Abstract
In this paper, we present a culture of A549 and MRC-5 spheroids in a microfluidic system. The aim of our work was to develop a good lung cancer model for the evaluation of drug cytotoxicity. Our research was focused on determining the progress of cell aggregation depending on such factors as the depth of culture microwells in the microdevices, a different flow rate of the introduced cell suspensions, and the addition of collagen to cell suspensions. We showed that these factors had a significant influence on spheroid formation. It was found that both MRC-5 and A549 cells exhibited higher aggregation in 500 μm microwells. We also noticed that collagen needs to be added to A549 cells to form the spheroids. Optimizing the mentioned parameters allowed us to form 3D lung tissue models in the microfluidic system during the 10-day culture. This study indicates how important an appropriate selection of the specified parameters is (e.g., geometry of the microwells in the microsystem) to obtain the spheroids characterized by high viability in the microfluidic system.
I. INTRODUCTION
In the last few decades, three dimensional (3D) cell culture has gained increasing recognition as an effective method for biological research.1–3 It is because of the fact that 3D cell culture mimics natural tissues more closely than two dimensional (2D) cell culture.4 In 2D cell culture, the cells adhere to the surface of a growth material and create a monolayer. This kind of cell growth does not simulate tissue in the human body. Metabolic pathways and gene expression of the cells in 2D cultures differ from those existing in the organism. Moreover, the cells attach to the growth surface creating an unnatural protein connection, simplified extracellular matrix (ECM).5,6 In 3D cell culture, cells attach to each other creating a spatial arrangement. Methods such as multicellular spheroids (MCs), hydrogels, and scaffolds are used to obtain a 3D cell culture. The usage of these methods allows us to form a 3D network of the ECM matrix and cell-cell interactions.7 3D cultures consist of a complex of proteins in their native configuration. It provides conditions similar to the natural environment of cell growth.8,9
Multicellular spheroids (MCs) are the most popular 3D cancer models.10,11 In this type of 3D culture, cells (one or various types) attach and aggregate to each other, finally forming spheroids. Spheroids are composed of three parts: A necrotic core (in the centre), cells at rest, and proliferating cells (the outer part).12 Such a distribution of the cells is associated with the exchange of nutrients, supply of oxygen, and removal of metabolic components.13 The morphology, cell interactions, and kinetics of cell growth in MCs are similar to an early, avascular stage of tumor. Moreover, cellular heterogeneity of tumors, i.e., an induction of proliferation gradients, a differentiation, histological structures, and an expression of antigens could be mimicked in MCs.14
Various techniques, e.g., the hanging drop method, culture in stirred bioreactors, and culture on hydrophobic substrates, are used to form multicellular spheroids.15–17 However, Lab-on-a-chip systems have gained popularity for spheroid cultures in the last few years.18–22 Various types of cancer cells were used for the creation of spheroids in the microsystems. For example, Hsiao et al. presented a microfluidic system for the formation of PC-3 prostate cancer spheroids.18 Ota et al. showed the formation of HepG2 spheroid cultures using micro-rotational flow.19 The hepatocyte spheroid culture was also obtained in a microfluidic system by Lee et al. They developed a spheroid-based 3D artificial Liver-chip with a perfusion function. Moreover, they used this chip to investigate the paracrine effects of HSCs (hematopoietic stem cells) on the growth of hepatocytes.20 The application of Lab-on-a-chip systems for spheroid cultures allows us to mimic the natural conditions of cell growth, i.e., the cells are specially arranged and the microchannel network mimics tumor vascularization. Furthermore, a high surface to volume ratio in a microscale corresponds to the ratio under in vivo conditions. In addition, the usage of a small volume of reagents allowed us to reduce waste and cost of the research.23
The formation of spheroids in Lab-on-a-chip systems is mainly based on the gravitational falling of the cells in the culture microchambers/microwells.20,22 Such microchambers/microwells have a flat bottom and they are fabricated using hydrophobic materials. It was noticed that such a property of a material enhanced cell aggregation. However, the flat bottom of microchambers contributed to the formation of non-regular spheroids. The parameters such as shape of a microchamber and type of a material used for the construction of the microsystem are important to form model MCs. Moreover, aggregation of the cells is dependent on the type of the tested cells and the composition of their membrane.24 The number and type of proteins present in the cell membrane have an influence on the degree of the cell aggregation. More than 50 proteins have been identified as agents involved in cell adhesion, e.g., integrins, selectins, and cadherins.25 Each of these proteins has a specific structure and different function in cell adhesion.26 Therefore, the optimization of spheroid formation from various cell types is important.
In this paper, we describe the formation of A549 and MRC-5 spheroids in a microfluidic system. The aim of our work was an investigation of these types of cells, because lung cancer is one of the most common tumors around the world. Moreover, there are no reports about culture of A549 or MRC-5 spheroids in the microfluidic systems. The investigation of lung cell lines in the microsystems was previously reported; however, all studies were based on a monolayer culture.27,28 Here, we present 10-day A549 and MRC-5 spheroid cultures as good models for further analysis, i.e., cell-cell interaction, cytotoxicity, and cell regeneration. Contrary to other works, we investigated the lung cell aggregation procedure and MC formation using various parameters: different depths of U-shaped culture microwells, different flow rates of the introduced cell suspensions, and addition of collagen to the cell suspensions. To our knowledge, this is the first presentation of such experiments.
II. MATERIALS AND METHODS
A. Microsystem design and fabrication
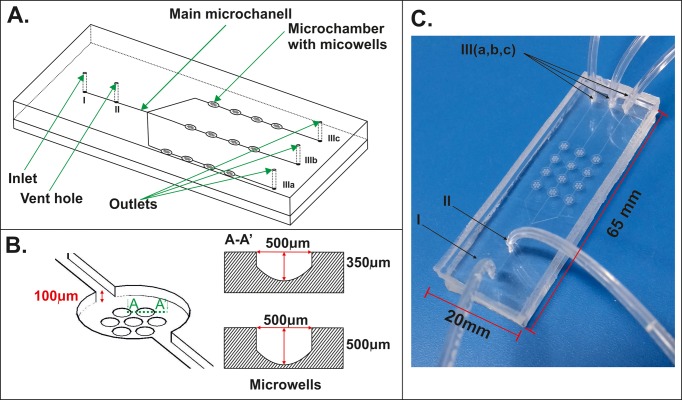
Figure 1 shows a geometry of the microsystem, which was used in our research. The microsystem consists of a network of microchannels (a width of 100 μm and a depth of 100 μm) and microchambers (a diameter of 2700 μm and a depth of 100 μm), dedicated for flow and culture of the cells. Spheroid culture microchambers were placed in 3 × 4 arrays. Rows of microchambers had a common inlet and three separate outlets (Figure 1(a)). Each microchamber contains seven U-shaped microwells, designated for the MC formation and culture. The U-shape geometry prevented the cell adhesion to the surface and enhanced the aggregation and formation of the spheroids. The microsystems with two different depths (500 μm and 350 μm) of U-shaped microwells were used in our studies (Figure 1(b)). The geometry of the microstructure was designed to provide isolation of a spheroid culture from shear stress caused by medium flow. In addition, the distribution of microchambers and microwells allowed us to obtain a reproducible size of the spheroids. The design of the microsystem also enables us to test three different concentrations of compounds in a single step. Moreover, the microsystem was equipped with a venting system (Figure 1(c)). Spheroids were protected from damage caused by air bubbles by using the venting system. This allowed us to obtain a high viability of MCs.
