stages of infection. However, our results show that the intensity of nnAb staining of bystander cells does not surpass that of bNAbs on productively infected cells. Moreover, we did not detect a significant elimination of bystander cells by nnAbs when we used the number of NK cells as a reference in our ADCC assay (not shown).
We cannot rule out that the 9 nnAbs tested here are not the most potent ones. However, we show that the prototypic anti-cluster A CD4i antibody A32 (22) does not display strong ADCC against cells infected by a virus isolated from one patient. Numerous other nnAbs have been isolated and tested in ADCC assays (57, 65, 67, 68, 71, 87). It will be worth determining whether they display a broader recognition of HIV-1-infected cells, since most previous studies were based on a relatively low number of primary cross-clade viral isolates or used gp120-coated cells as ADCC targets (24, 39, 50, 60, 65–70). Future experiments in animal models and in human cell cultures will also help evaluate the efficacy of nnAbs, used alone or in combination with other nnAbs, bNAbs, and CD4 mimetics, in the elimination of infected cells.
In summary, our data suggest that the breadth of recognition of HIV-1-infected cells by nnAbs is narrow and that bNAbs display a broader and higher ADCC capacity than do nnAbs.
MATERIALS AND METHODS
Cells and viruses.
The CEM-NKR-CCR5 cell line (referred to as CEM-NKR) was obtained from the NIH AIDS Reagent Program. NK cells were purified from human peripheral blood (obtained anonymously from the Etablissement Français du Sang [EFS]) by density gradient centrifugation followed by immunomagnetic selection (Miltenyi). The purity of NK cells was 90 to 98%. NK cells were maintained in complete medium, and interleukin-2 (IL-2) (50 IU/ml) was added the day before use. Virus stocks were prepared by the transfection of 293T cells (obtained from the ATCC) along with vesicular stomatitis virus G (VSV-G) to normalize infectivity (88). Cells were infected with NL4.3, NLAD8, and transmitted-founder HIV-1 strains (CH040, CH058, CH077, CH106, RHPA, THRO, REJO, and WITO; obtained from the NIH AIDS Reagent Program) as described previously (89). Viral inocula (25 to 100 ng of p24/106 cells) were adjusted to achieve similar levels of Gag+ cells (around 40 to 50%) at 48 h postinfection.
Antibodies and the CD4 mimetic.
Anti-Env nnAbs and bNAbs, as well as the isotypic control mGO53, were produced as recombinant monoclonal antibodies carrying the same human IgG1 Fc region by the cotransfection of 293T or 293F cells (obtained from the ATCC) as previously described (27). Antibodies were purified by batch/gravity-flow affinity chromatography using protein G-Sepharose 4 fast-flow beads (GE Healthcare). The CD4 mimetic (mCD4.2-PS1) was prepared by chemical synthesis and characterized as previously described (80).
ADCC assay.
The ADCC assay was performed as previously described (63). HIV-1-infected target CEM-NKR or primary CD4 T cells (obtained anonymously from the EFS) were stained by using the Far-Red cell tracker (Life Technologies). Totals of 2 × 104 to 5 × 104 targets were plated into U-bottom 96-well plates and incubated with antibodies (15 μg/ml) for 5 min at room temperature. Heterologous NK cells were added to each well (at a ratio of 1 CEM-NKR cell to 10 NK cells or 1 primary CD4 T cell to 1 NK cell). Plates were spun for 1 min at 300 × g to promote cell contacts and incubated at 37°C for 6 h (for primary CD4 T cells) or 4 h (for CEM-NKR cells). Cells were then stained for intracellular Gag as previously described (88). Data were acquired on a FACS Canto II instrument (BD Biosciences) or an Attune Nxt instrument (Life Technologies) and analyzed by using FlowJo software. The frequencies of Gag+ cells among Far-Red+ cells were determined. ADCC was calculated by using the following formula: 100 × (% of Gag+ target cells plus NK cells without antibody − % of Gag+ target cells plus effector cells with antibody)/(% of Gag+ target cells plus NK cells without antibody). Negative values were set to zero. In some experiments, we used the number of NK cells in the culture as a reference to specifically measure the disappearance of p24− cells.
Staining of HIV-1-infected cells with nnAbs and bNAbs.
Cells (0.5 × 104 to 2 × 104 cells per well) were incubated for 30 min at 37°C with anti-Env bNAbs or with an isotypic human IgG1 control (mGO53) at 15 μg/ml diluted in culture medium. Cells were then washed and incubated for 30 min at 4°C with anti-human IgG1(H+L) Alexa Fluor 647 (1:400 dilution; Life Technologies). Cells were then fixed with 4% paraformaldehyde (PFA) and processed for intracellular Gag staining.
Neutralization assay.
Neutralization of cell-free HIV-1 was measured by using the Tzm-BL cell line (obtained from the NIH AIDS Reagent Program) as previously described (90). Dose-response inhibition curves were drawn by fitting the data to sigmoid dose-response curves (variable slope) using GraphPad Prism software. The percentage of inhibition was defined as (% signal in nontreated target cells − % signal in bNAb-treated cells)/(% signal in nontreated target cells) × 100. The IC50 was calculated with GraphPad Prism.
Reactivation of HIV-1 from highly active antiretroviral therapy (HAART)-treated patients.
