Abstract
Oxaliplatin, a platinum-based anti-cancer drug, induces peripheral neuropathy as a side effect and causes cold hyperalgesia in cancer patients receiving anti-cancer chemotherapy. In oxaliplatin-treated mice, aluminum was accumulated in the dorsal root ganglia (DRG), and accumulated aluminum in DRG or other organs aggravated oxaliplatin-induced neuropathic pain. To investigate whether aluminum oxalate, which is the compound of aluminum and oxaliplatin, might be the peripheral neuropathy inducer, the withdrawal responses of mice to coldness, the expression of transient receptor potential ankyrin 1 and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assays in DRG were analyzed in mice administered with aluminum oxalate. In addition, the concentrations of aluminum in aluminum oxalate-treated mice were significantly increased compared to those of mice treated with aluminum chloride. To alleviate neuropathic pain, glutathione (GSH), known as an antioxidant and a metal chelator, was injected into oxaliplatin-treated mice. The concentrations of aluminum in the DRG were decreased by the chelation action of GSH. Taken together, behavioral and molecular analyses also supported that aluminum accumulation on the DRG might be a factor for neuropathic pain. This result also suggested that the aluminum chelation by GSH can provide an alleviatory remedy of neuropathic pain for cancer patients with oxaliplatin-induced neuropathic pain.
Keywords: Aluminum, glutathione, peripheral neuropathy, oxaliplatin, TRPA1
Introduction
Oxaliplatin, a third-generation platinum-based anti-cancer agent, is mainly used in the treatment of colorectal cancer [1]. It generates the cross-link DNA that leads to the replication failure of cancer cells, and has improved therapeutic effects [2,3]. However, peripheral neuropathy, a side effect of oxaliplatin, is extremely harsh for the patients and can cause them to give up treatment because of their low quality of life [4,5]. Peripheral neuropathy is caused by infection, diabetes, alcoholism, and neurotoxic chemicals such as anti-cancer drugs, and results in paresthesia such as tingling, electric shock-like and burning sensations in the hands and feet [6-9]. Oxaliplatin-induced peripheral neuropathy is divided into two kinds: the acute typeis characterized by cold hyperalgesia or allodynia right after treatment, often transiently, and the chronic type results in hardly reversible neuronal damage caused by continuous treatment [10]. Drugs used to treat peripheral neuropathy include pain relievers, anticonvulsants, antidepressants, and local anesthetics, but these just alleviate the symptoms to some extent regardless of the actual mechanism of neuropathy [11]. Ca-Mg infusions and vitamin E supplements have been tested as treatment components, but they could not completely remove the pain [4,12].
Aluminumis the most abundant metal on Earth’s surface, and most adults consume 3 mg of it every day on average [13,14]. When rats were exposed to aluminum, reactive oxygen species (ROS) increased, resulting in serious problems [15]. Not much is known about aluminum’s transporters or channels except for transferrin, due to its low bioavailability, but aluminum may be toxic in large quantities and has been reported to be associated with neurodegenerative diseases such as Alzheimer’s disease or Parkinson’s disease [16,17]. A compound such as deferoxamine can be used to chelate aluminum, but its side effects cannot be ignored [16].
There has been a report on the correlation between aluminum and oxaliplatin, which clarified that aluminum accumulated in the dorsal root ganglia (DRG) of oxaliplatin-treated mice [18]. This correlation was also revealed in the usage of oxaliplatin: aluminum-containing needles or infusion sets should not be used when injecting oxaliplatin due to the possibility of their degradation [19]. In this research, aluminum oxalate, a compound produced from the reaction of oxaliplatin and aluminum, was expected to be the main reason for the prohibition of simultaneous application.
The DRG, mentioned above, is a special part of the body in research on peripheral neuropathy. First, transient receptor potential ankyrin 1 (TRPA1) is expressed in the DRG [20]. TRPA1 is a calcium channel that is activated by noxious cold, and the expression of TRPA1 is increased by oxaliplatin and aluminum [18,20,21]. Second, DRG is vulnerable to external chemicals [22,23]. There are some barriers in our bodies, like the blood brain barrier (BBB) in the central nervous system (CNS) and the blood nerve barrier (BNB) in the peripheral nervous system (PNS), which block some outside substances, but the BNB is not as robust as the BBB [23]. Thus, DRG plays a key role in central and peripheral sensory regulation and is the therapeutic target of peripheral neuropathy [24].
Glutathione (GSH), the tripeptide yielded by combining cysteine, glutamic acid, and glycine, is mainly produced in the liver [25,26]. It is a powerful antioxidant that protects our bodies from ROS, anti-cancer agents, and heavy metals [27-29]. GSH is commonly used to alleviate neuropathic pain. When GSH was present in vitro, TRPA1 activation by oxaliplatin was reduced [30]. In addition, there have been studies in which GSH relieved oxaliplatin-induced neuropathy by preventing platinum from accumulating in the DRG [31,32].
In this paper, we investigated the influence of aluminum oxalate on peripheral neuropathy to evaluate the negative role of aluminum in oxaliplatin-induced peripheral neuropathy. Furthermore, we studied the effect of GSH on oxaliplatin-induced peripheral neuropathic mice in terms of aluminum concentrations and TRPA1 expression in the DRG.
Materials and methods
Animals
Four-week-old male ICR mice (20-22 g) (Daehan Bio Link, Eumseong, Korea) were acclimatized for one week in the normal conditions of the laboratory; feeds and water were freely accessible. All experiments were carried out in compliance with protocols approved by the Ethics Committee for Animal Experiments of the Sungkyunkwan University. All animals were adapted to the room’s environment for a week before the experiment (Approval Number: 12-37).
Chemicals
The control mice were treated with 5% dextrose solution (Joongwae Life Science Co., Ltd, Dangjin, Korea) and 0.9% NaCl (Daehan Pham Co., Ltd, Seoul, Korea), which are the vehicles of the chemicals used in the experiment. To determine the power of the aluminum oxalate complex, aluminum oxalate hydrate (4.61 mg/kg, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) was administered by intraperitoneal injection each day for five days, followed by five days of rest, which was repeated three times. Aluminum chloride hydrate (7 mg/kg, Sigma-Aldrich Korea Ltd, Yongin, Korea) and oxaliplatin (3 mg/kg, Boryung Co., Ltd, Seoul, Korea) were injected according to the same schedule [33]. With oxaliplatin as the positive control, the doses of aluminum oxalate and aluminum chloride were calculated to contribute equal amounts of aluminum to compare results by the two materials.
To examine the efficacy of GSH, oxaliplatin (3 mg/kg) was administered each day for five days, followed by five days of rest, for the first 20 days [34], and GSH (33 mg/kg, Kwangdong Pharmaceutical Co., Ltd, Seoul, Korea) was injected during the next 10 days at three-day intervals. All drugs were dissolved in appropriate solvents described in the provided usage, and the dates of administration are marked by black triangles (▲) in Figures 1A and 5A.
