ABSTRACT
In addition to their intended use, progesterone (P4)-based contraceptives promote anti-inflammatory immune responses, yet their effects on the outcome of infectious diseases, including influenza A virus (IAV) infection, are rarely evaluated. To evaluate their impact on immune responses to sequential IAV infections, adult female mice were treated with placebo or one of two progestins, P4 or levonorgestrel (LNG), and infected with a mouse-adapted H1N1 (maH1N1) virus. Treatment with P4 or LNG reduced morbidity but had no effect on pulmonary virus titers during primary H1N1 infection compared to placebo treatment. In serum and bronchoalveolar lavage fluid, total anti-IAV IgG and IgA titers and virus-neutralizing antibody titers but not hemagglutinin stalk antibody titers were lower in progestin-treated mice than placebo-treated mice. Females were challenged 6 weeks later with either an maH1N1 drift variant (maH1N1dv) or maH3N2 IAV. The level of protection following infection with the maH1N1dv was similar among all groups. In contrast, following challenge with maH3N2, progestin treatment reduced survival as well as the numbers and activity of H1N1- and H3N2-specific memory CD8+ T cells, including tissue-resident cells, compared with placebo treatment. In contrast to primary IAV infection, progestin treatment increased the titers of neutralizing and IgG antibodies against both challenge viruses compared with those achieved with placebo treatment. While the immunomodulatory properties of progestins protected immunologically naive female mice from the severe outcomes from IAV infection, it made them more susceptible to secondary challenge with a heterologous IAV, despite improving their antibody responses against a secondary IAV infection. Taken together, the immunomodulatory effects of progestins differentially regulate the outcome of infection depending on exposure history.
IMPORTANCE The impact of hormone-based contraceptives on the outcome of infectious diseases outside the reproductive tract is rarely considered. Using a mouse model, we have made the novel observation that treatment with either progesterone or a synthetic analog found in hormonal contraceptives, levonorgestrel, impacts sequential influenza A virus infection by modulating antibody responses and decreasing the numbers and activity of memory CD8+ T cells. Progestins reduced the antibody responses during primary H1N1 virus infection but increased antibody titers following a sequential infection with either an H1N1 drift variant or an H3N2 virus. Following challenge with an H3N2 virus, female mice treated with progestins experienced greater mortality with increased pulmonary inflammation and reduced numbers and activity of CD8+ T cells. This study suggests that progestins significantly affect adaptive immune responses to influenza A virus infection, with their effect on the outcome of infection depending on exposure history.
KEYWORDS: H1N1, H3N2, progesterone, levonorgestrel, memory CD8+ T cells, stalk antibody, tissue-resident memory cells, memory CD8+ T cells, women's health
INTRODUCTION
Adults worldwide are exposed to multiple influenza viruses and viral antigens during their lifetimes through both natural infection and vaccination. In the United States, since 2010, vaccination has been recommended for all individuals ages 6 months and older (1). By 6 years of age, all children have been exposed to at least one influenza virus, with infections with novel antigenically distinct viruses occurring every 5 to 10 years (2, 3). By adulthood, most individuals are no longer immunologically naive to influenza virus, which is rarely considered in animal models of influenza A virus (IAV) pathogenesis.
Protective immune responses to sequential IAV infections include cross-reactive antibody responses, including those involving broadly neutralizing antibodies which target the conserved stalk region of the hemagglutinin (HA) antigen, and CD8+ T cell responses, with the latter being the primary mechanism of cross protection against heterosubtypic influenza virus strains, as CD8+ T cells can recognize conserved viral proteins, including the nucleoprotein (NP) (4–6). In addition to circulating memory T cells and T central memory (TCM) cells that traffic through the lymph nodes, tissue-resident memory (TRM) cells promote local and immediate protection in the lungs and have the ability to expand rapidly, kill virus-infected cells, recruit circulating memory T cells, and release cytokines, with the result being that TRM cells are indispensable for heterosubtypic protection (7–9).
Several factors, including the sex, age, and reproductive status of the host, can influence adaptive immune responses and the outcome of IAV infection (10–12). During IAV infection, sex steroids, in general, and progesterone (P4), in particular, can alter the functioning of immune cells and respiratory epithelial cells to reduce pulmonary inflammation, improve pulmonary repair and function, and cause faster recovery from primary infection with IAV (13–15). Data from the Klein lab (13) and others (16–20) illustrate that P4 has broad anti-inflammatory properties, resulting in the reduced activity of NK and T cells, lower antibody responses, greater concentrations of transforming growth factor β, and increased numbers of regulatory T cells and regulatory Th17 cells with increased activity.
In addition to natural exposure to P4 through ovarian and placental production during reproductive cycles and pregnancy, respectively, females can be exposed to synthetic forms of P4 (i.e., progestins) through the use of hormone-based contraceptives, all of which contain some form of progestin (21). It is estimated that 88% of all adult women in the United States have been exposed to progestins in some form of contraceptives (22). Progestins, such as levonorgestrel (LNG), are more commonly used in contraceptives than P4 because they bind to the progesterone receptor with a greater affinity, have a longer half-life, and cause fewer side effects (23). Despite the broad use of progestins and their known anti-inflammatory properties, their effects on viral infections at mucosal sites outside the reproductive tract have not been adequately explored.
The goal of this study was to evaluate the effects of both P4 and LNG on adaptive immune responses and the outcome of sequential infection with an IAV drift variant or heterosubtypic IAV. We hypothesized that, similar to the effects of P4 treatment in ovariectomized mice, exposure to either P4 or LNG in ovary-intact mice would improve the outcome of a primary IAV infection in immunologically naive female mice by reducing the level of inflammation and the adaptive immune responses that contribute to immune-mediated pathology (13). Following a secondary challenge with either a drift variant or a heterosubtypic strain of IAV, we speculated that the reduced adaptive immune responses to the primary infection, while beneficial for recovery from the primary infection, may cause reduced protection against a subsequent infection. Our findings demonstrate that following treatment with either P4 or LNG, female mice were better protected from a primary IAV infection, despite generating lower antibody and memory CD8+ T cell responses. Following secondary infection with a heterosubtypic IAV but not an IAV drift variant, female mice treated with either P4 or LNG suffered a worse disease outcome from infection, which was likely mediated by insufficient memory T cell responses, as opposed to antibody responses from the primary infection.
RESULTS
Progesterone and levonorgestrel protect against primary IAV infection but reduce systemic and pulmonary antibody responses against IAV.
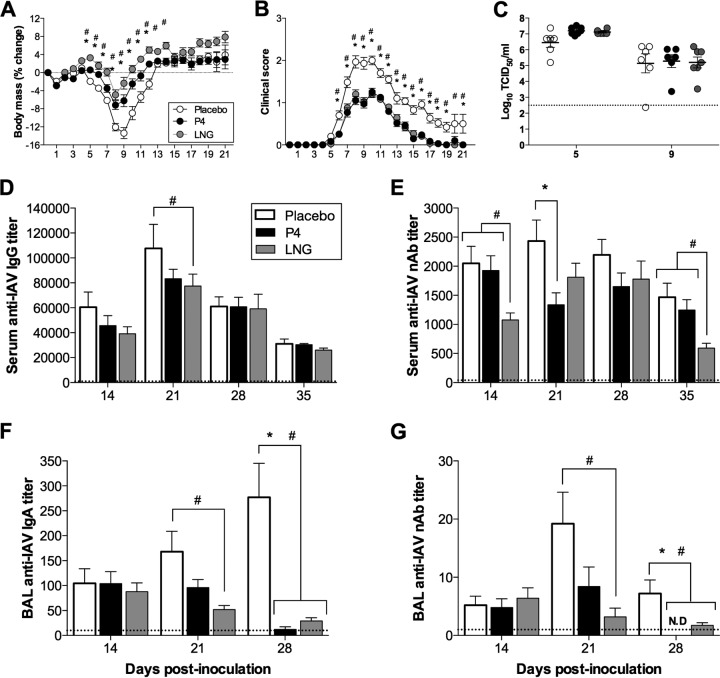
Adult female mice were treated with subcutaneous placebo, P4, or LNG pellets, which delivered a continuous dose of hormone over the course of 60 days, and were infected with a low dose of mouse-adapted A/California/04/09 H1N1 (ma2009 H1N1) virus. Similar to previously published results obtained with ovariectomized female mice (13), treatment of ovary-intact female mice with P4 reduced the rate of morbidity following IAV infection by decreasing body mass loss, hypothermia, and clinical disease compared to those achieved with placebo treatment (Fig. 1A and B and data not shown) (P < 0.05). One of the most common progestins (i.e., a synthetic analog of P4) used in hormonal contraceptives is LNG, which signals through the progesterone receptor with a greater affinity than P4 (24). Similar to the results obtained with P4 treatment, mice treated with LNG and infected with a low dose of ma2009 H1N1 virus had less morbidity than placebo-treated female mice during infection, including reduced body mass loss, hypothermia, and clinical signs (Fig. 1A and B and data not shown) (P < 0.05). Even though P4 and LNG treatment reduced the rates of morbidity, neither P4 nor LNG treatment significantly affected pulmonary virus titers prior to (5 days postinoculation [dpi]) or during (9 dpi) peak disease compared with those achieved with placebo treatment (Fig. 1C).
FIG 1.
P4 and LNG treatment reduced the rate of morbidity and the level of antibody production during primary H1N1 IAV infection. (A, B) Adult female mice were treated with placebo, P4, or LNG and inoculated intranasally with a low dose of ma2009 H1N1 virus. Mice were monitored daily for 21 dpi (as indicated on the y axis) for changes in body mass (A) and clinical disease (B). (C) Infectious virus titers in the lungs were measured prior to (5 dpi) and during (9 dpi) peak influenza disease (n = 6 to 7 mice per treatment). (D, E) Serum was collected at 14, 21, 28, and 35 dpi (as indicated on the y axis), and anti-ma2009 IgG titers were measured by ELISA (D) and ma2009-neutralizing antibody titers were analyzed by a neutralization assay (E) (n = 30 mice per treatment per time point). (F, G) BAL fluid was collected at 14, 21, and 28 dpi and analyzed for anti-ma2009 IgA titers (F) and neutralizing antibody titers (G) (n = 8 to 10 mice per treatment per time point). The stippled line represents the antibody levels for naive animals. Data represent the means ± SEMs from three independent replications. Significant differences between P4-treated and placebo-treated mice are represented by asterisks, and significant differences between the LNG-treated and placebo-treated mice are represented by pound signs. nAb, neutralizing antibody; N.D., not detectable.
