ABSTRACT
Vaccine-induced B cells differentiate along two pathways. The follicular pathway gives rise to germinal centers (GCs) that can take weeks to fully develop. The extrafollicular pathway gives rise to short-lived plasma cells (PCs) that can rapidly secrete protective antibodies within days of vaccination. Rabies virus (RABV) postexposure prophylaxis (PEP) requires rapid vaccine-induced humoral immunity for protection. Therefore, we hypothesized that targeting extrafollicular B cell responses for activation would improve the speed and magnitude of RABV PEP. To test this hypothesis, we constructed, recovered, and characterized a recombinant RABV-based vaccine expressing murine B cell activating factor (BAFF) (rRABV-mBAFF). BAFF is an ideal molecule to improve early pathways of B cell activation, as it links innate and adaptive immunity, promoting potent B cell responses. Indeed, rRABV-mBAFF induced a faster, higher antibody response in mice and enhanced survivorship in PEP settings compared to rRABV. Interestingly, rRABV-mBAFF and rRABV induced equivalent numbers of GC B cells, suggesting that rRABV-mBAFF augmented the extrafollicular B cell pathway. To confirm that rRABV-mBAFF modulated the extrafollicular pathway, we used a signaling lymphocytic activation molecule (SLAM)-associated protein (SAP)-deficient mouse model. In response to antigen, SAP-deficient mice form extrafollicular B cell responses but do not generate GCs. rRABV-mBAFF induced similar anti-RABV antibody responses in SAP-deficient and wild-type mice, demonstrating that BAFF modulated immunity through the extrafollicular and not the GC B cell pathway. Collectively, strategies that manipulate pathways of B cell activation may facilitate the development of a single-dose RABV vaccine that replaces current complicated and costly RABV PEP.
IMPORTANCE Effective RABV PEP is currently resource- and cost-prohibitive in regions of the world where RABV is most prevalent. In order to diminish the requirements for rabies immunoglobulin (RIG) and multiple vaccinations for effective prevention of clinical rabies, a more rapidly protective vaccine is needed. This work presents a successful approach to rapidly generate antibody-secreting PCs in response to vaccination by targeting the extrafollicular B cell pathway. We demonstrate that the improved early antibody responses induced by rRABV-mBAFF confer improved protection against RABV in a PEP model. Significantly, activation of the early extrafollicular B cell pathway, such as that demonstrated here, could improve the efficacy of vaccines targeting other pathogens against which rapid protection would decrease morbidity and mortality.
KEYWORDS: B cell activating factor (BAFF), antibody-based vaccine, extrafollicular B cell response, postexposure prophylaxis, rabies, vaccines, virus neutralizing antibodies
INTRODUCTION
Antibodies against rabies virus (RABV) glycoprotein (G), the only surface protein of RABV, are protective against pathogenic rabies (1–3). To prevent clinical symptoms of rabies after exposure to a potentially infectious animal, the World Health Organization recommends a standard RABV postexposure prophylaxis (PEP) protocol consisting of wound debridement, administration of rabies immunoglobulin (RIG), and five vaccinations (4). The current RABV vaccine induces protective anti-RABV G antibodies within 7 to 10 days (5). RABV PEP relies on the administration of RIG for rapid, passive protection during the delay between vaccination and production of protective virus neutralizing antibodies (VNAs) by the recipient (4, 6, 7).
Incomplete prevention of human RABV exposure coupled with inadequate access to medical intervention leads to over 55,000 deaths each year, despite the existence of highly successful reservoir control programs and effective PEP (8–11); this death toll is vastly underreported (12). At least 40% of deaths due to rabies occur in children 15 years of age or younger, making rabies the seventh most costly infectious disease in terms of disability-adjusted life years (3, 7).
Over 15 million people receive RABV PEP annually, with direct costs totaling an estimated $1.7 billion (13). This cost remains burdensome in areas of the world where the incidence of RABV exposures is highest. In Asia and Africa, where over 95% of RABV exposures occur, a course of PEP is estimated to cost $49 to $50, while the average worker earns only $1 to $2 per day (http://www.who.int/mediacentre/factsheets/fs099/en/). Additionally, RIG is prohibitively expensive and inconsistently available in regions with insufficient medical infrastructure (14–16). A vaccine that induces B cells to rapidly differentiate into antibody-secreting plasma cells (PCs) that produce protective antibodies could substantially reduce the financial burden and death toll of RABV by eliminating the necessity of RIG for successful PEP and by reducing the need for repeated vaccination.
Vaccine-induced B cells receive signals from cognate T cells in secondary lymphoid organs and then differentiate along two pathways (reviewed in reference 17). The follicular pathway gives rise to GCs which generate high-affinity memory B cells and long-lived PCs secreting high-affinity antibodies (18, 19). GC-derived PCs can take a week or more to fully develop. The extrafollicular pathway gives rise to short-lived PCs that secrete antigen-specific antibodies within days of antigen encounter (20). Extrafollicular expansion of activated B cells occurs in foci within the red pulp of the spleen or in the medullary cords of lymph nodes. Due to the speed with which extrafollicular B cells differentiate into PCs, we anticipated that activating the extrafollicular B cell pathway would improve the speed of protective immunity to RABV vaccination. With the goal of exploiting extrafollicular B cell responses to accelerate RABV vaccine-induced immunity, we hypothesized that expression of the tumor necrosis factor (TNF) family cytokine BAFF by a live recombinant RABV-based vaccine (rRABV-mBAFF) would induce rapid and robust anti-RABV-specific B cell responses and protection against pathogenic RABV more effectively than the parental vaccine, rRABV.
BAFF is a cytokine expressed mainly by cells of the innate immune system as well as by some T and B cells (21, 22). BAFF predominantly binds to receptors on B cells, making it an extremely attractive molecule to potentiate vaccine-induced B cell responses by linking innate and adaptive immunity. BAFF binds to BAFF receptor (BAFFR), B cell maturation antigen (BCMA), and transmembrane activator and calcium modulator and cyclophilin ligand interactor (TACI). These receptors are expressed on a wide range of differentiated B cells, including marginal-zone B cells, B1 B cells, follicular B cells, and CD138+ antibody-secreting PCs in secondary lymphoid organs. These receptors can also be detected on B cells at the site of infection, such as in the lungs, during influenza infection (23). Due to the specificity of receptor expression on B cells, BAFF has the ability to modulate a wide range of B cell functions, such as mediating B cell survival and proliferation (24–27), inducing and maintaining T and B cell responses, including antibody-secreting PCs, the most important effector B cell population in the context of protection against RABV infection, and increasing protective IgG antibody titers (28). BAFF has been demonstrated to enhance antibody-mediated protection in models of other infectious diseases, including HIV, influenza, pneumococcus, malaria, Trypanosoma cruzi (Chagas' disease), and respiratory syncytial virus (23, 29–33). BAFF influences B cell proliferation, differentiation, and long-term survival of antiviral PCs during recovery from alphaviral encephalomyelitis (34). This suggests BAFF influences B cell responses in the central nervous system (CNS) as well as in peripheral sites, which may help to protect against other neurotropic viruses.
In this report, we show that rRABV-mBAFF accelerates the kinetics and improves the magnitude of the anti-RABV G antibody response. Mice treated with rRABV-mBAFF were protected more efficiently in PEP settings than mice immunized with rRABV. Importantly, the speed by which BAFF exerts its effects can be attributed to the activation of extrafollicular B cells and not to early expansion of GC B cells. Collectively, our work presents the direct targeting of specific pathways of B cell activation as a promising approach to the development of a single-dose RABV vaccine that does not require RIG.
RESULTS
Construction, recovery, and characterization of a live attenuated RABV-based vaccine expressing functional murine BAFF.
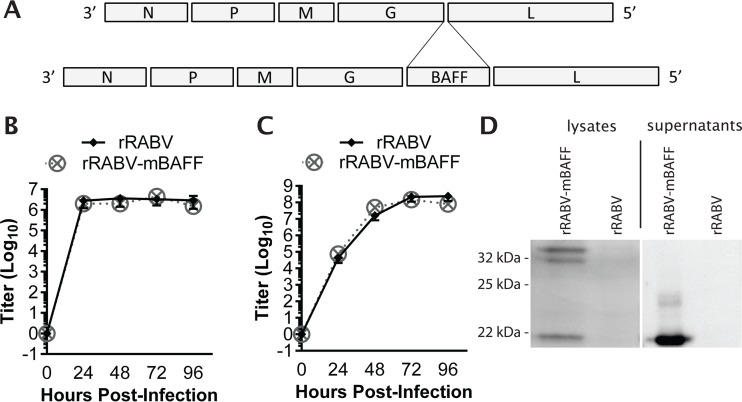
In order to demonstrate that BAFF expression would improve the immunogenicity and protection conferred by rabies vaccination, we constructed and recovered a recombinant, attenuated vaccine strain of RABV expressing murine baff, resulting in rRABV-mBAFF (Fig. 1A). The growth kinetics of rRABV-mBAFF were similar to those of the parental vector, rRABV (Fig. 1B and C), indicating insertion of this foreign gene within the RABV genome does not affect the growth of RABV in vitro.