For each patient, 50 ml of blood was harvested in the presence of EDTA. Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll gradient purification, and CD4 T cells were purified as described above. For the viral outgrowth assay, CD4 T cells were stimulated with PHA-M (2 mg/ml; Sigma-Aldrich) or anti-CD2,3 and -CD28 beads (1 bead for 2 cells; Miltenyi Biotech) with 100 IU/ml of IL-2 (R&D) at 1 × 106 cells/ml. Every 2 to 3 days, 1 ml of the supernatant was harvested and replaced with fresh medium. At the indicated time points, cells were evaluated for Gag expression and antibody binding by flow cytometry. Cells were used for ADCC experiments when the fraction of Gag+ cells was above 5%. The KB18v HIV-1 strain isolated from patient KB18 was amplified once in primary CD4+ T cells.
Data processing and statistical analysis.
Calculations were performed and figures were drawn by using Excel 2011 or GraphPad Prism 5.0. Statistical analysis was performed by using GraphPad Prism, with Wilcoxon matched-paired t tests or Mann-Whitney unpaired t tests. Spearman correlation coefficients (r) were calculated by using GraphPad Prism.
Ethics statement.
All patients were from the Hôpital Kremlin Bicêtre (Kremlin Bicêtre, France) under successful HAART (Table 2). Each participant provided written consent to participate in the study, which was approved by the regional investigational review board (IRB) (Comité de Protection des Personnes Ile-de-France VII, Paris, France) and performed according to European guidelines and the Declaration of Helsinki. All samples were anonymized. All subjects were adults.
ACKNOWLEDGMENTS
We thank members of the Virus and Immunity Unit for discussions and help and Daniel Aaron Donahue for critical readings of the manuscript. We thank patients who participated in the study and Cecile Goujard (Hôpital Kremlin Bicêtre) for supervision of the patients' enrollment. We thank the NIH AIDS Reagent Program for providing reagents. We thank Michel C. Nussenzweig (The Rockefeller University) for providing the antibody expression vectors.
O.S. was supported by grants from the Vaccine Research Institute (ANR-10-LABX-77), the Agence Nationale de Recherche sur le SIDA et les Hepatitis (ANRS) (AO 2015-2 CSS-2), the Labex Integrative Biology of Emerging Infectious Diseases program (ANR-10-LABX-62-IBEID), the FP7 program Hit Hidden HIV (Health-F3-2012-305762), and the Institut Pasteur. H.M. was supported by the European Research Council-Seventh Frame-Work Program (ERC-2013-StG 337146), the ANRS, the G5 Institut Pasteur Program, and The Milieu Intérieur Program (ANR1-101LABX-69-01).
REFERENCES
- 1.Klein F, Halper-Stromberg A, Horwitz JA, Gruell H, Scheid JF, Bournazos S, Mouquet H, Spatz LA, Diskin R, Abadir A, Zang T, Dorner M, Billerbeck E, Labitt RN, Gaebler C, Marcovecchio PM, Incesu R-B, Eisenreich TR, Bieniasz PD, Seaman MS, Bjorkman PJ, Ravetch JV, Ploss A, Nussenzweig MC. 2012. HIV therapy by a combination of broadly neutralizing antibodies in humanized mice. Nature 492:118–122. doi: 10.1038/nature11604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horwitz JA, Halper-Stromberg A, Mouquet H, Gitlin AD, Tretiakova A, Eisenreich TR, Malbec M, Gravemann S, Billerbeck E, Dorner M, Büning H, Schwartz O, Knops E, Kaiser R, Seaman MS, Wilson JM, Rice CM, Ploss A, Bjorkman PJ, Klein F, Nussenzweig MC. 2013. HIV-1 suppression and durable control by combining single broadly neutralizing antibodies and antiretroviral drugs in humanized mice. Proc Natl Acad Sci U S A 110:16538–16543. doi: 10.1073/pnas.1315295110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barouch DH, Whitney JB, Moldt B, Klein F, Oliveira TY, Liu J, Stephenson KE, Chang H-W, Shekhar K, Gupta S, Nkolola JP, Seaman MS, Smith KM, Borducchi EN, Cabral C, Smith JY, Blackmore S, Sanisetty S, Perry JR, Beck M, Lewis MG, Rinaldi W, Chakraborty AK, Poignard P, Nussenzweig MC, Burton DR. 2013. Therapeutic efficacy of potent neutralizing HIV-1-specific monoclonal antibodies in SHIV-infected rhesus monkeys. Nature 503:224–228. doi: 10.1038/nature12744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shingai M, Nishimura Y, Klein F, Mouquet H, Donau OK, Plishka R, Buckler-White A, Seaman M, Piatak M, Lifson JD, Dimitrov DS, Nussenzweig MC, Martin MA. 2013. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature 503:277–280. doi: 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moldt B, Rakasz EG, Schultz N, Chan-Hui P-Y, Swiderek K, Weisgrau KL, Piaskowski SM, Bergman Z, Watkins DI, Poignard P, Burton DR. 2012. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci U S A 109:18921–18925. doi: 10.1073/pnas.1214785109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gautam R, Nishimura Y, Pegu A, Nason MC, Klein F, Gazumyan A, Golijanin J, Buckler-White A, Sadjadpour R, Wang K, Mankoff Z, Schmidt SD, Lifson JD, Mascola JR, Nussenzweig MC, Martin MA. 2016. