Abstract
Intravenous immunoglobulin (IVIg) is used in the therapy of various autoimmune and inflammatory diseases. Recent studies in experimental models propose that anti-inflammatory effects of IVIg are mainly mediated by α2,6-sialylated Fc fragments. These reports further suggest that α2,6-sialylated Fc fragments interact with DC-SIGN+ cells to release IL-33 that subsequently expands IL-4-producing basophils. However, translational insights on these observations are lacking. Here we show that IVIg therapy in rheumatic patients leads to significant raise in plasma IL-33. However, IL-33 was not contributed by human DC-SIGN+ dendritic cells and splenocytes. As IL-33 has been shown to expand basophils, we analyzed the proportion of circulating basophils in these patients following IVIg therapy. In contrast to mice data, IVIg therapy led to basophil expansion only in two patients who also showed increased plasma levels of IL-33. Importantly, the fold-changes in IL-33 and basophils were not correlated and we could hardly detect IL-4 in the plasma following IVIg therapy. Thus, our results indicate that IVIg-induced IL-33 is insufficient to mediate basophil expansion in autoimmune patients. Hence, IL-33 and basophil-mediated anti-inflammatory mechanism proposed for IVIg might not be pertinent in humans.
Intravenous immunoglobulin (IVIg) is a therapeutic preparation of normal pooled immunoglobulin G (IgG) obtained from the plasma of several thousand healthy donors. High-dose IVIg (1–2 g/kg) is widely used in the treatment of various autoimmune and inflammatory diseases including Kawasaki disease, idiopathic thrombocytopenic purpura, Guillain-Barré syndrome, chronic inflammatory demyelinating polyneuropathy, myasthenia gravis, autoimmune blistering diseases, inflammatory myopathies, graft versus host disease and others1,2,3,4. The cellular and molecular mechanisms of action of IVIg in these diverse diseases remain incompletely understood. However, available evidence both from experimental and clinical studies provide an indicator that IVIg could benefit these diverse diseases via several mutually non-exclusive mechanisms2,5,6,7,8,9,10. These mechanisms include inhibition of activation and functions of innate immune cells such as dendritic cells (DCs), monocytes, macrophages and neutrophils; inhibition of pathogenic effector T cells such as Th1 and Th17 cells; expansion of regulatory T cells (Tregs); modulation of B cell responses; and inhibition of complement pathways. In addition, IVIg has been shown to inhibit inflammatory cytokines and to augment anti-inflammatory molecules such as IL-10 and IL-1 receptor antagonist11,12,13,14,15,16,17,18,19,20,21.
IgGs are glycoproteins and contain fragment antigen-binding (Fab) regions that recognize antigens, and fragment crystallizable (Fc) regions that exert effector functions upon binding to Fcγ receptors. The Fc fragments are glycosylated at Asn297 and recent studies in animal models advocate that anti-inflammatory effects of IVIg are mediated by a small fraction of antibodies that contain terminal α2,6-sialylated glycans at Asn297. It was proposed that α2,6-sialylated Fc fragments interact with dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin-positive (DC-SIGN+) innate cells to release IL-33, which subsequently expands IL-4-producing basophils22. However, translational insights on these observations are lacking. Therefore, we investigated whether high-dose IVIg therapy induces IL-33 production in autoimmune patients, which in turn would mediate basophil expansion and IL-4 responses.
Results
IVIg therapy induces IL-33 in autoimmune patients
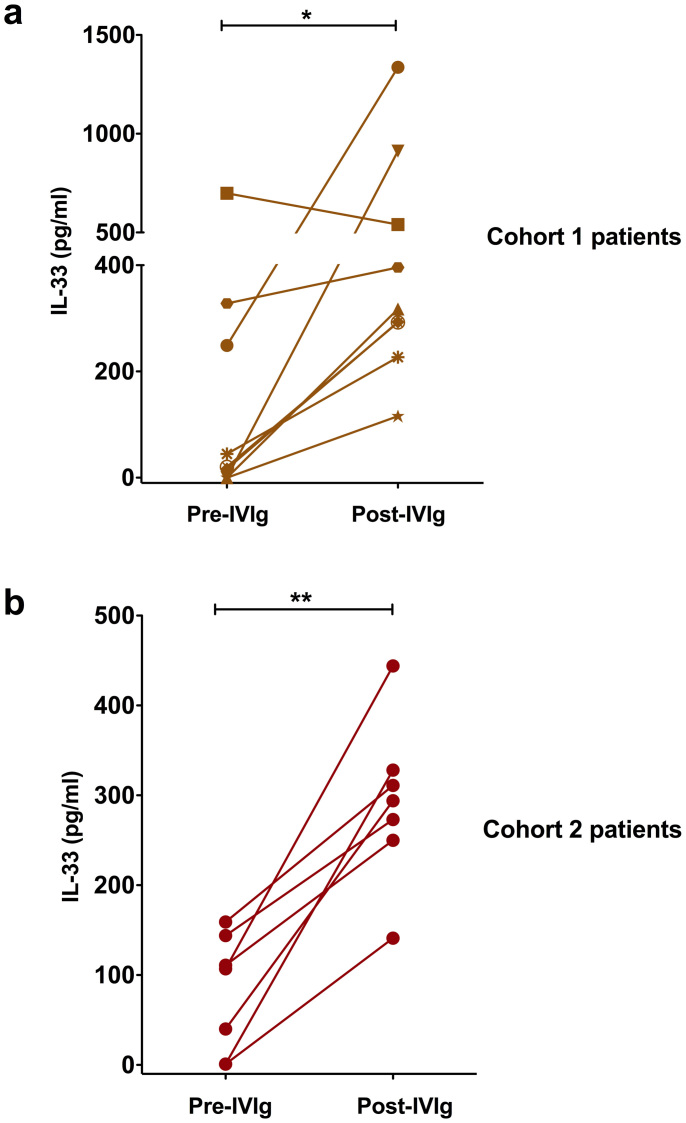
Previous work on the role of IL-33 in IVIg-mediated anti-inflammatory effects was performed in K/BxN serum-induced murine arthritis model. It should be noted that IVIg is not recommended for rheumatoid arthritis due to its inefficacy to relieve inflammation4. Therefore, K/BxN serum-induced murine arthritis model might not provide factual image of the mechanisms of IVIg in autoimmune patients. Earlier studies have indicated that IVIg therapy benefits patients with inflammatory myopathies1,4. Therefore, by using heparinized blood samples of these patients (cohort 1 patients), we first investigated the repercussion of IVIg therapy on the induction of IL-33. We found that, out of nine patients, six had minimal level of plasma IL-33 prior to IVIg therapy. The pre-IVIg plasma level of IL-33 was in the range of 150.75 ± 79.52 pg/ml (n = 9) (Fig. 1a). Following IVIg therapy, with an exception of one patient, all remaining patients had significant raise in plasma IL-33 and was in the range of 492.23 ± 130.30 pg/ml (n = 9) (Fig. 1a). However, the increase in IL-33 following IVIg therapy was heterogeneous and was varying from 1.2 to 911-fold.
