Abstract
Aberrant angiogenesis and vascular remodeling are the main features of idiopathic pulmonary fibrosis. Kallistatin is an anti-angiogenic peptide with known effects on endothelial cells. This study aimed to demonstrate that kallistatin has beneficial effects on bleomycin (BLM)-induced pulmonary fibrosis in a rat model by inhibiting angiogenesis. Twenty-five rats were randomly divided into five experimental groups: (A) Saline only (SA)-as the negative control, (B) BLM only (BLM)-as the model group, (C) BLM and 0.1 mg/kg kallistatin (L-Kal), (D) BLM and 0.5 mg/kg kallistatin (M-Kal), and (E) BLM and 2.5 mg/kg kallistatin (H-Kal). Fibrillar collagen was quantified by Masson’s trichrome and hematoxylin-eosin staining. Transforming growth factor-β1 (TGF-β1), α-smooth-muscle-actin (α-SMA) and microvascular density (MVD) were measured by immunohistochemistry. Vascular endothelial growth factor (VEGF), vascular endothelial growth factor receptor (VEGFR), and tumor necrosis factor-α (TNF-α) were assayed by Western immunoblotting or ELISA. Daily administration of kallistatin attenuated fibrosis in BLM-induced pulmonary fibrosis, as shown by histology. During inflammation from BLM-induced pulmonary fibrosis, kallistatin reduced the number of inflammatory cells infiltrating the bronchoalveolar lavage fluid. Kallistatin also inhibited VEGF expression and phosphorylation of VEGFR2 (Flk-1). In vitro, kallistatin blocked tube formation by inhibiting Flk-1 and GSK-3β phosphorylation. The results demonstrated that continuous administration of kallistatin attenuated BLM-induced pulmonary fibrosis and improved survival of BLM rats. Reducing pulmonary fibrosis was achieved by partial inhibition of pulmonary inflammation and angiogenesis.
Keywords: Kallistatin, bleomycin, pulmonary fibrosis, angiogenesis
Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive lung disease of unknown etiology. The pathogenesis of pulmonary fibrosis involves lung epithelial injury and aberrant proliferation of fibroblasts, and results in progressive pulmonary scarring and declining lung function [1,2]. Additionally, there is ample evidence to support the importance of platelet-derived growth factor (PDGF) in the lung of IPF [3,4]. Previous reports have shown that PDGF is expressed in the lung of IPF patients and in bronchoalveolar lavage fluid (BALF) of animal models, which supports the hypothesis that PDGF is involved in disease development [5,6]. In addition, vascular endothelial growth factor (VEGF) is a key regulator of angiogenesis, and its receptors are involved in the pathogenesis of pulmonary sarcoidosis and fibrosis [5-7]. The working hypothesis is that tyrosine kinase inhibitors of VEGFR will slow both the fibrotic process that is associated with PDGF signaling and angiogenic processes regulated by VEGF.
Kallistatin is a plasma protein and a novel human tissue kallikrein inhibitor [8]. Kallistatin exerts pleiotropic functions, such as displaying activity against angiogenesis, apoptosis, inflammation, and oxidative stress [9-12]. Plasma kallistatin levels are significantly reduced in patients with liver disease, and correlate with sepsis and disease severity in community-acquired pneumonia [13]. Furthermore, animal studies have previously shown that kallistatin inhibits inflammatory cell infiltration and oxidative stress in animal models of myocardial ischemia-reperfusion injury, chronic myocardial infarction and salt-induced renal injury [14-16]. In the carbon tetrachloride-induced liver injury rat model, liver damage brought on by reduced oxidative stress was attenuated by transgenic expression of kallistatin or administration of recombinant kallistatin [10,17]. Taken together, these findings reveal that kallistatin exerts protective functions by various bioactivities. However, the role of kallistatin in IPF has not yet been described.
In the present study, we provide evidence that kallistatin protects against idiopathic pulmonary fibrosis. Our results show that kallistatin levels in the BALF were associated with lung inflammation. Kallistatin prevents BLM-induced pulmonary fibrosis in rats by dampening aberrant angiogenesis, proinflammatory cytokine production and inhibition of Primary Bronchial Epithelial Cell (PBEC) tuber formation.
Material and methods
Animals and reagents
Male Sprague-Dawley (SD) rats (mean weight: 150-200 g) were obtained from the Experimental Center of the Medical Scientific Academy of Fujian, China. Animal studies were approved by the Animal Ethics Committee of Huaqiao University. The animals were cared for in accordance with protocols that were approved by the Animal Care and Use Committee of Huaqiao University. Rats were housed at 23 ± 2°C and 55 ± 5% humidity, with a 12 h light-dark cycle, and acclimatized for one week before commencing studies. Rodent food and water were provided adlibitum.
Bleomycin hydrochloride was purchased from Invitrogen (New York, USA), and dissolved in sterile PBS on the day of intratracheal instillation at a dose of 5 mg/kg. The rabbit anti-VEGF, rabbit anti-Flk-1, rabbit anti-P-Flk-1, rabbit anti-ERK1/2, rabbit anti-P-ERK, and rabbit anti-p38MAPK antibodies were obtained from Cell Signaling Technology (Boston, MA, USA). Both the IL-1β and TNF-α ELISA kits were purchased from R&D Systems (Minneapolis, USA). PVDF membranes and the Immobilon ECL detection system were purchased from Millipore Co (Billerica, MA, USA). Recombinant human kallistatin was expressed in Pichia pastoris strain GS115 and purified with a series of chromatographic steps, mainly Phenyl Superose and Heparin Sepharose FF chromatography.
Animal model of BLM-induced pulmonary fibrosis
Pulmonary fibrosis was induced in rats as previously described with some modifications [18]. Briefly, the rats were randomly divided into five groups, each group included five rats: (A) comprised the negative control group that was injected with saline only (SA group), (B) comprised the model group that was injected with BLM only (BLM group), (C) comprised the BLM plus 0.1 mg/kg kallistatin (L-Kal) group, (D) comprised the BLM plus 0.5 mg/kg kallistatin (M-Kal) group, and (E) comprised the BLM plus 2.5 mg/kg kallistatin (H-Kal) group. Bleomycin (5 mg/kg bodyweight) was intratracheally administrated to male ratsin a total volume of 100 μl PBS. Control groups received an equal volume of sterile PBS using the same procedures. Kallistatin was administered daily through subcutaneous injection during the entire course (i.e., from days 1-28) after BLM infusion. Lungs were harvested on day 29 for histological examination and hydroxyproline assay. Bronchoalveolar lavage (BAL) fluid was collected from a group of rats on day 29 and cell numbers were counted using a hemocytometer. Two hundred cells from each animal were counted and expressed as a percentage of the total cells. The protein permeability index (PPI) was calculated as described previously [19] and as follows: PPI = (BALF total protein/plasma total protein) × 100.
