Abstract
Alpinumisoflavone (AIF) is a naturally occurring flavonoid that is a major bioactive component of the medicinal plant Derris eriocarpa. In this study we evaluated the antimetastatic effect of AIF and investigated the underlying mechanism of action using in vitro and in vivo models of melanoma. We found that AIF impaired the metastatic potential of A375 and SK-MEL-1 human melanoma cells by promoting cell differentiation as assessed by melanin content, protoporphyrin IX accumulation, and tissue transglutaminase activity. In addition, AIF inhibited cell adhesion, migration, and invasion in melanoma cells. We found that AIF treatment decreased cyclooxygenase-2 (COX-2) expression, and COX-2 overexpression attenuated the inhibitory effects of AIF on the metastatic behaviors of melanoma cells. AIF dose-dependently increased microRNA-124 (miR-124) levels and decreased levels of sphingosine kinase 1 (SPHK1), a target of miR-124. In a mouse model of melanoma, AIF suppressed lung metastasis. Taken together, our findings suggest that AIF inhibits metastasis in melanoma by modulating COX-2 expression, at least in part, through targeting the miR-124/SphK1 axis. Our study provides evidence that AIF may be useful as an antimetastatic agent in the treatment of melanoma.
Keywords: Alpinumisoflavone, melanoma, metastasis, COX-2, miR-124, SPHK1
Introduction
Skin cancer, which is now the most common type of cancer in white populations [1], is traditionally classified as melanoma or nonmelanoma. Malignant melanoma is an aggressive and deadly skin cancer that is responsible for most skin cancer-related deaths [2]. According to the American Cancer Society, 9,710 melanoma-associated deaths occurred in the United States in 2014, and the number of new cases of invasive melanoma was estimated at 76,100 [3]. Furthermore, the incidence and mortality rates of melanoma have increased world-wide in the last 30 years, especially in young people [4]. If diagnosed and treated at an early stage, melanoma is curable; however, metastatic melanoma is difficult to treat. Most advanced melanomas respond poorly to radiotherapy and chemotherapy, and no currently available therapy effectively inhibits the metastatic spread of this cancer [5], resulting in the high mortality rate [6]. The development of novel agents that can decrease the metastatic potential of melanoma may represent an effective strategy for its prevention or treatment.
Risk factors for the development of melanoma included exposure to solar ultraviolet (UV) radiation, nevus count, skin phototype, family history of melanoma and, hypothetically, exposure to artificial light [7]. Of these factors, UV exposure is considered the most common cause of melanoma. Exposing the skin to UV irradiation upregulates the expression of cyclooxygenase-2 (COX-2), one of the two isoforms of COX [8]. In contrast to COX-1, which is constitutively expressed in most tissues and is involved in normal physiological functions, COX-2 is an inducible rate-limiting enzyme that catalyzes the conversion of arachidonic acid to prostaglandins and can be activated by extracellular stimuli such as UV radiation, hormones, cytokines, and tumor promoters [8,9]. The UV-induced upregulation of COX-2 and its products, prostaglandins, is associated with the development and progression of melanoma [10] because of their ability to decrease apoptosis and promote cancer invasion and metastasis [11-13]. Therefore, COX-2 is considered an important therapeutic target for melanoma.
Alpinumisoflavone (AIF) is a major bioactive component of Derris eriocarpa, a traditional Chinese medicinal plant that is widely distributed throughout the Yunnan, Guangxi, and Guizhou provinces of China. Previous studies have described various pharmacological activities of AIF including atheroprotective [14], estrogenic [15], and antibacterial actions [16]. In addition, AIF has been identified as a potential antineoplastic agent [17,18]. However, little is known about the effect of AIF on the metastatic potential of tumor cells. In this study, we investigated the effect of AIF on the metastatic potential of human melanoma cell lines and in a mouse model of melanoma. Our results showed that AIF impairs the metastatic potential of melanoma cells by downregulating COX-2 via the microRNA-124 (miR-124)/sphingosine kinase 1 (SPHK1) axis.
Materials and methods
Cell lines and cultures
The human melanoma cell lines A375 and SKMEL-1 and the murine melanoma cell line B16-F10 were obtained from the American Type Culture Collection (Rockville, MD, USA). The cells were grown in Dulbecco’s modified Eagle medium (DMEM, HyClone, Waltham, MA, USA) containing 10% fetal bovine serum (FBS, HyClone) and 1% antibiotic solution (100 mg/L streptomycin, 100 U/ml penicillin) at 37°C in a 5% CO2 atmosphere. Every 48 hours, the cells were detached with trypsin-EDTA solution (HyClone), resuspended at 1.5 × 105/ml, and replated.
Cell proliferation assay
In vitro cytotoxicity was determined with the MTT assay using A375 and SK-MEL-1 cells and the Cell Counting Kit-8 (Wako Chem., Shanghai, China) according to the manufacturer’s instructions. After a 24-hour incubation in various concentrations of AIF, cell viability was determined by measuring absorbance at 595 nm with an enzyme-linked immunosorbent assay reader (Tecan Group Ltd., Männedorf, Switzerland).
Determination of melanin content
Melanin content was determined as previously described [19]. The A375 cells were seeded in 6-well culture plates (5 × 105 cells/well in 2 ml DMEM) and incubated overnight to allow cells to adhere. The cells were then treated with 5 or 10 μM AIF for 48 hours, detached by using 0.25% trypsin-EDTA, and centrifuged for 15 minutes at 1200 × g at 4°C. The supernatant was removed, and the cell pellets were washed with phosphate buffer saline and lysed with 500 μl water. Cell lysates were clarified by centrifugation at 12,000 rpm for 5 minutes and disrupted by ultrasonic treatment at 4°C. The lysates were clarified by centrifugation at 12,000 rpm for 5 minutes at 37°C, and the pellet was washed with 10% trichloroacetic acid (500 μl) and 75% ethanol (500 μl) and then clarified again. The pellet was then incubated with 50 μl NaOH (1 mol/L) containing 10% DMSO for 1 hour at 80°C. Absorbance at 470 nm was measured using an enzyme-linked immunosorbent assay reader (Tecan Group Ltd.).
Determination of intracellular protoporphyrin IX concentration
Intracellular protoporphyrin IX (PpIX) concentration was measured as previously described [20]. After 24-hour treatment with 5 or 10 μM AIF, the A375 and SK-MEL-1 cells were washed three times with phosphate buffered saline (PBS) and harvested by trypsinization. After centrifugation at 800 × g for 5 min, the cells were resuspended in 0.5 ml PBS. Intracellular PpIX concentration was analyzed by flow cytometry (BD Biosciences FACScan, San Diego, CA, USA) using an excitation wavelength of 488 nm and emission wavelength of 650 nm and quantified with CellQuest software (BD Biosciences).