Figure 1. Consequence of IVIg therapy in autoimmune patients on the plasma level of IL-33.
(a) Heparinized blood samples were obtained from nine patients with inflammatory myopathies (Cohort 1 patients) before (Pre-IVIg) and 2-3 days after initiation of IVIg therapy (Post-IVIg). IL-33 (pg/ml) in the plasma was measured by ELISA. Each symbol in the graph represents individual patient. (b) IL-33 in the plasma of four inflammatory myopathies and three anti-neutrophil cytoplasmic antibody-associated vasculitis patients (Cohort 2 patients) before and post-IVIg therapy. The statistical significance as determined by two-tailed Student-t-test is indicated, where *, P < 0.05; **, P < 0.01.
To confirm these results, we analyzed the plasma samples from another cohort of patients with inflammatory myopathies (n = 4) or anti-neutrophil cytoplasmic antibody-associated vasculitis (n = 3) (cohort 2 patients). Importantly, these patients also showed significant increase in plasma IL-33 following IVIg therapy (Fig. 1b) thus confirming the results obtained with cohort 1 patients. The pre-IVIg plasma level of IL-33 was 80.43 ± 24.93 pg/ml (n = 7) that increased to 291.58 ± 34.40 pg/ml following IVIg therapy. Together, these results indicate that irrespective of pathologies, IVIg therapy in patients leads to increased plasma level of IL-33.
IVIg-induced IL-33 is not associated with an expansion of basophils
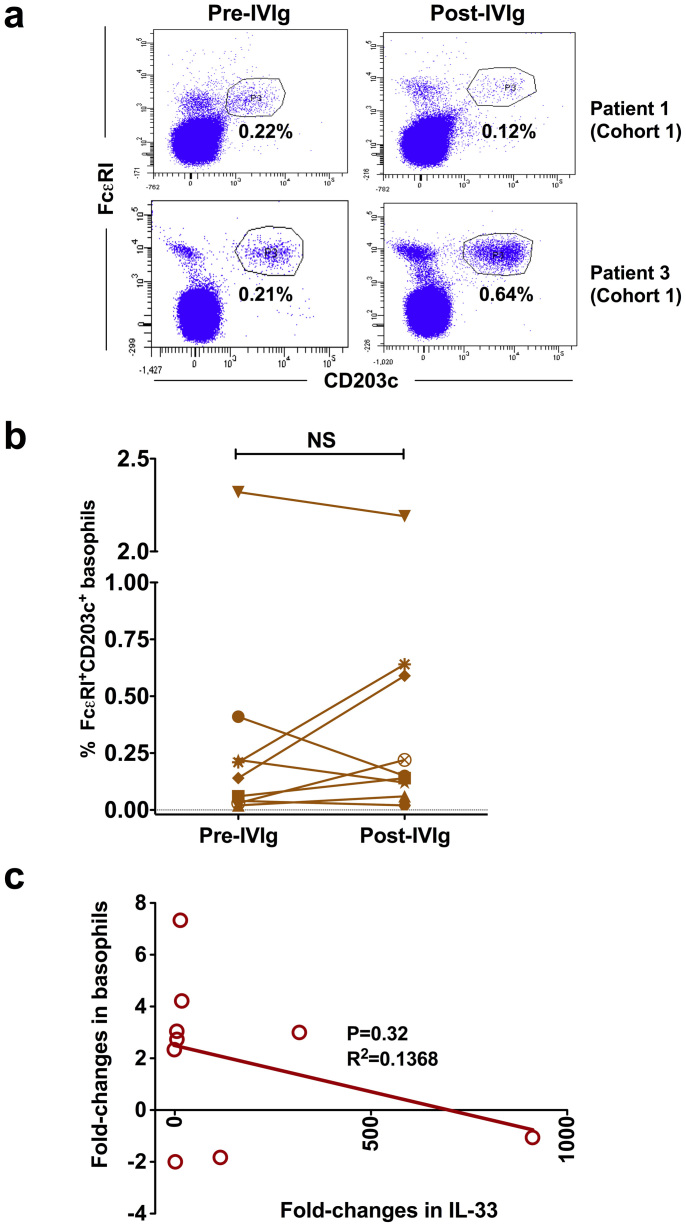
Basophils play a crucial role in the induction of Th2 responses23,24. Recent data from K/BxN serum-induced murine arthritis model suggest that IVIg-induced IL-33 promotes basophil expansion22. Therefore, we investigated changes in the circulating basophils following IVIg therapy in cohort 1 patients. Basophils were identified based on the expression of FcεRI and CD203c (Fig. 2a)25. In contrast to the results from murine model, we found that IVIg therapy leads to basophil expansion only in two patients who also showed increased plasma level of IL-33 (Fig. 2b). In other patients, basophils were either declined or unaltered. The changes in the proportion of basophils in the circulation following IVIg therapy were not statistically significant. Importantly, the fold-changes in IL-33 and basophils were not correlated (Fig. 2c). Also contrary to previous report22, we could hardly detect IL-4 in the plasma of patients following IVIg therapy. Thus, these results demonstrate that IVIg therapy in patients does not lead to an expansion of basophils. Of note, a recent data from murine models of collagen antibody-induced arthritis and K/BxN serum transfer arthritis also reveal that therapeutic effect of IVIg is independent of sialylation and basophils26.