FIG 1.
Construction, recovery, and characterization of a recombinant RABV-based vaccine expressing the murine BAFF gene (rRABV-mBAFF). (A, top) RABV is a molecular clone of the SAD-B19 vaccine strain of rabies. (A, bottom) RABV, which contains two unique restriction sites between the G and the L genes, was spliced with the gene encoding murine BAFF. BSR cells were exposed to rRABV or rRABV-mBAFF at an MOI of 5 for one-step growth kinetics (B) or at an MOI of 0.01 for multicycle growth kinetics (C). Aliquots of cell culture supernatants were collected and viral titers were determined in duplicate for each time point. (D) Expression of BAFF by rRABV-mBAFF was confirmed by Western blotting. BSR cells, which do not endogenously express BAFF, were infected with rRABV or rRABV-mBAFF and lysed 72 h later. Proteins were separated by SDS-PAGE and transferred to a polyvinylidene membrane, and immunodetection with antibodies specific for BAFF was performed. A protein of the expected size for full-length and cleaved soluble BAFF was detected in lysates of rRABV-mBAFF-exposed but not from rRABV-exposed BSR cells. In parallel, purified and concentrated BSR cell supernatants were analyzed by Western blotting. A protein of the expected size of soluble BAFF was detected in rRABV-mBAFF-exposed but not in rRABV-exposed BSR cell supernatant.
BAFF is expressed as a 34-kDa protein that is cleaved by furin proteases on the cell surface, resulting in soluble BAFF of the expected size, ∼21 kDa (24). Western blot analysis of cell lysates from BSR cells infected with rRABV or rRABV-mBAFF confirmed that rRABV-mBAFF expressed full-length BAFF, which was processed and cleaved into soluble BAFF (Fig. 1D). Western blot analysis of supernatants from rRABV-mBAFF-infected BSR cells demonstrated the presence of the mature, cleaved soluble BAFF. The results showed rRABV-mBAFF expressed mature BAFF that is secreted into the supernatant.
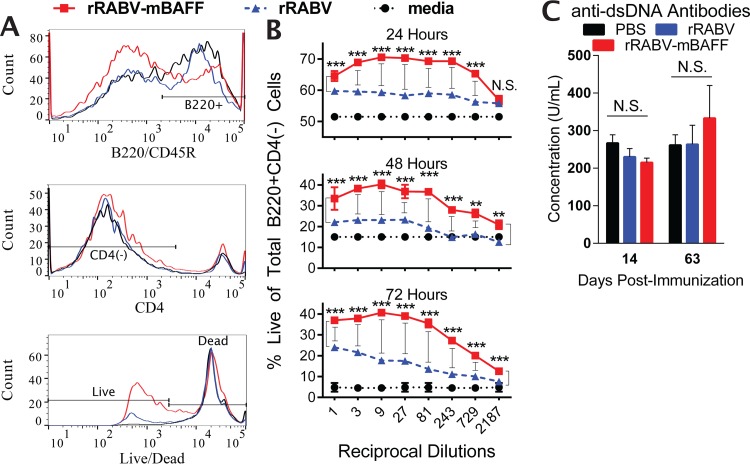
To ensure virally encoded, secreted BAFF was functional, primary murine splenocytes were cultured with serial dilutions of purified and concentrated supernatants from BSR cell cultures infected with rRABV or rRABV-mBAFF at a multiplicity of infection (MOI) of 0.1. At 24, 48, and 72 h, splenocytes were harvested, stained with Live/Dead aqua, α-B220 allophycocyanin-Cy7, and α-CD4 phycoerythrin (PE), and then analyzed by flow cytometry (Fig. 2A). Exposure of splenocytes to supernatant from rRABV-mBAFF-infected cells significantly increased the percentage of live B cells compared to exposure to supernatant from rRABV-infected cells (Fig. 2B), indicating virally encoded BAFF that is secreted into the supernatant of infected cells was functional.
FIG 2.
Cells infected with rRABV-mBAFF produce functional BAFF. Primary murine splenocytes were exposed to media or to serial dilutions of purified and concentrated supernatants from BSR cell cultures infected with rRABV or rRABV-mBAFF at an MOI of 0.1. After 24, 48, or 72 h, splenocytes were harvested, stained with Live/Dead aqua, α-B220 allophycocyanin-Cy7, and α-CD4 PE, and then analyzed by flow cytometry. (A) Representative gating strategies to identify live B220+ CD4− B cells are shown. (B) Quantitative data of the percentages of live total B220+ CD4− B cells are presented as the means ± standard errors of the means (SEM). n = 2 per time point, per dilution. An unpaired, two-tailed t test was used to compare groups (**, P < 0.01; ***, P < 0.001). (C) On day 0, C57BL/6 mice were immunized i.m. with 5 × 106 FFU/mouse rRABV or rRABV-mBAFF or 100 μl sterile PBS. On indicated days postimmunization, serum anti-double-stranded DNA antibody concentrations were determined by ELISA. Pooled sera were run in duplicate (n = 5/group) from two independent experiments. Unpaired, two-tailed t tests were used to compare groups (N.S., P > 0.05).
To ensure that transient expression of BAFF by rRABV-mBAFF did not induce autoimmunity, serum levels of anti-double-stranded DNA (dsDNA) antibodies were measured in mice immunized intramuscularly (i.m.) with 5 × 106 focus-forming units (FFU) of rRABV or rRABV-mBAFF, 50-fold more virus than that used in subsequent immunogenicity experiments. Mice injected with 100 μl sterile phosphate-buffered saline (PBS) i.m. served as a negative control. Indeed, no significant differences in anti-dsDNA antibody levels were detected between the groups at days 14 and 63 postimmunization (Fig. 2C) despite seroconversion against RABV G at these time points in all immunized mice (data not shown).
rRABV-mBAFF improves the speed and magnitude of anti-RABV G IgM and IgG responses in vivo compared to rRABV.
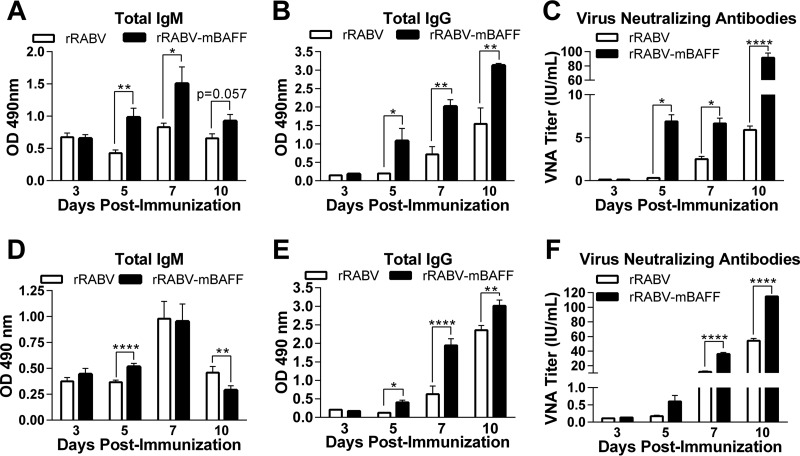
To evaluate the effect of BAFF expression on the immunogenicity of rabies vaccination, mice were immunized intraperitoneally (i.p.) with 105 FFU of rRABV or rRABV-mBAFF. The kinetics of the immune response were monitored by serum levels of total anti-RABV G IgM and IgG antibodies at various times postimmunization. rRABV-mBAFF induced significant levels of anti-RABV G IgM (Fig. 3A) and IgG (Fig. 3B) by day 5 postimmunization compared to mice immunized with rRABV alone. This trend continued for each time point tested. Consistent with the antibody levels determined by enzyme-linked immunosorbent assay (ELISA), significantly higher VNA titers were detected in mice immunized with rRABV-mBAFF as early as 5 days postimmunization than in mice immunized with rRABV (Fig. 3C). VNAs reached levels indicative of a satisfactory immunization (greater than 0.5 IU/ml) (https://www.cdc.gov/rabies/specific_groups/doctors/serology.html) by day 5 postimmunization in response to rRABV-mBAFF, whereas VNAs in response to rRABV did not reach this level until day 10 postimmunization. VNA titers in response to rRABV-mBAFF remained significantly higher through day 10 postimmunization relative to those induced by rRABV.