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature 533:105–109. doi: 10.1038/nature17677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caskey M, Klein F, Lorenzi JCC, Seaman MS, West AP, Buckley N, Kremer G, Nogueira L, Braunschweig M, Scheid JF, Horwitz JA, Shimeliovich I, Ben-Avraham S, Witmer-Pack M, Platten M, Lehmann C, Burke LA, Hawthorne T, Gorelick RJ, Walker BD, Keler T, Gulick RM, Fätkenheuer G, Schlesinger SJ, Nussenzweig MC. 2015. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 522:487–491. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lynch RM, Boritz E, Coates EE, DeZure A, Madden P, Costner P, Enama ME, Plummer S, Holman L, Hendel CS, Gordon I, Casazza J, Conan-Cibotti M, Migueles SA, Tressler R, Bailer RT, McDermott A, Narpala S, O'Dell S, Wolf G, Lifson JD, Freemire BA, Gorelick RJ, Pandey JP, Mohan S, Chomont N, Fromentin R, Chun T-W, Fauci AS, Schwartz RM, Koup RA, Douek DC, Hu Z, Capparelli E, Graham BS, Mascola JR, Ledgerwood JE, VRC 601 Study Team . 2015. Virologic effects of broadly neutralizing antibody VRC01 administration during chronic HIV-1 infection. Sci Transl Med 7:319ra206. doi: 10.1126/scitranslmed.aad5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheid JF, Horwitz JA, Bar-On Y, Kreider EF, Lu C-L, Lorenzi JCC, Feldmann A, Braunschweig M, Nogueira L, Oliveira T, Shimeliovich I, Patel R, Burke L, Cohen YZ, Hadrigan S, Settler A, Witmer-Pack M, West AP, Juelg B, Keler T, Hawthorne T, Zingman B, Gulick RM, Pfeifer N, Learn GH, Seaman MS, Bjorkman PJ, Klein F, Schlesinger SJ, Walker BD, Hahn BH, Nussenzweig MC. 2016. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature 535:556–560. doi: 10.1038/nature18929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bar KJ, Sneller MC, Harrison LJ, Justement JS, Overton ET, Petrone ME, Salantes DB, Seamon CA, Scheinfeld B, Kwan RW, Learn GH, Proschan MA, Kreider EF, Blazkova J, Bardsley M, Refsland EW, Messer M, Clarridge KE, Tustin NB, Madden PJ, Oden K, O'Dell SJ, Jarocki B, Shiakolas AR, Tressler RL, Doria-Rose NA, Bailer RT, Ledgerwood JE, Capparelli EV, Lynch RM, Graham BS, Moir S, Koup RA, Mascola JR, Hoxie JA, Fauci AS, Tebas P, Chun T-W. 2016. Effect of HIV antibody VRC01 on viral rebound after treatment interruption. N Engl J Med 375:2037–2050. doi: 10.1056/NEJMoa1608243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haynes BF, Shaw GM, Korber B, Kelsoe G, Sodroski J, Hahn BH, Borrow P, McMichael AJ. 2016. HIV-host interactions: implications for vaccine design. Cell Host Microbe 19:292–303. doi: 10.1016/j.chom.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rusert P, Kouyos RD, Kadelka C, Ebner H, Schanz M, Huber M, Braun DL, Hozé N, Scherrer A, Magnus C, Weber J, Uhr T, Cippa V, Thorball CW, Kuster H, Cavassini M, Bernasconi E, Hoffmann M, Calmy A, Battegay M, Rauch A, Yerly S, Aubert V, Klimkait T, Böni J, Fellay J, Regoes RR, Günthard HF, Trkola A, Swiss HIV Cohort Study. 2016. Determinants of HIV-1 broadly neutralizing antibody induction. Nat Med 22:1260–1267. doi: 10.1038/nm.4187. [DOI] [PubMed] [Google Scholar]
- 13.Mouquet H. 2014. Antibody B cell responses in HIV-1 infection. Trends Immunol 35:549–561. doi: 10.1016/j.it.2014.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Klein F, Diskin R, Scheid JF, Gaebler C, Mouquet H, Georgiev IS, Pancera M, Zhou T, Incesu R-B, Fu BZ, Gnanapragasam PNP, Oliveira TY, Seaman MS, Kwong PD, Bjorkman PJ, Nussenzweig MC. 2013. Somatic mutations of the immunoglobulin framework are generally required for broad and potent HIV-1 neutralization. Cell 153:126–138. doi: 10.1016/j.cell.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, Premsri N, Namwat C, de Souza M, Adams E, Benenson M, Gurunathan S, Tartaglia J, McNeil JG, Francis DP, Stablein D, Birx DL, Chunsuttiwat S, Khamboonruang C, Thongcharoen P, Robb ML, Michael NL, Kunasol P, Kim JH, MOPH-TAVEG Investigators . 2009. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 361:2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 16.Tomaras GD, Ferrari G, Shen X, Alam SM, Liao H-X, Pollara J, Bonsignori M, Moody MA, Fong Y, Chen X, Poling B, Nicholson CO, Zhang R, Lu X, Parks R, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Gilbert PB, Kim JH, Michael NL, Montefiori DC, Haynes BF. 2013. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci U S A 110:9019–9024. doi: 10.1073/pnas.1301456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao H- X, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. 2012. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chung AW, Kumar MP, Arnold KB, Yu WH, Schoen MK, Dunphy LJ, Suscovich TJ, Frahm N, Linde C, Mahan AE, Hoffner M, Streeck H, Ackerman ME, McElrath MJ, Schuitemaker H, Pau MG, Baden LR, Kim JH, Michael NL, Barouch DH, Lauffenburger DA, Alter G. 2015. Dissecting polyclonal vaccine-induced humoral immunity against HIV using systems serology. Cell 163:988–998. doi: 10.1016/j.cell.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung AW, Ghebremichael M, Robinson H, Brown E, Choi I, Lane S, Dugast A-S, Schoen MK, Rolland M, Suscovich TJ, Mahan AE, Liao L, Streeck H, Andrews C, Rerks-Ngarm S, Nitayaphan S, de Souza MS, Kaewkungwal J, Pitisuttithum P, Francis D, Michael NL, Kim JH, Bailey-Kellogg C, Ackerman ME, Alter G. 2014. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci Transl Med 6:228ra38. doi: 10.1126/scitranslmed.3007736. [DOI] [PubMed] [Google Scholar]