Figure 2. Changes in the proportion of circulating basophils of autoimmune patients following IVIg therapy.
Heparinized blood samples were obtained from cohort 1 patients with inflammatory myopathies before (Pre-IVIg) and 2–3 days after initiation of IVIg therapy (Post-IVIg). (a) Representative dot-plots showing basophils from cohort 1 patients gated positive for FcεRI and CD203c (b) Modulation of circulating basophils following IVIg therapy (n = 9). Basophils were analyzed in the whole blood by flow cytometry. The statistical significance as determined by two-tailed Student-t-test is indicated, where NS, non-significant. (c) The correlation between fold-changes in IL-33 and basophils following IVIg therapy.
DC-SIGN-positive human innate cells do not produce IL-33 upon IVIg exposure
DC-SIGN+ innate cells (or SIGN-R1+ cells in the murine spleen) were proposed to produce IL-33 upon interaction with α2,6-sialylated Fc fragments of IVIg22. By generating humanized DC-SIGN-transgenic mice, the authors found that these transgenic mice express DC-SIGN on DCs, macrophages and monocytes in the blood, bone marrow and spleen. Importantly, higher percentage of monocytes in these transgenic mice expressed DC-SIGN22.
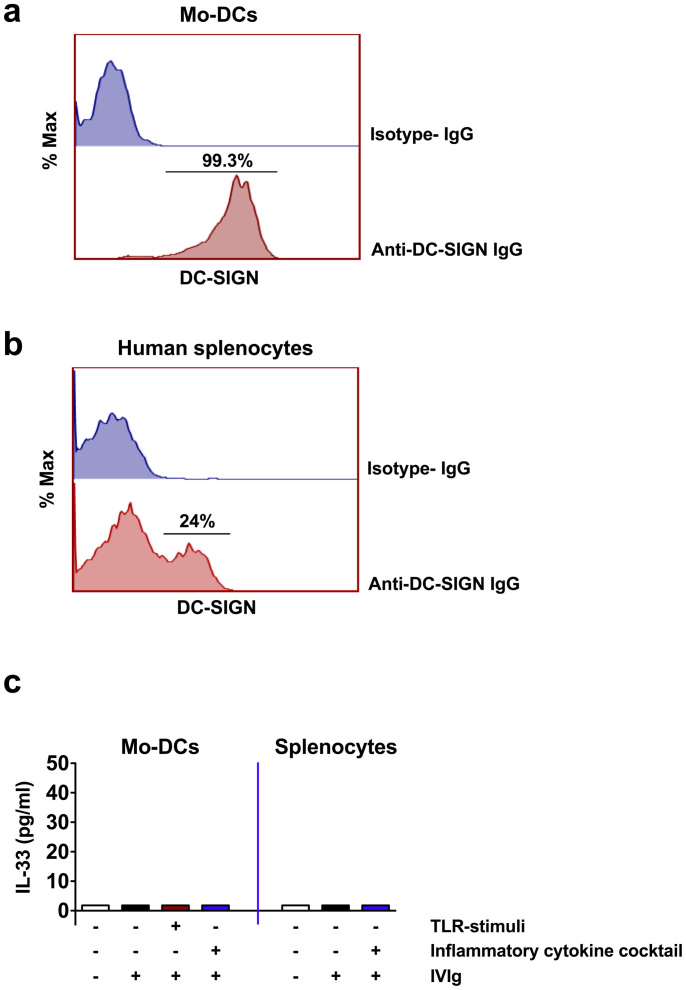
We analyzed the expression of DC-SIGN in human myeloid cells. Contrary to humanized DC-SIGN-transgenic mice, circulating human monocytes did not express DC-SIGN whereas its expression on macrophages was restricted to M2 type macrophages wherein up to 28% cells were positive for DC-SIGN. We could observe high expression of DC-SIGN (≈100%) only in monocyte-derived DCs (Mo-DCs) (Fig. 3a). In the human spleen, up to 24% splenocytes were positive for DC-SIGN (Fig. 3b).
Figure 3. Effect of IVIg on the IL-33 production from DC-SIGN+ human innate cells.
(a and b) Histograms showing the expression of DC-SIGN by healthy donor's monocyte-derived human dendritic cells (Mo-DCs) and splenocytes. (c) IVIg does not induce IL-33 from DC-SIGN+ human innate cells. Mo-DCs or human splenocytes (n = 5 donors) were exposed to IVIg either under non-inflammatory conditions or under inflammatory conditions (TLR-stimuli or inflammatory cytokine cocktail) for 48 hours. IL-33 in the culture supernatants was analyzed by ELISA.