FIG. 1.
(a) The scheme of the microfluidic system used for A549 and MRC-5 spheroid cultures. (b) The microchamber with cross-section of two different microwells. (c) The fabricated microsystem (I—inlet, II—vent hole, III—outlets).
The microsystem consists of two poly(dimethylsiloxane) (PDMS) (Sylgard 184; Dow Corning) layers. The bottom layer contains microchannels, microchambers, and microwells. The top layer has got five holes: an inlet, a vent hole, and three outlets. The microsystem was fabricated using a double casting technique described in the previous work.29 In short, the first replication master, with a concave microstructure, was made in poly(methylmethacrylate) (PMMA) using a computer numerically controlled (CNC) micromilling machine (Minitech Machinery Co.). Afterwards, PDMS was prepared by mixing the prepolymer with the curing agent with a weight ratio of 9:1. After degassing, the liquid mixture was poured over the PMMA master and cured at 70 °C for 2.5 h. Next, the crosslinked PDMS was peeled off from the PMMA master and the secondary PDMS master was received. The PDMS master was thermally aged at 100 °C for 48 h and it was used in the next replica moulding procedure. In this way, the bottom layer with the microstructure was obtained. The top layer was fabricated by pouring the liquid PDMS over a glass plate. After the curing step, the PDMS layer was peeled off and holes for tubings were drilled. Both polymer layers were bonded (for 35 s) using an oxygen plasma activation (Plasma-preen®II-973 (Plasmatic Systems, INC.)).
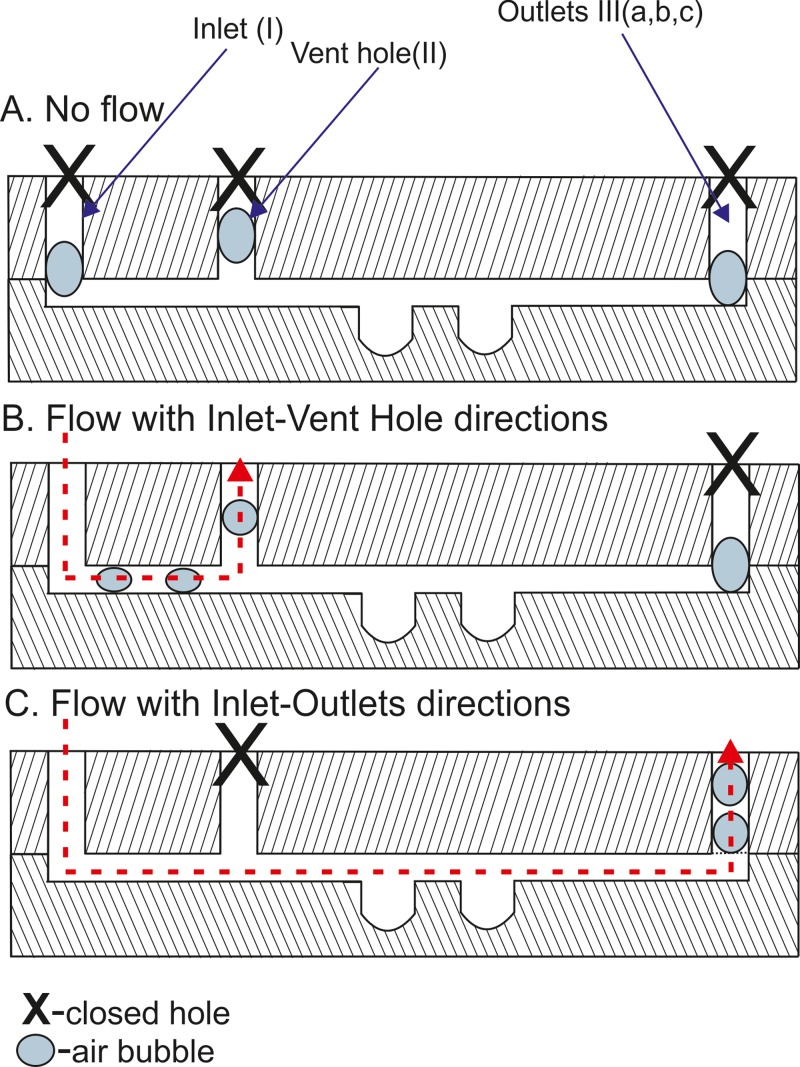
B. The venting system operation
The venting system was a hole, which was located in the main microchannel (Figure 1(a)). The principle of the venting system was based on the flow of the culture medium through the microsystem. This flow was controlled by sealing the microsystem holes. Before the cell introduction, all air bubbles were removed from the microchambers and the channel network. After cell seeding, when the cells were placed in culture microwells, all holes (Figure 2(a)) were closed. At this time, the cells aggregated and formed multicellular spheroids. In order to remove air bubbles, which usually accumulate in the holes, the inlet (I) and the vent hole (II) were opened. Next, the flow of culture medium in this direction (I–II) was started (Figure 2(b)). This enabled us to remove the residual air from these holes. Then, the vent hole (II) was sealed and three outlets (III a, b, and c) were opened (Figure 2(c)). This enabled the exchange of the culture medium in the microwells and the removal of residual air from outlets. Due to this operation, the fresh culture medium was transported to the multicellular spheroids without cell damage.
FIG. 2.
The operation of the venting system. (a) Flow is switched off. (b) Flow is applied between the Inlet and Vent Hole. (c) Flow is applied between Inlet and Outlets.