The serum titers of anti-ma2009 H1N1 virus IgG antibodies were measured at 14, 21, 28, and 35 dpi and did not differ between placebo- and P4-treated female mice but were transiently lower at 21 dpi following LNG treatment (Fig. 1D) (P < 0.05). The serum titers of neutralizing antibodies against the ma2009 virus measured at 14, 21, 28, and 35 dpi were significantly reduced in female mice treated with P4 (at 21 dpi) or LNG (at 14 and 35 dpi) compared to those in female mice treated with placebo (Fig. 1E) (P < 0.05). Progestins reduced the neutralizing antibody responses; however, the titers at 35 dpi remained at about the 1/640 dilution, which is high enough to protect mice against a homologous challenge (25). Antibody titers in bronchoalveolar lavage (BAL) fluid were also analyzed at 14, 21, and 28 dpi to assess the mucosal immune response at the local site of infection. The titers of both anti-ma2009 virus IgA and neutralizing antibodies against ma2009 in BAL fluid were reduced in female mice treated with either P4 (at 28 dpi) or LNG (at 21 and 28 dpi) compared to those in female mice treated with placebo (Fig. 1F and G) (P < 0.05). In summary, P4 and, to a greater extent, LNG protected female mice against IAV infection but significantly reduced the production of systemic and local antibodies during a primary infection.
Protection against an H1N1 drift variant and hemagglutinin stalk antibody responses are not affected by treatment with progestins.
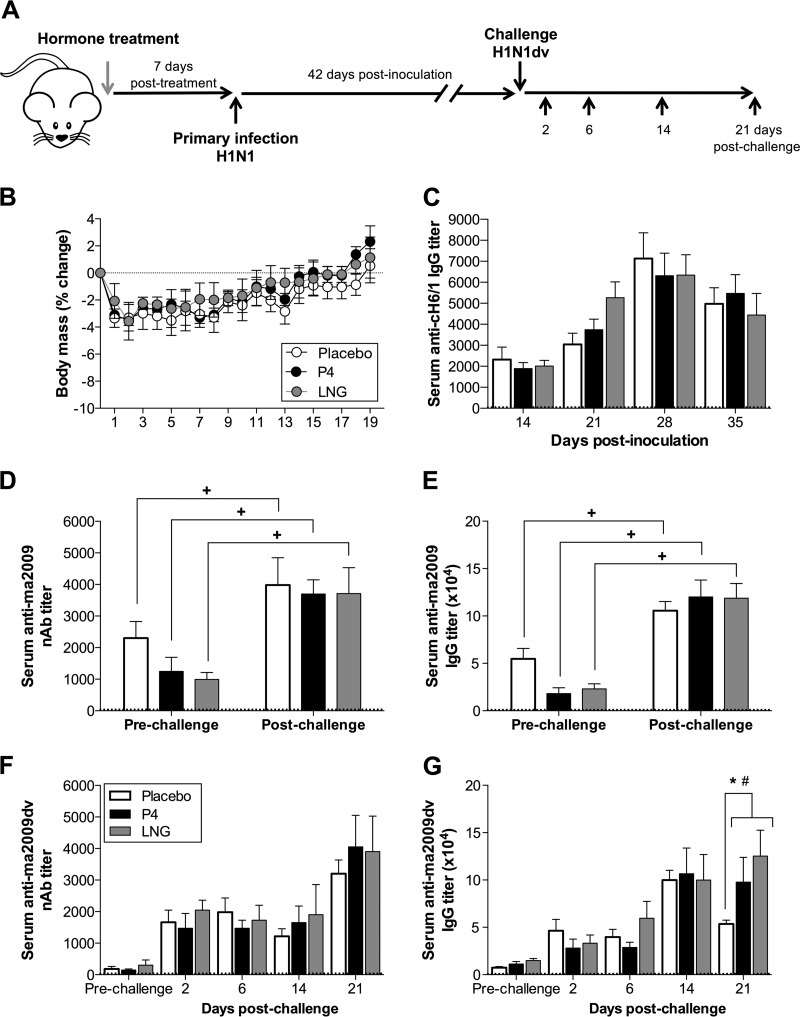
To assess whether the P4- and LNG-induced reductions in serum and, to a greater extent, BAL fluid antibody titers (Fig. 1D to G) could impact the outcome of sequential infection with a closely related H1N1 virus, female mice were treated with P4, LNG, or placebo, inoculated with the ma2009 H1N1 virus, and then challenged 6 weeks later with an H1N1 ma2009 drift variant (ma2009dv) which contained a mutation at position 166 in the HA head domain (K166Q) (26) (Fig. 2A). All female mice, regardless of hormone treatment, were equally protected against ma2009dv H1N1 (Fig. 2B). Hemagglutinin stalk-specific antibodies recognize the conserved stalk region of the HA protein of IAVs and contribute to cross protection against diverse IAVs (27, 28). To assess whether the titers of broadly neutralizing antibodies against the stalk of H1 may contribute to the similar levels of protection against ma2009dv observed in both placebo- and hormone-treated female mice following challenge, we measured the stalk-specific antibody responses in serum at several time points following the primary ma2009 H1N1 virus infection. Stalk antibody titers increased over time, peaking at 28 dpi in all female mice, and did not differ between placebo-treated and either P4- or LNG-treated female mice (Fig. 2C).
FIG 2.
Neither P4 nor LNG treatment altered protection following challenge with an ma2009 H1N1 drift variant in female mice. (A) Adult female mice were treated with placebo, P4, or LNG and inoculated intranasally with a low dose of the ma2009 H1N1 virus. Six weeks later, the mice were challenged intranasally with a high dose of an ma2009 drift variant (K166Q) H1N1 virus and euthanized at the indicated time points. (B) Mice (n = 10 mice per treatment) were monitored for changes in body mass for 21 days postchallenge (as indicated on the y axis). (C) The titers of broadly neutralizing stalk antibodies in serum collected at 14, 21, 28, and 35 days after primary infection were measured using a stalk antibody ELISA with a chimeric protein with an exotic HA head and conserved ma2009 H1N1 stalk (cH6/1) (n = 25 to 30 mice per treatment per time point). (D, E) The neutralizing antibody responses (D) and titers of IgG antibodies (E) against the primary ma2009 H1N1 virus were measured in serum collected prior to challenge (21 days after primary infection) and 21 days after challenge (n = 10 mice per treatment per time point). (F, G) Neutralizing antibody responses (F) and titers of IgG antibodies (G) against the ma2009dv H1N1 virus were measured in serum collected prior to challenge (21 days after primary infection) and 2, 6, 14, or 21 days after challenge (n = 10 mice per treatment per time point). The stippled line represents the antibody levels for naive animals. Data represent the means ± SEMs from two independent replications. Significant differences between P4-treated and placebo-treated female mice are represented by an asterisk, significant differences between LNG-treated and placebo-treated female mice are represented by a pound sign, and significant differences within a treatment group between the results before and after challenge are represented by plus signs.
To assess whether the P4-based treatments affected the antibody responses against the primary virus following the ma2009dv challenge, we measured serum anti-ma2009 virus-neutralizing antibody and IgG titers pre- and postchallenge with ma2009dv. Following challenge with a drift variant virus, antibody responses to the primary virus were boosted at 21 days postchallenge in all treatment groups (Fig. 2D and E) (P < 0.05). Among all treatment groups, despite minimal titers of preexisting serum neutralizing and IgG antibodies against the drift variant, following challenge with maH1N1dv, serum neutralizing antibodies to the drift variant were detectable as early as 2 days postchallenge and were associated with complete clearance of the virus from the lungs (Fig. 2F and data not shown). Serum anti-IAV IgG antibody responses against ma2009dv also increased as early as 2 days postchallenge but were lower in the placebo-treated than the P4- or LNG-treated female mice at 21 days postchallenge (Fig. 2G) (P < 0.05). Taken together, these data suggest that the P4-based treatments did not alter broadly neutralizing stalk antibody responses or protection against a drift variant of H1N1 but increased total IgG antibody responses following challenge with ma2009dv.
Progestins reduce protection against a heterologous IAV challenge while increasing the titers of antibodies against the challenge virus.
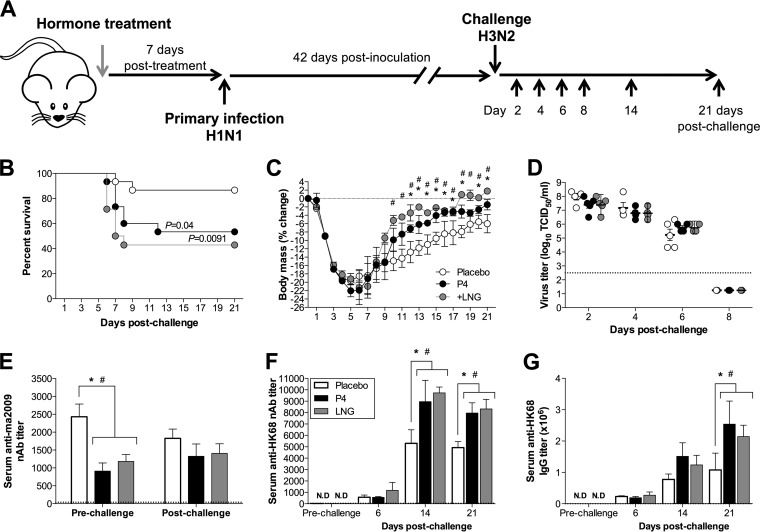
Treatment with either P4 or LNG did not affect the outcome of challenge with a homologous group 1 drift variant (Fig. 2B), but during influenza seasons, both the H1N1 and H3N2 subtypes circulate. H1 and H3 IAVs are antigenically distinct, belonging to the group 1 and group 2 HA phylogenetic groups, respectively (29). To analyze the effects of progestins on a heterologous IAV challenge with an HA group 2 IAV, female mice were treated with either P4, LNG, or placebo, infected with the ma2009 H1N1 virus, and challenged 6 weeks later with an maH3N2 virus, A/Hong Kong/2/68 (HK68), at a dose that is uniformly lethal in naive animals (data not shown). In contrast to the results obtained with primary IAV infection, placebo-treated female mice were protected against lethal maH3N2 virus challenge, with 87% (13/15) of placebo-treated, 53% (8/15) of P4-treated, and 43% (6/14) of LNG-treated female mice surviving the challenge (Fig. 3B) (P < 0.05). In the mice that survived the challenge, however, progestin treatment promoted faster recovery than placebo treatment did (Fig. 3C) (P < 0.05), a finding which is consistent with the effects during a primary infection (Fig. 1A and B). Treatment with either P4 or LNG did not alter H3N2 titers or clearance in the lungs (Fig. 3D).