Therefore, we explored if Mo-DCs secrete IL-33 upon IVIg treatment. In contrast to proposition by Ravetch and colleagues, we could detect secreted IL-33 from IVIg-exposed DC-SIGN+ Mo-DCs neither under non-inflammatory nor under inflammatory conditions (Fig. 3c). Similarly, despite the presence of DC-SIGN+ cells in the spleen, human splenocytes did not produce detectable levels of IL-33 upon IVIg exposure both under inflammatory and non-inflammatory conditions (Fig. 3c).
Discussion
Our results demonstrate that IVIg therapy induces IL-33 in autoimmune patients thus confirming the previous observation made in mice. However, IL-33 was not contributed either by splenic DC-SIGN+ cells or myeloid DCs. Also, the amount of IL-33 induced in the patients was not sufficient to expand basophils. It should be noted that the quantity of IL-33 protein induced in the mice following IVIg treatment was not presented in the previous report. In addition, significant amount of data on IVIg was indirect rather than direct demonstration of IVIg-mediated regulation of cytokine network22. Authors showed that IVIg induces about 12-fold increase in IL-33 mRNA level. However, the contribution of this increased IL-33 mRNA towards IL-33 protein is not clear. Considering five liters as total blood volume in adults, our results show that IVIg induces ≈2460 ± 650 ng of IL-33 (based on the data from cohort 1 patients). However, to demonstrate the role of IL-33 in IVIg-mediated anti-inflammatory effects, Anthony et al., injected 400 ng of IL-33 for four consecutive days22. As mouse weighing 25 g would have ≈1.5 ml of blood, based on the IL-33 data from patients, we could infer that the amount of exogenous IL-33 injected into the mice represents at least 540-times excess of IL-33 that otherwise induced by IVIg. This might explain why IVIg failed to induce expansion of basophils in the patients. Although in our study, patients' sample size was small, we included diseases such as inflammatory myopathies and vasculitis that were shown to benefit from IVIg therapy. Further investigations in a larger number of patients should confirm these observations.
The role of Fc-sialylation, DC-SIGN and Fcγ receptor IIB (FcγRIIB) in the anti-inflammatory effects of IVIg has been debated recently by several groups27. Mice and humans show wide variations in the expression pattern of FcγRs, and the phenotype and anatomical distribution of innate cells. Unlike mice, human innate cells express both activating FcγRIIA and inhibitory FcγRIIB. Therefore, the proposition that IVIg enhances FcγRIIB on effector macrophages of mice without having corresponding data on FcγRIIA might provide a biased picture on the mechanisms of IVIg. Gene array analysis could not confirm IVIg-mediated up-regulation of FcγRIIB in the patients with Kawasaki disease28. In line with this report, another recent study failed to demonstrate enhanced expression of FcγRIIB on monocytes following IVIg therapy in children with immune thrombocytopenia29. Also, FcγR polymorphisms did not predict response to IVIg in myasthenia gravis30. Although DC-SIGN promoter −336 A/G (rs4804803) polymorphism was associated with susceptibility of Kawasaki disease, this variant was found to be not associated with the occurrence of IVIg resistance31. Of note, treatment response in Kawasaki disease is apparently associated with sialylation levels of endogenous IgG but not therapeutic IVIg32. All these data thus questions the relevance of DC-SIGN-FcγRIIB pathway of anti-inflammatory mechanisms of IVIg in humans.
Several recent studies have challenged the concept of α2,6-sialylated Fc fragments-mediated anti-inflammatory mechanism of IVIg both in experimental models and in humans. IVIg could inhibit human Th17 cell differentiation and expansion independent of antigen presenting cells and hence independent of interaction of DC-SIGN and α2,6-sialylated Fc fragments13,14,15. Also, F(ab')2 fragments of IVIg exerted similar effects thus pointing towards dispensability of α2,6-sialylated Fc fragments in mediating the suppression of Th17 cells. We have demonstrated that DC-SIGN and α2,6-sialylated Fc fragment interaction is dispensable for the anti-inflammatory activity of IVIg on human DCs33. F(ab')2 fragments but not Fc fragments of IVIg were shown to mediate Treg expansion by inducing cyclooxygenase-2-mediated prostaglandin E2 secretion in human myeloid DCs and was dependent in part on DC-SIGN19. Similarly, sialylation-enriched F(ab')2 fragments could inhibit interferon-α production from toll-like receptor (TLR)7 and TLR9 stimulated human plasmacytoid DCs, although sialic acid itself was not required34.
In the previous reports, Ravetch and colleagues enriched sialic acid-containing IgG-Fc by using sialic acid-specific lectin Sambucus nigra agglutinin-based affinity fractionation22,35,36,37. However, by using same fractionation method, Guhr et al., showed that IVIg fractions depleted for the sialylated antibody fraction exert benefits in a murine model of passive-immune thrombocytopenia similar to that of intact IVIg. However, sialic acid-enriched IVIg fraction failed to enhance platelets count in this model38. Similar sialic-acid independent anti-inflammatory mechanisms were also reported in murine herpes simplex virus encephalitis model39. Further, Käsermann and colleagues showed that lectin fractionation of IVIg results in increased sialylation of Fab fragments but not Fc fragments. By using human whole blood stimulation assay either with lipopolysaccharide or phytohaemagglutinin, they further showed that anti-inflammatory effects of IVIg is associated with F(ab')2 fraction of IVIg40. In animal models of immune thrombocytopenia and multiple sclerosis, the beneficial effects of IVIg were independent of Fc or F(ab')2 -sialylation and FcγRIIB41,42,43,44. Based on these results, it was suggested that genetic background of the mice and dose of IVIg are the critical factors that determine the role of FcγRIIB in IVIg-mediated beneficial effects. In line with these observations, two studies have failed to demonstrate the direct interaction between sialylated IgG Fc fragment and DC-SIGN45,46. These data thus point out that α2,6-sialylated Fc fragment-DC-SIGN-FcγRIIB mechanism merely represents one of the several anti-inflammatory mechanisms of IVIg that were reported. Therefore, this anti-inflammatory pathway of IVIg might be operational in certain pathologies and experimental models and might not be considered as a universal mechanism.