Measurement of tissue transglutaminase 2 activity
To evaluate intracellular tissue transglutaminase (TG2) activity, A375 cells were plated on 100-mm plates (1 × 106/plate) and grown in the presence of [14C]-methylamine (0.5 μl/ml) with or without AIF. The incorporation of radiolabeled amine into cell proteins was measured with a scintillation counter (Beckman LS 5000 TD, Fullerton, CA, USA), and TG2 activity was analyzed as previously described [21].
Wound healing assay
The A375 and SK-MEL-1 cells were seeded into 24-well tissue culture plates (1 × 105 cells/well) and maintained at 37°C and 5% CO2 for 24 hours to allow cell adhesion and formation of a confluent monolayer. The monolayers were scored with a sterile pipette tip to leave a scratch approximately 0.4- to 0.5-mm wide and then washed with serum-free medium three times to remove dislodged cells. Wound closure was monitored by collecting digitized images at 0 and 24 hours after the scratch was performed using an inverted microscope (Motic China Group Co., Xiamen, China) and digital camera (Nikon, Tokyo, Japan). The digitized images were analyzed using ImageJ software.
Cell invasion assay
Cell invasion was evaluated using Transwell plates coated with Matrigel (8-μm pore size; BD Biosciences, San Jose, California) following the standard protocol. Cells were seeded into the 24-well plates (1 × 105/well) and starved overnight in serum-free medium. The cells were then trypsinized, washed three times in DMEM containing 1% FBS, resuspended in DMEM containing 1% FBS, and then added to the upper chamber of the Transwell plate (1 × 105 cells in 500 μl medium). Minimal Essential Medium with 10% FBS was added to the lower chamber as a chemoattractant. For control cells, medium containing 1% FBS was added to the lower chamber. After 24-hour incubation, the Matrigel and cells remaining in the upper chamber were removed with cotton swabs. Cells on the lower surface of the membrane were fixed in formaldehyde and stained with hematoxylin. The cells in at least five random microscopic fields (magnification, × 200) were counted and photographed.
Determination of miRNA and mRNA levels
TRIzol Reagent (Thermo Fisher Scientific, Waltham, MA) was used to extract total RNA from cultured cells. MiRNAs expression was quantified by quantitative reverse transcription PCR (qRT-PCR). Briefly, cDNA was obtained by using a High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA, USA) and amplified using the ABI 7500 System (Thermo Fisher Scientific) and a TaqMan PCR kit (Applied Biosystems, Carlsbad, CA) according to the manufacture’s instructions. Expression of miR-124 was normalized to that of U6 small nuclear RNA. COX-2 expression was determined by using primers synthesized based on published sequences [22]. First-strand cDNA was generated from 1 μg total RNA using Super M-MLV reverse transcriptase (BioTeke Co., Beijing, China). PCR amplification was carried out using SYBR Green master mix (Solarbio Co., Beijing, China), forward and reverse primers, and 10 ng template cDNA. COX-2 expression was normalized to that of glyceraldehyde-3-phosphate dehydrogenase (GADPH) and analyzed using the comparative ΔCt method (ABPrism software, Applied Biosystems).
Generation of plasmid constructs and cell lines overexpressing COX-2 or SPHK1
To generate the COX-2 overexpression vector, the COX-2 coding sequence was obtained by RT-PCR and cloned into a pCMV vector (Beyotime Institute of Biotechnology, Shanghai, China). The resulting plasmid was named pCMV-COX-2. Both A375 or SK-MEL-1 cells were transfected with pCMV-COX-2 or the empty pCMV vector as a control. Two days after transfection, stable clones were selected and maintained in medium containing G418. The SPHK1 overexpression vector was generated similarly. The cDNA was amplified and subcloned into the pCMV vector as previously described [23]. The A375 or SK-MEL-1 cells were then transfected with pCMV-SPHK1 or the empty pCMV vector. Overexpression of COX-2 and SPHK1 was verified by qRT-PCR and western blot analysis.
Silencing COX-2 or SPHK1 in melanoma cells
The siRNA oligos for COX-2 or SPHK1 gene knockdown were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Two different siRNA sequences and one scramble sequence (control) were subcloned into plasmid vector pGCsi-H1 according to the manufacturer’s instructions. These recombinant vectors were designated p-siRNA1, p-siRNA2, and p-siRNA-control, respectively. A375 or SK-MEL-1 cells from logarithmic growth phase cultures were seeded into 6-well plates (3 × 105 cells/well). The next day, the cells were transfected with p-siRNA1, p-siRNA2, or p-siRNA-control using Lipofectamine 2000 (Invitrogen, CA, USA) according to the manufacturer’s protocol. After 48 hours, transfection efficiency was evaluated under florescence microscopy. Stable transfection was verified by qRT-PCR and western blot analysis.
MiR-124 knockdown or overexpression
Melanoma cells were seeded into a 96-well plate, incubated overnight, and then transfected with lentiviral constructs for miR-124 overexpression (miR-124 mimic), miR-124 knockdown (miR-124 inhibitor), or control (miR-con) (Qiagen, Dusseldorf, Germany) using Lipofectamine-2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Transfection efficiency was confirmed by qRT-PCR analysis.
Western blotting analysis
Cells were harvested, lysed with ice-cold lysis buffer (Invitrogen) for 30 minutes on ice, and centrifuged at 4°C, 12,000 rpm for 5 minutes. The cell lysate was collected, and lysate proteins (50 μg) were separated by 10% SDS-PAGE and transferred onto PVDF membranes (Millipore, MA, USA). The proteins were probed with specific antibodies following the standard protocol. After washing with Tris-buffered saline with Tween 20, the blot was incubated with the secondary antibody for 2 hours. After washing the blot with Tris-buffered saline with Tween 20, signals were detected using chemiluminescent substrate (KPL, Guildford, UK) and analyzed using Bandscan software (Glyko, Novato, CA).
Construction of reporter plasmids and luciferase assay
The luciferase reporter plasmid was constructed as previously described [23]. A fragment containing the SPHK1 3’ untranslated region (UTR) was amplified by PCR from human genomic DNA and inserted into a pGL3 vector (Promega, Madison, WI) downstream of the stop codon of the firefly luciferase reporter gene to generate pGL3-3’UTR/SPHK1. For the luciferase assay, 293T cells were transiently cotransfected with 0.2 μg of the pGL3-3’UTR/SPHK1 construct, 0.02 μg of the control pRL-TK-Renilla luciferase reporter plasmid (Promega, Madison, WI) used for normalization, and 5 pmol of miR-124 mimic, miR-124 inhibitor, or miR-con. After 24 hours, the cells were lysed, and luciferase activity was determined with a luminometer using the Dual-Luciferase Reporter Assay Sys-tem (Promega), according to the manufacturer’s instructions.