Histological analysis
Lung tissues were fixed with 10% formalin and embedded in paraffin. Six micrometer sections of paraffin-embedded rat lung tissues were stained with hematoxylin and eosin (H&E) and Masson’s trichrome to examine the degree of fibrosis. The content of collagen (stained blue on a pale red background) was quantified using the histogram module of the Photoshop 8.0 software program (Adobe).
Hydroxyproline assay of lung tissues
To analyze the amount of collagen in rat lung tissues, hydroxyproline content was measured as previously described [20]. In brief, 100 mg of lung tissue was hydrolyzed in 1 ml lysis buffer solution (pH 7.4, 10 mM Tris-HCl, 0.1 mM EDTA-2Na, 10 mM sucrose, and 0.8% sodium chloride solution) at 100°C for 20 min. The hydroxyproline concentration was then determined according to the manufacturer’s instructions.
Immunohistochemistry
To detect the immunohistochemical localization of TGF-β1, α-SMA and CD34, we took sections from formalin-fixed, paraffin-embedded specimens that were then deparaffinized and rehydrated in decreasing concentrations of ethanol. All tissue sections were treated with fresh 3% hydrogen peroxide for 20 min, and then washed with PBS. The sections were sequentially incubated with 1% normal blocking serum for 30 min, and then with rabbit anti-rat TGF-β1 monoclonal antibody or rabbit anti-rat α-SMA monoclonal antibodies or rabbit anti-rat CD34 monoclonal antibody, and then incubated for 60 min, with appropriate HRP-conjugated goat anti-rabbit secondary antibody subsequently incubated for 60 min, using DAB as a substrate. All the incubation steps were performed at room temperature and three washes with PBS were applied between steps. Negative controls were obtained by omitting the primary antibodies. The sections that were counterstained with hematoxylin, were then mounted and observed under light microscopy by a blinded pathologist.
Enzyme-linked immunosorbent assay
The TNF-α concentration was measured in lung tissue lysates of BALF. Briefly, 100 mg of lung tissue from both control and experimental animals were homogenized in 2 ml lysate buffer (50 mM Tris-HCl (pH 7.4) containing 1% NP-40, 50 mM NaCl, 0.5 mM ethylene diamine tetraacetic acid (EDTA), and 100 mM phenylmethylsulfonyl fluoride (PMSF)). The protein concentration in each sample was determined by the bicinchoninic acid (BCA) assay according to the manufacturer’s instructions. TNF-α levels were measured by quantitative ELISA according to the manufacturer’s protocols (R&D Systems, Minneapolis, MN, USA).
Real-time PCR
Total RNA was extracted from lung tissues using Trizol (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Real-time PCR was performed in the ABI2720 system (Applied Biosystems) by the SYBR Green assay after reverse transcribing 1 μg of RNA with reverse transcriptase. All data were normalized to the level of β-actin mRNA. The primer pairs and expected lengths (in bp) were as follows (as defined as 5’ to 3’): VEGF (GenBank: NM_001110333.1, forward: GGCGAGCTGAGCGGCG GCAGCGGAG, reverse: AAGAGGTTTGAATATCAAATCCCAG; 115 bp) VEGFR2 (GenBank: NM_013062.1, forward: AGACACTGAGCATGGAAGAGGATTC, reverse: GGCCGGCTTTTTCGC TTGCTGTTCT; 155 bp) β-actin (GenBank: NM_031144.3, forward: CACCCGCGA GTACAACCTTC, reverse: CCCATACCCACCATCA-CACC; 207 bp). The cycling conditions were as follows: 95°C for 30 s, followed by 40 cycles of 95°C for 5 s, 65°C for 30 s, and 65°C for 15 s.
Western immunoblot
Protein samples (40 μg) were separated on precast 10% SDS polyacrylamide gels (SDS-PAGE). After electrophoresis, the proteins were transferred to PVDF membrane filters (Millipore Biotechnology Inc., USA). The membranes were blocked with BSA, then incubated overnight at 4°C with primary rabbit monoclonal phosphorylated ERK (p-ERK) and ERK (Cell Signaling, Beverly, MA, USA). After washing three times in TBS-T, horseradish peroxidase (HRP)-conjugated secondary antibodies were inoculated at a dilution of 1:5000 in TBS-T for 1 h at room temperature. After three additional washes with TBS-T, the immunoreactive bands were visualized with chemiluminescence reagent (ECL, Millipore Biotechnology Inc., USA) and quantified using Image lab3.0 (Hercules, CA, USA) Analysis Software.
Actions of kallistatin on tube formation
Primary Bronchial Epithelial Cells (PBECs, ATCC, PCS-300-010) were cultured in EGM-2 media (Lonza, Wakersfield, MD, USA), according to the manufacturer’s protocol. Briefly, a 24-well plate was coated with 200 μl of matrigel (BD Biosciences), which was allowed to polymerize and solidify at 37°C for 1 h. The PBECs were incubated in 6-well plate with EGM-2 medium containing 1% FBS for 6 h, following which, the cells were trypsinized before harvesting them. Cells were then resuspended in EBM-2 medium containing 1% FBS, and kallistatin was added to the cell mixture at a final concentration of 100 μg/ml at the same time. Cells were seeded onto the matrigel layer, and then VEGF165 (10 ng/ml) was added 1 h later. Blood-vessel-like tubules from six randomly chosen fields were counted and photographed under a microscope after 18 h post-treatment.
Statistical analysis
Results were shown as the mean ± SD. Comparisons among groups were performed by one-way ANOVA, followed by Scheffe’s post-hoc test. All statistical analyses were done using the IBM SPSS statistics 22 software program (IBM Corporation, Armonk, NY, USA). The criterion for statistical significance was set at an alpha value of P < 0.05.
Results
Effect of kallistatin on BLM-induced pulmonary fibrosis
We elucidated the effect of kallistatin on the BLM-induced pulmonary fibrosis model. Lung fibrosis was assessed by morphometry of Masson’s trichrome-stained lung sections and H&E staining, which demonstrated that BLM treatment significantly induced distortion of the lung (Figure 1A and 1B). The rats in the saline control group did not show any lung damage, and well-alveolised normal histology was observed. Lung injury was attenuated in the kallistatin-treated groups. Fibrillar collagen deposition as an indicator of lung fibrosis was determined by Masson’s trichrome staining. Injection of BLM induced bridging fibrosis in rats. By contrast, the pathological progression was attenuated by addition of kallistatin, which showed fewer and smaller fibrotic foci. Quantification of Masson’s trichrome staining showed obvious increases in collagen accumulation in BLM-induced fibrotic rats compared with the negative control group, while combined administration of kallistatin resulted in a dose-dependent decrease in the positive staining area (Figure 1C). These data support the view that kallistatin played an important role in reducing pathological collagen deposition and structural damage in the BLM-induced rat lung fibrosis model.