It was proposed that in humanized DC-SIGN-transgenic mice, DC-SIGN+ innate cells such as monocytes, macrophages and DCs produce IL-33 upon interaction with α2,6-sialylated Fc fragments of IVIg22. Recent reports show that IL-33 is an important player for the promotion of Th2 responses and activated DCs are one of the sources of this cytokine47,48. We found that unlike monocytes from humanized DC-SIGN-transgenic mice that were highly positive for DC-SIGN, human monocytes hardly express DC-SIGN. Further, human Mo-DCs despite expressing DC-SIGN, failed to produce IL-33 when exposed to IVIg either under non-inflammatory or inflammatory conditions. In wild type mice, it was suggested that α2,6-sialylated Fc fragments bind to SIGN-R1 expressed on splenic marginal zone macrophages35. Marginal zone macrophages are absent in human spleen and data from humans show that spleen is dispensable for the anti-inflammatory effects of IVIg. In line with this concept, by using a passive model of induced immune thrombocytopenia, it was shown that IVIg is fully functional in splenectomized mice although this report supported the sialic acid and SIGN-R1-dependent mechanisms of IVIg49. We found that despite the presence of DC-SIGN+ innate cells in the human spleen, IVIg could not induce IL-33 from the splenocytes. All these data indicate that spleen and DC-SIGN+ cells are dispensable for IVIg-mediated IL-33 induction in humans. Thus, the source of IL-33 in humans following IVIg therapy remains elusive. As IVIg is known to cause apoptosis of cells, we suggest that secondary necrosis of late stage apoptotic cells could release IL-3350,51,52. This process might depend on the signals provided by anti-Fas IgG or anti-Siglec IgG in the IVIg preparations rather than the repercussion of interaction of α2,6-sialylated Fc fragments with DC-SIGN53,54. In addition, IL-33 is also constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo55.
Methods
Patients
All experiments were performed in accordance with relevant guidelines and regulations. We obtained heparinized blood samples of nine patients (cohort 1 patients) with inflammatory myopathies (Table 1). Patients were aged 49.1 ± 15.2 years and include two men. Blood samples were obtained before and 2–3 days following initiation of IVIg therapy (CLAIRYG®, Laboratoire Français du Fractionnement et des Biotechnologies, France). Informed consent was obtained from all the patients. The study was approved by CPP-Ile-de-France VI, Groupe Hospitalier Pitié-Salpêtrière, Paris. In addition, we also analyzed plasma samples of seven rheumatic patients (cohort 2 patients) before and 2–3 days post-IVIg therapy (TEGELINE®, Laboratoire Français du Fractionnement et des Biotechnologies). The patients were aged 47 ± 5.8 years (four men) and include inflammatory myopathies and anti-neutrophil cytoplasmic antibody-associated vasculitis (Table 1).
Table 1. Summary of data for autoimmune rheumatic patients.
| Cohort 1 patients | |||||
|---|---|---|---|---|---|
| Number | Disease | Age (years) | Sex | IVIg | Additional treatments |
| 1 | Polymyositis | 59 | F | CLAIRYG® 1 g/kg | Methylprednisolone |
| 2 | Anti-SRP associated necrotizing myopathy | 27 | F | CLAIRYG® 1 g/kg | Prednisone, Methotrexate |
| 3 | Anti-HMGCR associated necrotizing myopathy | 62 | F | CLAIRYG® 0.5 g/kg | Prednisone, Methotrexate |
| 4 | Anti-HMGCR associated necrotizing myopathy | 61 | F | CLAIRYG® 1 g/kg | Prednisone, Methotrexate |
| 5 | Dermatomyositis | 52 | F | CLAIRYG® 1 g/kg | Prednisone, Methotrexate |
| 6 | Polymyositis associated with mixed connective tissue disease and Sjögren's syndrome | 41 | F | CLAIRYG® 1 g/kg | Prednisone, Methotrexate |
| 7 | Anti-SRP associated necrotizing myopathy | 40 | M | CLAIRYG® 1 g/kg | Prednisone, Methotrexate |
| 8 | Anti-Mi2 associated unclassified myositis | 30 | M | CLAIRYG® 1 g/kg | Prednisone, Methotrexate |
| 9 | Polymyositis and probable associated Sjögren's syndrome | 70 | F | CLAIRYG® 1 g/kg | Prednisone, Methotrexate |
SRP, Signal Recognition Particle; HMGCR, 3-hydroxy-3-methylglutaryl-CoA reductase.
SRP, Signal Recognition Particle; HMGCR, 3-hydroxy-3-methylglutaryl-CoA reductase.
Analysis of basophils
Red blood cells (RBCs) from heparinized blood samples of cohort 1 patients were depleted by using HetaSepTM (Stemcell Technologies Sarl, France) and nucleated cell suspension was obtained. Basophils were analyzed in RBC-depleted cell suspension by flow cytometry (LSR II, BD Biosciences, France) using fluorochrome-conjugated monoclonal antibodies to FcεRI (Miltenyi Biotec, France) and CD203c (eBioscience, France). Data were analyzed by FACSDiva™ software (BD Biosciences).