Figure 1.
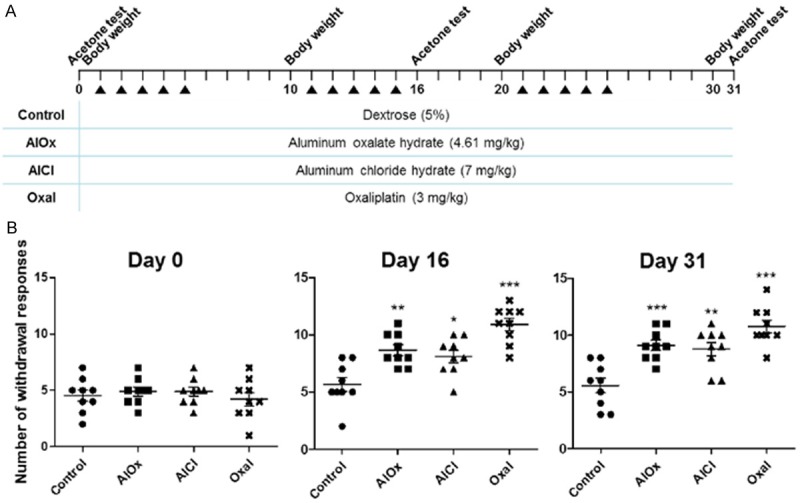
Cold hyperalgesia induced in mice treated with aluminum oxalate, aluminum chloride, or oxaliplatin. A. Injection schedule for each material by mouse group. Dates of acetone tests, body weight measurements, and the injections during 31 days are displayed at the top. The table shows the group names and concentrations corresponding to each chemical. B. The graphs of the acetone test results on days 0, 16, and 31. The x-axis is the group name, and the y-axis is the number of withdrawal responses of nine mice per group described by mean ± SEM. A p-value was calculated in comparison with the control group by one-way ANOVA: *P<0.05, **P<0.01, and ***P<0.001.
Figure 5.
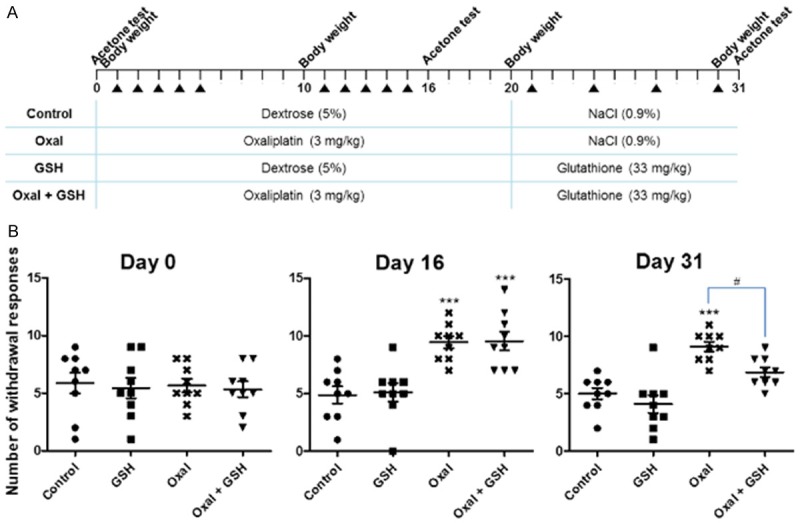
Alleviation of cold hyperalgesia by GSH. A. Injection schedule for each material by mouse group. Dates of acetone tests, body weight measurements, and the injections during 31 days are displayed at the top. The table shows the group names and concentrations corresponding to each chemical, and the treatment materials and injection intervals were changed after day 20. B. The graphs of the acetone test results on days 0, 16, and 31. The x-axis is the group name, and the y-axis is the number of withdrawal responses of nine mice per group described by means ± SEMs. Statistical significance was illustrated as ***P<0.001 compared to the Control group by one-way ANOVA, and as #P<0.05 compared to the Oxal group by one-way ANOVA.
Body weight
Body weight was measured every 10 days from the beginning day of the acetone test.
Acetone test
Each animal was covered with a transparent plastic box (8×8×5 cm) on the wire mesh floor. After 15 minutes or more for adaptation, a drop of acetone was sprayed onto a hind paw. We finished observing the reaction of the animal after 20 seconds if there were no withdrawal or paw-beating responses. If some reactions induced by cold appeared, monitoring lasted an additional 20 seconds. Responses were graded on a 4-point scale: 0, no response; 1, quick withdrawal, flick, or stamp of the paw; 2, prolonged withdrawal or repeated flicks (≥2) of the paw; 3, repeated flicking of the paw with licking directed at the ventral side of the paw. Acetone was applied three times alternately to each hind paw at 2-minute intervals. The final score was calculated by the addition of the six scores. All mice were randomized in this experiment [1,35]. The test was performed before starting the injections and repeated on days 16 and 31 to avoid the impact of the injection stress.
Quantitative real-time polymerase chain reaction
Animals were sacrificed after day 31, and the thoracic and lumbar DRGs were extracted. Total RNA was isolated using an easy-spinTM (DNA Free) Total RNA Extraction Kit (Intron Biotechnology, Co., Ltd., Seongnam, Korea) according to the manufacturer’s instructions. Then, cDNA was synthesized from 3 µg of total RNA using Moloney murine leukemia virus reverse transcriptase (Bioneer, Daejeon, Korea) and oligo dT primer. The reverse transcription conditions were five minutes at 65°C and 60 minutes at 37°C using a T100TM Thermal Cycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA). SYBR Premix Ex Taq (Takara Bio, Shiga, Japan) was used for qRT-PCR and the condition of the 40 reaction cycles was controlled by a Corbett Research Rotor-Gene 3000 (QIAGEN Korea Ltd., Heiden, Germany) as follows: 95°C for 10 seconds, 59°C for 15 seconds, and 72°C for 20 seconds. The house-keeping gene was β-actin and the primer sequences were as follows: β-actin, 5’-ACCCACACTGTGCCCATCTA-3’ (forward) [36], 5’-TAGAGCAACATAGCACAGCTTC-3’ (reverse); TRPA1, 5’-ACAATGCTCTGGAATGGGTTATC-3’ (forward), 5’-GTAGGAAGTTCATCCAGTAGAAG-3’ (reverse). The specific primers were designed referring to the National Center for Biotechnology Information: mouse β-actin [GenBank: NM00739] and mouse TRPA1 [GenBank: NM177781]. The control group was assigned a value of 1 to calculate the relative expression levels of TRPA1 of the experimental groups.