Mouse model of lung metastasis
All animal experiments were performed with the approval of the Ethics Committee of Luoyang Central Hospital affiliated with Zhengzhou University. To establish a mouse model of lung metastasis, B16-F10 melanoma cells (105 cells in 50 μl PBS) were injected into the tail vein of C57BL/6 mice. The mice were randomly assigned to three treatment groups and received AIF in saline (50 or 1000 mg/kg/day) or saline (control) intragastrically for consecutive 24 days. At the end of the experiment, the mice were sacrificed, and the lungs were dissected to count metastasized colonies.
Statistical analysis
All statistical analyses were performed using SPSS statistical software. Results are presented as mean ± standard deviation. Groups were compared by one-way analysis of variance, followed by Tukey’s post hoc test for multiple comparisons. Student’s t-test was used for single comparisons. P < 0.05 was considered significant.
Results
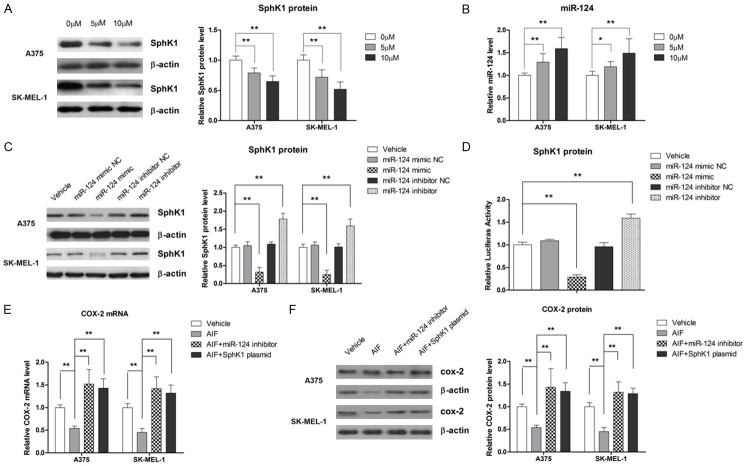
AIF inhibits melanoma cell proliferation
To assess the effect of AIF on cell viability, A375 and SK-MEL-1 cells were treated with 0, 2, 5, or 10 μM AIF for 24, 48, or 72 hours. As shown in Figure 1A, AIF suppressed the proliferation of both A375 and SK-MEL-1 cells in a dose- and time-dependent manner. At 2 μM, AIF did not significantly reduce the viability of either cell line, even when the incubation was prolonged to 72 hours. However, 24-hour incubation with 5 or 10 μg/ml AIF significantly inhibited cell growth. To evaluate the effect of AIF on the metastatic potential of melanoma cells, we used 2 and 5 μM AIF for further experiments.
Figure 1.
AIF reduces cell viability and promotes differentiation in A375 and SK-MEL-1 human melanoma cells. A. AIF treatment led to a time- and dose-dependent loss of cell viability. To determine the effect of AIF on cell differentiation, cells were treated with AIF at the indicated concentrations for 48 hours. B. AIF treatment increased the melanin content of cells. C. AIF treatment increased PpIX accumulation. D. AIF enhanced TG2 activity. *P < 0.05, **P < 0.01.
AIF stimulates differentiation and suppresses adhesion, migration, and invasion of human melanoma cells
Metastatic melanoma is known for its phenotypic diversity and loss of differentiation markers. Therefore, we evaluated the effect of AIF on TG2 activity, melanin synthesis, and PpIX accumulation as markers of differentiation [24]. After 48-hour treatment with 5 or 10 μM AIF, intracellular melanin content in A375 cells was increased by approximately 50% compared to vehicle-treated control cells (Figure 1B). AIF treatment also increased PpIX accumulation in the melanoma cells, as demonstrated by flow cytometry (Figure 1C). In addition, TG2 activity was significantly enhanced by AIF treatment (Figure 1D). Taken together, these findings demonstrate that AIF is able to stimulate differentiation in melanoma cell lines.
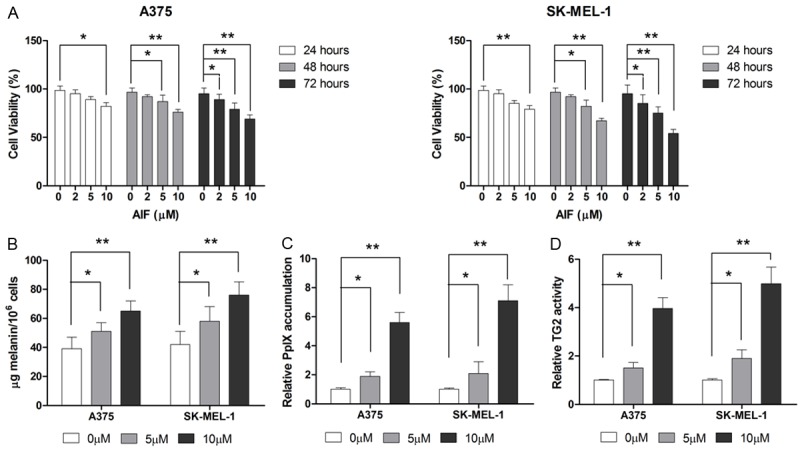
We next examined the effect of AIF on cell migration in A375 and SK-MEL-1 cells. As shown in Figure 2A, AIF suppressed cell migration in a dose- and time-dependent manner. We then evaluated the effect of AIF on cell invasion by using the transwell assay. As shown in Figure 2B, 48-hour incubation with 5 or 10 μM AIF significantly suppressed cell invasion. These results show that AIF impairs the metastatic potential of melanoma cells.
Figure 2.
AIF suppresses migration and invasion in A375 and SK-MEL-1 human melanoma cells. To demonstrate the effect of AIF on migration and invasion, cells were incubated with AIF at the indicated concentrations. A. Treatment with 5 or 10 μM AIF for 24 hours decreased cell migration. B. Treatment with 5 or 10 μM AIF for 48 hours suppressed cell invasion. *P < 0.05, **P < 0.01.