Figure 1.
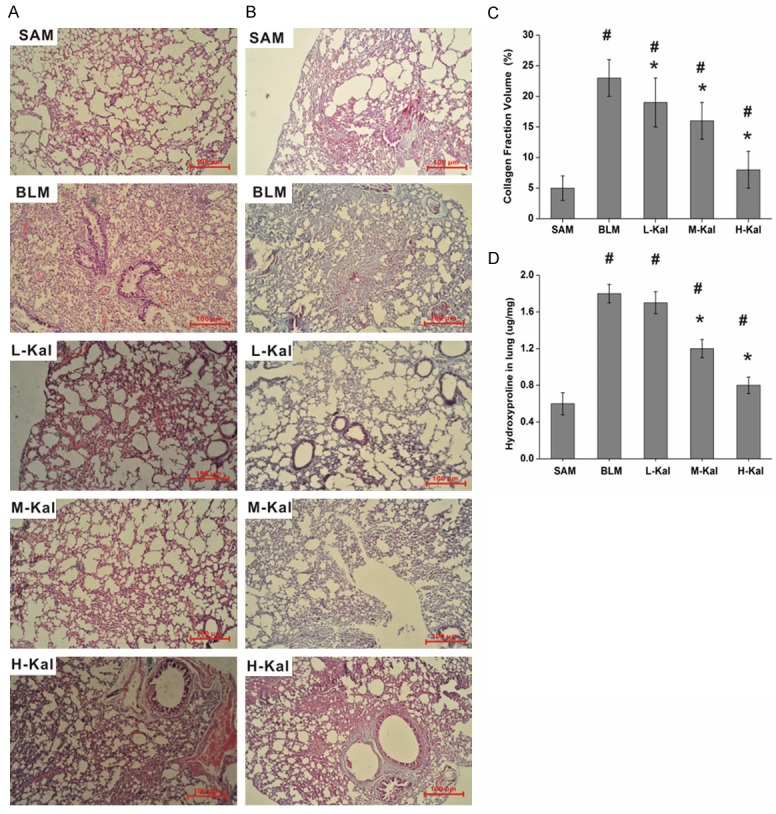
Kallistatin prevents BLM-induced injury to lung structures in rats. Representative images of H&E (A) or Masson’s trichrome (B) stained sections (original magnifications × 40). Scale bars = 100 μm. (C) Collagen deposition was evaluated by Masson’s trichrome staining and quantitated by image analysis. Data are expressed as mean ± SD (n = 5). (D) Collagen deposition was assessed by measuring the hydroxyproline content. #P < 0.05 vs. SA group; *P < 0.05 vs. BLM group.
The results were further confirmed by analysis of hydroxyproline content in the lung. Fibrosis, which represents the final result of prolonged lung injury, can be quantified by hydroxyproline analysis and is expressed as lung collagen content. Hydroxylation of prolines stabilizes collagen, and hydroxyproline content expression is a marker of fibrosis. The BLM-treated rats had significantly higher lung hydroxyproline content than did saline control rats. Whereas administration of kallistatin reduced the lung hydroxyproline content in BLM-induced lung fibrotic rats in a dose-dependent manner.
Effect of kallistatin on the inflammation response in BLM-induced pulmonary fibrosis
To investigate the effect of kallistatin on the inflammation response that was induced by BLM infusion in rats, we assessed inflammatory cell number and the levels of the pro-inflammatory cytokine TNF-α in the BALF. The total number of cells and the number of inflammatory cells were significantly increased in BLM-treated rats. However, the total number of cells, lymphocytes, neutrophils and macrophages were significantly decreased following kallistatin treatment (Figure 2A-D). Numerous inflammatory mediators have been identified to contribute to the pathogenesis of lung fibrosis. Therefore, we determined the expression profiles of several cytokines and chemokines in rats with BLM-induced pulmonary fibrosis. Our results showed that the levels of TNF-α and IL-1β in the BLAF were markedly decreased in rats receiving kallistatin as compared to control rats after BLM challenge (Figure 2E and 2F). Since vascular permeability plays a key role in leukocyte and protein leakage, we assessed the PPI as the ratio of BALF to plasma protein. In our study, the PPI was suppressed by kallistatin administration (Figure 2G).
Figure 2.
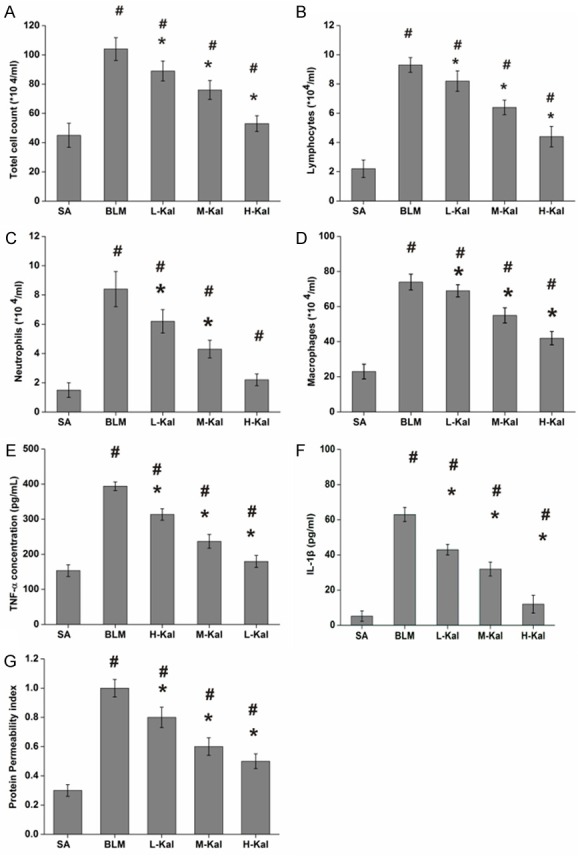
Effect of kallistatin on the inflammatory response in BLM-induced pulmonary fibrosis. Total cells (A), neutrophils (B), lymphocytes (C), and macrophage (D) counts in BALF rats and expression of TNF-α (E), and IL-1β (F) in BALF rats. (G) Representative changes in PPI, calculated as BALF total protein/plasma total protein × 100. Results are expressed as mean ± SD; n = 5 in each group. #P < 0.001 vs. SA group; *P < 0.05 vs. BLM group.
Effect of kallistatin on TGF-β1 and α-SMA expression in lung tissue
TGF-β1 and α-SMA are considered the most important fibroblast stimulators leading to pulmonary fibrosis. Therefore, we measured the levels of TGF-β1 and α-SMA in lung tissue by immunohistochemistry and Western immunoblotting. Both TGF-β1 and α-SMA levels were increased in the BLM group as compared the SA group. After kallistatin administration, the induction of TGF-β1 and α-SMA expression by BLM was decreased (Figure 3).