C. Cell culture
The human lung carcinoma cell line (A549) and the fetal lung fibroblast cell line (MRC-5) were used in this research. The cells were purchased through American Type Culture Collection and European Type Culture Collection, respectively. A549 cells were cultured in Dulbecco's Modified Eagle Medium (DMEM) medium (Sigma-Aldrich) containing 10%vol fetal bovine serum (Gibco), 1%vol 100 mM penicillin, and streptomycin (Sigma-Aldrich) and 1%vol of 25 mM Glutamax (Gibco). MRC-5 cells were cultured in MEM medium (Sigma-Aldrich) containing 10%vol fetal bovine serum (Gibco), 1%vol 100 mM penicillin, and streptomycin (Sigma-Aldrich), 1%vol of 25 mM Glutamax (Gibco), and 0,01% amino acid solution. The cells were cultured at 37 °C in a humidified atmosphere containing 5% CO2 (HeraCell 150, Thermo Scientific). Before the cell culture, the fabricated microsystems were sterilized. The double sterilization with 70%vol ethyl alcohol and UV light (Black Ray) were used. After that, the microsystems were filled with 0.5%weight solution of poly(vinyl alcohol) (Sigma-Aldrich) and incubated for 1.5 h. This prevented cell adhesion to the PDMS surface. Finally, the microsystems were filled with the culture medium and warmed by placing them into an incubator (37 °C, 5% CO2).
A549 and MRC-5 cell suspensions (density of 4 × 106 cells/ml) were prepared according to the conventional method.30 To improve the aggregation of the cells, A549 cell suspension with 0.001%vol collagen (0.1% solution in acetic acid, Collagen from calf skin, Sigma Aldrich) was prepared. The prepared cell suspensions were introduced into the sterilized microsystems with a flow rate of 10 μl min−1. Experiments were performed using the microsystems with two different depths of culture microwells: 350 μm and 500 μm. The microsystems with the introduced cells were sealed and placed into the incubator (37 °C, 5% CO2). The cell culture medium was exchanged daily over 3 days (4.0 μl min−1 for 20 min). The influence of the flow rate on the viability of the cells was evaluated in our research. For this purpose, the microsystem with 500 μm depth of microwells was used. Four different flow rates: 10 μl min−1, 12 μl min−1, 15 μl min−1, and 20 μl min−1 were used for the introduction of cell suspensions. Additionally, aggregation and viability of A549 and MRC-5 spheroids during the 10-day culture were evaluated. For this purpose, we used the microsystem with 500 μm deep microwells. In this case, the culture medium was exchanged daily. The peristaltic pumps (Ismatec Reglo-Digital MS-4/12) were used for introduction of all fluids and cells at each stage of the experiment.
D. Spheroid observations and measurements
The Monitoring of MC culture was carried out using an inverted fluorescence microscope coupled with a CCD camera (Olympus IX-71). The MC formation and the changes of their morphology were observed 1, 24, 48, and 72 h after cell seeding. For the microsystems with the best A549 and MRC-5 cell aggregation, the changes of the MC morphology were also observed during the 10-day spheroid culture. CellSens image analysis software (Olympus) was used for data acquisition and analysis. The effect of the MC formation was determined by two parameters: the cross-sectional area and sphericity. The cross-sectional area is the parameter which determines the quality of cell aggregation. The low value of this parameter indicates a high aggregation of the cells. The sphericity is approximately the square of the quotient of width and length. This parameter determines the degree of cell aggregation and the shape of the spheroids.31 For circular spheroids (full aggregation), the sphericity is nearing 1. In contrast, a value of the sphericity lower than 1 indicates poor aggregation of the cells. This kind of culture does not form MCs.
E. Fluorescence cell viability alamarBlue® assay
Fluorescence measurements of spheroid viability were performed using a Varian Cary Eclipse Fluorescence Spectrophotometer equipped with a Microplate Reader (Agilent). This measurement was carried out daily for the 10-day culture. Furthermore, it was also used to check the influence of the flow rate of the cell suspension introduced to the chip on the spheroid viability. The evaluation of the spheroid viability was based on the introduction of 10%vol alamarBlue® (AbD Serotec) solution (10 min, 4.0 μl min−1) to the microsystem with spheroid culture. Next, the microsystem was incubated for 20 min at 37 °C. After this time, the fluorescence measurements were performed. Due to the specially designed chip holder and the microchamber arrangement (the microsystem's microchambers correspond to the size and the location of the wells in a standard 384 well plate), the direct measurement of fluorescence intensity was possible using a microplate reader. After the measurement, the culture medium was exchanged (20 min, 4.0 μl min−1) and the microsystem was incubated for the next 24 h (37 °C, 5% CO2).
F. Image cell viability assay
Calcein AM and Propidium iodide (PI) staining was used to identify live and dead cells (Sigma Aldrich). This viability assay was performed 72 h after cell seeding. Furthermore, this test was also conducted on the last day of 10-day spheroid culture. The viability test was performed according to the previously described method.32 Shortly, 4 μl of Calcein-AM stock solution (2 mM) and 100 μl of PI solution (1mgml−1) were added to 1 ml of culture medium. Next, the spheroids were incubated with the test solution for 15 min. The double-stained cells were assessed using a microscope with a CDD camera (Olympus IX-71). The viability of the cells was determined by comparing the amount of living to dead cells.
G. Statistical analysis
The results of cross-sectional area, sphericity, and alamarBlue® represent the means and standard errors of five independent experiments (five separate microsystems). For one experiment, 10 spheroids were selected. Statistical analysis was performed using one way analysis of variance (ANOVA). Values of p less than 0.05 were considered as statistically significant (asterisks indicate p < 0.05).