- 20.Scheid JF, Mouquet H, Feldhahn N, Seaman MS, Velinzon K, Pietzsch J, Ott RG, Anthony RM, Zebroski H, Hurley A, Phogat A, Chakrabarti B, Li Y, Connors M, Pereyra F, Walker BD, Wardemann H, Ho D, Wyatt RT, Mascola JR, Ravetch JV, Nussenzweig MC. 2009. Broad diversity of neutralizing antibodies isolated from memory B cells in HIV-infected individuals. Nature 458:636–640. doi: 10.1038/nature07930. [DOI] [PubMed] [Google Scholar]
- 21.Pollara J, Bonsignori M, Moody MA, Pazgier M, Haynes BF, Ferrari G. 2013. Epitope specificity of human immunodeficiency virus-1 antibody dependent cellular cytotoxicity [ADCC] responses. Curr HIV Res 11:378–387. doi: 10.2174/1570162X113116660059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis GK, Finzi A, DeVico AL, Pazgier M. 2015. Conformational masking and receptor-dependent unmasking of highly conserved Env epitopes recognized by non-neutralizing antibodies that mediate potent ADCC against HIV-1. Viruses 7:5115–5132. doi: 10.3390/v7092856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santra S, Tomaras GD, Warrier R, Nicely NI, Liao H- X, Pollara J, Liu P, Alam SM, Zhang R, Cocklin SL, Shen X, Duffy R, Xia S-M, Schutte RJ, Pemble CW IV, Dennison SM, Li H, Chao A, Vidnovic K, Evans A, Klein K, Kumar A, Robinson J, Landucci G, Forthal DN, Montefiori DC, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Robb ML, Michael NL, Kim JH, Soderberg KA, Giorgi EE, Blair L, Korber BT, Moog C, Shattock RJ, Letvin NL, Schmitz JE, Moody MA, Gao F, Ferrari G, Shaw GM, Haynes BF. 2015. Human non-neutralizing HIV-1 envelope monoclonal antibodies limit the number of founder viruses during SHIV mucosal infection in rhesus macaques. PLoS Pathog 11:e1005042. doi: 10.1371/journal.ppat.1005042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veillette M, Désormeaux A, Medjahed H, Gharsallah N-E, Coutu M, Baalwa J, Guan Y, Lewis G, Ferrari G, Hahn BH, Haynes BF, Robinson JE, Kaufmann DE, Bonsignori M, Sodroski J, Finzi A. 2014. Interaction with cellular CD4 exposes HIV-1 envelope epitopes targeted by antibody-dependent cell-mediated cytotoxicity. J Virol 88:2633–2644. doi: 10.1128/JVI.03230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wyatt R, Moore J, Accola M, Desjardin E, Robinson J, Sodroski J. 1995. Involvement of the V1/V2 variable loop structure in the exposure of human immunodeficiency virus type 1 gp120 epitopes induced by receptor binding. J Virol 69:5723–5733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferrari G, Pollara J, Kozink D, Harms T, Drinker M, Freel S, Moody MA, Alam SM, Tomaras GD, Ochsenbauer C, Kappes JC, Shaw GM, Hoxie JA, Robinson JE, Haynes BF. 2011. An HIV-1 gp120 envelope human monoclonal antibody that recognizes a C1 conformational epitope mediates potent antibody-dependent cellular cytotoxicity (ADCC) activity and defines a common ADCC epitope in human HIV-1 serum. J Virol 85:7029–7036. doi: 10.1128/JVI.00171-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mouquet H, Scharf L, Euler Z, Liu Y, Eden C, Scheid JF, Halper-Stromberg A, Gnanapragasam PNP, Spencer DIR, Seaman MS, Schuitemaker H, Feizi T, Nussenzweig MC, Bjorkman PJ. 2012. Complex-type N-glycan recognition by potent broadly neutralizing HIV antibodies. Proc Natl Acad Sci U S A 109:E3268–E3277. doi: 10.1073/pnas.1217207109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mouquet H, Klein F, Scheid JF, Warncke M, Pietzsch J, Oliveira TYK, Velinzon K, Seaman MS, Nussenzweig MC. 2011. Memory B cell antibodies to HIV-1 gp140 cloned from individuals infected with clade A and B viruses. PLoS One 6:e24078. doi: 10.1371/journal.pone.0024078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheid JF, Mouquet H, Ueberheide B, Diskin R, Klein F, Oliveira TYK, Pietzsch J, Fenyo D, Abadir A, Velinzon K, Hurley A, Myung S, Boulad F, Poignard P, Burton DR, Pereyra F, Ho DD, Walker BD, Seaman MS, Bjorkman PJ, Chait BT, Nussenzweig MC. 2011. Sequence and structural convergence of broad and potent HIV antibodies that mimic CD4 binding. Science 333:1633–1637. doi: 10.1126/science.1207227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yasmeen A, Ringe R, Derking R, Cupo A, Julien J-P, Burton DR, Ward AB, Wilson IA, Sanders RW, Moore JP, Klasse PJ. 2014. Differential binding of neutralizing and non-neutralizing antibodies to native-like soluble HIV-1 Env trimers, uncleaved Env proteins, and monomeric subunits. Retrovirology 11:41. doi: 10.1186/1742-4690-11-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Astronomo RD, Santra S, Ballweber-Fleming L, Westerberg KG, Mach L, Hensley-McBain T, Sutherland L, Mildenberg B, Morton G, Yates NL, Mize GJ, Pollara J, Hladik F, Ochsenbauer C, Denny TN, Warrier R, Rerks-Ngarm S, Pitisuttithum P, Nitayapan S, Kaewkungwal J, Ferrari G, Shaw GM, Xia S-M, Liao H-X, Montefiori DC, Tomaras GD, Haynes BF, McElrath JM. 2016. Neutralization takes precedence over IgG or IgA isotype-related functions in mucosal HIV-1 antibody-mediated protection. EBioMedicine 14:97–111. doi: 10.1016/j.ebiom.2016.