Figure 3.
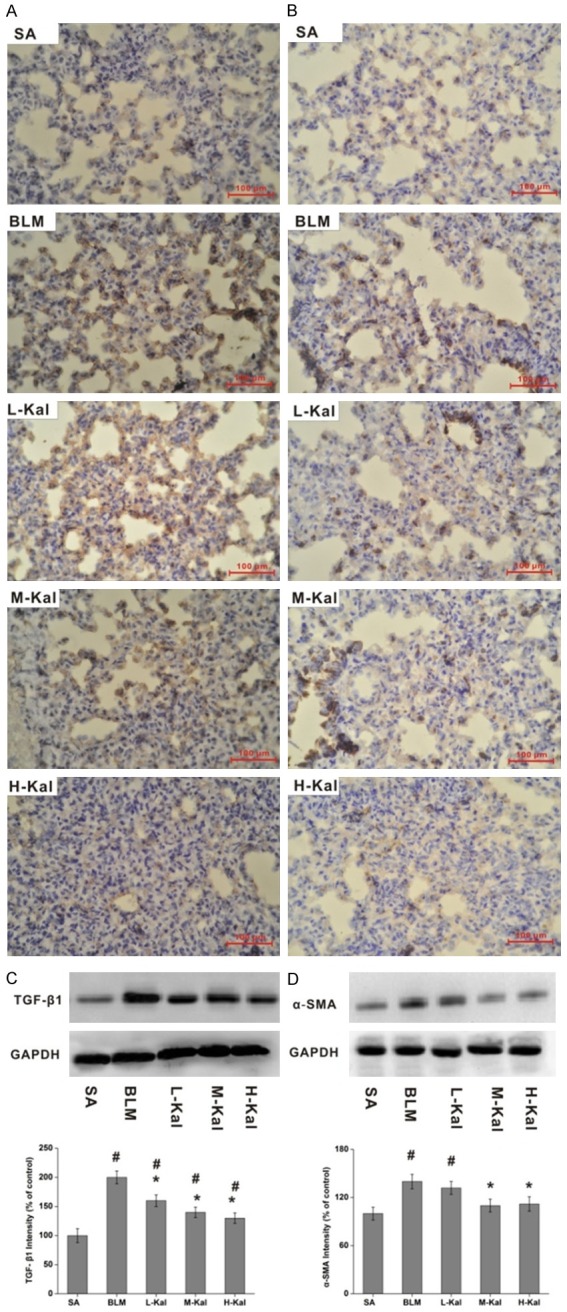
Kallistatin prevents BLM-induced lung fibrogenesis in rats. Representative images of immunohistochemical staining for TGF-β1 (A) and α-SMA (B) are shown (original magnification × 40) respectively. Western immunoblotting analysis and levels of TGF-β1 (C) and α-SMA (D) in lung from BLM alone or BLM plus kallistatin treated rats. Data are expressed as mean ± SD (n = 5). #P < 0.05 vs. SA group; *P < 0.05 vs. BLM group. Data from Western immunoblotting were confirmed, showing kallistatin-dependent abrogation of α-SMA and TGF-β1 expression. Western immunoblotting and immunohistochemistry results were consistent. House-keeping proteins like GAPDH are useful as loading controls for Western blotting and protein normalization.
Effect of kallistatin on MVD in BLM-treated rat lung tissues
It was previously proposed that IPF is associated with increased angiogenesis, and select reports showed that angiogenesis in fibrotic lungs may actually be decreased. To explore the effect of kallistatin on angiogenesis in BLM-induced pulmonary fibrosis, we quantified lung MVD by immunostaining for the endothelial marker CD34. The number of CD34-positive vessels was increased in BLM-treated lungs. In addition, kallistatin administration markedly decreased CD34-positive cell numbers as compared the BLM-only group (Figure 4).
Figure 4.
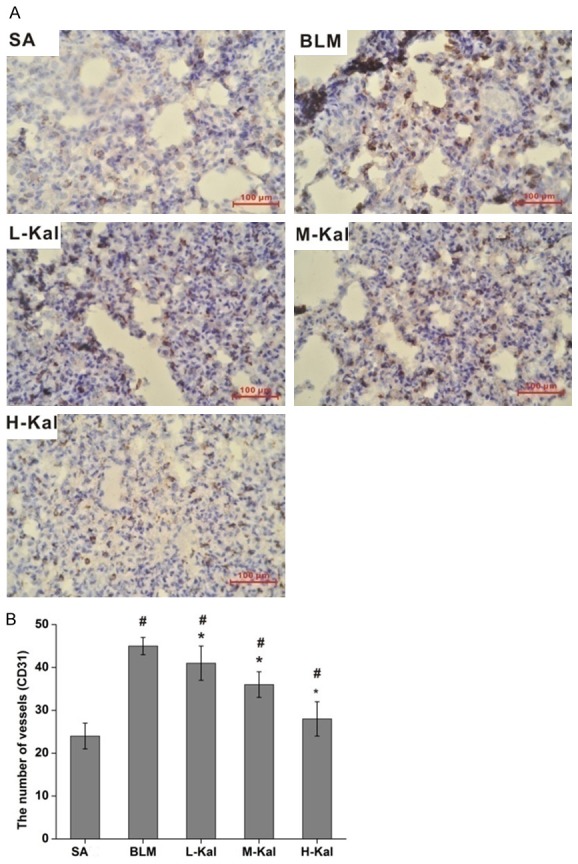
Effect of kallistatin on MVD in BLM-induced lung fibrosis. A. Immuno-histochemical staining of CD34 in lung sections. B. MVD was assayed by counting the number of microvessels per high-power field in lung sections that were stained with antibody targeted to CD34. Bar = 100 μm. #P < 0.05 vs. SA group; *P < 0.05 vs. BLM group.
Effect of kallistatin on VEGF/Flk-1 expression in BLM induced pulmonary fibrosis
VEGF expression is significantly increased after BLM instillation in lung tissues, which is measured by Western immunoblotting and real-time PCR. The VEGF levels in the kallistatin treated groups were lower than in the BLM group (Figure 5A and 5C). The Flk-1 mRNA from the BLM group was higher than that found in the saline group after BLM infusion. However, no difference was found between any of the kallistatin treatment and BLM groups in terms of the levels of Flk-1 mRNA (Figure 5B). Further, kallistatin administration efficiently inhibited the phosphorylation of Flk-1 as compared the BLM group (Figure 5D).
Figure 5.