Tissue preparation
Mouse L5 DRGs were dissected, fixed with 4% paraformaldehyde (Affymetrix, OH, USA), washed with phosphate buffered saline (PBS), impregnated with 15% sucrose, and then with 30% sucrose, each overnight at 4°C except for the washing step. The tissues were embedded in OCT compound (Sakura Finetek Japan, Tokyo, Japan), frozen at -80°C, and sectioned into 7-µm thicknesses in a cryostat on HistoBond® adhesive microscope slides (Marienfeld-Superior, Lauda-Königshofen, Germany).
Immunofluorescence
Tissue sections were dried sufficiently, washed three times with PBS until the OCT compound melted away, and blocked using 5% fetal bovine serum (GE Healthcare Life Sciences HyClone Laboratories, UT, USA) and 20 mg/ml bovine serum albumin (MP Biomedicals, Santa Ana, CA, USA) in PBS for two hours at room temperature. Immunostaining was conducted using the following antibodies: primary antibody named RbpAb to TRPA1 (1:200, ab68847; Abcam plc, Cambridge, UK) and secondary antibody named goat pAb to Rb IgG (TRITC) (1:500, ab6718; Abcam). Tissues were incubated with primary antibody overnight at 4°C and then with secondary antibody for two hours at room temperature in a dark room. The slides were washed before and after staining with the secondary antibody. Sections were mounted with Vectashield mounting medium for fluorescence with 4’,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Inc., Burlingame, CA, USA), and examined under an LSM700 confocal microscope (Carl Zeiss, Germany).
TUNEL assay
To test the neuronal destruction, the cell death levels of the DRG tissues were studied with the use of the in situ cell death detection kit (Roche Applied Science, PA, USA), according to the manufacturer’s instructions. Tissue sections were mounted with Vectashield mounting medium for fluorescence with DAPI (Vector Laboratories, Inc.) and observed under a confocal microscope (Carl Zeiss).
Metal concentrations
To examine the concentrations of heavy metals in the body, the DRGs, brains, and sera were dissected from the animals. Serum was separated by centrifugation for 30 minutes at 3000 rpm and 4°C after collecting 1 ml of blood by cardiac puncture. The DRGs and brains were washed with Hanks’ Balanced Salt Solution (Welgene Inc., Daegu, Korea) and again with double distilled water immediately after dissection. Measurements of the concentrations of five kinds of metal (Hg, Pb, Al, Pt, and Cd) were made by the Korea Research Institute of Analytical Technology (Daejeon, Korea) with ICP-MS.
Statistical analysis
Data were expressed as means ± standard error of the mean (SEM) graphs using GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA, USA). Statistically significant differences were evaluated by one-way or two-way analyses of variance depending on the case of fluorescence intensity measurement, and Bonferroni’s test was conducted for acetone test as the post-test. Significance levels were set at *P<0.05, **P<0.01, ***P<0.001, and #P<0.05.
Results
Cold hyperalgesia appeared after administering aluminum oxalate
To see whether aluminum oxalate-treated mice havecold hyperalgesia, we performed an acetone test. The acetone test is a behavioral test used as a scale for cold hyperalgesia. The experimental schedule is shown in Figure 1A. The mice in the AlOx group were injected with aluminum oxalate hydrate (4.61 mg/kg), and those in the AlCl group were injected with aluminum chloride hydrate (7 mg/kg) to determine the difference in the impact of an aluminum-oxaliplatin compound versus another form of aluminum. Also, the Oxal group (3 mg/kg of oxaliplatin) was set as a positive control, and the Control group (5% dextrose) was set as a negative control. Mice treated with aluminum oxalate had a significantly increased withdrawal response as compared to the Control group on days 16 and 31 (P<0.01 and P<0.001, respectively; Figure 1B). The AlCl group also showed pain in response to cold contrast to the Control group, but the increased pain degree was slightly lower than that in the AlOx group (P<0.05 and P<0.01). The Oxal group, which was the positive control, showed the most severe pain on days 16 and 31 (P<0.001 for both days), and thus, the acetone test was an accurate way to determine cold hyperalgesia.
Aluminum oxalate increased the expression of TRPA1 in the DRG
Each group of mice was sacrificed after 31 days of inoculation according to Figure 1A, and we investigated the expression of TRPA1 in the DRG by quantitative real-time polymerase chain reaction (qRT-PCR) and immunofluorescence. The RNA purity was 1.7-1.9 when measured by spectrophotometer. The TRPA1 mRNA expression level of the AlOx group was almost 2-fold higher than that of the Control group (P<0.05), and it was very close to that of the Oxal group (Figure 2A). This means that aluminum oxalate caused neuropathic pain similar to that caused by oxaliplatin, because TRPA1 is regarded as a marker gene of oxaliplatin-induced neuropathy. The AlCl group also had higher TRPA1 expression than the Control group did, but the difference was statistically insignificant. With confocal microscopy, TRPA1 expression in the L5 DRG was confirmed at a protein level through immunofluorescence. In line with the qRT-PCR results, a strong red signal was detected in the AlOx and AlCl groups, even if it was weaker than that found in the Oxal group (Figure 2B). In contrast, the DRG section of the Control was stained very little.
Figure 2.
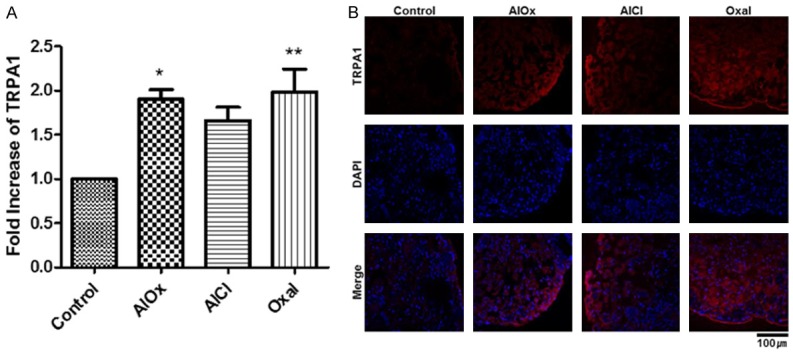
Hyper-expressed TRPA1 in mice treated with aluminum oxalate, aluminum chloride, or oxaliplatin. A. RNA expression levels of TRPA1, as analyzed by qRT-PCR. Total RNA was extracted fromthe DRGs of the Control (5% dextrose), AlOx (4.61 mg/kg), AlCl (7 mg/kg), and Oxal (3 mg/kg) groups. Statistical analyses were expressed as a graph of means ± SEMs (*P<0.05, **P<0.01). B. Immunofluorescence of TRPA1 in L5 DRG tissue. TRPA1 was red-colored with yellow arrows and nuclei were DAPI-stained in blue. The DRG tissue was magnified 40× with a confocal microscope, and the length of the scale bar is 100 μm.