FIG 3.
rRABV-mBAFF improves the speed and magnitude of anti-RABV G antibody responses. C57BL/6 mice were immunized i.p. (A, B, and C) or i.m. (D, E, and F) with a single dose of 105 FFU/mouse of rRABV or rRABV-mBAFF. On the indicated days postimmunization, RABV G-specific IgM (A and D) and IgG (B and E) antibodies were determined by ELISA. (C and F) VNA titers were determined by RFFIT on pooled serum of immunized mice. Neutralization titers, defined as the inverse of the highest serum dilution that neutralizes 50% of the challenge virus (challenge virus strain 11), were normalized to international units/milliliter (IU/ml) using the WHO antirabies virus antibody reference standard. (A, B, and C) n = 9, i.e., 3/group from 3 independent experiments; (D, E, and F) n = 3/group from a single experiment. An unpaired, two-tailed t test was used to compare groups (*, P < 0.05; **, P < 0.01; ****, P < 0.0001). OD 490 nm indicates the optical density at 490 nm.
The kinetics of serum antibody production was repeated as described for the i.p. experiments, except vaccine was administered i.m.; this route of administration delivers antigens to the draining lymph nodes specifically, whereas i.p. administration distributes antigen to the peritoneal B1 B cells, the spleen, and the draining lymph nodes. rRABV-mBAFF-immunized mice generated significant IgM levels (Fig. 3D) and IgG levels (Fig. 3E) by day 5 compared with rRABV. Significantly higher levels of IgM and IgG were maintained through day 5 and day 10 postimmunization, respectively, compared to those induced by rRABV. rRABV-mBAFF induced higher titers of VNAs (Fig. 3F) than did rRABV at all of the time points tested. Together, rRABV-mBAFF induced significantly faster and greater antibody responses than rRABV following two different immunization routes.
rRABV-mBAFF is more protective than rRABV in a model of PEP.
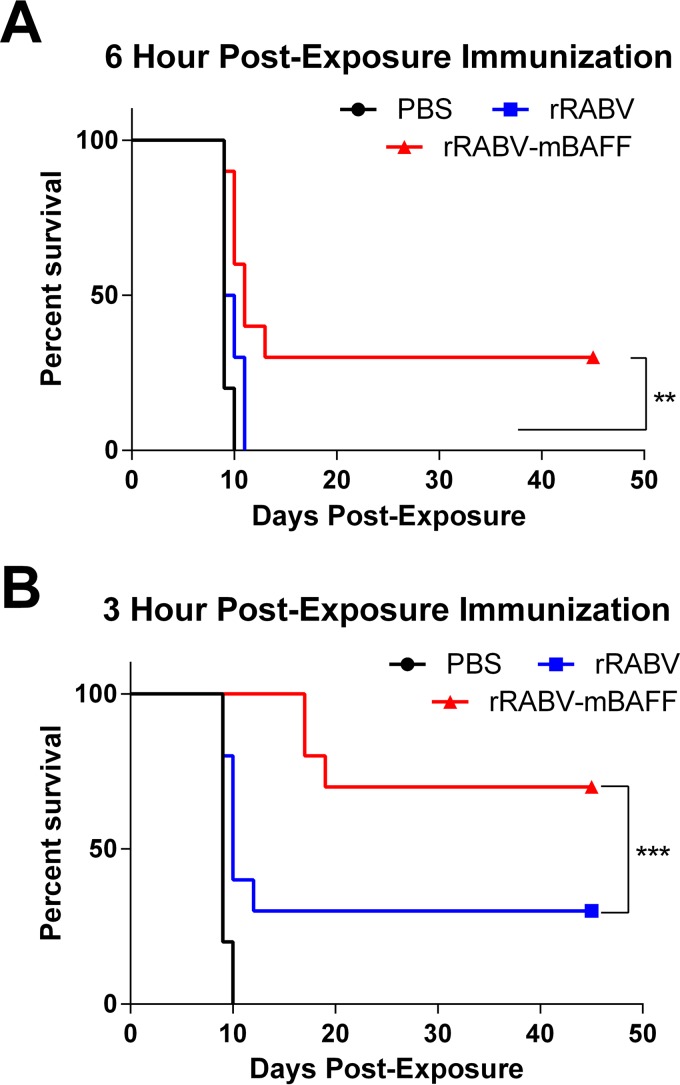
To test whether the increased speed and magnitude of the antibody response to rRABV-mBAFF compared to rRABV conferred improved protection in PEP settings, a well-described mouse model of rabies PEP was used (35, 36). Groups of female C57BL/6 mice (5 mice per group, two independent experiments, for n = 10/group) were challenged in the hind leg with 4 × 104 DOG4 virus on day 0 and then immunized i.m. with a single dose of 105 FFU of rRABV-mBAFF or rRABV or 100 μl PBS 12 h, 6 h (Fig. 4A), or 3 h (Fig. 4B) later. The pathogenic RABV DOG4 was isolated from brain tissue of a human rabies victim infected by a rabid dog (37). DOG4 is a relevant challenge virus because 90% of human exposures and 99% of the human deaths worldwide are attributed to dog rabies (13, 38). Furthermore, DOG4 virus kills mice around 12 to 15 days postinfection, making PEP studies feasible in mice (35, 39). Immunization with rRABV-mBAFF or rRABV 12 h after pathogenic challenge improved longevity relative to unimmunized mice to a similar extent (data not shown). Immunization 6 h after pathogenic challenge demonstrated improved survival in response to rRABV-mBAFF relative to rRABV, which was further improved by more prompt vaccination at 3 h postchallenge. In all, rRABV-mBAFF significantly protected more mice in PEP settings than mice immunized with rRABV.
FIG 4.
Postexposure protection of RABV-based vaccines. On day 0, C57BL/6 mice were challenged i.m. with 4 × 104 FFU/mouse of pathogenic DOG4 rabies virus and then immunized i.m. with 105 FFU/mouse rRABV or rRABV-mBAFF or 100 μl sterile PBS at 6 h postchallenge (A) or 3 h postchallenge (B). n = 10, i.e., 5/group from 2 independent experiments. Kaplan-Meier survival curves were analyzed by the log-rank test (**, P < 0.01; ***, P < 0.001).
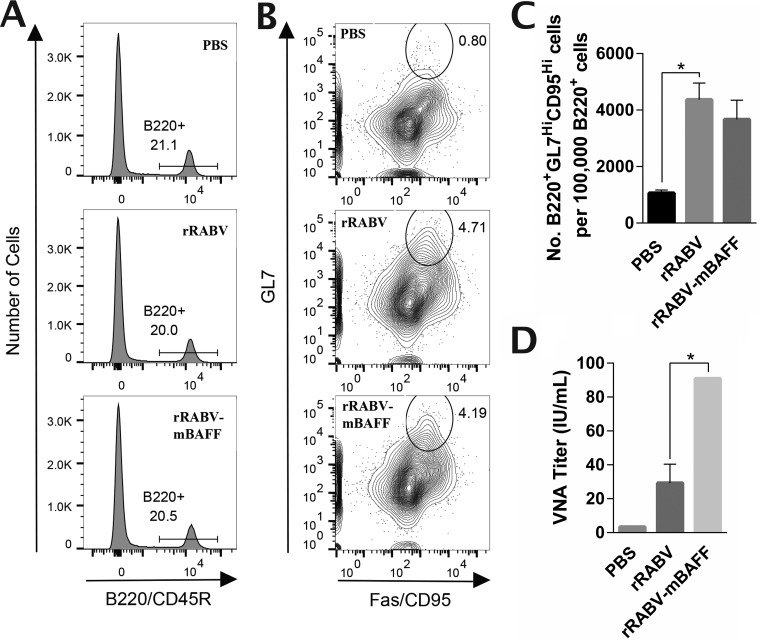
rRABV-mBAFF induces greater VNA titers without expanding GC B cell populations.
Improvement in anti-RABV G antibody responses as early as day 5 postimmunization with rRABV-mBAFF suggested that BAFF was not mediating these early improvements through GC B cells. This is consistent with our previous findings that recombinant rabies vaccines can induce antibody responses prior to the detection of GC B cells (40, 41). Evaluation of the GC B cell population (B220+ Fashi GL7hi) at day 7 postimmunization by flow cytometry (Fig. 5A and B) revealed no difference in the GC B cell populations between rRABV and rRABV-mBAFF (Fig. 5C) despite significantly higher VNA titers in response to rRABV-mBAFF (Fig. 5D).