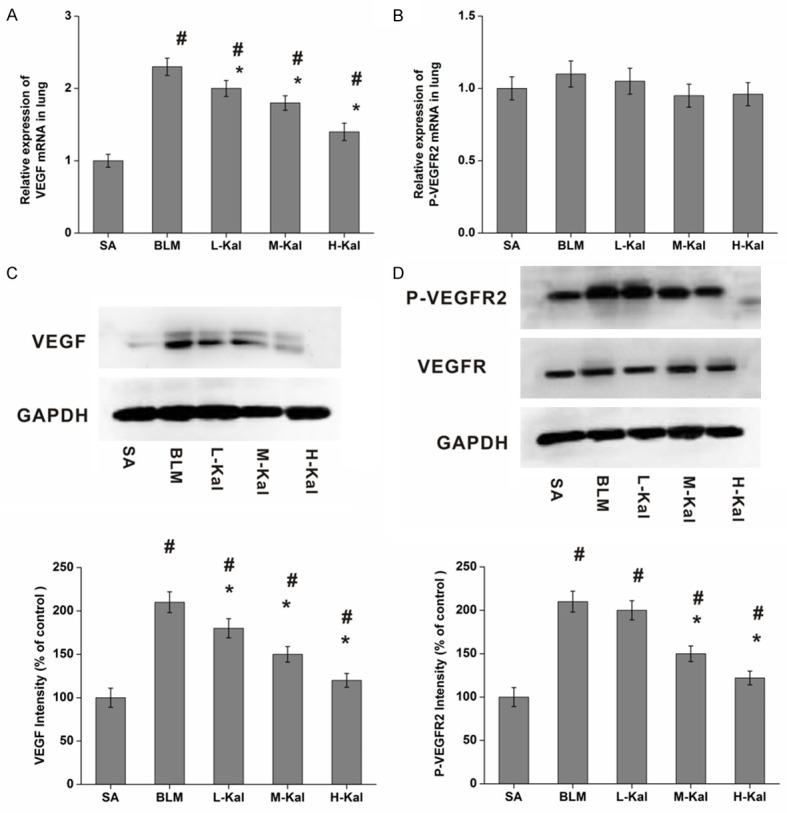
Effect of kallistatin on VEGF/VEGFR2 expression in BLM-induced pulmonary fibrosis. A. VEGF mRNA; B. VEGFR2 mRNA expression were measured by real-time PCR. C. VEGF; D. VEGFR2 expression were measured by Western immunoblotting. Results are expressed as mean ± SD, n = 5 in each group, #P < 0.05 vs. SA group; *P < 0.05 vs. BLM group. The house-keeping protein GAPDH was used as a loading control for Western blot and protein normalization.
Effect of kallistatin on ERK1/2 phosphorylation in BLM induced pulmonary fibrosis
Both ERK and MAPK activation play an important role in angiogenesis and inflammation during pulmonary fibrosis. We evaluated whether ERK1/2 and p38MAPK phosphorylation levels were altered by kallistatin administration in rat lungs after BLM challenge. Western immunoblotting showed that phosphorylated ERK1/2 and p38MAPK were significantly increased in BLM groups as compared with the saline groups. By contrast, kallistatin treatment resulted in a significant decrease in phosphorylated ERK1/2 and p38MAPK expression. However, no significant differences were observed in ERK1/2 and p38MAPK expression levels when comparing the kallistatin treated and BLM groups (Figure 6).
Figure 6.
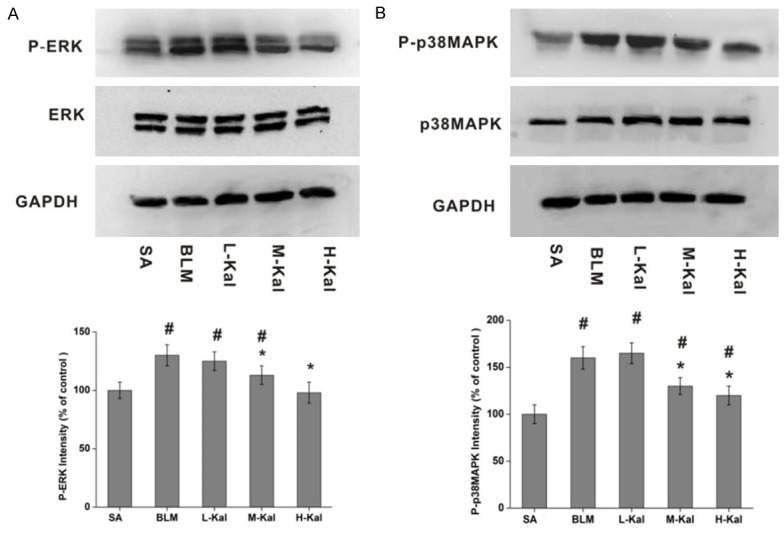
Kallistatin suppressed both ERK1/2 and p38MAPK phosphorylation. A. Western blotting analysis and quantification of ERK1/2 and P-ERK1/2 levels in different experimental groups. B. Western blotting and determination of the levels of p38MAPK and P-p38MAPK in different experimental groups. Data are expressed as mean ± SD (n = 5). #P < 0.05 vs. SA group; *P < 0.05 vs. BLM group.
Kallistatin suppressed VEGF165-induced tube formation of PBECs
The PBECs on matrigel matrices were stimulated with VEGF165, which promoted the differentiation of the cells into a tube form. Kallistatin effectively reduced the quantity of endothelial tubes that were induced by VEGF165 (Figure 7A). In vitro, kallistatin also suppressed VEGF and phosphorylated Flk-1 expression, but did not suppress Flk-1 expression (Figure 7B and 7C). Both the AKT and GSK-3β signal transduction pathways regulates angiogenesis, including protein synthesis, cell differentiation, apoptosis and cell survival. We evaluated the effects of kallistatin treatment on PBECs. The results showed that kallistatin suppressed GSK and AKT phosphorylation (Figure 7D and 7E), and kallistatin could block VEGF165-induced tube formation of PBECs.
Figure 7.