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alpert MD, Harvey JD, Lauer WA, Reeves RK, Piatak M, Carville A, Mansfield KG, Lifson JD, Li W, Desrosiers RC, Johnson RP, Evans DT. 2012. ADCC develops over time during persistent infection with live-attenuated SIV and is associated with complete protection against SIV(mac)251 challenge. PLoS Pathog 8:e1002890. doi: 10.1371/journal.ppat.1002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gómez-Román VR, Patterson LJ, Venzon D, Liewehr D, Aldrich K, Florese R, Robert-Guroff M. 2005. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J Immunol 174:2185–2189. doi: 10.4049/jimmunol.174.4.2185. [DOI] [PubMed] [Google Scholar]
- 34.Hidajat R, Xiao P, Zhou Q, Venzon D, Summers LE, Kalyanaraman VS, Montefiori DC, Robert-Guroff M. 2009. Correlation of vaccine-elicited systemic and mucosal nonneutralizing antibody activities with reduced acute viremia following intrarectal simian immunodeficiency virus SIVmac251 challenge of rhesus macaques. J Virol 83:791–801. doi: 10.1128/JVI.01672-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao P, Zhao J, Patterson LJ, Brocca-Cofano E, Venzon D, Kozlowski PA, Hidajat R, Demberg T, Robert-Guroff M. 2010. Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J Virol 84:7161–7173. doi: 10.1128/JVI.00410-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dugast A-S, Chan Y, Hoffner M, Licht A, Nkolola J, Li H, Streeck H, Suscovich TJ, Ghebremichael M, Ackerman ME, Barouch DH, Alter G. 2014. Lack of protection following passive transfer of polyclonal highly functional low-dose non-neutralizing antibodies. PLoS One 9:e97229. doi: 10.1371/journal.pone.0097229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Florese RH, Van Rompay KKA, Aldrich K, Forthal DN, Landucci G, Mahalanabis M, Haigwood N, Venzon D, Kalyanaraman VS, Marthas ML, Robert-Guroff M. 2006. Evaluation of passively transferred, nonneutralizing antibody-dependent cellular cytotoxicity-mediating IgG in protection of neonatal rhesus macaques against oral SIVmac251 challenge. J Immunol 177:4028–4036. doi: 10.4049/jimmunol.177.6.4028. [DOI] [PubMed] [Google Scholar]
- 38.Burton DR, Hessell AJ, Keele BF, Klasse PJ, Ketas TA, Moldt B, Dunlop DC, Poignard P, Doyle LA, Cavacini L, Veazey RS, Moore JP. 2011. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc Natl Acad Sci U S A 108:11181–11186. doi: 10.1073/pnas.1103012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moog C, Dereuddre-Bosquet N, Teillaud J-L, Biedma ME, Holl V, Van Ham G, Heyndrickx L, Van Dorsselaer A, Katinger D, Vcelar B, Zolla-Pazner S, Mangeot I, Kelly C, Shattock RJ, Le Grand R. 2014. Protective effect of vaginal application of neutralizing and nonneutralizing inhibitory antibodies against vaginal SHIV challenge in macaques. Mucosal Immunol 7:46–56. doi: 10.1038/mi.2013.23. [DOI] [PubMed] [Google Scholar]
- 40.Chung AW, Isitman G, Navis M, Kramski M, Center RJ, Kent SJ, Stratov I. 2011. Immune escape from HIV-specific antibody-dependent cellular cytotoxicity (ADCC) pressure. Proc Natl Acad Sci U S A 108:7505–7510. doi: 10.1073/pnas.1016048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ahmad R, Sindhu ST, Toma E, Morisset R, Vincelette J, Menezes J, Ahmad A. 2001. Evidence for a correlation between antibody-dependent cellular cytotoxicity-mediating anti-HIV-1 antibodies and prognostic predictors of HIV infection. J Clin Immunol 21:227–233. doi: 10.1023/A:1011087132180. [DOI] [PubMed] [Google Scholar]
- 42.Johansson SE, Rollman E, Chung AW, Center RJ, Hejdeman B, Stratov I, Hinkula J, Wahren B, Kärre K, Kent SJ, Berg L. 2011. NK cell function and antibodies mediating ADCC in HIV-1-infected viremic and controller patients. Viral Immunol 24:359–368. doi: 10.1089/vim.2011.0025. [DOI] [PubMed] [Google Scholar]