FIG 5.
rRABV-mBAFF induces greater VNA titers without expanding GC B cell populations. C57BL/6 mice were immunized i.m. with 100 μl sterile PBS (n = 2), 105 FFU rRABV (n = 4), or 105 FFU rRABV-mBAFF (n = 6). On day 7 postimmunization, sera and inguinal lymph nodes were collected. Lymph nodes were homogenized, and cells were stained with allophycocyanin-Cy7 α-B220, PE-Cy7 α-CD95, and FITC α-GL7 and analyzed by flow cytometry. (A) Representative gating strategies to identify B220+ B cells are shown. (B) Contour plots of the B220+ B cells were then subgated for CD95hi GL7hi GC B cells, with representative gates shown. (C) Quantitative data of the number of GC B cells per 100,000 B cells is presented as the means ± SEM. (D) VNA titers at day 7 postimmunization were determined by RFFIT on pooled serum. Neutralization titers were normalized to IU/ml using the WHO anti-rabies virus antibody reference standard. Unpaired, two-tailed t tests were used to compare groups (*, P < 0.05).
rRABV-mBAFF induces significantly faster and higher T cell-dependent extrafollicular antibody responses than rRABV.
BAFF has the ability to influence the development of both T cell-independent (TI) and T cell-dependent (TD) antibody responses (42), both of which can contribute to RABV PEP (41, 43). Because BAFF enhances anti-RABV immunity and protection in PEP settings without expanding GC B cell numbers (Fig. 5), we next wanted to determine whether expressing BAFF improved the TI pathway of B cell activation. Mice completely devoid of all CD4 T cells (41) were immunized with a single dose of 105 FFU of rRABV-mBAFF or rRABV i.p. BAFF did not improve antibody responses in a TI manner (data not shown), suggesting BAFF influences alternative B cell pathways in the context of rRABV vaccination.
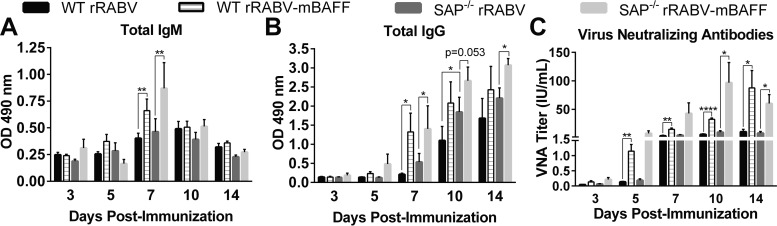
To confirm that BAFF was acting on B cells in extrafollicular foci, we employed a signaling lymphocytic activation molecule (SLAM)-associated protein (SAP)-deficient mouse model. SAP-deficient mice exhibit short-lived cognate T-B pairing without migration of stable T-B pairs into the follicle. SAP-deficient mice do not generate GCs in response to antigen but have intact extrafollicular responses (44–46). Using SAP-deficient mice, we evaluated the kinetics and magnitude of the extrafollicular antibody response induced by rRABV and rRABV-mBAFF (Fig. 6). rRABV induced similar IgM (Fig. 6A), IgG (Fig. 6B), and VNA levels (Fig. 6C) in SAP-deficient mice compared with wild-type mice, verifying that rRABV induces early antibodies primarily through the extrafollicular B cell pathway. Moreover, rRABV-mBAFF induces similar IgM (Fig. 6A), IgG (Fig. 6B), and VNA levels (Fig. 6C) in wild-type and SAP-deficient mice, confirming that BAFF modulates the extrafollicular B cell pathway.
FIG 6.
rRABV-mBAFF improves the speed and magnitude of extrafollicular anti-RABV G antibody responses in SAP-deficient mice. C57BL/6 (WT) or SAP-deficient (SAP) mice aged 7 to 10 weeks were immunized i.p. with a single dose of 105 FFU/mouse of rRABV or rRABV-mBAFF. On indicated days postimmunization, RABV G-specific IgM (A) and IgG (B) antibodies were determined by ELISA. (C) VNA titers were determined by the RFFIT on pooled serum of immunized mice. Neutralization titers were normalized to IU/ml using the WHO anti-rabies virus antibody reference standard. n = 6, i.e., 3/group from 2 independent experiments. An unpaired, two-tailed t test was used to compare groups (*, P < 0.05; **, P < 0.01; ****, P < 0.0001). OD 490 nm indicates optical density at 490 nm.
Compared to rRABV, rRABV-mBAFF induced significantly faster and higher IgM and IgG anti-RABV G antibody titers in SAP-deficient mice (Fig. 6A and B). Indeed, by day 5 postimmunization rRABV-mBAFF induced over 40-fold greater VNA titers in SAP-deficient mice than did rRABV (Fig. 6C). VNA titers remained 7- to 10-fold greater in response to rRABV-mBAFF than rRABV through day 14 postimmunization in SAP-deficient mice. Together, these data indicate that rRABV-mBAFF induces improved antibody responses through a TD extrafollicular pathway of B cell activation.
DISCUSSION
In this study, we hypothesized that modulating early pathways in B cell activation could improve RABV-based vaccination in PEP settings. To test this hypothesis, we constructed and recovered a recombinant RABV-based vaccine that expressed murine BAFF (rRABV-mBAFF). We confirmed that rRABV-mBAFF produced secreted, functional BAFF, and that expressing BAFF from rRABV did not alter its growth kinetics in vitro. We show that rRABV-mBAFF enhances the kinetics and magnitude of the anti-RABV G antibody response in mice. This is consistent with previous reports that BAFF is an effective adjuvant for viral and bacterial antigens, including HIV-1 envelope and pneumococcal surface adhesin A (29, 30). Our findings dovetail with reports that dampening the BAFF signaling axis is an effective strategy to prevent autoimmune diseases such as arthritis, systemic lupus erythematosus, and type II diabetes by diminishing the antibody production and survival of autoreactive B cells (47–49). While BAFF in the context of RABV vaccination supports desired B cell responses, we confirmed through monitoring of autoimmune antibodies, specifically serum anti-double-stranded DNA antibody levels, that the transient expression of BAFF did not induce associated autoimmune dysfunction (Fig. 2C).
It has been suggested that unique B cell subsets have differential contributions to follicular and extrafollicular responses (50–52). We immunized mice using two routes of inoculation in order to help elucidate the B cell subsets and/or compartment(s) relevant to the anti-RABV antibody response. i.m. inoculation exposes recirculating B2 cells in the draining lymph nodes to antigen, while i.p. inoculation also exposes peritoneal B1 B cells and splenic B2 cells to antigen (53). Despite the additional splenic and B1 B exposure to antigen following i.p. immunization, the responses to i.p. and i.m. vaccination with rRABV-mBAFF were similar, suggesting that BAFF was affecting B cell subsets common to both routes of immunization, excluding B1 B cells, in the improved antibody response.
Protection against pathogenic rabies is conferred by VNAs that neutralize infectious virions before they infect CNS neurons and induce an encephalitis which underlies clinical symptoms. A DOG4 challenge model of PEP was used to confirm that the BAFF-mediated improvement of the early antibody response conferred better protection against pathogenic rabies. The improved speed and magnitude of the protective antibody response to rRABV-mBAFF enhanced the protection of PEP relative to rRABV. Vaccination with rRABV-mBAFF 6 h postchallenge conferred protection similar to that of vaccination with rRABV 3 h postchallenge, suggesting that the window for successful PEP could be improved by vaccines that exploit early pathways of B cell activation.
Our data suggested that in the context of RABV vaccination, BAFF influences TD B cell responses, leaving rapid GC responses and extrafollicular responses as the potential pathways underlying the improved antibody response to rRABV-mBAFF. In order to decipher which of these B cell compartments BAFF affects, we evaluated the GC B cell population at a time point when rRABV-mBAFF induced a significantly higher anti-RABV antibody response than rRABV. The size of the GC B cell populations generated in response to rRABV and rRABV-mBAFF were found to be similar despite significantly higher VNA titers in rRABV-mBAFF-immunized mice, suggesting that BAFF was affecting non-GC B cells. This led us to evaluate the response in SAP-deficient mice that lack GC responses (44, 45). The improved antibody response to rRABV-mBAFF relative to rRABV was maintained in SAP-deficient mice, confirming that BAFF potentiates extrafollicular B cell responses.