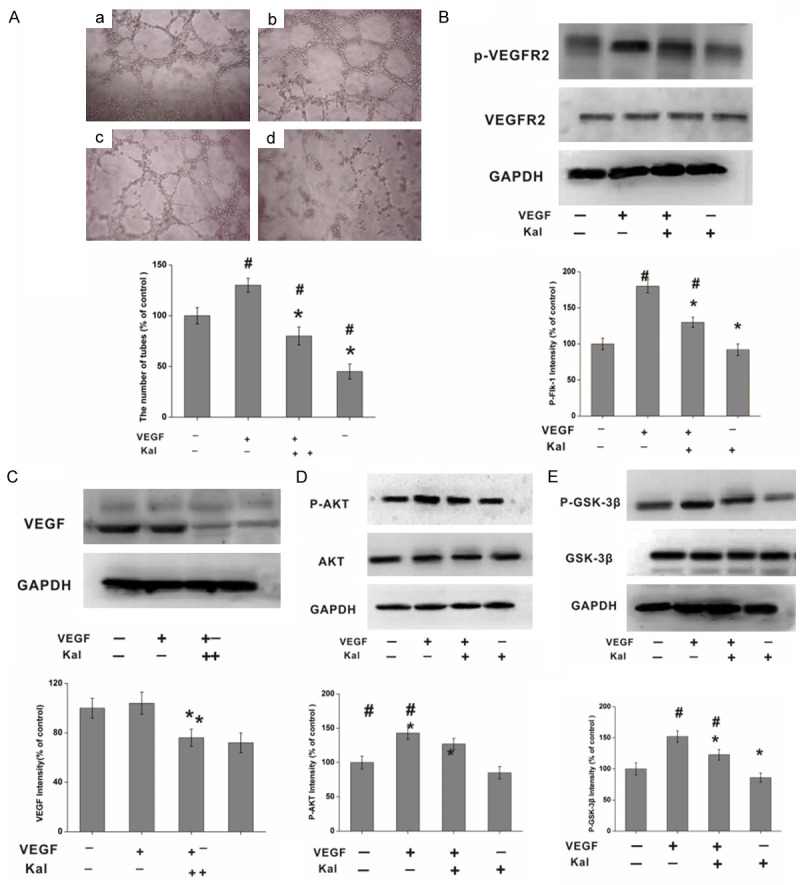
Effect of kallistatin on VEGF-induced tube formation of PBECs. (A) Effect of kallistatin on tube formation (a: PBECs; b: PBECs treated with VEGF; c: PBECs treated with kallistatin and VEGF; d: PBECs treated with kallistatin); (B) Effect of kallistatin on VEGFR2 phosphorylation; (C) Effect of kallistatin on VEGF expression; (D) Effect of kallistatin on AKT phosphorylation; (E) Effect of kallistatin on GSK-3β phosphorylation.
Discussion
IPF is an important health issue in humans, and the precise pathogenic mechanisms remain unknown. BLM can cause toxicity by generating an acute inflammatory response and fibrosis in a short period of time, whereas IPF has a slow and irreversible progression in patients. Histological hallmarks present in BLM-treated rats, including intra-alveolar buds, mural incorporation of collagen and obliteration of the alveolar space, are similar to that seen in IPF patients [21]. Increasing evidence has demonstrated that angiogenesis is important in the development and progression of pulmonary fibrosis [22-24].
In this study, kallistatin was administered in BLM-induced lung injury and fibrosis in rats, which demonstrated that kallistatin administration had the following effects: (1) decreased hydroxyproline content and collagen deposition; (2) reduced numbers of inflammatory cells present in the BALF and decreased production of pro-inflammatory and fibrotic cytokines like TNF-α and IL-1β; (3) reduced MVD and VEGF/P-Flk-1 expression; (4) inhibited ERK1/2 and p38MAPK activation; and (5) inhibited tube formation of PBECs. From these findings, we concluded that kallistatin might play an important role in the course of pulmonary fibrosis induced by BLM, which may be mediated by its potential regulatory effects on inhibiting the VEGF receptor and ERK1/2 activation.
Kallistatin is a pleiotropic cytokine which has anti-inflammatory and anti-oxidant properties, and may hold therapeutic promise in preventing many diseases, including cardiometabolic disorders [25], vascular injury [26,27], arthritis [28-30], cancer [11,31-33], kidneyfibrosis [16] and liver fibrosis [10]. Kallistatin gene delivery studies have shown significantly alleviated CCl4-induced oxidative stress and inflammatory responses, and reduced liver damage in mouse models [17]. In vitro, recombinant human kallistatin effectively inhibited myofibroblast-like activation of isolated rat HSCs and reduced the expression of α-SMA. In vivo, recombinant human kallistatin treatment significantly reduced the expression levels of TGF-β1 and α-SMA in the liver, and decreased the progression of fibrogenesis in a rat model [10]. Previous studies have demonstrated that kallistatin is an endogenous angiogenic inhibitor [9,33]. However, angiogenesis is the process of new capillary blood vessel growth from preexisting vasculature and is an important physiologic process during growth, tissue injury, repair, and healing. It has been elegantly demonstrated that capillary density is increased in IPF lungs in areas of minimal fibrosis or normal lung tissues, whereas it is decreased in the most extensive fibrotic lesions [34]. Fibroblastic foci in IPF tissues are devoid of blood vessels and are surrounded by disorganized capillaries.
Aberrant angiogenesis has been implicated in the development and progression of pulmonary fibrosis. Further evidence also strongly suggests a role for angiogenic vascular remodeling in pulmonary fibrosis, and emerging studies have indicated that angiogenesis is a central hallmark of the progression of IPF [35,36]. The presence of angiogenic chemokines in human IPF has been nicely described by Keane et al., who demonstrated that inhibition of inflammation and angiogenesis is an anti-fibrotic strategy in the model of bleomycin-induced pulmonary fibrosis [22,37].
Angiogenesis is regulated by a balance between the angiogenic and angiostatic regulators of blood vessel growth. VEGF is the principal angiogenic factor and is proven to be a proinflammatory and permeability-inducing factor in BLM-induced pulmonary fibrosis [35]. Based on this information, inhibition of the VEGF/VEGFR pathway in IPF may be protective against angiogenesis and fibrogenesis. Huang showed that kallistatin inhibits VEGF-mediated signaling by directly suppressing Flk-1 phosphorylation in HUVECs [11].
In our study, we found that the expression of VEGF and phosphorylated Flk-1 apparently decreased after kallistatin administration in the BLM-induced lung fibrosis model. Meanwhile, exacerbated MVD was significantly reduced. This finding supports a contribution of VEGF to the fibrotic process via angiogenesis induction [17]. Furthermore, transient protein leakage following BLM can aggravate the initial injury and thereby promote fibrogenesis. VEGF is a major enhancer of vascular permeability, and our study showed that kallistatin down-regulated the VEGF-related protein permeability index.
Prior to this study the main focus of research into the biological effects of kallistatin was on endothelial cells in angiogenesis and cancer biology [32,33,38]. The actions of kallistatin on endothelial cells has variously been shown to reduce migration, spreading and induce apoptosis. Moreover, for the first time, this study described the actions of kallistatin on primary bronchial epithelial cells.
In conclusion, this study demonstrated that elevated kallistatin levels in IPF rats can ameliorate BLM-induced pulmonary fibrosis but could not completely prevent the onset of this condition. Our findings elucidated that kallistatin blocked VEGF/Flk-1 signaling and diminished ERK1/2 and p38MAPK phosphorylation, and inhibited the increased extent of MVD. Moreover, kallistatin decreased TNF-α, IL-1β and TGF-β1 production, leukocyte trafficking and protein leakage. Finally, kallistatin levels correlated with the degree of impaired lung function and levels of inflammatory mediators that are associated with angiogenesis. Further investigation should be promoted to explore novel treatment strategies in patients with pulmonary fibrotic diseases.