Aluminum oxalate induced severe cell death in DRG tissue
To determine the toxicity of aluminum oxalate to the DRG, we carried out terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assays at the end of every injection and acetone test. TUNEL-stained cells (green) were the most common in the Oxal group (Figure 3A). The AlOx and AlCl groups also show signs of cell death, and the degree was slightly higher in the AlOx group. The signal was very small in the Control group. This means that the long-term injection of oxaliplatin accompanied the destruction of the DRG and caused neuropathic pain, and that this pain was also induced by aluminum oxalate. In addition, the general toxicity of the treatment materials was measured by checking body weights (Figure 3B). There were no significant differences among the Control, AlOx, and AlCl groups. However, the food intake and hair condition changed significantly in the group of mice treated with oxaliplatin, in accordance with decreasing average 4 g of body weight compared to 3 other groups (P<0.001).
Figure 3.
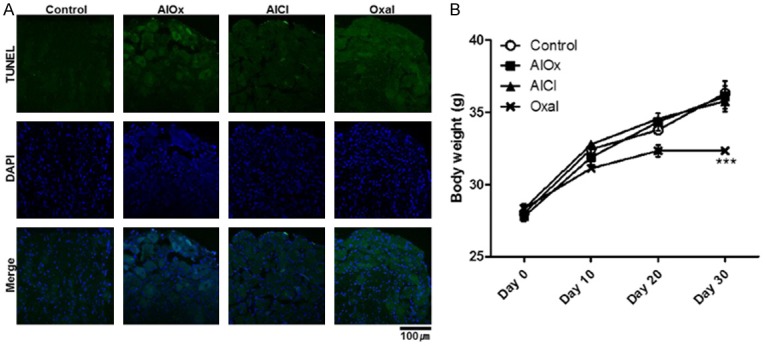
Increased cell death of DRG tissue and general toxicity. A. The cell death of DRG was examined by TUNEL assay. TUNEL-positive cells were green-colored with Alexa 488 and nuclei were blue-colored. The DRG tissue was magnified 40× with a confocal microscope, and the length of the scale bar is 100 μm. B. Body weight of mice every 10 days. The graph shows the means ± SEMs with ***P<0.001, which was analyzed by two-way ANOVA.
Aluminum oxalate accumulated the greatest amount of aluminum in the DRG and serum, but not in the brain
To confirm the correlation between the aluminum concentrations in the body and peripheral neuropathy, inductively coupled plasma mass spectrometry (ICP-MS) was performed on the aluminum (Al) and platinum (Pt) intended for the tissues of the four mice groups. The DRG of the AlOx group showed an increased concentration of Al (8.73 mg/kg), which was greater than those of the other groups such as the AlCl (3.52 mg/kg) and Oxal (0.49 mg/kg) groups (Figure 4). Specifically, it was possible to detect the accumulation of Al in the DRG of the Oxal group that had not been treated with Al. This means that oxalate acted as an aluminum attractant, which corresponds with the acetone test results. However, in the brain, all three of the groups treated with chemicals showed lower concentrations of Al than the Control group did (4.36 mg/kg). Foreign materials were not likely to flow into the brain due to the BBB and instead were eliminated from the brain. The aluminum accumulation pattern of the serum was similar to that of the DRG. Although the concentrations of Pt in both the DRG and serum of the Oxal group could be detected, this was not possible in the brain because of the BBB.
Figure 4.
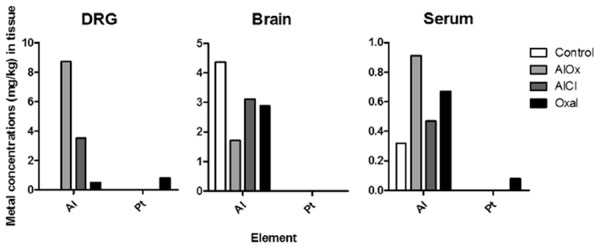
Metal concentrations of murine DRG, brain, and serum. Accumulated amounts of aluminum and platinum in the body were measured by ICP-MS, and concentrations were calculated by the weight of metal/tissue. The table at the bottom shows the limits of quantification of ICP-MS, and any amounts lower than these could not be measured.
GSH alleviated cold hyperalgesia
To investigate the effects of GSH, which is used to mitigate the side effects of anticancer drugs, acetone tests were carried out. Figure 5A shows the schedule of injections, acetone tests, and body weight measurements with the test substances administered for each group. The mice treated with GSH after oxaliplatin showed a significant decline in their withdrawal responses on day 31 when compared to the group of mice treated only with oxaliplatin (Figure 5B). The Oxal and Oxal + GSH groups had similar reaction increases on day 16, because those groups were treated only with oxaliplatin up to day 16. However, on day 31, a noteworthy difference was observed in the results between two groups treated with the vehicle alone or with GSH. This suggests that GSH functioned as a palliative of cold hyperalgesia, the main side effect of oxaliplatin. Mice that were only treated with GSH did not display significant differences from the Control group.
GSH eased the overexpression of TRPA1 by oxaliplatin
Both qRT-PCR and immunofluorescence were performed to determine the expression levels of TRPA1 in the DRG after every injection and behavioral analysis. First, the RNA expression of TRPA1 in the Oxal group showed a significant difference when compared to the Control, which was designated as 1 (P<0.05), but that of the Oxal + GSH group did not (Figure 6A). Furthermore, the protein expression of TRPA1 in L5 DRG showed exactly the same pattern with the qRT-PCR results (Figure 6B).
Figure 6.
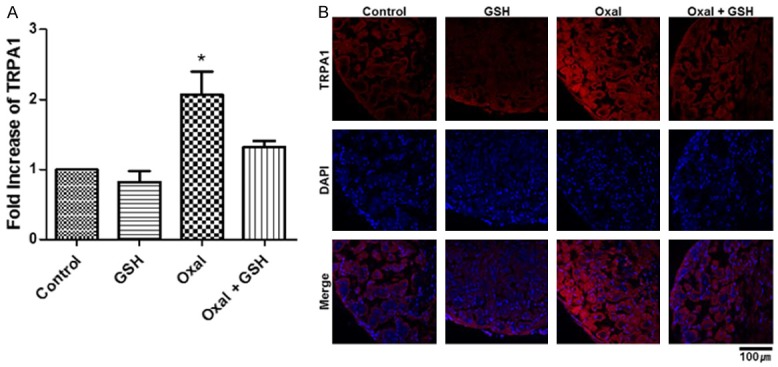
Mitigation of oxaliplatin-derived TRPA1 overexpression by treatment with GSH. A. Level of TRPA1 RNA expression. Statistical analysis was conveyed as a graph of means ± SEMs (*P<0.05). B. Immunofluorescence of TRPA1 protein in DRG tissue. TRPA1 was stained with red color and indicated with yellow arrows, and nuclei were DAPI-stained. The DRG section was magnified 40× with a confocal microscope, and the length of the scale bar is 100 μm.