- 43.Chung AW, Navis M, Isitman G, Wren L, Silvers J, Amin J, Kent SJ, Stratov I. 2011. Activation of NK cells by ADCC antibodies and HIV disease progression. J Acquir Immune Defic Syndr 58:127–131. doi: 10.1097/QAI.0b013e31822c62b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lambotte O, Ferrari G, Moog C, Yates NL, Liao H-X, Parks RJ, Hicks CB, Owzar K, Tomaras GD, Montefiori DC, Haynes BF, Delfraissy J-F. 2009. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS 23:897–906. doi: 10.1097/QAD.0b013e328329f97d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lambotte O, Pollara J, Boufassa F, Moog C, Venet A, Haynes BF, Delfraissy J-F, Sáez-Cirión A, Ferrari G. 2013. High antibody-dependent cellular cytotoxicity responses are correlated with strong CD8 T cell viral suppressive activity but not with B57 status in HIV-1 elite controllers. PLoS One 8:e74855. doi: 10.1371/journal.pone.0074855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von Bredow B, Arias JF, Heyer LN, Gardner MR, Farzan M, Rakasz EG, Evans DT. 2015. Envelope glycoprotein internalization protects human and simian immunodeficiency virus-infected cells from antibody-dependent cell-mediated cytotoxicity. J Virol 89:10648–10655. doi: 10.1128/JVI.01911-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arias JF, Heyer LN, von Bredow B, Weisgrau KL, Moldt B, Burton DR, Rakasz EG, Evans DT. 2014. Tetherin antagonism by Vpu protects HIV-infected cells from antibody-dependent cell-mediated cytotoxicity. Proc Natl Acad Sci U S A 111:6425–6430. doi: 10.1073/pnas.1321507111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alvarez RA, Hamlin RE, Monroe A, Moldt B, Hotta MT, Rodriguez Caprio G, Fierer DS, Simon V, Chen BK. 2014. HIV-1 Vpu antagonism of tetherin inhibits antibody-dependent cellular cytotoxic responses by natural killer cells. J Virol 88:6031–6046. doi: 10.1128/JVI.00449-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pham TN, Lukhele S, Hajjar F, Routy J-P, Cohen RA. 2014. HIV Nef and Vpu protect HIV-infected CD4+ T cells from antibody-mediated cell lysis through down-modulation of CD4 and BST2. Retrovirology 11:15. doi: 10.1186/1742-4690-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Veillette M, Coutu M, Richard J, Batraville L-A, Dagher O, Bernard N, Tremblay C, Kaufmann DE, Roger M, Finzi A. 2015. The HIV-1 gp120 CD4-bound conformation is preferentially targeted by antibody-dependent cellular cytotoxicity-mediating antibodies in sera from HIV-1-infected individuals. J Virol 89:545–551. doi: 10.1128/JVI.02868-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pham TNQ, Lukhele S, Dallaire F, Perron G, Cohen ÉA. 2016. Enhancing virion tethering by BST2 sensitizes productively and latently HIV-infected T cells to ADCC mediated by broadly neutralizing antibodies. Sci Rep 6:37225. doi: 10.1038/srep37225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu C-L, Murakowski DK, Bournazos S, Schoofs T, Sarkar D, Halper-Stromberg A, Horwitz JA, Nogueira L, Golijanin J, Gazumyan A, Ravetch JV, Caskey M, Chakraborty AK, Nussenzweig MC. 2016. Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science 352:1001–1004. doi: 10.1126/science.aaf1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Halper-Stromberg A, Lu C-L, Klein F, Horwitz JA, Bournazos S, Nogueira L, Eisenreich TR, Liu C, Gazumyan A, Schaefer U, Furze RC, Seaman MS, Prinjha R, Tarakhovsky A, Ravetch JV, Nussenzweig MC. 2014. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell 158:989–999. doi: 10.1016/j.cell.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, Ravetch JV. 2014. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell 158:1243–1253. doi: 10.1016/j.cell.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hessell AJ, Hangartner L, Hunter M, Havenith CEG, Beurskens FJ, Bakker JM, Lanigan CMS, Landucci G, Forthal DN, Parren PWHI, Marx PA, Burton DR. 2007. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 449:101–104. doi: 10.1038/nature06106. [DOI] [PubMed] [Google Scholar]
- 56.Ding S, Veillette M, Coutu M, Prévost J, Scharf L, Bjorkman PJ, Ferrari G, Robinson JE, Stürzel C, Hahn BH, Sauter D, Kirchhoff F, Lewis GK, Pazgier M, Finzi A. 2016. A highly conserved residue of the HIV-1 gp120 inner domain is important for antibody-dependent cellular cytotoxicity responses mediated by anti-cluster A antibodies. J Virol 90:2127–2134. doi: 10.1128/JVI.02779-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guan Y, Pazgier M, Sajadi MM, Kamin-Lewis R, Al-Darmarki S, Flinko R, Lovo E, Wu X, Robinson JE, Seaman MS, Fouts TR, Gallo RC, DeVico AL, Lewis GK. 2013. Diverse specificity and effector function among human antibodies to HIV-1 envelope glycoprotein epitopes exposed by CD4 binding. Proc Natl Acad Sci U S A 110:E69–E78. doi: 10.1073/pnas.1217609110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holl V, Peressin M, Decoville T, Schmidt S, Zolla-Pazner S, Aubertin A-M, Moog C. 2006. Nonneutralizing antibodies are able to inhibit human immunodeficiency virus type 1 replication in macrophages and immature dendritic cells. J Virol 80:6177–6181. doi: 10.1128/JVI.02625-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.von Bredow B, Arias JF, Heyer LN, Moldt B, Le K, Robinson JE, Zolla-Pazner S, Burton DR, Evans DT. 2016. Comparison of antibody-dependent cell-mediated cytotoxicity and virus neutralization by HIV-1 Env-specific monoclonal antibodies. J Virol 90:6127–6139. doi: 10.1128/JVI.00347-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Richard J, Veillette M, Brassard