Acknowledgements
This research is supported by National Natural Science Foundation of China (81502687, 81371669, 31302190, 81270734), Natural Science Foundation of Fujian Province in China (2017J01548); Project of Science and Technology of Quanzhou (2016Z070, 2016N006); Key laboratory for the Chemistry and Molecular Engineering of Medicinal Resources, Ministry of Education of China (No.CMEMR-2015-B06).
Disclosure of conflict of interest
None.
Abbreviations
- IPF
idiopathic pulmonary fibrosis
- BALF
bronchoalveolar lavage fluids
- BLM
bleomycin
- MVD
microvascular density
- PBECs
Primary Bronchial Epithelial Cells
- TGF-β1
transforming growth factor-β1
- TNF-α
tumor necrosis factor-α
- VEGFR
vascular endothelial growth factor
- VEGF
vascular endothelial growth factor
- α-SMA
α-smooth-muscle-actin
References
- 1.Xia H, Diebold D, Nho R, Perlman D, Kleidon J, Kahm J, Avdulov S, Peterson M, Nerva J, Bitterman P, Henke C. Pathological integrin signaling enhances proliferation of primary lung fibroblasts from patients with idiopathic pulmonary fibrosis. J Exp Med. 2008;205:1659–1672. doi: 10.1084/jem.20080001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Selman M, Pardo A. Idiopathic pulmonary fibrosis: an epithelial/fibroblastic cross-talk disorder. Respir Res. 2002;3:3. doi: 10.1186/rr175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aono Y, Kishi M, Yokota Y, Azuma M, Kinoshita K, Takezaki A, Sato S, Kawano H, Kishi J, Goto H, Uehara H, Izumi K, Nishioka Y. Role of platelet-derived growth factor/platelet-derived growth factor receptor axis in the trafficking of circulating fibrocytes in pulmonary fibrosis. Am J Respir Cell Mol Biol. 2014;51:793–801. doi: 10.1165/rcmb.2013-0455OC. [DOI] [PubMed] [Google Scholar]
- 4.Nishioka Y, Azuma M, Kishi M, Aono Y. Targeting platelet-derived growth factor as a therapeutic approach in pulmonary fibrosis. J Med Invest. 2013;60:175–183. doi: 10.2152/jmi.60.175. [DOI] [PubMed] [Google Scholar]
- 5.Iyer AK, Ramesh V, Castro CA, Kaushik V, Kulkarni YM, Wright CA, Venkatadri R, Rojanasakul Y, Azad N. Nitric oxide mediates bleomycin-induced angiogenesis and pulmonary fibrosis via regulation of VEGF. J Cell Biochem. 2015;116:2484–2493. doi: 10.1002/jcb.25192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antoniou KM, Soufla G, Proklou A, Margaritopoulos G, Choulaki C, Lymbouridou R, Samara KD, Spandidos DA, Siafakas NM. Different activity of the biological axis VEGF-Flt-1 (fms-like tyrosine kinase 1) and CXC chemokines between pulmonary sarcoidosis and idiopathic pulmonary fibrosis: a bronchoalveolar lavage study. Clin Dev Immunol. 2009;2009:537929. doi: 10.1155/2009/537929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farkas L, Farkas D, Ask K, Moller A, Gauldie J, Margetts P, Inman M, Kolb M. VEGF ameliorates pulmonary hypertension through inhibition of endothelial apoptosis in experimental lung fibrosis in rats. J Clin Invest. 2009;119:1298–1311. doi: 10.1172/JCI36136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chao J, Schmaier A, Chen LM, Yang Z, Chao L. Kallistatin, a novel human tissue kallikrein inhibitor: levels in body fluids, blood cells, and tissues in health and disease. J Lab Clin Med. 1996;127:612–620. doi: 10.1016/s0022-2143(96)90152-3. [DOI] [PubMed] [Google Scholar]
- 9.Miao RQ, Agata J, Chao L, Chao J. Kallistatin is a new inhibitor of angiogenesis and tumor growth. Blood. 2002;100:3245–3252. doi: 10.1182/blood-2002-01-0185. [DOI] [PubMed] [Google Scholar]
- 10.Huang X, Wang X, Lv Y, Xu L, Lin J, Diao Y. Protection effect of kallistatin on carbon tetrachloride-induced liver fibrosis in rats via antioxidative stress. PLoS One. 2014;9:e88498. doi: 10.1371/journal.pone.0088498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang KF, Yang HY, Xing YM, Lin JS, Diao Y. Recombinant human kallistatin inhibits angiogenesis by blocking VEGF signaling pathway. J Cell Biochem. 2014;115:575–584. doi: 10.1002/jcb.24693. [DOI] [PubMed] [Google Scholar]
- 12.Shen B, Smith RS Jr, Hsu YT, Chao L, Chao J. Kruppel-like factor 4 is a novel mediator of Kallistatin in inhibiting endothelial inflammation via increased endothelial nitric-oxide synthase expression. J Biol Chem. 2009;284:35471–35478. doi: 10.1074/jbc.M109.046813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin WC, Lu SL, Lin CF, Chen CW, Chao L, Chao J, Lin YS. Plasma kallistatin levels in patients with severe community-acquired pneumonia. Crit Care. 2013;17:R27. doi: 10.1186/cc12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chao J, Yin H, Yao YY, Shen B, Smith RS Jr, Chao L. Novel role of kallistatin in protection against myocardial ischemia-reperfusion injury by preventing apoptosis and inflammation. Hum Gene Ther. 2006;17:1201–1213. doi: 10.1089/hum.2006.17.1201. [DOI] [PubMed] [Google Scholar]
- 15.Gao L, Yin H, S Smith R Jr, Chao L, Chao J. Role of kallistatin in prevention of cardiac remodeling after chronic myocardial infarction. Lab Invest. 2008;88:1157–1166. doi: 10.1038/labinvest.2008.85. [DOI] [PubMed] [Google Scholar]
- 16.Shen B, Hagiwara M, Yao YY, Chao L, Chao J. Salutary effect of kallistatin in salt-induced renal injury, inflammation, and fibrosis via antioxidative stress. Hypertension. 2008;51:1358–1365. doi: 10.1161/HYPERTENSIONAHA.107.108514. [DOI] [PubMed] [Google Scholar]