GSH reduced the degree of cell death in DRG, which induced by oxaliplatin
After the 31 days of the experiment described in Figure 5A, the mice were sacrificed to examine how much influence GSH had on the survival rate of the DRG cells. GSH was found to reduce oxaliplatin-induced cell death, and it did not have a negative effect on DRG when administered alone, as confirmed by TUNEL assay (Figure 7A). This expresses that GSH slowed the DRG cell death, but the degree was not as effective as reducing the expression of TRPA1. Figure 7B illustrates the body weight recovery effect of GSH. There were significant differences (P<0.001) between the Control group and both the Oxal and Oxal + GSH groups on day 20, when the GSH treatment had not yet started, but the Oxal + GSH group was thoroughly recovered and did not exhibit a difference from the Control group after day 30, when the injections were completed. The administration of GSH alone did not affect body weight.
Figure 7.
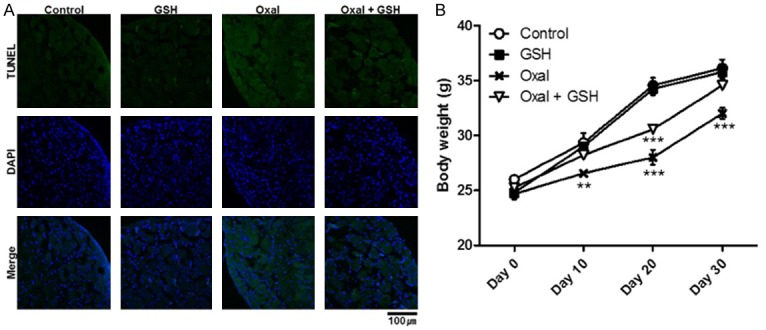
Slightly decreased cell death of DRG tissue and general toxicity. A. The cell death of DRG was evaluated by TUNEL assay. TUNEL-positive cells were green-colored with Alexa 488 and nuclei were blue-colored. The DRG section was magnified 40× with a confocal microscope, and the length of the scale bar is 100 mm. B. Body weights of mice every 10 days. The graph shows means ± SEMs with **P<0.01 and ***P<0.001, as analyzed by two-way ANOVA.
GSH decreased the concentrations of aluminum and platinum in the bodies of the mice
To determine the correlation between the amount of aluminum in the body and peripheral neuropathy, ICP-MS was carried out, targeting the DRG, brain, and serum, after all injections were completed. Analysis showed that oxaliplatin built up aluminum in the DRG, while GSH treated group showed that the amount of aluminum reduced more than 0.1 mg/kg of it (Figure 8). This also supports that the aluminum deposited in the DRG was correlated with neuropathic pain and that the dispersion of aluminum by GSH was associated with pain relief. Platinum was decreased relatively less (0.03 mg/kg). On the other hand, aluminum was reduced in the brain by oxaliplatin (3.65 mg/kg), and was further reduced by GSH (6.98 mg/kg). The aluminum concentrations in the serum were similar to those in the DRG. We could not detect platinum in the brain or serum.
Figure 8.
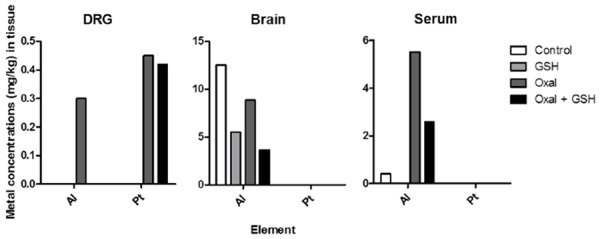
Aluminum concentrations of murine DRG, brain, and serum were reduced by GSH administration. The graphs show the accumulated amounts of aluminum and platinum in the body as measured by ICP-MS, and the concentrations were calculated by the weight of metal/tissue. The table at the bottom shows the limits of quantification of ICP-MS, and any amounts lower than these could not be measured. All of the tissues described in this figure were harvested on the day after the last injection of GSH had been completed.
Discussion
Aluminum is a nonessential metal, and it was long considered virtually innocuous to humans [37]. However, aluminum is now recognized as a highly neurotoxic agent, and at high amounts, aluminum inhibits the prenatal and postnatal development of the brain in humans and experimental animals [37]. There is lots of information about the neurotoxic effects of aluminum, but there have been few reports about neuropathy, even though humans intake an average of 3-100 mg aluminum through their daily diet [38]. Our previous study [18] reported that aluminum levels were relatively high in some cancer patients who suffering from neuropathic pain based on clinical observations. They suggested that aluminum accumulation in the DRG in the course of oxaliplatin treatment could exacerbate neuropathic pain. ICP-MS analysis showed a significant increase in aluminum concentrations in the DRG of mice treated with aluminum chloride and oxaliplatin compared to those treated with aluminum chloride alone. Therefore, these findings suggested that aluminum accumulation in the DRG may exacerbate neuropathic pain in oxaliplatin-treated mice.
In this study, we showed that aluminum oxalate could induce neuropathic pain, and this implies that a patient could experience a dangerous impact of aluminum in the body when taking oxaliplatin. Aluminum oxalate was identified by the acetone test as a substance that causes cold hyperalgesia. Cold hyperalgesia is a typical side effect of oxaliplatin. According to one report, among oxaliplatin metabolites, dichloro(1,2-diaminocyclohexane)platinum is the cause of mechanical allodynia, and oxalate is referred to as the cause of cold hyperalgesia [39,40]. This proves that aluminum oxalate may cause cold hyperalgesia and shows a negative of aluminum in the body: it could bind to oxalate when oxaliplatin is injected.
In addition, aluminum oxalate caused TRPA1 expression and cell death in murine DRG, and overall, it might be said to act similarly to oxaliplatin. TRPA1 is a channel that is activated by noxious cold and by chemicals such as cinnamaldehyde and mustard oil; like those, oxaliplatin also increases the TRPA1 activation [20,39,41]. Further research is needed in vitro to investigate whether aluminum oxalate is the activating material of TRPA1. Also, when injecting aluminum according to the method of one study [33] in the form of aluminum oxalate, we could not observe general toxicity in the mice in the Oxal group, even if the DRG cells were terribly destroyed. Oxaliplatin generated DRG cell disruption similarly to aluminum oxalate, and simultaneously caused neuropathic pain. Platinum in a platinum-based chemotherapeutic agent cannot pass through the BBB, but has an affinity to peripheral neurons, which leads platinum to accumulate in the DRG and destroy it [42]. Based on this, the cell death of the DRG could be said to be associated with neuropathic pain, and the results that aluminum oxalate showed were the same as those that oxaliplatin showed. In particular, the accumulation of aluminum in the DRG was doubled when mice were treated with the oxalate form rather than the chloride form. This signifies that if aluminum and oxalate are injected together, neuropathic pain may become more serious.