Generation of monocyte-derived DCs
Buffy coats from the healthy donors were purchased from Centre Necker-Cabanel, Etablissement Français du Sang (EFS), Paris, France. Institut National de la Santé et de la Recherche Médicale-EFS ethical committee permission (N°12/EFS/079) was obtained for the use of buffy coats of healthy donors. Peripheral blood mononuclear cells (PBMCs) were purified from the buffy coats by density gradient centrifugation using Ficoll-paque PREMIUM (GE healthcare, France). CD14+ monocytes were isolated from PBMCs by using CD14 microbeads (Miltenyi Biotec). Purified monocytes were then cultured for 6 days in RPMI-1640 medium plus 10% fetal calf serum (FCS) containing cytokines GM-CSF (1000 IU/106 cells) and IL-4 (500 IU/106 cells) (both from Miltenyi Biotec) to obtain DCs56. The purity of DCs was >98%. DC-SIGN expression on Mo-DCs was examined by flow cytometry using fluorochrome-conjugated monoclonal antibodies (BD Biosciences) and data were analyzed by FACSDiva™ and FlowJo softwares (Tree Star, USA).
Isolation of human splenocytes
The remnant human spleen sections from individuals submitted for pathological diagnosis were obtained from service d'anatomie pathologique, Hôpital Européen Georges Pompidou, Paris, France. Only healthy spleen tissues were used for the research purpose. Since the study did not require additional sampling, an approval from an ethics committee was not required under French law according to the article L.1121-1 of the public health code. The article states that: The research organized and performed on human beings in the development of biological knowledge and medical research are permitted under the conditions laid down in this book and are hereinafter referred to by the term “biomedical research”. The article further states that it does not imply under conditions: For research in which all actions are performed and products used in the usual way, without any additional or unusual diagnostic procedure or surveillance.
The spleen sections were collected in RPMI 1640 medium supplemented with 100 IU/ml penicillin, 100 μg/ml streptomycin, and 10% FCS. Single-cell suspension of splenocytes was obtained by mechanical disaggregation of spleen tissue pieces by using gentleMACS dissociator (Miltenyi Biotec) followed by filtration through 70-μm nylon membrane filter (BD Biosciences). Splenocytes were then subjected to Ficoll-Paque PREMIUM density gradient centrifugation to obtain mononuclear cells. DC-SIGN expression on the splenocytes was investigated by flow cytometry using fluorochrome-conjugated monoclonal antibodies and data were analyzed by FACSDiva™ and FlowJo softwares.
Stimulation of cells
Mo-DCs (0.5 × 106/ml) were cultured in RPMI 1640-10% FCS containing GM-CSF and IL-4 in a 12-well plate. The cells were then exposed to IVIg (25 mg/ml) for 48 hours to analyze the effect of IVIg on IL-33 production under non-inflammatory conditions. In parallel, Mo-DCs were stimulated with either TLR4 ligand lipopolysaccharide (100 ng/ml/0.5 × 106 cells) (Sigma-Aldrich, France) or inflammatory cytokine cocktail (10 ng/ml each of IL-1β, IL-6 and TNF-α, all from ImmunoTools, Germany)57. After four hours, IVIg was added and cultures were maintained for 48 hours to analyze the effect of IVIg on IL-33 production under inflammatory conditions.
Similarly, splenocytes (0.5 × 106/ml) were cultured in RPMI 1640-10% FCS for 48 hours either alone or with IVIg. In addition, splenocytes were also stimulated with inflammatory cytokine cocktail and IVIg was added to the cultures after four hours. The cultures were maintained for 48 hours.
Quantification of cytokines
IL-33 in the plasma samples of the patients and in cell-free culture supernatants was quantified by ELISA (R&D systems, France). IL-4 in the plasma was also measured by ELISA (R&D systems).
Statistical analysis
Data was analyzed using Prism 5 software (GraphPad software). Two-tailed Student's t-test was used to determine the statistical significance of the data. Values of P < 0.05 were considered as statistically significant.
Acknowledgments
This study was supported by Institut National de la Santé et de la Recherche Médicale (INSERM), Centre National de la Recherche Scientifique (CNRS), Université Pierre et Marie Curie, Université Paris Descartes and European Community's Seventh Framework Programme [FP7/2007–2013] under grant agreement HEALTH-2010.2.4.5-2 ALLFUN. We also thank Laboratoire Français du Fractionnement et des Biotechnologies, France for the support.
Footnotes
The authors declare no competing financial interests.
Author Contributions J.B. designed the research, M.S., C.S., P.H., M.S.M., E.S-V., L.G. & M.L. performed the experiments, M.S., P.H., M.S.M., S.V.K. & J.B. analyzed the data, Y.S., L.M. & O.B. provided blood samples of the patients, P.B. provided the spleen tissues, J.B. wrote the paper and all authors reviewed and approved the manuscript.