FIG 3.
P4 and LNG treatments reduced survival but increased antibody titers following challenge with a heterosubtypic H3N2 influenza A virus. (A) Adult female mice were treated with placebo, P4, or LNG and inoculated intranasally with a low dose of ma2009 H1N1 virus. Six weeks later, ma2009 H1N1-infected mice were challenged intranasally with a high dose of HK68 H3N2 virus and euthanized at the indicated days postchallenge. (B, C) These female mice (n = 15 mice per treatment) were monitored for changes in mortality (B) and body mass (C) for 21 days postchallenge (as indicated on the y axis). (D) Infectious virus titers in the lungs were measured at 2, 4, 6, and 8 days postchallenge (n = 5 to 8 mice per treatment per day). (E) The titers of neutralizing antibodies against the primary ma2009 H1N1 virus were measured in serum collected prior to challenge (21 days after primary infection) and 21 days after challenge (n = 10 mice per treatment per time point). (F, G) Neutralizing antibody responses (F) and titers of IgG antibodies (G) against the HK68 H3N2 virus were measured in serum collected prior to challenge (21 days after primary infection) and 6, 14, or 21 days after challenge (n = 10 mice per treatment per time point). The stippled line represents the antibody levels for naive animals. Data represent the means ± SEMs from two independent replications. Significant differences between P4-treated and placebo-treated female mice are represented by asterisks, and significant differences between LNG-treated and placebo-treated female mice are represented by pound signs.
To determine whether the humoral immune responses during the heterosubtypic challenge were affected by progestins in the mice that survived the challenge, neutralizing and total IgG antibody responses against the primary ma2009 IAV and the secondary heterologous HK68 IAV were measured both prior to and after challenge. In contrast to the neutralizing antibody titers after ma2009dv challenge (Fig. 2D), when female mice were challenged with a heterosubtypic maH3N2 virus, the titers of neutralizing antibody against the primary H1N1 IAV (i.e., the ma2009 H1N1 virus) were not altered (Fig. 3E). Preexisting serum neutralizing antibodies against HK68 were not detected in the serum prior to the challenge in any of the treatment groups (Fig. 3F), but the titers of antibodies against HK68 were significantly greater in P4- and LNG-treated female mice than placebo-treated female mice at 14 and 21 days postchallenge (Fig. 3F) (P < 0.05). Similarly, serum anti-HK68 IgG antibodies were not detected prior to challenge, but at 21 days postchallenge their titers were significantly greater in both P4- and LNG-treated mice than in placebo-treated female mice (Fig. 3G) (P < 0.05). Although treatment with progestins reduced the neutralizing antibody responses to primary IAV infection, these hormones significantly increased the neutralizing antibody responses against a sequential infection with an antigenically unrelated IAV. Taken together, these data illustrate that in the context of a heterologous challenge, progestins increase the antibody responses to sequential influenza A virus infection, without having an effect on antibodies against the primary virus, which may be beneficial for vaccine responses.
Progestins increase pulmonary immunopathology following challenge with a heterologous IAV.
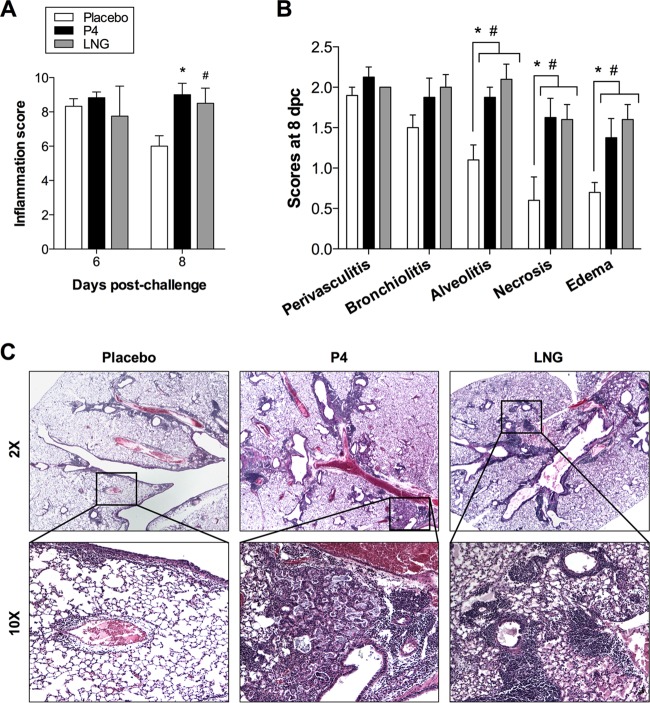
Because the titers of antibodies against HK68 early after challenge were not different between the placebo- and progestin-treated female mice (Fig. 3E), we sought to identify other possible mechanisms of differential mortality between the placebo- and progestin-treated female mice following a heterologous challenge. We analyzed the pulmonary immunopathology in female mice that were treated with either P4, LNG, or placebo, infected with ma2009 H1N1 virus, and challenged 6 weeks later with an maH3N2 virus. The lungs were inflated and fixed at 6 and 8 days postchallenge to encompass the average day of death for female mice in each of the progestin treatment groups (average day of death for P4- and LNG-treated mice, 7.8 ± 0.7 and 6.6 ± 0.2, respectively). No differences in overall inflammation scores across the treatment groups were observed at day 6 after H3N2 challenge, but by day 8 postchallenge, female mice treated with either P4 or LNG had greater inflammation, as characterized by increased alveolitis, necrosis, and edema, than placebo-treated female mice (Fig. 4A to C) (P < 0.05). Taken together, these data indicate that the increased mortality following a heterologous H3N2 challenge in progestin-treated female mice may be caused by excessive immunopathology in the lungs at the time of death.
FIG 4.
P4 and LNG treatments increased pulmonary immunopathology following challenge with a heterosubtypic H3N2 influenza A virus. Adult female mice were treated with placebo, P4, or LNG and inoculated intranasally with a low dose of ma2009 H1N1 virus. Six weeks later, the mice were challenged intranasally with a high dose of HK68 H3N2 virus. (A) H&E-stained lung sections collected at 6 and 8 days postchallenge were scored for inflammation, given as a cumulative score for perivasculitis, bronchiolitis, alveolitis, edema, and necrosis. (B) Separate scores at 8 days postchallenge (dpc) for perivasculitis, bronchiolitis, alveolitis, edema, and necrosis are shown. (C) Representative images of overall inflammation (2× magnification) and focused areas (10× magnification) with cellular infiltration and edema are shown (n = 5 mice per treatment per day). Data represent the means ± SEMs from two independent replications. Significant differences between P4-treated and placebo-treated female mice are represented by asterisks, and significant differences between LNG-treated and placebo-treated female mice are represented by pound signs.
Progestins reduce memory CD8+ T cell responses against a heterologous IAV challenge.
Although antibody responses to the primary ma2009 H1N1 infection were significantly lower in P4- and LNG-treated female mice than placebo-treated female mice, antibody-mediated immunity is not the primary mechanism of cross protection against different groups of IAVs, which possess distinct surface antigens but share common core proteins that could be recognized by T cells. Populations of memory T cells mediate cross protection against different groups of IAVs (4–6, 30) and were analyzed at several time points prior to and after challenge with the H3N2 IAV to determine if suppressed memory T cell numbers and activity in progestin-treated female mice may underlie the increased susceptibility to heterosubtypic infection. Treatment with either P4 or LNG did not alter the numbers of total virus-specific CD4+ T cell, Th2 cell, or Th17 cell subsets in the lungs but reduced the numbers of Th1 cells after challenge (Table 1). The frequencies of total CD8+ T cells and naive CD8+ T cells also were not significantly different between placebo-, P4-, and LNG-treated mice prior to or after heterosubtypic IAV challenge (Table 1).
TABLE 1.