In subsequent experiments depending on injection schedules (Figure 5A), GSH had the effects of removing aluminum and relieving neuropathy. The injection of GSH lowered the scores of the acetone test and the expression levels of TRPA1. TRPA1 is activated by GSH-sensitive molecules such as ROS and inactivated by GSH [30], but the decreased expression of TRPA1 due to GSH was surprisingly proved in this study. In addition, GSH reduced the concentration of aluminum in the body, especially in the DRG, which is believed to correlate with peripheral neuropathy. Because aluminum deposited in the DRG induced peripheral neuropathy, and the pain was worse when aluminum and oxaliplatin were used in combination treatments [18], aluminum chelation is required in chemotherapy. The risk of exposure to aluminum is high these days, and research into related illnesses is also thriving [43]. GSH prevented the accumulation of platinum in the DRG as well as chelating aluminum from the brain and serum [44,45]. We also investigated whether GSH could chelate aluminum from the DRG and if GSH could reduce the neuropathy in mice treated with oxaliplatin.
In Figure 8, the aluminum was accumulated in the DRG, brain, and serum of the mice treated with oxaliplatin, and GSH reduced the aluminum accumulation in all organs. Specifically, aluminum was not detected in the DRG after GSH treatment. The aluminum chelation effect by GSH was effective in the DRG. Further research using aluminum tracing materials is needed to determine why and how aluminum was more effectively removed in the DRG by GSH. In contrast to aluminum, platinum was mostly accumulated and detected in the DRG. There was no detectable platinum in either the brain or serum.
In normal, aluminum-exposed humans and in aluminum-treated experimental animals, aluminum was distributed unevenly to various tissues [46]. In general, about one-half of the total amount of aluminum in the body is accumulated in the skeleton, and about one-fourth is in the lungs [47]. The brain is also an important accumulation site of aluminum in the body. Aluminum toxicity is well documented, but the mechanism of action is poorly understood [48]. The toxic effects of aluminum on the brain and other organs are relatively well established [49], but there is little information on its molecular cytotoxicity and cellular mechanisms [50]. Aluminum uptake and efflux in blood plasma and brain extracellular fluid have been investigated. The BBB is the primary route for brain aluminum uptake. The brain uptake mechanism of aluminum transferrin may be accomplished by transferrin receptor-mediated endocytosis, but this is still controversial [51]. Recent epidemiological, neuropathological, and biochemical studies have suggested a possible link between the neurotoxicity of aluminum and the pathogenesis of Alzheimer’s disease [52]. Interestingly, by the data shown in this study, high amounts of aluminum were accumulated in the brains of the control mice group. This may have been caused by the intake of food containing high amounts of aluminum. The dietary food for the mice contained 52.22 µg/g of aluminum, and most of the mice usually ate more than 4 g of food per day. Therefore, the mice ate a lot of aluminum during this experiment. Yokel and Robert [51] also revealed that once aluminum entered the brain, it persisted in the brain for a long period. They estimated that its half-life ranges from 20% are greater than its ordinary half-life. In addition, the amount of aluminum in bone, which maintained the majority of the body burden, may influence the amount of brain aluminum.
In our previous study [18], we demonstrated that aluminum accumulation aggravated the peripheral neuropathy induced by oxaliplatin through the activation of TRPA1 and induction of cell death in the DRG. Therefore, we proposed that oxaliplatin-induced peripheral neuropathy may be alleviated by agents that chelate aluminum. In another previous study, pre-treatment with GSH before a challenge with oxaliplatin was effective to reduce chemotherapy-induced neuropathic pain, but there was no explanation of relevance of this phenomenon to aluminum [30]. In this work, the results of the GSH chelation experiment strongly support the causal relationship between aluminum accumulation in the DRG and peripheral neuropathy induced in the experiments by injecting aluminum oxalate. Especially in the DRG, aluminum was detected when oxaliplatin was injected, but it was not detected when GSH was administered. However, in the brain and serum, aluminum was detected both when oxaliplatin was injected and when oxaliplatin and GSH were co-administered, even though the amounts of aluminum were reduced when GSH was injected (Figure 8). This result implies that the aluminum in the serum might be effluxed from highly accumulated aluminum in the bones or other tissues. Therefore, aluminum chelation by GSH was not sufficient to eliminate it. Aluminum in the brain can be explained by the low efficient efflux rate of aluminum via the BBB. However, these explanations should be investigated further with more intensive research. This study provided the information to alleviate chemotherapy-induced neuropathic pain by using GSH treatment for the effective removal of aluminum.
This work demonstrated that aluminum oxalate alone caused symptoms similar to oxaliplatin-induced peripheral neuropathy, and aluminum chelation using GSH could be the alternative medication for reducing the complex of aluminum: oxaliplatin-induced peripheral neuropathy. Taken together, this strongly indicated the importance of the side effect of aluminum in the mice model, which was the oxaliplatin-induced neuropathy. Therefore, intensive research into more effective methods to remove aluminum in the body is required.
The reason why the internal aluminum content of the control mice was higher than that of humans was their large intake of aluminum [53]. Since the food used in this study contained 52.22 µg/g of aluminum and most of the mice ate more than 4 g of food per day, the mice ate a lot of aluminum as compared to humans.
The results with GSH support the causal relationship between aluminum and peripheral neuropathy that was suggested in the previous experiments using aluminum oxalate, and therefore, we can try to approach neuropathy treatment using aluminum chelation. In a previous study, treatment with GSH before oxaliplatin administration was effective to reduce chemotherapy-induced neuropathic pain, but there was no explanation of the relevance to aluminum [31]. Therefore, this study provides the necessity of trying GSH for the effective removal of aluminum through various treatment methods.
Conclusions
This paper demonstrated that aluminum oxalate caused symptoms similar to oxaliplatin-induced peripheral neuropathy, and showed for the first time that aluminum chelation using GSH could be the solution. This indicates the importance of aluminum in the study of oxaliplatin-induced neuropathy, and thus, active research into effective methods of removing aluminum in the body is required.
Acknowledgements
This work was supported by the National Research Foundation of Korea (2013R1A2A2A04014661, Chung-KyoonAuh).
Disclosure of conflict of interest
None.