COX-2 is involved in the inhibitory effect of AIF on the metastatic potential of melanoma cells
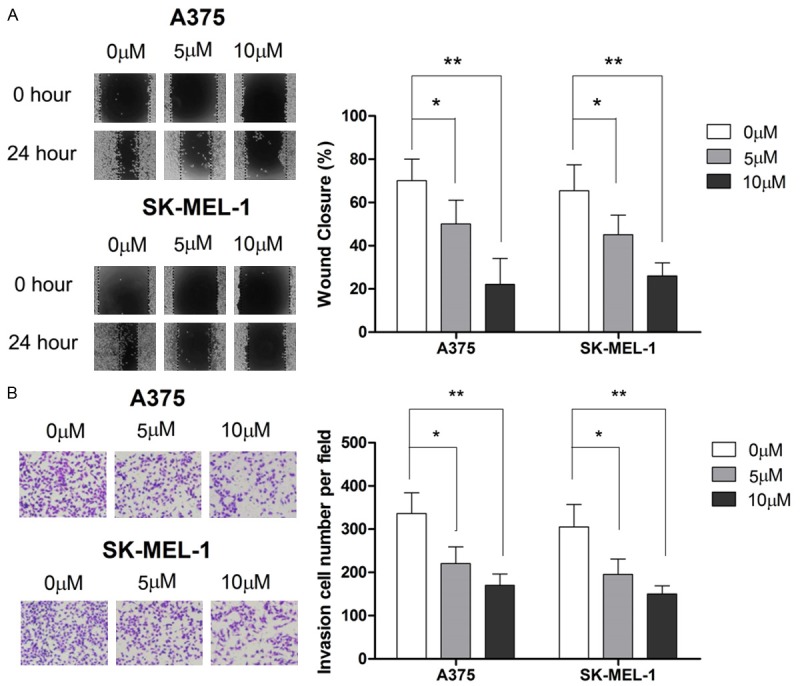
Given the pivotal role of COX-2 in the metastasis of melanoma, we asked whether COX-2 was involved in the suppressive effect of AIF on the metastatic potential of melanoma cells. Our results show that AIF downregulated COX-2 expression in melanoma cells at both the mRNA and protein levels (Figure 3A, 3B). To further explore the role of COX-2 in AIF-induced differentiation and inhibition of cell adhesion, migration, and invasion, we transfected A375 and SKMEL-1 cells with a COX-2 overexpression plasmid or siRNA targeting COX-2. As shown in Figure 3C-E, siRNA knockdown of COX-2 significantly increased intracellular melanin synthesis, PpIX accumulation, and TG2 activity, whereas COX-2 overexpression decreased these markers of differentiation. AIF treatment significantly attenuated COX-2 overexpression in A375 and SK-MEL-1 cells, indicating that that AIF promotes cell differentiation, at least in part, through the modulation of COX-2 expression. Similarly, siRNA knockdown of COX-2 significantly decreased cell migration and invasion, whereas COX-2 overexpression abrogated the inhibitory effect of AIF on these metastatic behaviors (Figure 4A, 4B). Collectively, our results suggest that the downregulation of COX-2 is involved in the inhibitory effect of AIF on the metastatic potential of melanoma cells.
Figure 3.
AIF promotes cell differentiation by suppressing COX-2 expression. Cells were incubated with AIF at the indicated concentrations for 24 hours. A. AIF treatment dose-dependently decreased COX-2 mRNA levels. B. AIF treatment dose-dependently decreased COX-2 protein levels. C. Transfection of a COX-2 overexpression plasmid attenuated the effect of AIF on melanin content. D. COX-2 overexpression attenuated AIF-induced PpIX accumulation. E. COX-2 overexpression attenuated the effect of AIF on TG2 activity. **P < 0.01 vs. control, ^^P < 0.01 vs. AIF.
Figure 4.
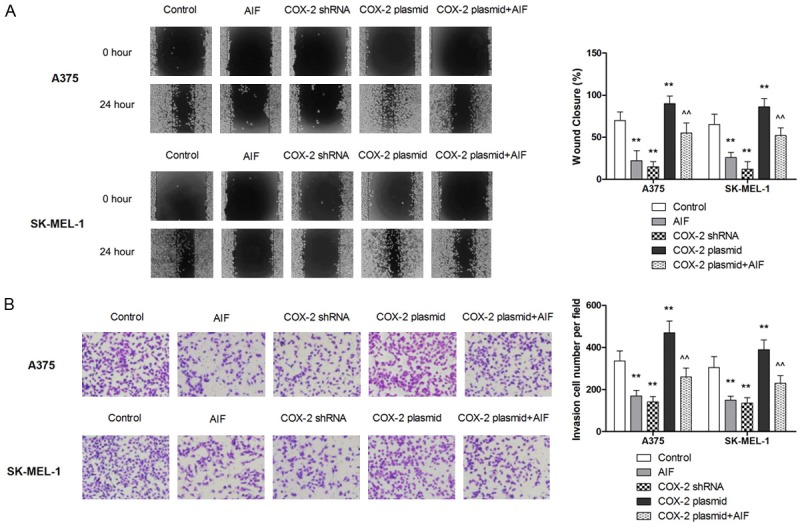
The anti-invasive effect of AIF in melanoma cells is mediated by downregulating COX-2 expression. Melanoma cells were incubated with AIF at the indicated concentrations for 24 hours. A. COX-2 overexpression attenuated the inhibitory effect of AIF on cell migration. B. COX-2 overexpression attenuated the inhibitory effect of AIF on cell invasion. **P < 0.01 vs. control, ^^P < 0.01 vs. AIF.
AIF modulates COX-2 expression by targeting miR-124/SPHK1 expression
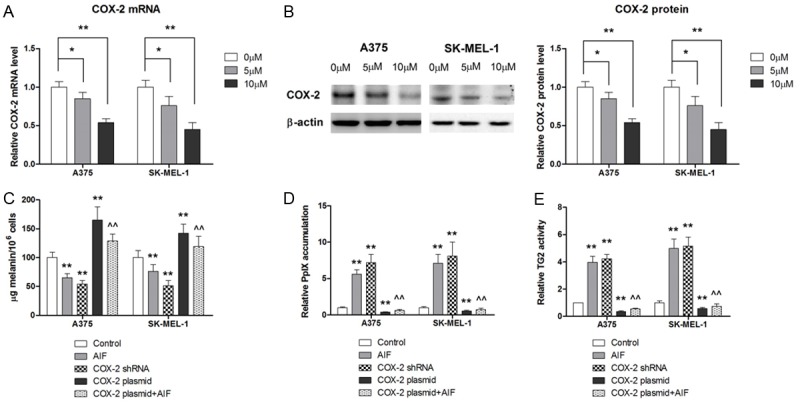
SPHK1 has been identified as a therapeutic target in melanoma [25] and has been reported to regulate COX-2 transcription [26,27]. Therefore, we evaluated whether AIF modulated COX-2 expression by suppressing SPHK1 activity. As shown in Figure 5A, western blot analysis showed that AIF treatment decreased SPHK1 protein levels. Since a number of flavonoid compounds are able to regulate miRNAs in cancer cells [28,29], two computational algorithms, TargetScan [30] and microRNA.org [31], were used in combination to search for miRNAs that might mediate the effect of AIF on SPHK1 in melanoma. Of the three miRNAs identified by this analysis (miR-122, miR-124, and miR-411), AIF treatment affected only the expression of miR-124 in melanoma cells (Figure 5B). Therefore, we examined whether SPHK1 was a direct target of miR-124 in melanoma cells. As shown in Figure 5C, miR-124 overexpression significantly decreased SPHK1 expression in melanoma cells, whereas miR-124 knockdown significantly increased SPHK1 expression. Similarly, results of the luciferase reporter assay showed that miR-124 overexpression significantly decreased SPHK1 gene transcription, whereas miR-124 knockdown significantly increased SPHK1 transcription, compared with controls (Figure 5D). Moreover, knockdown of miR-124 or overexpression of SPHK1 attenuated the ability of AIF to decrease COX-2 mRNA and protein levels (Figure 5E and 5F). We next examined the involvement of miR-124 and SPHK1 in the antimetastatic effect of AIF. Our results showed that miR-124 knockdown or SPHK1 overexpression significantly attenuated the antimetastatic effect of AIF (Figure 6A-E). Taken together, our results showed that AIF attenuates the metastatic potential of melanoma cells by downregulating COX-2 via the miR-124/SPHK 1 axis.