Total numbers of virus-specific CD4+ and CD8+ T cells recognizing ma2009 in the lungs of female mice following challengea
| Total cells | Treatment | No. of virus-specific CD8+ T cells on the following days postchallenge: |
||
|---|---|---|---|---|
| 0 | 2 | 6 | ||
| CD4+ T cells | Placebo | 8,230 ± 662 | 6,829 ± 1,480 | 32,752 ± 3,067 |
| P4 | 8,093 ± 1,720 | 9,511 ± 1,951 | 29,904 ± 3,816 | |
| LNG | 7,018 ± 857 | 7,017 ± 1,424 | 27,204 ± 3,200 | |
| IFN-γ+ CD4+ T cells | Placebo | 86.35 ± 9.2 | 185.7 ± 32.3 | 883 ± 114 |
| P4 | 93.04 ± 21.1 | 244 ± 35.1 | 551.6 ± 101* | |
| LNG | 83.63 ± 12.2 | 159.8 ± 35.3 | 454 ± 81.3# | |
| IL-4+ CD4+ T cells | Placebo | 19.72 ± 5.6 | 8.71 ± 4.0 | 22.84 ± 6.1 |
| P4 | 28.36 ± 10.8 | 14.88 ± 7.8 | 26.75 ± 5.4 | |
| LNG | 20.72 ± 7.6 | 5.96 ± 2.8 | 18.63 ± 4.4 | |
| IL-17+ CD4+ T cells | Placebo | 46.76 ± 14.2 | 26.62 ± 9.5 | 34.24 ± 11.0 |
| P4 | 67.85 ± 24.5 | 33.64 ± 7.7 | 29.27 ± 11.3 | |
| LNG | 42.76 ± 24.2 | 41.68 ± 11.3 | 31.31 ± 12.3 | |
| CD8+ T cells | Placebo | 8,958 ± 823 | 11,275 ± 2,590 | 55,003 ± 13,269 |
| P4 | 9,798 ± 400 | 15,512 ± 5,861 | 44,293 ± 7,757 | |
| LNG | 12,192 ± 2,273 | 12,215 ± 1,289 | 45,214 ± 9,833 | |
| Naive CD8+ T cells | Placebo | 4,413 ± 608 | 3,500 ± 1,271 | 5,530 ± 380 |
| P4 | 4,372 ± 1,272 | 2,365 ± 650 | 5,633 ± 814 | |
| LNG | 2,815 ± 817 | 3,360 ± 283 | 4,800 ± 1,126 | |
Adult female mice were treated with placebo, progesterone (P4), or levonorgestrel (LNG) and inoculated intranasally with a low dose of ma2009 H1N1 virus. Six weeks later, the mice were challenged intranasally with a high dose of HK68 H3N2 virus. Lung single-cell suspensions were harvested for flow cytometry analysis at 42 days after primary infection (i.e., prior to challenge; referred to as day 0 postchallenge) and at days 2 and 6 days postchallenge and stimulated ex vivo with ma2009-specific antigen in the presence of brefeldin A. The total numbers of live T cells were analyzed. Data represent means ± standard errors of the means from two independent replications (n = 8 mice per treatment per day). Significant differences between P4-treated and placebo-treated female mice are represented by an asterisk and boldface, and significant differences between LNG-treated and placebo-treated female mice are represented by a pound sign and boldface.
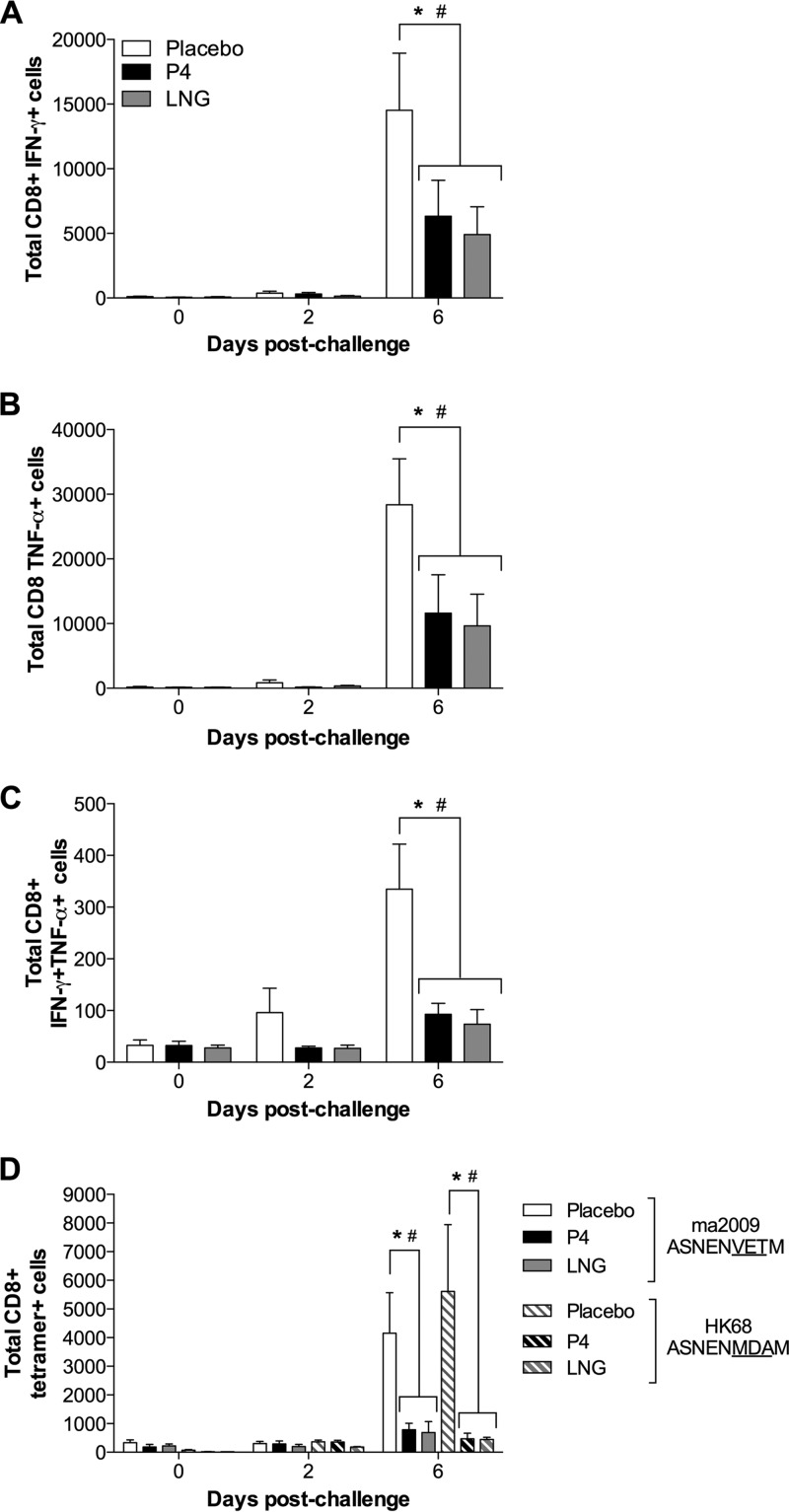
Following challenge with a heterosubtypic maH3N2, the frequency of most virus-specific CD8+ T cell populations in the lungs increased over time, regardless of hormone treatment (Fig. 5A to D). Treatment of female mice with either P4 or LNG significantly reduced the total number of ma2009 virus-specific CD8+ T cells producing gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), or both cytokines compared to treatment with placebo at 6 days postchallenge (Fig. 5A to C) (P < 0.05). The numbers of tetramer-specific CD8+ T cells that recognized either the ma2009-specific (i.e., primary IAV) or HK68-specific (i.e., secondary IAV) immunodominant NP peptides also were lower at 6 days postchallenge in female mice treated with either P4 or LNG than in female mice treated with placebo (Fig. 5D) (P < 0.05).
FIG 5.
Treatment with either P4 or LGN reduced the numbers of virus-specific CD8+ T cells in the lungs of female mice challenged with a heterosubtypic H3N2 influenza A virus. Adult female mice were treated with placebo, P4, or LNG and inoculated intranasally with a low dose of ma2009 H1N1 virus. Six weeks later, the mice were challenged intranasally with a high dose of HK68 H3N2 virus. Lung single-cell suspensions were harvested for flow cytometry analysis at 42 days after primary infection (i.e., prior to challenge; labeled day 0 postchallenge) and at days 2 and 6 days postchallenge and stimulated ex vivo with an ma2009-specific antigen in the presence of brefeldin A. The total numbers of live CD8+ T cells expressing IFN-γ (A), TNF-α (B), and both IFN-γ and TNF-α (C) and the total numbers of live tetramer-specific CD8+ T cells (D) were quantified. Data represent the means ± SEMs from two independent replications (n = 8 mice per treatment per day). Significant differences between P4-treated and placebo-treated female mice are represented by asterisks, and significant differences between LNG-treated and placebo-treated female mice are represented by pound signs.
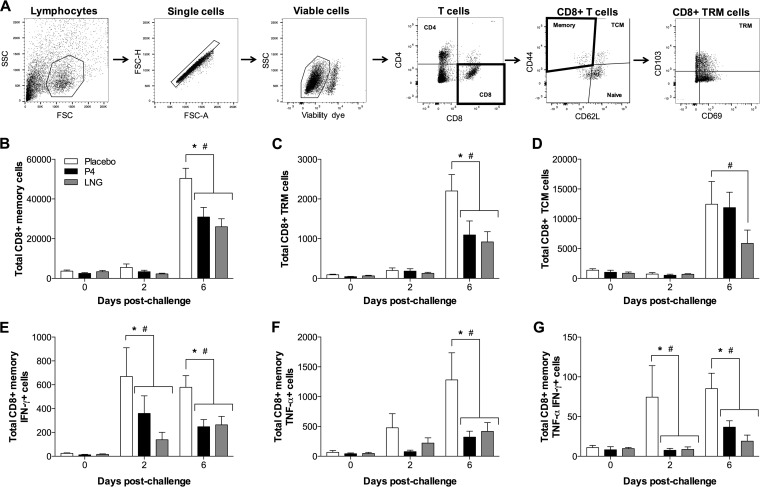
The total numbers of CD8+ memory (CD44+) T cells following ex vivo stimulation with a peptide from ma2009 were also decreased at 6 days following heterosubtypic IAV challenge in the female mice treated with either P4 or LNG compared to those in mice treated with the placebo (Fig. 6A and B). This included both CD8+ tissue-resident memory (TRM; CD8+ CD44+ CD69+ CD103+) and CD8+ T central memory (TCM; CD8+ CD44+ CD62L+) cells (Fig. 6C and D) (P < 0.05). The total numbers of virus-specific memory CD8+ T cells producing IFN-γ with or without TNF-α production at days 2 and 6 postchallenge and memory CD8+ T cells producing TNF-α at day 6 postchallenge were also significantly decreased in progestin-treated female mice compared to female mice treated with placebo (Fig. 6E to G) (P < 0.05). Similarly, when memory CD8+ T cells were stimulated ex vivo with an HK68 peptide, the numbers of TNF-α-producing (TNF-α+) CD8+ T cells, IFN-γ-producing (IFN-γ+) and TNF-α-producing (TNF-α+) CD8+ T cells, memory CD8+ T cells, CD8+ TRM cells, and CD8+ TCM cells were lower in the lungs of P4- and LNG-treated female mice than the lungs of placebo-treated female mice at day 2, day 6, or both days postchallenge (Table 2) (P < 0.05). Taken together, these data illustrate that IAV-specific memory CD8+ T cell numbers and activity but not total CD8+ or CD4+ T cell numbers were significantly reduced by progestin treatment during the heterosubtypic challenge.