References
- Dalakas M. C. Intravenous immunoglobulin in autoimmune neuromuscular diseases. JAMA 291, 2367–2375 (2004). [DOI] [PubMed] [Google Scholar]
- Kazatchkine M. D. & Kaveri S. V. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N. Engl. J. Med. 345, 747–755 (2001). [DOI] [PubMed] [Google Scholar]
- Arnson Y., Shoenfeld Y. & Amital H. Intravenous immunoglobulin therapy for autoimmune diseases. Autoimmunity 42, 553–560 (2009). [DOI] [PubMed] [Google Scholar]
- Bayry J., Negi V. S. & Kaveri S. V. Intravenous immunoglobulin therapy in rheumatic diseases. Nat. Rev. Rheumatol. 7, 349–359 (2011). [DOI] [PubMed] [Google Scholar]
- Schwab I. & Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat. Rev. Immunol. 13, 176–189 (2013). [DOI] [PubMed] [Google Scholar]
- Seite J. F., Shoenfeld Y., Youinou P. & Hillion S. What is the contents of the magic draft IVIg? Autoimmun. Rev. 7, 435–439 (2008). [DOI] [PubMed] [Google Scholar]
- Tha-In T., Bayry J., Metselaar H. J., Kaveri S. V. & Kwekkeboom J. Modulation of the cellular immune system by intravenous immunoglobulin. Trends Immunol. 29, 608–615 (2008). [DOI] [PubMed] [Google Scholar]
- Ballow M. The IgG molecule as a biological immune response modifier: Mechanisms of action of intravenous immune serum globulin in autoimmune and inflammatory disorders. J. Allergy Clin. Immunol. 127, 315–323 (2011). [DOI] [PubMed] [Google Scholar]
- Jordan S. C., Toyoda M. & Vo A. A. Intravenous immunoglobulin a natural regulator of immunity and inflammation. Transplantation 88, 1–6 (2009). [DOI] [PubMed] [Google Scholar]
- Semple J. W. et al. Intravenous immunoglobulin prevents murine antibody- mediated acute lung injury at the level of neutrophil reactive oxygen species (ROS) production. PLoS One 7, e31357 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayry J. et al. Intravenous immunoglobulin abrogates dendritic cell differentiation induced by interferon-alpha present in serum from patients with systemic lupus erythematosus. Arthritis Rheum. 48, 3497–3502 (2003). [DOI] [PubMed] [Google Scholar]
- Aubin E., Lemieux R. & Bazin R. Indirect inhibition of in vivo and in vitro T-cell responses by intravenous immunoglobulins due to impaired antigen presentation. Blood 115, 1727–1734 (2010). [DOI] [PubMed] [Google Scholar]
- Maddur M. S. et al. Inhibition of differentiation, amplification, and function of human TH17 cells by intravenous immunoglobulin. J. Allergy Clin. Immunol. 127, 823–830 e821–827 (2011). [DOI] [PubMed] [Google Scholar]
- Maddur M. S., Kaveri S. V. & Bayry J. Comparison of different IVIg preparations on IL-17 production by human Th17 cells. Autoimmun. Rev. 10, 809–810 (2011). [DOI] [PubMed] [Google Scholar]
- Maddur M. S. et al. Inhibitory effect of IVIG on IL-17 production by Th17 cells is independent of anti-IL-17 antibodies in the immunoglobulin preparations. J. Clin. Immunol. 33, S62–S66 (2013). [DOI] [PubMed] [Google Scholar]
- Kessel A. et al. Intravenous immunoglobulin therapy affects T regulatory cells by increasing their suppressive function. J. Immunol. 179, 5571–5575 (2007). [DOI] [PubMed] [Google Scholar]
- De Groot A. S. et al. Activation of natural regulatory T cells by IgG Fc- derived peptide “Tregitopes”. Blood 112, 3303–3311 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massoud A. H. et al. Intravenous immunoglobulin attenuates airway inflammation through induction of forkhead box protein 3-positive regulatory T cells. J. Allergy Clin. Immunol. 129, 1656–1665 e1653 (2012). [DOI] [PubMed] [Google Scholar]
- Trinath J. et al. Intravenous immunoglobulin expands regulatory T cells via induction of cyclooxygenase-2-dependent prostaglandin E2 in human dendritic cells. Blood 122, 1419–1427 (2013). [DOI] [PubMed] [Google Scholar]
- Seite J. F., Goutsmedt C., Youinou P., Pers J. O. & Hillion S. Intravenous immunoglobulin induces a functional silencing program similar to anergy in human B cells. J. Allergy Clin. Immunol. 133, 181–188 e181–189 (2014). [DOI] [PubMed] [Google Scholar]
- Massoud A. H. et al. Dendritic cell immunoreceptor: a novel receptor for intravenous immunoglobulin mediates induction of regulatory T cells. J. Allergy Clin. Immunol. 133, 853–863 e855 (2014). [DOI] [PubMed] [Google Scholar]
- Anthony R. M., Kobayashi T., Wermeling F. & Ravetch J. V. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature 475, 110–113 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voehringer D. Protective and pathological roles of mast cells and basophils. Nat. Rev. Immunol. 13, 362–375 (2013). [DOI] [PubMed] [Google Scholar]
- Otsuka A. et al. Basophils are required for the induction of Th2 immunity to haptens and peptide antigens. Nat. Commun. 4, 1739, 10.1038/ncomms2740 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M. et al. Circulating human basophils lack the features of professional antigen presenting cells. Sci. Rep. 3, 1188, 10.1038/srep01188 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell I. K. et al. Therapeutic effect of IVIG on inflammatory arthritis in mice is dependent on the Fc portion and independent of sialylation or basophils. J. Immunol. 192, 5031–5038 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gunten S. et al. IVIG pluripotency and the concept of Fc-sialylation: challenges to the scientist. Nat. Rev. Immunol. 14, 349 (2014). [DOI] [PubMed] [Google Scholar]
- Abe J. et al. Gene expression profiling of the effect of high-dose intravenous Ig in patients with Kawasaki disease. J. Immunol. 174, 5837–5845 (2005). [DOI] [PubMed] [Google Scholar]
- Shimomura M. et al. Intravenous immunoglobulin does not increase FcγRIIB expression levels on monocytes in children with immune thrombocytopenia. Clin. Exp. Immunol. 169, 33–37 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett C. et al. Fcγ receptor polymorphisms do not predict response to intravenous immunoglobulin in myasthenia gravis. J. Clin. Neuromuscul. Dis. 14, 1–6 (2012). [DOI] [PubMed] [Google Scholar]