- 17.Diao Y, Zhao XF, Lin JS, Wang QZ, Xu RA. Protection of the liver against CCl4-induced injury by intramuscular electrotransfer of a kallistatin-encoding plasmid. World J Gastroenterol. 2011;17:111–117. doi: 10.3748/wjg.v17.i1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cahill EF, Kennelly H, Carty F, Mahon BP, English K. Hepatocyte growth factor is required for mesenchymal stromal cell protection against bleomycin-induced pulmonary fibrosis. Stem Cells Transl Med. 2016;5:1307–1318. doi: 10.5966/sctm.2015-0337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perkins GD, Chatterjie S, McAuley DF, Gao F, Thickett DR. Role of nonbronchoscopic lavage for investigating alveolar inflammation and permeability in acute respiratory distress syndrome. Crit Care Med. 2006;34:57–64. doi: 10.1097/01.ccm.0000190197.69945.c5. [DOI] [PubMed] [Google Scholar]
- 20.Chung EJ, McKay-Corkum G, Chung S, White A, Scroggins BT, Mitchell JB, Mulligan-Kehoe MJ, Citrin D. Truncated plasminogen activator inhibitor-1 protein protects from pulmonary fibrosis mediated by irradiation in a murine model. Int J Radiat Oncol Biol Phys. 2016;94:1163–1172. doi: 10.1016/j.ijrobp.2015.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Usuki J, Fukuda Y. Evolution of three patterns of intra-alveolar fibrosis produced by bleomycin in rats. Pathol Int. 1995;45:552–564. doi: 10.1111/j.1440-1827.1995.tb03503.x. [DOI] [PubMed] [Google Scholar]
- 22.Keane MP, Belperio JA, Arenberg DA, Burdick MD, Xu ZJ, Xue YY, Strieter RM. IFN-gamma-inducible protein-10 attenuates bleomycin-induced pulmonary fibrosis via inhibition of angiogenesis. J Immunol. 1999;163:5686–5692. [PubMed] [Google Scholar]
- 23.Burdick MD, Murray LA, Keane MP, Xue YY, Zisman DA, Belperio JA, Strieter RM. CXCL11 attenuates bleomycin-induced pulmonary fibrosis via inhibition of vascular remodeling. Am J Respir Crit Care Med. 2005;171:261–268. doi: 10.1164/rccm.200409-1164OC. [DOI] [PubMed] [Google Scholar]
- 24.Ou XM, Li WC, Liu DS, Li YP, Wen FQ, Feng YL, Zhang SF, Huang XY, Wang T, Wang K, Wang X, Chen L. VEGFR-2 antagonist SU5416 attenuates bleomycin-induced pulmonary fibrosis in mice. Int Immunopharmacol. 2009;9:70–79. doi: 10.1016/j.intimp.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Zhu H, Chao J, Kotak I, Guo D, Parikh SJ, Bhagatwala J, Dong Y, Patel SY, Houk C, Chao L. Plasma kallistatin is associated with adiposity and cardiometabolic risk in apparently healthy African American adolescents. Metabolism. 2013;62:642–646. doi: 10.1016/j.metabol.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu Y, Bledsoe G, Hagiwara M, Shen B, Chao L, Chao J. Depletion of endogenous kallistatin exacerbates renal and cardiovascular oxidative stress, inflammation, and organ remodeling. Am J Physiol Renal Physiol. 2012;303:F1230–1238. doi: 10.1152/ajprenal.00257.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin H, Gao L, Shen B, Chao L, Chao J. Kallistatin inhibits vascular inflammation by antagonizing tumor necrosis factor-alpha-induced nuclear factor kappaB activation. Hypertension. 2010;56:260–267. doi: 10.1161/HYPERTENSIONAHA.110.152330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang CR, Chen SY, Wu CL, Liu MF, Jin YT, Chao L, Chao J. Prophylactic adenovirus-mediated human kallistatin gene therapy suppresses rat arthritis by inhibiting angiogenesis and inflammation. Arthritis Rheum. 2005;52:1319–1324. doi: 10.1002/art.20991. [DOI] [PubMed] [Google Scholar]
- 29.Hsieh JL, Shen PC, Shiau AL, Jou IM, Lee CH, Teo ML, Wang CR, Chao J, Chao L, Wu CL. Adenovirus-mediated kallistatin gene transfer ameliorates disease progression in a rat model of osteoarthritis induced by anterior cruciate ligament transection. Hum Gene Ther. 2009;20:147–158. doi: 10.1089/hum.2008.096. [DOI] [PubMed] [Google Scholar]
- 30.Wang CR, Chen SY, Shiau AL, Wu CL, Jou IM, Chao L, Chao J. Upregulation of kallistatin expression in rheumatoid joints. J Rheumatol. 2007;34:2171–2176. [PubMed] [Google Scholar]
- 31.Miao RQ, Chen V, Chao L, Chao J. Structural elements of kallistatin required for inhibition of angiogenesis. Am J Physiol Cell Physiol. 2003;284:C1604–1613. doi: 10.1152/ajpcell.00524.2002. [DOI] [PubMed] [Google Scholar]
- 32.Diao Y, Ma J, Xiao WD, Luo J, Li XY, Chu KW, Fung P, Habib N, Farzaneh F, Xu RA. Inhibition of angiogenesis and HCT-116 xenograft tumor growth in mice by kallistatin. World J Gastroenterol. 2007;13:4615–4619. doi: 10.3748/wjg.v13.i34.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang KF, Huang XP, Xiao GQ, Yang HY, Lin JS, Diao Y. Kallistatin, a novel anti-angiogenesis agent, inhibits angiogenesis via inhibition of the NF-kappaB signaling pathway. Biomed Pharmacother. 2014;68:455–461. doi: 10.1016/j.biopha.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 34.Ebina M, Shimizukawa M, Shibata N, Kimura Y, Suzuki T, Endo M, Sasano H, Kondo T, Nukiwa T. Heterogeneous increase in CD34-positive alveolar capillaries in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2004;169:1203–1208. doi: 10.1164/rccm.200308-1111OC. [DOI] [PubMed] [Google Scholar]
- 35.Selman M, Pardo A, Richeldi L, Cerri S. Emerging drugs for idiopathic pulmonary fibrosis. Expert Opin Emerg Drugs. 2011;16:341–362. doi: 10.1517/14728214.2011.565049. [DOI] [PubMed] [Google Scholar]
- 36.Cao B, Guo Z, Zhu Y, Xu W. The potential role of PDGF, IGF-1, TGF-beta expression in idiopathic pulmonary fibrosis. Chin Med J (Engl) 2000;113:776–782. [PubMed] [Google Scholar]
- 37.Keane MP, Belperio JA, Moore TA, Moore BB, Arenberg DA, Smith RE, Burdick MD, Kunkel SL, Strieter RM. Neutralization of the CXC chemokine, macrophage inflammatory protein-2, attenuates bleomycin-induced pulmonary fibrosis. J Immunol. 1999;162:5511–5518. [PubMed] [Google Scholar]
- 38.Jiang X, Li H, Qiao H, Jiang H, Xu R, Sun X. Combining kallistatin gene therapy and meloxicam to treat hepatocellular carcinoma in mice. Cancer Sci. 2009;100:2226–2233. doi: 10.1111/j.1349-7006.2009.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