FIG 6.
Treatment with P4 or LGN reduced the virus-specific memory CD8+ T cell responses in the lungs of female mice challenged with a heterosubtypic H3N2 influenza A virus. Adult female mice were treated with placebo, P4, or LNG and inoculated intranasally with a low dose of ma2009 H1N1 virus. Six weeks later, the mice were challenged intranasally with a high dose of HK68 H3N2 virus. Lung single-cell suspensions were harvested for flow cytometry analysis at 42 days after primary infection (i.e., prior to challenge; labeled day 0 postchallenge) and at 2 and 6 days postchallenge and stimulated ex vivo with an ma2009-specific NP antigen in the presence of brefeldin A. (A) Cells were gated on lymphocytes (side scatter [SSC] versus forward scatter [FSC]) and doublets (FSC-H versus FSC-A), and dead cells (viability dye positive) were excluded. CD8+ or CD4+ T cells were determined from the live cell gate. Memory T cells (CD44+ CD62L−), T central memory (TCM) cells (CD44+ CD62+), and naive CD8+ T cells (CD44− CD62L+) were gated on the CD8+ T cell subset. Tissue-resident memory (TRM) cells were gated on the basis of their expression of CD103 and CD69 from the CD44+ CD62L− memory T cell gate. (B to G) The total numbers of live memory CD8+ T cells (B), TRM CD8+ T cells (C), TCM cells (D), and memory CD8+ T cells expressing IFN-γ (E), TNF-α (F), or both IFN-γ and TNF-α (G) were quantified. Data represent the means ± SEMs from two independent replications (n = 8 mice per treatment per day). Significant differences between P4-treated and placebo-treated female mice are represented by asterisks, and significant differences between the LNG-treated and placebo-treated female mice are represented by pound signs.
TABLE 2.
Total numbers of virus-specific CD8+ T cells recognizing HK68 in the lungs of female mice following challengea
| Total T cells | Treatment | No. of virus-specific CD8+ T cells on the following days postchallenge: |
||
|---|---|---|---|---|
| 0 | 2 | 6 | ||
| IFN-γ+ CD8+ T cells | Placebo | 73.3 ± 17.3 | 246.7 ± 92.7 | 12,183 ± 4,127 |
| P4 | 52.5 ± 11.6 | 352.9 ± 124.9 | 6,993 ± 31.22 | |
| LNG | 65.3 ± 13.1 | 235.7 ± 85.6 | 7,204 ± 125.6 | |
| TNF-α+ CD8+ T cells | Placebo | 209.4 ± 78.6 | 1,125 ± 582 | 26,323 ± 8,405 |
| P4 | 171.0 ± 42.0 | 207.1 ± 25.74 | 10,609 ± 5,160* | |
| LNG | 120.2 ± 39.6 | 1,296 ± 586.7 | 12,849 ± 5,691# | |
| IFN-γ+ TNF-α+ CD8+ T cells | Placebo | 37.5 ± 8.8 | 74.7 ± 39.3 | 262.8 ± 41.9 |
| P4 | 28.9 ± 4.6 | 25.39 ± 4.5 | 107.0 ± 23.6* | |
| LNG | 21.7 ± 3.8 | 53.56 ± 43.4 | 66.09 ± 12.2# | |
| Memory CD8+ T cells | Placebo | 3,462 ± 566.2 | 5,395 ± 1,793 | 47,865 ± 5,875 |
| P4 | 2,523 ± 627.0 | 3,439 ± 765.4 | 26,862 ± 5,207* | |
| LNG | 3,384 ± 556.5 | 4,548 ± 1,152 | 23,661 ± 4,660# | |
| TRM CD8+ T cells | Placebo | 82.0 ± 14.6 | 167.6 ± 66.8 | 1,843 ± 449.3 |
| P4 | 51.7 ± 18.2 | 176.0 ± 53.6 | 826.6 ± 318.6* | |
| LNG | 64.3 ± 9.8 | 152.5 ± 46.0 | 710.8 ± 258.4# | |
| TCM CD8+ T cells | Placebo | 1,765 ± 401.3 | 842.0 ± 297.0 | 12,804 ± 2,494 |
| P4 | 1,261 ± 277.9 | 617.0 ± 206.1 | 8,660 ± 2,075* | |
| LNG | 1,404 ± 259.6 | 1,174 ± 121.8 | 5,641 ± 710.0# | |
| IFN-γ+ CD8+ memory T cells | Placebo | 21.0 ± 7.1 | 365.1 ± 164.7 | 444.3 ± 96.3 |
| P4 | 11.9 ± 4.6 | 342.5 ± 120.3 | 258.8 ± 98.8 | |
| LNG | 17.0 ± 3.9 | 232.9 ± 119.3 | 242.0 ± 52.0 | |
| TNF-α+ CD8 memory T cells | Placebo | 106.5 ± 48.8 | 706.9 ± 376.2 | 1,071 ± 209.1 |
| P4 | 60.1 ± 14.7 | 81.3 ± 26.8* | 373.8 ± 94.1* | |
| LNG | 45.2 ± 16.5 | 398.7 ± 259.8 | 320.0 ± 84.9# | |
| IFN-γ+ TNF-α+ CD8 memory T cells | Placebo | 12.1 ± 2.1 | 79.2 ± 50.3 | 45.9 ± 10.4 |
| P4 | 7.7 ± 1.1 | 11.15 ± 3.8* | 20.96 ± 8.2 | |
| LNG | 7.7 ± 2.9 | 28.2 ± 16.8# | 16.5 ± 5.3 | |
Adult female mice were treated with placebo, progesterone (P4), or levonorgestrel (LNG) and inoculated intranasally with a low dose of ma2009 H1N1 virus. Six weeks later, the mice were challenged intranasally with a high dose of HK68 H3N2 virus. Lung single-cell suspensions were harvested for flow cytometry analysis at 42 days after primary infection (i.e., prior to challenge; labeled as day 0 postchallenge) and at days 2 and 6 days postchallenge and stimulated ex vivo with HK68-specific antigen in the presence of brefeldin A. The total numbers of live T cells were analyzed. Data were analyzed by a two-way ANOVA followed by Tukey tests and represent the means ± standard errors of the means from two independent replications (n = 8 mice per treatment per day). Significant differences between P4-treated and placebo-treated female mice are represented by asterisks and boldface, and significant differences between LNG-treated and placebo-treated female mice are represented by pound signs and boldface.
DISCUSSION
A significant majority of women in the United States are exposed to some form of progestin in either hormone contraceptives or hormone replacement therapy (22). Concurrently, women (and men) are exposed to novel strains of IAVs approximately every 5 to 10 years over the life course (3). The impacts of progestins on the outcome and responses to infectious diseases at mucosal sites outside the reproductive tract are rarely considered. In the present study, we show that while both P4 and LNG improved the outcome of IAV infection in immunologically naive female mice, these hormones significantly reduced the memory CD8+ T cell responses and thereby increased the susceptibility of the mice to heterosubtypic challenge with novel strains of IAV. Surprisingly and possibly beneficially for vaccine responses, both P4 and LNG improved the antibody responses to subsequent IAV challenges.
Following H1N1 infection and subsequent challenge with an H1N1 drift variant, placebo-, P4-, and LNG-treated female mice were equally protected, despite the low preexisting antibody responses to ma2009dv and the reduced antibody responses to the primary H1N1 infection in progestin-treated female mice. The K166Q mutation is located in the globular head region of the HA, with the stalk regions of both the ma2009 H1N1 and the ma2009dv H1N1 viruses being conserved (26). To address whether the protection that was found following a drift variant virus challenge was due to antibody responses to the stalk region of the HA, we measured broadly neutralizing antibody responses to the conserved stalk region of the H1 HA using a chimeric HA containing an exotic H6 head and the stalk from the ma2009 H1N1 HA. Broadly neutralizing antibodies target epitopes within the HA stem region that are conserved within each phylogenetic HA group. In our study, broadly neutralizing stalk antibody responses were not affected by hormone treatment, which may explain why there was equal protection against another group 1 virus and complete clearance of the virus by 2 days postchallenge (28, 31). Additionally, the neutralizing antibody response to the drift variant was boosted as early as 2 days postchallenge with the drift variant virus and may explain why these female mice were fully protected.
Following H1N1 infection and subsequent challenge with a heterologous H3N2 strain of IAV, female mice treated with either P4 or LNG suffered greater mortality between 6 and 8 days postchallenge. The increased susceptibility to the secondary heterologous IAV challenge was not caused by the effects of either P4 or LNG on virus replication or clearance but was associated with increased pulmonary immunopathology, including edema, alveolitis, and necrosis, and impaired memory CD8+ T cell responses. Memory CD8 T cells are indispensable in the control of heterosubtypic IAV infection, and TRM cells, in particular, are the primary mediator of protection against secondary IAV challenge. Studies in mice show that if the entry of any nonresident, circulating, memory T cells to the lungs is blocked, then the presence of tissue-embedded TRM cells alone is sufficient to induce heterosubtypic immunity (9). In the current study, reduced virus-specific memory CD8+ T cell and CD8+ TRM cell activity was associated with an inability to survive heterosubtypic IAV challenge in P4- or LNG-treated mice. Although the total numbers of pulmonary CD4+ or CD8+ T cells following H3N2 virus challenge in female mice were not affected by progestins, the expansion of virus-specific CD8+ T cells that recognize both H1N1 and H3N2 IAVs was restricted in progestin-treated female mice compared to placebo-treated female mice. Furthermore, the expansion of memory CD8+ T cell subsets occurred following stimulation ex vivo with either the H1N1 or H3N2 NP immunodominant peptide, which, despite a 3-amino-acid difference, is conserved across group 1 and group 2 viruses and elicits similar memory CD8+ T cell responses.