Our results show that SAP is dispensable for the early adaptive response to rabies vaccination. Moreover, BAFF expression improves the anti-RABV G antibody response independent of SAP. Despite previously reported defects in class switch recombination (CSR) in SAP-deficient B cells (54, 55), CSR occurs similarly in SAP-deficient and wild-type PCs in response to rRABV, as evidenced by the transition from IgM to IgG antibodies in the serum.
Since SAP-deficient mice exhibit decreased stability of T-B interactions, it might be expected that the TD response to rRABV would be muted in this model. However, the antibody response to rRABV in these mice was sustained at levels comparable to that induced in wild-type mice. Lymph node-resident dendritic cells (DCs) may have compensated for the diminished T cell help in SAP-deficient mice. DC-secreted BAFF reportedly supports T cell costimulation of B cells (56, 57). Furthermore, DCs have been shown to support the growth and increase the generation of PCs from extrafollicular responses (58–60). Antigen-presenting cells (APCs) offer another route of compensation; in a porcine model of foot-and-mouth disease virus, APC-secreted BAFF supports TD antiviral antibody responses under conditions of limited T cell help, similar to what occurs in the SAP-deficient model (57). Vaccine-encoded BAFF could influence the anti-RABV antibody response through the same mechanisms as APC- or DC-secreted BAFF, modulating downstream B cell outcomes.
While BAFF may influence the TD B cell response to rabies vaccination through indirect mechanisms such as those just discussed, the potential for BAFF to directly affect B cells is well documented. BAFF signaling through BAFF-R, expressed on all mature B cells, improves B cell survival and induces proliferation (25, 61, 62). Consequently, increased availability of BAFF following immunization with rRABV-mBAFF may improve the survival and/or increase the proliferation of B cells. Further work is needed to determine whether BAFF expression modulates the repertoire produced by the PCs and whether the PCs induced by rRABV-mBAFF are functionally different from those developed in response to rRABV. Additionally, identification of the receptor(s) involved in modulating the early TD extrafollicular B cell response would be relevant for future vaccine development. The unique expression patterns of BAFF-binding receptors and the growing understanding of their complex interactions with BAFF present a potent avenue for exploitation in the development of future vaccines.
Our results collectively demonstrate that in the context of rabies vaccination, BAFF improves the kinetics and magnitude of TD extrafollicular B cell responses. The current understanding of the BAFF signaling axis indicates that this strategy could be leveraged to improve vaccine-induced antibody responses against other infectious diseases. For example, the extrafollicular response to influenza vaccination plays a significant role in protection (63, 64). Amplifying the extrafollicular response in such settings would improve the protection conferred by vaccinations which occur in close temporal proximity to pathogen exposure, potentially decreasing mortality in high-risk populations. Additionally, the ability to boost extrafollicular responses could be of significant clinical importance in the control of pathogens which do not evoke T cell help, such as pneumococci and meningococci, against which the extrafollicular pathway is responsible for generating adaptive immunity.
MATERIALS AND METHODS
Recombinant RABV-based vaccine construction and recovery.
rRABV is a recombinant RABV-based vaccine vector and a molecular clone of the SAD-B19 vaccine strain of RABV (65, 66). To construct rRABV expressing BAFF, the BAFF gene of Mus musculus (GenBank accession number BC106841) was amplified by reverse transcription-PCR (RT-PCR) of lipopolysaccharide (LPS)-stimulated murine splenocytes from C57BL/6 mice (The Jackson Laboratory) with Taq polymerase (Invitrogen) using forward primer JPMRP-37 (5′-TTTCGTACGATTATGGATGAGTCTGCAAAGACC-3′) (BsiWi is underlined) and reverse primer JPMRP-38 (5′-AAAGCTAGCTTACAGCAGTTTTAGGGCACC-3′) (NheI is underlined). The restriction enzymes BsiWI and NheI (New England BioLabs) were used to digest and insert this RT-PCR product into the rRABV plasmid, resulting in recombinant RABV plasmid encoding murine BAFF (pRABV-mBAFF). Infectious virus was recovered as described previously (65, 67, 68), resulting in rRABV-mBAFF. Viral stocks were grown on BSR cells, concentrated, and purified over a 20% sucrose cushion and reconstituted in sterile 1× phosphate-buffered saline (PBS).
One- and multistep growth curves.
Growth kinetics of rRABV and rRABV-mBAFF were determined in parallel as previously described (69). Briefly, BSR cells were seeded at 5.0 × 105 cells/well in 6-well plates (Corning, Inc.) in Dulbecco's modified Eagle's medium (DMEM) containing 5% heat-inactivated fetal bovine serum (FBS)–1% penicillin/streptomycin and infected 24 h later with rRABV or rRABV-mBAFF at a multiplicity of infection (MOI) of 5 or 0.01 for one-step or multicycle growth curves, respectively. After a 1-h incubation, cells were extensively washed with 1× PBS and supplemented with DMEM. Tissue culture supernatants (100 μl) were harvested at the indicated time points. Titers of infectious supernatants were determined in duplicate using BSR cells.
Western blotting.
BSR cells were infected with rRABV or rRABV-BAFF at an MOI of 0.1. Cells were incubated at 37°C with 5% CO2 overnight, washed three times with 1× PBS, supplemented with serum-free medium, and grown for another 3 days. Supernatants were harvested, cellular debris was pelleted out, and virus was removed from supernatants by ultracentrifugation (1 h, 24,000 rpm at 4°C). Purified supernatants were incubated overnight with 80 mol/liter β-propriolactone (Sigma-Aldrich) to inactivate any residual virus and then concentrated 100× using a 10-kDa centrifugal filter (Merck Millipore, Ltd.). Cells were washed extensively with 1× PBS and lysed with radioimmunoprecipitation assay (RIPA) buffer (Sigma-Aldrich), and cellular debris was pelleted out. Proteins were separated via 10% SDS-PAGE, transferred to a polyvinylidene fluoride membrane (Thermo Fisher Scientific), and blocked with 5% nonfat milk powder in 1× PBS overnight. The membrane was probed for 1 h with polyclonal goat IgG anti-murine BAFF primary antibody (R&D Systems) at a dilution of 1:2,500 in PBS–0.05% Tween 20. The membrane was then incubated for 1 h with donkey anti-goat IgG horseradish peroxidase-conjugated secondary antibody diluted 1:20,000 in PBS–0.05% Tween 20. The membrane was developed using ECL Western blotting substrate (Pierce).
In vitro splenocyte stimulation by rRABV-mBAFF supernatant and flow cytometry.
Spleens were collected from 6- to 10-week-old C57BL/6 mice (The Jackson Laboratory) and homogenized, red blood cells were lysed, and splenocytes were cultured in 96-well flat-bottomed plates at a density of 2.5 × 105 cells/well for 24, 48, or 72 h in serial 3-fold dilutions of 100× concentrated supernatant from BSR cells infected with rRABV or rRABV-mBAFF at an MOI of 0.1, with a starting dilution of 1:10 in RPMI-based splenocyte medium (68, 70). Medium alone served as a negative control. At the indicated time, cells were harvested, stained with Live/Dead fixable aqua (Invitrogen), blocked with CD16/32 Fc block (BD Biosciences) in PBS containing 2% FBS (fluorescence-activated cell sorting [FACS] buffer), and then stained for the markers B220/CD45R (allophycocyanin-Cy7; clone RA3-6B2; BD Biosciences) and CD4 (phycoerythrin; clone GK1.5; BD Biosciences). Samples were fixed in 2% paraformaldehyde and immediately analyzed on a BD LSR Fortessa flow cytometer. Data were analyzed using FlowJo (Treesoft) and Prism 5 (GraphPad) software.
Evaluation of anti-dsDNA antibody responses by ELISA.
Groups of 5 female 6- to 10-week-old C57BL/6 mice (The Jackson Laboratory) were immunized intramuscularly (i.m.) with a single dose of 5 × 106 FFU/mouse of rRABV or rRABV-mBAFF or 100 μl sterile 1× PBS. On days 14 and 62 postimmunization, blood was collected and serum was isolated for analysis. RABV G-specific IgG antibodies were determined by ELISA as described previously (40, 71–73). Anti-dsDNA levels were determined by ELISA on pooled sera from each immunized group diluted 1:100 in 1× PBS and plated in duplicate using a mouse anti-dsDNA Ig (total A+G+M) ELISA kit (Alpha Diagnostic International), run according to the manufacturer's protocol. A positive index for anti-dsDNA antibodies was calculated based on the values from mice immunized with PBS. Anti-dsDNA antibody levels were analyzed using one-way analysis of variance (ANOVA) with Tukey's posttest in Prism 5 (GraphPad) software.