III. RESULTS AND DISCUSSION
A. Multicellular spheroid formation
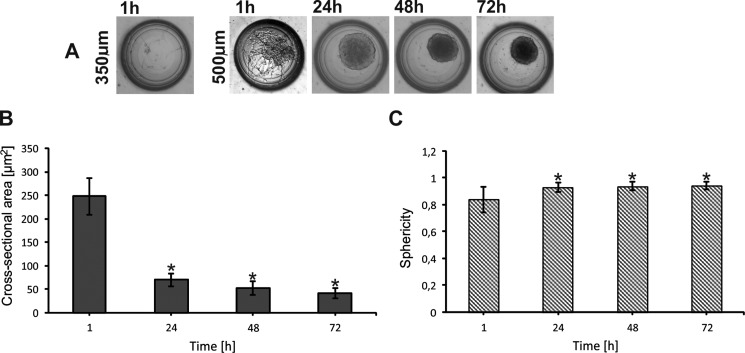
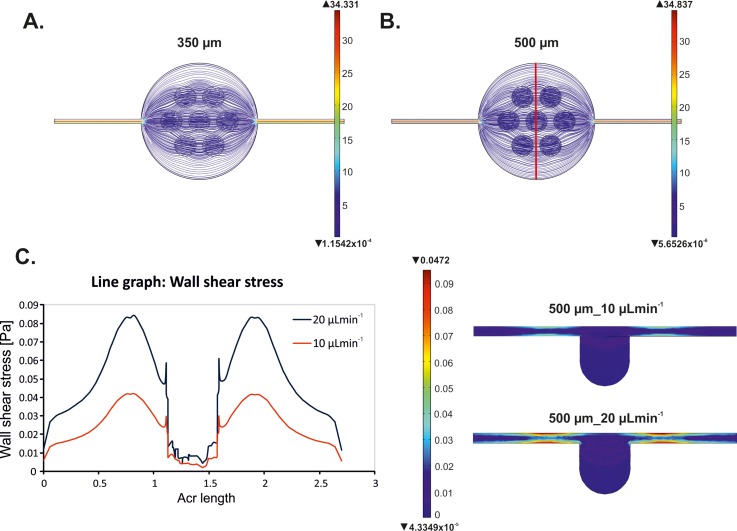
The aggregation of both A549 and MRC-5 cells was monitored using a microscope (Olympus IX-71). CellSens Dimension image analysis software was used for data acquisition and analysis. We observed large differences in cell cultures in 350 μm and 500 μm deep microwells. Figure 3(a) presents the aggregation of MRC-5 cells 72 h after cell seeding. We noticed that the usage of small microwells (a depth of 350 μm) prevented MRC-5 spheroid formation. MRC-5 cells were not observed in the microwells 1 h after cell introduction (Figure 3(a)). The formation of MRC-5 spheroids 24 h after cell introduction into the 500 μm deep microwells was observed. During the next hours of the culture, the aggregation of MRC-5 cells increased in these microwells. It was noticed that for 72 h of spheroid culture, the cross-sectional area decreased 5 times in relation to 1 h of the culture (Figure 3(b)) and in further days it remained at a constant level. In addition, the value of sphericity of MRC-5 spheroids was closest to 1. It indicated that a good model of MRC-5 cell spheroids can be obtained in this kind of microsystem (Figure 3(c)). All obtained results for both cross-sectional area and sphericity measurements were statistically significant. The aggregation of MRC-5 in the tested microwells could be associated with the cell size and the distribution of medium flow in the microchamber. To verify the culture medium flow in the designed microchambers, a computer modeling simulation using the Microelectromechanical Systems (MEMS) Simulations Module of COMSOL MULTIPHYSICS software was carried out. Figures 4(a) and 4(b) present the flow distribution in both microchambers with 350 μm and 500 μm deep microwells. The simulation was conducted for 10 μl min−1 flow rate at the inlet. A similar fluid value was observed in both tested microchambers; however, fluid trajectory was different. We noticed that the fluid mainly spreads above the microwells in the microchambers with 500 μm deep microwells. So a higher number of MRC-5 cells could be introduced into the 500 μm than 350 μm deep microwells. A suitable number of the cells and cell-cell interactions are essential for spheroid formation. Such conditions could not be observed in the small microwells; therefore, spheroids did not form. Additionally, we supposed that the remaining single cells were washed out from the small microwells.
FIG. 3.
MRC-5 cell aggregation. (a) Microwells (500 and 350 μm) with MC culture. (b) Changes of the cross-sectional area during MRC-5 cell culture. (c) Changes of sphericity during MRC5 cell culture. Error bars are calculated as the standard deviation of the spheroid population. The reference values for the statistical analysis are the results obtained 1 h after cell introduction. Asterisks indicate p < 0.05.
FIG. 4.
Results of COMSOL MULTIPHYSICS simulation. Fluid flow modelling in microchambers with (a) 350 μm deep microwells. (b) 500 μm deep microwells at a flow rate of 10 μl min−1 at the inlet. (c) Wall shear stress profiles in the tested microchambers with 500 μm deep microwells for flow rates of 10 μl min−1 and 20 μl min−1. The place in which the wall stress measurement was marked using red line in (b). Left side—line graph of wall shear stress distribution. Right side—visualization of the distribution of wall shear stress in a single microwell.
The cell-based assays for drug development and testing have become an important topic in toxicology studies. In the case of testing potential anti-cancer drugs, an important step is to determine their effect not only on tumor cells, but also on normal cells. Many reports about this type of research can be found in the literature.33,34 Zuco et al. evaluated the activity of betulinic acid, in comparison with doxorubicin, on different human neoplastic and non-neoplastic cell lines. In the case of betulinic acid, growth inhibition in all tumor cell lines occurred. Although cytotoxic activity of doxorubicin was evident for all tested cell lines, betulinic acid was not toxic for normal cells.33 The study of apoptosis of normal and tumor cells has also been carried out by another group. Jo et al. checked the influence of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) on hepatocytes from rat, mouse, rhesus monkey, and human livers. Jo et al. showed that TRAIL induced apoptosis in normal human hepatocytes but not in hepatocytes isolated from the other species.34 These results indicate that verification of a new compound activity not only on tumor but also on normal cell lines is an important stage of cytotoxicity research. Therefore, in our research we wanted to create a model of normal tissue, which would reflect conditions similar to in vivo. In the literature, the spheroids composed of normal cells are found very rarely. However, when the cytotoxic tests on tumor spheroids are conducted, the same tests on normal cultures under similar environmental conditions should be performed.