Figure 5.
AIF represses COX-2 expression by modulating miR-124/SPHK1 signaling. Cells were pretreated for 24 hours with AIF (10 μM unless otherwise noted). A. AIF treatment dose-dependently decreased SPHK1 protein levels in melanoma cells. B. AIF treatment dose-dependently increased mIR-124 expression in melanoma cells. C. MiR-124 knockdown significantly attenuated the suppressive effects of AIF on SPHK1 expression, as demonstrated by western blot analysis. D. Overexpression of miR-124 significantly decreased SPHK1 transcription, as assessed by the luciferase reporter assay. E. Both MiR-124 knockdown and SPHK1 overexpression significantly attenuated the suppressive effect of AIF on COX-2 mRNA expression. F. Both MiR-124 knockdown and SPHK1 overexpression significantly attenuated the suppressive effect of AIF on COX-2 protein expression. **P < 0.01 vs. control, ^^P < 0.01 vs. AIF.
Figure 6.
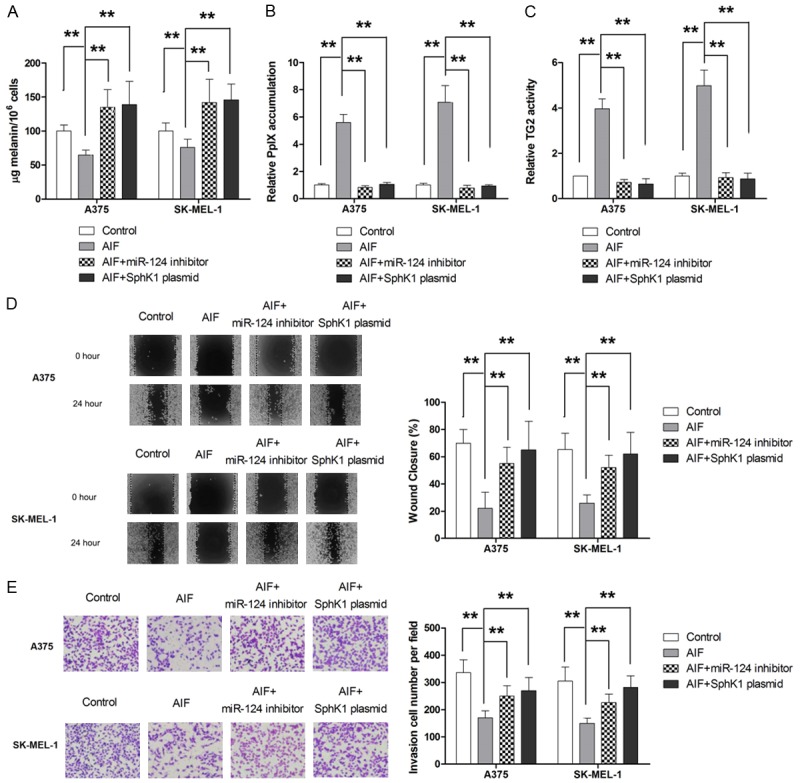
MiR-124/SPHK1 signaling is involved in the antimetastatic effect of AIF. Cells were pretreated for 24 hours with AIF (10 μM if not otherwise noted). A. COX-2 overexpression attenuated the effect of AIF on melanin content in melanoma cells. B. Both MiR-124 knockdown and SPHK1 overexpression significantly attenuated the effect of AIF on PpIX accumulation. C. Both MiR-124 knockdown and SPHK1 overexpression significantly attenuated the effect of AIF on TG2 activity. D. Both MiR-124 knockdown and SPHK1 overexpression significantly attenuated AIF-mediated inhibition of cell migration. E. Both MiR-124 knockdown and SPHK1 overexpression significantly attenuated AIF-mediated inhibition of cell invasion. **P < 0.01.
AIF suppresses melanoma lung metastasis in a mouse xenograft model
The antimetastatic effect of AIF was then evaluated in a mouse model of melanoma. Our results showed that AIF decreased the number of metastatic pulmonary nodules compared to that of control mice (Figure 7B). As shown in Figure 7C, results of qRT-PCR and western blot analysis showed that AIF decreased COX-2 and SPHK1 expression and increased miR-124 expression in metastatic tissues, supporting our in vitro results.
Figure 7.
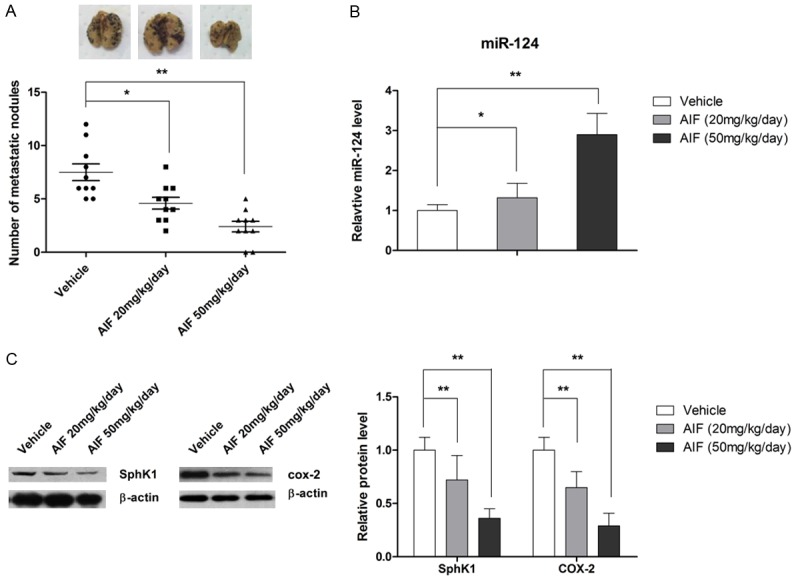
AIF suppresses B16-F10 murine melanoma cell lung metastasis in vivo. Xenograft-bearing mice were treated for 24 days with vehicle or AIF (20 or 50 mg/kg/day) by intragastric administration. Representative images of lung metastasis and the number of metastatic nodules in the lungs are shown. A. AIF significantly suppressed lung metastasis in vivo. B. AIF increased miR-124 expression in vivo. C. AIF treatment decreased expression levels of COX-2 and SPHK1 in vivo. *P < 0.05 (n = 8).