Evaluation of early antibody responses by ELISA and RFFIT.
Groups of 3 female 6- to 10-week-old C57BL/6 mice (The Jackson Laboratory) were immunized intraperitoneally (i.p.) or i.m. with a single dose of 105 FFU of rRABV or rRABV-mBAFF. On days 3, 5, 7, and 10 postimmunization, blood was collected and serum was isolated for analysis. RABV G-specific IgG and IgM antibodies were determined by ELISA and reported at a 1:50 dilution as described previously (40, 71–73). VNA titers of pooled, heat-inactivated sera were determined using the rapid fluorescent focus inhibition test (RFFIT) as described previously (40, 71).
PEP protection.
For PEP protection (35), 6- to 10-week-old female C57BL/6 mice (The Jackson Laboratory) were challenged i.m. with 4 × 104 FFU of DOG4 RABV, a gift from Matthias Schnell. This dose of DOG4 RABV was chosen because it is lethal to all unimmunized mice. At either 3, 6, or 12 h postchallenge with DOG4, mice were immunized i.m. with 105 FFU of rRABV or rRABV-mBAFF. Alternatively, control mice received 100 μl sterile 1× PBS i.m. For 4 weeks postchallenge, the mice were weighed daily and monitored for clinical signs of RABV, the onset of which was a terminal endpoint.
Evaluation of GC B cells by flow cytometry.
Female 6- to 10-week-old mice were immunized i.m. with 100 μl sterile 1× PBS (n = 2) or 105 FFU rRABV (n = 4) or rRABV-mBAFF (n = 6). On day 7 postimmunization, serum and inguinal lymph nodes were isolated for analysis. Lymph nodes were homogenized, red blood cells were lysed, and lymphocytes were blocked with CD16/32 Fc block (BD Biosciences) in FACS buffer. Lymphocytes were then stained for the markers B220/CD45R (allophycocyanin-Cy7; clone RA3-6B2; BD Biosciences), Fas/CD95 (PE-Cy7; clone Jo2; BD Biosciences), and T&B cell activation antigen (fluorescein isothiocyanate; clone GL7; BD Biosciences). Samples were fixed in 2% paraformaldehyde and immediately analyzed on a BD LSRII flow cytometer. Data were analyzed using FlowJo (Treesoft) and Prism 5 (GraphPad) software. VNA titers of pooled, heat-inactivated sera were determined using the RFFIT as described previously (40, 71).
Evaluation of extrafollicular antibody responses in SAP-deficient mice.
Groups of 3 female 6- to 10-week-old SAP-deficient mice (B6.129S6-Sh2d1atm1Pls/J; The Jackson Laboratory) (74) were immunized i.p. with a single dose of 105 FFU of rRABV or rRABV-mBAFF. Groups of 3 female 6- to 10-week-old C57BL/6 mice (The Jackson Laboratory) were immunized i.p. with a single dose of 105 FFU of rRABV or rRABV-mBAFF in parallel. On days 3, 5, 7, 10, and 14 postimmunization, blood was collected and serum was isolated for analysis. RABV G-specific IgG and IgM antibodies were determined by ELISA and reported at a 1:50 dilution as described previously (40, 71). VNA titers of pooled, heat-inactivated sera were determined using the RFFIT as described previously (40, 71).
ACKNOWLEDGMENTS
We thank Jianke Zhang and Lei Yu of the Sidney Kimmel Cancer Center Flow Cytometry Facility at Thomas Jefferson University for their help and guidance with the flow cytometry experiments.
REFERENCES
- 1.Turner GS. 1978. Immunoglobulin (IgG) and (IgM) antibody responses to rabies vaccine. J Gen Virol 40:595–604. doi: 10.1099/0022-1317-40-3-595. [DOI] [PubMed] [Google Scholar]
- 2.Johnson N, Cunningham AF, Fooks AR. 2010. The immune response to rabies virus infection and vaccination. Vaccine 28:3896–3901. doi: 10.1016/j.vaccine.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 3.McGettigan JP. 2010. Experimental rabies vaccines for humans. Expert Rev Vaccines 9:1177–1186. doi: 10.1586/erv.10.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Publication. 2010. Rabies vaccines: WHO position paper–recommendations. Vaccine 28:7140–7142. doi: 10.1016/j.vaccine.2010.08.082. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard-Rohner G, Pulickal AS, Jol-van der Zijde CM, Snape MD, Pollard AJ. 2009. Appearance of peripheral blood plasma cells and memory B cells in a primary and secondary immune response in humans. Blood 114:4998–5002. doi: 10.1182/blood-2009-03-211052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilde H, Khawplod P, Hemachudha T, Sitprija V. 2002. Postexposure treatment of rabies infection: can it be done without immunoglobulin? Clin Infect Dis 34:477–480. doi: 10.1086/324628. [DOI] [PubMed] [Google Scholar]
- 7.Gacouin A, Bourhy H, Renaud JC, Camus C, Suprin E, Thomas R. 1999. Human rabies despite postexposure vaccination. Eur J Clin Microbiol Infect Dis 18:233–235. doi: 10.1007/s100960050269. [DOI] [PubMed] [Google Scholar]
- 8.McGettigan JP, David F, Figueiredo MD, Minke J, Mebatsion T, Schnell MJ. 2014. Safety and serological response to a matrix gene-deleted rabies virus-based vaccine vector in dogs. Vaccine 32:1716–1719. doi: 10.1016/j.vaccine.2014.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belotto A, Leanes LF, Schneider MC, Tamayo H, Correa E. 2005. Overview of rabies in the Americas. Virus Res 111:5–12. doi: 10.1016/j.virusres.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 10.Lembo T, Hampson K, Kaare MT, Ernest E, Knobel D, Kazwala RR, Haydon DT, Cleaveland S. 2010. The feasibility of canine rabies elimination in Africa: dispelling doubts with data. PLoS Negl Trop Dis 4:e626. doi: 10.1371/journal.pntd.0000626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davlin SL, VonVille HM. 2012. Canine rabies vaccination and domestic dog population characteristics in the developing world: a systematic review. Vaccine 30:3492–3502. doi: 10.1016/j.vaccine.2012.03.069. [DOI] [PubMed] [Google Scholar]
- 12.Cleaveland S, Fèvre EM, Kaare M, Coleman PG. 2002. Estimating human rabies mortality in the United Republic of Tanzania from dog bite injuries. Bull World Health Organ 80:304–310. [PMC free article] [PubMed] [Google Scholar]
- 13.Hampson K, Coudeville L, Lembo T, Sambo M, Kieffer A, Attlan M, Barrat J, Blanton JD, Briggs DJ, Cleaveland S, Costa P, Freuling CM, Hiby E, Knopf L, Leanes F, Meslin F-X, Metlin A, Miranda ME, Müller T, Nel LH, Recuenco S, Rupprecht CE, Schumacher C, Taylor L, Vigilato MAN, Zinsstag J, Dushoff J. 2015. Estimating the global burden of endemic canine rabies. PLoS Negl Trop Dis 9:e0003709. doi: 10.1371/journal.pntd.0003709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hampson K, Cleaveland S, Briggs D. 2011. Evaluation of cost-effective strategies for rabies post-exposure vaccination in low-income countries. PLoS Negl Trop Dis 5:e982. doi: 10.1371/journal.pntd.0000982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knobel DL, Cleaveland S, Coleman PG, Fèvre EM, Meltzer MI, Miranda MEG, Shaw A, Zinsstag J, Meslin F-X. 2005. Re-evaluating the burden of rabies in Africa and Asia. Bull World Health Organ 83:360–368. [PMC free article] [PubMed] [Google Scholar]
- 16.Müller T, Dietzschold B, Ertl H, Fooks AR, Freuling C, Fehlner-Gardiner C, Kliemt J, Meslin FX, Rupprecht CE, Tordo N, Wanderler AI, Kieny MP, Kieny MP. 2009. Development of a mouse monoclonal antibody cocktail for post-exposure rabies prophylaxis in humans. PLoS Negl Trop Dis 3:e542. doi: 10.1371/journal.pntd.0000542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vinuesa CG, Tangye SG, Moser B, Mackay CR. 2005. Follicular B helper T cells in antibody responses and autoimmunity. Nat Rev Immunol 5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 18.Benner R, Hijmans W, Haaijman JJ. 1981. The bone marrow: the major source of serum immunoglobulins, but still a neglected site of antibody formation. Clin Exp Immunol 46:1–8. [PMC free article] [PubMed] [Google Scholar]