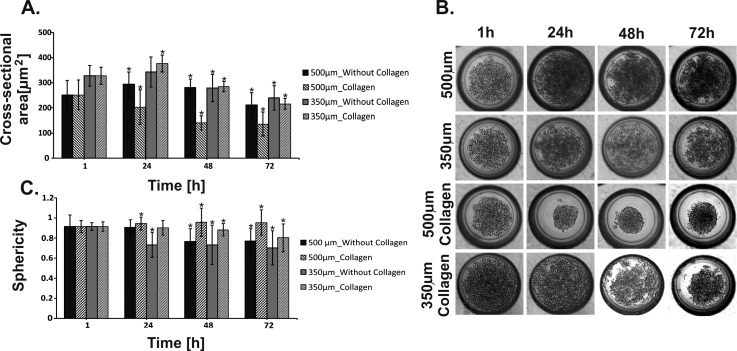
A549 cell aggregation was noticed in two tested microsystems (microwells depth: 500 μm and 350 μm) (Figure 5) 1 h after cell introduction. Similar trends of spheroid formation were observed in both tested microsystems. Cell aggregation reduction was observed in the next hours. The cells did not form circular spheroids, but they were loosely connected to each other. We suppose that this can be due to the small amount of adhesive proteins included in the cell membrane.24 An increase of the cross-sectional area (Figure 5(a)) (after 24 h) could be caused by the dispersion of the cells in the microwells, associated with a lack of cell aggregation. On the third day (72 h) of the cell culture, the cross-sectional area decreased in the microsystems with both 500 μm and 350 μm deep microwells. This could be caused by the washing out of single cells, which were located in the upper part of the microwells. Single cells and their small aggregates can be removed more easily under a flow of culture medium than the full aggregated spheroids. These experiments showed that the creation of A549 MCs in the microsystems (with 500 and 350 μm deep microwells) was rather impossible. Therefore, we decided to improve cell-cell interaction by adding additional proteins. For this purpose, A549 cell suspension with collagen (0.001%vol) was prepared. Collagen is the main structural protein in ECM of animal tissues; therefore, it was used in our experiments.35 The results of this study are presented in Figure 5(b). It was observed that the addition of collagen allowed the formation of fully aggregated spheroids. However, in deep microwells (500 μm), A549 cells formed spheroids with the highest efficiency. This was also proven by analysing the value of both the cross-sectional area and sphericity (Figures 5(a) and 5(c)). The cross-sectional area decreased every day for microwells with a depth of 500 μm (all obtained results were statistically significant). Within three days of the cell culture, it decreased by 40% and did not change after that. Moreover, the sphericity of A549 spheroids was higher for the culture with collagen than without it. In microwells with a depth of 350 μm, the cross-sectional area of spheroids increased during the first 24 h. Then, a decrease was observed in both with and without collagen cultures. However, sphericity was higher for the A549 cells' cultures with collagen. Based on the presented results, we can conclude that the addition of collagen allowed the creation of a good model of A549 spheroids in microwells with a depth of 500 μm. All presented results are the average from five microsystems with spheroid cultures. 10 spheroids were selected from each microsystem, so each set of data were calculated for 50 spheroids.
FIG. 5.
A549 cell aggregation. (a) Changes of the cross-sectional area during A549 cell culture with and without collagen. (b) Microwells (500 and 350 μm) 1, 24, 48, and 72 h after A549 cell seeding. MC formation with and without collagen. (c) Changes of the sphericity during A549 cell culture with and without collagen. Error bars are calculated as the standard deviation of the spheroid population. The reference values for statistical analysis are the results obtained 1 h after cell introduction. Asterisks indicate p < 0.05.
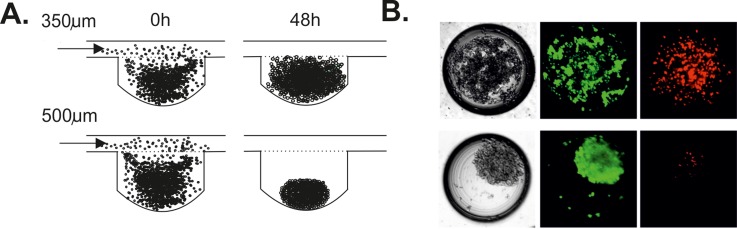
Our investigation showed that the depth of the microwells had an influence on the formation of A549 spheroids. Aggregation of A549 cells in microwells was different for the cultures with and without collagen. The cell aggregation in both types of the microwells was increased by adding collagen to the A549 cell suspension. A549 cell aggregation was higher in microwells with a depth of 500 μm than 350 μm. We suppose that it could be from the differing supply of nutrients to spheroids in the microwells with a different depth. When nutrients did not reach the cells, the cells died and lost their ability to aggregate.20 The same density of cell suspensions and the same flow rate were used during the experiments in the microsystem with different microwell depths. This enabled the comparison of how the depth of microwells influenced the spheroid formation. The fluid flow simulation showed that fluid spreads mainly above the microwells in the microchambers with 500 μm deep microwells. For A549 cells, the aggregates were formed in both types of microwells; however, more compact spheroids were noticed in deeper microwells. (Figures 6(a) and 6(b)). It could result from the different size of the A549 and MRC-5 cells. The level of metabolic waste was higher in 350 μm deep microwells, which could influence the viability of the cells. Therefore, A549 cell viability was higher for the cells cultured in the microsystems with deeper microwells (Figure 6(b)). Compact spheroids could be more resistant to shear stress, so in small microwells A549 cells were exposed to higher shear stress. Consequently, A549 cells died and lost their ability to aggregate (Figure 6(b)).
FIG. 6.
(a) The scheme of the A549 cell aggregation in microwells with a depth of 350 and 500 μm. (b) A549 cell viability in microwells with a depth of 350 and 500 μm (red objects—dead cells, green objects—live cells).
Our study also showed that this type of cell has an influence on MC formation. MRC-5 cells aggregated in microwells without additional components. However, MRC-5 spheroid growth is limited by the depth of the microwells. A549 cells are unable to aggregate spontaneously in the developed microsystems. It was found that A549 cells needed an external factor to create spheroids. The adhesive properties of cells play the main role in cell aggregation. Aggregation of the cells is obtained by the interaction between integrins present on the surface of the cell membrane and proteins secreted by the cells or added purposely to the culture medium.36 The ability of the aggregation of various cell types depends on both the amount of matrix proteins secreted into medium culture and the volume of integrins in cell membranes.37 MRC-5 cells have the ability to produce collagen.35 Collagen produced by MRC-5 cells probably allowed for rapid cell aggregation and long term spheroid culture. A549 cells did not produce these adhesive proteins; therefore, the addition of proteins was necessary to form A549 spheroids.