N, Iyer SS, Roger M, Martin L, Pazgier M, Schön A, Freire E, Routy J-P, Smith AB, Park J, Jones DM, Courter JR, Melillo BN, Kaufmann DE, Hahn BH, Permar SR, Haynes BF, Madani N, Sodroski JG, Finzi A. 2015. CD4 mimetics sensitize HIV-1-infected cells to ADCC. Proc Natl Acad Sci U S A 112:E2687–E2694. doi: 10.1073/pnas.1506755112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Richard J, Veillette M, Ding S, Zoubchenok D, Alsahafi N, Coutu M, Brassard N, Park J, Courter JR, Melillo B, Smith AB, Shaw GM, Hahn BH, Sodroski J, Kaufmann DE, Finzi A. 2016. Small CD4 mimetics prevent HIV-1 uninfected bystander CD4+ T cell killing mediated by antibody-dependent cell-mediated cytotoxicity. EBioMedicine 3:122–134. doi: 10.1016/j.ebiom.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Richard J, Pacheco B, Gohain N, Veillette M, Ding S, Alsahafi N, Tolbert WD, Prévost J, Chapleau J-P, Coutu M, Jia M, Brassard N, Park J, Courter JR, Melillo B, Martin L, Tremblay C, Hahn BH, Kaufmann DE, Wu X, Smith AB, Sodroski J, Pazgier M, Finzi A. 2016. Co-receptor binding site antibodies enable CD4-mimetics to expose conserved anti-cluster A ADCC epitopes on HIV-1 envelope glycoproteins. EBioMedicine 12:208–218. doi: 10.1016/j.ebiom.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bruel T, Guivel-Benhassine F, Amraoui S, Malbec M, Richard L, Bourdic K, Donahue DA, Lorin V, Casartelli N, Noël N, Lambotte O, Mouquet H, Schwartz O. 2016. Elimination of HIV-1-infected cells by broadly neutralizing antibodies. Nat Commun 7:10844. doi: 10.1038/ncomms10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moldt B, Schultz N, Dunlop DC, Alpert MD, Harvey JD, Evans DT, Poignard P, Hessell AJ, Burton DR. 2011. A panel of IgG1 b12 variants with selectively diminished or enhanced affinity for Fcγ receptors to define the role of effector functions in protection against HIV. J Virol 85:10572–10581. doi: 10.1128/JVI.05541-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alsmadi O, Tilley SA. 1998. Antibody-dependent cellular cytotoxicity directed against cells expressing human immunodeficiency virus type 1 envelope of primary or laboratory-adapted strains by human and chimpanzee monoclonal antibodies of different epitope specificities. J Virol 72:286–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Forthal DN, Landucci G, Gorny MK, Zolla-Pazner S, Robinson WE. 1995. Functional activities of 20 human immunodeficiency virus type 1 (HIV-1)-specific human monoclonal antibodies. AIDS Res Hum Retroviruses 11:1095–1099. doi: 10.1089/aid.1995.11.1095. [DOI] [PubMed] [Google Scholar]
- 67.Tyler DS, Stanley SD, Zolla-Pazner S, Gorny MK, Shadduck PP, Langlois AJ, Matthews TJ, Bolognesi DP, Palker TJ, Weinhold KJ. 1990. Identification of sites within gp41 that serve as targets for antibody-dependent cellular cytotoxicity by using human monoclonal antibodies. J Immunol 145:3276–3282. [PubMed] [Google Scholar]
- 68.Bonsignori M, Pollara J, Moody MA, Alpert MD, Chen X, Hwang K-K, Gilbert PB, Huang Y, Gurley TC, Kozink DM, Marshall DJ, Whitesides JF, Tsao C-Y, Kaewkungwal J, Nitayaphan S, Pitisuttithum P, Rerks-Ngarm S, Kim JH, Michael NL, Tomaras GD, Montefiori DC, Lewis GK, DeVico A, Evans DT, Ferrari G, Liao H-X, Haynes BF. 2012. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J Virol 86:11521–11532. doi: 10.1128/JVI.01023-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trkola A, Purtscher M, Muster T, Ballaun C, Buchacher A, Sullivan N, Srinivasan K, Sodroski J, Moore JP, Katinger H. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol 70:1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hezareh M, Hessell AJ, Jensen RC, van de Winkel JG, Parren PW. 2001. Effector function activities of a panel of mutants of a broadly neutralizing antibody against human immunodeficiency virus type 1. J Virol 75:12161–12168. doi: 10.1128/JVI.75.24.12161-12168.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Williams KL, Cortez V, Dingens AS, Gach JS, Rainwater S, Weis JF, Chen X, Spearman P, Forthal DN, Overbaugh J. 2015. HIV-specific CD4-induced antibodies mediate broad and potent antibody-dependent cellular cytotoxicity activity and are commonly detected in plasma from HIV-infected humans. EBioMedicine 2:1464–1477. doi: 10.1016/j.ebiom.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mabuka J, Nduati R, Odem-Davis K, Peterson D, Overbaugh J. 2012. HIV-specific antibodies capable of ADCC are common in breastmilk and are associated with reduced risk of transmission in women with high viral loads. PLoS Pathog 8:e1002739. doi: 10.1371/journal.ppat.1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien J-P, Wang S-K, Ramos A, Chan-Hui P-Y, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong C-H, Phogat S, Wrin T, Simek MD, Protocol G Principal Investigators, Koff WC, Wilson IA, Burton DR, Poignard P. 2011. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477:466–470. doi: 10.1038/nature10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Walker LM, Phogat SK, Chan-Hui P-Y, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Protocol G Principal Investigators, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smalls-Mantey A, Doria-Rose N, Klein R, Patamawenu A, Migueles SA, Ko S-Y, Hallahan CW, Wong H, Liu B, You L, Scheid J, Kappes JC, Ochsenbauer C, Nabel GJ, Mascola JR, Connors M. 2012. Antibody-dependent cellular cytotoxicity against primary HIV-infected CD4+ T cells is directly associated with the magnitude of surface IgG binding. J Virol 86:8672–8680. doi: 10.1128/JVI.00287-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sourisseau M, Sol-Foulon N, Porrot F, Blanchet F, Schwartz O. 2007. Inefficient human immunodeficiency virus replication in mobile lymphocytes. J Virol 81:1000–1012. doi: 10.1128/JVI.01629-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garcia JV, Miller AD. 1991. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature 350:508–511. doi: 10.1038/350508a0. [DOI] [PubMed] [Google Scholar]
- 78.Willey RL, Maldarelli F, Martin MA, Strebel K. 1992. Human immunodeficiency virus type 1 Vpu protein induces rapid degradation of CD4. J Virol 66:7193–7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Salazar-Gonzalez JF, Salazar MG, Keele BF, Learn GH, Giorgi EE, Li H, Decker JM, Wang S, Baalwa J, Kraus MH, Parrish NF, Shaw KS, Guffey MB, Bar KJ, Davis KL, Ochsenbauer-Jambor C, Kappes JC, Saag MS, Cohen MS, Mulenga J, Derdeyn CA, Allen S, Hunter E, Markowitz M, Hraber P, Perelson AS, Bhattacharya T, Haynes BF, Korber BT, Hahn BH, Shaw GM. 2009. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J Exp Med 206:1273–1289. doi: 10.1084/jem.20090378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ariën KK, Baleux F, Desjardins D, Porrot F, Coïc Y-M, Michiels J, Bouchemal K, Bonnaffé D, Bruel T, Schwartz O, Le Grand R, Vanham G, Dereuddre-Bosquet N, Lortat-Jacob H. 2016. CD4-mimetic sulfopeptide conjugates display sub-nanomolar anti-HIV-1 activity and protect macaques against a SHIV162P3 vaginal challenge. Sci Rep 6:34829. doi: 10.1038/srep34829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Connell BJ, Baleux F, Coïc Y-M, Clayette P, Bonnaffé D, Lortat-Jacob H. 2012. A synthetic heparan sulfate-mimetic peptide conjugated to a mini CD4 displays very high anti-HIV-1 activity independently of coreceptor usage. Chem Biol 19:131–139. doi: 10.1016/j.chembiol.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 82.Decker JM, Bibollet-Ruche F, Wei X, Wang S, Levy DN, Wang W, Delaporte E, Peeters M, Derdeyn CA, Allen S, Hunter E, Saag MS, Hoxie JA, Hahn BH, Kwong PD, Robinson JE, Shaw GM. 2005. Antigenic conservation and immunogenicity of the HIV coreceptor binding site. J Exp Med 201:1407–1419. doi: 10.1084/jem.20042510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gohain N, Tolbert WD, Orlandi C, Richard J, Ding S, Chen X, Bonsor DA, Sundberg EJ, Lu W, Ray K, Finzi A, Lewis GK, Pazgier M. 2016. Molecular basis for epitope recognition by non-neutralizing anti-gp41 antibody F240. Sci Rep 6:36685. doi: 10.1038/srep36685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Guttman M, Cupo A, Julien J-P, Sanders RW, Wilson IA, Moore JP, Lee KK. 2015. Antibody potency relates to the ability to recognize the closed, pre-fusion form of HIV Env. Nat Commun 6:6144. doi: 10.1038/ncomms7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lewis GK. 2014. Role of Fc-mediated antibody function in protective immunity against HIV-1. Immunology 142:46–57. doi: 10.1111/imm.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mengistu M, Ray K, Lewis GK, DeVico AL. 2015. Antigenic properties of the human immunodeficiency virus envelope glycoprotein gp120 on virions bound to target cells. PLoS Pathog 11:e1004772. doi: 10.1371/journal.ppat.1004772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Forthal DN, Moog C. 2009. Fc receptor-mediated antiviral antibodies. Curr Opin HIV AIDS 4:388–393. doi: 10.1097/COH.0b013e32832f0a89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Casartelli N, Sourisseau M, Feldmann J, Guivel-Benhassine F, Mallet A, Marcelin A-G, Guatelli J, Schwartz O. 2010. Tetherin restricts productive HIV-1 cell-to-cell transmission. PLoS Pathog 6:e1000955. doi: 10.1371/journal.ppat.1000955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ayinde D, Bruel T, Cardinaud S, Porrot F, Prado JG, Moris A, Schwartz O. 2015. SAMHD1 limits HIV-1 antigen presentation by monocyte-derived dendritic cells. J Virol 89:6994–7006. doi: 10.1128/JVI.00069-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Malbec M, Porrot F, Rua R, Horwitz J, Klein F, Halper-Stromberg A, Scheid JF, Eden C, Mouquet H, Nussenzweig MC, Schwartz O. 2013. Broadly neutralizing antibodies that inhibit HIV-1 cell to cell transmission. J Exp Med 210:2813–2821. doi: 10.1084/jem.20131244. [DOI] [PMC free article] [PubMed] [Google Scholar]