References
- 1.Thibault K, Calvino B, Dubacq S, Roualle-de-Rouville M, Sordoillet V, Rivals I, Pezet S. Cortical effect of oxaliplatin associated with sustained neuropathic pain: exacerbation of cortical activity and down-regulation of potassium channel expression in somatosensory cortex. Pain. 2012;153:1636–1647. doi: 10.1016/j.pain.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 2.Lersch C, Schmelz R, Eckel F, Erdmann J, Mayr M, Schulte-Frohlinde E, Quasthoff S, Grosskreutz J, Adelsberger H. Prevention of oxaliplatin-induced peripheral sensory neuropathy by carbamazepine in patients with advanced colorectal cancer. Clin Colorectal Cancer. 2002;2:54–58. doi: 10.3816/CCC.2002.n.011. [DOI] [PubMed] [Google Scholar]
- 3.Lehky T, Leonard G, Wilson R, Grem J, Floeter M. Oxaliplatin-induced neurotoxicity: acute hyperexcitability and chronic neuropathy. Muscle Nerve. 2004;29:387–392. doi: 10.1002/mus.10559. [DOI] [PubMed] [Google Scholar]
- 4.Amptoulach S, Tsavaris N. Neurotoxicity caused by the treatment with platinum analogues. Chemother Res Pract. 2011;2011:843019. doi: 10.1155/2011/843019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McWhinney SR, Goldberg RM, McLeod HL. Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther. 2009;8:10–16. doi: 10.1158/1535-7163.MCT-08-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. 2009;32:1–32. doi: 10.1146/annurev.neuro.051508.135531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 8.Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004;110:628–638. doi: 10.1016/j.pain.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Ammendola A, Tata M, Aurilio C, Ciccone G, Gemini D, Ammendola E, Ugolini G, Argenzio F. Peripheral neuropathy in chronic alcoholism: a retrospective cross-sectional study in 76 subjects. Alcohol Alcohol. 2001;36:271–275. doi: 10.1093/alcalc/36.3.271. [DOI] [PubMed] [Google Scholar]
- 10.Jaggi AS, Singh N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology. 2012;291:1–9. doi: 10.1016/j.tox.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 11.Hariprasad M, Razdan R. A review on animal models of peripheral neuropathy. Int J Cur Biomed Phar Res. 2011;1:193–200. [Google Scholar]
- 12.Gamelin L, Boisdron-Celle M, Delva R, Guerin-Meyer V, Ifrah N, Morel A, Gamelin E. Prevention of oxaliplatin-related neurotoxicity by calcium and magnesium infusions: a retrospective study of 161 patients receiving oxaliplatin combined with 5-Fluorouracil and leucovorin for advanced colorectal cancer. Clin Cancer Res. 2004;10:4055–4061. doi: 10.1158/1078-0432.CCR-03-0666. [DOI] [PubMed] [Google Scholar]
- 13.Gura KM. Aluminum contamination in products used in parenteral nutrition: has anything changed? Nutrition. 2010;26:585–594. doi: 10.1016/j.nut.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Kumar V, Gill KD. Aluminium neurotoxicity: neurobehavioural and oxidative aspects. Arch Toxicol. 2009;83:965–978. doi: 10.1007/s00204-009-0455-6. [DOI] [PubMed] [Google Scholar]
- 15.Bhalla P, Dhawan DK. Protective role of lithium in ameliorating the aluminium-induced oxidative stress and histological changes in rat brain. Cell Mol Neurobiol. 2009;29:513–521. doi: 10.1007/s10571-008-9343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crisponi G, Nurchi VM, Bertolasi V, Remelli M, Faa G. Chelating agents for human diseases related to aluminium overload. Coordination Chemistry Reviews. 2012;256:89–104. [Google Scholar]
- 17.Exley C, House ER. Aluminium in the human brain. Monatsh Chem. 2011;142:357–363. [Google Scholar]
- 18.Park JH, Chae J, Roh K, Kil EJ, Lee M, Auh CK, Lee MA, Yeom CH, Lee S. Oxaliplatin-induced peripheral neuropathy via TRPA1 stimulation in mice dorsal root ganglion is correlated with aluminum accumulation. PLoS One. 2015;10:e0124875. doi: 10.1371/journal.pone.0124875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shirao K, Matsumura Y, Yamada Y, Muro K, Gotoh M, Boku N, Ohtsu A, Nagashima F, Sano Y, Mutoh M, Tanigawara Y. Phase I study of single-dose oxaliplatin in Japanese patients with malignant tumors. Jpn J Clin Oncol. 2006;36:295–300. doi: 10.1093/jjco/hyl016. [DOI] [PubMed] [Google Scholar]
- 20.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 21.Descoeur J, Pereira V, Pizzoccaro A, Francois A, Ling B, Maffre V, Couette B, Busserolles J, Courteix C, Noel J, Lazdunski M, Eschalier A, Authier N, Bourinet E. Oxaliplatin-induced cold hypersensitivity is due to remodelling of ion channel expression in nociceptors. EMBO Mol Med. 2011;3:266–278. doi: 10.1002/emmm.201100134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sapunar D, Kostic S, Banozic A, Puljak L. Dorsal root ganglion - a potential new therapeutic target for neuropathic pain. J Pain Res. 2012;5:31–38. doi: 10.2147/JPR.S26603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang XM, Lehky TJ, Brell JM, Dorsey SG. Discovering cytokines as targets for chemotherapy-induced painful peripheral neuropathy. Cytokine. 2012;59:3–9. doi: 10.1016/j.cyto.2012.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krames ES. The role of the dorsal root ganglion in the development of neuropathic pain. Pain Med. 2014;15:1669–1685. doi: 10.1111/pme.12413. [DOI] [PubMed] [Google Scholar]
- 25.Gutierrez-Gutierrez G, Sereno M, Miralles A, Casado-Saenz E, Gutierrez-Rivas E. Chemotherapy-induced peripheral neuropathy: clinical features, diagnosis, prevention and treatment strategies. Clin Transl Oncol. 2010;12:81–91. doi: 10.1007/S12094-010-0474-z. [DOI] [PubMed] [Google Scholar]
- 26.Pastore A, Federici G, Bertini E, Piemonte F. Analysis of glutathione: implication in redox and detoxification. Clin Chim Acta. 2003;333:19–39. doi: 10.1016/s0009-8981(03)00200-6. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Wei G, Chen J. Glutathione: a review on biotechnological production. Appl Microbiol Biotechnol. 2004;66:233–242. doi: 10.1007/s00253-004-1751-y. [DOI] [PubMed] [Google Scholar]