The ability of CD8+ T cells to rapidly expand and produce antiviral cytokines, including TNF-α and IFN-γ, which can have synergistic effects, is crucial for both the clearance of the virus and the resolution of inflammation (32, 33). Mice with IFN-γ-deficient CD8+ T cells or complete TNF-α knockout mice have extensive inflammation and lung pathology with impairment of lung function following IAV infection, demonstrating the importance of these cytokines not only in the clearance of the virus but also in the resolution of the infection-induced lung pathology (34, 35). The production of TNF-α and IFN-γ by effector and memory CD8+ T cells in response to heterosubtypic IAV challenge was significantly impaired in female mice treated with either P4 or LNG. Consequently, both P4- and LNG-treated female mice had increased pulmonary inflammation and lung pathology immediately following heterosubtypic IAV challenge. Progestins may prevent the production of IFN-γ and TNF-α by IAV-specific CD8+ T cells by inhibiting signaling pathways in these cells. When bound to the progesterone receptor, progestins can directly interfere with the NF-κB pathway and the mitogen-activated protein kinase pathway, respectively, which control the transcription of TNF-α and IFN-γ (36–39), suggesting a potential mechanism for the progestin-mediated reduction of IFN-γ and TNF-α within IAV-specific CD8+ T cells.
The impacts of progestins on sequential exposure and memory responses to viral infection have been evaluated in only a few vaccine studies, mostly in the context of herpes simplex virus (HSV) infection. Following HSV-2 vaccination, progestin treatment decreases protection against a challenge, reduces virus-specific IgG and IgA antibody levels, and increases virus shedding (40, 41). Several studies have illustrated that progestins inhibit antibody production (17, 42), but most studies considered this effect only after an exposure to antigen in immunologically naive animals. Consistent with these findings, in the current study, progestins significantly reduced the titers of neutralizing and virus-specific IgG and IgA in both the serum and the BAL fluid of naive female mice infected with IAV. A secondary virus infection with a drift variant virus resulted in a boost in the titers of antibodies against the primary virus in all treatment groups. However, the titers of both neutralizing and total IgG antibodies against the secondary challenge virus were significantly higher in P4- and LNG-treated female mice than placebo-treated female mice. Whether progestins, binding to either the progesterone receptor or the glucocorticoid receptor in B cells, have differential effects on the transcriptional activity of naive versus memory B cells to directly alter antibody production (i.e., reduce the levels of antibody production in naive B cells and increase the levels of antibody production in memory B cells) must be considered in future studies. Following secondary challenge with a drift variant of H1N1, progestin treatment reduced the antibody responses to a primary IAV infection and increased the antibody responses to a secondary IAV challenge compared with placebo treatment, which may be beneficial in the context of vaccination.
The current usage of hormonal contraceptives is on the rise, with over 100 million women worldwide taking some form of progestin-based hormonal contraceptives. These hormone-based contraceptives are listed by the World Health Organization (WHO) as an essential medication because of their role in preventing excessive pregnancies and maternal morbidity. Additionally, in light of concerns about infectious diseases that can be transmitted from mother to fetus (e.g., Zika virus), the WHO, the Centers for Disease Control and Prevention in the United States, and other national health agencies recommend the use of hormonal contraceptives to prevent pregnancies and the transmission of infections to the fetus. In this study, we demonstrate that exposure to progestins in ovary-intact female mice has beneficial effects on primary infection with IAV but detrimental effects on the generation and activation of memory CD8 T cell responses and the outcome of secondary infection. The impact of these responses in humans should be considered in human surveillance studies as well as vaccine trials to determine if contraceptives alter the outcome of IAV infection and responses to vaccination in women.
MATERIALS AND METHODS
Animals.
Adult (age, 7 to 9 weeks) female C57BL/6 mice were purchased from Charles River Laboratories (Frederick, MD) and housed at up to 5 mice per microisolator cage under standard biosafety level 2 housing conditions, with food and water being provided ad libitum. All animal procedures were approved by the Johns Hopkins University Animal Care and Use Committee under animal protocol M015H236. At 8 to 12 weeks of age, the mice were anesthetized with an intramuscular injection of a ketamine (80 mg/kg of body weight) and xylazine (8 mg/kg) cocktail, and hormones were administered in the form of a subcutaneous dorsal implant: (i) 15 mg progesterone (P4) 60-day-release pellets (Innovative Research of America), (ii) 5 mm levonorgestrel (LNG; Sigma) in a silastic capsule (inside diameter, 0.040 in; outside diameter, 0.085 in), or (iii) a placebo as an empty silastic capsule. The capsules were equilibrated in sterile physiological saline at 37°C overnight prior to implantation. The doses of P4 and LNG increase the P4 concentrations, uterine horn mass, or both to within the physiological range for young nonpregnant female mice (13, 43, 44).
Virus infection, quantification, and purification.
Mouse-adapted influenza A virus A/California/04/09 (ma2009; H1N1; generated by Andrew Pekosz from a published sequence [45]) and a mouse-adapted A/California/04/09 drift variant (ma2009dv; H1N1) containing the K166Q mutation of the HA sequence (generated by reverse genetics) were used. Viral RNA was extracted (QIAamp viral RNA kit; Qiagen) from existing ma2009 IAV and transcribed into cDNA (with SuperScript III reverse transcriptase; Invitrogen). HA-specific cDNA was PCR amplified and purified on a 1% agarose gel. Purified HA cDNA was digested and cloned into the pHH21 plasmid vector (46). The K166Q HA mutation (26) was introduced by site-directed mutagenesis (QuikChange Lightning site-directed mutagenesis kit; Agilent Technologies). Viruses whose genomes encoded the K166Q HA mutation were generated entirely from cDNA using a 12-plasmid rescue system (46, 47). The HA sequence of the rescued virus was confirmed by sequencing the coding region of the HA gene. Viral stocks were generated by infecting Madin-Darby canine kidney (MDCK) cells at a multiplicity of infection of 0.01, and the infected cell supernatant was collected at 72 h postinfection. A/Hong Kong/2/68 (HK68; H3N2) was given to us courtesy of Innocent N. Mbawuike. All three viruses were used in these studies, and infectious virus titers were determined using the 50% tissue culture infective dose (TCID50) assay. For infections, mice were anesthetized with a ketamine (80 mg/kg) and xylazine (8 mg/kg) cocktail and inoculated intranasally with 30 μl of a low dose of the ma2009 virus (0.04 50% mouse lethal dose [MLD50]) or mock infected with Dulbecco modified Eagle medium (DMEM) alone. For challenge experiments, animals were inoculated with 30 μl of a lethal dose of HK68 (162 MLD50s in 30 μl of DMEM) or ma2009dv (32 MLD50s) at 6 weeks following the initial infection.
For virus quantification, a TCID50 assay was used. In that assay, log10 dilutions of lung homogenates were plated onto a monolayer of MDCK cells in replicates of 6 for 6 days at 32°C. Cells were stained with naphthol blue black (Sigma-Aldrich) and scored for cytopathic effects. The titer (the TCID50) was calculated according to the Reed-Muench method. For virus purification, viruses were grown in MDCK cells at 37°C for 4 days and pelleted by centrifugation on a 20% sucrose gradient in a Beckman SW28TI rotor at 26,000 rpm for 1 h at 4°C. The virus pellet was resuspended in 1× phosphate-buffered saline (PBS), protein was quantified by a bicinchoninic acid assay (Pierce), and aliquots were stored at −80°C.
Morbidity and mortality studies.
Clinical scores as well as body mass, rectal temperature, and survival were recorded daily over the course of the study. Clinical disease scores for IAV-infected mice were based on four parameters, with one point being given for each of the following: dyspnea, piloerection, hunched posture, and the absence of an escape response (13).
Serum and BAL fluid sample collection.
Serum and bronchoalveolar lavage (BAL) fluid samples were collected at relevant time points to measure antibody and neutralizing antibody titers. Mice were bled from the cheek for the survival experiments or from the retro-orbital sinus for terminal procedures. Serum was heat inactivated at 56°C for 30 min and stored at −80°C. For BAL fluid collection, the mice were euthanized by cervical dislocation and the lungs were lavaged twice with 0.5 ml of a 0.9% saline solution. The BAL fluid was centrifuged at 500 × g for 10 min to remove cells and debris, and the supernatant was collected, heat inactivated at 56°C for 30 min, and stored at −80°C.
Histopathology and immunohistochemistry.
Lungs were inflated at constant pressure, fixed in Z-fix fixative (Anatech) for at least 48 h, embedded in paraffin, cut into 5-μm sections, and mounted on glass slides. Tissue sections were stained with hematoxylin and eosin (H&E) and used to evaluate lung inflammation. Histopathological scoring was performed by a single veterinary pathologist blind to the treatment, and scores given on a scale ranging from 0 to 3 (0, no inflammation; 1, mild inflammation; 2, moderate inflammation; 3, severe inflammation) were used for the following parameters: bronchiolitis, alveolitis, vasculitis, perivasculitis, necrosis, consolidation, and edema (13). The percentage of lesioned areas within each tissue section was also evaluated. Images were taken using a Nikon Eclipse E800 microscope.
Antibody neutralization.
Serially diluted serum was mixed with 100 TCID50s of virus (ma2009 or HK68) for 1 h at room temperature and used to infect quadruplicate wells of confluent MDCK cells for 24 h at 37°C. After 16 to 18 h of incubation, the inoculum was removed, the cells were washed with 1× PBS (with calcium and magnesium), and fresh medium was added. The cells were incubated for 6 days at 32°C and then fixed with 4% formaldehyde and stained with naphthol blue black for 6 h. The titer was calculated as the highest serum dilution that eliminated virus cytopathic effects in 2 out of 4 wells per dilution.
Anti-influenza ELISA.