- 19.Smith KG, Hewitson TD, Nossal GJ, Tarlinton DM. 1996. The phenotype and fate of the antibody-forming cells of the splenic foci. Eur J Immunol 26:444–448. doi: 10.1002/eji.1830260226. [DOI] [PubMed] [Google Scholar]
- 20.MacLennan ICM, Toellner K-M, Cunningham AF, Serre K, Sze DM-Y, Zúñiga E, Cook MC, Vinuesa CG. 2003. Extrafollicular antibody responses. Immunol Rev 194:8–18. doi: 10.1034/j.1600-065X.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 21.Mackay F, Schneider P. 2009. Cracking the BAFF code. Nat Rev Immunol 9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 22.Rickert RC, Jellusova J, Miletic AV. 2011. Signaling by the tumor necrosis factor receptor superfamily in B-cell biology and disease. Immunol Rev 244:115–133. doi: 10.1111/j.1600-065X.2011.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolf AI, Mozdzanowska K, Quinn WJ, Metzgar M, Williams KL, Caton AJ, Meffre E, Bram RJ, Erickson LD, Allman D, Cancro MP, Erikson J. 2011. Protective antiviral antibody responses in a mouse model of influenza virus infection require TACI. J Clin Investig 121:3954–3964. doi: 10.1172/JCI57362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider P, Mackay F, Steiner V, Hofmann K, Bodmer J-L, Holler N, Ambrose C, Lawton P, Bixler S, Acha-Orbea H, Valmori D, Romero P, Werner-Favre C, Zubler RH, Browning JL, Tschopp J. 1999. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med 189:1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Batten M, Groom J, Cachero TG, Qian F, Schneider P, Tschopp J, Browning JL, Mackay F. 2000. BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med 192:1453–1466. doi: 10.1084/jem.192.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mackay F, Browning JL. 2002. BAFF: a fundamental survival factor for B cells. Nat Rev Immunol 2:465–475. doi: 10.1038/nri844. [DOI] [PubMed] [Google Scholar]
- 27.Fu L, Lin-Lee Y-C, Pham LV, Tamayo AT, Yoshimura LC, Ford RJ. 2009. BAFF-R promotes cell proliferation and survival through interaction with IKK and NF-κB/c-Rel in the nucleus of normal and neoplastic B-lymphoid cells. Blood 113:4627–4636. doi: 10.1182/blood-2008-10-183467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen L, Ran MJ, Shan XX, Cao M, Cao P, Yang XM, Zhang SQ. 2009. BAFF enhances B-cell-mediated immune response and vaccine-protection against a very virulent IBDV in chickens. Vaccine 27:1393–1399. doi: 10.1016/j.vaccine.2008.12.040. [DOI] [PubMed] [Google Scholar]
- 29.Dosenovic P, Soldemo M, Scholz JL, O'Dell S, Grasset EK, Pelletier N, Karlsson MCI, Mascola JR, Wyatt RT, Cancro MP, Karlsson Hedestam GB. 2012. BLyS-mediated modulation of naive B cell subsets impacts HIV Env-induced antibody responses. J Immunol 188:6018–6026. doi: 10.4049/jimmunol.1200466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gor DO, Ding X, Li Q, Sultana D, Mambula SS, Bram RJ, Greenspan NS. 2011. Enhanced immunogenicity of pneumococcal surface adhesin A (PsaA) in mice via fusion to recombinant human B lymphocyte stimulator (BLyS). Biol Direct 6:9. doi: 10.1186/1745-6150-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanagavelu SK, Snarsky V, Termini JM, Gupta S, Barzee S, Wright JA, Khan WN, Kornbluth RS, Stone GW. 2012. Soluble multi-trimeric TNF superfamily ligand adjuvants enhance immune responses to a HIV-1 Gag DNA vaccine. Vaccine 30:691–702. doi: 10.1016/j.vaccine.2011.11.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bermejo DA, Amezcua-Vesely MC, Montes CL, Merino MC, Gehrau RC, Cejas H, Acosta-Rodríguez EV, Gruppi A. 2010. BAFF mediates splenic B cell response and antibody production in experimental Chagas disease. PLoS Negl Trop Dis 4:e679. doi: 10.1371/journal.pntd.0000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reed JL, Welliver TP, Sims GP, McKinney L, Velozo L, Avendano L, Hintz K, Luma J, Coyle AJ, Welliver RC. 2009. Innate immune signals modulate antiviral and polyreactive antibody responses during severe respiratory syncytial virus infection. J Infect Dis 199:1128–1138. doi: 10.1086/597386. [DOI] [PubMed] [Google Scholar]
- 34.Metcalf TU, Baxter VK, Nilaratanakul V, Griffin DE. 2013. Recruitment and retention of B cells in the central nervous system in response to alphavirus encephalomyelitis. J Virol 87:2420–2429. doi: 10.1128/JVI.01769-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faber M, Li J, Kean RB, Hooper DC, Alugupalli KR, Dietzschold B. 2009. Effective preexposure and postexposure prophylaxis of rabies with a highly attenuated recombinant rabies virus. Proc Natl Acad Sci U S A 106:11300–11305. doi: 10.1073/pnas.0905640106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li J, Ertel A, Portocarrero C, Barkhouse DA, Dietzschold B, Hooper DC, Faber M. 2012. Postexposure treatment with the live-attenuated rabies virus (RV) vaccine TriGAS triggers the clearance of wild-type RV from the central nervous system (CNS) through the rapid induction of genes relevant to adaptive immunity in CNS tissues. J Virol 86:3200–3210. doi: 10.1128/JVI.06699-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dietzschold B, Morimoto K, Hooper DC, Smith JS, Rupprecht CE, Koprowski H. Genotypic and phenotypic diversity of rabies virus variants involved in human rabies: implications for postexposure prophylaxis. J Hum Virol 3:50–57. [PubMed] [Google Scholar]
- 38.Coetzee P, Nel LH. 2007. Emerging epidemic dog rabies in coastal South Africa: a molecular epidemiological analysis. Virus Res 126:186–195. doi: 10.1016/j.virusres.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 39.Preuss MAR, Faber M-L, Tan GS, Bette M, Dietzschold B, Weihe E, Schnell MJ. 2009. Intravenous inoculation of a bat-associated rabies virus causes lethal encephalopathy in mice through invasion of the brain via neurosecretory hypothalamic fibers. PLoS Pathog 5:e1000485. doi: 10.1371/journal.ppat.1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cenna J, Hunter M, Tan GS, Papaneri AB, Ribka EP, Schnell MJ, Marx PA, McGettigan JP. 2009. Replication-deficient rabies virus-based vaccines are safe and immunogenic in mice and nonhuman primates. J Infect Dis 200:1251–1260. doi: 10.1086/605949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dorfmeier CL, Lytle AG, Dunkel AL, Gatt A, McGettigan JP. 2012. Protective vaccine-induced CD4(+) T cell-independent B cell responses against rabies infection. J Virol 86:11533–11540. doi: 10.1128/JVI.00615-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Do RK, Hatada E, Lee H, Tourigny MR, Hilbert D, Chen-Kiang S. 2000. Attenuation of apoptosis underlies B lymphocyte stimulator enhancement of humoral immune response. J Exp Med 192:953–964. doi: 10.1084/jem.192.7.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dorfmeier CL, Shen S, Tzvetkov EP, McGettigan JP. 2013. Reinvestigating the role of IgM in rabies virus postexposure vaccination. J Virol 87:9217–9222. doi: 10.1128/JVI.00995-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cannons JL, Qi H, Lu KT, Dutta M, Gomez-Rodriguez J, Cheng J, Wakeland EK, Germain RN, Schwartzberg PL. 2010. Optimal germinal center responses require a multistage T cell:B cell adhesion process involving integrins, SLAM-associated protein, and CD84. Immunity 32:253–265. doi: 10.1016/j.immuni.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qi H, Cannons JL, Klauschen F, Schwartzberg PL, Germain RN. 2008. SAP-controlled T-B cell interactions underlie germinal centre formation. Nature 455:764–769. doi: 10.1038/nature07345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, Schwartzberg PL, Cook MC, Walters GD, Vinuesa CG. 2009. Follicular helper T cells are required for systemic autoimmunity. J Exp Med 206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woo YJ, Yoon BY, Jhun JY, Oh HJ, Min SW, Cho La M, Park SH, Kim HY, Min JK. 2011. Regulation of B cell activating factor (BAFF) receptor expression by NF-ΚB signaling in rheumatoid arthritis B cells. Exp Mol Med 43:350–357. doi: 10.3858/emm.2011.43.6.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mariño E, Walters SN, Villanueva JE, Richards JL, Mackay CR, Grey ST. 2014. BAFF regulates activation of self-reactive T cells through B-cell dependent mechanisms and mediates protection in NOD mice. Eur J Immunol 44:983–993. doi: 10.1002/eji.201344186. [DOI] [PubMed] [Google Scholar]