Discussion
Recent studies have described natural products that exert anticancer effects in melanoma [32]. In this study we showed that the naturally occurring flavonoid AIF suppresses metastasis in in vitro and in vivo models of melanoma. In addition, we found that COX-2 expression, mediated by the miR-124/SPHK1 signaling pathway, was important for the antimetastatic effect of AIF in melanoma.
COX-2 catalyzes the production of prostaglandins, which play a central role in cancer invasion and metastasis, making COX-2 a promising target for the prevention of metastatic melanoma [10]. In the present study we found that treatment with AIF impairs the metastatic potential of melanoma cells and is associated with decreased COX-2 expression. COX-2 is involved in a variety of biological activities in cancer cells. For example, Carpi et al. reported that COX-2 inhibition results in apoptosis and cell cycle arrest in A375 human melanoma cells [33], induces autophagy in cervical cancer cells [34], and exerts antiangiogenic effects in tumor endothelial and vascular progenitor cells in a mouse tumor model [35]. Therefore, by repressing COX-2, AIF may induce apoptosis and autophagy and inhibit angiogenesis in melanoma. Given that melanoma is a highly immunogenic tumor [36], AIF may also induce protective immunity against melanoma [37] because of the immunomodulatory function of COX-2 [38].
SPHK1 is an evolutionarily conserved lipid kinase that has been implicated in the development of a variety of various cancers including melanoma [39]. In glioma cells, SPHK1 knockdown induces apoptosis through decreased PI3K/Akt signaling [40]. In addition, targeting SPHK1 using siRNA or chemotherapeutic agents suppresses tumor cell growth in breast, prostate, and brain cancers [41-43]. In the context of melanoma, an early study by Bektas et al. reported that high SPHK1 activity counteracts ceramide-mediated cell death in human melanoma cells [44]. Madhunapantula et al. showed that targeting SPHK1 using siRNA or the pharmacological inhibitor SKI-I decreased anchorage-dependent and -independent growth and sensitized melanoma cells to apoptosis-inducing agents [25]. Moreover, a recent study reported that SPHK1 modulates communication between melanoma cells and dermal fibroblasts [45]. In the present study, we provide evidence that SPHK1 plays a role in the metastasis of melanoma. Collectively, these findings highlight the potential of SPHK1 as a therapeutic target in melanoma.
MicroRNAs (miRNAs or miRs) are highly conserved noncoding RNAs (14-24 nucleotides) that are widely present in mammalian genomes. They regulate gene expression by mediating the degradation of target mRNA or inhibition of mRNA translation [46]. Accumulating evidence shows that miRNA dysregulation occurs at various stages of melanoma progression and is associated with patient prognosis [47,48]. A number of miRNAs act as tumor suppressors in melanoma. For instance, miR-137 regulates melanocyte differentiation by repressing microphthalmia-associated transcription factor (MITF) expression [48] and exerts antitumor effects in melanoma cells by regulating MITF, c-Met, Y-box-binding protein 1, and enhancer of zeste homolog 2 (EZH2) [49]. In addition, MITF and EZH2 are targets of the tumor suppressor miR-101 [50]. However, several miRNAs play oncogenic roles in melanoma. For example, miR-135a promotes the progression of malignant melanoma through the regulation of FOXO1 [51]. In addition, miR-21 is upregulated in advanced melanoma [52,53] and regulates the metastatic behavior of B16 melanoma cells by inhibiting the tumor suppressor PDCD4 [54]. However, little is known about the role of miR-124 in melanoma. In the present study, we found that upregulation of miR-124 by AIF was associated with impaired metastasis, suggesting that miR-124 functions as a tumor suppressor in melanoma.
In conclusion, our study shows that the inhibition of COX-2 contributes to the antimetastatic effect of AIF in melanoma. We found that AIF modulates COX-2 expression, at least in part, through targeting the miR-124/SPHK1 axis. Our results suggest the potential of AIF as an anticancer agent in melanoma.
Disclosure of conflict of interest
None.
References
- 1.Leiter U, Garbe C. Epidemiology of melanoma and nonmelanoma skin cancer--the role of sunlight. Adv Exp Med Biol. 2008;624:89–103. doi: 10.1007/978-0-387-77574-6_8. [DOI] [PubMed] [Google Scholar]
- 2.Maddodi N, Setaluri V. Role of UV in cutaneous melanoma. Photochem Photobiol. 2008;84:528–536. doi: 10.1111/j.1751-1097.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 3.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 4.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 5.Gogas HJ, Kirkwood JM, Sondak VK. Chemotherapy for metastatic melanoma: time for a change? Cancer. 2007;109:455–464. doi: 10.1002/cncr.22427. [DOI] [PubMed] [Google Scholar]
- 6.Eberle J, Kurbanov BM, Hossini AM, Trefzer U, Fecker LF. Overcoming apoptosis deficiency of melanoma-hope for new therapeutic approaches. Drug Resist Updat. 2007;10:218–234. doi: 10.1016/j.drup.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Kvaskoff M, Weinstein P. Are some melanomas caused by artificial light? Med Hypotheses. 2010;75:305–311. doi: 10.1016/j.mehy.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Rundhaug JE, Fischer SM. Cyclo-oxygenase-2 plays a critical role in UV-induced skin carcinogenesis. Photochem Photobiol. 2008;84:322–329. doi: 10.1111/j.1751-1097.2007.00261.x. [DOI] [PubMed] [Google Scholar]
- 9.Meeran SM, Punathil T, Katiyar SK. IL-12 deficiency exacerbates inflammatory responses in UV-irradiated skin and skin tumors. J Invest Dermatol. 2008;128:2716–2727. doi: 10.1038/jid.2008.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denkert C, Kobel M, Berger S, Siegert A, Leclere A, Trefzer U, Hauptmann S. Expression of cyclooxygenase 2 in human malignant melanoma. Cancer Res. 2001;61:303–308. [PubMed] [Google Scholar]