Enzyme-linked immunosorbent assay (ELISA) plates (Microlon 96-well high-binding plates; Greiner Bio-One) were coated with 100 ng of purified virus overnight at 4°C in carbonate buffer (pH 9.6). For IgG ELISAs, the plates were washed 3 times with PBST (1× PBS plus 0.1% Tween 20 [Sigma]) and blocked for at least 1 h at 37°C with 10% dry milk powder in 1× PBS. The plates were washed 3 times prior to the addition of serially diluted serum to the plates for 1 h at 37°C at a starting dilution of 1:1,000. Anti-mouse horseradish peroxidase (HRP)-conjugated secondary IgG (1:5,000; Thermo) was added, and the plates were incubated for 1 h at 37°C. The plates were washed 3 times with PBST, and the reactions were developed with 3,3′,5,5′-tetramethylbenzidine (TMB; BD Biosciences) and stopped using 1 N HCl. The absorbance at 450 nm of the plates was read on a plate reader. For IgA ELISAs, the plates were washed 3 times with 1× Tris-buffered saline (TBS) plus 0.1% Tween (TBST) and blocked for at least 1 h at 37°C with 10% dry milk powder in 1× TBS. The plates were washed 3 times prior to the addition of serially diluted BAL fluid to the plates for 1 h at 37°C at a starting dilution of 1:1. Anti-mouse alkaline phosphatase (AP) secondary IgA (1:2,000; Southern Biotech) was added, and the plates were incubated for 1 h at 37°C. The plates were washed 3 times with TBST, and the reactions were developed with p-nitrophenyl phosphate (PNPP) substrate (Thermo) and stopped using 2 N NaOH. The absorbance at 405 nm of the plates was read on a plate reader. To determine the antibody titer, a cutoff value was determined by multiplying the average optical density (OD) values for the negative controls at each dilution by 3. The titer for the sample was calculated as the highest serum dilution with an OD value above the cutoff.
Stalk antibody ELISA.
Flat-bottom Immuno 4HBX 96-well plates (Thermo) were coated overnight at 4°C with 100 ng of recombinant HA using the chimeric cH6/1 protein (with the A/mallard/Sweden/81/02 H6 head and a A/California/04/09 stalk domain, generated as described previously [48]) in carbonate buffer (pH 9.4). The plates were washed 3 times with PBST and blocked for at least 1 h at room temperature with 3% goat serum (Gibco) and 0.5% milk powder in PBST. The plates were washed 3 times prior to the addition of serially diluted (2-fold dilution) samples, which were added at a starting dilution of 1:100. Samples were incubated for 2 h at room temperature and washed, anti-mouse IgG secondary antibody conjugated to peroxidase (HRP; 1:3,000; catalog number A9044; Sigma) was added, and the plates were incubated for 1 h at room temperature. The plates were washed 3 times and developed with o-phenylenediamine dihydrochloride (OPD; Sigma), and the reactions were stopped after 10 min using 3 M HCl. The absorbance at 490 nm of the plates was read on a plate reader. To determine the antibody titer, a cutoff value was determined by calculating the average OD values for the negative controls for each plate and adding the standard deviation. The titer for the sample was calculated as the highest serum dilution with an OD value above the cutoff.
Flow cytometry analysis of T cells.
Lungs were excised, and single-cell suspensions were generated following red blood cell lysis. The total numbers of viable cells were determined using a hemocytometer and trypan blue (Invitrogen) exclusion, and the cells were resuspended at 1 × 106 cells/ml in RPMI 1640 (Cellgro) supplemented with 10% fetal bovine serum (Fisher Scientific) and 1% penicillin-streptomycin. For IAV-specific T cell enumeration, cells were cultured for 5 h with IAV peptide antigen (for CD8, NP from residues 366 to 374 [NP366–374]; for CD4, HA from residues 211 to 255 and NP from residues 311 to 325; ProImmune), phorbol myristate acetate (Sigma), and ionomycin (Sigma) or medium alone (unstimulated) in medium containing brefeldin A (GolgiPlug; BD Biosciences). The viability of cells was determined by use of a fixable Live/Dead aqua viability dye (Invitrogen), and Fc receptors were blocked using anti-CD16/32 (BD Biosciences). The T cell populations were stained with the following antibodies: fluorescein isothiocyanate (FITC)-conjugated anti-CD3 (antibody 17A2; BD Biosciences), Alexa Fluor 700-conjugated anti-CD4 (antibody RM4-5; BD Biosciences), peridinin chlorophyll protein-Cy5.5-conjugated anti-CD8 (antibody 53-6.7; eBioscience), allophycocyanin (APC)-conjugated anti-CD44 (antibody IM7; BD Biosciences), eVolve 605-conjugated anti-CD62L (antibody MEL-14; eBioscience), eFluor 450-conjugated anti-CD69 (antibody H1.2F3; eBioscience), phycoerythrin (PE)-conjugated anti-CD103 (antibody M290; BD Biosciences), PE-conjugated DbNP366–374 tetramer for ma2009 (ASNENVETM; NIH Tetramer Core Facility), and BV421-conjugated DbNP366–374 tetramer for HK68 (ASNENMDAM; NIH Tetramer Core Facility). Intracellular staining with PeCy7-conjugated anti-IFN-γ (antibody XMG1.2; BD Biosciences), FITC-conjugated anti-TNF-α (antibody MP6-XT22; BD Biosciences), BV412-conjugated anti-interleukin-4 (anti-IL-4; antibody 11B11; BD Biosciences), or APC-conjugated IL-17 (eBio17B7; eBioscience) was performed following permeabilization and fixation with Cytofix/Cytoperm and Perm/Wash buffer (BD Biosciences). Data were acquired using a Fortessa fluorescence-activated cell sorter (with FACSDiva software) and analyzed using FlowJo (v.10) software (Tree Star, Inc.). Total cell counts were determined based on the percentages of live cells in the live cell gate multiplied by the total live cell counts acquired prior to staining by the trypan blue exclusion counts obtained on a hemocytometer.
Statistical analyses.
Morbidity and clinical data were analyzed with a multivariate analysis of variance followed by planned comparisons. Antibody titers, virus titers, and histopathological data were analyzed using two-way analyses of variance (ANOVAs) or t tests, and significant interactions were further analyzed using the Tukey method for pairwise multiple comparisons. Survival was analyzed using a Kaplan-Meier survival curve, followed by a log-rank test. Mean differences were considered statistically significant if P was <0.05.
ACKNOWLEDGMENTS
We thank the members of the Sabra Klein and Andrew Pekosz laboratories for ongoing discussions about these data, Andrew Pekosz for feedback on an earlier draft, and two anonymous reviewers for encouraging feedback. We also thank Ariana Hirsh, Katherine Fenstermacher, and Hsuan Liu for technical assistance with antigen production and development of the H1N1 drift variant.
REFERENCES
- 1.Grohskopf LA, Sokolow LZ, Olsen SJ, Bresee JS, Broder KR, Karron RA. 2015. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices, United States, 2015-16 influenza season. Am J Transplant 15:2767–2775. doi: 10.1111/ajt.13505. [DOI] [PubMed] [Google Scholar]
- 2.Bodewes R, de Mutsert G, van der Klis FR, Ventresca M, Wilks S, Smith DJ, Koopmans M, Fouchier RA, Osterhaus AD, Rimmelzwaan GF. 2011. Prevalence of antibodies against seasonal influenza A and B viruses in children in Netherlands. Clin Vaccine Immunol 18:469–476. doi: 10.1128/CVI.00396-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kucharski AJ, Lessler J, Read JM, Zhu H, Jiang CQ, Guan Y, Cummings DA, Riley S. 2015. Estimating the life course of influenza A(H3N2) antibody responses from cross-sectional data. PLoS Biol 13:e1002082. doi: 10.1371/journal.pbio.1002082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yewdell JW, Bennink JR, Smith GL, Moss B. 1985. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc Natl Acad Sci U S A 82:1785–1789. doi: 10.1073/pnas.82.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ulmer JB, Fu TM, Deck RR, Friedman A, Guan L, DeWitt C, Liu X, Wang S, Liu MA, Donnelly JJ, Caulfield MJ. 1998. Protective CD4+ and CD8+ T cells against influenza virus induced by vaccination with nucleoprotein DNA. J Virol 72:5648–5653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wrammert J, Koutsonanos D, Li GM, Edupuganti S, Sui J, Morrissey M, McCausland M, Skountzou I, Hornig M, Lipkin WI, Mehta A, Razavi B, Del Rio C, Zheng NY, Lee JH, Huang M, Ali Z, Kaur K, Andrews S, Amara RR, Wang Y, Das SR, O'Donnell CD, Yewdell JW, Subbarao K, Marasco WA, Mulligan MJ, Compans R, Ahmed R, Wilson PC. 2011. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J Exp Med 208:181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wakim LM, Gupta N, Mintern JD, Villadangos JA. 2013. Enhanced survival of lung tissue-resident memory CD8(+) T cells during infection with influenza virus due to selective expression of IFITM3. Nat Immunol 14:238–245. doi: 10.1038/ni.2525. [DOI] [PubMed] [Google Scholar]