- 28.Jansen BA, Brouwer J, Reedijk J. Glutathione induces cellular resistance against cationic dinuclear platinum anticancer drugs. J Inorg Biochem. 2002;89:197–202. doi: 10.1016/s0162-0134(02)00381-1. [DOI] [PubMed] [Google Scholar]
- 29.Cobbett C, Goldsbrough P. Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Annu Rev Plant Biol. 2002;53:159–182. doi: 10.1146/annurev.arplant.53.100301.135154. [DOI] [PubMed] [Google Scholar]
- 30.Nassini R, Gees M, Harrison S, De Siena G, Materazzi S, Moretto N, Failli P, Preti D, Marchetti N, Cavazzini A, Mancini F, Pedretti P, Nilius B, Patacchini R, Geppetti P. Oxaliplatin elicits mechanical and cold allodynia in rodents via TRPA1 receptor stimulation. Pain. 2011;152:1621–1631. doi: 10.1016/j.pain.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 31.Cascinu S, Catalano V, Cordella L, Labianca R, Giordani P, Baldelli AM, Beretta GD, Ubiali E, Catalano G. Neuroprotective effect of reduced glutathione on oxaliplatin-based chemotherapy in advanced colorectal cancer: a randomized, double-blind, placebo-controlled trial. J. Clin. Oncol. 2002;20:3478–3483. doi: 10.1200/JCO.2002.07.061. [DOI] [PubMed] [Google Scholar]
- 32.Holmes J, Stanko J, Varchenko M, Ding H, Madden VJ, Bagnell CR, Wyrick SD, Chaney SG. Comparative neurotoxicity of oxaliplatin, cisplatin, and ormaplatin in a Wistar rat model. Toxicol Sci. 1998;46:342–351. doi: 10.1006/toxs.1998.2558. [DOI] [PubMed] [Google Scholar]
- 33.Guo CH, Hsu GS, Chuang CJ, Chen PC. Aluminum accumulation induced testicular oxidative stress and altered selenium metabolism in mice. Environ Toxicol Pharmacol. 2009;27:176–181. doi: 10.1016/j.etap.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Ta LE, Low PA, Windebank AJ. Mice with cisplatin and oxaliplatin-induced painful neuropathy develop distinct early responses to thermal stimuli. Mol Pain. 2009;5:9. doi: 10.1186/1744-8069-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flatters SJ, Bennett GJ. Ethosuximide reverses paclitaxel- and vincristine-induced painful peripheral neuropathy. Pain. 2004;109:150–161. doi: 10.1016/j.pain.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 36.Ta LE, Bieber AJ, Carlton SM, Loprinzi CL, Low PA, Windebank AJ. Transient receptor potential vanilloid 1 is essential for cisplatin-induced heat hyperalgesia in mice. Mol Pain. 2010;6:15. doi: 10.1186/1744-8069-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sorenson JR, Campbell IR, Tepper LB, Lingg RD. Aluminum in the environment and human health. Environmental Health Perspectives. 1974;8:3–95. doi: 10.1289/ehp.7483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lione A. The prophylactic reduction of aluminium intake. Food Chem Toxicol. 1983;21:103–109. doi: 10.1016/0278-6915(83)90277-6. [DOI] [PubMed] [Google Scholar]
- 39.Zhao M, Isami K, Nakamura S, Shirakawa H, Nakagawa T, Kaneko S. Acute cold hypersensitivity characteristically induced by oxaliplatin is caused by the enhanced responsiveness of TRPA1 in mice. Mol Pain. 2012;8:55. doi: 10.1186/1744-8069-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sakurai M, Egashira N, Kawashiri T, Yano T, Ikesue H, Oishi R. Oxaliplatin-induced neuropathy in the rat: involvement of oxalate in cold hyperalgesia but not mechanical allodynia. Pain. 2009;147:165–174. doi: 10.1016/j.pain.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Högestätt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 42.Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249:9–17. doi: 10.1007/pl00007853. [DOI] [PubMed] [Google Scholar]
- 43.Crisponi G, Fanni D, Gerosa C, Nemolato S, Nurchi VM, Crespo-Alonso M, Lachowicz JI, Faa G. The meaning of aluminium exposure on human health and aluminium-related diseases. Biomol Concepts. 2013;4:77–87. doi: 10.1515/bmc-2012-0045. [DOI] [PubMed] [Google Scholar]
- 44.Cavaletti G, Minoia C, Schieppati M, Tredici G. Protective effects of glutathione on cisplatin neurotoxicity in rats. Int J Radiat Oncol Biol Phys. 1994;29:771–776. doi: 10.1016/0360-3016(94)90565-7. [DOI] [PubMed] [Google Scholar]
- 45.Sharma P, Ahmad Shah Z, Kumar A, Islam F, Mishra KP. Role of combined administration of Tiron and glutathione against aluminum-induced oxidative stress in rat brain. J Trace Elem Med Biol. 2007;21:63–70. doi: 10.1016/j.jtemb.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 46.Yokel RA, McNamara PJ. Aluminium toxicokinetics: an updated minireview. Pharmacol Toxicol. 2001;88:159–167. doi: 10.1034/j.1600-0773.2001.d01-98.x. [DOI] [PubMed] [Google Scholar]
- 47.Ganrot PO. Metabolism and possible health effects of aluminum. Environ Health Perspect. 1986;65:363. doi: 10.1289/ehp.8665363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han J, Han J, Dunn MA. Effect of dietary aluminum on tissue nonheme iron and ferritin levels in the chick. Toxicology. 1999;142:97–109. doi: 10.1016/s0300-483x(99)00119-5. [DOI] [PubMed] [Google Scholar]
- 49.Yokel RA. The toxicology of aluminum. Neurotoxicology. 2000;21:813–828. [PubMed] [Google Scholar]
- 50.Lévesque L, Mizzen CA, McLachlan DR, Fraser PE. Ligand specific effects on aluminum incorporation and toxicity in neurons and astrocytes. Brain Res. 2000;877:191–202. doi: 10.1016/s0006-8993(00)02637-8. [DOI] [PubMed] [Google Scholar]
- 51.Yokel RA. The pharmacokinetics and toxicology of aluminum in the brain. Current Inorganic Chemistry. 2012;2:54–63. [Google Scholar]
- 52.Kawahara M, Kato M, Kuroda Y. Effects of aluminum on the neurotoxicity of primary cultured neurons and on the aggregation of β-amyloid protein. Brain Res Bull. 2001;55:211–217. doi: 10.1016/s0361-9230(01)00475-0. [DOI] [PubMed] [Google Scholar]
- 53.Aguilar F, Autrup H, Barlow S, Castle L, Crebelli R, Dekant W, Engel K, Gontard N, Gott D, Grilli S. Safety of aluminium from dietary intake scientific opinion of the panel on food additives, flavourings, processing aids and food contact materials (AFC) The EFSA Journal. 2008;754:1–34. doi: 10.2903/j.efsa.2008.754. [DOI] [PMC free article] [PubMed] [Google Scholar]