- 11.Liao Z, Mason KA, Milas L. Cyclo-oxygenase-2 and its inhibition in cancer: is there a role? Drugs. 2007;67:821–845. doi: 10.2165/00003495-200767060-00001. [DOI] [PubMed] [Google Scholar]
- 12.Singh T, Vaid M, Katiyar N, Sharma S, Katiyar SK. Berberine, an isoquinoline alkaloid, inhibits melanoma cancer cell migration by reducing the expressions of cyclooxygenase-2, prostaglandin E(2) and prostaglandin E(2) receptors. Carcinogenesis. 2011;32:86–92. doi: 10.1093/carcin/bgq215. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Reich R, Martin GR. Identification of arachidonic acid pathways required for the invasive and metastatic activity of malignant tumor cells. Prostaglandins. 1996;51:1–17. doi: 10.1016/0090-6980(95)00154-9. [DOI] [PubMed] [Google Scholar]
- 14.Mvondo MA, Njamen D, Kretzschmar G, Imma Bader M, Tanee Fomum S, Wandji J, Vollmer G. Alpinumisoflavone and abyssinone V 4’-methylether derived from Erythrina lysistemon (Fabaceae) promote HDL-cholesterol synthesis and prevent cholesterol gallstone formation in ovariectomized rats. J Pharm Pharmacol. 2015;67:990–996. doi: 10.1111/jphp.12386. [DOI] [PubMed] [Google Scholar]
- 15.Mvondo MA, Njamen D, Tanee Fomum S, Wandji J. Effects of alpinumisoflavone and abyssinone V-4’-methyl ether derived from Erythrina lysistemon (Fabaceae) on the genital tract of ovariectomized female Wistar rat. Phytother Res. 2012;26:1029–1036. doi: 10.1002/ptr.3685. [DOI] [PubMed] [Google Scholar]
- 16.Chukwujekwu JC, Van Heerden FR, Van Staden J. Antibacterial activity of flavonoids from the stem bark of Erythrina caffra thunb. Phytother Res. 2011;25:46–48. doi: 10.1002/ptr.3159. [DOI] [PubMed] [Google Scholar]
- 17.Kuete V, Mbaveng AT, Nono EC, Simo CC, Zeino M, Nkengfack AE, Efferth T. Cytotoxicity of seven naturally occurring phenolic compounds towards multi-factorial drug-resistant cancer cells. Phytomedicine. 2016;23:856–863. doi: 10.1016/j.phymed.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Namkoong S, Kim TJ, Jang IS, Kang KW, Oh WK, Park J. Alpinumisoflavone induces apoptosis and suppresses extracellular signal-regulated kinases/mitogen activated protein kinase and nuclear factor-kappaB pathways in lung tumor cells. Biol Pharm Bull. 2011;34:203–208. doi: 10.1248/bpb.34.203. [DOI] [PubMed] [Google Scholar]
- 19.Tian S, He PY, Zhang JZ, Chen Z. Effect of kappa elastin on melanogenesis in A375 human melanoma cells and its related mechanism. Chin Med J (Engl) 2012;125:4088–4092. [PubMed] [Google Scholar]
- 20.Pichichero E, Cicconi R, Mattei M, Canini A. Chrysin-induced apoptosis is mediated through p38 and Bax activation in B16-F1 and A375 melanoma cells. Int J Oncol. 2011;38:473–483. doi: 10.3892/ijo.2010.876. [DOI] [PubMed] [Google Scholar]
- 21.Tabolacci C, Lentini A, Mattioli P, Provenzano B, Oliverio S, Carlomosti F, Beninati S. Antitumor properties of aloe-emodin and induction of transglutaminase 2 activity in B16-F10 melanoma cells. Life Sci. 2010;87:316–324. doi: 10.1016/j.lfs.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Dikshit B, Irshad K, Madan E, Aggarwal N, Sarkar C, Chandra PS, Gupta DK, Chattopadhyay P, Sinha S, Chosdol K. FAT1 acts as an upstream regulator of oncogenic and inflammatory pathways, via PDCD4, in glioma cells. Oncogene. 2013;32:3798–3808. doi: 10.1038/onc.2012.393. [DOI] [PubMed] [Google Scholar]
- 23.Xia J, Wu Z, Yu C, He W, Zheng H, He Y, Jian W, Chen L, Zhang L, Li W. miR-124 inhibits cell proliferation in gastric cancer through down-regulation of SPHK1. J Pathol. 2012;227:470–480. doi: 10.1002/path.4030. [DOI] [PubMed] [Google Scholar]
- 24.Ickowicz Schwartz D, Gozlan Y, Greenbaum L, Babushkina T, Katcoff DJ, Malik Z. Differentiation-dependent photodynamic therapy regulated by porphobilinogen deaminase in B16 melanoma. Br J Cancer. 2004;90:1833–1841. doi: 10.1038/sj.bjc.6601760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madhunapantula SV, Hengst J, Gowda R, Fox TE, Yun JK, Robertson GP. Targeting sphingosine kinase-1 to inhibit melanoma. Pigment Cell Melanoma Res. 2012;25:259–274. doi: 10.1111/j.1755-148X.2012.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawamori T, Osta W, Johnson KR, Pettus BJ, Bielawski J, Tanaka T, Wargovich MJ, Reddy BS, Hannun YA, Obeid LM, Zhou D. Sphingosine kinase 1 is up-regulated in colon carcinogenesis. FASEB J. 2006;20:386–388. doi: 10.1096/fj.05-4331fje. [DOI] [PubMed] [Google Scholar]
- 27.Pettus BJ, Bielawski J, Porcelli AM, Reames DL, Johnson KR, Morrow J, Chalfant CE, Obeid LM, Hannun YA. The sphingosine kinase 1/sphingosine-1-phosphate pathway mediates COX-2 induction and PGE2 production in response to TNF-alpha. FASEB J. 2003;17:1411–1421. doi: 10.1096/fj.02-1038com. [DOI] [PubMed] [Google Scholar]
- 28.Selvi RB, Swaminathan A, Chatterjee S, Shanmugam MK, Li F, Ramakrishnan GB, Siveen KS, Chinnathambi A, Zayed ME, Alharbi SA, Basha J, Bhat A, Vasudevan M, Dharmarajan A, Sethi G, Kundu TK. Inhibition of p300 lysine acetyltransferase activity by luteolin reduces tumor growth in head and neck squamous cell carcinoma (HNSCC) xenograft mouse model. Oncotarget. 2015;6:43806–43818. doi: 10.18632/oncotarget.6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Guo Q, Chen J, Chen Z. Quercetin Enhances cisplatin sensitivity of human osteosarcoma cells by modulating microRNA-217-KRAS Axis. Mol Cells. 2015;38:638–642. doi: 10.14348/molcells.2015.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–798. doi: 10.1016/s0092-8674(03)01018-3. [DOI] [PubMed] [Google Scholar]