- Yu H. R. et al. DC-SIGN (CD209) promoter -336 A/G (rs4804803) polymorphism associated with susceptibility of Kawasaki disease. Sci. World J. 2012, 634835 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata S. et al. Treatment response in kawasaki disease is associated with sialylation levels of endogenous but not therapeutic intravenous immunoglobulin G. PLoS One 8, e81448 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayry J., Bansal K., Kazatchkine M. D. & Kaveri S. V. DC-SIGN and α2,6-sialylated IgG Fc interaction is dispensable for the anti-inflammatory activity of IVIg on human dendritic cells. Proc. Natl. Acad. Sci. U S A. 106, E24 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedeman A. E. et al. Contrasting mechanisms of interferon-alpha inhibition by intravenous immunoglobulin after induction by immune complexes versus Toll-like receptor agonists. Arthritis Rheum. 65, 2713–2723 (2013). [DOI] [PubMed] [Google Scholar]
- Anthony R. M., Wermeling F., Karlsson M. C. & Ravetch J. V. Identification of a receptor required for the anti-inflammatory activity of IVIG. Proc. Natl. Acad. Sci. U S A. 105, 19571–19578 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony R. M. et al. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science 320, 373–376 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko Y., Nimmerjahn F. & Ravetch J. V. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science 313, 670–673 (2006). [DOI] [PubMed] [Google Scholar]
- Guhr T. et al. Enrichment of sialylated IgG by lectin fractionation does not enhance the efficacy of immunoglobulin G in a murine model of immune thrombocytopenia. PLoS One 6, e21246 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakrishna C., Newo A. N., Shen Y. W. & Cantin E. Passively administered pooled human immunoglobulins exert IL-10 dependent anti- inflammatory effects that protect against fatal HSV encephalitis. PLoS Pathog. 7, e1002071 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasermann F. et al. Analysis and functional consequences of increased Fab- sialylation of intravenous immunoglobulin (IVIG) after lectin fractionation. PLoS One 7, e37243 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leontyev D. et al. Sialylation-independent mechanism involved in the amelioration of murine immune thrombocytopenia using intravenous gammaglobulin. Transfusion 52, 1799–1805 (2012). [DOI] [PubMed] [Google Scholar]
- Leontyev D., Katsman Y. & Branch D. R. Mouse background and IVIG dosage are critical in establishing the role of inhibitory Fcgamma receptor for the amelioration of experimental ITP. Blood 119, 5261–5264 (2012). [DOI] [PubMed] [Google Scholar]
- Othy S. et al. Intravenous gammaglobulin inhibits encephalitogenic potential of pathogenic T cells and interferes with their trafficking to the central nervous system, implicating sphingosine-1 phosphate receptor 1-mammalian target of rapamycin axis. J. Immunol. 190, 4535–4541 (2013). [DOI] [PubMed] [Google Scholar]
- Othy S. et al. Sialylation may be dispensable for reciprocal modulation of helper T cells by intravenous immunoglobulin. Eur. J. Immunol. (epub ahead of print) 10.1002/eji.201444440 (2014). [DOI] [PubMed] [Google Scholar]
- Crispin M., Yu X. & Bowden T. A. Crystal structure of sialylated IgG Fc: implications for the mechanism of intravenous immunoglobulin therapy. Proc. Natl. Acad. Sci. U S A. 110, E3544–3546 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Vasiljevic S., Mitchell D. A., Crispin M. & Scanlan C. N. Dissecting the molecular mechanism of IVIg therapy: the interaction between serum IgG and DC-SIGN is independent of antibody glycoform or Fc domain. J. Mol. Biol. 425, 1253–1258 (2013). [DOI] [PubMed] [Google Scholar]
- Grotenboer N. S., Ketelaar M. E., Koppelman G. H. & Nawijn M. C. Decoding asthma: translating genetic variation in IL33 and IL1RL1 into disease pathophysiology. J. Allergy Clin. Immunol. 131, 856–865 (2013). [DOI] [PubMed] [Google Scholar]
- Williams J. W. et al. Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat. Commun. 4, 2990, 10.1038/ncomms3990 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab I., Biburger M., Kronke G., Schett G. & Nimmerjahn F. IVIg- mediated amelioration of ITP in mice is dependent on sialic acid and SIGNR1. Eur. J. Immunol. 42, 826–830 (2012). [DOI] [PubMed] [Google Scholar]
- Bonilla W. V. et al. The alarmin interleukin-33 drives protective antiviral CD8+ T cell responses. Science 335, 984–989 (2012). [DOI] [PubMed] [Google Scholar]
- Cayrol C. & Girard J. P. The IL-1-like cytokine IL-33 is inactivated after maturation by caspase-1. Proc. Natl. Acad. Sci. U S A. 106, 9021–9026 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraldsen G., Balogh J., Pollheimer J., Sponheim J. & Kuchler A. M. Interleukin-33 - cytokine of dual function or novel alarmin? Trends Immunol. 30, 227–233 (2009). [DOI] [PubMed] [Google Scholar]
- Prasad N. K. et al. Therapeutic preparations of normal polyspecific IgG (IVIg) induce apoptosis in human lymphocytes and monocytes: a novel mechanism of action of IVIg involving the Fas apoptotic pathway. J. Immunol. 161, 3781–3790 (1998). [PubMed] [Google Scholar]
- von Gunten S. et al. Immunologic and functional evidence for anti-Siglec-9 autoantibodies in intravenous immunoglobulin preparations. Blood 108, 4255–4259 (2006). [DOI] [PubMed] [Google Scholar]
- Moussion C., Ortega N. & Girard J. P. The IL-1-like cytokine IL-33 is constitutively expressed in the nucleus of endothelial cells and epithelial cells in vivo: a novel ‘alarmin’? PLoS One 3, e3331 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddur M. S. et al. Human B cells induce dendritic cell maturation and favour Th2 polarization by inducing OX-40 ligand. Nat. Commun. 5, 4092, 10.1038/ncomms5092 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banchereau J. et al. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18, 767–811 (2000). [DOI] [PubMed] [Google Scholar]