B. The influence of a flow rate on cell viability in spheroids
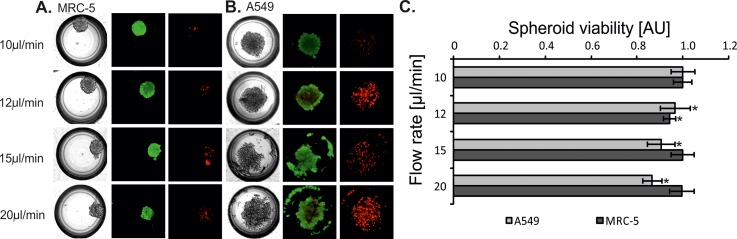
Fully created spheroids of MRC-5 and A549 cells were obtained in microsystems with microwells with a depth of 500 μm. Additionally, to form A549 spheroids the cells need the addition of collagen. For these culture conditions, additional experiments were performed. The influence of the introduced cell suspension's flow rate on the cell viability was evaluated. MRC-5 and A549 cell suspensions were introduced into the microsystems at four different flow rates: 10 μl min–1, 12 μl min–1, 15 μl min−1, and 20 μl min−1. Studies using propidium iodide and calcein AM were performed 24 h after the introduction of cell suspensions. The viability and the aggregation of MRC-5 cells were the same for each of the applied flow rates (Figure 7(a)). However, it was observed that A549 cell viability decreased with the increase of flow rate (Figure 7(b)). These results were also confirmed by alamarBlue assay® (Figure 7(c)). Moreover, a flow rate of the introduced cells had an influence on A549 spheroid formation. If a flow rate was high, the cell aggregation and sphericity of A549 spheroids were low. We supposed that this could be caused by the generation of shear stress. In the literature, there is information that shear stress is proportional to the flow rate of the culture medium.38 To identify the value of the wall shear stress in various areas of the microchambers, we simulated the distribution of this parameter in the microchambers with 500 μm deep microwells. The simulation was conducted for two different flow rates at the inlet (10 μl min−1 and 20 μl min−1). The wall shear stress generated in the microchambers was directly dependent on the fluid trajectory. The comparison of the wall shear stress for different flow rates (the same microchamber geometry) showed that the highest wall shear stress was observed around the microwells. The usage of 20 μl/min flow rate generated about double wall shear stress than a flow rate of 10 μl/min (Figures 4(c) and 4(d)). Shear stress is a factor which has an adverse effect on the cells. It causes the reduction of cell viability, changes their morphology and has an effect on the ion permeability through the cell membranes.39 Shear stress caused by too high of a cell suspension flow flow also influences the aggregation of the cells and the formation of spheroids. Apoptotic and necrotic cells do not aggregate and they cannot create a spheroid structure. MRC-5 cells formed fully aggregated spheroids for each tested flow rate of the introduced cell suspension. Moreover, in every case (with different flow rates) the MRC-5 spheroids were characterized by high viability. Our results showed that MRC-5 (non-malignant) spheroids were more resistant to the flow of the medium than A549 (carcinoma) spheroids. It can be caused by the differences in their morphology. MRC-5 cells have got fibroblast morphology, while A549 cells have got epithelial morphology. The epithelial cells exposed to the fluid shear stress change their shape, regulation, and structure of the microfilament network.40,41 The differences in MRC-5 and A549 resistance to shear stress can also be confirmed by high abilities of MRC-5 cells to aggregate. It seems that cells which formed stable spheroids are more resistant to various external factors.42,43
FIG. 7.
(a) MRC-5 and (b) A549 aggregation and viability depending on a flow rate of the introduced cell suspensions. Tests performed in the microsystem with microwells with a depth of 500 μm. A suspension of (a) MRC5 cells without collagen. (b) A549 cells with collagen. Green objects are the life cells/spheroids (stained with Calcein AM) Red objects are the dead cells/spheroids (stained with Propidium iodide). (c) Spectrofluorimetric measurement of A549 and MRC-5 spheroid viability. The bars of the fluorescence intensity were normalized to 1. The reference values for statistical analysis are the results obtained for 10 μl min−1. Asterisks indicate p < 0.05.
It was found that during the introduction of cell suspension the flow rate had an influence on MC formation. The highest flow rate caused shear stress, which influenced A549 cell viability. Shear stress also had an effect on the aggregation of cells and sphericity of spheroids. A549 and MRC-5 viability and aggregation in the presented microsystem depend on the type and the morphology of the tested cells. Cell properties may cause resistance or a lack there of on a fluid rate. Therefore, an important step in studies about Cell-on-a-Chip is the selection of a medium flow rate and cell suspension.
C. Cell aggregation and viability during 10-day culture
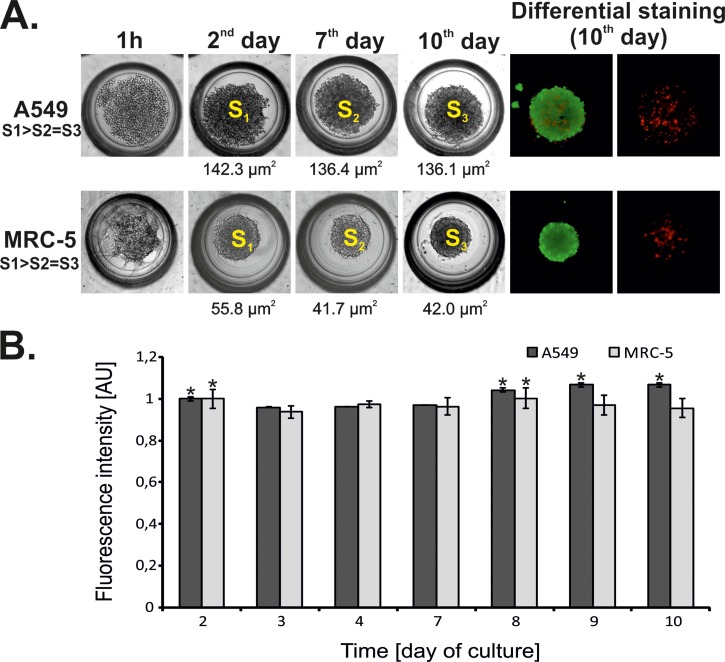
The next step of our research was a study of A549 and MRC-5 aggregation during a 10-day spheroid culture. For this purpose, the microsystem with 500 μm depth of microwells was used. In the case of A549, the culture with the addition of collagen was chosen. The cross-sectional area did not change from the 3rd day in both A549 and MRC-5 spheroids (Figure 8(a)). Furthermore, both types of spheroid culture (cancer/normal) exhibited high viability during the 10-day culture, which was confirmed by both differential staining and alamarBlue® assay (Figure 8(b)). These results confirmed that the presented method of A549 and MRC-5 cell aggregation in the microsystem allowed us to obtain long-term A549 and MRC-5 spheroid cultures.
FIG. 8.