- 31.John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human MicroRNA targets. PLoS Biol. 2004;2:e363. doi: 10.1371/journal.pbio.0020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Guo Z, Wu N, Xu W, Han L, Li N, Han Y. Two novel naphthalene glucosides and an anthraquinone isolated from Rumex dentatus and their antiproliferation activities in four cell lines. Molecules. 2012;17:843–850. doi: 10.3390/molecules17010843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carpi S, Fogli S, Romanini A, Pellegrino M, Adinolfi B, Podesta A, Costa B, Da Pozzo E, Martini C, Breschi MC, Nieri P. AM251 induces apoptosis and G2/M cell cycle arrest in A375 human melanoma cells. Anticancer Drugs. 2015;26:754–762. doi: 10.1097/CAD.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 34.Wang IT, Chou SC, Lin YC. Zoledronic acid induces apoptosis and autophagy in cervical cancer cells. Tumour Biol. 2014;35:11913–11920. doi: 10.1007/s13277-014-2460-5. [DOI] [PubMed] [Google Scholar]
- 35.Muraki C, Ohga N, Hida Y, Nishihara H, Kato Y, Tsuchiya K, Matsuda K, Totsuka Y, Shindoh M, Hida K. Cyclooxygenase-2 inhibition causes antiangiogenic effects on tumor endothelial and vascular progenitor cells. Int J Cancer. 2012;130:59–70. doi: 10.1002/ijc.25976. [DOI] [PubMed] [Google Scholar]
- 36.Shimanovsky A, Jethava A, Dasanu CA. Immune alterations in malignant melanoma and current immunotherapy concepts. Expert Opin Biol Ther. 2013;13:1413–1427. doi: 10.1517/14712598.2013.827658. [DOI] [PubMed] [Google Scholar]
- 37.Toomey D, Conroy H, Jarnicki AG, Higgins SC, Sutton C, Mills KH. Therapeutic vaccination with dendritic cells pulsed with tumor-derived Hsp70 and a COX-2 inhibitor induces protective immunity against B16 melanoma. Vaccine. 2008;26:3540–3549. doi: 10.1016/j.vaccine.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Padol IT, Hunt RH. Association of myocardial infarctions with COX-2 inhibition may be related to immunomodulation towards a Th1 response resulting in atheromatous plaque instability: an evidence-based interpretation. Rheumatology (Oxford) 2010;49:837–843. doi: 10.1093/rheumatology/kep225. [DOI] [PubMed] [Google Scholar]
- 39.Shida D, Takabe K, Kapitonov D, Milstien S, Spiegel S. Targeting SphK1 as a new strategy against cancer. Curr Drug Targets. 2008;9:662–673. doi: 10.2174/138945008785132402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guan H, Song L, Cai J, Huang Y, Wu J, Yuan J, Li J, Li M. Sphingosine kinase 1 regulates the Akt/FOXO3a/Bim pathway and contributes to apoptosis resistance in glioma cells. PLoS One. 2011;6:e19946. doi: 10.1371/journal.pone.0019946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kapitonov D, Allegood JC, Mitchell C, Hait NC, Almenara JA, Adams JK, Zipkin RE, Dent P, Kordula T, Milstien S, Spiegel S. Targeting sphingosine kinase 1 inhibits Akt signaling, induces apoptosis, and suppresses growth of human glioblastoma cells and xenografts. Cancer Res. 2009;69:6915–6923. doi: 10.1158/0008-5472.CAN-09-0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sarkar S, Maceyka M, Hait NC, Paugh SW, Sankala H, Milstien S, Spiegel S. Sphingosine kinase 1 is required for migration, proliferation and survival of MCF-7 human breast cancer cells. FEBS Lett. 2005;579:5313–5317. doi: 10.1016/j.febslet.2005.08.055. [DOI] [PubMed] [Google Scholar]
- 43.Edsall LC, Cuvillier O, Twitty S, Spiegel S, Milstien S. Sphingosine kinase expression regulates apoptosis and caspase activation in PC12 cells. J Neurochem. 2001;76:1573–1584. doi: 10.1046/j.1471-4159.2001.00164.x. [DOI] [PubMed] [Google Scholar]
- 44.Bektas M, Jolly PS, Muller C, Eberle J, Spiegel S, Geilen CC. Sphingosine kinase activity counteracts ceramide-mediated cell death in human melanoma cells: role of Bcl-2 expression. Oncogene. 2005;24:178–187. doi: 10.1038/sj.onc.1208019. [DOI] [PubMed] [Google Scholar]
- 45.Albinet V, Bats ML, Huwiler A, Rochaix P, Chevreau C, Segui B, Levade T, Andrieu-Abadie N. Dual role of sphingosine kinase-1 in promoting the differentiation of dermal fibroblasts and the dissemination of melanoma cells. Oncogene. 2014;33:3364–3373. doi: 10.1038/onc.2013.303. [DOI] [PubMed] [Google Scholar]
- 46.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mueller DW, Rehli M, Bosserhoff AK. miRNA expression profiling in melanocytes and melanoma cell lines reveals miRNAs associated with formation and progression of malignant melanoma. J Invest Dermatol. 2009;129:1740–1751. doi: 10.1038/jid.2008.452. [DOI] [PubMed] [Google Scholar]
- 48.Segura MF, Belitskaya-Levy I, Rose AE, Zakrzewski J, Gaziel A, Hanniford D, Darvishian F, Berman RS, Shapiro RL, Pavlick AC, Osman I, Hernando E. Melanoma MicroRNA signature predicts post-recurrence survival. Clin Cancer Res. 2010;16:1577–1586. doi: 10.1158/1078-0432.CCR-09-2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luo C, Tetteh PW, Merz PR, Dickes E, Abukiwan A, Hotz-Wagenblatt A, Holland-Cunz S, Sinnberg T, Schittek B, Schadendorf D, Diederichs S, Eichmuller SB. miR-137 inhibits the invasion of melanoma cells through downregulation of multiple oncogenic target genes. J Invest Dermatol. 2013;133:768–775. doi: 10.1038/jid.2012.357. [DOI] [PubMed] [Google Scholar]
- 50.Luo C, Merz PR, Chen Y, Dickes E, Pscherer A, Schadendorf D, Eichmuller SB. MiR-101 inhibits melanoma cell invasion and proliferation by targeting MITF and EZH2. Cancer Lett. 2013;341:240–247. doi: 10.1016/j.canlet.2013.08.021. [DOI] [PubMed] [Google Scholar]
- 51.Ren JW, Li ZJ, Tu C. MiR-135 post-transcriptionally regulates FOXO1 expression and promotes cell proliferation in human malignant melanoma cells. Int J Clin Exp Pathol. 2015;8:6356–6366. [PMC free article] [PubMed] [Google Scholar]
- 52.Satzger I, Mattern A, Kuettler U, Weinspach D, Niebuhr M, Kapp A, Gutzmer R. microRNA-21 is upregulated in malignant melanoma and influences apoptosis of melanocytic cells. Exp Dermatol. 2012;21:509–514. doi: 10.1111/j.1600-0625.2012.01510.x. [DOI] [PubMed] [Google Scholar]
- 53.Jiao J, Fan Y, Zhang Y. Expression and clinicopathological significance of microRNA-21 and programmed cell death 4 in malignant melanoma. J Int Med Res. 2015;43:672–678. doi: 10.1177/0300060515583707. [DOI] [PubMed] [Google Scholar]
- 54.Yang CH, Yue J, Pfeffer SR, Handorf CR, Pfeffer LM. MicroRNA miR-21 regulates the metastatic behavior of B16 melanoma cells. J Biol Chem. 2011;286:39172–39178. doi: 10.1074/jbc.M111.285098. [DOI] [PMC free article] [PubMed] [Google Scholar]