- 8.Laidlaw BJ, Zhang N, Marshall HD, Staron MM, Guan T, Hu Y, Cauley LS, Craft J, Kaech SM. 2014. CD4+ T cell help guides formation of CD103+ lung-resident memory CD8+ T cells during influenza viral infection. Immunity 41:633–645. doi: 10.1016/j.immuni.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu T, Hu Y, Lee YT, Bouchard KR, Benechet A, Khanna K, Cauley LS. 2014. Lung-resident memory CD8 T cells (TRM) are indispensable for optimal cross-protection against pulmonary virus infection. J Leukoc Biol 95:215–224. doi: 10.1189/jlb.0313180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.vom Steeg LG, Klein SL. 2016. SeXX matters in infectious disease pathogenesis. PLoS Pathog 12:e1005374. doi: 10.1371/journal.ppat.1005374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson DP, Huber SA, Moussawi M, Roberts B, Teuscher C, Watkins R, Arnold AP, Klein SL. 2011. Sex chromosome complement contributes to sex differences in coxsackievirus B3 but not influenza A virus pathogenesis. Biol Sex Differ 2:8. doi: 10.1186/2042-6410-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein SL, Flanagan KL. 2016. Sex differences in immune responses. Nat Rev Immunol 16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 13.Hall OJ, Limjunyawong N, Vermillion MS, Robinson DP, Wohlgemuth N, Pekosz A, Mitzner W, Klein SL. 2016. Progesterone-based therapy protects against influenza by promoting lung repair and recovery in females. PLoS Pathog 12:e1005840. doi: 10.1371/journal.ppat.1005840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.vom Steeg LG, Vermillion MS, Hall OJ, Alam O, McFarland R, Chen H, Zirkin BR, Klein SL. 2016. Age and testosterone mediate influenza pathogenesis in male mice. Am J Physiol Lung Cell Mol Physiol 311:L1234–L1244. doi: 10.1152/ajplung.00352.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robinson DP, Lorenzo ME, Jian W, Klein SL. 2011. Elevated 17beta-estradiol protects females from influenza A virus pathogenesis by suppressing inflammatory responses. PLoS Pathog 7:e1002149. doi: 10.1371/journal.ppat.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piccinni MP, Giudizi MG, Biagiotti R, Beloni L, Giannarini L, Sampognaro S, Parronchi P, Manetti R, Annunziato F, Livi C. 1995. Progesterone favors the development of human T helper cells producing Th2-type cytokines and promotes both IL-4 production and membrane CD30 expression in established Th1 cell clones. J Immunol 155:128–133. [PubMed] [Google Scholar]
- 17.Canellada A, Blois S, Gentile T, Margni Idehu RA. 2002. In vitro modulation of protective antibody responses by estrogen, progesterone and interleukin-6. Am J Reprod Immunol 48:334–343. doi: 10.1034/j.1600-0897.2002.01141.x. [DOI] [PubMed] [Google Scholar]
- 18.Butts CL, Shukair SA, Duncan KM, Bowers E, Horn C, Belyavskaya E, Tonelli L, Sternberg EM. 2007. Progesterone inhibits mature rat dendritic cells in a receptor-mediated fashion. Int Immunol 19:287–296. doi: 10.1093/intimm/dxl145. [DOI] [PubMed] [Google Scholar]
- 19.Arruvito L, Giulianelli S, Flores AC, Paladino N, Barboza M, Lanari C, Fainboim L. 2008. NK cells expressing a progesterone receptor are susceptible to progesterone-induced apoptosis. J Immunol 180:5746–5753. doi: 10.4049/jimmunol.180.8.5746. [DOI] [PubMed] [Google Scholar]
- 20.Jones LA, Kreem S, Shweash M, Paul A, Alexander J, Roberts CW. 2010. Differential modulation of TLR3- and TLR4-mediated dendritic cell maturation and function by progesterone. J Immunol 185:4525–4534. doi: 10.4049/jimmunol.0901155. [DOI] [PubMed] [Google Scholar]
- 21.Petitti DB. 2003. Clinical practice. Combination estrogen-progestin oral contraceptives. N Engl J Med 349:1443–1450. [DOI] [PubMed] [Google Scholar]
- 22.Daniels K, Daugherty J, Jones J. 2014. Current contraceptive status among women aged 15-44: United States, 2011-2013. NCHS Data Brief 2014:1–8. [PubMed] [Google Scholar]
- 23.Evans G, Sutton EL. 2015. Oral contraception. Med Clin North Am 99:479–503. doi: 10.1016/j.mcna.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Sitruk-Ware R. 2006. New progestagens for contraceptive use. Hum Reprod Update 12:169–178. [DOI] [PubMed] [Google Scholar]
- 25.Lorenzo ME, Hodgson A, Robinson DP, Kaplan JB, Pekosz A, Klein SL. 2011. Antibody responses and cross protection against lethal influenza A viruses differ between the sexes in C57BL/6 mice. Vaccine 29:9246–9255. doi: 10.1016/j.vaccine.2011.09.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Linderman SL, Chambers BS, Zost SJ, Parkhouse K, Li Y, Herrmann C, Ellebedy AH, Carter DM, Andrews SF, Zheng NY, Huang M, Huang Y, Strauss D, Shaz BH, Hodinka RL, Reyes-Teran G, Ross TM, Wilson PC, Ahmed R, Bloom JD, Hensley SE. 2014. Potential antigenic explanation for atypical H1N1 infections among middle-aged adults during the 2013-2014 influenza season. Proc Natl Acad Sci U S A 111:15798–15803. doi: 10.1073/pnas.1409171111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, Goudsmit J, Wilson IA. 2009. Antibody recognition of a highly conserved influenza virus epitope. Science 324:246–251. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krammer F, Palese P. 2013. Influenza virus hemagglutinin stalk-based antibodies and vaccines. Curr Opin Virol 3:521–530. doi: 10.1016/j.coviro.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Air GM. 1981. Sequence relationships among the hemagglutinin genes of 12 subtypes of influenza A virus. Proc Natl Acad Sci U S A 78:7639–7643. doi: 10.1073/pnas.78.12.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kreijtz JH, Bodewes R, van Amerongen G, Kuiken T, Fouchier RA, Osterhaus AD, Rimmelzwaan GF. 2007. Primary influenza A virus infection induces cross-protective immunity against a lethal infection with a heterosubtypic virus strain in mice. Vaccine 25:612–620. doi: 10.1016/j.vaccine.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 31.Ellebedy AH, Ahmed R. 2012. Re-engaging cross-reactive memory B cells: the influenza puzzle. Front Immunol 3:53. doi: 10.3389/fimmu.2012.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuwano K, Kawashima T, Arai S. 1993. Antiviral effect of TNF-alpha and IFN-gamma secreted from a CD8+ influenza virus-specific CTL clone. Viral Immunol 6:1–11. doi: 10.1089/vim.1993.6.1. [DOI] [PubMed] [Google Scholar]
- 33.Thomas PG, Keating R, Hulse-Post DJ, Doherty PC. 2006. Cell-mediated protection in influenza infection. Emerg Infect Dis 12:48–54. doi: 10.3201/eid1201.051237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiley JA, Cerwenka A, Harkema JR, Dutton RW, Harmsen AG. 2001. Production of interferon-gamma by influenza hemagglutinin-specific CD8 effector T cells influences the development of pulmonary immunopathology. Am J Pathol 158:119–130. doi: 10.1016/S0002-9440(10)63950-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Damjanovic D, Divangahi M, Kugathasan K, Small CL, Zganiacz A, Brown EG, Hogaboam CM, Gauldie J, Xing Z. 2011. Negative regulation of lung inflammation and immunopathology by TNF-alpha during acute influenza infection. Am J Pathol 179:2963–2976. doi: 10.1016/j.ajpath.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rincon M, Flavell RA, Davis RJ. 2001. Signal transduction by MAP kinases in T lymphocytes. Oncogene 20:2490–2497. doi: 10.1038/sj.onc.1204382. [DOI] [PubMed] [Google Scholar]
- 37.Hardy DB, Janowski BA, Corey DR, Mendelson CR. 2006. Progesterone receptor plays a major antiinflammatory role in human myometrial cells by antagonism of nuclear factor-kappaB activation of cyclooxygenase 2 expression. Mol Endocrinol 20:2724–2733. doi: 10.1210/me.2006-0112. [DOI] [PubMed] [Google Scholar]
- 38.Gerondakis S, Fulford TS, Messina NL, Grumont RJ. 2014. NF-kappaB control of T cell development. Nat Immunol 15:15–25. doi: 10.1038/ni.2785. [DOI] [PubMed] [Google Scholar]
- 39.Lei K, Georgiou EX, Chen L, Yulia A, Sooranna SR, Brosens JJ, Bennett PR, Johnson MR. 2015. Progesterone and the repression of myometrial inflammation: the roles of MKP-1 and the AP-1 system. Mol Endocrinol 29:1454–1467. doi: 10.1210/me.2015-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gillgrass AE, Ashkar AA, Rosenthal KL, Kaushic C. 2003. Prolonged exposure to progesterone prevents induction of protective mucosal responses following intravaginal immunization with attenuated herpes simplex virus type 2. J Virol 77:9845–9851. doi: 10.1128/JVI.77.18.9845-9851.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhavanam S, Snider DP, Kaushic C. 2008. Intranasal and subcutaneous immunization under the effect of estradiol leads to better protection against genital HSV-2 challenge compared to progesterone. Vaccine 26:6165–6172. doi: 10.1016/j.vaccine.2008.08.045. [DOI] [PubMed] [Google Scholar]
- 42.Pauklin S, Petersen-Mahrt SK. 2009. Progesterone inhibits activation-induced deaminase by binding to the promoter. J Immunol 183:1238–1244. doi: 10.4049/jimmunol.0803915. [DOI] [PubMed] [Google Scholar]
- 43.Flurkey K, Gee DM, Sinha YN, Wisner JR Jr, Finch CE. 1982. Age effects on luteinizing hormone, progesterone and prolactin in proestrous and acyclic C57BL/6j mice. Biol Reprod 26:835–846. doi: 10.1095/biolreprod26.5.835. [DOI] [PubMed] [Google Scholar]
- 44.Finch CE, Holinka CF. 1982. Aging and uterine growth during implantation in C57BL/6J mice. Exp Gerontol 17:235–241. doi: 10.1016/0531-5565(82)90030-4. [DOI] [PubMed] [Google Scholar]
- 45.Ye J, Sorrell EM, Cai Y, Shao H, Xu K, Pena L, Hickman D, Song H, Angel M, Medina RA, Manicassamy B, Garcia-Sastre A, Perez DR. 2010. Variations in the hemagglutinin of the 2009 H1N1 pandemic virus: potential for strains with altered virulence phenotype? PLoS Pathog 6:e1001145. doi: 10.1371/journal.ppat.1001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCown MF, Pekosz A. 2005. The influenza A virus M2 cytoplasmic tail is required for infectious virus production and efficient genome packaging. J Virol 79:3595–3605. doi: 10.1128/JVI.79.6.3595-3605.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takeda M, Pekosz A, Shuck K, Pinto LH, Lamb RA. 2002. Influenza A virus M2 ion channel activity is essential for efficient replication in tissue culture. J Virol 76:1391–1399. doi: 10.1128/JVI.76.3.1391-1399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Margine I, Palese P, Krammer F. 2013. Expression of functional recombinant hemagglutinin and neuraminidase proteins from the novel H7N9 influenza virus using the baculovirus expression system. J Vis Exp 2013:e51112. doi: 10.3791/51112. [DOI] [PMC free article] [PubMed] [Google Scholar]