(a) Microscopic analysis of A549 and MRC-5 spheroids. The morphological changes in spheroids during long-term culture and the results of differential staining at the last day of the culture. (b) The results of spectrofluorimetric monitoring of A549 and MRC-5 spheroids during long term spheroid culture. The bars of the fluorescence intensity were normalized to 1 on the second day of the culture. The reference values for statistical analysis are the spheroid fluorescence intensity on the 2nd day of the culture. Asterisks indicate p < 0.05.
In Lab-on-a-chip systems, long-term cultures of various types of spheroids were performed.17,18,22 For example, a 7-day culture of PC-3 and 14-day culture of both HepG2 and MCF-7 spheroids were maintained in the microsystems.17,18 However, A549 and MRC-5 spheroids were only studied in macroscale.44–46 For instance, a 12-day culture of an A549 spheroid culture was performed using an air- and liquid-interface.45 This model was used for the evaluation of drug cytotoxicity.45 The disadvantage of such a spheroid model included different spheroid dimensions as well as the inability to measure exactly the same spheroid twice. Here, we present the investigation of A549 and MRC-5 spheroid formation in the microfluidic system. It was found that the construction and dimensions of the microwells influenced spheroid formation. Spheroids with a high degree of sphericity and repeatable dimensions were obtained in the microsystem. Moreover, the parameters of the microsystem enabled the observation of size and morphology changes of a single spheroid. There is no information about an influence of external factors on spheroid formation and viability in the literature. Contrary to other works, here the flow rate of the introduced cell suspension, microwell size, and the addition of collagen were optimized. Finally, the lung spheroids showed high viability during the 10-day culture. The obtained A549 and MRC-5 spheroids can be a good model for further analysis, i.e., cytotoxic studies of a new compound or evaluation of various new therapeutic procedures.
IV. CONCLUSION
Cell lines differ from each other in many aspects. These differences can be related to cell nature (cancer/normal), morphology, and metabolic abilities. During the cell culture in the microfluidic system mentioned above factors can influence cell growth. Formation of multicellular spheroids in the Lab-on-a-chip also depends on cell type. Therefore, in our work we optimized the formation of A549 (malignant) and MRC-5 (non-malignant) spheroids in the microfluidic system. It was found that cell aggregation and spheroid formation depend on various factors (i.e., cell type, geometry, and depth of the culture microwell, a flow rate). We noticed that MRC-5 cells have high ability to aggregate. It results from the fact that MRC-5 cells produce collagen, a protein which is present in the extracellular matrix. We also observed that A549 cells did not aggregate spontaneously. To obtain spherical A549 spheroids, the addition of collagen to the cell suspension was necessary. Moreover, our study demonstrated that viability as well as aggregation of A549 cells is related to both the flow rate, which was used during the introduction of cell suspension and the depth of the microwell. To conclude, our study shows how important an appropriate selection of the specified parameters is to obtain long-term spheroid cultures in the microfluidic system. Our results could be used during the fabrication of Lab-on-a-chip systems, in which the cell culture microenvironment mimics natural cell growth.
ACKNOWLEDGMENTS
This work has been supported by Warsaw University of Technology.
This work was financially supported within a frame of SONATA 5 Program No. UMO-2013/09/D/ST5/03887.
References
- 1. Li Z., Sun H., Zhang J., Zhang H., Meng F., and Cui Z., PLoS One , e72612 (2013). 10.1371/journal.pone.0072612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Frey O., Misun P. M., Rothe J., and Hierlemann A., Procedia Eng. , 96 (2014). 10.1016/j.proeng.2014.11.274 [DOI] [Google Scholar]
- 3. Ramaiahgari S. C., den Braver M. W., Herpers B., Terpstra V., Commandeur J. N., van de Water B., and Price L. S., Arch. Toxicol. , 1083 (2014) 10.1007/s00204-014-1215-9. [DOI] [PubMed] [Google Scholar]
- 4. Edmondson R., Broglie J. J., Adcock A. F., and Yang L., Assay Drug Dev. Technol. , 207 (2014). 10.1089/adt.2014.573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. J. M. McKim, Jr. , Comb. Chem. High Throughput Screening , 188 (2010). 10.2174/138620710790596736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bhise N. S., Gray R. S., Sunshine J. C., Htet S., Ewald A. J., and Green J. J., Biomaterials , 8088 (2010). 10.1016/j.biomaterials.2010.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Saias L., Gomes A., Cazales M., Ducommun B., and Lobjois V., Cancer Res. , 2426 (2015). 10.1158/0008-5472.CAN-14-3534 [DOI] [PubMed] [Google Scholar]
- 8. Ziółkowska K., Kwapiszewski R., and Brzózka Z., New J. Chem. , 979 (2011). 10.1039/c0nj00709a [DOI] [Google Scholar]
- 9. Dubessya C., Merlin J. L., Marchal C., and Guillemin F., Crit. Rev. Oncol. Hematol. , 179 (2000). 10.1016/S1040-8428(00)00085-8 [DOI] [PubMed] [Google Scholar]
- 10. Wua M. H., Huang S. B., Cui Z., Cui Z., and Lee G. B., Sens. Actuators, B , 231 (2008). 10.1016/j.snb.2007.07.145 [DOI] [Google Scholar]
- 11. Trédan O., Galmarini C. M., Patel K., and Tannock I. F., J. Natl. Cancer Inst. , 1441 (2007). 10.1093/jnci/djm135 [DOI] [PubMed] [Google Scholar]