- 49.Figgett WA, Deliyanti D, Fairfax KA, Quah PS, Wilkinson-Berka JL, Mackay F. 2015. Deleting the BAFF receptor TACI protects against systemic lupus erythematosus without extensive reduction of B cell numbers. J Autoimmun 61:9–16. doi: 10.1016/j.jaut.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 50.Klinman NR, Linton PJ. 1990. The generation of B-cell memory: a working hypothesis. Curr Top Microbiol Immunol 159:19–35. [DOI] [PubMed] [Google Scholar]
- 51.Oliver AM, Martin F, Gartland GL, Carter RH, Kearney JF. 1997. Marginal zone B cells exhibit unique activation, proliferative and immunoglobulin secretory responses. Eur J Immunol 27:2366–2374. doi: 10.1002/eji.1830270935. [DOI] [PubMed] [Google Scholar]
- 52.Lane PJ, Gray D, Oldfield S, MacLennan IC. 1986. Differences in the recruitment of virgin B cells into antibody responses to thymus-dependent and thymus-independent type-2 antigens. Eur J Immunol 16:1569–1575. doi: 10.1002/eji.1830161216. [DOI] [PubMed] [Google Scholar]
- 53.Parker RJ, Hartman KD, Sieber SM. 1981. Lymphatic absorption and tissue disposition of liposome-entrapped [14C]adriamycin following intraperitoneal administration to rats. Cancer Res 41:1311–1317. [PubMed] [Google Scholar]
- 54.Al-Alem U, Li C, Forey N, Relouzat F, Fondanèche M-C, Tavtigian SV, Wang Z-Q, Latour S, Yin L. 2005. Impaired Ig class switch in mice deficient for the X-linked lymphoproliferative disease gene Sap. Blood 106:2069–2075. doi: 10.1182/blood-2004-07-2731. [DOI] [PubMed] [Google Scholar]
- 55.Crotty S, Kersh EN, Cannons J, Schwartzberg PL, Ahmed R. 2003. SAP is required for generating long-term humoral immunity. Nature 421:282–287. doi: 10.1038/nature01318. [DOI] [PubMed] [Google Scholar]
- 56.Huard B, Arlettaz L, Ambrose C, Kindler V, Mauri D, Roosnek E, Tschopp J, Schneider P, French LE. 2004. BAFF production by antigen-presenting cells provides T cell co-stimulation. Int Immunol 16:467–475. doi: 10.1093/intimm/dxh043. [DOI] [PubMed] [Google Scholar]
- 57.Huard B, Schneider P, Mauri D, Tschopp J, French LE. 2001. T cell costimulation by the TNF ligand BAFF. J Immunol 167:6225–6231. doi: 10.4049/jimmunol.167.11.6225. [DOI] [PubMed] [Google Scholar]
- 58.Chappell CP, Draves KE, Giltiay NV, Clark EA. 2012. Extrafollicular B cell activation by marginal zone dendritic cells drives T cell-dependent antibody responses. J Exp Med 209:1825–1840. doi: 10.1084/jem.20120774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.García de Vinuesa C, MacLennan IC, Holman M, Klaus GG. 1999. Anti-CD40 antibody enhances responses to polysaccharide without mimicking T cell help. Eur J Immunol 29:3216–3224. [DOI] [PubMed] [Google Scholar]
- 60.García De Vinuesa C, Gulbranson-Judge A, Khan M, O'Leary P, Cascalho M, Wabl M, Klaus GG, Owen MJ, MacLennan IC. 1999. Dendritic cells associated with plasmablast survival. Eur J Immunol 29:3712–3721. [DOI] [PubMed] [Google Scholar]
- 61.Ng LG, Sutherland APR, Newton R, Qian F, Cachero TG, Scott ML, Thompson JS, Wheway J, Chtanova T, Groom J, Sutton IJ, Xin C, Tangye SG, Kalled SL, Mackay F, Mackay CR. 2004. B cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF costimulation of circulating T and B cells. J Immunol 173:807–817. doi: 10.4049/jimmunol.173.2.807. [DOI] [PubMed] [Google Scholar]
- 62.Hsu BL, Harless SM, Lindsley RC, Hilbert DM, Cancro MP. 2002. Cutting edge: BLyS enables survival of transitional and mature B cells through distinct mediators. J Immunol 168:5993–5996. doi: 10.4049/jimmunol.168.12.5993. [DOI] [PubMed] [Google Scholar]
- 63.Gerhard W, Mozdzanowska K, Furchner M, Washko G, Maiese K. 1997. Role of the B-cell response in recovery of mice from primary influenza virus infection. Immunol Rev 159:95–103. doi: 10.1111/j.1600-065X.1997.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 64.Mozdzanowska K, Furchner M, Zharikova D, Feng J, Gerhard W. 2005. Roles of CD4+ T-cell-independent and -dependent antibody responses in the control of influenza virus infection: evidence for noncognate CD4+ T-cell activities that enhance the therapeutic activity of antiviral antibodies. J Virol 79:5943–5951. doi: 10.1128/JVI.79.10.5943-5951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schnell MJ, Foley HD, Siler CA, McGettigan JP, Dietzschold B, Pomerantz RJ. 2000. Recombinant rabies virus as potential live-viral vaccines for HIV-1. Proc Natl Acad Sci U S A 97:3544–3549. doi: 10.1073/pnas.97.7.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Conzelmann KK, Cox JH, Schneider LG, Thiel HJ. 1990. Molecular cloning and complete nucleotide sequence of the attenuated rabies virus SAD B19. Virology 175:485–499. doi: 10.1016/0042-6822(90)90433-R. [DOI] [PubMed] [Google Scholar]
- 67.McGettigan JP, Sarma S, Orenstein JM, Pomerantz RJ, Schnell MJ. 2001. Expression and immunogenicity of human immunodeficiency virus type 1 Gag expressed by a replication-competent rhabdovirus-based vaccine vector. J Virol 75:8724–8732. doi: 10.1128/JVI.75.18.8724-8732.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Norton JE, Lytle AG, Shen S, Tzvetkov EP, Dorfmeier CL, McGettigan JP. 2014. ICAM-1-based rabies virus vaccine shows increased infection and activation of primary murine B cells in vitro and enhanced antibody titers in-vivo. PLoS One 9:e87098. doi: 10.1371/journal.pone.0087098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McGettigan JP, Naper K, Orenstein J, Koser M, McKenna PM, Schnell MJ. 2003. Functional human immunodeficiency virus type 1 (HIV-1) Gag-Pol or HIV-1 Gag-Pol and env expressed from a single rhabdovirus-based vaccine vector genome. J Virol 77:10889–10899. doi: 10.1128/JVI.77.20.10889-10899.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lytle AG, Norton JE, Dorfmeier CL, Shen S, McGettigan JP. 2013. B cell infection and activation by rabies virus-based vaccines. J Virol 87:9097–9110. doi: 10.1128/JVI.00800-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cenna J, Tan GS, Papaneri AB, Dietzschold B, Schnell MJ, McGettigan JP. 2008. Immune modulating effect by a phosphoprotein-deleted rabies virus vaccine vector expressing two copies of the rabies virus glycoprotein gene. Vaccine 26:6405–6414. doi: 10.1016/j.vaccine.2008.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dorfmeier CL, Tzvetkov EP, Gatt A, McGettigan JP. 2013. Investigating the role for IL-21 in rabies virus vaccine-induced immunity. PLoS Negl Trop Dis 7:e2129. doi: 10.1371/journal.pntd.0002129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dunkel A, Shen S, LaBranche CC, Montefiori D, McGettigan JP. 2015. A bivalent, chimeric rabies virus expressing simian immunodeficiency virus envelope induces multifunctional antibody responses. AIDS Res Hum Retrovir 31:1126–1138. doi: 10.1089/aid.2014.0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Czar MJ, Kersh EN, Mijares LA, Lanier G, Lewis J, Yap G, Chen A, Sher A, Duckett CS, Ahmed R, Schwartzberg PL. 2001. Altered lymphocyte responses and cytokine production in mice deficient in the X-linked lymphoproliferative disease gene SH2D1A/DSHP/SAP. Proc Natl Acad Sci U S A 98:7449–7454. doi: 10.1073/pnas.131193098. [DOI] [PMC free article] [PubMed] [Google Scholar]