- 12. Kim J. W., Ho W. J., and Wu B. M., Anticancer Res. , 3237 (2011). [PubMed] [Google Scholar]
- 13. Shweiki D., Neeman M., Itin A., and Keshet E., Proc. Natl. Acad. Sci. , 768 (1995). 10.1073/pnas.92.3.768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hirschhaeuser F., Menne H., Dittfeld C., West J., Mueller-Kliesera W., and Kunz-Schughart L. A., J. Biotechnol. , 3 (2010). 10.1016/j.jbiotec.2010.01.012 [DOI] [PubMed] [Google Scholar]
- 15. Schwarz R. P., Goodwin T. J., and Wolf D. A., J. Tissue Cult. Methods , 51 (1992). 10.1007/BF01404744 [DOI] [PubMed] [Google Scholar]
- 16. Wozniak M. and Keely P., Biol. Proced. Online , 144 (2005). 10.1251/bpo112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Torisawa Y., Takagi A., Nashimoto Y., Yasukawa T., Shiku H., and Matsue T., Biomaterials , 559 (2007). 10.1016/j.biomaterials.2006.08.054 [DOI] [PubMed] [Google Scholar]
- 18. Hsiao A. Y., Torisawa Y., Tung Y., Sud S., Taichman R. S., Pienta K. J., and Takayama S., Biomaterials , 3020 (2009). 10.1016/j.biomaterials.2009.02.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ota H., Yamamoto R., Deguchi K., Tanaka Y., Kazoe Y., Sato Y., and Miki N., Sens. Actuators, B , 359 (2010). 10.1016/j.snb.2009.11.061 [DOI] [Google Scholar]
- 20. Lee S. A., No D. Y., Kang E., Ju J., Kim D. S., and Lee S. H., Lab Chip , 3529 (2013). 10.1039/c3lc50197c [DOI] [PubMed] [Google Scholar]
- 21. Yeh C. H., Tsai S. H., Wu L. W., and Lin L. C., Lab Chip , 2583 (2011). 10.1039/c1lc20113a [DOI] [PubMed] [Google Scholar]
- 22. Lee K., Kim C., Bang J., Kim Y., Lee S., Ahn B., Kang J. Y., and Oh K. W., in 14th International Conference on Miniaturized Systems for Chemistry and Life Sciences, 3–7 October 2010, Groningen, The Netherlands (2010). [Google Scholar]
- 23. Karp J. M., Yeh J., Eng G., Fukuda J., Blumling J., Suh K. Y., Cheng J., Mahdavi A., Borenstein J., Langer R., and Khademhosseini A., Lab Chip , 786 (2007). 10.1039/b705085m [DOI] [PubMed] [Google Scholar]
- 24. Joseph-Silverstein J. and Silverstein R. L., Cancer Invest. , 176 (1998). 10.3109/07357909809050034 [DOI] [PubMed] [Google Scholar]
- 25. Li G., Satyamoorthy K., and Herlyn M., Cancer Res. , 3819 (2001). [PubMed] [Google Scholar]
- 26. Yamada K. M. and Geiger B., Curr. Opin. Cell Biol. , 76 (1997). 10.1016/S0955-0674(97)80155-X [DOI] [PubMed] [Google Scholar]
- 27. Jastrzebska E., Bulka M., Rybicka N., and Zukowski K., Sens. Actuators, B , 1356 (2015). 10.1016/j.snb.2015.08.022 [DOI] [Google Scholar]
- 28. Jastrzebska (Jedrych) E., Grabowska-Jadach I., Chudy M., Dybko A., and Brzozka Z., Biomicrofluidics , 44116 (2012). 10.1063/1.4771966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ziółkowska K., Żukowski K., Chudy M., Dybko A., and Brzózka Z., in Proceedings of the MicroTAS (2011), p. 1164. [Google Scholar]
- 30. Ziółkowska K., Kwapiszewski R., Stelmachowska A., Chudy M., Dybko A., and Brzózka Z., Sens. Actuators, B , 908 (2012). 10.1016/j.snb.2012.07.045 [DOI] [Google Scholar]
- 31. Zanoni M., Piccinini F., Arienti C., Zamagni A., Santi S., Polico R., Bevilacqua A., and Tesei A., Sci. Rep. , 19103 (2016). 10.1038/srep19103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kwapiszewska K., Michalczuk A., Rybka M., Kwapiszewski R., and Brzózka Z., Lab Chip , 2096 (2014). 10.1039/C4LC00291A [DOI] [PubMed] [Google Scholar]
- 33. Zuco V., Supino R., Righetti S. C., Cleris L., Marchesi E., Gambacorti-Passerini C., and Formelli F., Cancer Lett. , 17 (2002). 10.1016/S0304-3835(01)00718-2 [DOI] [PubMed] [Google Scholar]
- 34. Jo M., Kim T. H., Seol D. W., Esplen J. E., Dorko K., Billiar T. R., and Strom S. C., Nat. Med. , 564 (2000). 10.1038/75045 [DOI] [PubMed] [Google Scholar]
- 35. Houck J. C., Sharma V. K., Patel Y. M., and Gladner J. A., Biochem. Pharmacol. , 2081 (1968). 10.1016/0006-2952(68)90182-2 [DOI] [PubMed] [Google Scholar]
- 36. Lin R. Z. and Chang H., Biotechnol. J. , 1172 (2008). 10.1002/biot.200700228 [DOI] [PubMed] [Google Scholar]
- 37. Lin R. Z., Chou L. F., Chien C. C., and Chang H., Cell Tissue Res. , 411 (2006). 10.1007/s00441-005-0148-2 [DOI] [PubMed] [Google Scholar]
- 38. Franke R. P., Grafe M., Mittermayer C., and Drenckhahn D., Nature , 648 (1984). 10.1038/307648a0 [DOI] [PubMed] [Google Scholar]
- 39. Frangos J. A., McIntire L. V., and Eskin S. G., Biotechnol. Bioeng. , 1053 (1988). 10.1002/bit.260320812 [DOI] [PubMed] [Google Scholar]
- 40. Malek A. M. and Izumo S., J. Cell Sci. , 713 (1996). [DOI] [PubMed] [Google Scholar]
- 41. Seebach J., Dieterich P., Luo F., Schillers H., Vestweber D., Oberleithner H., Galla H. J., and Schnittler H. J., Lab. Invest. , 1819 (2000). 10.1038/labinvest.3780193 [DOI] [PubMed] [Google Scholar]
- 42. Cody N. A., Zietarska M., Filali-Mouhim A., Provencher D. M., Mes-Masson A. M., and Tonin P. N., BMC Med. Genomics , 34 (2008). 10.1186/1755-8794-1-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gaedtke L., Thoenes L., Culmsee C., Mayer B., and Wagner E., J. Proteome Res. , 4111 (2007). 10.1021/pr0700596 [DOI] [PubMed] [Google Scholar]
- 44. Ekert J. E., Johnson K., Strake B., Pardinas J., Jarantow S., Perkinson R., and Colter D. C., PLoS One , e92248 (2014). 10.1371/journal.pone.0092248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Meenach S. A., Tsoras A. N., McGarry R. C., Mansour H. M., Hilt J. Z., and Anderson K. W., Int. J. Oncol. , 1701 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Perard M., Tricot-Doleux S., Pellen-Mussi P., Meary F., and Pérez F., Bull Group Int. Rech. Sci. Stomatol. Odontol. , 42 (2011). [PubMed] [Google Scholar]